Abstract
Vision is the sense humans rely on most to navigate the world and survive. A tremendous amount of research has focused on understanding the neural circuits for vision and the developmental mechanisms that establish them. The eye-to-brain, or “retinofugal” pathway remains a particularly important model in these contexts because it is essential for sight, its overt anatomical features relate to distinct functional attributes and those features develop in a tractable sequence. Much progress has been made in understanding the growth of retinal axons out of the eye, their selection of targets in the brain, the development of laminar and cell type-specific connectivity within those targets, and also dendritic connectivity within the retina itself. Moreover, because the retinofugal pathway is prone to degeneration in many common blinding diseases, understanding the cellular and molecular mechanisms that establish connectivity early in life stands to provide valuable insights into approaches that re-wire this pathway after damage or loss. Here we review recent progress in understanding the development of retinofugal pathways and how this information is important for improving visual circuit regeneration.
Introduction
Understanding how the nervous system ‘wires up’ is one of the central quests of biology. More than 100 years ago, Cajal initiated work to understand how nerve cells grow out their processes and connect with each other-in an effort to understand how to ‘generate’ the nervous system [1]. Cajal also proposed that, in order to understand how to regenerate the nervous system after injury, one should look to the normal course of developmental events that established them in the first place [2]. Here we review recent progress exploring how a particular pathway - the connections linking the eyes to the brain-initially grow out their axons and target appropriate brain areas, topographic locations, and laminar depths within their targets-all of which are necessary for light-driven percepts and behaviors. We also discuss recent work showing how developmental mechanisms can be leveraged towards regeneration of visual connections and visual function.
The eye-to-brain pathway: basic features and developmental emergence
The eye-to-brain (or ‘retinofugal’) pathway consists of the axons from retinal ganglion cells (RGCs), the output neurons of the eye. There are ~30 RGC types, each of which responds best to a particular feature in the visual environment by virtue of the cell-type specific connections it collects on its dendrites within the retina [3**, 4**], and its connections to 1, or as many as 4, of the ~40 subcortical retinorecipient nuclei in the brain [5*]. These specific axonal connections in turn drive conscious perception of visual scenes (‘sight’) and also non-image-forming visual functions that support sight such as eye movements and pupil reflexes. Some RGC connections also drive other non-image-forming visual functions that influence the brain and body over long time scales, including entrainment of circadian rhythms and hormone secretion [6**].
The retinofugal pathway, in addition to being experimentally accessible, has all of the anatomical and functional features one could wish for in a system of study where the goal is to understand the mechanisms of neural circuit development and regeneration. It has distinguishable cell types that require specification and whose dendritic targeting patterns are both visible and meaningful. It exhibits long-and short-range axon targeting specificity, and also within-target specificity such as topographic and eye-specific organization crucial for accurate representation of the outside world. Therefore, this pathway has remained a prominent focus of developmental neurobiologists for > 50 years. Moreover, the retinofugal pathway is vulnerable to many common neurodegenerative disease such as glaucoma [7], stroke, and head trauma [8]. Together, all of these features continue to provide a platform for addressing how to generate and regenerate eye-to-brain connections after injury, both for the sake of reversing blindness and as a general model for CNS injury.
RGC axon growth out of the eye and down the optic nerve
RGCs are born in the ventricular zone of the nascent eye-cup, where they are endowed with the expression of different transcription factors that segregate them into distinct functional types [9*, 10**, 11, 12]. RGCs extend axons as they migrate into the inner retina [13–15]. The trajectory of these axons away from the periphery and toward the optic nerve head, where they can exit the eye, is governed both by the inhibitory influence of chondroitin-sulfate proteoglycans (CSPGs) expressed around the border of the eye-cup and by the attractive influence of Netrin-1 (Fig 1A) [16, 17]. Netrin-1 is expressed at the center of the eye and attracts RGC axons through interactions with the Netrin-1 receptor Deleted in colorectal cancer (Dcc) expressed by RGC axons [17].
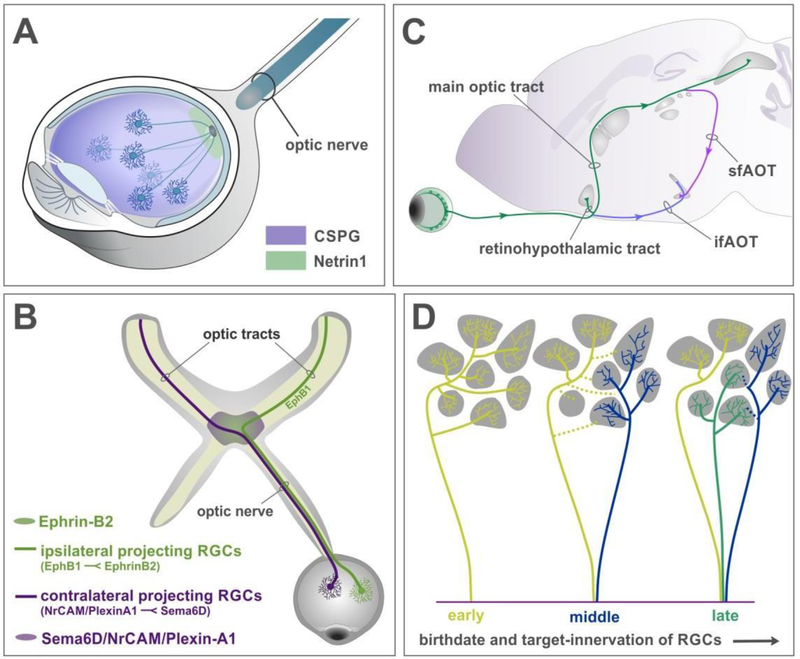
Figure 1: RGC axon pathfinding and target selection.
(A) Retinal ganglion cells extend axons away from the periphery due to repulsive influences from chondroitin sulfate proteoglycans (CSPG, purple) and grow towards the optic disk. Netrin 1 expressed by glial cells at the optic disc (green) provides local attractive cues and enables axons to exit the eye, into the optic nerve.
(B) Schematic of the optic nerve reaching the optic chiasm, a midline choice point for RGC axons. EphrinB2 expressed at the midline repels EphB1 expressing RGC axons to form ipsilateral projections (green), while Sema6D/NrCAM/PlexinA1 complex directs other RGCs to cross at the chiasm and form the contralateral projections (magenta).
(C) Schematic showing the anatomical position of the eye, optic nerve, and optic tracts projecting to targets in the brain. After crossing the chiasm, the contralateral projection ascends into the brain to form the main optic tract (green). A smaller bundle projects into the SCN at the base of the hypothalamus, forming the retinohypothalamic tract. Two bundles deviate from the tract however – the inferior fasciculus of the accessory optic tract (ifAOT, purple), extends at the base of the brain to project to the MTN. While another bundle continues from the main optic tract and dives down to form the superior fasciculus of the AOT (sfAOT, pink).
(D) Birth order of RGCs determines their target-selection and exploration. Early-born RGCs extend axon branches into many targets (yellow lines). Axons born shortly after (blue) extend to a few different targets while the early born axons retract some connections (yellow dotted lines). The later-born axons project directly to their targets without much exploration (green).
The neurogenic birthdates of different RGC types vary in time [18*, 19], as do the birthdates of RGCs programmed to extend their axons to the contralateral versus the ipsilateral side of the brain [20*, 21]. However, in general RGC axons obey a common growth program as they extend out of each eye and form the developing optic nerves. Once they exit, individual RGC axon mingles with axons of other RGC types from the same eye and course toward the optic chiasm in a tight bundle at the base of the brain (Fig 1B-D).
RGC axon tract segregation and pathfinding
When RGC axons reach the optic chiasm at the base of the brain, they orient to the midline and either cross (“decussate”) or remain ipsilateral, a binary decision that is crucial for establishment of visuotopic and binocular visual maps in downstream central targets [21]. Decussation of SoxC expressing RGCs [22**] is mediated in large part by the expression of a Sema6D/Nr-CAM/Plexin-A1 receptor complex that promotes growth in NrCAM-PlexinA1-expressing contralateral projecting RGCs [23]. The RGC axons that project ipsilaterally do so because they express EphB1, which transduces a repellant signal from ephrinB2 expressed by midline radial glial cells [24*]. The EphB1 expression in ipsilateral-projecting cells is driven by the transcription factor Zic2 (Fig 1B) [25]. Interestingly, species with eyes located in more frontal positions express Zic2 in larger populations of RGCs [26]. Moreover, cell autonomous factors mediate fasciculation of axons within the optic tract such that ipsilateral and contralateral projections remain segregated in the optic nerve and tract [27*].
After they navigate the chiasm, RGC axons select 1 of 3 tracts to reach their targets (Fig 1C). First, a limited number of RGC axons (originating from M1 and M2 type intrinsically photosensitive RGCs-so-called because they act as photoreceptors [28]) project into the hypothalamus to innervate the master circadian pacemaker: the ‘suprachiasmatic nucleus’ (SCN). In doing so, they form the “retinohypothalamic tract” (Fig 1C). This connection is crucial for a myriad of non-image-forming functions such as linking of endogenous arousal and hormone secretion rhythms with the environmental light-dark cycle [reviewed in: 6**]. A second population of RGC axons, upward-selective On-type direction selective RGCs that express Spig1 [29*], depart the posterior optic chiasm and travel in a tight fascicle along the base of the brain in what is termed the “inferior fasciculus of the accessory optic tract” (ifAOT) (Fig 1C). This tract innervates the dorsal aspect of the medial terminal nucleus (MTNd), a structure made up of a narrow column of neurons residing at the base of the midbrain-hindbrain border. While the molecular factors that enable RGC axons to innervate the MTN have been identified (see below), the signals that direct upward-sensing On-DSGCs to leave the chiasm and embark on their trajectory along the base of the brain remain unknown.
The third trajectory for RGC axons to reach their targets is the major one. It includes ~30 functionally distinct subtypes, and by sheer numbers, represents ~90% of eye-to-brain connections. After navigating the chiasm, these RGC axons ascend dorso-caudally from the base of the diencephalon toward the dorsal thalamus and midbrain to form the main optic tract (Fig 1C). RGC axons emerging from the main optic tract are actively repelled from entering the diencephalon and non-visual nuclei by Slit/Robo interactions [30]. Technically, there is also a fourth optic tract in which downward sensing On-DSGCs depart the main optic tract just anterior to the SC and dive ventrally, forming the “superior fasciculus of the AOT” (sfAOT) and innervating the ventral division of the medial terminal nucleus (vMTN) at the base of the brainstem (Fig 1C).
Different RGC types destined to innervate different brain targets are born and send out axons at different developmental stages, such that early-born axons are able to pioneer and sample many targets, whereas later deployed axons have fewer options for targets to innervate and therefore exhibit correspondingly less target-sampling and refinement (Fig 1D) [18*].
Retinal ganglion cell axon-target matching
The process of axon-target matching reflects the mechanisms by which different RGC types that encode functionally distinct features in the visual world, such as directional motion or overall luminance, connect to appropriate brain targets in order to process those features into the correct perceptual or behavioral events. For example, RGCs that sense directional motion project to targets involved in conscious sight, such as the dorsal lateral geniculate nucleus (dLGN), whereas RGCs that detect overall levels of ambient luminance project to targets such as the olivary pretectal nucleus (OPN), which is involved in generating pupil reflexes (reviewed in: 6**, 31*]. Axon-target matching is a process involving dynamic interactions between pre- and post-synaptic components. The various retinorecipient targets along the optic tract undergo maturation during the same time when RGC axons arrive in their vicinity. In general, retinofugal maturation proceeds in a caudal-to-rostral manner-the most distal RGC target, the midbrain superior colliculus (SC), matures before the dLGN, and other targets follow suit. In fact, many RGC axons first grow all the way to SC, bypassing the >20 retinorecipient targets that reside between the chiasm and SC (Fig 1C, 1D), before innervating more proximal targets [18*, 32], perhaps because those targets do not yet harbor the full array of cell types and signals required for accurate wiring. It is also worth pointing out that, in the mouse, most (~90%) of RGCs connect to the SC and therefore all RGC inputs to targets such as the dLGN reflect the elaboration of RGC axon collaterals that also project to the SC [33**]. Thus, individual axons target multiple brain structures separated by other RGC targets, mainly through a process of highly regulated collateralization and not primary growth cone termination [18*, 32].
In recent years, the mechanisms underlying the process of target-selective collateralization have started to become clear. Osterhout et al., [34**] discovered that target selection by subsets of RGCs is facilitated by adhesive interactions: the classical cadherins mediate homophilic interactions between RGCs and target cells that express Cdh6 [34**], whereas contactin-4 (CNTN4) is involved in the assembly of a parallel pathway consisting of ‘forward sensing On-DSGCs’ that project to the nucleus of the optic tract (NOT) - a circuit element essential for horizontal image stabilizing eye movements [35**] (Fig 2).
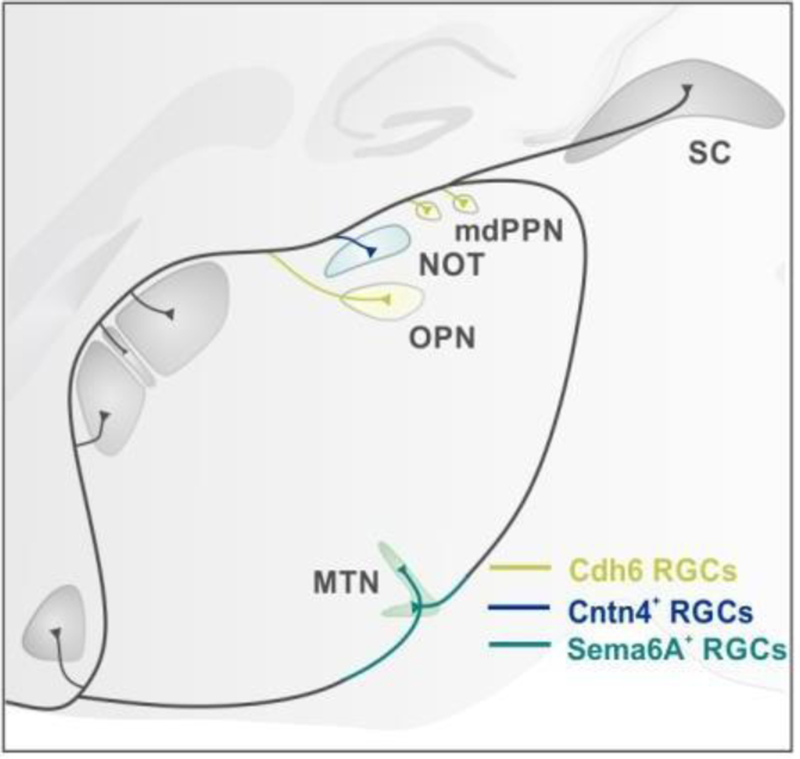
Figure 2: Molecular mechanisms regulate axon projections to specific targets.
Cadherin 6 (Cdh6) RGCs (yellow) grow to Cdh6 expressing target cells in the OPN. Contactin-4 (CNTN4+) expressing RGCs (blue) act with their co-receptor amyloid precursor protein (APP) to regulate branch formation of direction-selcective ganglion cells (DSGC) in the NOT. Sema6A expressing DSGCs (green) enter the MTN by interacting with Plexin A2/A4 expressed by cells in the MTN.
In a related set of pathways, the On-DSCGs that target the MTNd and MTNv also rely on adhesive interactions between Sema6A (acting as a receptor) expressed by On-DSGC axons and plexinA2/4 (acting as a ligand) expressed by MTNd/v neurons (Fig 2) [**36]. In the dorsal thalamus, reelin, an extracellular glycoprotein present as a gradient in target tissues, promotes target-specific innervation via LRP8 and VLDLR [37**, 38*, 39]. Thus, multiple parallel retinofugal pathways rely on adhesive interactions to achieve axon-target specificity. Recently, Seabrook et al., showed that early genetic removal of the RGCs that target the OPN did not result in NOT-projecting RGCs arborizing in the OPN, even though NOT-projecting RGC axons extend past the OPN en route to their target [40*]. Collectively, the model of RGC axon-target selection that has emerged is one in which axon growth and exploration of targets is the default mode early in development, with RGC targeting being tightly regulated by specific ligand-receptor pairs and unaltered by axon competition.
Targeting the correct topographic zone
Upon arriving at their targets, RGCs map to the location within the target appropriate for their topographic address in the retina. The basic rule is that RGCs that are neighbors in the retina project to neighboring regions in the target [41]. This process is mediated largely by repellent interactions between Eph receptors expressed by RGCs that transduce signals from ephrin ligands, although reverse signaling occurs as well [41]. Members of the ephrinA-EphA family mediate mapping along the nasal-temporal axis [42], and there is some evidence that ephrinB-EphB signaling mediates mapping along the dorsal-ventral axis [e.g., 43]. Other signals for mapping the medial-lateral axis include Wnt-Ryk [44]. It should be noted that not all topographic sorting of RGC axons relies on axon-target interactions; as they approach their targets, RGCs located in the dorsal versus ventral location of the retina sort from one another within the optic tract [45*]. Also, neural activity in the form of spontaneous retinal waves [46] drives refinement of topographic mapping. If these waves are quieted, or their patterns altered [42, 47, 48], RGC axons still map to the correct general area but the arbor termination zone becomes diffuse [49]. The interplay between guidance molecules and activity in this system is complementary; when all ephrin/Eph interactions are eliminated by genetic knockouts, RGC axons map to the wrong locations but still form focal termination zones, whereas removal of both ephrin/Eph’s and activity causes complete disruption of topographic targeting and diffuse arborizations [48, 49].
Directing RGC dendrites and axons to appropriate laminar depth
The functional integrity of retinofugal connections is also constrained by intra-retinal wiring events. In the retina, the location and architecture that neuronal dendrites adopt dictates the type and pattern of pre-synaptic inputs from amacrine and bipolar cells that are available to them. The specificity of these inputs essentially determines to which features in the outside world a given RGC (and therefore its target neuron in the brain) will respond. Intra-retinal cell-type-specific connectivity is, in large part, determined by the RGC dendritic laminar depth in the inner plexiform layer (Fig 3A) [3**, 4**]. RGC axons, too, must select the correct laminar depth within their targets, and in doing so, bias the number, type and location of postsynaptic neurons to which they connect [50]. The process of laminar depth selection appears to be independent of topographic mapping, since altering spontaneous neural activity or ephrin signaling disrupts precise topographic fidelity but not the depth to which RGCs of a given type projects [33**, 51]. In fish, elegant work from Baier and co-workers shows that slit1 interactions with type IV collagen expressed at the pial surface of the tectum is essential for laminar RGC axon targeting (52**, 53*). The molecular signals that promote RGC axon laminar-specific targeting in mammals remain unknown- a crucial gap that needs to be resolved.
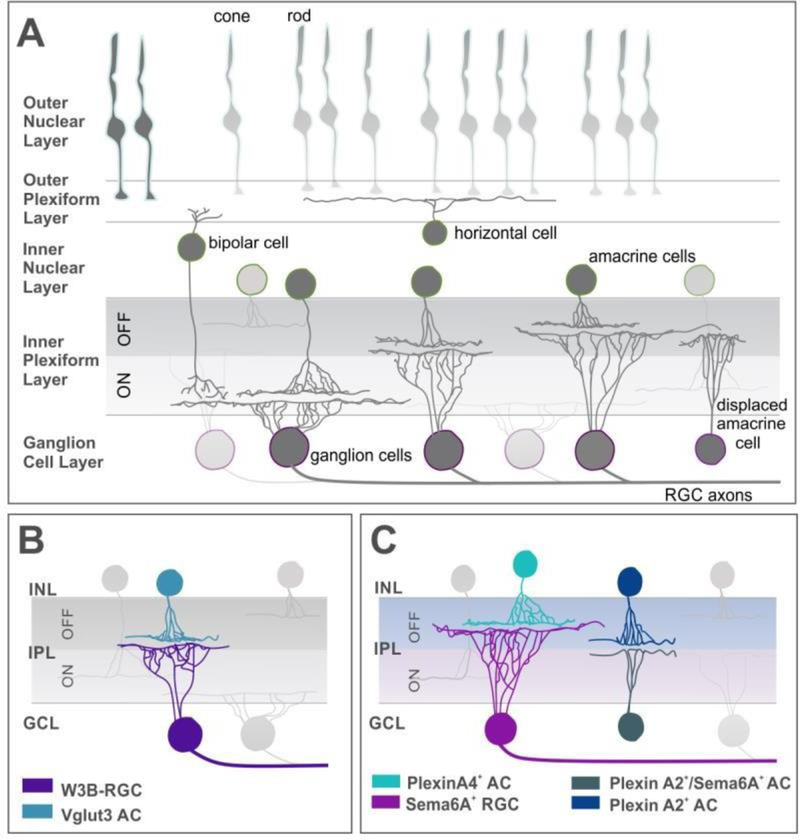
Figure 3: Laminar specification of RGC dendrites in the inner plexiform layer.
(A) Schematic showing the layers of the mouse retina and the different types of cells present in each layer.
(B) A particular type of RGC, the W3B-RGC (blue) dendrites receive inputs from VGlut3 (vesicular glutamate transporter 3) amacrine cells (purple). Both W3B-RGCs and VG3-AC express sidekick 2 (Sdk2), thus binding via homophilic interactions.
(C) Sema6A expressing RGCs (pink) received dendritic inputs from PlexinA4 expressing dopaminergic amacrine cells (light blue) in the OFF sublamina of the inner plexiform layer. OFF starburst amacrine cells (SAC) expressing Plexin A2 (dark blue) are repelled by Sema6A-PlexinA2 expressing ON SACs (teal) thus specifying laminar depth in SACs.
The signals directing RGC dendrites to their correct layers, on the other hand, have been extensively described and include repellant interactions between specific semaphorins and plexins that restrict RGC dendrites to particular depths in the IPL [54**, 55] - (Fig 3B, 3C) [56]. There is still much work to do, however, in order to figure out how the incredible degree of target and within-target specificity is achieved, and how inputs from different RGC types are combined and transformed to yield coherent behavioral and perceptual outputs.
Eye-specific segregation
Contralateral versus ipsilateral-projecting RGC axons are segregated from one another in every retinofugal target in which they converge, except in the SCN where they overlap, at least at the overt scale (they may be segregated onto individual cells). This segregation emerges during development from a state in which axons from the two eyes initially overlap [57]. The segregation process requires spontaneous waves of neural activity in the retina [58, 59] driven by acetylcholine and ephrin-A/EphA interactions to define where the eye-specific zones,-which reflect retinotopically-matched positions from the two eyes, will reside within each target [60, 61] [reviewed in: 47].
Key cell types and mechanisms in the segregation process include astroglial and microglial ‘engulfment’ of weaker synapses that encroach upon the opposite eye-specific zone, and recruitment of immune system proteins such as complement [62**] and MEGF10 [63**] [reviewed in: 64].
Regeneration of eye-to-brain connections
As bona fide central nervous system (CNS) neurons, mammalian RGCs lack the capacity to regenerate [2, 65**, 66*]. Most traumatic injuries and diseases that damage the retina or the optic nerve eventually lead to RGC degeneration; the axons whither and eventually the entire cell dies. Similarly, degenerative diseases that cause RGC damage either directly or indirectly, such as glaucoma, result in irreversible vision loss [7].
RGCs in cold-blooded vertebrates such as fish and lizards readily regenerate and even re-establish accurately mapped connections [67]. To understand the barriers to mammalian RGC regeneration, the field has looked to both cold-blooded vertebrates and developmental mechanisms in mammals. Generally speaking, barriers to RGC regeneration fall into two categories: extrinsic and intrinsic factors [68*]. In terms of extrinsic factors, after injury, scarring accumulates at the lesion site, and while some scar-related factors can aid repair [69*], scarring is generally restrictive for regeneration due to the inflammatory cytokines and physical barriers to axon passage that it creates [69*, 70*]. In addition, myelin present in most nerve tracts (including the optic nerve and tract) maintains factors that prohibits RGC axon growth. Intrinsic barriers include the slowing of RGC axon growth as a function of age, injury-induced death and lack of RGC replenishment. This last point is essential. Whereas fish naturally produce more RGCs as they age and their eyes grow [71], after development the number of mammalian RGCs is fixed and injury reduces those numbers. Thus, maintaining RGC viability after injury is a time-pressured limitation on post-injury regeneration and reformation of synapses with brain targets.
Maintaining cell survival and capacity to regenerate
A prerequisite for axon regeneration by endogenous RGCs is that they remain viable long enough following injury to allow for a regeneration-inducing intervention to work. However, very quickly after axon crush or transection more than half of RGCs die, just as in development [72], and eventually lead to death of all RGCs. Why are RGCs so susceptible to axotomy-induced death, and what can be done to increase RGC viability? Possible answers come from the fact that during neural development activity is crucial for RGC sustenance [72, 73]. In their exploration of the role played by neural activity in optic nerve regeneration, Lim et al., (2016) showed that suppressing electrical activity after nerve crush adversely impacts RGC survival [74**] (Fig 4A). Others showed that subsets of RGC types, the melanopsin-expressing ipRGCs, as well as the alpha RGCs that express osteopontin, insulin-like-growth factor1 (IGF1) and high levels of mTOR [75**], together can account for almost all the RGCs that survive axotomy and remain viable 2 weeks after nerve crush [76–78]. However, new findings demonstrate that other RGC subsets are endowed with factors that promote survival capabilities; overexpression of the transcription factor Sox11 promotes non-alpha-RGC survival and regeneration following optic nerve injury while, somewhat paradoxically, it preferentially kills alpha-RGCs [79*]. This suggests that threshold levels of Sox11 may be crucial for RGC survival and raises the possibility that alpha-RGCs typically survive axotomy because they have already elevated endogenous Sox11 levels.
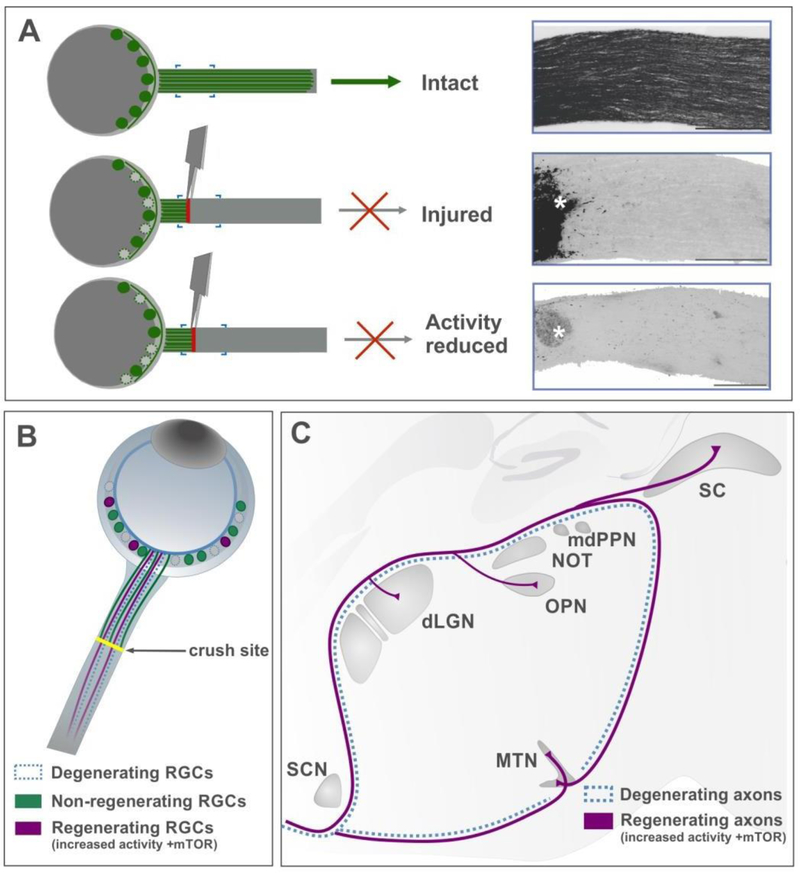
Figure 4: Developmentally informed regenerative strategies.
(A) Schematic to show how injury impacts the optic nerve. Injured RGCs start dying within two weeks and cannot regenerate their axons without therapeutic intervention. Silencing neural activity using chemogenetic approaches, reduces the survival of RGCs.
(B) Combinatorial approaches that increase neural activity and mTOR signaling in RGCs (pink circles) promote regenerated axons to reinnervate visual targets in the brain (pink lines).
The success with which RGCs re-extend and connect axons to any one target after injury plays an important role in determining sustenance of the cell itself; if an RGC is unable to re-form connections, the lack of target-derived trophic factors signals cell death [80]. Several groups have tested different strategies to promote re-extension of axons after injury [81*, 82]. Interestingly, most of these hinge on re-activating developmental growth pathways and/or suppressing the growth-inhibiting pathways that characterize RGCs. Manipulations that have proved successful in this regard include increasing inflammatory factors such as oncomodulin [83*], increasing insulin signaling [84], or inhibiting negative regulators of growth such as PTEN (phosphatase/tensin homolog) [65**]. Other inhibitors of axon growth include the transcription factors SOCS3 (suppressor of cytokine signaling 3) [85, 86*], Klf 4 and Klf 9 [87]. Increasing positive regulators of cell growth, including mammalian target of rapamycin (mTOR), ciliary neurotrophic factor (CNTF), doublecortin-like kinases (DCLK2) [88] or the transcription factors Klf 6 and Klf7 can also shift RGCs into a growth mode [87].
Further, increasing RGC electrical activity using chemogenetic techniques [74**], or a combination of increasing activity and growth promoting pathways, have been shown to have synergistic effects on RGC axon growth. And while no single manipulation has proven to be a “magic bullet” for regeneration, combining enhancement of developmental growth programs while at the same time inhibiting growth suppression pathways leads to modest long-distance RGC axon re-extension [74**, 86*, 89**]. One thing to note is that, even in studies where successful regeneration is induced, RGCs continue dying, further emphasizing that if RGCs are to be induced to regenerate in large numbers, approaches to sustain RGC survival must be introduced as well.
Re-targeting the correct brain nuclei, reforming myelination and synapses after injury
A longstanding question has been: if RGCs can regenerate, will they navigate to visual targets, and if so, to the correct ones? Apparently the answer is yes; much as it is in lizards and fish [71, 90*]. Two papers, De Lima et al., [89**] and Lim et al., [74**], show that when RGCs are triggered to regenerate after injury, their axons innervate visual targets in the brain and still actively avoid non-visual targets, as they did during development (Fig 4B). Others showed that when lesions are more distal to the optic nerve [86*], target specific regeneration still occurs, but in the absence of neural activity, myelination of the regenerated fibers does not. This inadequate functional transmission from the regenerated axons/synapses could explain why some, but not all, visual functions were restored in these mice.
One approach that has yet to gain traction in the field of visual regeneration is the use of guidance cues to promote axon re-extension. Axon guidance cues provide a particularly important regenerative mechanism that couples intrinsic and extrinsic promoters of growth during development. Perhaps a strategy that utilizes neural activity and guidance cues will be key in promoting sufficient and accurate regeneration of axons in order to achieve complete functional restoration of vision. However, comparison between developmental and regenerative roles of neural activity, indicates that although the same genes and mechanistic features may be reactivated in adulthood, the outcomes could vary. Thus, a caveat is that the context in which a guidance cue is activated is relevant if it is to be reused in regeneration.
Conclusions
The assembly of eye-to-brain connections is now a fairly well understood process in terms of the overall sequence of events. However, given the diversity of RGC types, targets and their functional roles, quite a bit more work remains to be done. Retinal cell-type-specific RNA profiling [91, 92] is likely to unveil new candidate molecules on both the RGC and target sides that regulate retinofugal connectivity, and also new cell types and patterns of connectivity. Advanced labeling, ultrastructural and functional microscopy methods [93] will no doubt advance this pursuit even further. The field of retinofugal regeneration is gaining ground thanks to progress in understanding developmental mechanisms and the development of genetic and viral approaches used to label and manipulate specific RGCs subsets [e.g., 94, *40]. Using these approaches to provide factors that stimulate survival and re-extension of specific RGC axons will allow for higher resolution views of the regeneration process, compared to labeling RGCs en mass.
Given the key roles of target derived cues and overall developmental events that direct retinofugal connectivity, the role of target neurons in the regeneration process also deserves study; one wonders, for example, if increasing neural activity in distal targets retrogradely increases responses to trophic support, as it does during development, thus promoting regeneration. Therefore, we view the implications of developmentally informed regenerative strategies for therapeutic interventions as an extremely exciting area likely to yield major progress in the near future.
ACKNOWLEDGEMENTS:
We thank Onkar Dhande and Tania Seabrook for helpful comments. Support was provided by NIH/NEI R01 EY026100, NIH/NEI R01 EY027713, NIH U01 EY27261 and The Glaucoma Research Foundation to ADH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ramón y Cajal S. 1995. Histology of the nervous system of man and vertebrates New York: Oxford University Press. [Google Scholar]
- 2.Ramon y Cajal S. 1928. Degeneration and Regeneration of the Nervous System New York: Oxford University Press, Reprinted 1991. [Google Scholar]
- 3.**.Baden T, Berens P, Franke K, Rosόn MR, Bethge M, Euler T. 2016. The functional diversity of retinal ganglion cells in the mouse. Nature 529: 345–50.A comprehensive exploration of the functional types of RGCs in mouse retina, achieved by calcium imaging all the RGCs in a large patch of explanted retina. A technical tour-de-force, this approach expanded beyond targeted recordings and previous anatomical studies in mouse.
- 4.**.Roska B, Werblin F. 2001. Vertical interactions across ten parallel stacked representations in the mammalian retina. Nature 410: 583–587.Using patch-clamp recordings from whole-mount rabbit retinas, the authors show that the inner plexiform layer is organized into at least ten parallel stacks of strata that all different RGCs to receive distinct amacrine and bipolar inputs. These neural inputs act in a distinct spatial and temporal fashion and are mediated by feedback inhibition, indicating that the interactions between strata occur in a vertical fashion.
- 5.*.Morin LP, Studholme KM. 2014. Retinofugal projections in the mouse. J. Comp. Neurol 522: 3733–53.This is the most comprehensive paper to date on the number, location and pattern of retinorecipient targets in mouse. Using intraocular injections of cholera-toxin β subunit, the authors compare and contrast the visual projections in the mouse with that of the hamster and the rat.
- 6.**.Dhande OS, Stafford BK, Lim JA, Huberman AD. 2015. Contributions of retinal ganglion cells to subcortical visual processing and behaviors. Annu Rev Vis Sci 1: 291–328.This is an in-depth review that provides detailed information about the different types, morphological and functional classification of ganglion cells as well as their projections in the brain and the functional roles (where known) of those projections. Summary of the known anatomical pathways, their functions and genetic markers for each are included.
- 7.Calkins DJ. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog. Retin. Eye Res 31: 702–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.London A, Benhar I, Schwartz M. 2013. The retina as a window to the brain – from eye research to CNS disorders. Nat. Rev. Neurol 9: 44–53. [DOI] [PubMed] [Google Scholar]
- 9.*.Liu J, Reggiani JDS, Laboulaye MA, Pandey S, Chen B, Rubenstein JLR, Krishnaswamy A, Sanes JR. 2018. Tbr1 instructs laminar patterning of retinal ganglion cell dendrites. Nature Neuroscience 21: 659–670.This paper identifies a transcription factor Tbr1, expressed by four RGC types, as a regulator of laminar specification. They show that Tbr1 expressing RGCs specifically target the outer strata of the inner plexiform layer. This study elegantly demonstrates the sufficiency and requirement of Tbr1 and identifies its downstream effectors.
- 10.**.Peng YR, Tran NM, Krishnaswamy A, Kostadinov D, Martersteck EM, Sanes JR. 2017. Satb1 regulated contactin5 to pattern dendrites of a mammalian retinal ganglion cell. Neuron 95: 869–883.The authors show that the transcription factor Satb1 regulates the expression of the adhesion molecule contactin5 and in doing so, selectively controls patterning of ON-OFF direction selective ganglion cell (ooDSGC) dendrites. In the absence of Satb1, ooDSGCs lose their characteristic bistratified ON and OFF arbors in the inner plexiform layer and only retain their OFF inputs, thus losing their ON responses.
- 11.Badea TC, Cahill H, Ecker J, Hattar S, Nathans J. 2009. Distinct roles of transcription factors brn3a and brn3b in controlling the development, morphology, and function of retinal ganglion cells. Neuron 61: 852–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De La Huerta I, Kim IJ, Voinescu PE, Sanes JR. 2012. Direction-selective retinal ganglion cells arise from molecularly specified multipotential progenitors. Pro Natl Acad Sci 109: 17663–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stuermer CAO, Bastmeyer M. 2000. The retinal axon’s pathfinding to the optic disk. Progress in Neurobiology 62: 197–214. [DOI] [PubMed] [Google Scholar]
- 14.Zelina P, Avci HX, Thelen K, Pollerberg GE. 2005. The cell adhesion molecule NrCAM is crucial for growth cone behavior and pathfinding of retinal ganglion cell axons. Development 132: 3609–18. [DOI] [PubMed] [Google Scholar]
- 15.Randlett O, Poggi L, Zolessi FR, Harris WA. 2011. The oriented emergence of axons from retinal ganglion cells is directed by laminin contact in vivo. Neuron 70: 266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brittis PA, Canning DR, Silver J. 1992. Chondroitin sulfate as a regulator of neuronal patterning in the retina. Science 255: 733–736. [DOI] [PubMed] [Google Scholar]
- 17.Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier-Lavigne M, Sretavan DW. 1997. Netrin-1 and DCC mediate axon guidance locally at the optic disc: Loss of function leads to optic nerve hypoplasia. Neuron 19: 575–589. [DOI] [PubMed] [Google Scholar]
- 18.*.Osterhout JA, El-Danaf RN, Nguyen PL, Huberman AD. 2014. Birthdate and outgrowth timing predict cellular mechanisms of axon target matching in the developing visual pathway. Cell Rep 8: 1006–17.This paper identifies the molecular and cellular mechanisms that dictate RGC axon-target matching. Its describes a temporal strategy by which RGCs find and select their targets, demonstrating that early born RGCs innervate multiple targets and then refine their connections while later-born RGCs are more selective in choosing and entering their targets perhaps as a result of having fewer targets to choose from.
- 19.Bhansali P, Rayport I, Rebsam A, Mason C. 2014. Delayed neurogenesis leads to altered specification of ventrotemporal retinal ganglion cells in albino mice. Neural Dev 9: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.*.Soares CA, Mason CA. 2017. Transient ipsilateral retinal ganglion cell projections to the brain: extent, targeting and disappearance. Develop. Neurobiol 75: 1385–1401.This study demonstrates how ipsilaterally projecting RGCs form transient projections that innervate the SC and dLGN. These results are particularly interesting because they provide a spatiotemporal resolution of ipsilateral and contralateral axon extension in the tract and show that although the ipsilateral projections are the first to enter the optic tract and comprise a higher number relative to contralateral projections, most of these transient ipsilateral projections disappear before they reach the targets.
- 21.Petros TJ, Rebsam A, Mason CA. 2008. Retinal axon growth at the optic chiasm. To cross or not to cross. Annu. Rev. Neurosci 31: 295–315. [DOI] [PubMed] [Google Scholar]
- 22.**.Kuwajima T, Soares CA, Sitko AA, Lefebvre V, Mason C. 2017. SoxC transcription factors promote contralateral retinal ganglion cell differentiation and axon guidance in the mouse visual system. Neuron 93: 1110–25.This study identifies a transcription factor SoxC as involved in specifying the differentiation of contralateral-projecting RGCs by Notch-repressive signaling. SoxC genes (Sox 4, 11 and 12) further promote midline crossing of contralateral RGCs by regulating Plexin-A1 and NrCAM expression, thus identifying a novel factor that controls contralateral RGC identity.
- 23.Kuwajima T, Yoshida Y, Takegahara N, Petros TJ, Kumanogoh A, Jessell TM, Sakurai T, Mason C. 2012. Optic chiasm presentation of Semaphorin 6D in the context of Plexin-A1 and Nr-CAM promotes retinal axon midline crossing. Neuron 74: 676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.*.Williams SE, Mann F, Erskine L, Sakurai T, Wei S, Rossi DJ, Gale NW, Holt CE, Mason CA, Henkemeyer M. 2003. Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron 6: 919–935.The authors show that ephrin-B2 is expressed by radial glial cells at the optic chiasm midline in the mouse. EphB1 is exclusively expressed by RGC axons originating from the ventrotemporal retina and transduces the repulsive signals of Ephrin-B2, thus forming the ipsilateral projections.
- 25.Lee R, Petros TJ, Mason CA. 2008. Zic2 regulates retinal ganglion cell axon avoidance of ephrinB2 through inducing expression of the guidance receptor EphB1. J Neuroscience 28: 5910–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herrera E, Brown L, Aruga J, Rachel RA, Dolen G, Mikoshiba K, Brown S, Mason CA. 2003. Zic2 patterns binocular vision by specifying the uncrossed retinal projection. Cell 5: 545–557. [DOI] [PubMed] [Google Scholar]
- 27.*.Sitko AA, Kuwajima T, Mason CA. 2018. Eye-specific segregation and differential fasciculation of developing retinal ganglion cell axons in the mouse visual pathway. J Comp Neurol 526: 1077–1096.This study focuses on the retinogeniculate pathway to demonstrate the segregation of ipsi vs contralateral RGC axons in the optic tract. Ipsilateral axons self-fasciculate more than contralateral axons suggesting that fasciculation may be a mode of segregation and organization of RGC axons within the tract. This study also highlights the fact that fasciculation is an important step in axon-target finding and therefore in the development of neural circuits.
- 28.Berson DM, Dunn FA, Takao M. 2002. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295: 1070–73. [DOI] [PubMed] [Google Scholar]
- 29.*.Yonehara K, Shintani T, Suzuki R, Sakuta H, Takeuchi Y, Nakamura-Yonehara K, Noda M. 2008. Expression of SPIG1 reveals development of a retinal ganglion cell subtype projecting to the medial terminal nucleus in the mouse. PLoS ONE 3: e1533.This study identified that a subset of RGCs, the ON-DSGCs project exclusively to the MTN. It helped clarify the features of the mouse RGCs that project to the MTN and illustrated the distinct fascicles that innervate the MTN. One of the earliest genetic labeling studies of specific RGC subtypes.
- 30.Ringstedt T, Braisted JE, Brose K, Kidd T, Goodman C, Tessier-Lavigne M, O’Leary DDM. 2000. Slit inhibition of retinal axon growth and its role in retinal axon pathfinding and innervation patterns in the diencephalon. J Neurosc 20: 4983–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.*.Seabrook TA, Burbridge TJ, Crair MC, Huberman AD. 2017. Architecture, function and assembly of the mouse visual system. Annu. Rev. of Neurosc 40: 499–538.This review provides a detailed account of the retinotopic and other functional maps in the mouse visual system, as well as how the functional maps between the retina, dLGN, SC and visual cortex are interconnected and form.
- 32.Godement P, Salaun J, IMbert M. 1984. Prenatal and postnatal development of retinogeniculate and retinocollicular projections in the mouse. J. Comp. Neurol 230: 552–575. [DOI] [PubMed] [Google Scholar]
- 33.**.Huberman AD, Manu M, Koch SM, Susman MW, Lutz AB, Ullian EM, Baccus SA, Barres SA. 2008. Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron 59: 425–438.This study identifies a transgenic mouse line that labels a mosaic of cells in the retina, the transient OFF-α RGCs and show that these cells project only to the dLGN and SC. Further, they show that columnar and laminar depths of axonal projections in the targets is regulated by spontaneous activity mediated refinement.
- 34.**.Osterhout JA, Josten N, Yamada J, Pan F, Wu SW, Nguyen PL, Panagiotakos G, Inoue Yu, Egusa SF, Volgyi B, Inoue T, Bloomfield SA, Barres BA, Berson DM, Feldheim DA, Huberman AD. 2011. Cadherin-6 mediates axon-target matching in a non-image-forming visual circuit. Neuron 71: 632–639.The authors demonstrate a role for classical Cadherins in promoting wiring specificity in the mouse visual system. Cdh3-GFP RGC axons that express Cdh6 grow and connect to subcortical targets that also express Cdh6. In mutants lacking Cdh6, the Cdh3-GFP RGC axons grow past their normal targets and form ectopic terminations indicating that Cdh6 interactions are necessary for proper termination of RGC axons within specific targets.
- 35.**.Osterhout JA, Stafford BK, Nguyen PL, Yoshihara Y, Huberman AD. 2015. Contactin-4 mediates axon-target specificity and functional development of the accessory optic system. Neuron 86: 985–999.This study highlights the importance of mechanisms that regulate axonal arbor formation and their role in ultimately determining behavioral functions. They specifically focus on the accessory optic system and identify that Cntn4 and its co-receptor amyloid precursor protein (APP), both of which are expressed by RGC axons are required for specifying arborization inside an AOS target, the nucleus of the optic tract.
- 36.**.Sun LO, Brady CM, Cahill H, Al-Khindi T, Sakuta H, Dhande OS, Noda M, Huberman AD, Nathans J, Kolodkin AL. 2015. Functional assembly of accessory optic system circuitry critical for compensatory eye movements. Neuron 86: 971–984.This paper identifies the molecular mechanisms that direct direction selective ganglion cells to innervate the MTN. The authors show that in a surprising reverse signaling system, Sema6A expressing RGC axons require PlexinA2/A4, expressed by target cells in the MTN. In mice mutant for either the ligand or the receptor, the axons still reach the MTN but are unable to innervate the target.
- 37.**.Su J, Haner CV, Imbery TE, Brooks JM, Morhardt DR, Gorse K, Guido W, Fox MA. 2011. Reelin is required for class-specific retinogeniculate training. J Neurosci 31: 575–586.This study shows that reelin is required to direct ipRGC axons into the vLGN and IGL. Reelin is highly enriched in the vLGN and IGL compared to the dLGN. As a result, in reelin mutants, the retinal projections are reduced in the vLGN and almost absent in the IGL. These results are particularly interesting as they reveal the involvement of molecular cues in the segregation of RGC axons to different but very closely situated retinorecipient nuclei.
- 38.*.Su J, Klemm MA, Josephson AM, Fox MA. 2013. Contributions of VLDLR and LRP8 in the establishment of retinogeniculate projections. Neural Dev 8:11.3.This study examines the role of reelin receptors VLDLR and LRP8 in directing retinogeniculate targeting. The authors show that a subset of RGC axons that normally target the IGL are misrouted to the dorsomedial dLGN in the absence of both receptors; however this study shows that these functions are likely independent of reelin, thus suggesting involvement of other molecules.
- 39.Di Donato V, De Santis F, Albadri S, Auer TO, Duroure K, Charpentier M, Concordet JP, Genhardt C, Del Bene F. 2018. An attractive reelin gradient establishes synaptic lamination in the vertebrate visual system. Neuron 97: 1049–62. [DOI] [PubMed] [Google Scholar]
- 40.*.Seabrook TA, Dhande OS, Ishiko N, Wooley VP, Nguyen PL, Huberman AD. 2017. Strict independence of parallel and poly-synaptic axon-target matching during visual reflex circuit assembly. Cell Rep 21: 3049–64.The authors identify that Tph2cre mouse line can be used to label luminance-sensing RGC axons that project to the OPN and mdPPN. Further, this study compares two populations of RGC axons that project either to image-forming or non-image-forming centers in the pretectum and find that, after genetic ablations of one population, the other remaining RGC axons project through and past vacant target sites, retaining their targeting specificity. Additionally, functional wiring of downstream targets for the pupil reflex remains unaffected by afferent depletion to the primary target in this circuit (the OPN) indicating independent retention of individual components in a poly-synaptic circuit.
- 41.Feldheim DA, O’Leary DDM. 2010. Visual map development: Bidirectional signaling, bifunctional guidance molecules, and competition. Cold Spring Harb Perspect Biol 2:a001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfeiffenberger C, Yamada J, Feldheim DA. 2006. Ephrin-As and patterned retinal activity act together in the development of topographic maps in the primary visual system. J Neurosc 26:12873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McLaughlin T, Hindges R, Yates PA, O’Leary DDM. 2003. Bifunctional action of Ephrin-B1 as a repellent and attractant to control bidirectional branch extension in dorsal-ventral retinotopic mapping. Development 130: 2407–18. [DOI] [PubMed] [Google Scholar]
- 44.Schmitt AM, Shi J, Wolf AM, Lu CC, King LA, Zou Y. 2006. Wnt-Ryk signaling mediates medial-lateral retinotectal topographic mapping. Nature 439: 31–37. [DOI] [PubMed] [Google Scholar]
- 45.*.Plas DT, Lopez JE, Crair MC. 2005. Pretarget sorting of retinocollicular axons in the mouse. J Comp. Neurol 491: 305–319.The results from this study show that RGC axons are retinotopically organized for dorso-ventral RGCs, within the optic tract. Dorso-ventral axons re-establish their retinotopy within the tract well before the axon terminals reach the target. Importantly, the naso-temporal axons do not which may explain the varying severity of various EphA (associated with N-T mapping) vs EphB (associated with D-V mapping) mutations in other studies. These results indicate that RGC axons are organized in a meaningful way based on their origin in the retina as well as chronological order of extension.
- 46.Meister M, Wong RO, Baylor DA, Shatz CJ. 1991. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science 252: 939–943. [DOI] [PubMed] [Google Scholar]
- 47.Huberman AD, Feller MB, Chapman B. 2008. Mechanisms underlying development of visual maps and receptive fields. Annu. Rev. Neurosci 31: 479–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cang J, Wang L, Stryker MP, Feldheim DA. Roles of Ephrin-As and structured activity in the development of functional maps in the superior colliculus. J Neurosc 28:11015–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dhande OS, Hua EW, Guh E, Yeh J, Bhatt S, Zhang Y, Ruthazer ES, Feller MB, Crair MC. 2011. Development of single retinofugal axon arbors in normal and β2 knock-out mice. J Neuroscience 31: 3384–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong KY, Kim I- J, Sanes JR. 2011. Stereotyped axonal arbors of retinal ganglion cell subsets in the mouse superior colliculus. J. Comp. Neurol 519: 1691–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Owens MT, Feldheim DA, Stryker MP, Triplett JW. 2015. Stochastic interaction between neural activity and molecular cues in the formation of topographic maps. Neuron 87: 1261–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.**.Xiao T, Staub W, Robles E, Gosse NJ, Cole GJ, Baier H. 2011. Assembly of lamina-specific neuronal connections by slit bound to type IV collagen. Cell 146: 164–176.The results from this study show that Slit1 in the zebrafish tectum binds to a type IV collagen and is localized on the basement membrane. Further, by interacting with Robo2-expressing RGC axons Slit1 directs axons to specific lamina, an effect that is disrupted by overexpressing Slit1. This study also highlights the importance of axon-target interactions in lamina-specific positioning.
- 53.*.Robles E, Filosa A, Baier H. 2013. Precise lamination of retinal axons generates multiple parallel input pathways in the tectum. J Neurosc 33: 5027–39.This study examined laminar targeting by generating brainbow zebrafish models. They show that while each laminae receives inputs from multiple subtypes of RGCs, the dendrites from each subtype of RGC has a specific, stereotyped laminar position.
- 54.**.Matsuoka RL, Nguyen-Ba-Charvet KT, Parray A, Badea TC, Chedotal A, Kolodkin A. 2011. Transmembrane semaphorin signaling controls laminar stratification in the mammalian retina. Nature 470: 259–263.This study examines the molecular mechanisms that direct RGC dendrites to specific laminar positions and show that Sema6A and Plexin-A4 are expressed in a complementary manner in the inner plexiform layer. In PlexinA4 knockout mice, amacrine cells normally extending branches in the outermost OFF layer are disrupted and instead extend branches to the ON layer. Thus Sema6A repels Plexin A4 expressing amacrine and ganglion cells in a non-cell autonomous manner.
- 55.Sun LO, Jiang Z, Rivlin-Etzion M, Hand R, Brady CM, Matsuoka RL, Yau KW, Kolodkin AL. 2013. On and off retinal circuit assembly by divergent molecular mechanisms. Science 342: 1241974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krishnaswamy A, Yamagata M, Duan X, Hong KY, Sanes JR. 2015. Sidekick 2 directs formation of a retinal circuit that detects differential motion. Nature 524: 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jaubert-Miazza L, Green E, Lo FS, Bui K, Mills J, Guido W. 2005. Structural and functional composition of the developing retinogeniculate pathway in the mouse. Vis Neurosci 22:661–76 [DOI] [PubMed] [Google Scholar]
- 58.Katz LC, Shatz CJ. 1996. Synaptic activity and the construction of cortical circuits. Science 274: 1133–38. [DOI] [PubMed] [Google Scholar]
- 59.Torborg CL, Hansen KA, Feller MB. 2005. High frequency, synchronized bursting drives eye-specific segregation of retinogeniculate projections. Nat. Neurosci 8: 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huberman AD, Murray KD, Warland DK, Feldheim DA, Chapman B. 2005. Ephrin-As mediate targeting of eye-specific projections to the lateral geniculate nucleus. Nat. Neurosci 8: 1013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pfeiffenberger C, Cutforth T, Woods G, Yamada J, Rentería RC, Copenhagen DR, Flanagan JG, Feldheim DA. 2005. Ephrin A-s and neural activity are required for eye-specific patterning during retinogeniculate mapping. Nat Neurosci 8:1022–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.**.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. 2007. The classical complement cascade mediates CNS synapse elimination. Cell 131:1164–78.The authors show that the components of the complement cascade, expressed by astroglia and neurons during development, control refinement of the retinogeniculate projections by eliminating unwanted synapses. This paper is particularly interesting as it establishes a strong model of the immune system and nervous system working together to refine retinogeniculate projections – astrocytes upregulate C1q in ganglion cells to tag unwanted synapses and activate the complement cascade.
- 63.**.Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, Smith SJ, Barres BA. 2013. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504: 394–400.This paper describes another important role for astrocytes in synapse elimination that is dependent on neural activity. The results implicate two phagocytic pathways MEGF10 and MERTK in the engulfment of unwanted synapses, especially during the refinement of retinogeniculate projections.
- 64.Zuchero JB, Barres BA. 2015. Glia in mammalian development and disease. Development 142: 3805–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.**.Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. 2008. Promoting axon regeneration in the adults CNS by modulation for the PTEN/mTOR pathway. Science 322: 963–966.The results from this study showed that by knocking down PTEN, an inhibitor of mammalian target of rapamycin (mTOR), RGC axons can regenerate after optic nerve injury, thus firmly planting the idea that developmental mechanisms can be reused to promote regeneration of the CNS.
- 66.*.Goldberg JL, Klassen MP, Hua Y, Barres BA. 2002. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science 296: 1860–64.This paper sheds light on the mechanisms that curb the growth capacity of RGCs. Amacrine cells signal the maturation of RGCs causing them to irreversibly lose their axonal growth ability while increasing their dendritic growth, expanding our understanding of the mechanisms by which extrinsic signals can regulate axon growth potential and why the growth capacity of axons differs in the mature CNS.
- 67.Murray M, Edwards M. 1982. A quantitative study of the reinnervation of the goldfish optic tectum following optic nerve crush. J. Comp. Neurol 209: 363–373. [DOI] [PubMed] [Google Scholar]
- 68.*.Laha B, Stafford BK, Huberman AD. 2017. Regenerating optic pathways from the eye to the brain. Science 356:1031–34.This review outlines the different intrinsic and extrinsic barriers that hinder regeneration in the visual system and provides a summary of the most recent findings on ways to overcome these in the experimental and clinical context.
- 69.*.Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV. 2016. Astrocyte scar formation aids central nervous system axon regeneration. Nature 532: 195–200.This study addresses the purpose of the astrocytic scar formation following a CNS injury. Using mouse models that prevent the formation of scar, they show that the presence of astrocytic scar can be beneficial for axon regeneration after an injury and demonstrate that the cells in lesion site express several genes that promote axon growth.
- 70.*.Silver J, Miller JH. 2004. Regeneration beyond the glial scar. Nature Reviews Neuroscience 5: 146–156.An important review that features the physical, chemical and inflammatory aspects of glial scarring. Sheds light on some of the varying effects of glial scarring on regenerative potential as in [ref. 66]
- 71.Becker C, Becker T. 2007. Growth and pathfinding of regenerating axons in the optic projection of adult fish. J Neuroscience Research 85: 2793–99. [DOI] [PubMed] [Google Scholar]
- 72.Lipton SA. 1986. Blockade if electrical activity promotes the death of mammalian retinal ganglion cells in culture. Pro Natl Acad Sci 83: 9774–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.**.Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GTA, Barres BA. 2002. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron 33: 689–702.This study shows that RGC axon growth is stimulated by a combination of trophic factors and electrical activity and is one of the first examples to show the involvement neurotrophins and electrical activity in visual system development.
- 74.**.Lim JH, Stafford BK, Nguyen PL, Lien BV, Wang C, Zukor K, He Z, Huberman AD. 2016. Neural activity promotes long-distance, target-specific regeneration of adult retinal axons. Nat Neurosci 19: 1073–84.This study shows that a combinatorial approach of increasing mTOR signaling and neural activity can promote significant regeneration of RGC axons well past the chiasm and to different targets in the brain. The results from this study highlights the role of neural activity in promoting regeneration and restoring functional behaviors.
- 75.**.Duan X, Qiao M, Bei F, Kim IJ, He Z, Sanes JR. 2015. Subtype-specific regeneration of retinal ganglion cells following axotomy: effects of osteopontin and mTOR signaling. Neuron 85: 1244–56.The authors show that α-RGCs comprise the majority of regenerating axons after axotomy, perhaps owing to the intrinsically high levels of mTOR they express. This study demonstrates subtype-specific survival capabilities of RGCs and identifies α-RGCs as a robust regenerating group, which is particularly useful in identifying factors that promote survival and/or regeneration.
- 76.Perez de Sevilla Muller L, Sargoy A, Rodriguez AR, Brecha NC. 2014. Melanopsin ganglion cells are the most resistant retinal ganglion cell type to axonal injury in the rat retina. PLoS ONE 9, e93274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cui Q, Ren C, Sollars PJ, Pickard GE, So KF. 2015. The injury resistant ability of melanopsin-expressing intrinsically photosensitive retinal ganglion cells. Neuroscience 284: 845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sanchez-Migallon MC, Valiente-Soriano FJ, Nadal-Nicolas FM, Pierdomenico JD, Vidal-Sanz M, Agudo-Barriuso M. 2018. Survival of melanopsin expressing retinal ganglion cells long term after optic nerve trauma in mice. Exp. Eye Research 174: 93–97. [DOI] [PubMed] [Google Scholar]
- 79.*.Norsworthy MW, Bei F, Kawaguchi R, Wang Q, Tran NM, Li Y, Brommer B, Zhang Y, Wang C, Sanes JR, Coppola G, He Z. 2017. Sox11 expression promotes regeneration of some retinal ganglion cell types but kills others. Neuron 94: 1112–20.This study demonstrates the role of Sox11 in optic nerve regeneration. In a surprising result, the authors show that overexpression of Sox11 kills alpha RGCs, which normally constitute the surviving population of RGCs in other treatments including PTEN knockdown, while simultaneously increasing survival of other subtypes of RGCs. A particularly interesting feature of this study is to note that not all developmental mechanisms can be beneficial in regeneration.
- 80.Shen S, Wiemelt AP, McMorris AF, Barres BA. 1999. Retinal ganglion cells lose trophic responsiveness after axotomy. Neuron 23: 285–295. [DOI] [PubMed] [Google Scholar]
- 81.*.Crair MC, Mason CA. 2017. Reconnecting eye to brain. J Neurosc 36: 10707–22.This review discusses recent findings regarding visual system regeneration and provides a detailed account of different approaches discussing their success as well as limitations. The authors also discuss gaps in the field that should be addressed, thus providing a comprehensive summary of the state of visual system repair.
- 82.Benowitz LI, He Z, Goldberg JL. 2015. Reaching the brain: advances in optic nerve regeneration. Exp. Neurol 287: 365–373. [DOI] [PubMed] [Google Scholar]
- 83.*.Yin Y, Henzl MT, Lorber B, Nakazawa T, Thomas TT, Jiang F, Langer R, Benowitz LI. 2006. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nature Neuroscience 9: 843–852.This study identifies oncomodulin as a key factor to manipulate in regeneration. The authors show that oncomodulin is secreted by macrophages and binds to RGCs in a cyclic AMP dependent manner to promote axon growth.
- 84.Agostinone J, Alarcon-Martinez L, Gamlin C, Yu WQ, Wong ROL, Di Polo A. 2018. Insulin signaling promotes dendrite and synapse regeneration and restores circuit function after axonal injury. Brain 141: 1963–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith PD, Sun F, Park KK, Cai B, Wang C, Kuwako K, Marinez-Carrasco I, Connolly L, He Z. 2009. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron 64: 617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.*.Bei F, Lee HH, Liu X, Gunner G, Jin H, Ma L, Wang C, Hou L, Hensch TK, Frank E, Sanes JR, Chen C, Fagiolini M, He Z. 2016. Restoration of visual function by enhancing conduction in regenerated axons. Cell 164: 219–232.This study shows examines the functional sufficiency of regenerating RGC axons and finds that regenerating axons are not myelinated. By using a potassium-channel blocker 4-AP, action potential conductance was improved in regenerating axons thus leading to behavioral recovery.
- 87.Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL. 2009. KLF family members regulate intrinsic axon regeneration ability. Science 326: 298–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nawabi H, Belin S, Cartoni R, Williams PR, Wang C, Latremoliere A, Wang X, Zhu J, Taub DG, Fu X, Yu B, Gu X, Woolf CJ, Liu JS, Gabel CV, Steen JA, He Z. 2015. Doublecortin-like kinases promote neuronal survival and induce growth cone reformation via distinct mechanisms. Neuron 88: 704–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.**.De Lima S, Koriyama Y, Kurimoto T, Oliveira JT, Yin Y, Li Y, Gilbert HY, Fagiolini M, Martinez AM, Benowitz L. 2012. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Pro Natl Acad Sci 109: 9149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.*.Beazley LD, Rodger J, Chen P, Tee LBG, Stirling RV, Taylor AL, Dunlop SA. 2003. Training on a visual task improves the outcome of optic nerve regeneration. J Neurotrauma 20: 1263–70.This study examines optic nerve regeneration in lizards and shows that visual training improved both the precision of topographic projections as well as functional restoration after injury.
- 91.Rheaume BA, Jereen A, Bolisetty M, Sajid MS, Yang Y, Renna K, Sun L, Robson P, Trakhtenberg EF. 2018. Single cell transcriptome profiling of retinal ganglion cells identifies cellular subtypes. Nature Comm 9: 2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shekhar K, Lapan SW, Whitney IE, Tran NM, Macosko EZ, Kowalczyk M, Adiconis X, Levin JZ, Nemesh J, Goldman M, McCarroll SA, Cepko CL, Regev A, Sanes JR. 2016. Comprehensive classification of retinal bipolar neurons by single-cell transcriptomics. Cell 166: 1308–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morgan JL, Berger DR, Wetzel AW, Lichtman JW. 2016. The fuzzy-logic of network connectivity in mouse visual thalamus. Cell 165: 192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tang JCY, Rudolph S, Dhande OS, Abraira VE, Choi S, Lapan SW, Drew IR, Drokhlyansky E, Huberman AD, Regehr WG, Cepko CL. 2015. Cell type-specific manipulation with GFP-dependent Cre recombinase. Nat. Neurosci 18: 1334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]