Abstract
Background
Platinum‐based therapy, including cisplatin, carboplatin, oxaliplatin or a combination of these, is used to treat a variety of paediatric malignancies. One of the most significant adverse effects is the occurrence of hearing loss or ototoxicity. In an effort to prevent this ototoxicity, different otoprotective medical interventions have been studied. This review is the third update of a previously published Cochrane Review.
Objectives
To assess the efficacy of medical interventions to prevent hearing loss and to determine possible effects of these interventions on antitumour efficacy, toxicities other than hearing loss and quality of life in children with cancer treated with platinum‐based therapy as compared to placebo, no additional treatment or another protective medical intervention.
Search methods
We searched the Cochrane Central Register of Controlled Trials, MEDLINE (PubMed) and Embase (Ovid) to 8 January 2019. We handsearched reference lists of relevant articles and assessed the conference proceedings of the International Society for Paediatric Oncology (2006 up to and including 2018), the American Society of Pediatric Hematology/Oncology (2007 up to and including 2018) and the International Conference on Long‐Term Complications of Treatment of Children and Adolescents for Cancer (2010 up to and including 2015). We scanned ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch) for ongoing trials (on 2 January 2019).
Selection criteria
Randomized controlled trials (RCTs) or controlled clinical trials (CCTs) evaluating platinum‐based therapy with an otoprotective medical intervention versus platinum‐based therapy with placebo, no additional treatment or another protective medical intervention in children with cancer.
Data collection and analysis
Two review authors independently performed the study selection, data extraction, risk of bias assessment and GRADE assessment of included studies, including adverse effects. We performed analyses according to the Cochrane Handbook for Systematic Reviews of Interventions.
Main results
We identified two RCTs and one CCT (total number of participants 149) evaluating the use of amifostine versus no additional treatment in the original version of the review; the updates identified no additional studies. Two studies included children with osteosarcoma, and the other study included children with hepatoblastoma. Children received cisplatin only or a combination of cisplatin and carboplatin, either intra‐arterially or intravenously. Pooling of results of the included studies was not possible. From individual studies the effect of amifostine on symptomatic ototoxicity only (i.e. National Cancer Institute Common Toxicity Criteria version 2 (NCICTCv2) or modified Brock grade 2 or higher) and combined asymptomatic and symptomatic ototoxicity (i.e. NCICTCv2 or modified Brock grade 1 or higher) were uncertain (low‐certainty evidence). Only one study including children with osteosarcoma treated with intra‐arterial cisplatin provided information on tumour response, defined as the number of participants with a good or partial remission. The available‐data analysis (data were missing for one participant), best‐case scenario analysis and worst‐case scenario analysis showed a difference in favour of amifostine, although the certainty of evidence for this effect was low. There was no information on survival for any of the included studies. Only one study, including children with osteosarcoma treated with intra‐arterial cisplatin, provided data on the number of participants with adverse effects other than ototoxicity grade 3 or higher (on NCICTCv2 scale). There was low‐certainty evidence that grade 3 or 4 vomiting was higher with amifostine (risk ratio (RR) 9.04, 95% confidence interval (CI) 1.99 to 41.12). The effects on cardiotoxicity and renal toxicity grade 3 or 4 were uncertain (low‐certainty evidence). None of the studies evaluated quality of life.
In the recent update, we also identified one RCT including 109 children with localized hepatoblastoma evaluating the use of sodium thiosulfate versus no additional treatment. Children received intravenous cisplatin only (one child also received carboplatin). There was moderate‐certainty evidence that both symptomatic ototoxicity only (i.e. Brock criteria grade 2 or higher) and combined asymptomatic and symptomatic ototoxicity (i.e. Brock criteria grade 1 or higher) was lower with sodium thiosulfate (combined asymptomatic and symptomatic ototoxicity: RR 0.52, 95% CI 0.33 to 0.81; symptomatic ototoxicity only: RR 0.39, 95% CI 0.19 to 0.83). The effect of sodium thiosulfate on tumour response (defined as number of participants with a complete or partial response at the end of treatment), overall survival (calculated from time of randomization to death or last follow‐up), event‐free survival (calculated from time of randomization until disease progression, disease relapse, second primary cancer, death, or last follow‐up, whichever came first) and adverse effects other than hearing loss and tinnitus grade 3 or higher (according to National Cancer Institute Common Toxicity Criteria Adverse Effects version 3 (NCICTCAEv3) criteria) was uncertain (low‐certainty evidence for all these outcomes). Quality of life was not assessed.
We found no eligible studies for possible otoprotective medical interventions other than amifostine and sodium thiosulfate and for other types of malignancies.
Authors' conclusions
At the moment there is no evidence from individual studies in children with osteosarcoma or hepatoblastoma treated with different platinum analogues and dosage schedules that underscores the use of amifostine as an otoprotective intervention as compared to no additional treatment. Since pooling of results was not possible and the evidence was of low certainty, no definitive conclusions can be made. Since we found only one RCT evaluating the use of sodium thiosulfate in children with localized hepatoblastoma treated with cisplatin, no definitive conclusions on benefits and harms can be drawn. It should be noted that 'no evidence of effect', as identified in this review, is not the same as 'evidence of no effect'. We identified no eligible studies for other possible otoprotective medical interventions and other types of malignancies, so no conclusions can be made about their efficacy in preventing ototoxicity in children treated with platinum‐based therapy. More high‐quality research is needed.
Plain language summary
Medicines to prevent hearing loss in children receiving platinum chemotherapy for cancer
Review question
We reviewed the evidence of the effectiveness of any medical intervention to prevent hearing loss in children with cancer treated with platinum‐based therapy (i.e. including the anticancer drugs cisplatin, carboplatin, oxaliplatin or a combination of these). We also looked at anticancer effectiveness, side effects other than hearing loss and quality of life.
Background
Platinum‐based chemotherapy, including cisplatin, carboplatin, oxaliplatin or a combination of these, is used in the treatment of different types of childhood cancer. Unfortunately, one of the most important side effects of platinum chemotherapy is hearing loss. This can occur not only during treatment but also years after the end of treatment. Although it is not life‐threatening, the loss of hearing, especially during the first three years of life, may lead to difficulties with school performance and psychosocial functioning. Prevention of platinum‐induced hearing loss is thus very important and might improve the quality of life of children undergoing cancer treatment and those who have survived treatment with platinum‐based chemotherapy.
Study characteristics
The evidence is current to January 2019.
We found two randomized studies (clinical studies where people are randomly put into one of two or more treatment groups) and one controlled study (clinical studies where people are put into one of two or more treatment groups but this is not done in a random way) (149 participants), all comparing amifostine with no additional treatment. Two studies included children with osteosarcoma (a type of bone cancer), the other study included children with hepatoblastoma (a type of liver cancer). Combining the results of the included studies was not possible. It is not clear how long participants were monitored.
We also found one randomized study (109 children with localized hepatoblastoma) comparing sodium thiosulfate with no additional treatment. Half of the participants were monitored for more than four years.
Key results
At the moment there is no evidence from individual studies showing that the use of amifostine prevents hearing loss. Only one study reported results on cancer response and side effects, so we could make no definitive conclusions. None of the studies assessed survival and quality of life. Hearing loss seemed to be lower with the use of sodium thiosulfate, but the effect of sodium thiosulfate on cancer response and side effects was uncertain. We identified no adequate studies for other possible drugs to prevent hearing loss and for other types of cancer. Before definitive conclusions can be made about the usefulness of possible medicines to prevent hearing loss (amifostine, sodium thiosulfate or another medicine) in children treated with platinum chemotherapy more high‐quality research is needed.
Quality of the evidence
The quality of the evidence was moderate (for hearing loss with sodium thiosulfate) to low (for all other outcomes (results)). The quality of the evidence was limited because of issues with the study design (for all outcomes) and small numbers of participants in each study (for all outcomes except hearing loss with sodium thiosulfate).
Summary of findings
Background
Description of the condition
Platinum‐based therapy, including cisplatin, carboplatin, oxaliplatin or a combination of these, is used to treat a variety of paediatric malignancies. Unfortunately one of the most significant adverse effects is the occurrence of hearing loss (ototoxicity). It usually manifests as bilateral, symmetrical, sensorineural hearing loss first affecting the higher frequencies (6000 Hz or higher) (McHaney 1983), and is often accompanied by tinnitus (Reddel 1982).
There is a wide variation in the reported frequency of platinum‐induced hearing loss, but one Cochrane systematic review described the frequency to be as high as 90.1% (Van As 2016a). The hearing loss not only develops during platinum‐based therapy but also years after completion of the therapy (Bertolini 2004; Knight 2005). This might be explained by the prolonged retention of platinum in the body; up to 20 years after treatment circulating platinum is still detectable in the plasma (Gietema 2000). Platinum‐induced hearing loss seems to be irreversible and worsening of hearing loss occurs during follow‐up (McHaney 1983; Bertolini 2004).
Different risk factors have been identified, such as the type of platinum analogue used. Cisplatin seems to cause substantially more hearing loss than carboplatin and the highest incidence of hearing loss was in people who received both cisplatin and carboplatin (Bertolini 2004; Dean 2008). The ototoxicity of oxaliplatin as compared to the other platinum analogues is not as well established but oxaliplatin seems to be the least ototoxic (Eloxatin SPC). Furthermore, the incidence of platinum‐induced hearing loss seems to be dose‐dependent, increasing with higher cumulative doses (McHaney 1983; Schell 1989; Bertolini 2004; Li 2004), and with higher individual doses (Reddel 1982; Li 2004). Also, bolus injections are more ototoxic than longer infusion durations (Reddel 1982), although Cochrane systematic reviews did not confirm this (Van As 2014a; Van As 2016b; Van As 2018). Additional risk factors are cranial radiotherapy (Schell 1989), younger age (Schell 1989; Li 2004), genetic variants (Ross 2009; Grewal 2010) and other host‐specific factors (Veal 2001), impaired renal function at the time of platinum treatment (Skinner 2004), and other ototoxic drugs such as aminoglycosides (Skinner 2004; Jenney 2005) and furosemide (Gallagher 1979).
Description of the intervention
In an effort to prevent or reduce platinum‐induced hearing loss, extensive research has been devoted to the identification of medical interventions capable of ameliorating this adverse effect. Cisplatin interacts with cochlear tissues such as the outer hair cells of the organ of Corti, stria vascularis, spiral ligament and spiral ganglionic cells to generate a reactive oxygen species (ROS) response while also depleting the antioxidant enzyme system that would scavenge and neutralize this increase in superoxides. Cisplatin accumulates in the cochlear tissue, integrates into DNA, and causes inefficient and dysfunctional protein and enzyme synthesis. The cochlea, because of its unique anatomical position and isolation, is practically a closed system and is therefore unable to flush out the accumulated toxins with the rapid pace of their generation. This results in ROS overload and a decreased antioxidant system leading to irreversible cell injury (Rybak 2007; Rybak 2009). Thus, antioxidants such as amifostine (Gallegos‐Castorena 2007; Fouladi 2008) and sodium thiosulfate (Freyer 2009) might be good treatment options against platinum‐induced hearing loss. Furthermore, other medical interventions such as neurotrophins, A1 adenosine receptors and dexamethasone have been studied (Rybak 2009).
Why it is important to do this review
Although platinum‐induced hearing loss is not life‐threatening, loss of hearing, especially during the first three years of life and even when only borderline to mild, can have important implications. It can negatively impact speech and language development, which may lead to difficulties with school performance and psychosocial functioning (Gregg 2004; Skinner 2004; Dean 2008).
Prevention of platinum‐induced hearing loss is thus very important and might improve the quality of life of childhood cancer patients and survivors treated with platinum‐based therapy.
This is the third update of the first systematic review evaluating all medical interventions for the prevention of platinum‐induced hearing loss in children with cancer (Van As 2012; Van As 2014b; Van As 2016c).
Objectives
To assess the efficacy of medical interventions to prevent hearing loss and to determine possible effects of these interventions on antitumour efficacy, toxicities other than hearing loss and quality of life in children with cancer treated with platinum‐based therapy as compared to placebo, no additional treatment or another protective medical intervention.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) or controlled clinical trials (CCTs) evaluating platinum‐based therapy together with a protective medical intervention versus platinum‐based therapy with placebo, no additional treatment or another protective medical intervention in children with cancer.
Types of participants
Children (aged 0 to 18 years at diagnosis) with any type of childhood malignancy. Studies including both children and adults were only eligible for inclusion in this review if the majority of participants were children (i.e. either more than 90% children or the maximal age did not exceed 22 years).
Types of interventions
Platinum‐based therapy together with a protective medical intervention versus platinum‐based therapy with placebo, no additional treatment or another protective medical intervention.
Treatment other than with cisplatin, carboplatin, oxaliplatin or a combination of these and the investigated protective medical intervention should have been the same in both treatment groups, including radiotherapy to the head or neck, or both. In both treatment groups, the same platinum analogue(s) should have been given with the same infusion duration and individual dose. In the design of the study, it should have been the intention to treat (ITT) both treatment groups with the same cumulative dose of cisplatin, carboplatin, oxaliplatin or a combination of these.
Types of outcome measures
Outcomes listed here were not used as criteria for including studies, but were the outcomes of interest within studies identified for inclusion.
Primary outcomes
Hearing loss (as defined by the authors of the original studies).
Tinnitus (as defined by the authors of the original studies).
Survival (overall survival and event‐free survival as defined by the authors of the original study).
Secondary outcomes
Tumour response (complete and partial remission as defined by the authors of the original study).
Adverse effects other than hearing loss and tinnitus (grade 3 or higher according to the criteria used by the authors of the original study).
Quality of life (as defined by the authors of the original study).
Search methods for identification of studies
We imposed no language restrictions. Cochrane Childhood Cancer ran the searches in the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and Embase for the original version of the review and the first and second update, the clinical librarian at the medical library of the Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands ran the searches in CENTRAL, MEDLINE and Embase for the third update; the review authors ran all other searches.
Electronic searches
We searched the following electronic databases: CENTRAL (the Cochrane Library, 2018, Issue 12), MEDLINE in PubMed (from 1945 to 8 January 2019) and Embase in Ovid (from 1980 to 8 January 2019).
The appendices show the search strategies for the different electronic databases (using a combination of controlled vocabulary and text words) (Appendix 1; Appendix 2; Appendix 3).
Searching other resources
We located information about trials not registered in CENTRAL, MEDLINE or Embase, either published or unpublished, by searching the reference lists of relevant articles and review articles. We assessed the conference proceedings of the International Society for Paediatric Oncology (SIOP) (from 2006 up to and including 2018), the American Society of Pediatric Hematology/Oncology (ASPHO) (from 2007 up to and including 2018) and the International Conference on Long‐Term Complications of Treatment of Children and Adolescents for Cancer (from 2010 up to and including 2015) (Appendix 4 shows search strategies). We scanned ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch) for ongoing trials (both searched 2 January 2019; Appendix 5 shows search strategies).
Data collection and analysis
Selection of studies
After employing the search strategy, two review authors independently identified studies meeting the inclusion criteria for this review. We resolved discrepancies between authors by discussion and needed no third‐party arbitration. We obtained in full any study that seemed to meet the inclusion criteria on the grounds of the title or abstract, or both, for closer inspection. We clearly stated the details of the reasons for exclusion of any study considered for the review. We included a flow diagram of the selection of studies. When multiple reports of one study were identified, we collated the full‐text results.
Data extraction and management
Two review authors independently performed data extraction using standardized forms. We extracted data on the characteristics of participants (such as age, stage of disease and renal function), interventions (such as route of delivery, dose and timing of the protective medical intervention, information on the received antineoplastic treatment and possible other ototoxic drugs such as aminoglycosides and furosemide), outcome measures, length of follow‐up, details of funding sources and the declaration of interests for each included study. We resolved discrepancies between authors by discussion and needed no third‐party arbitration.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias in the included studies (i.e. selection bias, performance bias, detection bias (for each outcome separately), attrition bias (for each outcome separately), reporting bias and other potential sources of bias). We used the risk of bias items as described in the module of Cochrane Childhood Cancer (Module CCG), which are based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved discrepancies between authors by discussion and needed no third‐party arbitration. The risk of bias in the included studies was taken into account in the interpretation of the review's results.
Measures of treatment effect
We analysed dichotomous variables using risk ratios (RR). For the assessment of survival, we used the generic inverse variance function of the Review Manager 5 software (Review Manager 2014) to combine logs of the hazard ratios (HRs). All results were presented with the corresponding 95% confidence interval (CI).
Dealing with missing data
We attempted to contact the authors of the studies awaiting classification, but received no responses. We attempted to contact the study authors with regard to missing data for data extraction and risk of bias assessment. The primary author of Katzenstein 2009 told us that they were in the process of writing a final manuscript. Some of our requested data might be in there but at the time he was unable to provide us with the additional data. During the updates of this review this manuscript was not yet available. Brock 2018 was unable to provide additional information at the time of our request, but possibly it will be able in the future. We received no additional information from Gallegos‐Castorena 2007 and Petrilli 2002. We extracted the data by the allocated intervention, irrespective of compliance with the allocated intervention, in order to allow an ITT analysis. If this was not possible, we stated this and performed an as treated or available‐data analysis.
Assessment of heterogeneity
Since pooling of results was not possible, the assessment of heterogeneity (both by visual inspection of the forest plots and by a formal statistical test for heterogeneity, that is, the I2 statistic (Higgins 2011)), was not applicable.
Assessment of reporting biases
In addition to the evaluation of reporting bias as described in the Assessment of risk of bias in included studies section, we planned to assess reporting bias by constructing a funnel plot when there was a sufficient number of included studies (i.e. at least 10 studies included in a meta‐analysis) because otherwise the power of the test is too low to distinguish chance from real asymmetry (Higgins 2011). Since pooling of results was not possible, this was not applicable.
Data synthesis
We entered data into the Review Manager 5 software provided by Cochrane (Review Manager 2014); we performed analyses according to the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We included outcome measures only if it was the intention of the study to perform the necessary assessments in all randomized participants (so not optional, or only performed in some centres). When the results of a particular outcome measure were available for less than 50% of the participants of a study, due to the associated high risk of attrition bias, we did not report the results of this outcome measure. We performed pooling of results only if both treatment groups were comparable, including the definition of ototoxicity that was used. We summarized studies for which pooling of results was not possible descriptively. We used a fixed‐effect model throughout the review. For outcomes where there was only one study, we were unable to calculate an RR if one of the treatment groups experienced no events and used the Fischer's exact test instead. We calculated all RRs and hazard ratios (HRs), 95% CIs and P values mentioned in the results in Review Manager 5 (Review Manager 2014), with the exception of the Fischer's exact P value (calculated in GraphPad).
For each comparison, we prepared a 'Summary of findings' table using GRADEpro software (GRADEpro for the first and second updates; GRADEpro GDT for the third update), in which we presented the following outcomes: ototoxicity (i.e. hearing loss or tinnitus, or both), tumour response, survival (overall and event‐free), adverse effects other than ototoxicity (grade 3 or higher) and quality of life. Two review authors independently assessed the certainty of the evidence (i.e. very low, low, moderate or high certainty) for each outcome using the five GRADE considerations: study limitations, inconsistency, indirectness, imprecision and publication bias.
Subgroup analysis and investigation of heterogeneity
We planned to analyse data separately for participants treated with cisplatin, carboplatin, oxaliplatin or combinations of these platinum analogues. However, since pooling of results was not possible, subgroup analyses were not applicable.
Sensitivity analysis
Since pooling of results was not possible, sensitivity analyses for risk of bias items (i.e. excluding studies with a high risk of bias and studies for which the risk of bias was unclear, and comparing the results of studies with a low risk of bias with the results of all available studies; sensitivity analyses would only have been performed if at least two studies remained in the analysis after exclusion of the studies with a high or unclear risk of bias) were not applicable.
Results
Description of studies
Results of the search
We ran searches of the electronic databases CENTRAL, MEDLINE (PubMed) and Embase (Ovid) in December 2011 for the original version of this review. This search yielded 573 references. Following initial screening of the titles, abstracts, or both, we excluded 551 references that clearly did not meet all criteria required for considering studies for this review. We assessed the 22 remaining references in full, of which three fulfilled all the criteria for considering studies for this review and were thus eligible for inclusion. One reference described an ongoing study and we excluded the remaining 18 references for the reasons described in the Characteristics of excluded studies table. Scanning the reference lists of included articles and reviews and the conference proceedings did not identify any additional eligible studies. By scanning the ongoing trials databases, we identified one additional ongoing trial.
For the first update, we ran searches of CENTRAL, MEDLINE (PubMed) and Embase (Ovid) in March 2014 yielding 138 references, which were added to the search results from December 2011. Initial screening of the titles, abstracts, or both, excluded all 138 references as they clearly did not meet the inclusion criteria. Scanning the reference lists of relevant articles, the conference proceedings and the ongoing trials registers did not identify any additional eligible studies. At the time of this update no publications of the ongoing trials identified in the original version of the review were available.
For the second update, we ran searches of CENTRAL, MEDLINE (PubMed) and Embase (Ovid) in July 2016 yielding 79 references (60 references after we removed duplicates). Initial screening of titles, abstracts, or both excluded 57 references as they clearly did not meet the inclusion criteria. We assessed the three remaining references in full; two were conference proceedings describing the two ongoing studies identified in the original version of the review (which were moved from the Characteristics of ongoing studies table to the Characteristics of studies awaiting classification table), the other publication did not meet the inclusion criteria (Characteristics of excluded studies table). Scanning the reference lists of relevant articles, conference proceedings and ongoing trials registers did not identify any additional eligible studies.
For the third update, we ran searches of CENTRAL, MEDLINE (PubMed) and Embase (Ovid) in January 2019 yielding 157 references (135 references after we removed duplicates). Initial screening of titles, abstracts, or both excluded 125 references as they clearly did not meet the inclusion criteria. We assessed the 10 remaining references in full. Four publications did not meet the inclusion criteria (Characteristics of excluded studies table). Three publications (describing two different studies) did not provide enough information to assess eligibility for this review and we did not succeed in contacting the authors (Characteristics of studies awaiting classification table); two of these publications described the Children's Oncology Group study already included in the previous version of the review as a study awaiting classification. The final three publications were conference proceedings describing the SIOPEL 6 (Société Internationale d'Oncologie Pédiatrique – Epithelial Liver Tumor Study Group) study already included in the previous version of the review as a study awaiting classification. While searching for full‐text publications not yet included in the electronic database search of the two studies awaiting classification included in the previous version of the review we identified the full‐text publication of the SIOPEL 6 study; this publication was eligible for inclusion. Scanning the reference lists of relevant articles and conference proceedings did not identify any additional eligible studies. By scanning the ongoing trial registers, we identified one ongoing trial; as we are not yet certain that it fulfils all eligibility criteria and contacting the investigators for additional information was not successful this study is included in the Characteristics of studies awaiting classification table.
In summary, the review included four studies. We also identified three studies awaiting classification. See Figure 1 for a flow diagram of the selection of studies for this systematic review.
1.
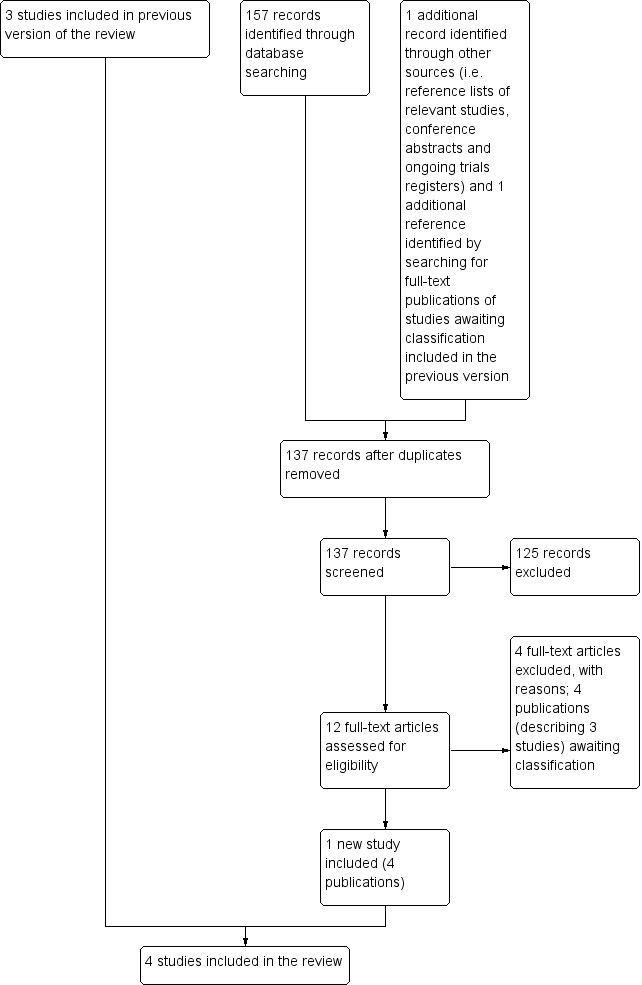
Flow diagram of selection of studies.
Included studies
The characteristics of the included studies are summarized below. For more detailed information, see the Characteristics of included studies table.
Amifostine
We identified two RCTs (Gallegos‐Castorena 2007; Katzenstein 2009) and one CCT (Petrilli 2002) evaluating amifostine as a possible otoprotective intervention. The total number of participants included in these studies was 149: 72 participants received amifostine and 77 participants received no otoprotective intervention. Note that the presented participants in Katzenstein 2009 were considered in a special interim analysis of the incidence of toxicity; the total number of eligible participants was unclear. Participants were aged between 0 and 22 years. All studies gave amifostine 740 mg/m2 in a 15‐minute infusion immediately prior to the platinum doses; for more detailed information, see the Characteristics of included studies table. Two studies diagnosed participants with an osteosarcoma (Petrilli 2002; Gallegos‐Castorena 2007); the other study diagnosed participants with hepatoblastoma (Katzenstein 2009). In one study, participants received cisplatin (Gallegos‐Castorena 2007), in one study, participants received a combination of cisplatin and carboplatin (Petrilli 2002), and in one study, participants received either cisplatin or a combination of cisplatin and carboplatin depending on the stage of disease and randomization (Katzenstein 2009). For detailed information on the cumulative platinum doses, individual platinum doses, platinum infusion durations, routes of delivery and other agents included in the chemotherapeutic protocols see the Characteristics of included studies table. Regarding other ototoxic drugs, in two studies, participants received anthracyclines (i.e. doxorubicin) (Petrilli 2002; Gallegos‐Castorena 2007), and in one study, some of the participants received vincristine (Katzenstein 2009); no study stated if participants received gentamycin or furosemide. In two studies, participants did not have prior hearing dysfunction and pretreatment renal impairment (Petrilli 2002; Katzenstein 2009), whereas in the other study this was unclear. In none of the studies did participants receive prior platinum treatment, prior radiotherapy to the head and neck region or prior cranial surgery. None of the studies reported genetic variants of platinum ototoxicity. Finally, none of the studies reported the length of follow‐up.
Sodium thiosulfate
We identified one RCT evaluating sodium thiosulfate as a possible otoprotective intervention (Brock 2018). The total number of participants was 109: 57 participants received sodium thiosulfate and 52 participants received no otoprotective intervention. Participants were aged between 0.1 and 8.2 years. Sodium thiosulfate 20 g/m2 was given as an intravenous infusion over 15 minutes, six hours after the end of cisplatin infusion; for more detailed information, see the Characteristics of included studies table. All participants were diagnosed with hepatoblastoma. All participants received cisplatin; one participant also received carboplatin. For detailed information on the cumulative platinum doses, individual platinum doses, platinum infusion durations, route of delivery and other agents included in the chemotherapeutic protocols see the Characteristics of included studies table. Regarding other ototoxic drugs, 21 participants received anthracyclines (i.e. doxorubicin), none of the participants received vincristine. It was not stated if participants received gentamycin or furosemide. It was unclear if participants had prior hearing dysfunction; none of the participants had pretreatment renal impairment. Participants had not received prior platinum treatment, prior radiotherapy to the head and neck region or prior cranial surgery. Genetic variants of platinum ototoxicity were not reported. The median follow‐up was 4.33 years; final audiometry was performed at a median of 3 years (range 0.25 to 6.9 years) after randomization.
Risk of bias in included studies
See the 'Risk of bias' section of the Characteristics of included studies table and Figure 2 for the exact scores per study and the support for the judgements made.
2.
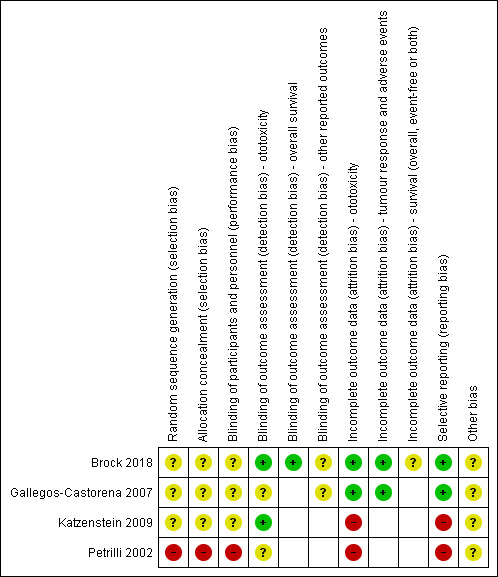
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
For evaluating selection bias, we assessed random sequence generation and allocation concealment.
Amifostine
Both random sequence generation and allocation concealment, and thus the risk of selection bias, were unclear in two studies (Gallegos‐Castorena 2007; Katzenstein 2009). In the other study, there was a high risk of selection bias; there was no randomization since this was a CCT (Petrilli 2002).
Sodium thiosulfate
Both random sequence generation and allocation concealment, and thus the risk of selection bias, were unclear (Brock 2018).
Blinding
For evaluating performance bias, we assessed blinding of participants and personnel. For evaluating detection bias, we evaluated blinding of outcome assessors for all separate outcomes.
Amifostine
In two studies, the risk of performance bias was unclear (Gallegos‐Castorena 2007; Katzenstein 2009). In the other study, the risk of performance bias was high; participants treated with amifostine were consecutive participants so blinding was not possible (Petrilli 2002). For ototoxicity the risk of detection bias was low in one study (Katzenstein 2009), and unclear in two studies (Petrilli 2002; Gallegos‐Castorena 2007). Only one study evaluated response rate and adverse effects; the risk of detection bias was unclear for both these outcomes (Gallegos‐Castorena 2007).
Sodium thiosulfate
The risk of performance bias was unclear (Brock 2018). For ototoxicity and overall survival, the risk of detection bias was low;, for event‐free survival, response rate and adverse effects the risk of detection bias was unclear.
Incomplete outcome data
For evaluating attrition bias, we assessed incomplete outcome data for all outcomes separately.
Amifostine
In one study, the risk of attrition bias was low for all outcomes, that is, ototoxicity, response rate and adverse effects (Gallegos‐Castorena 2007). In the other two studies the risk of attrition bias was high for the reported outcome, that is, ototoxicity (Petrilli 2002; Katzenstein 2009).
Sodium thiosulfate
The risk of attrition bias was low for ototoxicity, response rate and adverse effects; for overall and event‐free survival, it was unclear (Brock 2018).
Selective reporting
For evaluating reporting bias, we assessed selective reporting.
Amifostine
In one study, the risk of reporting bias was low (Gallegos‐Castorena 2007). In the other two studies, the risk of reporting bias was high (Petrilli 2002; Katzenstein 2009).
Sodium thiosulfate
The risk of reporting bias was low (Brock 2018).
Other potential sources of bias
For evaluating other potential sources of bias, we assessed the following items: block randomization in unblinded trials, baseline imbalance between treatment groups related to outcome (prior ototoxic treatment, age, sex, prior hearing loss), difference in ototoxic drugs other than platinum analogue between treatment groups (furosemide, gentamycin, anthracyclines, vincristine), difference in cumulative platinum dose between treatment groups, difference in length of follow‐up between treatment groups, difference in impaired renal function at the time of platinum treatment between treatment groups, and if an insensitive instrument was used to evaluate ototoxicity.
Amifostine
In all three studies, the risk of other potential sources of bias was unclear. For a more detailed description of all different items see the 'Risk of bias' section of the Characteristics of included studies table.
Sodium thiosulfate
The risk of other potential sources of bias was unclear. For a more detailed description of all different items see the 'Risk of bias' section of the Characteristics of included studies table.
Effects of interventions
Summary of findings for the main comparison. Amifostine compared to no otoprotective intervention for the prevention of platinum‐induced hearing loss in children with cancer.
| Amifostine compared to no otoprotective intervention for the prevention of platinum‐induced hearing loss in children with cancer | ||||||
| Patient or population: children with cancer treated with platinum‐based therapy Settings: paediatric oncology departments Intervention: amifostine Comparison: no otoprotective intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No otoprotective intervention | Amifostine | |||||
| Ototoxicity (i.e.hearing loss or tinnitus, or both) | ||||||
|
Ototoxicity according to NCICTCv2 criteria with intra‐arterial platinum (combined asymptomatic and symptomatic disease)
Exact test method not reported Follow‐up not mentioned |
769 per 1000a | 992 per 1000 (723 to 1000) | RR 1.29 (0.94 to 1.77) | 28 (1 study) | ⊕⊕⊝⊝ Lowb,c | When only looking at symptomatic disease there was also no significant difference between treatment groups (RR 0.87, 95% CI 0.14 to 5.32; GRADE assessment identical to combined asymptomatic and symptomatic disease analysis) |
|
Ototoxicity according to NCICTCv2 criteria with intravenous platinum (combined asymptomatic and symptomatic disease)
Objective and subjective audiometric evaluations were performed, no further information provided Follow‐up not mentioned |
789 per 1000a | 821 per 1000 (600 to 1000) | RR 1.04 (0.76 to 1.44) | 36 (1 studyd) | ⊕⊕⊝⊝ Lowc,e | For 3/39 children included in the study (all in the amifostine group) there were no data on ototoxicity. The RR reported here resulted from the available‐data analysis. Intention‐to‐treat analyses (i.e. best‐case and worst‐case scenarios) also showed no significant difference between the treatment groups. When only looking at symptomatic disease there was also no significant difference between treatment groups (available‐data analysis: RR 0.87, 95% CI 0.14 to 5.32; intention‐to‐treat analyses (i.e. best‐case and worst‐case scenarios) also showed no significant difference between treatment groups). The GRADE assessment for the worst‐case and best‐case scenarios and the symptomatic disease‐only analysis was identical to that of the 'available‐data' analysis for the combined asymptomatic and symptomatic disease analysis. |
|
Ototoxicity according to modified Brock criteria with intravenous platinum (combined asymptomatic and symptomatic disease)
Audiograms were performed, but no further information provided Follow‐up not mentioned |
556 per 1000a | 594 per 1000 (411 to 861) | RR 1.07 (0.74 to 1.55) | 82 (1 study) | ⊕⊕⊝⊝ Lowc,f | It should be noted that these 82 children were part of a larger study group; they were considered in a special interim analysis of the incidence of toxicity. The total number of eligible participants was unclear and as a result we were unable to perform an intention‐to‐treat analysis. Also, we were unable to check if the ototoxicity results were available for at least 50% of the eligible participants. In the 'Methods' section, we stated that if that was not the case, we would not report the results due to the associated high risk of attrition bias. However, we decided to give this study the benefit of the doubt. When only looking at symptomatic disease, there was also no significant difference between treatment groups (RR 1.00, 95% CI 0.57 to 1.75; GRADE assessment identical to combined asymptomatic and symptomatic disease analysis). |
| Survival | ||||||
| Survival (overall and event‐free) – not reported | — | — | — | — | — | No information on overall and event‐free survival |
| Tumour response | ||||||
|
Tumour response with intra‐arterial platinum (good and partial remission) Follow‐up not mentioned |
583 per 1000a | 933 per 1000 (566 to 1000) | RR 1.6 (0.97 to 2.63) | 27 (1 study) | ⊕⊕⊝⊝ Lowb,c | For 1/28 children included in the study (in the control group) there were no data on tumour response. The RR reported here resulted from the available‐data analysis. Intention‐to‐treat analyses also showed no significant difference between the treatment groups in the best‐case scenario, but in the worst‐case scenario there was a significant difference in favour of amifostine (GRADE assessment identical to available‐data analysis). Due to the nature of this outcome (number of participants with a remission) a high event rate is favourable. The studies using intravenous platinum did not report on this outcome. |
| Adverse effects other than ototoxicity | ||||||
|
Renal toxicity/vomiting/ cardiotoxicity (all grade ≥ 3 according to NCICTCv2 criteria) with intra‐arterial platinum Follow‐up not mentioned |
Renal toxicity: no significant difference between treatment groups (Fischer's exact test P = 0.21) Vomiting: significant difference in favour of the control group (RR 9.04, 95% CI 1.99 to 41.12) Cardiotoxicity: none of the participants in this study experienced cardiac toxicity grade 3 or 4) |
— | 28 (1 study) | ⊕⊕⊝⊝ Lowb,c | The studies using intravenous platinum did not provide adequate data on adverse effects. | |
| Quality of life | ||||||
| Quality of life – not reported | — | — | — | — | — | No information on quality of life |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NCICTCv2: National Cancer Institute Common Toxicity Criteria version 2; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aThe assumed risk was based on the prevalence in the control group of the included study. bPresence of selection bias, performance bias, detection bias and other bias was unclear; low risk of attrition bias and reporting bias (downgraded one level). cAs this was a small study with a total number of events fewer than 300 (the threshold rule‐of‐thumb value stated in the GRADEpro software (GRADEpro)), we downgraded one level. dThis was a controlled clinical trial. eHigh risk of selection bias, performance bias, attrition bias and reporting bias; unclear risk of detection bias and other bias (downgraded one level). fHigh risk of attrition and reporting bias; unclear risk of selection bias, performance bias and other bias; low risk of detection bias (downgraded one level).
Summary of findings 2. Sodium thiosulfate compared to no otoprotective intervention for the prevention of platinum‐induced hearing loss in children with cancer.
| Sodium thiosulfate compared to no otoprotective intervention for the prevention of platinum‐induced hearing loss in children with cancer | ||||||
| Patient or population: children with cancer treated with platinum‐based therapy Setting: paediatric oncology departments Intervention: sodium thiosulfate Comparison: no otoprotective intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no otoprotective intervention | Risk with sodium thiosulfate | |||||
| Ototoxicity (i.e.hearing loss or tinnitus, or both) | ||||||
| Ototoxicity according to Brock criteria with intravenous platinum (combined asymptomatic and symptomatic disease) Assessed with: pure‐tone audiometry Follow‐up: median 3 years | 630 per 1000a | 328 per 1000 (208 to 511) | RR 0.52 (0.33 to 0.81) | 101 (1 RCT) | ⊕⊕⊕⊝ Moderateb,c | For 8/109 children included in the study (2 in the sodium thiosulfate group and 6 in the control group), there were no data on ototoxicity. The RR reported here results from the available‐data analysis. Intention‐to‐treat analyses (i.e. best‐case and worst‐case scenarios) also showed a significant difference in favour of the sodium thiosulfate group. When only looking at symptomatic disease results were similar (RR 0.39, 95% CI 0.19 to 0.83; GRADE assessment identical to combined asymptomatic and symptomatic disease analysis); intention‐to‐treat analyses (i.e. best‐case and worst‐case scenarios) also showed a significant difference in favour of the sodium thiosulfate group. The GRADE assessment for the worst‐case and best‐case scenarios and the symptomatic disease only analysis was identical to that of the available‐data analysis for the combined asymptomatic and symptomatic disease analysis. |
| Survival | ||||||
| Overall survival with intravenous platinum (i.e. mortality/number of participants deceased) Follow‐up: 6 years | 77 per 1000a | 34 per 1000 (2 to 374) | HR 0.43 (0.03 to 5.85) | 109 (1 RCT) | ⊕⊕⊝⊝ Lowe,f | The follow‐up of 6 years as mentioned in the Outcome column was the time point from the survival curve used to obtain the risk in the control group; the overall follow‐up was median 4.33 years. It was unclear if this was an intention‐to‐treat analysis. Note that due to limitations of the software in this table overall survival was presented as mortality/number of participants deceased. |
| Event‐free survival with intravenous platinum (i.e. number of participants with disease progression, disease relapse, second primary cancer or death) Follow‐up: 6 years | 212 per 1000a | 183 per 1000 (84 to 369) | HR 0.85 (0.37 to 1.94) | 109 (1 RCT) | ⊕⊕⊝⊝ Lowe,g | The follow‐up of 6 years as mentioned in the Outcome column was the time point from the survival curve used to obtain the risk in the control group; the overall follow‐up was median 4.33 years. Unclear if this was an intention‐to‐treat analysis. Note that due to limitations of the software in this table event‐free survival was presented as number of participants with an event. |
| Tumour response | ||||||
| Tumour response with intravenous platinum (complete and partial remission) Follow‐up: median 4.33 years | 941 per 1000a | 998 per 1000 (922 to 1000) | RR 1.06 (0.98 to 1.15) | 108 (1 RCT) | ⊕⊕⊝⊝ Lowd,e | For 1/109 children included in the study (in the control group) there were no data on tumour response. The RR reported here resulted from the available‐data analysis. Intention‐to‐treat analyses (i.e. best‐case and worst‐case scenarios) also showed no significant difference between the treatment groups (GRADE assessment identical to available‐data analysis). Due to the nature of this outcome (number of participants with a remission) a high event rate is favourable. |
| Adverse effects other than ototoxicity | ||||||
| Adverse effects other than ototoxicity (≥ grade 3 according to NCICTCAEv3 criteria) with intravenous platinum Follow‐up: median 4.33 years | There were no significant differences between treatment groups in febrile neutropenia grade 3, infection grade 3, hypomagnesaemia grade 3, vomiting grade 3, nausea grade 3, anaemia grade 3 or 4, leukopenia grade 3, neutropenia grade 3 or 4, thrombocytopenia grade 3 or 4, gastrointestinal event (not reported if grade 3 or 4), elevated liver enzyme level grade 3 or 4, elevated serum glucose level grade 3, hypermagnesaemia grade 3, left ventricular systolic dysfunction grade 3 or 4, renal event grade 3 or 4, allergy grade 3, hypernatraemia grade 3, hypophosphataemia grade 3, hyperkalaemia grade 3, dyspnoea grade 3, hypokalaemia grade 3 or 4. | — | 109 (1 RCT) | ⊕⊕⊝⊝ Lowd,e | — | |
| Quality of life | ||||||
| Quality of life – not reported | — | — | — | — | — | No information on quality of life. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; NCICTCAEv3: National Cancer Institute Common Toxicity Criteria version 3; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aThe risk with no otoprotective intervention was based on the prevalence in the control group of the included study. bPresence of selection bias, performance bias and other bias was unclear; low risk of detection bias, attrition bias and reporting bias (downgraded one level). cWe did not downgrade for imprecision. It was a small study but the effect was large and the confidence interval was below no effect. dPresence of selection bias, performance bias, detection bias and other bias was unclear; low risk of attrition bias and reporting bias (downgraded one level). eAs this was a small study with a total number of events fewer than 300 (the threshold rule‐of‐thumb value stated in the GRADEpro software (GRADEpro), we downgraded one level. fPresence of selection bias, performance bias, attrition bias and other bias unclear; low risk of detection bias and reporting bias (downgraded one level). gPresence of selection bias, performance bias, detection bias, attrition bias and other bias; low risk of reporting bias (downgraded one level).
Amifostine
We identified two RCTs (Gallegos‐Castorena 2007; Katzenstein 2009) and one CCT (Petrilli 2002) evaluating amifostine as a possible otoprotective intervention. Not all articles allowed data extraction for all end points (see the Characteristics of included studies table for a more detailed description of the extractable end points from each article). We calculated all RRs, 95% CIs and P values mentioned below in Review Manager 5 (Review Manager 2014), with the exception of the Fischer's exact P value (calculated in GraphPad). Reasons for downgrading the level of evidence are provided in Table 1.
Ototoxicity (i.e. hearing loss or tinnitus, or both)
We extracted data on ototoxicity from all three eligible trials (Petrilli 2002; Gallegos‐Castorena 2007; Katzenstein 2009). It was not possible to pool the results of this outcome. Two studies used a comparable definition but in one study participants received their platinum treatment intra‐arterially (Gallegos‐Castorena 2007), and in the other it was given intravenously (Petrilli 2002). Due to the potential influence of this difference on the occurrence of ototoxicity, pooling was not possible. The other study initially used the same definition as the other two trials but during the study it was decided that using that definition substantially underestimated the true incidence of significant hearing loss and it was decided to use another definition instead (Katzenstein 2009). The authors were unable to provide results using the initial definition and, therefore, pooling was not possible. For the definitions used in the different studies, see Table 3 (for Petrilli 2002; Gallegos‐Castorena 2007) and Table 4 (for Katzenstein 2009).
1. NCICTC version 2 'Inner ear and hearing' *.
| Grade | Description |
| 0 | Normal |
| 1 | Hearing loss on audiometry only |
| 2 | Tinnitus or hearing loss, not requiring hearing aid or treatment |
| 3 | Tinnitus or hearing loss, correctable with hearing aid or treatment |
| 4 | Severe uni‐ or bilateral hearing loss (deafness), not correctable |
NCICTC: National Cancer Institute Common Toxicity Criteria.
*from NCICTC v2.
2. Modified Brock criteria for the classification of hearing loss*.
| Grade of hearing loss | Description | Potential clinical effects on hearing |
| 0 | ≤ 20 dB at 1, 2 and 4 kHz | None |
| 1a | > 40 dB at any frequency from 6 kHz to 12 kHz | Measurable |
| 1b | > 20 dB but ≤ 40 dB at any frequency from 3 kHz to 5 kHz | Measurable |
| 2a | > 40 dB at any frequency from 3 kHz to 5 kHz | Noticeable |
| 2b | > 20 dB but ≤ 40 dB at 2 kHz | Noticeable |
| 3 | > 40 dB at 2 kHz | Correctable with hearing aids |
| 4 | > 40 dB at 1 kHz | Speech comprehension deficits even with hearing aids |
dB: decibel; kHz: kilohertz.
*from Katzenstein 2009.
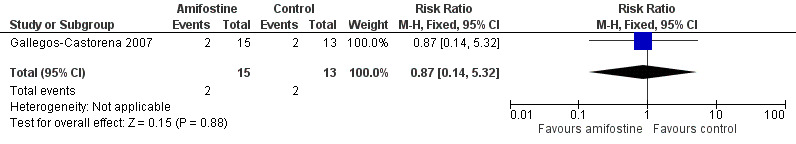
We extracted data on ototoxicity (defined as National Cancer Institute (NCI) Common Toxicity Criteria (CTC) version 2 (NCICTCv2)) with the use of intra‐arterial platinum chemotherapy from one study with 28 participants (Gallegos‐Castorena 2007). All 15 participants randomized to amifostine had asymptomatic or symptomatic ototoxicity (i.e. grade 1 and higher); and 10/13 participants in the control group. The analysis showed no significant difference between the treatment groups (RR 1.29, 95% CI 0.94 to 1.77; P = 0.11; Figure 3; low‐certainty evidence). There were two cases of symptomatic ototoxicity (i.e. grade 2 and higher) among 15 participants randomized to amifostine and two cases among the 13 participants in the control group. The analysis showed no significant difference between the treatment groups (RR 0.87, 95% CI 0.14 to 5.32; P = 0.88; Figure 4; low‐certainty evidence). It should be noted that both analyses included the participants who experienced symptomatic ototoxicity.
3.
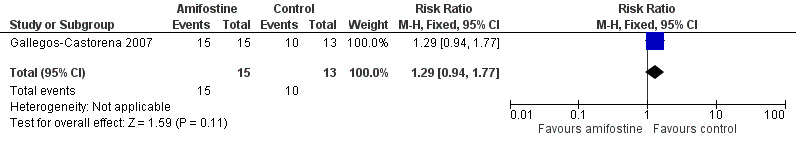
Forest plot of comparison: 1 Amifostine versus no otoprotective intervention, outcome: 1.1 Ototoxicity according to NCICTCv2 criteria with intra‐arterial platinum (combined asymptomatic and symptomatic disease).
4.
Forest plot of comparison: 1 Amifostine versus no otoprotective intervention, outcome: 1.4 Ototoxicity according to NCICTCv2 criteria with intra‐arterial platinum (symptomatic disease).
We extracted data on ototoxicity (defined as NCICTCv2) with the use of intravenous platinum chemotherapy from one study with 39 participants (Petrilli 2002). For 3/20 participants in the amifostine group, there were no ototoxicity data available. The available‐data analysis of asymptomatic or symptomatic ototoxicity (i.e. grade 1 and higher) showed no significant difference between the treatment groups (RR 1.04, 95% CI 0.76 to 1.44; P = 0.80; Figure 5; low‐certainty evidence); there were 14 cases among the 17 available participants in the amifostine group and 15 cases among the 19 control participants. ITT analyses (data not shown) also showed no significant difference between the treatment groups: the RR for the worst‐case scenario (i.e. 17 cases among 20 participants in the amifostine group) was 1.08 (95% CI 0.80 to 1.45; P = 0.63; low‐certainty evidence), while the RR for the best‐case scenario (i.e. 14 cases among 20 participants in the amifostine group) was 0.89 (95% CI 0.61 to 1.28; P = 0.52; low‐certainty evidence). The available‐data analysis of symptomatic ototoxicity (i.e. grade 2 or higher) showed no significant difference between the treatment groups (RR 1.32, 95% CI 0.83 to 2.10; P = 0.24; Figure 6; low‐certainty evidence); there were 13 cases among the 17 available participants in the amifostine group and 11 cases among the 19 control participants. ITT analyses (data not shown) also showed no significant difference between the treatment groups: the RR for the worst‐case scenario (i.e. 16 cases among 20 participants in the amifostine group) was 1.38 (95% CI 0.89 to 2.15; P = 0.15; low‐certainty evidence), while the RR for the best‐case scenario (i.e. 13 cases among 20 participants in the amifostine group) was 1.12 (95% CI 0.68 to 1.85; P = 0.65; low‐certainty evidence). It should be noted that both analyses included the participants who experienced symptomatic ototoxicity.
5.
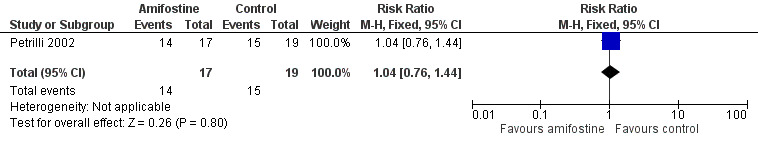
Forest plot of comparison: 1 Amifostine versus no otoprotective intervention, outcome: 1.2 Ototoxicity according to NCICTCv2 criteria with intravenous platinum (combined asymptomatic and symptomatic disease).
6.
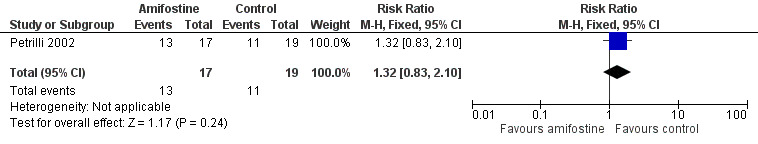
Forest plot of comparison: 1 Amifostine versus no otoprotective intervention, outcome: 1.5 Ototoxicity according to NCICTCv2 criteria with intravenous platinum (symptomatic disease).
We extracted data on ototoxicity (defined as modified Brock criteria) with the use of intravenous platinum chemotherapy from one study; the presented interim analysis included 82 participants (Katzenstein 2009). It should be noted that these 82 children were part of a larger study group; they were considered in a special interim analysis of the incidence of toxicity. The total number of eligible participants was unclear and as a result we were unable to perform an ITT analysis. Also, we were unable to check if the ototoxicity results were available for at least 50% of the eligible participants. In the Methods section, we stated that if that was not the case we would not report the results due to the associated high risk of attrition bias; however, we decided to give this study the benefit of the doubt. There were 22 cases of asymptomatic or symptomatic ototoxicity (i.e. grade 1a and higher) among 37 participants randomized to amifostine and 25 cases among the 45 participants in the control group. The analysis showed no significant difference between the treatment groups (RR 1.07, 95% CI 0.74 to 1.55; P = 0.72; Figure 7; low‐certainty evidence). There were 14 cases of symptomatic ototoxicity (i.e. grade 2a and higher) among 37 participants randomized to amifostine and 17 cases among the 45 participants in the control group. The analysis showed no significant difference between treatment groups (RR 1.00, 95% CI 0.57 to 1.75; P = 1.00; Figure 8; low‐certainty evidence). It should be noted that both analyses included the participants who experienced symptomatic ototoxicity.
7.
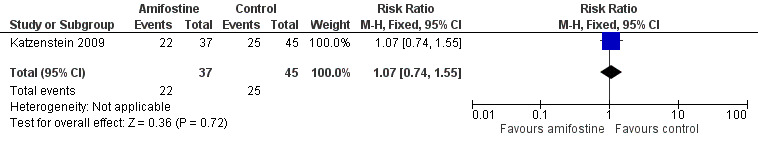
Forest plot of comparison: 1 Amifostine versus no otoprotective intervention, outcome: 1.3 Ototoxicity according to modified Brock criteria (combined asymptomatic and symptomatic disease).
8.
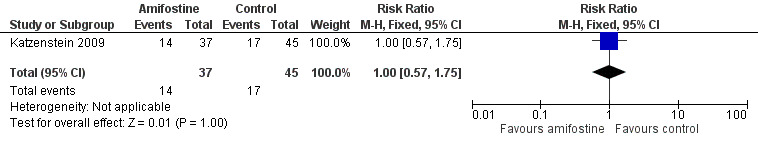
Forest plot of comparison: 1 Amifostine versus no otoprotective intervention, outcome: 1.6 Ototoxicity according to modified Brock criteria (symptomatic disease).
Survival
Gallegos‐Castorena 2007 and Petrilli 2002 provided no information on survival (i.e. event‐free survival and overall survival). Katzenstein 2009 did not provide survival data for the 82 participants included in the interim analysis presented in this manuscript.
Tumour response
Note that due to the nature of this outcome (i.e. the number of participants with a remission) a high event rate is favourable. Therefore, in the figures of the analyses 'favours control' is on the left and 'favours amifostine' is on the right, as opposed to the figures of the other analyses.
One study with 28 participants provided data on tumour response (defined as number of participants with a complete, good or partial response) with the use of intra‐arterial platinum chemotherapy (Gallegos‐Castorena 2007). For one of the 13 participants in the control group there were no response data. The available‐data analysis of tumour response showed no significant difference between the treatment groups (RR 1.60, 95% CI 0.97 to 2.63; P = 0.06; low‐certainty evidence); there were 14 remissions among the 15 participants randomized to amifostine and seven remissions in the 12 control participants. The ITT analysis (data not shown) for the best‐case scenario (i.e. eight remissions among 13 participants in the control group) also showed no significant difference between the treatment groups (RR 1.52, 95% CI 0.97 to 2.38; P = 0.07; low‐certainty evidence), while the ITT analysis (data not shown) for the worst‐case scenario (i.e. seven remissions among 13 participants in the control group) showed a significant difference in favour of the amifostine group (RR 1.73, 95% CI 1.03 to 2.92; P = 0.04; low‐certainty evidence).
Petrilli 2002 and Katzenstein 2009 provided no information on tumour response.
Adverse effects other than hearing loss and tinnitus (grade 3 or higher)
Since all participants receiving chemotherapy will experience adverse effects, we decided to analyse only the severe and life‐threatening effects. We defined these as grade 3 or higher.
In Gallegos‐Castorena 2007 (using intra‐arterial platinum chemotherapy; adverse effects according to NCICTCv2 criteria), there was a significant difference in favour of the control group in the occurrence of vomiting grade 3 or 4. All 15 participants in the amifostine group and 1/13 participants in the control group experienced vomiting grade 3 or 4 (RR 9.04, 95% CI 1.99 to 41.12; P = 0.004). None of the participants in this study experienced cardiac toxicity grade 3 or 4. We were unable to calculate an RR for renal toxicity grade 3 or 4 since one group experienced no events, but there was no significant difference between treatment groups; 0/15 of the participants in the amifostine group and 2/13 participants in the control group experienced renal toxicity grade 3 or 4 (Fischer's exact test P = 0.21). The certainty of evidence was low for all assessed adverse effects. They also provided data on amifostine‐related toxicity although without stating the exact grading. However, in this review only toxicities evaluated in both treatment groups were eligible so we did not include these toxicities.
The adverse effects other than hearing loss and tinnitus (grade 3 or higher) that Petrilli 2002 and Katzenstein 2009 reported could not be included in this review. Petrilli 2002 provided data for both treatment groups but as the number of infusions with toxicity present not as the number of participants with toxicity. As a result, we could not adequately analyse these data. They provided data on amifostine infusion‐related toxicity, although without stating the exact grading for most toxicities, in (part of) the amifostine group. However, in this review only toxicities evaluated in both treatment groups were eligible, so we did not include these toxicities. Katzenstein 2009 provided data on adverse effects in both treatment groups but as the number of courses with toxicity present and not as the number of participants with toxicity. Furthermore, it was not clear if the presented data regarded only the 82 participants included in the interim analysis presented in this manuscript. As a result, we could not adequately analyse these adverse effects data.
Quality of life
None of the studies evaluated quality of life (Petrilli 2002; Gallegos‐Castorena 2007; Katzenstein 2009).
Sodium thiosulfate
We identified one RCT evaluating sodium thiosulfate as a possible otoprotective intervention (Brock 2018). Data extraction was not possible for all end points (see Characteristics of included studies table for a more detailed description of the extractable end points from each article). We calculated all RRs, HRs, 95% CIs and P values mentioned below in Review Manager 5 (Review Manager 2014), with the exception of the Fischer's exact P value (calculated in GraphPad). Reasons for downgrading the level of evidence are provided in Table 2.
Ototoxicity (i.e. hearing loss or tinnitus, or both)
We extracted data on hearing loss (defined using Brock criteria; Table 5) with the use of intravenous platinum chemotherapy from one study with 109 participants (Brock 2018). The study evaluated hearing loss in 101/109 children (in the sodium thiosulfate group, two participants were not tested; in the control group, six participants were not tested). The available‐data analysis of asymptomatic or symptomatic ototoxicity (i.e. grade 1 and higher) showed a significant difference in favour of the sodium thiosulfate group (RR 0.52, 95% CI 0.33 to 0.81; P = 0.003; Figure 9; moderate‐certainty evidence); there were 18 cases among the 55 available participants in the sodium thiosulfate group and 29 cases among the 46 available control participants. ITT analyses (data not shown) also showed significant differences in favour of the sodium thiosulfate group: the RR for the worst‐case scenario (i.e. 20 cases among 57 participants in the sodium thiosulfate group and 33 cases among 52 participants in the control group) was 0.55 (95% CI 0.37 to 0.83; P = 0.005; moderate‐certainty evidence), while the RR for the best‐case scenario (i.e. 18 cases among 57 participants in the sodium thiosulfate group and 29 cases among the 52 participants in the control group) was 0.57 (95% CI 0.36 to 0.89; P = 0.01; moderate‐certainty evidence). The available‐data analysis of symptomatic ototoxicity (i.e. grade 2 or higher) showed a significant difference in favour of the sodium thiosulfate group (RR 0.39, 95% CI 0.19 to 0.83; P = 0.01; Figure 10; moderate‐certainty evidence); there were eight cases among the 55 available participants in the sodium thiosulfate group and 17 cases among the 46 available control participants. ITT analyses (data not shown) also showed significant differences in favour of the sodium thiosulfate group: the RR for the worst‐case scenario (i.e. 10 cases among 57 participants in the sodium thiosulfate group and 21 cases among the 52 participants in the control group) was 0.43 (95% CI 0.23 to 0.83; P = 0.01; moderate‐certainty evidence), while the RR for the best‐case scenario (i.e. 8 cases among 57 participants in the sodium thiosulfate group and 17 cases among the 52 participants in the control group) was 0.43 (95% CI 0.20 to 0.89; P = 0.03; moderate‐certainty evidence). It should be noted that both analyses included the participants who experienced symptomatic hearing loss.
3. Brock criteria for the classification of hearing loss*.
| Bilateral hearing loss | Grade | Designation |
| < 40 dB at all frequencies | 0 | Minimal |
| ≥ 40 dB at 8 kHz only | 1 | Mild |
| ≥ 40 dB at ≥ 4 kHz | 2 | Moderate |
| ≥ 40 dB at ≥ 2 kHz | 3 | Marked |
| ≥ 40 dB at ≥ 1 kHz | 4 | Severe |
dB: decibel; kHz: kilohertz.
*from Brock 2018.
9.
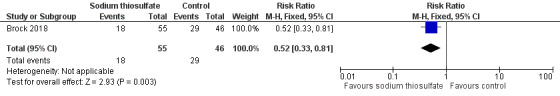
Forest plot of comparison: 2 Sodium thiosulfate versus no otoprotective intervention, outcome: 2.1 Ototoxicity according to Brock criteria with intravenous platinum (combined asymptomatic and symptomatic disease).
10.
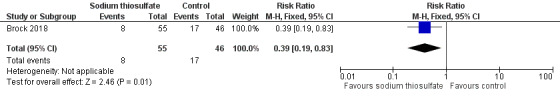
Forest plot of comparison: 2 Sodium thiosulfate versus no otoprotective intervention, outcome: 2.2 Ototoxicity according to Brock criteria with intravenous platinum (symptomatic disease).
Survival
We extracted data on overall survival (calculated from time of randomization to death or last follow‐up) with the use of intravenous platinum chemotherapy from one study with 109 participants (Brock 2018). For the assessment of survival, we used the generic inverse variance function of Review Manager 5 to combine logs of the HRs. We used Parmar's method to obtain the necessary data for the analysis (Parmar 1998); it was unclear if this was an ITT analysis. The HR showed no significant difference between the treatment groups (HR 0.43, 95% CI 0.03 to 5.85; P = 0.53; low‐certainty evidence). Six children died; in the sodium thiosulfate group, there were two deaths due to disease; in the control group, there were four deaths: one due to surgical complications, one due to cardiac arrest after treatment with paclitaxel following progression and two due to disease.
We extracted data on event‐free survival (calculated from time of randomization until disease progression, disease relapse, second primary cancer, death or last follow‐up, whichever came first) with the use of intravenous platinum chemotherapy from one study with 109 participants (Brock 2018). For the assessment of survival, we used the generic inverse variance function of Review Manager 5 to combine logs of the HRs. We used Parmar's method to obtain the necessary data for the analysis (Parmar 1998); it was unclear if this was an ITT analysis. The HR showed no significant difference between the treatment groups (HR 0.85, 95% CI 0.37 to 1.94; P = 0.70; low‐certainty evidence).
Tumour response
Note that due to the nature of this outcome (i.e. the number of participants with a remission), a high event rate is favourable. Therefore, in the figures of the analyses 'favours control' is on the left and 'favours sodium thiosulfate' is on the right, as opposed to the figures of the other analyses.
We extracted data on tumour response (defined as number of participants with a complete or partial response at the end of treatment) with the use of intravenous platinum chemotherapy from one study with 109 participants (Brock 2018). For 1/52 participants in the control group, there were no response data. The available‐data analysis of tumour response showed no significant difference between the treatment groups (RR 1.06, 95% CI 0.98 to 1.15; P = 0.12; low‐certainty evidence); all participants in the sodium thiosulfate group had a remission and there were 48 remissions among the 51 available participants in the control group. ITT analyses (data not shown) also showed no significant difference between the treatment groups: the RR for the best‐case scenario (i.e. 49 remissions among 52 participants in the control group) was RR 1.06 (95% CI 0.98 to 1.14; P = 0.12; low‐certainty evidence), while the RR for the worst‐case scenario (i.e. 48 remissions among 52 participants in the control group) was 1.08 (95% CI 0.99 to 1.18; P = 0.07; low‐certainty evidence).
Adverse effects other than hearing loss and tinnitus (grade 3 or higher)
Since all participants receiving chemotherapy will experience adverse effects, we decided to analyse only the severe and life‐threatening effects. We defined these as grade 3 or higher. In cases where the grade of an adverse effect was unclear or only results for one treatment group were available, we did not include these toxicities.
We extracted data on adverse effects other than hearing loss and tinnitus grade 3 or higher (according to NCICTCAEv3 criteria; serious adverse events were defined in accordance with the harmonized tripartite guidelines for Good Clinical Practice) with the use of intravenous platinum chemotherapy from one study with 109 participants (Brock 2018). Based on the provided information, we assumed that all 109 children were evaluated; number of participants with an adverse effect in both treatment groups that are not reported here are shown in Analysis 2.6. There were no significant differences between treatment groups for febrile neutropenia grade 3 (RR 0.73, 95% CI 0.31 to 1.71; P = 0.47), infection grade 3 (RR 0.74, 95% CI 0.40 to 1.39; P = 0.35), hypomagnesaemia grade 3 (RR 0.91, 95% CI 0.06 to 14.22; P = 0.95), vomiting grade 3 (RR 1.82, 95% CI 0.35 to 9.55; P = 0.48), nausea grade 3 (RR 0.61, 95% CI 0.11 to 3.50; P = 0.58), anaemia grade 3 or 4 (RR 1.25, 95% CI 0.55 to 2.8; P = 0.59), leukopenia grade 3 (RR 0.91, 95% CI 0.13 to 6.24; P = 0.93), neutropenia grade 3 or 4 (RR 1.52, 95% CI 0.59 to 3.90; P = 0.38), thrombocytopenia grade 3 or 4 (RR 0.91, 95% CI 0.13 to 6.24; P = 0.93), gastrointestinal event (not reported if grade 3 or 4; RR 1.37, 95% CI 0.24 to 7.87; P = 0.73), elevated liver enzyme level grade 3 or 4 (RR 0.61, 95% CI 0.18 to 2.04; P = 0.42), elevated serum glucose level grade 3 (RR 0.46, 95% CI 0.04 to 4.88; P = 0.52) and hypermagnesaemia grade 3 (RR 2.28, 95% CI 0.46 to 11.25; P = 0.31). None of the participants in this study experienced left ventricular systolic dysfunction grade 3 or 4 and renal event grade 3 or 4. We were unable to calculate an RR for some adverse effects since one group experienced no events, but there were no significant differences between treatment groups: 0/57 participants in the sodium thiosulfate group and 0/52 participants in the control group experienced allergy grade 3 (Fischer's exact test P = 0.48); 1/57 participants in the sodium thiosulfate group and 0/52 participants in the control group experienced hypernatraemia grade 3 (Fischer's exact test P = 1.00); 5/57 participants in the sodium thiosulfate group and 0/52 participants in the control group experienced hypophosphataemia grade 3 (Fischer's exact test P = 0.06); 0/57 participants in the sodium thiosulfate group and 2/52 participants in the control group experienced hyperkalaemia grade 3 (Fischer's exact test P = 0.23); 0/57 participants in the sodium thiosulfate group and 1/52 participants in the control group experienced dyspnoea grade 3 (Fischer's exact test P = 0.48); 5/57 participants in the sodium thiosulfate group and 0/52 participants in the control group experienced hypokalaemia grade 3 or 4 (Fischer's exact test P = 0.06). The certainty of evidence was low for all assessed adverse effects.
2.6. Analysis.
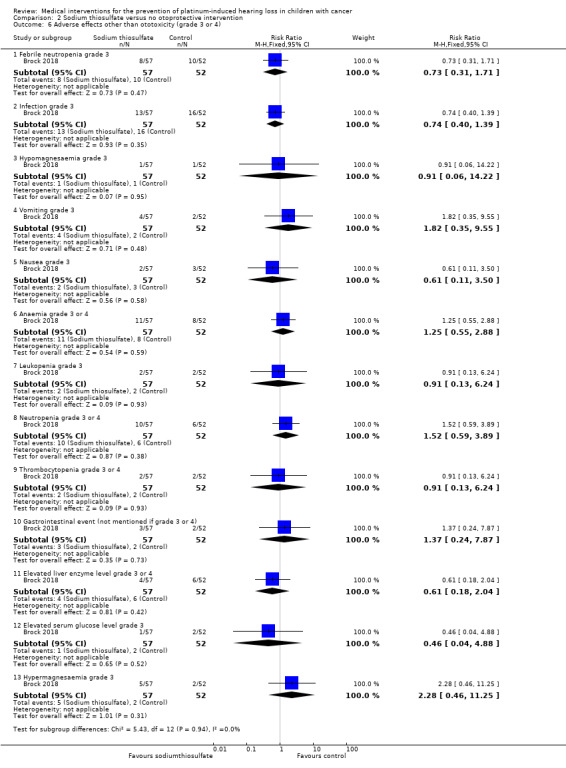
Comparison 2 Sodium thiosulfate versus no otoprotective intervention, Outcome 6 Adverse effects other than ototoxicity (grade 3 or 4).
Quality of life
Brock 2018 did not evaluate quality of life.
Discussion
Summary of main results
Platinum‐based therapy, including cisplatin, carboplatin, oxaliplatin or a combination of these, is used to treat a variety of paediatric malignancies. One of the most significant adverse effects is the occurrence of hearing loss or ototoxicity (McHaney 1983). Although it is not life‐threatening, loss of hearing, especially during the first three years of life and even when only borderline to mild, can have important implications. It can negatively impact speech and language development, which may lead to difficulties with school performance and psychosocial functioning (Gregg 2004; Skinner 2004; Gurney 2007; Dean 2008). Prevention of platinum‐induced hearing loss is thus very important. This is the third update of the first systematic review evaluating all medical interventions for the prevention of platinum‐induced hearing loss in children with cancer (Van As 2012; Van As 2016c).
To ascertain the efficacy of an otoprotective medical intervention adequately the best study design, provided that the design and execution are correct, is an RCT in which the only difference between the intervention group and control group is the use of the otoprotective medical intervention. CCTs can also provide reliable information, keeping in mind their limitations, but we did not include other study designs (including historical control groups) in this review due to the high risk of bias associated with such designs.
We identified three studies evaluating the use of amifostine versus no otoprotective intervention, two RCTs and one CCT; the updates identified no new studies. Two studies included children with osteosarcoma, the other study included children with hepatoblastoma. Participants received cisplatin only or a combination of cisplatin and carboplatin, either administered intra‐arterially or intravenously. Unfortunately, as is explained in the results, pooling of the results of the included studies was not possible. However, in all individual studies there was no significant difference in symptomatic ototoxicity only (i.e. NCICTCv2 or modified Brock criteria grade 2 or higher) and combined asymptomatic and symptomatic ototoxicity (i.e. NCICTCv2 or modified Brock criteria grade 1 or higher) between participants treated with or without amifostine. None of the included studies provided a description of the exact tests that were used to evaluate ototoxicity so we could not comment on their appropriateness (e.g. if age‐specific tests were used or if participants were checked for otitis media, which is common in this age group (Brock 1991). An important question regarding any otoprotective medical intervention during platinum treatment is whether the otoprotective drug could decrease the ototoxicity by platinum agents without reducing the antitumour efficacy (i.e. the tumour response and survival) and without negative effects on toxicities other than ototoxicity. Only one study provided information on tumour response, defined as the number of participants with a complete, good or partial remission (Gallegos‐Castorena 2007). This study included children with osteosarcoma treated with intra‐arterial cisplatin. The available‐data analysis (there were no response data available for one participant in the control group), best‐case scenario analysis and worst‐case scenario analysis all showed a difference in favour of the amifostine group but only in the worst‐case scenario analysis (i.e. the participant with missing data did not have a remission) was this difference significant (P = 0.04). There was no information on survival for any of the included study populations. However, Katzenstein 2009 did provide data on event‐free survival, defined as the period from the date chemotherapy was started until evidence of an event (progressive disease, death, diagnosis of a second malignant neoplasm) or date of last contact, whichever occurred first, for 184 participants enrolled in this study (instead of only the 82 participants included in the toxicity interim analysis); these 184 participants were not the complete study group. There was no significant difference between the treatment groups (P = 0.22). Only one study, including children with osteosarcoma treated with intra‐arterial cisplatin, provided data on the number of participants with adverse effects other than ototoxicity grade 3 or higher (Gallegos‐Castorena 2007; according to NCICTCv2 scale). There was a significant difference in favour of the control group in the occurrence of vomiting grade 3 or 4 (RR 9.04, 95% CI 1.99 to 41.12; P = 0.004). None of the participants in this study experienced cardiac toxicity grade 3 or 4. There was no significant difference between the treatment groups for renal toxicity grade 3 or 4. Finally, none of the studies evaluated quality of life (see Table 1).
In this update, we also identified one RCT including children with localized hepatoblastoma evaluating the use of sodium thiosulfate versus no otoprotective intervention. Participants received cisplatin only (one child also received carboplatin) administered intravenously. There was a significant difference in both symptomatic ototoxicity only (i.e. Brock criteria grade 2 or higher) and combined asymptomatic and symptomatic ototoxicity (i.e. Brock criteria grade 1 or higher) in favour of participants treated with sodium thiosulfate. Age‐appropriate tests were used and chronic otitis media was taken into account. There was no significant difference between the treatment groups in tumour response (defined as number of participants with a complete or partial response at the end of treatment), overall survival (calculated from time of randomization to death or last follow‐up), event‐free survival (calculated from time of randomization until disease progression, disease relapse, second primary cancer, death or last follow‐up, whichever came first) and adverse effects other than hearing loss and tinnitus grade 3 or higher (according to NCICTCAEv3 criteria). The study did not assess quality of life (see Table 2).
In this review, we tried to perform ITT analyses since they provide the most realistic and unbiased answer to the question of clinical effectiveness (Lachin 2000). However, for Katzenstein 2009 an ITT analysis was not possible; the presented toxicity interim analysis included 82 participants who were part of a larger study group and the total number of eligible participants was unclear. Therefore, we performed an 'available‐data' analysis for this study. Also, we were unable to check if the ototoxicity results of this study were available for at least 50% of the eligible participants. In the Methods section, we stated that if that was not the case we would not report the results due to the associated high risk of attrition bias. However, we decided to give this study the benefit of the doubt. For Brock 2018, it was unclear if the survival analyses were ITT.
In our previous update, we identified another study evaluating cisplatin chemotherapy with or without sodium thiosulfate in children with different types of tumours, such as childhood liver cancer, germ cell tumour, medulloblastoma, neuroblastoma and osteosarcoma. Even though this study has been published in full since that time (Freyer 2017), as not enough information is available to judge if treatment was the same in both treatment groups it remains unclear if this study is eligible for inclusion in our review, but it does not seem likely. Two other studies have been added as awaiting classification; one studying pantoprazole (Fox 2018) and one studying OTO‐104 (NCT02997189). Finally, we are still awaiting the final publication of the study by Katzenstein 2009.
For other possible otoprotective medical interventions and other types of malignancies, we found no eligible studies.
Overall completeness and applicability of evidence
'No evidence of effect', as identified in this review, is not the same as 'evidence of no effect'. The reason that no significant difference between treatment groups was identified could be due to the number of participants included in these studies being too small to detect a difference (i.e. low power). Also, hearing loss develops not only during platinum‐based therapy but also years after completion of the therapy (Bertolini 2004; Knight 2005), so the length of follow‐up could have been too short to detect a difference between the treatment groups. Unfortunately most of the included studies did not mention the length of follow‐up.
Quality of the evidence
The certainty of the evidence was low for almost all evaluated outcomes; we downgraded one level each for both imprecision and study limitations. Evidence was graded as moderate only for ototoxicity in the sodium thiosulfate study (downgraded one level because of study limitations). Often bias could not be ruled out due to lack of reporting. However, at this time this is the best available evidence, based on RCTs and CCTs, evaluating amifostine and sodium thiosulfate as an otoprotective medical intervention in children treated with platinum chemotherapy.
Furthermore, although according to the protocol for the included studies participants in both treatment groups should have received the same platinum dosage schedule, none of the included studies reported the exact cumulative platinum dose received by the participants in both treatment groups. One study stated that the actually received doses did not differ significantly between treatment groups (Petrilli 2002), but in the other studies it was unclear if the participants in the intervention and control groups received similar cumulative platinum doses. If participants in the control group received a higher cumulative platinum dose than participants in the intervention group this could have led to an overestimation of the otoprotective effect of the intervention (and vice versa). This uncertainty should also be kept in mind when interpreting the results of the secondary outcomes (response rate and adverse effects). The same is true for impaired renal function at the time of platinum treatment, and the use of other ototoxic drugs such as aminoglycosides (anthracyclines, gentamycin), vincristine and furosemide (Gallagher 1979; Skinner 2004; Jenney 2005; Meyer 2009). It was unclear if there were important imbalances between the treatment groups regarding these factors.
Potential biases in the review process
This systematic review used a very broad search strategy for identifying eligible studies. Thus, although it is unlikely that we missed eligible studies, it is never possible to completely rule out reporting bias. The search strategy included search terms for ototoxicity and as a result it is possible that for outcomes other than hearing loss and tinnitus more studies are available than the ones identified in this review.
Authors' conclusions
Implications for practice.
At the moment there is no evidence from the individual studies (two randomized controlled trials (RCTs) and one controlled clinical trial (CCT)) in children with osteosarcoma and hepatoblastoma treated with different platinum analogues and dosage schedules which underscores the use of amifostine as an otoprotective intervention as compared to no additional treatment. Since pooling of results was not possible, and the certainty of the evidence was low, no definitive conclusions can be made. It should be noted that 'no evidence of effect', as identified in this review, is not the same as 'evidence of no effect'. Based on the currently available evidence (low certainty) we are unable to draw conclusions on the benefits and harms of amifostine.
Since only one RCT evaluating the use of sodium thiosulfate in children with localized hepatoblastoma treated with cisplatin was identified, no definitive conclusions can be drawn. However, there was a significant difference in the occurrence of ototoxicity in favour of sodium thiosulfate (moderate‐certainty evidence), while there were no differences between treatment groups in antitumour efficacy and adverse effects (low‐certainty evidence). It should be noted that 'no evidence of effect', as identified in this review, is not the same as 'evidence of no effect'. Based on the currently available evidence (moderate to low certainty), we are unable to draw definitive conclusions on the benefits and harms of sodium thiosulfate.
For other possible otoprotective medical interventions and other types of malignancies we identified no RCTs or CCTs, so no conclusions can be made about their efficacy in preventing ototoxicity in children treated with platinum‐based therapy. Based on the currently available evidence we are unable to draw conclusions.
Implications for research.
Before definitive conclusions can be made about the efficacy of possible otoprotective medical interventions in children (amifostine, sodium thiosulfate or another intervention) treated with platinum‐based therapy, more high‐quality research is needed. Future trials should preferably be RCTs. They should preferably be performed in homogeneous study populations (with regard to, for example, tumour diagnosis and stage of disease) as otherwise for example no conclusions on antitumour efficacy can be made. Furthermore, future trials should have a long‐term follow‐up, i.e. study participants should not only be followed during treatment, but as platinum‐induced ototoxicity can also develop many years after the end of treatment as long after the end of treatment as possible. Also, valid outcome definitions (including ototoxicity, antitumour efficacy, adverse effects and quality of life) should be used. Appropriate age‐specific hearing tests should be used to assess ototoxicity (Clemens 2019), and it should be described how exactly these tests were performed. Possible risk factors for ototoxicity should be taken into account. The number of included participants should be sufficient to obtain the power needed for the results to be reliable.
What's new
| Date | Event | Description |
|---|---|---|
| 31 January 2019 | New citation required and conclusions have changed | Summary of most important changes in the update: One new randomized controlled trial addressing sodium thiosulfate in children with hepatoblastoma treated with cisplatin was included (this possible otoprotective agent was not yet addressed in the earlier versions of this review) |
| 8 January 2019 | New search has been performed | The search for eligible studies was updated to January 2019 |
History
Protocol first published: Issue 7, 2011 Review first published: Issue 5, 2012
| Date | Event | Description |
|---|---|---|
| 8 July 2016 | New citation required but conclusions have not changed | Unfortunately, no new studies could be included in this second update of the review. As a result the conclusions have not changed. |
| 8 July 2016 | New search has been performed | The search for eligible studies was updated to July 2016. |
| 2 April 2014 | New search has been performed | The search for eligible studies was updated to March 2014. |
| 2 April 2014 | New citation required but conclusions have not changed | Unfortunately, no new studies could be included in this update of the review. As a result the conclusions have not changed. However, as opposed to the original version of the review we have now included a summary of findings table. |
Acknowledgements
We thank FS van Etten‐Jamaludin (clinical librarian at the medical library of the Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands) for running the searches in CENTRAL, MEDLINE and Embase for the third update of this review. We thank Dr H Katzenstein and Dr PR Brock for responding to our request for additional information on their studies. We also thank Dr JB Dean (Pediatric Specialists of Virginia, George Washington University School of Medicine, USA) who kindly agreed to peer review this latest update of our review. We also thank 'Stichting Kinderen Kankervrij' (KiKa), Netherlands, for the financial support that made it possible to perform this systematic review. Finally, we would like to thank the Editorial Base of Cochrane Childhood Cancer for their advice and support. The Editorial Base of Cochrane Childhood Cancer has been funded by KiKa and is located in the Prinsess Máxima Center for Pediatric Oncology, Utrecht, the Netherlands.
Appendices
Appendix 1. Search strategy for Cochrane Central Register of Controlled Trials (CENTRAL)
1. For Hearing loss, we used the following text words in the original version of the review and the first update:
Deafness OR hearing loss OR Loss, Hearing OR hearing disorders OR auditory OR hearing impairment OR hearing impairments OR hearing impairment* OR hear* OR audiologic OR audiometry OR audiometr* OR audiogram OR ototoxicology OR ototoxic* OR hypoacusis OR hypoacuses OR hypoacus* OR ototoxicity OR deaf* OR cochleotoxicity
For the second and third update, we optimized this search strategy by excluding "hear*".
2. For Cisplatin, we used the following text words:
Cisplatin OR cis‐Diamminedichloroplatinum(II) OR Platinum Diamminodichloride OR Diamminodichloride, Platinum OR cis‐Platinum OR cis Platinum OR Dichlorodiammineplatinum OR cis‐Diamminedichloroplatinum OR cis Diamminedichloroplatinum OR cis‐Dichlorodiammineplatinum(II) OR Platinol OR Platidiam OR Platino OR NSC‐119875 OR Biocisplatinum OR CDDP OR CACP OR cisplatin* OR abiplatin OR neoplatin OR cis‐DDP
3. For Carboplatin, we used the following text words:
Carboplatin OR cis‐Diammine(cyclobutanedicarboxylato)platinum II OR CBDCA OR Carbosin OR Pharmachemie Brand of Carboplatin OR Carbotec OR Columbia Brand of Carboplatin OR Ercar OR Almirall Brand of Carboplatin OR JM‐8 OR JM 8 OR JM8 OR Neocarbo OR Neocorp Brand of Carboplatin OR NSC‐241240 OR NSC 241240 OR NSC241240 OR Paraplatin OR Carboplat OR Paraplatine OR Bristol‐Myers Squibb Brand of Carboplatin OR Platinwas OR Chiesi Brand of Carboplatin OR Ribocarbo OR ribosepharm Brand of Carboplatin OR Blastocarb OR Lemery Brand of Carboplatin OR Nealorin OR Prasfarma Brand of Carboplatin OR carboplatin* OR Platinum OR Platinum Compounds OR platinum*
4. For Oxaliplatin and other platinum compounds, we used the following text words:
Oxaliplatin OR oxaliplatin* OR oxaliplatine OR platinum(II)‐1,2‐cyclohexanediamine oxalate OR 1,2‐diaminocyclohexane platinum oxalate OR oxalato‐(1,2‐cyclohexanediamine)platinum II OR cis‐oxalato‐(trans‐l)‐1,2‐diaminocyclohexane‐platinum(II) OR Eloxatine OR Eloxatin OR oxaliplatin, (SP‐4‐2‐(1S‐trans))‐isomer OR oxaliplatin, (SP‐4‐3‐(cis))‐isomer OR ACT 078 OR ACT‐078 OR oxaliplatin, (SP‐4‐2‐(1R‐trans))‐isomer OR 63121‐00‐6 OR 61825‐94‐3 OR dacotin OR dacplat OR jm‐83 OR l‐ohp OR oxalatoplatinum OR rp 54780 OR sr‐96669 OR Platinum OR Platinum Compounds OR platinum* OR organoplatinum compounds
5. For Children, the following we used the following text words in the original version of the review and the first update:
Infant OR infan* OR newborn OR newborn* OR new‐born* OR baby OR baby* OR babies OR neonat* OR perinat* OR postnat* OR child OR child* OR schoolchild* OR schoolchild OR school child OR school child* OR kid OR kids OR toddler* OR adolescent OR adoles* OR teen* OR boy* OR girl* OR minors OR minors* OR underag* OR under ag* OR juvenil* OR youth* OR kindergar* OR puberty OR puber* OR pubescen* OR prepubescen* OR prepuberty* OR pediatrics OR pediatric* OR paediatric* OR peadiatric* OR schools OR nursery school* OR preschool* OR pre school* OR primary school* OR secondary school* OR elementary school* OR elementary school OR high school* OR highschool* OR school age OR schoolage OR school age* OR schoolage* OR infancy
For the second and third update, we used the following text words: infan* OR newborn* OR new‐born* OR perinat* OR neonat* OR baby OR baby* OR babies OR toddler* OR minors OR minors* OR boy OR boys OR boyfriend OR boyhood OR girl* OR kid OR kids OR child OR child* OR children* OR schoolchild* OR schoolchild OR school child OR school child* OR adolescen* OR juvenil* OR youth* OR teen* OR under*age* OR pubescen* OR pediatrics OR pediatric* OR paediatric* OR peadiatric* OR school OR school* OR prematur* OR preterm*
Final search 1 AND (2 OR 3 OR 4) AND 5
The search was performed in title, abstract or keywords
*=zero or more characters
Appendix 2. Search strategy for PubMed
1. ForHearing loss, we used the following MeSH headings and text words in the original version of the review and the first update:
Deafness OR hearing loss OR Loss, Hearing OR hearing disorder OR hearing disorders OR auditory OR hearing impairment OR hearing impairments OR hearing impairment* OR hear* OR audiology OR audiologic OR audiometry OR audiometr* OR audiogram OR audiography OR ototoxicology OR ototoxic* OR hypoacusis OR hypoacuses OR hypoacus* OR ototoxicity OR deaf* OR cochleotoxicity
For the second and third update, we optimized this search strategy by excluding "hear*".
2. For Cisplatin, we used the following MeSH headings and text words:
Cisplatin OR cis‐Diamminedichloroplatinum(II) OR Platinum Diamminodichloride OR Diamminodichloride, Platinum OR cis‐Platinum OR cis Platinum OR Dichlorodiammineplatinum OR cis‐Diamminedichloroplatinum OR cis Diamminedichloroplatinum OR cis‐Dichlorodiammineplatinum(II) OR Platinol OR Platidiam OR Platino OR NSC‐119875 OR Biocisplatinum OR CDDP OR CACP OR cisplatin* OR abiplatin OR neoplatin OR cis‐DDP
3. ForCarboplatin, we used the following MeSH headings and text words:
Carboplatin OR cis‐Diammine(cyclobutanedicarboxylato)platinum II OR CBDCA OR Carbosin OR Pharmachemie Brand of Carboplatin OR Carbotec OR Columbia Brand of Carboplatin OR Ercar OR Almirall Brand of Carboplatin OR JM‐8 OR JM 8 OR JM8 OR Neocarbo OR Neocorp Brand of Carboplatin OR NSC‐241240 OR NSC 241240 OR NSC241240 OR Paraplatin OR Carboplat OR Paraplatine OR Bristol‐Myers Squibb Brand of Carboplatin OR Platinwas OR Chiesi Brand of Carboplatin OR Ribocarbo OR ribosepharm Brand of Carboplatin OR Blastocarb OR Lemery Brand of Carboplatin OR Nealorin OR Prasfarma Brand of Carboplatin OR carboplatin*
4. For Oxaliplatin and other platinum compounds, we used the following MeSH headings and text words:
Oxaliplatin OR oxaliplatin* OR 1,2‐diamminocyclohexane(trans‐1)oxolatoplatinum(II) OR oxaliplatine OR platinum(II)‐1,2‐cyclohexanediamine oxalate OR 1,2‐diaminocyclohexane platinum oxalate OR oxalato‐(1,2‐cyclohexanediamine)platinum II OR cis‐oxalato‐(trans‐l)‐1,2‐diaminocyclohexane‐platinum(II) OR Eloxatine OR Eloxatin OR oxaliplatin, (SP‐4‐2‐(1S‐trans))‐isomer OR oxaliplatin, (SP‐4‐3‐(cis))‐isomer OR ACT 078 OR ACT‐078 OR oxaliplatin, (SP‐4‐2‐(1R‐trans))‐isomer OR 63121‐00‐6 OR 61825‐94‐3 OR dacotin OR dacplat OR jm‐83 OR l‐ohp OR oxalatoplatinum OR rp 54780 OR sr‐96669 OR Platinum OR Platinum Compounds OR platinum* OR organoplatinum compounds [mh]
5. ForChildren, we used the following MeSH headings and text words in the original version of the review and the first update:
Infant OR infan* OR newborn OR newborn* OR new‐born* OR baby OR baby* OR babies OR neonat* OR child OR child* OR schoolchild* OR schoolchild OR school child OR school child* OR kid OR kids OR toddler* OR adolescent OR adoles* OR teen* OR boy* OR girl* OR minors OR minors* OR underag* OR under ag* OR juvenil* OR youth* OR kindergar* OR puberty OR puber* OR pubescen* OR prepubescen* OR prepuberty* OR pediatrics OR pediatric* OR paediatric* OR peadiatric* OR schools OR nursery school* OR preschool* OR pre school* OR primary school* OR secondary school* OR elementary school* OR elementary school OR high school* OR highschool* OR school age OR schoolage OR school age* OR schoolage* OR infancy OR schools, nursery OR infant, newborn
For the second and third update, we used the following MeSH headings and text words: infan* OR newborn* OR new‐born* OR perinat* OR neonat* OR baby OR baby* OR babies OR toddler* OR minors OR minors* OR boy OR boys OR boyfriend OR boyhood OR girl* OR kid OR kids OR child OR child* OR children* OR schoolchild* OR schoolchild OR school child[tiab] OR school child*[tiab] OR adolescen* OR juvenil* OR youth* OR teen* OR under*age* OR pubescen* OR pediatrics[mh] OR pediatric* OR paediatric* OR peadiatric* OR school[tiab] OR school*[tiab] OR prematur* OR preterm* (Leclercq 2013)
6. ForRCTs/CCTs, we used the following MeSH headings and text words in the original version of the review and the first update:
(Randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) AND humans[mh]
For the second and third update, we used the following MeSH headings and text words: (Randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (animals [mh] NOT humans [mh]) (Higgins 2011)
Final search 1 AND (2 OR 3 OR 4) AND 5 AND 6
[pt = publication type; tiab = title, abstract; sh = subject heading; mh = MeSH term; *=zero or more characters; RCT = randomized controlled trial; CCT = controlled clinical trial]
Appendix 3. Search strategy for Embase (Ovid)
1. For Hearing loss, we used the following Emtree terms and text words in the original version of the review and the first update:
1. exp hearing impairment/ 2. (deafness or deaf$ or hearing impairment or hearing impairments or hearing impairment$).mp. 3. hearing loss.mp. or exp hearing loss/ 4. exp hearing disorder/ 5. (hearing disorder or hearing disorders).mp. 6. hear$.mp. 7. auditory.mp. 8. exp audiology/ or audiologic$.mp. 9. exp audiometry/ 10. (audiometry or audiometr$ or audiogram).mp. 11. exp audiography/ 12. (ototoxicology or ototoxic$ or ototoxicity).mp. 13. exp OTOTOXICITY/ 14. exp HYPOACUSIS/ 15. (hypoacusis or hypoacuses or hypoacus$).mp. 16. cochleotoxicity.mp. 17. or/1‐16
For the second and third update, we optimized this search strategy by excluding "hear$".
2. For Cisplatin, we used the following Emtree terms and text words:
1. exp CISPLATIN DERIVATIVE/ or exp CISPLATIN/ or cisplatin.mp. 2. cis‐Diamminedichloroplatinum.mp. 3. Platinum Diamminodichloride.mp. 4. (cis‐Platinum or cis Platinum or Dichlorodiammineplatinum or cis‐Diamminedichloroplatinum or cis Diamminedichloroplatinum or cis‐Dichlorodiammineplatinum).mp. 5. (Platinol or Platidiam or Platino or NSC‐119875 or Biocisplatinum or CDDP or CACP).mp. 6. (cisplatin$ or abiplatin or neoplatin or cis‐DDP).mp. 7. or/1‐6
3. For Carboplatin, we used the following Emtree terms and text words:
1. carboplatin.mp. or exp CARBOPLATIN/ 2. (CBDCA or Carbosin or Carbotec or Ercar).mp. 3. (JM‐8 or JM 8 or JM8).mp. 4. (NSC‐241240 or NSC 241240 or NSC241240).mp. 5. (Neocarbo or Paraplatin or Carboplat or Paraplatine).mp. 6. (Platinwas or Ribocarbo or Blastocarb or nealorin).mp. 7. (carboplatin$ or Platinum or Platinum Compounds or platinum$).mp. 8. or/1‐7
4. For Oxaliplatin and other platinum compounds, we used the following Emtree terms and text words:
1. Oxaliplatin.mp. or exp OXALIPLATIN/ 2. (oxaliplatin$ or oxaliplatine).mp. 3. 1,2‐diaminocyclohexane platinum oxalate.mp. or exp platinum 1,2 diaminocyclohexane/ 4. (Eloxatine or Eloxatin).mp. 5. ("ACT 078" or ACT‐078).mp. 6. (dacotin or dacplat or jm‐83 or l‐ohp or oxalatoplatinum or rp 54780 or sr‐96669).mp. 7. (oxalato 1,2 cyclohexanediamine platinum or platinum 1,2 cyclohexanediamine oxalate or platinum 1,2 diaminocyclohexane oxalate or platinum oxalate 1,2 diaminocyclohexane).mp. 8. transplastin.mp. 9. Organoplatinum Compounds.mp. or exp platinum complex/ 10. 61825‐94‐3.rn. 11. or/1‐10
5. For Children, we used the following Emtree terms and text words in the original version of the review and the first update:
1. infant/ or infancy/ or newborn/ or baby/ or child/ or preschool child/ or school child/ 2. adolescent/ or juvenile/ or boy/ or girl/ or puberty/ or prepuberty/ or pediatrics/ 3. primary school/ or high school/ or kindergarten/ or nursery school/ or school/ 4. or/1‐3 5. (infant$ or newborn$ or (new adj born$) or baby or baby$ or babies or neonate$ or perinat$ or postnat$).mp. 6. (child$ or (school adj child$) or schoolchild$ or (school adj age$) or schoolage$ or (pre adj school$) or preschool$).mp. 7. (kid or kids or toddler$ or adoles$ or teen$ or boy$ or girl$).mp. 8. (minors$ or (under adj ag$) or underage$ or juvenil$ or youth$).mp. 9. (puber$ or pubescen$ or prepubescen$ or prepubert$).mp. 10. (pediatric$ or paediatric$ or peadiatric$).mp. 11. (school or schools or (high adj school$) or highschool$ or (primary adj school$) or (nursery adj school$) or (elementary adj school) or (secondary adj school$) or kindergar$).mp. 12. or/5‐11 13. 4 or 12
For the second and third update, we used the following Emtree terms and text words:
1. infan$.mp. 2. (newborn$ or new‐born$).mp. 3. (perinat$ or neonat$).mp. 4. baby/ 5. (baby or baby$ or babies).mp. 6. toddler$.mp. 7. (minors or minors$).mp. 8. (boy or boys or boyfriend or boyhood).mp. 9. girl$.mp. 10. (kid or kids).mp. 11. child/ 12. (child or child$ or children$).mp. 13. school child/ 14. (schoolchild$ or schoolchild).mp. 15. (school child or school child$).ti,ab. 16. (adolescen$ or youth$ or teen$).mp. 17. (juvenil$ or under$age$).mp. 18. pubescen$.mp. 19. exp pediatrics/ 20. (pediatric$ or paediatric$ or peadiatric$).mp. 21. (school or school$).mp. 22. (prematur$ or preterm$).mp. 23. or/1‐22
6. For RCTs/CCTs, we used the following Emtree terms and text words in the original version of the review and the first update:
1. Randomized Controlled Trial/ 2. Controlled Clinical Trial/ 3. randomized.ti,ab. 4. placebo.ti,ab. 5. randomly.ti,ab. 6. trial.ti,ab. 7. groups.ti,ab. 8. drug therapy.sh. 9. or/1‐8 10. Human/ 11. 9 and 10
For the second and third update, we used the following Emtree terms and text words:
1. Randomized Controlled Trial/ 2. Controlled Clinical Trial/ 3. (randomized or randomised).ti,ab. 4. placebo.ti,ab. 5. randomly.ti,ab. 6. trial.ti,ab. 7. groups.ti,ab. 8. drug therapy.sh. 9. or/1‐8 10. animals/ not human/ 11. 9 not 10
Final search: 1 AND (2 OR 3 OR 4) AND 5 AND 6
[mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name; sh = subject heading; ti,ab = title, abstract; / = Emtree term; $=one or more characters; RCT = randomized controlled trial; CCT = controlled clinical trial]
Appendix 4. Search strategy for conference proceedings
As the 2014 editions of the International Society for Paediatric Oncology (SIOP) and the American Society of Pediatric Hematology/Oncology (ASPHO) conference proceedings were already included in the search of the electronic databases no separate search strategy was needed. For all other editions the pdf files were assessed using these terms: cisplatin, carboplatin, oxaliplatin, platinum, platidiam, CDDP, CACP, DDP, CBDCA, eloxatin, dacotin, dacplat, carbosin, carbotec, ercar, neocarbo, platin, carboplat, ribocarbo, blastocarb, nealorin. The conference proceedings of the International Conference on Long‐Term Complications of Treatment of Children and Adolescents for Cancer were only available on paper, so no search strategy could be used.
Appendix 5. Search strategy for ongoing trials registers
For the International Standard Randomized Controlled Trial Number (ISRCTN) Register (www.isrctn.com), we used the following search strategy:
(cisplatin OR carboplatin OR oxaliplatin OR platinum OR CDDP OR CACP OR DDP OR CBDCA OR platin) AND (deaf OR hearing OR audi OR ototoxic) AND (child OR pediatric OR paediatric OR infant OR neonate OR adolescent). We used the advanced search option for studies with date applied between 17‐3‐2014 and 12‐7‐2016 (earlier results were already included in the previous versions of this review; in the third update this register was not included).
For ClinicalTrials.gov, we used the following search strategy:
(cisplatin OR carboplatin OR oxaliplatin OR platinum OR CDDP OR CACP OR DDP OR CBDCA OR platin) AND (deaf OR hearing OR audi OR ototoxic) AND (child OR pediatric OR paediatric OR infant OR neonate OR adolescent) in combination with the interventional studies (at study type). We used the advanced search option for studies first received/first posted between 12‐7‐2016 and 02‐01‐2019 (earlier results were already included in the previous versions of this review).
For the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), we used the following search strategy (first included in the third update):
1. Cisplatin or carboplatin or oxaliplatin in interventions field 2. Child or pediatric or paediatric in title field 3. Limit trials in children We used the advanced search option with recruitment status 'recruiting' and date of registration between 24 May 2016 and 2 January 2019.
Data and analyses
Comparison 1. Amifostine versus no otoprotective intervention.
1.1. Analysis.
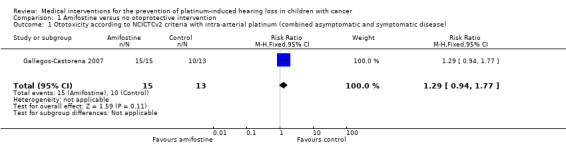
Comparison 1 Amifostine versus no otoprotective intervention, Outcome 1 Ototoxicity according to NCICTCv2 criteria with intra‐arterial platinum (combined asymptomatic and symptomatic disease).
1.2. Analysis.
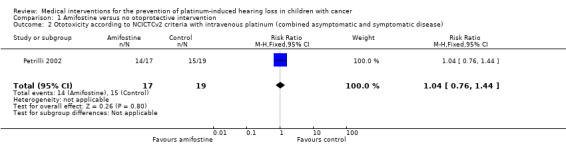
Comparison 1 Amifostine versus no otoprotective intervention, Outcome 2 Ototoxicity according to NCICTCv2 criteria with intravenous platinum (combined asymptomatic and symptomatic disease).
1.3. Analysis.
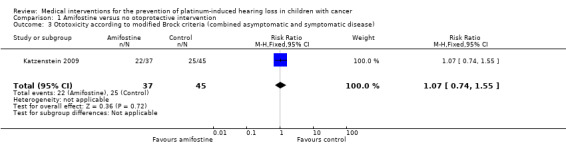
Comparison 1 Amifostine versus no otoprotective intervention, Outcome 3 Ototoxicity according to modified Brock criteria (combined asymptomatic and symptomatic disease).
1.4. Analysis.
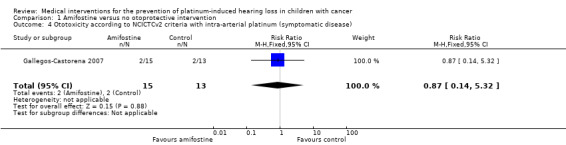
Comparison 1 Amifostine versus no otoprotective intervention, Outcome 4 Ototoxicity according to NCICTCv2 criteria with intra‐arterial platinum (symptomatic disease).
1.5. Analysis.
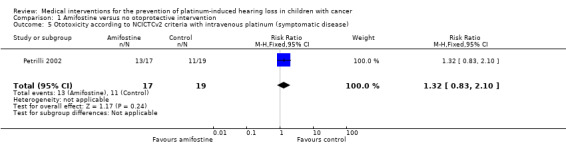
Comparison 1 Amifostine versus no otoprotective intervention, Outcome 5 Ototoxicity according to NCICTCv2 criteria with intravenous platinum (symptomatic disease).
1.6. Analysis.
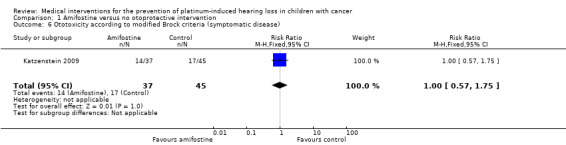
Comparison 1 Amifostine versus no otoprotective intervention, Outcome 6 Ototoxicity according to modified Brock criteria (symptomatic disease).
1.7. Analysis.
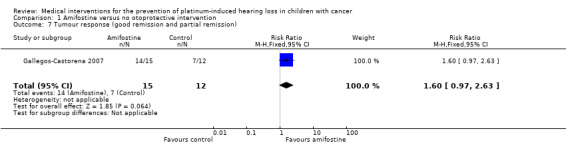
Comparison 1 Amifostine versus no otoprotective intervention, Outcome 7 Tumour response (good remission and partial remission).
1.8. Analysis.
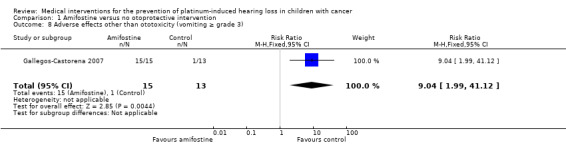
Comparison 1 Amifostine versus no otoprotective intervention, Outcome 8 Adverse effects other than ototoxicity (vomiting ≥ grade 3).
Comparison 2. Sodium thiosulfate versus no otoprotective intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ototoxicity according to Brock criteria with intravenous platinum (combined asymptomatic and symptomatic disease) | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.33, 0.81] |
| 2 Ototoxicity according to Brock criteria with intravenous platinum (symptomatic disease) | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.19, 0.83] |
| 3 Overall survival (Parmar's method was used to obtain the necessary data for the analysis) | 1 | 109 | Hazard Ratio (Fixed, 95% CI) | 0.43 [0.03, 5.85] |
| 4 Event‐free survival (Parmar's method was used to obtain the necessary data for the analysis) | 1 | 109 | Hazard Ratio (Fixed, 95% CI) | 0.85 [0.37, 1.94] |
| 5 Tumour response (complete and partial remission) | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.98, 1.15] |
| 6 Adverse effects other than ototoxicity (grade 3 or 4) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Febrile neutropenia grade 3 | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.31, 1.71] |
| 6.2 Infection grade 3 | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.40, 1.39] |
| 6.3 Hypomagnesaemia grade 3 | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.06, 14.22] |
| 6.4 Vomiting grade 3 | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.35, 9.55] |
| 6.5 Nausea grade 3 | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.11, 3.50] |
| 6.6 Anaemia grade 3 or 4 | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.55, 2.88] |
| 6.7 Leukopenia grade 3 | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.13, 6.24] |
| 6.8 Neutropenia grade 3 or 4 | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.59, 3.89] |
| 6.9 Thrombocytopenia grade 3 or 4 | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.13, 6.24] |
| 6.10 Gastrointestinal event (not mentioned if grade 3 or 4) | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.24, 7.87] |
| 6.11 Elevated liver enzyme level grade 3 or 4 | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.18, 2.04] |
| 6.12 Elevated serum glucose level grade 3 | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.04, 4.88] |
| 6.13 Hypermagnesaemia grade 3 | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [0.46, 11.25] |
2.1. Analysis.
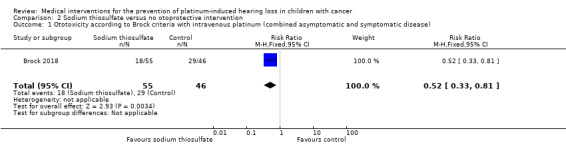
Comparison 2 Sodium thiosulfate versus no otoprotective intervention, Outcome 1 Ototoxicity according to Brock criteria with intravenous platinum (combined asymptomatic and symptomatic disease).
2.2. Analysis.
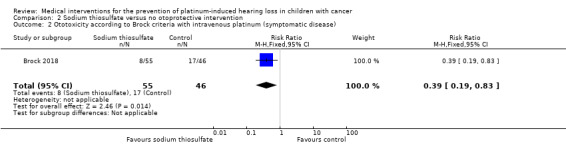
Comparison 2 Sodium thiosulfate versus no otoprotective intervention, Outcome 2 Ototoxicity according to Brock criteria with intravenous platinum (symptomatic disease).
2.3. Analysis.
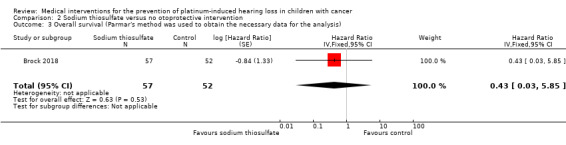
Comparison 2 Sodium thiosulfate versus no otoprotective intervention, Outcome 3 Overall survival (Parmar's method was used to obtain the necessary data for the analysis).
2.4. Analysis.
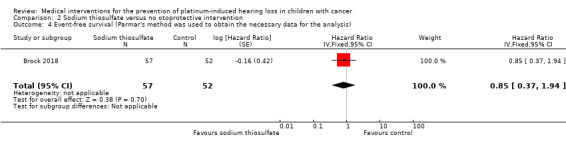
Comparison 2 Sodium thiosulfate versus no otoprotective intervention, Outcome 4 Event‐free survival (Parmar's method was used to obtain the necessary data for the analysis).
2.5. Analysis.
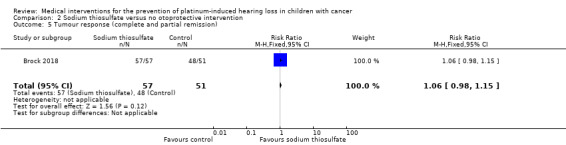
Comparison 2 Sodium thiosulfate versus no otoprotective intervention, Outcome 5 Tumour response (complete and partial remission).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brock 2018.
| Methods | Randomized controlled trial; method of randomization not clear; stratified for age at randomization (≤ 15 months vs > 15 months), tumour extent (PRETEXT score I or II vs III), and country Performed in the UK, Ireland, France, Italy, Belgium, Australia, Japan, Spain, New Zealand, USA, Switzerland and Denmark (52 centres in 12 countries); children were enrolled from 2007 to 2014 and treated on the SIOPEL 6 protocol. |
|
| Participants | 109 children (mean age nm (range 0.1–8.2 years); 59 boys and 50 girls) with standard‐risk hepatoblastoma (PRETEXT stage I or II (72 participants) and PRETEXT stage III without evidence of extrahepatic disease (37 participants), well‐balanced between treatment groups; primary disease) treated with radical surgery (attempted after 4 courses or if the tumour was considered unresectable postponed until the end of treatment; procedure nm) and chemotherapy (i.e. intravenous cisplatin (cumulative dose nm, but according to protocol participants should have received 480 mg/m2; individual platinum dose 80 mg/m2; infusion duration: continuous over 6 hours; doses were adjusted in children weighing < 10 kg, no further information provided), 21 children received 1–6 courses of doxorubicin (9 children/30 courses in cisplatin only group (5 for progressive disease, 4 for other reasons mainly at the request of the surgeon); 12 children/28 courses in the cisplatin and sodium thiosulfate group (4 children for progressive disease, 8 children for other reasons mainly at the request of the surgeon), no information on cumulative doses provided; 1 child in the cisplatin only group changed to SIOPEL 4 treatment at the request of the surgeon because of venous thrombosis which besides doxorubicin and cisplatin also includes carboplatin (no further information provided)). Other ototoxic drugs: anthracyclines yes for some participants (see doxorubicin earlier), vincristine no, gentamycin nm, furosemide nm. No prior platinum treatment. No prior radiotherapy to head or neck, or both. No prior cranial surgery. Prior hearing dysfunction nm (baseline tests were performed, but results nm). No pretreatment renal impairment (at diagnosis, a glomerular filtration rate < 75% of the lower limit of the normal range for age (< 60 mL per minute per 1.73 m2 in children > 2 years of age) was an exclusion criterion). | |
| Interventions | Sodium thiosulfate (20 g/m2 (cumulative dose nm, but according to protocol 120 g/m2; doses were adjusted in children weighing < 10 kg, no further information provided; intravenous infusion over 15 minutes, 6 hours after the end of cisplatin infusion) (57 children) vs no otoprotective intervention (52 children) | |
| Outcomes |
Ototoxicity (according to Brock criteria: see also Table 5); pure‐tone audiometry performed in children aged ≥ 3.5 years. Information on acute otitis media was not collected as this was unlikely to affect sensory‐hearing loss produced by changes in the cochlea. Information on chronic otitis media, fluid in the middle ear or glue ear (which would cause conductive hearing loss) was collected at each hearing test by tympanogram/impedance. In order to accept the Brock criteria grading, the tympanogram/impedance had to be normal. When this was not the case and fluid in the middle ear at the time of testing was suspected, the test had to be repeated 3 months later. Overall survival (calculated from time of randomization to death or last follow‐up) Event‐free survival (calculated from time of randomization until disease progression, disease relapse, second primary cancer, death or last follow‐up, whichever came first). Relapse was defined as recurrent tumour detected on imaging and a serial elevation in the serum alpha‐fetoprotein level (≥ 3 consecutive rising values at a minimum of weekly intervals) or as recurrent tumour detected on imaging, with a normal serum alpha‐fetoprotein level, and histologically confirmed on biopsy. Response rate (complete remission at the end of treatment defined as no evidence of tumour on imaging, partial remission at the end of treatment defined as residual tumour or an alpha‐fetoprotein level above the age‐standardized upper limit of the normal range). Adverse effects grade 3 or higher (according to NCICTCAEv3 criteria; serious adverse events were defined in accordance with the harmonized tripartite guidelines for Good Clinical Practice). |
|
| Notes | Median follow‐up 4.33 years. Final evaluation conducted once all surviving children had reached the age of 3.5 years. Final audiometry performed at median 3 years (range 0.25–6.9 years) after randomization. Age and gender in intervention and control groups were well balanced: in the cisplatin and sodium thiosulfate group median age 1.1 years (range 0.1–8.2 years) and 30 boys (53%); in the cisplatin group median age 1.1 years (range 0.25–5.85 years) and 29 boys (56%). Cumulative cisplatin dose per treatment group nm Genetic variants nm Use of prophylactic antiemetic agents was reported; no further information including the use in both treatment groups was provided. Sodium thiosulfate was supplied free of charge by Fennec Pharmaceuticals, which had no role in the design of the trial, the collection or analysis of the data, or the writing of the manuscript. This study was further supported by Cancer Research UK, La Ligue Contre le Cancer, Krebsforschung Schweiz–Swiss Cancer Research, and the Children's Cancer Research Trust New Zealand, and by grants (R01‐CA137488, R01‐CA199111 and 2R13 CA086959) from the National Institutes of Health, the Veterans Affairs Merit Review Grant, and the Walter S. and Lucienne Driskill Foundation. No information on the influence of those funders was provided. It was stated that Oregon Health and Science University (OHSU), the Portland Veterans Affairs Medical Center (PVAMC), and the Department of Veterans Affairs have a financial interest in Fennec Pharmaceuticals, a company that may have a commercial interest in the results of this research and technology. 1 of the authors who is an inventor of technology licensed to Fennec Pharmaceuticals, has divested himself of all potential earnings. One of the authors reported receiving grant support from Fennec, holding pending patents (U.S. patent numbers, 11/273,723 and 15/284,950), licensed to Fennec Pharmaceuticals, on the administration of a thiol‐based chemoprotectant compound, and holding patents (U.S. patent numbers 7,022,315; 1328253; 60118172 and 2001253919), licensed to Fennec Pharmaceuticals, on the administration of a thiol‐based chemoprotectant compound; he has divested himself of all financial interest regarding these patents. It was stated that "in no patient did the addition of doxorubicin reduce the size of the tumor further." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated that participants were randomly assigned to sodium thiosulfate or no additional treatment, but no further information on the methods of randomization provided. |
| Allocation concealment (selection bias) | Unclear risk | Stated that participants were randomly assigned to sodium thiosulfate or no additional treatment, but no further information on the methods of randomization provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding of participants and personnel provided. |
| Blinding of outcome assessment (detection bias) ‐ ototoxicity All outcomes | Low risk | For hearing loss, outcome assessors were blinded (quote: "The trial data were blinded for the audiology central reviewer") |
| Blinding of outcome assessment (detection bias) ‐ overall survival All outcomes | Low risk | No information on blinding of outcome assessors provided, but as blinding is not relevant for the outcome overall survival we judged this outcome at low risk of detection bias. |
| Blinding of outcome assessment (detection bias) ‐ other reported outcomes All outcomes | Unclear risk | No information on blinding of outcome assessors provided for event‐free survival, response rate and adverse effects other than ototoxicity. |
| Incomplete outcome data (attrition bias) ‐ ototoxicity | Low risk | Hearing loss evaluated in 101/109 children (in the sodium thiosulfate group, 1 child died before the definitive hearing test and 1 child was not tested; in the control group, 4 children died before the definitive hearing test and 2 children were not tested; the reasons 3 children were not tested were audiometry not feasible for health reasons (2 children) and parent refusal (1 child)). |
| Incomplete outcome data (attrition bias) ‐ tumour response and adverse events | Low risk | For tumour response, 1/52 participants in the control group was not evaluated (reason nm); based on the provided information, we assumed all participants were evaluated for adverse effects. |
| Incomplete outcome data (attrition bias) ‐ survival (overall, event‐free or both) | Unclear risk | Insufficient information provided to adequately judge this outcome. |
| Selective reporting (reporting bias) | Low risk | A protocol was mentioned in the manuscript and all expected outcomes were reported. |
| Other bias | Unclear risk |
Block randomization in unblinded trials: unclear (information on both method of randomization and blinding of participants and care providers and for most outcomes blinding of outcome assessors was not provided). Baseline imbalance between treatment groups related to outcome (prior ototoxic treatment, age, sex, prior hearing loss or a combination of these): no prior ototoxic treatment, age and sex were well balanced, prior hearing loss unclear (baseline tests were performed, but results nm). Difference in ototoxic drugs other than platinum analogue between treatment groups (furosemide, gentamycin, anthracyclines, vincristine): cumulative anthracycline dose nm, furosemide and gentamycin nm; vincristine not given. Difference in cumulative platinum dose between treatment groups: cumulative dose unclear, but according to protocol participants in both treatment groups should have received the same dose. Difference in length of follow‐up between treatment groups: unclear (length of follow‐up nm for either group). Difference in impaired renal function at time of platinum treatment between treatment groups: unclear. An insensitive instrument was used to evaluate ototoxicity: no (used pure tone audiometry). |
Gallegos‐Castorena 2007.
| Methods | Randomized controlled trial; method of randomization not clear Performed in Mexico; participants were diagnosed with osteosarcoma between March 1999 and December 2002. |
|
| Participants | 28 children (mean age 11.6 years (range 7 to 15 years); 14 boys and 14 girls) with osteosarcoma (stage nm, but in 5 participants metastatic disease, no significant differences in stage of disease between treatment groups; primary disease) treated with surgery (procedure and location nm) and chemotherapy (i.e. intra‐arterial cisplatin (cumulative dose nm, but according to protocol participants should have received 600 mg/m2; individual platinum dose 150 mg/m2; infusion duration nm) and doxorubicin (cumulative dose nm, but according to protocol participants should have received 150 mg/m2)). Other ototoxic drugs: anthracyclines yes (see doxorubicin earlier), vincristine no, gentamycin nm, furosemide nm. No prior platinum treatment. No prior radiotherapy to head or neck, or both. No prior cranial surgery. Prior hearing dysfunction nm (baseline tests were performed, but results nm). Pretreatment renal impairment nm (baseline tests were performed, but results nm). | |
| Interventions | Amifostine (740 mg/m2/dose (cumulative dose nm, but according to protocol 2960 mg/m2); intravenous infusion under sedation over 15 minutes immediately prior to each cisplatin dose) (15 participants) vs no otoprotective intervention (13 participants) | |
| Outcomes |
Ototoxicity (according to WHO criteria; stated that for the evaluated parameters they did not differ from the NCI system; see also Table 3); audiometry/tympanometry performed (exact instrument used nm). Response rate (complete/good remission defined as > 90% necrosis after tumourectomy; partial remission defined as 60–90% necrosis after tumourectomy). Adverse effects grade 3 or higher (according to WHO criteria; stated that for the evaluated parameters they did not differ from the NCI system). |
|
| Notes | Length of follow‐up nm Age and gender in intervention and control group nm, but stated that the groups were not statistically different. Cumulative cisplatin dose per treatment group nm Genetic variants nm All participants received ondansetron 4 mg/m2/dose 3 times a day and dexamethasone 6 mg/m2/day to prevent nausea and vomiting. Influence of funders unclear (funding nm); no declaration of interest of the authors provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated that participants were randomly assigned to amifostine or no additional treatment, but no further information on the methods of randomization provided. |
| Allocation concealment (selection bias) | Unclear risk | Stated that participants were randomly assigned to amifostine or no additional treatment, but no further information on the methods of randomization provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding of participants and personnel provided. |
| Blinding of outcome assessment (detection bias) ‐ ototoxicity All outcomes | Unclear risk | No information on blinding of outcome assessors provided for ototoxicity. |
| Blinding of outcome assessment (detection bias) ‐ other reported outcomes All outcomes | Unclear risk | No information on blinding of outcome assessors provided for response rate and adverse effects other than ototoxicity. |
| Incomplete outcome data (attrition bias) ‐ ototoxicity | Low risk | Ototoxicity evaluated in all participants. |
| Incomplete outcome data (attrition bias) ‐ tumour response and adverse events | Low risk | All reported outcomes evaluated in all participants, with the exception of response rate, but there was only 1 participant from the control group missing (no histological examination). |
| Selective reporting (reporting bias) | Low risk | No protocol mentioned in the manuscript (and we did not search for it), but all expected outcomes reported. |
| Other bias | Unclear risk |
Block randomization in unblinded trials: unclear (information on both method of randomization and blinding not provided). Baseline imbalance between treatment groups related to outcome (prior ototoxic treatment, age, sex, prior hearing loss or a combination): prior ototoxic treatment nm, age and sex balanced, prior hearing loss unclear (baseline tests were performed, but results nm). Difference in ototoxic drugs other than platinum analogue between treatment groups (furosemide, gentamycin, anthracyclines, vincristine): cumulative anthracycline dose unclear, but according to protocol participants in both treatment groups should have received the same dose; furosemide and gentamycin nm; vincristine not given Difference in cumulative platinum dose between treatment groups: cumulative dose unclear, but according to protocol participants in both treatment groups should have received the same dose Difference in length of follow‐up between treatment groups: unclear (length of follow‐up nm) Difference in impaired renal function at time of platinum treatment between treatment groups: unclear An insensitive instrument was used to evaluate ototoxicity: unclear (exact test method nm) |
Katzenstein 2009.
| Methods | Randomized controlled trial; method of randomization unclear Unclear where this study was performed; unclear when participants were diagnosed (study was opened in March 1999) |
|
| Participants | 82 children (age range 0–11 years; 44 boys and 38 girls) with hepatoblastoma (21 stage I non‐pure fetal histology or II, 61 stage III or IV; participants with stage I pure fetal histology were not eligible for this study; unclear if there were significant differences in stage of disease between treatment groups; primary disease) treated with surgery (procedure and location nm) and chemotherapy. Stage I and II participants received 4 cycles of intravenous cisplatin, C5V; participants with stage III or IV were either randomized to 6 cycles of C5V or 6 cycles of intravenous CC (see notes). Cumulative platinum doses nm, but total intended platinum dose in stage I/II participants was cisplatin 400 mg/m2 (12 mg/kg if < 1 year of age); individual platinum dose 100 mg/m2; infusion duration 4 hours. In stage III/IV participants treated with C5V, the total intended platinum dose was cisplatin 600 mg/m2 (18 mg/kg if < 1 year of age); individual platinum dose 100 mg/m2; infusion duration 4 hours and in stage III/IV participants treated with CC the total intended platinum dose was cisplatin 600 mg/m2 (18 mg/kg if < 1 year of age); individual platinum dose 100 mg/m2; infusion duration 4 hours and the total intended carboplatin dose was 3640 mg/m2 (120 mg/kg if < 10 kg bodyweight); individual platinum dose 700 mg/m2; infusion duration 1 hour. In case of C5V: cumulative 5‐FU doses were nm, but according to protocol participants should have received 600 mg/m2 per cycle (so total 2400 mg/m2 in stage I/II participants and total 3600 mg/m2 in stage III/IV participants); cumulative vincristine doses nm, but according to protocol participants should have received 4.5 mg/m2 per cycle (so total in stage I/II participants 18 mg/m2 and total in stage III/IV participants 27 mg/m2). Other ototoxic drugs: anthracyclines no, vincristine yes for some participants (see above), gentamycin nm, furosemide nm. No prior platinum treatment. No prior radiotherapy to head or neck, or both. No prior cranial surgery. No prior hearing dysfunction (on audiogram or auditory brainstem responses before therapy). No pretreatment renal impairment (i.e. adequate organ function documented at time of study enrolment) | |
| Interventions | Amifostine (740 mg/m2/dose (cumulative dose nm, according to the text of the article amifostine was given immediately before cisplatin, but in the abstract it was mentioned that it was given prior to each administration of a platinum agent, i.e. both before CC); intravenous infusion given over 15 minutes immediately prior to cisplatin) (37 participants) vs no otoprotective intervention (45 participants). In the amifostine group, 7 participants received CC and 30 participants received C5V; in the control group, 11 participants received CC and 34 participants received C5V. |
|
| Outcomes | Ototoxicity (according to modified Brock criteria: see notes and Table 4); audiograms were performed, further information nm | |
| Notes | These 82 children were part of a larger study group. They were considered in a special interim analysis of the incidence of toxicity. The total number of eligible participants was unclear Stage III and IV randomization to CC was suspended in January 2002 when the projected improvement in long‐term outcome associated with CC was excluded statistically as possible outcome of this trial. In November 2003, it was decided that using the CTC criteria substantially underestimated the true incidence of significant hearing loss (4% grade 3 or 4 ototoxicity); it was decided that the subgroup of 82 participants should be evaluated using modified Brock criteria. Length of follow‐up nm Age and gender in intervention and control group nm, it was not stated if the groups were statistically different or not. Cumulative cisplatin/carboplatin dose per treatment group nm Genetic variants nm All participants received granulocyte colony stimulating factor Influence of funders unclear; this study was supported by U10 CA98543 and U10 CA98413 (Children's Oncology Group) and U10 CA029139 (Pediatric Oncology Group) from the NCI, National Institutes of Health. It was stated that 1 of the authors has acted as a consultant or in an advisory role for Ziopharm. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated that participants were randomly assigned to amifostine or no additional treatment, but no further information on the methods of randomization provided. |
| Allocation concealment (selection bias) | Unclear risk | Stated that participants were randomly assigned to amifostine or no additional treatment, but no further information on the methods of randomization provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding of participants and personnel provided. |
| Blinding of outcome assessment (detection bias) ‐ ototoxicity All outcomes | Low risk | For ototoxicity outcome, assessors were blinded. |
| Incomplete outcome data (attrition bias) ‐ ototoxicity | High risk | The participants reported in this manuscript were only a subgroup of all included participants (unclear how many participants were originally included in this study). |
| Selective reporting (reporting bias) | High risk | There was no protocol mentioned in the manuscript (and we did not search for it). However, response was nm, while in the methods section alpha fetoprotein levels and imaging were mentioned. Also, definition of hearing loss was adjusted during the study and only results for the last definition were reported. |
| Other bias | Unclear risk |
Block randomization in unblinded trials: unclear (information on both method of randomization and blinding of participants and care providers not provided). Baseline imbalance between treatment groups related to outcome (prior ototoxic treatment, age, sex, prior hearing loss or a combination): prior ototoxic treatment nm, age and sex nm in different treatment groups, no prior hearing loss. Difference in ototoxic drugs other than platinum analogue between treatment groups (furosemide, gentamycin, anthracyclines, vincristine): furosemide and gentamycin nm; anthracyclines not given; vincristine unclear if balanced in different treatment groups. Difference in cumulative platinum dose between treatment groups: unclear if balanced between treatment groups Difference in length of follow‐up between treatment groups: unclear (length of follow‐up nm) Difference in impaired renal function at time of platinum treatment between treatment groups: unclear An insensitive instrument was used to evaluate ototoxicity: unclear (audiograms were performed, but no further information provided). |
Petrilli 2002.
| Methods | Controlled clinical trial (39 consecutive participants registered in the same protocol: the first 18 participants and the final participant did not receive an otoprotective intervention, whereas the 20 participants inbetween did); Brazilian Bone Tumor Study Group Protocol IV Performed in Brazil; participants were diagnosed with osteosarcoma between June 1996 and December 1997 |
|
| Participants | 39 children (mean age 14.4 years (range 5 to 22 years); 27 males and 12 females) with osteosarcoma (stage nm, participants were either metastatic or not, no significant differences in stage of disease between treatment groups; primary disease) treated with surgery (procedure and location nm) and intravenous chemotherapy (i.e. cisplatin (cumulative dose nm, but according to protocol participants should have received 500 mg/m2; individual dose 100 mg/m2; infusion duration nm), carboplatin (cumulative dose nm, but according to protocol participants should have received 2500 mg/m2; individual dose 500 mg/m2; infusion duration nm), doxorubicin (cumulative dose nm, but according to protocol participants should have received 360 mg/m2) and ifosfamide (cumulative dose nm, but according to protocol participants should have received 45 g/m2); for all 4 chemotherapeutic agents the actual received doses were not statistically significant different than the protocol doses). Other ototoxic drugs: anthracyclines yes (see doxorubicin earlier), vincristine no, gentamycin nm, furosemide nm. No prior platinum treatment. No prior radiotherapy to head or neck, or both. No prior cranial surgery. No prior hearing dysfunction (baseline tests were performed and without normal hearing participants were not eligible for this study). No pretreatment renal impairment (baseline tests were performed and without normal audiometric evaluations at diagnosis participants were not eligible for this study) | |
| Interventions | Amifostine (740 mg/m2/dose (cumulative dose nm, but according to protocol 7400 mg/m2); 15‐minute infusion (intra‐arterial or intravenous nm) immediately prior to every CC dose) (20 participants) vs no otoprotective intervention (19 participants) | |
| Outcomes | Ototoxicity (according to NCICTC version 2 criteria: see also Table 3); objective and subjective audiometric evaluations were performed, no further information provided. | |
| Notes | Length of follow‐up nm Age and gender in intervention and control group nm, but stated that the groups were not statistically different. Cumulative CC dose per treatment group nm (for more information see information under header Participants) Genetic variants nm All participants received ondansetron 0.15 mg/kg per dose 3 times a day, dexamethasone 6 mg/m2/day, metoclopramide 1 mg/kg/day and dimenhydrinate 2 mg/kg/day to prevent nausea and vomiting. Influence of funders unclear (funding nm); no declaration of interest of the authors provided. This was a pilot study for a larger randomized study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled clinical trial, so no randomization performed. |
| Allocation concealment (selection bias) | High risk | Controlled clinical trial, so no randomization performed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants with amifostine were consecutive participants, so blinding not possible. |
| Blinding of outcome assessment (detection bias) ‐ ototoxicity All outcomes | Unclear risk | No information on blinding of outcome assessors provided for ototoxicity. |
| Incomplete outcome data (attrition bias) ‐ ototoxicity | High risk | 3/20 participants in the intervention group missing for almost all outcomes (1 incomplete outcome data, 1 lost to follow‐up, 1 nm). |
| Selective reporting (reporting bias) | High risk | There was no protocol mentioned in the manuscript (and we did not search for it), but not all expected outcomes were reported (e.g. response rate and hypocalcaemia were missing, while for hypocalcaemia it was even stated in the discussion of this article that this is 1 of the most common adverse effects of amifostine) |
| Other bias | Unclear risk |
Block randomization in unblinded trials: not applicable, this was not a randomized trial. Baseline imbalance between treatment groups related to outcome (prior ototoxic treatment, age, sex, prior hearing loss or a combination): prior ototoxic treatment nm, age and sex were balanced, no prior hearing loss. Difference in ototoxic drugs other than platinum analogue between treatment groups (furosemide, gentamycin, anthracyclines, vincristine): cumulative anthracycline dose unclear, but according to protocol participants in both treatment groups should have received the same dose and the actual received doses were not statistically significant different than the protocol doses; furosemide and gentamycin nm; vincristine not given. Difference in cumulative platinum dose between treatment groups: cumulative dose unclear, but according to protocol participants in both treatment groups should have received the same dose and the actual received doses were not statistically significantly different than the protocol doses. Difference in length of follow‐up between treatment groups: unclear (length of follow‐up nm) Difference in impaired renal function at time of platinum treatment between treatment groups: unclear An insensitive instrument was used to evaluate ototoxicity: unclear (objective and subjective audiometric evaluations were performed, no further information provided). |
5‐FU: 5‐fluorouracil; C5V: 5‐fluorouracil plus vincristine; CC: cisplatin and carboplatin; CTC: Common Toxicity Criteria; NCI: National Cancer Institute; NCICTCAEv3: National Cancer Institute Common Toxicity Criteria Adverse Effects version 3; nm: not mentioned; PRETEXT: Pretreatment Extent of Disease; SIOPEL: Société Internationale d'Oncologie Pédiatrique – Epithelial Liver Tumor Study Group; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Biswas 2017 | Not RCT or CCT, but a commentary (no additional studies identified). |
| Crabb 2017 | Study included only adults. |
| Doolittle 2001 | Study with historical controls; both children and adults (maximum age 67 years) included (children not presented separately). |
| Elsendoorn 2001 | Same study as Weijl 2004 (describing the first 27 participants). |
| Fouladi 2008 | Study with historical controls; both children and adults (maximum age 20.16 years) included (children not presented separately); difference in cranial radiotherapy between treatment groups. |
| Geoerger 2005 | No otoprotective intervention evaluated. |
| Grau 1996 | Both children and adults (maximum age 73 years) included (children not presented separately); unclear if other treatment was the same in the different treatment groups. |
| Gurney 2014 | Not RCT or CCT. |
| Killock 2018 | Not RCT or CCT, but a research highlight (no additional studies identified). |
| Kingston 1986 | No otoprotective intervention evaluated. |
| Knight 2008 | Not RCT or CCT, but a case report; no otoprotective intervention. |
| Ladenstein 2010 | Ototoxicity not evaluated. |
| Mahoney 1982 | Same study as Mahoney 1983. |
| Mahoney 1983 | Not RCT or CCT; no otoprotective intervention evaluated. |
| Marina 2005 | Study with historical controls; unclear if same cumulative platinum dose, infusion duration, individual dose and other treatment in the different treatment groups. |
| McHaney 1983 | Not RCT or CCT; no otoprotective intervention evaluated. |
| Meyer 2009 | Not RCT or CCT, but a review (no additional studies identified). |
| Piel 1974 | No otoprotective intervention evaluated. |
| Sarafraz 2018 | Both children and adults (maximum age 60 years) included (children not presented separately); unclear if other treatment was the same in the different treatment groups. |
| Skinner 2006 | Not RCT or CCT, but a commentary (no additional studies identified). |
| Spunt 2007 | Not RCT or CCT; both children and adults (maximal age 21 years) included (children not presented separately). |
| Sullivan 2009 | Not RCT or CCT, but an editorial (no additional studies identified). |
| Weijl 2004 | Both children and adults (maximum age 69 years) included (children not presented separately); significant difference in individual platinum dose between the different treatment groups, unclear if platinum infusion duration and other treatment was the same in the different treatment groups. |
CCT: controlled clinical trial; RCT: randomized controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Fox 2018.
| Methods | Randomized cross‐over design; method of randomization not clear. |
| Participants | 12 children (median age 12.8 years; range 5.6–19 years) with newly diagnosed osteosarcoma. |
| Interventions | Cisplatin (120 mg/m2 per cycle; 4 cycles in total) with or without concurrent pantoprazole (either with cycles 1 and 2 or with cycles 3 and 4; 1.6 mg/kg over 4 hours intravenously), doxorubicin and methotrexate. |
| Outcomes | Objective hearing loss assessed using audiograms; tinnitus and subjective hearing loss assessed using questionnaires. |
| Notes | Not enough information available to judge if treatment was the same in both treatment groups and as only cycles 1 and 2 will be eligible for our review we need to have data for these 2 cycles separately; including participant characteristics and outcome data. Not enough information on ototoxicity results available. We have contacted the corresponding author but not yet received a response so it is not yet clear if this study is eligible for inclusion in this review. |
Freyer 2017.
| Methods | Centrally computer‐generated randomization using permuted blocks of 4; initially stratified by age and duration of cisplatin infusion, previous cranial irradiation added later. |
| Participants | 125 children (aged 1–18 years) with hepatoblastoma, germ cell tumour, medulloblastoma, central nervous system primitive neuroectodermal tumour, neuroblastoma, osteosarcoma or other cancer types. |
| Interventions | Cisplatin‐containing chemotherapy with or without sodium thiosulfate; 16 g/m2 sodium thiosulfate was given over 15 minutes intravenously 6 hours after each cisplatin dose; cisplatin was planned to be given with a cumulative dose of ≥ 200 mg/m2 and an infusion duration of ≤ 6 hours (median cumulative dose in sodium thiosulfate group 393 mg/m2 and in control group 387 mg/m2). |
| Outcomes | Hearing loss according to American Speech‐Language‐Hearing Association criteria using audiometry. |
| Notes | Not enough information available to judge if treatment was the same in both treatment groups. We have contacted the corresponding author but not yet received a response so it is unclear if this study is eligible for inclusion in this review. |
NCT02997189.
| Methods | Method of randomization unclear. |
| Participants | 12 participants aged 6 months to 21 years with neuroblastoma, hepatoblastoma, osteosarcoma or extracranial germ cell tumours. |
| Interventions | OTO‐104 (dexamethasone 12 mg) given by intratympatic administration prior to cisplatin‐based therapy (cumulative cisplatin dose ≥ 200 mg/m2). |
| Outcomes | Hearing function according to SIOP‐Boston ototoxicity scale. Study terminated based on negative efficacy results in people with Ménière's disease. |
| Notes | The only information currently available (as of 2 January 2019) was from the clinical.gov website and, based on that, unclear if the study is eligible for inclusion in the review. We contacted the study chair/sponsor but not yet received a response. |
Differences between protocol and review
In the protocol we stated that "Children (aged 0 to 18 years at diagnosis) with any type of childhood malignancy" were eligible for inclusion; in order not to exclude relevant data we have added the following: "Studies including both children and adults were only eligible for inclusion in this review if the majority of participants were children (i.e. either more than 90% children or the maximal age did not exceed 22 years)".
In the update, we included 'Summary of findings' tables.
For the second update, the Information Specialist of Cochrane Childhood Cancer optimized the search strategy as described in the appendices.
In the third update, we included the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) in our search, but we did not search the International Standard Randomized Controlled Trial Number (ISRCTN) Register anymore (which is in line with Cochrane standards). We also made some small changes and clarifications to be in line with the latest version of the Cochrane MECIR standards and the Cochrane Childhood Cancer standards (such as including information on declarations of interest in included studies and clarifying that sensitivity analyses would only have been performed if at least two studies remained in the analysis after exclusion of the studies with a high or unclear risk of bias).
We made all changes in consultation with Cochrane Childhood Cancer.
Contributions of authors
JvA: identified the studies meeting the inclusion criteria; performed data extraction, risk of bias assessment and GRADE assessment of the included studies; analysed data and interpreted results; wrote and revised the manuscript.
HvdB: provided a clinical perspective; critically reviewed the manuscript.
EvD: developed the search strategy in collaboration with Cochrane Childhood Cancer; identified the studies meeting the inclusion criteria; searched for unpublished and ongoing studies; performed data extraction, risk of bias assessment and GRADE assessment of the included studies; analysed data and interpreted results; wrote and revised the manuscript.
All authors approved the final version.
Sources of support
Internal sources
No sources of support supplied
External sources
Stichting Kinderen Kankervrij (KiKa), Netherlands.
Declarations of interest
JvA: none.
HvdB: none.
EvD: none.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Brock 2018 {published data only (unpublished sought but not used)}
- Brock P, Childs M, Rajput K, Maibach R, Brugieres L, Roebuck D, et al. Two year results of a randomised phase iii trial for standard risk hepatoblastoma (SR‐HB) SIOPEL 6; cisplatin and sodium thiosulfate (STS) vs cisplatin alone (O‐110). Pediatric Blood and Cancer 2016:S33. [Google Scholar]
- Brock P, Pritchard J, Roebuck D, Dicks‐Mireaux C, Bellman S, Rajput K. Improving outcomes for children with hepatoblastoma, increasing the cure rate, reducing the toxicity of treatment and monitoring and preventing hearing loss: thirty years of international collaboration pioneered from GOSH. Archives of Disease in Childhood 2017;102 (Suppl 3):A5. [Google Scholar]
- Brock PR, Childs M, Rajput K, Maibach R, Roebuck D, Sullivan MJ, et al. Two‐year results of clinical efficacy of cisplatin in combination with sodium thiosulfate (STS) vs cisplatin alone in a randomized phase III trial for standard risk hepatoblastoma (SR‐HB): SIOPEL 6. Journal of Clinical Oncology 2016;34 (suppl 15). [Google Scholar]
- Brock PR, Maibach R, Childs M, Rajput K, Roebuck D, Sullivan MJ, et al. Sodium thiosulfate for protection from cisplatin‐induced hearing loss. New England Journal of Medicine 2018;378(25):2376‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maibach R, Childs M, Rajput K, Neuwelt EA, Roebuck D, Sullivan MJ, et al. SIOPEL 6: a multicenter open‐label randomized phase III trial of the efficacy of sodium thiosulphate (STS) in reducing ototoxicity in patients receiving cisplatin (Cis) monotherapy for standard‐risk hepatoblastoma (SR‐HB). Journal of Clinical Oncology 2014;5s:abstract TPS10094. [Google Scholar]
Gallegos‐Castorena 2007 {published data only}
- Gallegos‐Castorena S, Martínez‐Avalos A, Mohar‐Betancourt A, Guerrero‐Avendaño G, Zapata‐Tarrés M, Medina‐Sansón A. Toxicity prevention with amifostine in pediatric osteosarcoma patients treated with cisplatin and doxorubicin. Pediatric Hematology and Oncology 2007;24(6):403‐8. [DOI] [PubMed] [Google Scholar]
Katzenstein 2009 {published data only (unpublished sought but not used)}
- Katzenstein HM, Chang KW, Krailo M, Chen Z, Finegold MJ, Rowland J, et al. Amifostine does not prevent platinum‐induced hearing loss associated with the treatment of children with hepatoblastoma: a report of the Intergroup Hepatoblastoma Study P9645 as a part of the Children's Oncology Group. Cancer 2009;115(24):5828‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petrilli 2002 {published data only}
- Petrilli AS, Oliveira DT, Ginani VC, Kechichian R, Dishtchekenian A, Medeiros Roque Filho W, et al. Use of amifostine in the therapy of osteosarcoma in children and adolescents. Journal of Pediatric Hematology/Oncology 2002;24(3):188‐91. [DOI] [PubMed] [Google Scholar]
- Petrilli AS, Camargo B, Filho VO, Bruniera P, Brunetto AL, Jesus‐Garcia R, et al. Results of the Brazilian Osteosarcoma Treatment Group Studies III and IV: prognostic factors and impact on survival. Journal of Clinical Oncology 2006;24(7):1161‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Biswas 2017 {published data only}
- Biswas B, Ganguly S, Ghosh J, Batra A. Cisplatin‐induced hearing loss in children with cancer. National Medical Journal of India 2017;30(6):327‐8. [DOI] [PubMed] [Google Scholar]
Crabb 2017 {published data only}
- Crabb SJ, Martin K, Abab J, Ratcliffe I, Thornton R, Lineton B, et al. COAST (cisplatin ototoxicity attenuated by aspirin trial): a phase II double‐blind, randomised controlled trial to establish if aspirin reduces cisplatin induced hearing‐loss. European Journal of Cancer 2017;87:75‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doolittle 2001 {published data only}
- Doolittle ND, Muldoon LL, Brummett RE, Tyson RM, Lacy C, Bubalo JS, et al. Delayed sodium thiosulfate as an otoprotectant against carboplatin‐induced hearing loss in patients with malignant brain tumors. Clinical Cancer Research 2001;7(3):493‐500. [PubMed] [Google Scholar]
Elsendoorn 2001 {published data only}
- Elsendoorn TJ, Weijl NI, Mithoe S, Zwinderman AH, Dam F, Zwart FA, et al. Chemotherapy‐induced chromosomal damage in peripheral blood lymphocytes of cancer patients supplemented with antioxidants or placebo. Mutation Research 2001;498(1‐2):145‐58. [DOI] [PubMed] [Google Scholar]
Fouladi 2008 {published data only}
- Fouladi M, Chintagumpala M, Ashley D, Kellie S, Gururangan S, Hassall T, et al. Amifostine protects against cisplatin‐induced ototoxicity in children with average‐risk medulloblastoma. Journal of Clinical Oncology 2008;26(22):3749‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geoerger 2005 {published data only}
- Geoerger B, Vassal G, Doz F, O'Quigley J, Wartelle M, Watson AJ, et al. Dose finding and O6‐alkylguanine‐DNA alkyltransferase study of cisplatin combined with temozolomide in paediatric solid malignancies. British Journal of Cancer 2005;93(5):529‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grau 1996 {published data only}
- Grau JJ, Estape J, Cuchi MA, Firvida JL, Blanch JL, Ascaso C. Calcium supplementation and ototoxicity in patients receiving cisplatin. British Journal of Clinical Pharmacology 1996;42(2):233‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurney 2014 {published data only}
- Gurney JG, Bass JK, Onar‐Thomas A, Huang J, Chintagumpala M, Bouffet E, et al. Evaluation of amifostine for protection against cisplatin‐induced serious hearing loss in children treated for average‐risk or high‐risk medulloblastoma. Neuro‐Oncology 2014;16(6):848‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Killock 2018 {published data only}
- Killock D. Sodium thiosulfate halves the risk of cisplatin‐induced hearing loss. Nature Reviews Clinical Oncology 2018;15(9):533. [DOI] [PubMed] [Google Scholar]
Kingston 1986 {published data only}
- Kingston JE, Abramovich S, Billings RJ. Assessment of the effect of chemotherapy and radiotherapy on the auditory function of children with cancer. Clinical Otolaryngology and Allied Sciences 1986;11:403‐9. [DOI] [PubMed] [Google Scholar]
Knight 2008 {published data only}
- Knight K. Hearing loss in pediatric cancer survivors treated with cisplatin. Oncology (Williston Park) 2008;22(4 Suppl Nurse Edition):35‐7. [PubMed] [Google Scholar]
Ladenstein 2010 {published data only}
- Ladenstein R, Valteau CD, Brock P, Yaniv I, Castel V, Laureys G, et al. Randomized trial of prophylactic granulocyte colony‐stimulating factor during rapid COJEC induction in pediatric patients with high‐risk neuroblastoma: the European HR‐NBL1/SIOPEN study. Journal of Clinical Oncology 2010;28:3516‐24. [DOI] [PubMed] [Google Scholar]
Mahoney 1982 {published data only}
- Mahoney DH, Weaver T, Steuber CP, Starling KA. Cis‐platinum (CDDP) ototoxicity in pediatric patients. Proceedings of the American Society of Clinical Oncology 1982:26. [Google Scholar]
Mahoney 1983 {published data only}
- Mahoney DH Jr, Weaver T, Steuber CP, Starling KA. Ototoxicity with cisplatin therapy. Journal of Pediatrics 1983;103(6):1006‐7. [DOI] [PubMed] [Google Scholar]
Marina 2005 {published data only}
- Marina N, Chang KW, Malogolowkin M, London WB, Frazier AL, Womer RB, et al. Amifostine does not protect against the ototoxicity of high‐dose cisplatin combined with etoposide and bleomycin in pediatric germ‐cell tumors: a Children's Oncology Group study. Cancer 2005;104(4):841‐7. [DOI] [PubMed] [Google Scholar]
McHaney 1983 {published data only}
- McHaney VA, Thibadoux G, Hayes FA, Green AA. Hearing loss in children receiving cisplatin chemotherapy. Journal of Pediatrics 1983;102(2):314‐7. [DOI] [PubMed] [Google Scholar]
Meyer 2009 {published data only}
- Meyer AK, Young NM. Auditory late effects of chemotherapy. Cancer Treatment and Research 2009;150:195‐213. [DOI] [PubMed] [Google Scholar]
Piel 1974 {published data only}
- Piel IJ, Meyer D, Perlia CP, Wolfe VI. Effects of cis‐diamminedichloroplatinum (NSC‐119875) on hearing function in man. Cancer Chemotherapy Reports 1974;58(6):871‐5. [PubMed] [Google Scholar]
Sarafraz 2018 {published data only}
- Sarafraz Z, Ahmadi A, Daneshi A. Transtympanic Injections of N‐acetylcysteine and dexamethasone for prevention of cisplatin‐induced ototoxicity: double blind randomized clinical trial. International Tinnitus Journal 2018;22(1):40‐5. [DOI] [PubMed] [Google Scholar]
Skinner 2006 {published data only}
- Skinner R. Preventing platinum‐induced ototoxicity in children – is there a potential role for sodium thiosulfate?. Pediatric Blood and Cancer 2006;47(2):120‐2. [DOI] [PubMed] [Google Scholar]
Spunt 2007 {published data only}
- Spunt SL, Freeman BB III, Billups CA, McPherson V, Khan RB, Pratt CB, et al. Phase I clinical trial of oxaliplatin in children and adolescents with refractory solid tumors. Journal of Clinical Oncology 2007;25(16):2274‐80. [DOI] [PubMed] [Google Scholar]
Sullivan 2009 {published data only}
- Sullivan MJ. Hepatoblastoma, cisplatin, and ototoxicity: good news on deaf ears. Cancer 2009;115(24):5623‐6. [DOI] [PubMed] [Google Scholar]
Weijl 2004 {published data only}
- Weijl NI, Elsendoorn TJ, Lentjes EG, Hopman GD, Wipkink‐Bakker A, Zwinderman AH, et al. Supplementation with antioxidant micronutrients and chemotherapy‐induced toxicity in cancer patients treated with cisplatin‐based chemotherapy: a randomised, double‐blind, placebo‐controlled study. European Journal of Cancer 2004;40(11):1713‐23. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Fox 2018 {published data only (unpublished sought but not used)}
- Fox E, Levin K, Zhu Y, Segers B, Balamuth N, Womer R, et al. Pantoprazole, an inhibitor of the organic cation transporter 2, does not ameliorate cisplatin‐related ototoxicity or nephrotoxicity in children and adolescents with newly diagnosed osteosarcoma treated with methotrexate, doxorubicin, and cisplatin. Oncologist 2018;23(7):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freyer 2017 {published data only (unpublished sought but not used)}
- Freyer DR. Erratum for "Effects of sodium thiosulfate versus observation on development of cisplatin‐induced hearing loss in children with cancer (ACCL0431): a multicentre, randomised, controlled, open‐label, phase 3 trial [Lancet Oncology 2017]". Lancet Oncology 2017;18(6):e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyer DR. The effects of sodium thiosulfate (STS) on cisplatin‐induced hearing loss: a report from the Children's Oncology Group. Journal of Clinical Oncology 2014;5s:abstract 10017. [Google Scholar]
- Freyer DR, Chen L, Krailo MD, Knight K, Villaluna D, Bliss B, et al. Effects of sodium thiosulfate versus observation on development of cisplatin‐induced hearing loss in children with cancer (ACCL0431): a multicentre, randomised, controlled, open‐label, phase 3 trial. Lancet Oncology 2017;18(1):63‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02997189 {published data only (unpublished sought but not used)}
- NCT02997189. Study of OTO‐104 in subjects at risk from cisplatin‐induced hearing loss (NCT02997189). clinicaltrials.gov/ct2/show/NCT02997189 (first received 19 December 2016).
Additional references
Bertolini 2004
- Bertolini P, Lassalle M, Mercier G, Raquin MA, Izzi G, Corradini N, et al. Platinum compound‐related ototoxicity in children: long‐term follow‐up reveals continuous worsening of hearing loss. Journal of Pediatric Hematology/Oncology 2004;26(10):649‐55. [DOI] [PubMed] [Google Scholar]
Brock 1991
- Brock PR, Bellman SC, Yeomans EC, Pinkerton CR, Pritchard J. Cisplatin ototoxicity in children: a practical grading system. Medical and Pediatric Oncology 1991;19(4):295‐300. [DOI] [PubMed] [Google Scholar]
Clemens 2019
- Clemens E, Heuvel‐Eibrink MM, Mulder RL, Kremer LC, Hudson MM, Skinner R, et al. Recommendations for ototoxicity surveillance for survivors of childhood, adolescent, and young adult cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCare consortium. Lancet Oncology 2019;20(1):e29‐e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dean 2008
- Dean JB, Hayashi SS, Albert CM, King AA, Karzon R, Hayashi RJ. Hearing loss in pediatric oncology patients receiving carboplatin‐containing regimens. Journal of Pediatric Hematology/Oncology 2008;30(2):130‐4. [DOI] [PubMed] [Google Scholar]
Eloxatin SPC
- Eloxatin SPC. Eloxatin summary of product characteristics. www.sanofi‐aventis.co.uk/products/Eloxatin_SPC.pdf (assessed 2 March 2010).
Freyer 2009
- Freyer DR, Sung L, Reaman GH. Prevention of hearing loss in children receiving cisplatin chemotherapy. Journal of Clinical Oncology 2009;27(2):317‐8. [DOI] [PubMed] [Google Scholar]
Gallagher 1979
- Gallagher KL, Jones JK. Furosemide‐induced ototoxicity. Annals of Internal Medicine 1979;91(1):744‐5. [DOI] [PubMed] [Google Scholar]
Gietema 2000
- Gietema JA, Meinardi MT, Messerschmidt J, Gelevert T, Alt F, Uges DR, et al. Circulating plasma platinum more than 10 years after cisplatin treatment for testicular cancer. Lancet 2000;355(9209):1075‐6. [DOI] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- McMaster University (developed by Evidence Prime). GRADEprofiler 3.6.1. Hamilton (ON): McMaster University (developed by Evidence Prime), 2011.
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
GraphPad [Computer program]
- GraphPad Software. GraphPad. San Diego (CA): GraphPad Software, 2018.
Gregg 2004
- Gregg RB, Wiorek LS, Arvedson JC. Pediatric audiology: a review. Pediatrics in Review 2004;25(7):224‐33. [DOI] [PubMed] [Google Scholar]
Grewal 2010
- Grewal S, Merchant T, Reymond R, McInerney M, Hodge C, Shearer P. Auditory late effects of childhood cancer therapy: a report from the Children's Oncology Group. Pediatrics 2010;125(4):e938‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurney 2007
- Gurney JG, Tersak JM, Ness KK, Landier W, Matthay KK, Schmidt ML, Children's Oncology Group. Hearing loss, quality of life, and academic problems in long‐term neuroblastoma survivors: a report from the Children's Oncology Group. Pediatrics 2007;120(5):e1229‐36. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jenney 2005
- Jenney ME. Late effects of cancer treatment and current protective measures. In: Voûte PA, Barett A, Stevens MC, Caron HN editor(s). Cancer in Children. 5th Edition. Oxford: Oxford University Press, 2005:123‐37. [Google Scholar]
Knight 2005
- Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. Journal of Clinical Oncology 2005;23(34):8588‐96. [DOI] [PubMed] [Google Scholar]
Lachin 2000
- Lachin JM. Statistical considerations in the intent‐to‐treat principle. Controlled Clinical Trials 2000;21:167‐89. [DOI] [PubMed] [Google Scholar]
Leclercq 2013
- Leclercq E, Leeflang MM, Dalen EC, Kremer LC. Validation of search filters for identifying pediatric studies in PubMed. Journal of Pediatrics 2013;162(3):629‐34. [DOI] [PubMed] [Google Scholar]
Li 2004
- Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. European Journal of Cancer 2004;40(16):2445‐51. [DOI] [PubMed] [Google Scholar]
Module CCG
- Kremer LC, Leclercq E, Noorman JK, Jellema P, Dalen EC. Cochrane Childhood Cancer. childhoodcancer.cochrane.org/ (accessed prior to 2 May 2019).
NCICTC v2
- National Cancer Institute. National Cancer Institute Common Toxicity Criteria version 2. ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed prior to 2 May 2019).
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Reddel 1982
- Reddel RR, Kefford RF, Grant JM, Coates AS, Fox RM, Tattersall MH. Ototoxicity in patients receiving cisplatin: importance of dose and method of drug administration. Cancer Treatment Reports 1982;66(1):19‐23. [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ross 2009
- Ross CJ, Katzov‐Eckert H, Dubé MP, Brooks B, Rassekh SR, Barhdadi A, et al. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nature Genetics 2009;41(12):1345‐9. [DOI] [PubMed] [Google Scholar]
Rybak 2007
- Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanisms of cisplatin‐induced ototoxicity and prevention. Hearing Research 2007;226(1‐2):157‐67. [DOI] [PubMed] [Google Scholar]
Rybak 2009
- Rybak LP, Mukherjea D, Jajoo S, Ramkumar V. Cisplatin ototoxicity and protection: clinical and experimental studies. Tohoku Journal of Experimental Medicine 2009;219(3):177‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schell 1989
- Schell MJ, McHaney VA, Green AA, Kun LE, Hayes FA, Horowitz M, et al. Hearing loss in children and young adults receiving cisplatin with or without prior cranial irradiation. Journal of Clinical Oncology 1989;7(6):754‐60. [DOI] [PubMed] [Google Scholar]
Skinner 2004
- Skinner R. Best practice in assessing ototoxicity in children with cancer. European Journal of Cancer 2004;40(16):2352‐4. [DOI] [PubMed] [Google Scholar]
Van As 2014a
- As JW, Berg H, Dalen EC. Different infusion durations for preventing platinum‐induced hearing loss in children with cancer. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD010885.pub2] [DOI] [PubMed] [Google Scholar]
Van As 2016a
- As JW, Berg H, Dalen EC. Platinum‐induced hearing loss after treatment for childhood cancer. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD010181.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van As 2016b
- As JW, Berg H, Dalen EC. Different infusion durations for preventing platinum‐induced hearing loss in children with cancer. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD010885.pub3] [DOI] [PubMed] [Google Scholar]
Van As 2018
- As JW, Berg H, Dalen EC. Different infusion durations for preventing platinum‐induced hearing loss in children with cancer. Cochrane Database of Systematic Reviews 2018, Issue 7. [DOI: 10.1002/14651858.CD010885.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Veal 2001
- Veal GJ, Dias C, Price L, Parry A, Errington J, Hale J, et al. Influence of cellular factors and pharmacokinetics on the formation of platinum‐DNA adducts in leukocytes of children receiving cisplatin therapy. Clinical Cancer Research 2001;7(8):2205‐12. [PubMed] [Google Scholar]
References to other published versions of this review
Van As 2011
- As JW, Berg H, Dalen EC. Medical interventions for the prevention of platinum‐induced hearing loss in children with cancer. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD009219] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van As 2012
- As JW, Berg H, Dalen EC. Medical interventions for the prevention of platinum‐induced hearing loss in children with cancer. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD009219.pub2] [DOI] [PubMed] [Google Scholar]
Van As 2014b
- As JW, Berg H, Dalen EC. Medical interventions for the prevention of platinum‐induced hearing loss in children with cancer. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD009219.pub3] [DOI] [PubMed] [Google Scholar]
Van As 2016c
- As JW, Berg H, Dalen EC. Medical interventions for the prevention of platinum‐induced hearing loss in children with cancer. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD009219.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]


