Abstract
Bovine respiratory disease is the most costly disease facing the cattle industry. Increasing resistance to antimicrobial treatment has been presented as a significant contributing factor, often through summarized susceptibility testing data. We assessed the relationship between previous antimicrobial treatment and antimicrobial susceptibility results from isolates of Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni cultured from bovine respiratory cases submitted to the Iowa State University Veterinary Diagnostic Laboratory from 2013 to 2015. Antimicrobial susceptibility data from 1,251 bacterial isolates were included for analysis. More bacterial isolates from cattle that received antimicrobial treatment showed resistance compared to isolates from untreated cattle, and the percentage of resistant isolates increased as the number of antimicrobial treatments increased. Resistance to enrofloxacin, spectinomycin, tilmicosin, and tulathromycin was present in >75% of M. haemolytica isolates from cattle that had received 3 or more antimicrobial treatments; resistance to each of those 4 antimicrobials was present in ≤10% of M. haemolytica isolates from untreated cattle. Similar but less dramatic trends were apparent for isolates of P. multocida and H. somni. The percentage of multi-drug resistant bacterial isolates also increased with the number of treatments. Results of our study suggest that previous antimicrobial treatment may have a profound effect on antimicrobial susceptibility testing. Summarized susceptibility results from diagnostic laboratories should not be used to make generalized statements regarding trends in antimicrobial resistance without providing context regarding antimicrobial treatment history.
Keywords: Antimicrobial resistance, bovine respiratory disease, Histophilus, Mannheimia, Pasteurella, susceptibility testing
Introduction
Bovine respiratory disease (BRD) is one of the most important and costly diseases facing the beef cattle industry.5,14 Many factors can contribute to the development of BRD, such as shipping distance, stress from weaning, and commingling cattle from multiple sources.13 Despite the development of new antimicrobials designed to treat bacterial agents involved in BRD, the impact of treatment failures and mortality caused by BRD remains high.9 Increasing antimicrobial resistance has also been discussed as a major component regarding the success or failure of treatment.3,16 Increased resistance to antimicrobials impacts the ability to effectively treat BRD, and retreatment has been associated with poor clinical outcomes and increased cost of production.10
Several national programs exist in the United States (National Antimicrobial Resistance Monitoring System for Enteric Bacteria) and Europe (Swedish Veterinary Antimicrobial Resistance Monitoring, Danish Integrated Antimicrobial Resistance Monitoring and Research Programme) to monitor and report on antimicrobial resistance. Antimicrobial resistance data are also often presented through summarized veterinary diagnostic laboratory (VDL) antimicrobial susceptibility data.4,8,11,17 Data from VDLs encompass a large number of cases from regional locations, and test results are easily searched and summarized. Summarized results of individual tests may not take into account important factors within each individual case that may affect the external validity of those results. Variables such as previous antimicrobial treatment or multiple treatments may have a significant effect on susceptibility testing results.7 Other variables such as the age of the animal and type of cattle operation in which it has been housed (e.g., pasture or feedlot) can provide important contextual information when interpreting testing results.
Our objectives were to (1) find and record pertinent information, particularly previous antimicrobial treatment history, from BRD case submissions, and (2) to compare the antimicrobial treatment history with antimicrobial susceptibility testing results from isolates of Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni derived from the lung.
Materials and methods
Submissions to the Iowa State University Veterinary Diagnostic Laboratory (ISU-VDL) from January 1, 2013, to December 2, 2015, were included in our study. Bacterial isolates and their corresponding case history information were included in the study if the following criteria were met: 1) the submitted samples were from a bovine field case (research cases were excluded), 2) M. haemolytica, P. multocida, or H. somni were isolated via routine culture, 3) the sample that yielded the isolate was from the lower respiratory tract (lung, pleural surface, bronchoalveolar lavage fluid), 4) antimicrobial susceptibility testing results were available, and (5) the submission form stated a history of respiratory disease and/or evidence of pneumonia was described in autopsy findings or on histologic evaluation of lung tissue.
A single researcher reviewed the submission forms for each case. The number of antimicrobial treatments and specific antimicrobials used, non-antimicrobial treatments, facility type, clinical signs, autopsy findings, number of animals at risk, age, and weight was recorded. Finalized case report information such as microscopic evidence of pneumonia was also noted. Case information was classified as “unknown” if the information was not provided or unclear. Only isolates from submissions that explicitly stated no antimicrobials had been administered were assigned the treatment history classification of “none.” Isolates from cases in which information regarding antimicrobial treatments was not given or was unclear (i.e., “many” or “everything”) were classified as “unknown.” Isolates from cases that contained a treatment history indicating treatment with 1 or 2 antimicrobial compounds were classified as “1 treatment” or “2 treatments,” respectively. Isolates from cases with treatment histories indicating 3 or more antimicrobials were administered were classified as “3+ treatments.”
Susceptibility testing was performed according to standard laboratory methods based on Clinical and Laboratory Standards Institute (CLSI) recommendations.1 Briefly, the selected culture was grown overnight, and a broth dilution was inoculated on a standard 96-well susceptibility plate (BOPO6F, Thermo Scientific, Oakwood Village, OH) using an automated inoculation system (Sensititre AIM, Thermo Scientific). Susceptibility plates were read using a manual system (Sensititre Vizion system, Thermo Scientific) following an 18–24 h incubation.
Given that not all antimicrobial compounds included on the standard susceptibility plate have CLSI-validated interpretive breakpoints, only those with CLSI-approved breakpoints2 for respiratory disease caused by M. haemolytica were included in our study. The antimicrobials included were ceftiofur, danofloxacin, enrofloxacin, florfenicol, oxytetracycline, spectinomycin, tilmicosin, and tulathromycin. Established breakpoints are not provided by CLSI for tilmicosin against P. multocida, and for tilmicosin and danofloxacin against H. somni in BRD; however, they were included in our study utilizing the CLSI breakpoints for M. haemolytica.
Multi-drug resistance (MDR) was evaluated using susceptibility results to the antimicrobials included in our study. Bacterial isolates with resistance to 3 or more antimicrobial classes were classified as MDR. For the purposes of our study, antimicrobial susceptibility results to enrofloxacin and tulathromycin were used to represent their respective antimicrobial class. Results for danofloxacin and tilmicosin were excluded from the MDR analysis. Descriptive analysis of the data was carried out (Excel 2010, Microsoft, Redmond, WA), and prevalence ratios were calculated along with 95% confidence intervals and p values (MedCalc, https://goo.gl/2oUJFY) to describe and evaluate the association between MDR and treatment history. To assess a linear trend in the prevalence of MDR for isolates with a treatment history, an extended Mantel–Haenszel chi square test for linear trend including a continuity correction was conducted, rejecting the null hypothesis if the p value was <0.001.
Results
A total of 1,251 bacterial isolates met the defined study criteria: 540 isolates of M. haemolytica, 404 isolates of P. multocida, and 307 isolates of H. somni (Table 1). Isolates were obtained from 1,031 individual animals under 989 separate case submissions by 378 veterinarians. Individual cases occasionally included multiple animals, and 2 or more bacterial species were occasionally cultured from an individual sample. Although isolates represented cases from 21 states, ~71% of the isolates were derived from cases from within Iowa, with another 21% from states bordering Iowa.
Table 1.
Number of qualifying bacterial isolates of Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni from each calendar year.
| Species | 2013 | 2014 | 2015 | All years |
|---|---|---|---|---|
| M. haemolytica | 148 | 202 | 190 | 540 |
| P. multocida | 82 | 136 | 186 | 404 |
| H. somni | 73 | 120 | 114 | 307 |
| Total | 303 | 458 | 490 | 1,251 |
The largest classification by antimicrobial treatment history was “unknown” (Table 2), representing 37.3% of isolates. Approximately 47.1% of isolates were derived from animals that had received at least 1 antimicrobial treatment; 15.5% had a case history that explicitly stated no antimicrobials had been administered.
Table 2.
Number of qualifying bacterial isolates classified by number of antimicrobial treatments administered as indicated on case submission form.
| No. of antimicrobial treatments | Mannheimia haemolytica | Pasteurella multocida | Histophilus somni | Total |
|---|---|---|---|---|
| None | 80 | 67 | 47 | 194 |
| 1 | 121 | 92 | 63 | 276 |
| 2 | 101 | 60 | 50 | 211 |
| 3+ | 45 | 22 | 36 | 103 |
| Unknown | 193 | 163 | 111 | 467 |
The number of different antimicrobials used, as indicated by the submitting veterinarian on the submission form, in most cases was 1–3 with a high of 7. Sixteen different antimicrobials were reported in treatment histories in our study. The most commonly used antimicrobials were tulathromycin (239 isolates), enrofloxacin (158), florfenicol (150), and ceftiofur (144). Sixty-two percent of all isolates came from cattle within a feedlot or confinement, 13% were on pasture at the time of sampling, and 3.3% of isolates came from dairy cattle. The remainder of isolates had an environment classification of “unknown.”
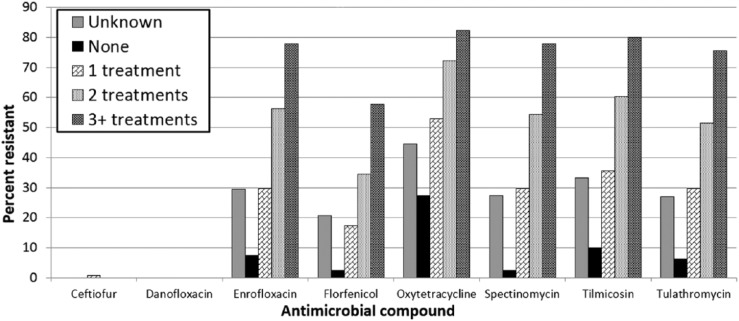
Across all 3 bacterial species, the percentage of resistant isolates increased as the number of antimicrobial treatments increased. The most dramatic differences between isolates from animals that received no antimicrobial treatment and those from animals that received ≥3 antimicrobial treatments were seen in M. haemolytica (Fig. 1). The percentage of isolates resistant to enrofloxacin, spectinomycin, tilmicosin, and tulathromycin was ≤10% in isolates from untreated animals but >75% in isolates from animals treated with 3 or more antimicrobials. Similar but less-pronounced differences in resistance were present for florfenicol and oxytetracycline, and little to no resistance was present to ceftiofur and danofloxacin regardless of treatment history.
Figure 1.
Percentage of Mannheimia haemolytica isolates classified as resistant to selected antimicrobials determined by antimicrobial susceptibility testing, grouped by the number of antimicrobial treatments reported on the submission history.
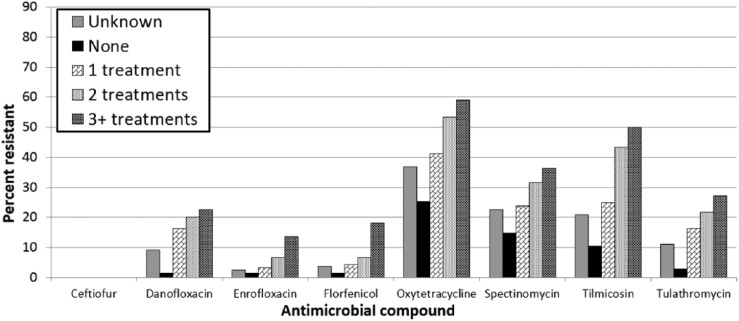
The percentage of antimicrobial resistance in isolates of P. multocida (Fig. 2) and H. somni (Fig. 3) was lower compared to isolates of M. haemolytica. However, similar trends of higher percentages of resistant isolates with an increase in the number of antimicrobial treatments as well as low percentages of resistance in isolates from untreated animals were apparent. Isolates from animals with an unknown treatment history had consistently higher rates of resistance than untreated isolates of all 3 bacterial species. In vitro resistance to ceftiofur was rare: 5 of 1,251 isolates (0.4%) were found to be resistant to ceftiofur.
Figure 2.
Percentage of Pasteurella multocida isolates classified as resistant to selected antimicrobials determined by antimicrobial susceptibility testing, grouped by the number of antimicrobial treatments reported on the submission history.
Figure 3.
Percentage of Histophilus somni isolates classified as resistant to selected antimicrobials determined by antimicrobial susceptibility testing, grouped by the number of antimicrobial treatments reported on the submission history.
The percentage of isolates from animals that did not receive antimicrobial treatment that were pan-susceptible (i.e., classified as susceptible to all 6 antimicrobial classes) was 69.1%, by far the highest percentage based on the number of antimicrobial treatments (Fig. 4), with 4.6% of isolates from untreated animals classified as MDR. In contrast, only 19.4% of isolates from animals treated with 3 or more antimicrobials were pan-susceptible; 47.6% of those isolates were classified as MDR. The percentage of isolates resistant to 3 or more antimicrobial classes increased as the number of antimicrobial treatments increased. The ratio of the prevalence of MDR in isolates from treated animals to those from untreated animals increased as the number of antimicrobial treatments increased (Table 3). There was evidence to support rejection of the null hypothesis for the linear trend test (χ2 = 93, degrees of freedom = 1, p < 0.0001). This result is consistent with the data that show an increase in the prevalence ratio as the number of reported treatments in the history increases.
Figure 4.
Percentage of isolates of Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni classified by the number of antimicrobial classes with resistant classifications, grouped by the number of antimicrobial treatments reported on the submission history (“Unknown” excluded).
Table 3.
Comparison of the prevalence of multi-drug resistance in isolates of Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni from treated and untreated animals.
| Antimicrobial treatment | Isolates resistant to at least 3 classes | Total isolates | Prevalence ratio* | p value |
|---|---|---|---|---|
| None | 9 | 194 | Referent | |
| 1 | 55 | 276 | 4.30 (2.18–8.48) | <0.0001 |
| 2 | 81 | 211 | 8.27 (4.27–16.02) | <0.0001 |
| 3+ | 49 | 103 | 10.25 (5.25–20.03) | <0.0001 |
| Unknown | 89 | 467 | 4.11 (2.11–7.99) | <0.0001 |
Numbers in parentheses are 95% confidence intervals. Prevalence ratio is the prevalence of a bacterial isolate from an animal in the given treatment group being classified as resistant to 3 or more of the 6 antimicrobial classes included in our study compared to the prevalence of a bacterial isolate from an untreated animal being classified as resistant to 3 or more of the 6 antimicrobial classes included in our study. The referent category for all ratios is a history of antibiotic treatments. Linear trend test (χ2 = 93, degrees of freedom = 1, p < 0.0001).
The inclusion of other potentially important information on case submission forms, such as age, weight, and autopsy findings, was also assessed. Eighty-six percent of isolates came from submissions that included autopsy findings, 80.7% included the number of other animals at risk of disease, 78.5% included history about the environment in which the animals were housed, 82% included an estimated weight, and 75.2% included information regarding age. Only 6% of submissions lacked information regarding both weight and age.
Discussion
The results of our study show that prior antimicrobial treatment was associated with an increased percentage of antimicrobial resistance in bacterial isolates from cases of BRD. Rates of resistance increased as the number of antimicrobials an animal received increased. These findings demonstrate that antimicrobial treatment history may have a significant impact on antimicrobial susceptibility. Prior treatment history is important when analyzing antimicrobial susceptibility results in individual cases; this contextual information is no less important when presenting summarized susceptibility data.
In previously untreated animals, the percentage of resistant isolates was ≤15% to all but 1 antimicrobial (oxytetracycline), and ~70% of these isolates were susceptible to all antimicrobial classes included in our study. These data show that overall antimicrobial resistance is low in cattle that have not been treated. Other factors are undoubtedly involved in the efficacy of antimicrobial treatment. The higher rates of resistance in isolates from treated animals also correlate with studies that show an increase in BRD treatment failures following multiple antimicrobial treatments (Busby D. Tri-county steer carcass futurity data. Proc Ann Conf Am Assoc Bovine Pract; 2010; 71–81).6,12
Fewer than 5% of isolates from cattle that did not receive an antimicrobial treatment were classified as MDR. In contrast, over 47% of isolates from animals with 3 or more previous antimicrobial treatments were classified as MDR. A 2013 study reported an increase in the number of MDR M. haemolytica isolates over a 3-y period.8 However, that study did not take into account previous antimicrobial treatment. The results of our study suggest that an increase in VDL submissions involving cattle receiving antimicrobial treatment or an increased number of antimicrobial treatments may account for a portion of the perceived change in MDR from year to year.
Over one-third of all isolates in our study originated from cases with an unknown treatment history. The percentage of case histories that do not include other important information, such as the type and size of cattle operation or the age and weight of the animal, also may affect the validity and usefulness of summarized VDL data. Summarized antimicrobial susceptibility results need to include some context as to what other factors may have impacted those results. Instead of presenting summarized data with little-to-no context, a more accurate measure of the change in antimicrobial resistance may be to track resistance in untreated animals (i.e., resistance that would affect the efficacy of the first antimicrobial treatment). This would eliminate the effect that previous treatments would have on the resistance or susceptibility of bacterial isolates to specific antimicrobials, provide a better gauge of changes in resistance trends, and allow researchers a more accurate look into predisposing factors to BRD that may be more important than characteristics of the bacteria themselves.15
Limitations of our study include a potential lack of external validity, with a large percentage of cases from one part of the country. Additional studies should be completed in other areas to assess potential regional variation. We also only assessed in vitro antimicrobial susceptibility, which may not be directly correlated with in vivo efficacy in all cases. We relied on the accuracy of information included on submission forms, not on detailed clinical trial records, and information regarding the treatment dose was also unavailable. All isolates included in our study were obtained from cattle that died with evidence of BRD. Isolates from cattle with BRD that survived with or without the benefit of treatment are not represented. This would support our statement that summarized VDL data should not be viewed as an accurate representation of overall antimicrobial resistance trends. Another limitation is that our study only includes data from cases submitted from 2013 to 2015. Hence, inferences about changes in resistance patterns were not possible.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial disk dilution susceptibility tests for bacteria isolated from animals; approved standard. 4th ed. Wayne, PA: CLSI, 2013. CLSI document VET01-A4. [Google Scholar]
- 2. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial disk dilution susceptibility tests for bacteria isolated from animals; second informational supplement. Wayne, PA: CLSI, 2013. CLSI document VET01-S2. [Google Scholar]
- 3. DeDonder KD, Apley MD. A literature review of antimicrobial resistance in Pathogens associated with bovine respiratory disease. Anim Health Res Rev 2015;16:125–134. [DOI] [PubMed] [Google Scholar]
- 4. Fales WH, et al. Antimicrobial resistance among Pasteurella spp recovered from Missouri and Iowa cattle with bovine respiratory disease complex. J Am Vet Med Assoc 1982;181:477–479. [PubMed] [Google Scholar]
- 5. Griffin D. Economic impact associated with respiratory disease in beef cattle. Vet Clin North Am Food Anim Pract 1997;13:367–377. [DOI] [PubMed] [Google Scholar]
- 6. Hoar BR, et al. A comparison of the clinical field efficacy and safety of florfenicol and tilmicosin for the treatment of undifferentiated bovine respiratory disease of cattle in western Canada. Can Vet J 1998;39:161–166. [PMC free article] [PubMed] [Google Scholar]
- 7. Kanwar N, et al. Effects of ceftiofur and chlortetracycline treatment strategies on antimicrobial susceptibility and on tet(A), tet(B), and bla CMY-2 resistance genes among E. coli isolated from the feces of feedlot cattle. PLoS One 2013;8:e80575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lubbers BV, Hanzlicek GA. Antimicrobial multidrug resistance and coresistance patterns of Mannheimia haemolytica isolated from bovine respiratory disease cases—a three-year (2009–2011) retrospective analysis. J Vet Diagn Invest 2013;25:413–417. [DOI] [PubMed] [Google Scholar]
- 9. Miles DG, Rogers KC. BRD control: tying it all together to deliver value to the industry. Anim Health Res Rev 2014;15:186–188. [DOI] [PubMed] [Google Scholar]
- 10. Pinchak WE, et al. Morbidity effects on productivity and profitability of stocker cattle grazing in the Southern Plains. J Anim Sci 2004;82:2773–2779. [DOI] [PubMed] [Google Scholar]
- 11. Portis E, et al. A ten-year (2000–2009) study of antimicrobial susceptibility of bacteria that cause bovine respiratory disease complex—Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni—in the United States and Canada. J Vet Diagn Invest 2012;24:932–944. [DOI] [PubMed] [Google Scholar]
- 12. Rooney KA, et al. Efficacy of tulathromycin compared with tilmicosin and florfenicol for the control of respiratory disease in cattle at high risk of developing bovine respiratory disease. Vet Ther 2005;6:154–166. [PubMed] [Google Scholar]
- 13. Sanderson MW, et al. Risk factors for initial respiratory disease in United States’ feedlots based on producer-collected daily morbidity counts. Can Vet J 2008;49:373–378. [PMC free article] [PubMed] [Google Scholar]
- 14. Smith RA. Impact of disease on feedlot performance: a review. J Anim Sci 1998:76:272–274. [DOI] [PubMed] [Google Scholar]
- 15. Timsit E, et al. Transmission dynamics of Mannheimia haemolytica in newly-received beef bulls at fattening operations. Vet Microbiol 2013;161:295–304. [DOI] [PubMed] [Google Scholar]
- 16. Watts JL, Sweeney MT. Antimicrobial resistance in bovine respiratory disease pathogens: measures, trends, and impact on efficacy. Vet Clin North Am Food Anim Pract 2010;26:79–88. [DOI] [PubMed] [Google Scholar]
- 17. Welsh RD, et al. Isolation and antimicrobial susceptibilities of bacterial pathogens from bovine pneumonia: 1994–2002. J Vet Diagn Invest 2004;16:426–431. [DOI] [PubMed] [Google Scholar]