Abstract
Purpose
To investigate the correlation between corneal densitometry, corneal topographic parameters, and corneal biomechanical properties in keratoconus.
Methods
A total of 76 eyes of 76 keratoconus patients were enrolled in this cross-sectional study. Corneal densitometry and topography were measured using Pentacam HR. Corneal biomechanical properties were measured using CorVis ST.
Results
The corneal densitometry values of the anterior 0 to 2 and 2 to 6 mm layers significantly correlated with the maximum keratometry values (R = 0.373, P = 0.001 and R = 0.276, P = 0.016, respectively), thinnest corneal thickness values (R = −0.331, P = 0.003 and R = −0.234, P = 0.042, respectively), anterior corneal elevation (R = 0.392, P < 0.001 and R = 0.323, P = 0.004, respectively), and posterior corneal elevation (R = 0.450, P < 0.001 and R = 0.367, P = 0.001, respectively). The stiffness parameter-applanation time 1 (SP-A1) significantly correlated with the corneal densitometry values for the anterior 0 to 2 mm (R = −0.397, P < 0.001), anterior 2 to 6 mm (R = −0.331, P = 0.004), central 0 to 2 mm (R = −0.306, P = 0.007), central 2 to 6 mm (R = −0.228, P = 0.048), posterior 2 to 6 mm (R = −0.243, P = 0.035), total 0 to 2 mm (R = −0.291, P = 0.011), and total 2 to 6 mm (R = −0.295, P = 0.010) layers.
Conclusions
The corneal densitometry values correlated with the severity of keratoconus and the SP-A1 values.
Translational Relevance
Corneal densitometry values may serve as markers to predict the severity of keratoconus.
Keywords: biomechanics, corneal structure, CorVis ST, keratoconus, Pentacam HR
Introduction
Diagnosis of keratoconus remains a challenge and a significant area of interest. Corneal tomography remains the diagnostic modality of choice. The sensitivity and specificity of screening for keratoconus has improved significantly with the advances in corneal imaging.1–3 Pentacam HR (Oculus, Wetzlar, Germany), an anterior segment analyzer, is based on the Scheimpflug principle. The attached Scheimpflug camera rotates 2.5 circuits to yield 25 tomographic images. Subsequently, it reconstructs the anterior segment of the target eye, thereby yielding measurements of the dimensions of the anterior segment. Pentacam HR also performs corneal densitometry by evaluating gray scale units, which reflect the corneal transparency (range, 0–100).4 Corneal Visualization Scheimpflug Technology (CorVis ST) also uses a high-speed Scheimpflug camera and provides a series of deformation parameters, such as applanation time, applanation length, applanation velocity, deformation and deflection amplitude, peak distance, stiffness parameter-applanation time 1 (SP-A1), Corvis Biomechanical index, and tomography and biomechanical index.5,6 These parameters serve as markers for the corneal biomechanical properties. Studies have indicated significant differences in the corneal biomechanical parameters between keratoconic and normal eyes.7–9 A Corvis biomechanical index of >0.50 is able to classify 98.8% of the cases of keratoconus correctly with a sensitivity of 98.4% and specificity of 100%.10 A tomography and biomechanical index of >0.79 has 100% sensitivity and specificity for detecting clinical ectasia.6,11 The extent of decrease in the biomechanical strength correlates with the severity of keratoconus.12 Likewise, the corneal densitometry has been shown to be significantly increased in keratoconic eyes.4
We investigated the correlations between the corneal densitometry and biomechanics in patients with keratoconus.
Subjects and Methods
In this cross-sectional study, keratoconus was diagnosed using an anterior segment analyzer (Pentacam HR; Oculus) based on the Amsler-Krumeich grading system. Patients with keratoconus of stages 1 to 3 or forme fruste keratoconus (cornea with no abnormal findings on slit-lamp examinations and corneal topography, with keratoconus of the fellow eye) were enrolled from the Eye and ENT Hospital of Fudan University. In total, 76 eyes of 76 participants (50 men, 26 women; mean age, 23.93 ± 6.81 years) were included. This study adhered to the tenets of the Declaration of Helsinki and was approved by the ethics committee of the hospital. Informed consent was obtained from all the participants.
Ophthalmologic Examination
Each patient underwent corneal tomography examination using the anterior segment analyzer Pentacam HR. The corneal biomechanical parameters were assessed using CorVis ST (Oculus). All measurements were obtained by a single examiner (YS). The corneal tomography images were acquired in the sitting position. Participants were required to keep their eyes wide open and to place their chins on the chin rest during the examination. The examiner maneuvered the joystick based on the image on the monitor. When the camera was aimed at the corneal apex, the images were captured automatically.
Statistical Analyses
Statistical analyses were performed using SPSS Version 20 (IBM, Armonk, NY). All data were tested for normality using the Kolmogorov-Smirnov test. A mixed linear model with Bonferroni-adjusted post hoc comparisons was used to analyze the differences in the corneal densitometry values in different locations. Pearson's correlation tests were performed to examine the correlations between the scale values, which fit a normal distribution. Spearman's correlation tests were used to determine the correlations between data with a skewed distribution or ranked ordinal data. P < 0.05 was considered statistically significant.
Results
The corneal densitometry values over the annulus of 2 to 6 mm followed a skewed distribution. The mean corneal densitometry values of each layer over the 0 to 2 and 2 to 6 mm annulus are listed in Tables 1 and 2. The main corneal tomographic data and corneal deformation parameters are listed in Tables 3 and 4, respectively.
Table 1.
Mean Corneal Densitometry Values of Anterior, Central, Posteriorm and Total Layers Over Annuli of 0 to 2 mm
|
Location, ϕ 0–2 mm |
Mean |
Standard Deviation |
Range |
| Anterior layer | 20.85 | 3.02 | 15.80–31.50 |
| Center layer | 13.14 | 1.61 | 10.60–18.00 |
| Posterior layer | 10.43 | 1.66 | 6.90–14.50 |
| Total cornea | 14.81 | 1.85 | 12.40–21.30 |
ϕ, annulus; mm, millimeter.
Table 2.
Mean Corneal Densitometry Values of Anterior, Central, Posterior, and Total Layers Over Annuli of 2 to 6 mm
|
Location, ϕ 2–6 mm |
Mean |
Standard Deviation |
Range |
| Anterior layer | 18.00 | 2.28 | 15.00–26.80 |
| Center layer | 11.58 | 1.45 | 9.40–15.80 |
| Posterior layer | 10.46 | 1.43 | 8.30–14.30 |
| Total cornea | 13.34 | 1.60 | 11.10–18.90 |
Table 3.
Corneal Tomographic Parameters of Study Participants Using Pentacam HR
|
Parameters |
Mean |
Standard Deviation |
Range |
| K1 (D) | 46.63 | 5.06 | 39.60–59.80 |
| K2 (D) | 49.98 | 6.08 | 40.90–68.00 |
| Km (D) | 48.15 | 5.32 | 40.30–63.70 |
| Kmax (D) | 56.25 | 9.41 | 41.80–88.10 |
| CCT (μm) | 495.3 | 37.7 | 413.0–604.0 |
| TCT (μm) | 477.0 | 44.0 | 369.0–583.0 |
| ACE (μm) | 23.9 | 15.3 | 0.0–76.0 |
| PCE (μm) | 50.1 | 28.9 | 0.0–150.0 |
K1, Flat Keratometry; K2, Steep Keratometry; Km, Mean Keratometry; CCT, Central Corneal Thickness; PCE, Posterior Central Elevation; D, diopter; μm, micron.
Table 4.
Corneal Deformation Parameters of Study Participants Measured on Corvis ST
|
Deformation Parameters |
Mean |
Standard Deviation |
Range |
| AT1 (ms) | 6.88 | 0.30 | 6.29–7.71 |
| ATh (ms) | 16.61 | 0.53 | 15.25–17.79 |
| AT2 (ms) | 21.80 | 0.42 | 20.65–22.56 |
| AL1 (mm) | 1.88 | 0.29 | 1.24–2.56 |
| AL2 (mm) | 1.59 | 0.40 | 0.63–2.15 |
| AV1 (m/s) | 0.17 | 0.03 | 0.11–0.24 |
| AV2 (m/s) | −0.29 | 0.05 | −0.44 to −0.19 |
| IOP (mmHg) | 14.11 | 2.68 | 4.77–21.00 |
| bIOP (mmHg) | 15.29 | 2.63 | 5.41–22.80 |
| PD (mm) | 4.99 | 0.26 | 4.06–5.57 |
| R (mm) | 5.16 | 0.77 | 2.90–6.68 |
| DA (mm) | 1.16 | 0.12 | 0.87–1.54 |
| CCT (μm) | 494.8 | 37.1 | 415–614 |
| DeA (mm) | 1.02 | 0.12 | 0.66–1.37 |
| DAR | 5.66 | 1.35 | 3.60–13.00 |
| IR | 12.47 | 2.17 | 8.90–18.40 |
| ARTh | 228.91 | 79.85 | 83.00–421.50 |
| SP-A1 | 61.14 | 20.21 | 15.90–123.50 |
| BAD-D | 8.03 | 4.87 | 0.05–23.67 |
| CBI | 0.93 | 0.18 | 0.00–1.00 |
| TBI | 0.94 | 0.20 | 0.01–1.00 |
AT1, Applanation Time (time 1); ATh, Applanation Time (highest ); AT2, Applanation Time (time 2); AL1, Applanation Length (time 1); AL2, Applanation Length (time 2); AV1, Applanation Velocity (time 1); AV2, Applanation Velocity (time 2); IOP, intraocular pressure; bIOP, biomechanically corrected IOP; PD, peak distance; R, radius of concave curvature; DA, deformation amplitude; CCT, central corneal thickness; DeA, deflection amplitude; IR, integrated radius; ARTh, Ambrósio's relational thickness; SP-A1, stiffness parameter at first applanation; BAD-D, Belin/Ambrósio enhanced ectasia display; CBI, Corvis biomechanical index; TBI, tomography and biomechanical index); ms, millisecond; mm, millimeter; m/s, meter per second; mmHg, millimeters of mercury (1mmHg, 133.32 Pascals).
Corneal Densitometry
A significant difference in the distribution of the densitometric values (Fig. 1) was detected between the anterior (first 120 μm of the corneal thickness) and posterior (last 60 μm of the corneal thickness) layers over the 0 to 2 and 2 to 6 mm annulus (F = 308.258, P < 0.001). Bonferroni post hoc comparisons revealed that the corneal densitometry value of the anterior cornea over the 0 to 2 mm annulus (anterior 0–2 mm layer) was significantly higher (post hoc P < 0.001) than that over the 2 to 6 mm annulus (anterior 2–6 mm layer).
Figure 1.
The densitometric distribution from the anterior to the posterior layers over the annuli of 0 to 2 and 2 to 6 mm of a cornea with keratoconus.
Correlation Between the Densitometric and Tomographic Parameters
Figure 2A demonstrates that the maximum keratometry (Kmax) values significantly correlated with the densitometry values obtained from the anterior layer over the 0 to 2 mm (R = 0.373, P = 0.001) and 2 to 6 mm (R = 0.276, P = 0.016) annulus, while Figure 2B shows that the thinnest corneal thickness (TCT) values significantly correlated with the densitometry values of the anterior 0 to 2 mm (R = −0.331, P = 0.003), anterior 2 to 6 mm (R = −0.234, P = 0.042), central 0 to 2 mm (R = −0.256, P = 0.026), and total 0 to 2 mm (R = −0.230, P = 0.045) layers. Moreover, Figure 2C indicates that the anterior corneal elevation (ACE) values remarkably correlated with the densitometry values of the anterior 0 to 2 mm (R = 0.392, P < 0.001), anterior 2 to 6 mm (R = 0.323, P = 0.004), central 0 to 2 mm (R = 0.232, P = 0.043), total 0 to 2 mm (R = 0.244, P = 0.033), and total 2 to 6 mm (R = 0.241, P = 0.036) layers. In addition, Figure 2D illustrates that the posterior corneal elevation (PCE) values considerably correlated with the densitometry values of the anterior 0 to 2 mm (R = 0.450, P < 0.001), anterior 2 to 6 mm (R = 0.323, P = 0.004), total 0 to 2 mm (R = 0.260, P = 0.023), and total 2 to 6 mm (R = 0.267, P = 0.020) layers.
Figure 2.
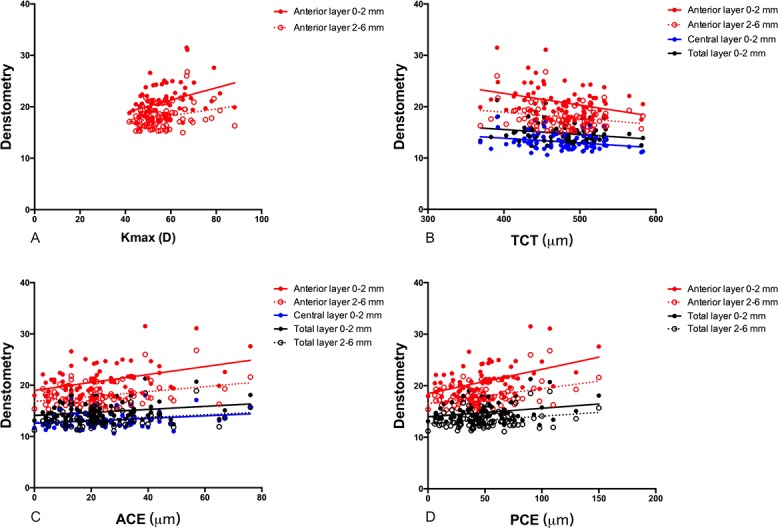
(A) The correlations between the Kmax and corneal densitometry values obtained in the anterior layer over the annuli of 0 to 2 and 2 to 6 mm (R = 0.373, P = 0.001, and R = 0.276, P = 0.016, respectively). (B) The correlations between the thinnest corneal thickness values and the corneal densitometry values of the anterior 0 to 2 mm (R = −0.331, P = 0.003), anterior 2 to 6 mm (R = −0.234, P = 0.042), central 0 to 2 mm (−0.256, P = 0.026), and total 0 to 2 mm (R = −0.230, P = 0.045) layers. (C) The correlations between the anterior corneal elevation values and the corneal densitometry values of anterior 0 to 2 mm (R = 0.392, P < 0.001), anterior 2-6mm (R = 0.323, P = 0.004), central 0 to 2 mm (R = 0.232, P = 0.043), total 0 to 2 mm (R = 0.244, P = 0.033), and total 2 to 6 mm (R = 0.241, P = 0.036) layers. (D) The correlations between the posterior corneal elevation values and the corneal densitometry values of anterior 0 to 2 mm (R = 0.450, P < 0.001), anterior 2 to 6 mm (R = 0.323, P = 0.004), total 0 to 2 mm (R = 0.260, P = 0.023), and total 2 to 6 mm (R = 0.267, P = 0.020) layers.
Correlation Between the Corneal Densitometry and Corneal Biomechanical Properties
The SP-A1 value significantly correlated with the densitometry values of the anterior 0 to 2 mm (R = −0.397, P < 0.001), anterior 2 to 6 mm (R = −0.331, P = 0.004), central 0 to 2 mm (R = −0.306, P = 0.007), central 2 to 6 mm (R = −0.228, P = 0.048), posterior 2 to 6 mm (R = −0.243, R = 0.035), total 0 to 2 mm (R = −0.291, P = 0.011), and total 2 to 6 mm (R = −0.295, P = 0.010) layers (Fig. 3).
Figure 3.
The correlations between the stiffness parameter-applanation time 1 values and the corneal densitometry values obtained in the anterior layer over the annuli of 0 to 2 and 2 to 6 mm, the central layer over the annuli of 0 to 2 and 2 to 6 mm, the posterior layer over the annuli of 2 to 6 mm, and the total layer over the annuli of 0 to 2 and 2 to 6 mm (anterior 0–2 mm, R = −0.397, P < 0.001; anterior layer 2–6 mm, R = −0.331, P = 0.004; central layer 0–2 mm, R = −0.306, P = 0.007; central layer 2–6 mm, R = −0.228, P = 0.048; posterior layer 2–6 mm, R = −0.243, R = 0.035; total layer 0–2 mm, R = −0.291, P = 0.011, and total layer 2–6 mm, R = −0.295, P = 0.010, respectively).
Discussion
Corneal densitometry, which also is known as corneal backscatter, relates to corneal transparency and is influenced by changes in corneal histology.13 It was first measured using a slit-lamp photometer with a pin-light attachment.14 Scheimpflug cameras allow for objective evaluation of the densitometry.15 It is noteworthy that for normal eyes, the corneal densitometry decreases from the anterior to posterior layers of the cornea. However, it does not show any relationship with the corneal keratometry.16 We observed that the distribution of the corneal densitometry values was similar to that of normal eyes. However, unlike normal eyes, the densitometry values of the anterior 0 to 2 and 2 to 6 mm layers significantly correlated with the Kmax values.16 In addition, we also noticed that the densitometry values of the anterior 0 to 2 mm, anterior 2 to 6 mm, and total 0 to 2 mm layers correlated with the thinnest corneal thickness, anterior corneal elevation, and posterior corneal elevation. This indicated that the severity of keratoconus may be correlated with the elevation of the corneal densitometry values, especially in the anterior layer. Elevated corneal densitometry also has been reported in the pathogenesis of various ocular surface disorders, which may compromise the corneal transparency, including keratitis,17 endothelial abnormality,18 and pseudoexfoliation syndrome.19 Misalignment of the corneal collagen has been noted in keratoconus.4 Further, periodic acid−Schiff-positive nodules, Z-shaped cracks caused by ruptures in the Bowman's layer,20 and wound healing reactions, which triggers fibronectin degeneration in the extracellular matrix,21 may be the key causes related to the compromised corneal transparency, leading to an increase in the densitometry values.
SP-A1 is a parameter related to corneal rigidity. It is defined as the ratio of the pressure loading (imposed by the air pulse) on the cornea to the displacement of the corneal apex (from the undeformed state to the first applanation). The SP-A1 value has been reported to be lower in thin than in normal corneas.10 In our study, the SP-A1 values were negatively correlated with the corneal densitometry values. This implies that, among patients with keratoconus, increased corneal densitometry values may indicate compromised corneal stiffness. Molecular biology studies have reported that enzyme activation has a key role in the degradation of the corneal stroma and in corneal thinning, thus affecting corneal stiffness.22 An increased anterior and posterior surface elevation at the thinnest point of the cornea leads to the formation of a cone as well as an increase in the corneal keratometry.23,24 The progressive increase in corneal irregularities, decrease in corneal thickness, and steeping of corneal curvature might underlie the correlations between corneal densitometry, SP-A1, and Kmax.
The limitations of our study are as follows. Firstly, the sample size was small. Secondly, a comparative group with normal eyes was absent. However, our main purpose was to investigate the potential correlations between the densitometric and biomechanical parameters in keratoconic eyes rather than to compare the difference in the densitometry values between patients with keratoconus and the normal population. Thirdly, the randomly enrolled patients with keratoconus were not classified based on the location of the cone apex. This might have affected the distribution of the corneal densitometry over the entire cornea. Further studies are needed to determine the difference in distribution of the corneal densitometry among patients with keratoconus with different types of cones.
In conclusion, we showed that the corneal densitometry values may correlate with the severity of keratoconus and the SP-A1 values in keratoconus eyes. The increased corneal densitometry values may allow the compromised corneal biomechanics in keratoconus to be predicted.
Acknowledgments
Supported in part by the National Natural Science Foundation of China for Young Scholars (Grants 81600762 and 81500753), the National Natural Science Foundation of China (Grants 81570879 and 81770955), and the Project of Shanghai Science and Technology (Grants 17411950200 and 17411950201).
Disclosure: Y. Shen, None; T. Han, None; V. Jhanji, None; J. Shang, None; J. Zhao, None; M. Li, None; X. Zhou, None
References
- 1.de Sanctis U, Loiacono C, Richiardi L, Turco D, Mutani B, Grignolo FM. Sensitivity and specificity of posterior corneal elevation measured by Pentacam in discriminating keratoconus/subclinical keratoconus. Ophthalmology. 2008;115:1534–1539. doi: 10.1016/j.ophtha.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Swartz T, Marten L, Wang M. Measuring the cornea: the latest developments in corneal topography. Curr Opin Ophthalmol. 2007;18:325–333. doi: 10.1097/ICU.0b013e3281ca7121. [DOI] [PubMed] [Google Scholar]
- 3.Tejwani S, Shetty R, Kurien M, Dinakaran S, Ghosh A, Sinha Roy A. Biomechanics of the cornea evaluated by spectral analysis of waveforms from ocular response analyzer and Corvis-ST. PLoS One. 2014;9:e97591. doi: 10.1371/journal.pone.0097591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen Y, Jian W, Sun L, et al. One-year follow-up of changes in corneal densitometry after accelerated (45 mW/cm2) transepithelial corneal collagen cross-linking for keratoconus: a retrospective study. Cornea. 2016;35:1434–1440. doi: 10.1097/ICO.0000000000000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen Y, Chen Z, Knorz MC, Li M, Zhao J, Zhou X. Comparison of corneal deformation parameters after SMILE, LASEK, and femtosecond laser-assisted LASIK. J Refract Surg. 2014;30:310318. doi: 10.3928/1081597X-20140422-01. [DOI] [PubMed] [Google Scholar]
- 6.Ambrosio R, Jr, Lopes BT, Faria-Correia F, et al. Integration of Scheimpflug-based corneal tomography and biomechanical assessments for enhancing ectasia detection. J Refract Surg. 2017;33:434–443. doi: 10.3928/1081597X-20170426-02. [DOI] [PubMed] [Google Scholar]
- 7.Tian L, Huang YF, Wang LQ, et al. Corneal biomechanical assessment using corneal visualization scheimpflug technology in keratoconic and normal eyes. J Ophthalmol. 2014;2014:147516. doi: 10.1155/2014/147516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopes B, Ramos I, Ambrosio R., Jr Corneal densitometry in keratoconus. Cornea. 2014;33:1282–1286. doi: 10.1097/ICO.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg J, Katz T, Lucke K, Frings A, Druchkiv V, Linke SJ. Screening for keratoconus with new dynamic biomechanical in vivo Scheimpflug analyses. Cornea. 2015;34:1404–1412. doi: 10.1097/ICO.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 10.Vinciguerra R, Ambrosio R, Jr, Elsheikh A, et al. Detection of keratoconus with a new biomechanical index. J Refract Surg. 2016;32:803–810. doi: 10.3928/1081597X-20160629-01. [DOI] [PubMed] [Google Scholar]
- 11.Nash IS, Greene PR, Foster CS. Comparison of mechanical properties of keratoconus and normal corneas. Exp Eye Res. 1982;35:413–424. doi: 10.1016/0014-4835(82)90040-9. [DOI] [PubMed] [Google Scholar]
- 12.Johnson RD, Nguyen MT, Lee N, Hamilton DR. Corneal biomechanical properties in normal, forme fruste keratoconus, and manifest keratoconus after statistical correction for potentially confounding factors. Cornea. 2011;30:516–523. doi: 10.1097/ICO.0b013e3181f0579e. [DOI] [PubMed] [Google Scholar]
- 13.Jester JV, Moller-Pedersen T, Huang J, et al. The cellular basis of corneal transparency: evidence for ‘corneal crystallins'. J Cell Sci. 1999;112:613–622. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- 14.Olsen T. Light scattering from the human cornea. Invest Ophthalmol Vis Sci. 1982;23:81–86. [PubMed] [Google Scholar]
- 15.Smith GT, Brown NA, Shun-Shin GA. Light scatter from the central human cornea. Eye (Lond) 1990;4:584–588. doi: 10.1038/eye.1990.81. [DOI] [PubMed] [Google Scholar]
- 16.Garzon N, Poyales F, Illarramendi I, et al. Corneal densitometry and its correlation with age, pachymetry, corneal curvature, and refraction. Int Ophthalmol. 2017;37:1263–1268. doi: 10.1007/s10792-016-0397-y. [DOI] [PubMed] [Google Scholar]
- 17.Otri AM, Fares U, Al-Aqaba MA, Dua HS. Corneal densitometry as an indicator of corneal health. Ophthalmology. 2012;119:501–508. doi: 10.1016/j.ophtha.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 18.Tekin K, Sekeroglu MA, Kiziltoprak H, Yilmazbas P. Corneal densitometry in healthy corneas and its correlation with endothelial morphometry. Cornea. 2017;36:1336–1342. doi: 10.1097/ICO.0000000000001363. [DOI] [PubMed] [Google Scholar]
- 19.Cankaya AB, Tekin K, Inanc M. Effect of pseudoexfoliation on corneal transparency. Cornea. 2016;35:1084–1088. doi: 10.1097/ICO.0000000000000852. [DOI] [PubMed] [Google Scholar]
- 20.Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28:293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 21.Kenney MC, Nesburn AB, Burgeson RE, Butkowski RJ, Ljubimov AV. Abnormalities of the extracellular matrix in keratoconus corneas. Cornea. 1997;16:345–351. [PubMed] [Google Scholar]
- 22.Kenney MC, Chwa M, Atilano SR, et al. Increased levels of catalase and cathepsin V/L2 but decreased TIMP-1 in keratoconus corneas: evidence that oxidative stress plays a role in this disorder. Invest Ophthalmol Vis Sci. 2005;46:823–832. doi: 10.1167/iovs.04-0549. [DOI] [PubMed] [Google Scholar]
- 23.Kenney MC, Chwa M, Opbroek AJ, Brown DJ. Increased gelatinolytic activity in keratoconus keratocyte cultures. A correlation to an altered matrix metalloproteinase-2/tissue inhibitor of metalloproteinase ratio. Cornea. 1994;13:114–124. doi: 10.1097/00003226-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Kao WW, Vergnes JP, Ebert J, Sundar-Raj CV, Brown SI. Increased collagenase and gelatinase activities in keratoconus. Biochem Biophys Res Commun. 1982;107:929–936. doi: 10.1016/0006-291x(82)90612-x. [DOI] [PubMed] [Google Scholar]




