Abstract
Cytokines are pivotal mediators of the immune response, and their coordinated expression protects host tissue from excessive damage and oxidant stress. Nevertheless, the development of lung pathology, including asthma, chronic obstructive pulmonary disease, and ozone-induced lung injury, is associated with oxidant stress; as evidence, there is a significant increase in levels of the modified guanine base 7,8-dihydro-8-oxoguanine (8-oxoG) in the genome. 8-OxoG is primarily recognized by 8-oxoguanine glycosylase 1 (OGG1), which catalyzes the first step in the DNA base excision repair pathway. However, oxidant stress in the cell transiently halts enzymatic activity of substrate-bound OGG1. The stalled OGG1 facilitates DNA binding of transactivators, including NF-κB, to their cognate sites to enable expression of cytokines and chemokines, with ensuing recruitments of inflammatory cells. Hence, defective OGG1 will modulate the coordination between innate and adaptive immunity through excessive oxidant stress and cytokine dysregulation. Both oxidant stress and cytokine dysregulation constitute key elements of oncogenesis by KRAS, which is mechanistically coupled to OGG1. Thus, analysis of the mechanism by which OGG1 modulates gene expression helps discern between beneficial and detrimental effects of oxidant stress, exposes a missing functional link as a marker, and yields a novel target for lung cancer.
Keywords: Kirsten-Ras, OGG1, Lung cancer, Innate immune response, Metastasis, Reactive oxygen species
1. Introduction
New molecular targeted therapies and an improvement in molecular diagnostics have marked the progress in lung cancer treatment (Hiley et al., 2016; Lee, 2017; Waqar & Morgensztern, 2017). Nevertheless an effective framework is still needed for the use of new targeted therapies, especially those that address interaction between tumors and the immune system (Hirsch et al., 2017). At the same time, in spite of intensified use of next-generation sequencing and improved selection of test samples, identification of druggable genomic alterations is reaching a plateau (Li et al., 2017; Alegre et al., 2016). However, in the non-coding genome, a vast genetic and epigenetic landscape exists, which encompasses functions that include chromatin remodeling and coordination of the expression of genes that affect the composition and function of the tissue microenvironment (Pan et al., 2016, Pan, Zhu et al., 2016; Vlahopoulos et al., 2015).
The lung is an organ that provides a dynamic interface with the environment, and lung inflammation is coupled to restoration of tissue barrier integrity under the coordinating function of cytokines, with the most notable example being the protein tumor necrosis factor alpha (TNFα) (Damjanovic et al., 2011; Quinn et al., 2017). TNFα is a pluripotent inflammatory cytokine that elicits diverse effects on cells, including generation of reactive oxygen species (ROS), mediation of innate immune response (IIR) and activation of both oncogenes, and tumor suppressors (S. Vlahopoulos, Boldogh, Casola, & Brasier, 1999; Wang et al., 2014; Balkwill & Joffroy, 2010). TNFα and TNFα-induced ROS activate many signaling mediators, including the protein complex Nuclear Factor-kappa B (NF-κB) to induce expression of genes that encode cytokines, chemokines, adhesion molecules, and matrix-degrading enzymes, implicated in metastasis (Acharyya et al., 2012; Kim et al., 2017).
Dysregulation of ROS generation is an essential factor in cancer development driven by the oncogene Kirsten (K)-RAS (Jinesh, Sambandam, Vijayaraghavan, Balaji, & Mukherjee, 2017; Park et al., 2014). Nevertheless, a mutant, recombinant version of TNFα has shown measurable therapeutic impact as a prospective biological agent during treatment of lung cancer patients (X. Ma et al., 2015). This suggests that at least one part of the TNFα-induced signal pathways elicits antineoplastic activity, while another part of the pathway has the opposite effect. Hence, intervention in mechanisms that are involved in both cancer progression and inflammation will require further molecular analysis, to consolidate their translational and clinical application. To this end, rapid TNF-induced expression of several inflammatory genes requires docking of the enzyme 8-oxoguanine glycosylase 1 (OGG1). This enzyme has been linked to lung tissue homeostasis and cancer through epidemiological studies, and through the analysis of cellular and animal models that we discuss here. These studies are a step toward differentiating between beneficial and detrimental pathways elicited by ROS in lung cancer progression.
2. Dysregulation of NF-κB target genes is linked to the immunopathology of non-small cell lung cancer
ROS have multiple effects on gene regulation (Kietzmann, Petry, Shvetsova, Gerhold, & Görlach, 2017). The main ROS function on inflammatory gene expression is via the induction of the NF-κB-dependent network. NF-κB is heterodimer of Rel-family proteins that in non-transformed and transformed cells under homeostatic conditions is tethered in the cytoplasm by association with inhibitory kappa B (IκB) proteins. The IκB proteins thereby act as direct inhibitors (Brasier, 2006).
The “canonical” NFκB pathway is initiated by either activated cell surface receptors, including TNF superfamily receptors, or the cytoplasmic pattern recognition receptor RIG-I (retinoic acid-inducible gene I), which activate IKK, the rate-limiting enzyme complex that phosphorylates iκBα to induce its degradation. RIG-I can also activate NF-κB via a “noncanonical” signal pathway in an IKKγ-independent manner that converges on the NF-κB inducing kinase (NIK)-IKKα complex. The latter initiates NFκB2/p100 processing to release sequestered RelB/p52 and RelA complexes from a cytoplasmic NFκB2/p100 precursor (Liu, Li, Garofalo, & Brasier, 2008). Combined computational and biochemical studies indicate that the extent of NF-κB-responsive expression of Nfkbia, which encodes IκBα, inversely correlates with cross-talk between canonical and noncanonical NF-κB pathways. The Nfkbia promoter shows enhanced responsiveness to NF-κB activation in macrophages compared to that in fibroblasts (Chatterjee et al., 2016). Thereby, the insulation of canonical and noncanonical NF-κB pathways by IκBα limits the deleterious effects of prolonged macrophage-mediated inflammation.
During NF-κB activation IκBα is released from cytoplasmic stores, and ROS induce phosphorylation of RelA at Ser276. This phosphorylation is performed by the enzyme MSK-1 in the nucleus, (Jamaluddin, Tian, Boldogh, Garofalo, & Brasier, 2009) or by a cytoplasmic IKK-associated catalytic subunit of protein kinase A (Christian, Smith, & Carmody, 2016; Jamaluddin, Wang, Boldogh, Tian, & Brasier, 2007). Phosphorylation at Ser276 causes RelA acetylation by CREB-binding protein (CBP)/p300 at Lys-310. This causes RelA association with the positive transcriptional elongation factor (PTEF-b), the epigenetic reader (and histone acetyltransferase) bromodomain 4 (BRD4), and cyclin-dependent kinase (CDK9) (Brasier et al., 2011; Nowak et al., 2008; Tian et al., 2013. BRD4 with CDK9 increase transcriptional elongation of paused RNA polymerase II to activate expression of specific gene cohorts that include genes encoding chemokines. The NF-κB-CDK9-BRD4 complex thereby induces expression of cohorts of “immediate early genes” for IIR, including IL-8 (interleukin-8, or CXCL8) and Groβ (CXCL2), encoding chemokines, which are cytokines involved in leukocyte chemotaxis and activation (Brasier et al., 2011; Nowak et al., 2008; Tian et al., 2013). Therefore pattern recognition receptors use ROS-induced signal pathways that cause NF-κB phosphorylation to trigger IIR.
The most notable failure of the immune response is tumor recurrence, as this is exactly a phenomenon that the immune system is aimed to prevent. With respect to lung cancer recurrence, the chemokine CXCL1 has shown significant association with recurrence after surgical removal of non-small-cell lung carcinoma (NSCLC) (Spaks, 2017). The patients that relapsed had a substantially higher (>10-fold) concentration of CXCL1 in peripheral and tumor draining blood samples, compared with those patients that did not relapse. The concentration of CXCL1 is critical for recruitment of Tregs in NSCLC (P < .01), which is inversely correlated with patient survival (P < .001) (Lv et al., 2014). In the lung, natural killer cells (NK), a key component of T-helper type 1 (Th1) inflammation, release CXCL1 (Hoegl et al., 2017). In turn, CXCL1 induces chemotaxis of Treg cells into the malignant pleural effusion, which allows the tumor to escape the immune response (Lv et al., 2014). This could lead to the surprising association of Th1 cell accumulations in the lung tissue with negative prognosis. Indeed, high titer of tissue-infiltrating Th1 cells is associated with poor prognosis in NSCLC, whereby the malignant tumor is protected from the immune system. This paradox is explained by the increased frequency of Th2 cells in the tumor nest, which is followed by Th17 cells at the tumor boundary, and Th1 cells in the rest of the tissue (Huang, Shen, Huang, Ling, & Zhang, 2017).
Thus, a transition between Th1, Th17, and Th2 cells toward the tumor nest is associated with a poor prognosis. Indeed, evidence is mounting that aberrant regulation between Th1 and Th17 is critical in NSCLC, and this defect is particularly evident in aberrant expression of the cytokine IL-17 that contributes to a poor disease prognosis (Joerger, Finn, Cuffe, Byrne, & Gray, 2016; Xu, Yu, Zhan, & Zhang, 2014; Zhang, Han, Fang, & Ma, 2012). In adipose-derived stem cells, IL-17 activates the transcription factor signal transducer and activator of transcription (STAT1), which enhances expression of the NF-κB target gene that encodes cytokine IL-6. In turn IL-6 stimulates neighboring lung cancer cells to activate kinase JAK2, which phosphorylates and activates transcription factor STAT3, and thereby generates a paracrine circuit of cytokine activity that shapes the local tissue microenvironment (Guo, Jin, & Chen, 2014; Lu et al., 2016). As a result, both STAT1 and STAT3 are active in different cell types within the same tumor microenvironment. In this example adipose-derived stem cells activate STAT1 and lung cancer cells STAT3. These types of molecular interactions can generate a tissue niche suitable for growth of KRAS mutant lung cancer cells, where T-cell mediated antitumor immunity is suppressed (Akbay et al., 2017; Caetano et al., 2016).
In regard to the Th1 and Th2 cytokines, early-stage NSCLC is characterized by a lower concentration of TNFα (Decotiis et al., 2016). In general, however, circulating concentrations of interleukin (IL)-IL23, IL-17A, IL-17F, IL-22, and TNFα were found to be significantly higher in the patients with NSCLC compared with the healthy controls (P < .05). Furthermore, higher TNFα, IL-23, IL-17A, IL-17F, and IL-22 are found in stages I-IIIA of NSCLC, compared to stages IIIB-IV (Liao et al., 2015). Namely in later stages of the disease, Th17-type cells are gradually replaced by immunosuppressive Treg cells that secrete TGFβ1 and IL-10 (Duan et al., 2015). Chemotaxis of Tregs to the tumor nest can be explained by their expression of IL8-receptors (Eikawa et al., 2010).
Which succession of events leads to the gradual change in the types of host cells that protect the tumor? The cytokine environment might play a key role in the differentiation of Th17 and Treg cells in NSCLC. TGFβ recruits and polarizes M2 macrophages, increases expression of MMPs, and contributes to Th17 differentiation and the release of IL-17, subsequently facilitating recruitment of IL17RA and IL17RC expressing macrophages in lung cancer (Szebeni, Vizler, Kitajka, & Puskas, 2017). IL-17 is a negative prognostic factor for overall survival in NSCLC (Zeng et al., 2015; Wang et al., 2017). Within a given study cohort, in addition to IL-17, circulating IL-6 is also a negative prognostic factor for NSCLC (Silva et al., 2017). Analysis of bronchoalveolar lavage fluid gives a significant correlation between the proportions of Tregs with IL-17A concentration (Kwiecien, Stelmaszczyk-Emmel, Polubiec-Kownacka, Dziedzic, & Domagala-Kulawik, 2017).
Moreover, circulating Th17 cells and Treg cells were increased in samples derived from patients with NSCLC, albeit with a negative correlation between Treg cells and Th17 cells. This supports a concept of gradual replacement of Th17 with Treg. Furthermore, in patients with NSCLC, the frequency of Th17 cells positively correlated with IL-1β, IL-6 and IL-23 and the frequency of Treg cells positively correlated with TGFβ1 and IL-10. Marking systemic disease, the Th17/Treg ratio was significantly higher in patients with NSCLC than in healthy controls. As proof of principle, in patients with NSCLC the Th17/Treg ratio positively correlated with the carcinoembryonic antigen (CEA) concentration, a protein that is normally present only at very low levels in body fluids (Duan et al., 2015).
Thus, these changes in cytokine expression and cell types indicate that NSCLC is characterized by a defect in the network that links innate and adaptive immunity. This defect leads to the formation of an immunosuppressive microenvironment that enables cancer progression and facilitates the development of characteristic pathological sequelae linked to deregulation of inflammatory genes.
In a mouse model of chronic obstructive pulmonary disease (COPD), chronic NF-κB activation generates an immunosuppressive microenvironment in the lungs that leads to lung adenomas (Zaynagetdinov et al., 2016). Sustained activation of the NF-κB pathway links COPD to lung cancer through generation and maintenance of an inflammatory environment consisting of alternatively activated macrophages and regulatory T cells. A tetracycline-inducible transgenic mouse model that conditionally expresses IKKβ in the airway epithelium led to sustained NF-κB activity, chronic inflammation, emphysema, and later to lung adenomas. During lung inflammation there was evidence for a substantial increase in M2-polarized macrophages and CD4+/CD25+/FoxP3+ Tregs. Depletion of alveolar macrophages in these mice reduced Tregs, increased lung CD8+ lymphocytes, and reduced tumor numbers following treatment with the carcinogen urethane. Alveolar macrophages supported increased generation of inducible Foxp3+ Tregs ex vivo through expression of TGFβ and IL-10. Targeting of TGFβ and IL-10 reduced the ability of alveolar macrophages to induce Foxp3 expression on T cells in these mice (Zaynagetdinov et al., 2016).
In human epidemiology, development of COPD increases circulating TNFα (Gan, Man, Senthilselvan, & Sin, 2004; Shahriary, Panahi, Shirali, & Rahmani, 2017). In comparison with COPD, cancer development in the case of NSCLC is associated with a decline in levels of the cytokines that favor the immune response. This is supported by the observation that the interferon-γ/IL-10 ratio was lower in cancer patients as compared to COPD patients, consistent with a cytokine milieu favouring tumor tolerance, while circulating TNFα remained increased (Eide et al., 2016).
3. Molecular drivers of inflammation-induced signaling in lung cancer
Inflammation-induced signaling has been recognized as a key factor in lung carcinogenesis, especially in cancers driven by the proto-oncogene KRAS (Akbay et al., 2017; Kitajima, Thummalapalli, & Barbie, 2016). At the molecular level, transcription factor NF-κB is a core component of K-Ras driven carcinogenesis (Kim et al., 2016). In neoplastic tissue NF-κB and its activator TRAF6 are pivotal mediators of malignant cell growth and RAS-induced carcinogenesis (Finco et al., 1997; He, Huang, Lin, & Ye, 2016; Starczynowski et al., 2011). At the cellular level, both upstream kinases IKKα and IKKβ contribute to the development of oncogenic properties of lung cells with mutant KRAS (Bassères, Ebbs, Cogswell, & Baldwin, 2014). In cells from lung tumors containing mutated KRAS, nuclei stained positive for RelA (Tang et al., 2006). RelA is implicated in tumor-stroma interactions and correlates strongly with the severity of tumor infiltration by inflammatory cells in NSCLC patients (Giopanou et al., 2015). This could explain the finding that RelA suppresses antitumor immunity in the mouse during Ras-induced oncogenesis (Wang, Ratnam, Byrd, & Guttridge, 2014).
Studies suggest that inflammation, and in particular the IIR, drives KRAS mutant lung cancer and has extensive impact on transformed cells (Gong et al., 2016). In the lung cancer cell line A 549, lysine residue 310 acetylation of RelA is essential for stability of nuclear RelA, interaction with BRD4, TNF-α-mediated NF-κB activation, NF-κB-dependent target gene expression, proliferation and transformation potential. Consequently, inhibition of BRD4 suppresses tumorigenicity of A549 cells in mice with severe combined immunodeficiency (Zou et al., 2014). NF-κB and BRD4 have specific gene targets that enable metastasis, including matrix met alloproteinases (MMPs) (Xiao et al., 2016). NSCLC shows increased potential for metastasis in proportion to the expression of MMPs. MMP-9, also known as type IV collagenase, is involved in the degradation of the main constituents of the extracellular matrix and basement membrane. MMP-9 increases NSCLC invasiveness that facilitates metastasis (Cai et al., 2015; Gallelli et al., 2014). An effective response of NSCLC to chemotherapy is associated with a decrease in serum concentration of MMP-9 (Qiao et al., 2016). It is important to note that through MMP-9 the lung can also be transformed into a metastatic site for cancers originating in other organs (Hiratsuka et al., 2002). Metastasis to the lung is especially prevalent in childhood lung cancer, which is 5:1 more metastatic than primary (Dishop & Kuruvilla, 2008).
Therefore inflammation qualifies as a lung cancer promoter, based not only on epidemiological data, but also based on in vitro and in vivo study models. Specifically in cancer, the feedback coordination between mechanisms that promote inflammation and the checkpoints that limit tissue damage from inflammation is defective (Vlahopoulos et al., 2015). This dysregulation of inflammatory mediators affects a number of cell types. In lung cancer, aberrant induction of paracrine circuits that link cancer cells to their proximal untransformed cells, including stem cells, mesenchymal cells, and cells of the myeloid/monocyte/macrophage lineage, causes continuous high expression of abnormal clusters of cytokine combinations (Guo et al., 2014; Lu et al., 2016). Sera from lung cancer patients can contain high concentrations of both inflammatory and anti-inflammatory cytokines. This abnormal combination impedes the function of dendritic cells that are essential in the immune response against the tumor, thus generating an immunosuppressive microenvironment (Li, Fang, et al., 2017). High concentrations of abnormal cytokine clusters are a type of variable that can be measured clinically when the effect is systemic.
In fact, combined differences in the abundance of specific inflammatory cytokines and other types of signaling mediators can distinguish between benign healthy controls, benign pulmonary disease and early stages of lung cancer with variable success. Data resulting from relevant studies range from no association to a statistically significant link to cancer and its prognosis (Liu et al., 2016; Pan, Zhou, et al., 2016). The primary reason for this variability in the success of inflammatory biomarkers in lung cancer is the fact that, while inflammation requires a complex interaction between organs and the immune system, cancer develops abnormal paracrine interference between different cell types, where diverse cytokines can have redundant effects on cancer cells.
In particular, in K-Ras-driven lung cancer, there is mounting evidence of abnormal feedback loops between signaling pathways of transcription factors STAT3 and NF-κB (Kitajima et al., 2016). This is very important, as KRAS mutations although common for many years were considered undruggable, but renewed hope has been given by analysis of the signaling mechanism (Kilgoz, Bender, Scandura, Viale, & Taneri, 2016). Aberrant feedback between NF-κB and STAT3 is a key factor that transforms a tissue niche into a microenvironment which mobilizes stromal cells to foster cancer cells (Vlahopoulos et al., 2015). RelA Ser 276 phosphorylation drives expression of both the STAT1 and STAT3 genes (Martincuks et al., 2017). Their protein products, STAT1 and STAT3 are highly activated by tyrosine phosphorylation during severe COPD (Yew-Booth et al., 2015). Independently from activating KRAS mutations, in cancer cells STAT1 and STAT3 have the capacity to increase expression of antiapoptotic genes (Radhakrishnan et al., 2017). Antiapoptotic genes have an important function, as they protect cancer cells from K-Ras-induced cell death (Tran et al., 2011). Furthermore, STAT1 and STAT3 drive expression of checkpoint molecules that block T-cell antitumor response, such as programmed death ligand 1 (PD-L1) (Chen, Jiang, Jin, & Zhang, 2016; Concha-Benavente et al., 2016; Fujita et al., 2015; Wang et al., 2017; Xiong, Ma, Wu, Lin, & Tu, 2014). In NSCLC, mutant EGFR activates NF-κB to induce expression of PD-L1, to elicit cancer resistance to checkpoint inhibitor drug treatment (Lin, Cheng, Yang, Li, & Zhu, 2015; Remon & Besse, 2016). As a result, lung cancers with KRAS or EGFR mutations can express high levels of PD-L1 (Sumimoto, Takano, Teramoto, & Daigo, 2016). PD-L1 expression is one of the most frequent immune system checkpoint aberrations in cancer, and constitutes a major block in T-cell mediated antitumor immunity. Even chimeric antigen receptor-directed T-cell (CART) activity is improved by blocking the PD-1 response of the T-cells (Liu et al., 2016; Nuovo, Folcik, & Magro, 2017).
On the other hand, the lung cancer cell microenvironment produces factors that protect the tumor from the immune system. Cancer cells recruit, through their secretions, macrophages that are later polarized into an M2-like phenotype (Li et al., 2016; Spaks, 2017). In the lung, M2-polarized macrophages induce proliferation and increase autophagy in blood vessel endothelial cells, causing enhanced vascular permeability and abnormal angiogenesis (Li et al., 2016). The M2 phenotype also produces mediators that promote immunosuppression, metastasis and angiogenesis, such as transforming growth factor beta (TGFβ) vascular endothelial growth factor (VEGF) and prostaglandin E2, which support lung carcinogenesis (Nguyen, Berim, & Agrawal, 2014). ROS produced by cancer cells and tumor-infiltrating leukocytes, including myeloid-derived suppressor cells, tumor-associated macrophages, and Tregs, suppress the immune responses (Chen, Song, Zhang, & Zhang, 2016). In lung cancer, even inflammatory macrophages of the M1 phenotype show decreased antitumor capacity, which can be explained by impaired interactions with other cell types, especially T-cells (Schoenhals et al., 2017; Vlahopoulos, 2017). While low levels of ROS activate T cells and anti-CD3 induced phosphorylation of extracellular signal-regulated ERK pathway, high ROS levels reduce T cell immune responses by inhibiting recognition between T cell receptor (TCR) and the MHC-peptide complex (Devadas, Zaritskaya, Rhee, Oberley, & Williams, 2002; Liu, Wei, Guo, & Zhou, 2015).
Inside the cell, ROS increase formation of reversible disulfide bonds between cysteine residues, a mechanism that inactivates important protein phosphatases (Bonham & Vacratsis, 2009). These phosphatases inactivate mitogen-activated protein (MAP) kinases that have essential roles in cell proliferation and expression of cytokine genes. As an example protein phosphatase MKP-1 dephosphorylates and inactivates stress-activated protein kinases p38 and JNK (Moosavi, Prabhala, & Ammit, 2017). Therefore, ROS normally enable the transient, reversible activation of MAP kinases, which allows the cell to return to kinase basal activity within a given time frame after stimulation (Irani, Herzlinger, & Finkel, 1994; Sundaresan, Yu, Ferrans, Irani,& Finkel, 1995). Notably, excessively high activity of p38 has been noted in asthma and COPD (Barnes, 2016). In vitro, mutant, constitutively active K-Ras protein has the capacity to activate p38 and ROS persistently, and cause cell transformation (Floyd et al., 2005; Park et al., 2014; Simanshu, Nissley, & McCormick, 2017). Evidently in cancer the response of cells to inflammatory stimuli and ROS is not identical to normal cells. Therefore, the difference among asthma, COPD and lung cancer can most likely be traced in the altered regulation of gene expression.
To bring all of the above in perspective, it can be summarized that in cancer, the extent and duration of cytokine expression determines the impact of both innate and adaptive immunity on tissue function and tumor persistence (Manning & Nemunaitis, 2017; Vlahopoulos et al.,2015 . The impact of inflammation is more pervasive in lung cancer due to the physiological function of the cell lineages that are involved in carcinogenesis. A pivotal difference between cancer-infiltrated tissue and the rest of the host, is that cancer cells express abnormal combinations of cytokines and their cognate receptors, leading to disruption of tissue structure and function (Akbay et al., 2017; Liu et al., 2016; Vlahopoulos, 2017; Vlahopoulos et al., 2015).
4. OGG1 mediates key ROS effects in lung inflammation: critical impact on NF-κB
Increased generation of ROS leads to oxidant stress, causing several types of modification on the DNA. Most notable is the oxidation of guanine base into the modified base 7,8-dihydro-8-oxoguanine (8-oxoG) due to guanine’s lowest redox potential among the four nucleobases (Dizdaroglu, 1985; Steenken & Jovanovic, 1997). Genomic 8-oxoG is primarily recognized by OGG1, which proceeds with two types of function. The first function acts on the regulatory regions of expressed genes, and facilitates transcription factor binding (Ba et al., 2014; Pan et al., 2017). The second OGG1 function initiates the DNA base excision repair pathway (BER), via its glycosylase and apurinic (AP) lyase activity, which generates a single-strand nick 3′ to the AP site via a beta elimination reaction mechanism (Mitra et al., 2002). Research has shown that AP-sites are processed by apurinic/apyrimidinic endonuclease 1 (APE1) to form polymerase-ready 3’OH residues. Gap filling can involve 1-nucleotide incorporation by DNA polymerase β (Polβ) in the short-patch repair sub-pathway or the displacement synthesis of 2–8 nucleotides by either Polβ or replicative DNA polymerase δ in the long-patch repair sub-pathway (Dizdaroglu, 2005; Fleming, Ding, & Burrows, 2017; Hazra, Hill, Izumi, & Mitra, 2001; Whitaker, Schaich, Smith, Flynn, & Freudenthal, 2017). (Fig. 1)
Fig. 1.
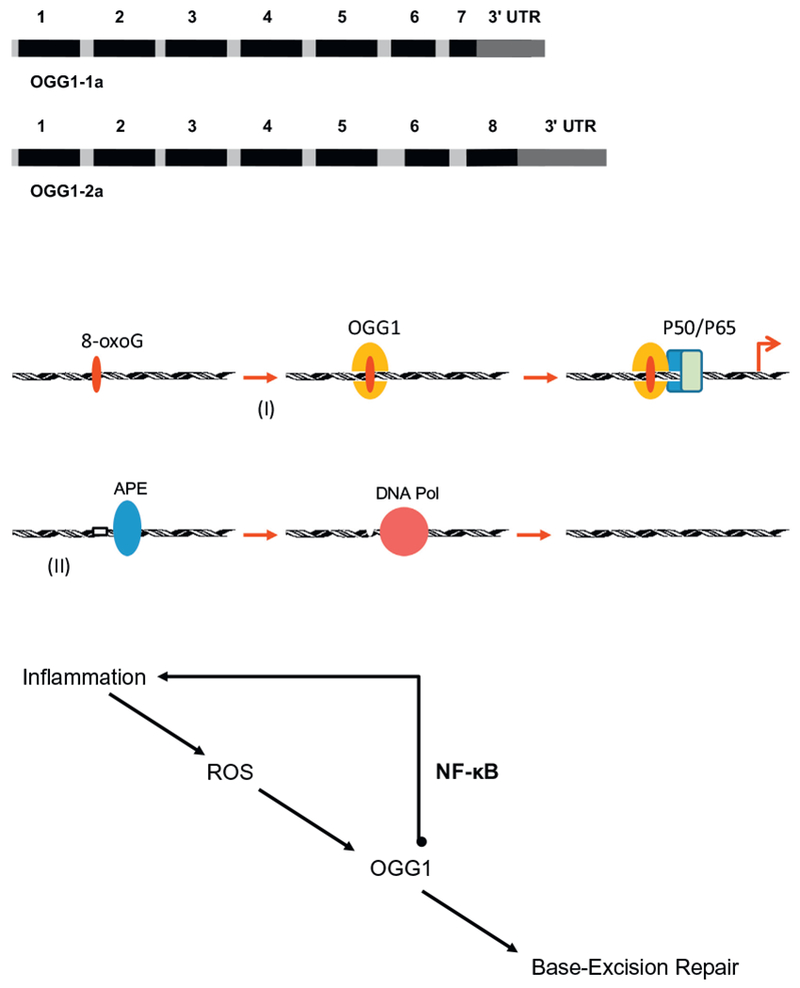
(a) Two functional isoforms of OGG1. The predominant form of OGG1 is 1a, which localizes both to the nucleus and mitochondria. The OGG1 isoform 2a mainly localizes to the mitochondria. (b) Two distinct phases of OGG1 activity on inflammatory gene promoters with the 8-oxoG lesion: I) DNA-binding and recruitment of transacting factors including NF-κB. Not shown are the DNA structural changes that OGG1 causes and the proteins that participate in the induction of transcriptional activity. II) Catalytic activity of OGG1 leaves an abasic site, which is processed by apurinic/apyrimidinic endonuclease1 (APE1). The single nucleotide gap is filled by DNA polymerase β and the DNA strand is sealed by ligases. Several essential interacting proteins are not shown in this depiction. (c) Impact of the interaction between NF-κB and OGG1 on inflammation. By recruiting NF-κB, OGG1 reprograms nuclear chromatin for inflammatory gene expression.
What is important in respect to the physiological function of OGG1, is that cells lacking CUX1 homeodomain protein 1 (CUX1), which stimulates the glycosylase and AP lyase activities of OGG1, can proliferate normally in the presence of 3% oxygen, while they senesce when placed in 20% oxygen (Ramdzan et al., 2015). Hence the OGG1-initiated DNA BER is essential for tissues that are exposed to high concentrations of oxygen. This makes OGG1 uniquely relevant to the lung, which is one of the few organs exposed to atmospheric oxygen tension. It is important to note that during mammalian development the lung gradually adapts from normoxia to atmospheric oxygen tension (Gao et al., 2016). In the neonatal lung, endothelial cell survival depends on basal, constitutive activity of NF-κB. In contrast, excessive NF-κB activity in fet al lung macrophage cells disrupts airway morphogenesis (Blackwell et al., 2011). The optimal physiological development and function of the lungs therefore depends on the control of NF-κB activity and downstream intracellular inflammatory signal pathways (Shahzad, Radajewski, Chao, Bellusci, & Ehrhardt, 2016).
Expression of the Ogg1 gene gives rise to several isoforms. Alternative splicing of the C-terminal region of this gene classifies splice variants into two major groups, type 1 and type 2, depending on the last exon of the sequence (Ogawa, Watanabe, Shoji, & Furihata, 2015). Type 1 splice variants end with exon 7, and type 2 variants end with exon 8. All variants share the N-terminal region, which contains an amino acid sequence acting as a mitochondrial targeting signal that is essential for mitochondrial localization (Nishioka et al., 1999). The predominant form of OGG1 is 1a, which also localizes to the nucleus through an amino acid sequence acting as a nuclear localization signal at its C terminus. An OGG1 isoform that is mainly encountered in mitochondria is 2a (Nishioka et al., 1999) (Fig. 1). Pathological changes in the lungs during asthma, COPD, and ozone-induced lung injury are associated with oxidant stress, evident by increased generation of 8-oxoG in the genome, which is recognized and then repaired by OGG1–1 both in nuclear and mitochondrial compartments (Bohr, 2002; Yang et al., 2014; Zhang et al., 2017; Ba, Aguilera-Aguirre, Sur, & Boldogh, 2015; Campalans, Amouroux, Bravard, Epe, & Radicella, 2007; Ogawa et al., 2015; Lee et al., 2017).
Oxidant stress elicited by TNFα in cells causes transient and reversible oxidation at cysteines of OGG1, which inhibits base excision of 8-oxoG. Although OGG1 is transiently stalled at 8-oxoG it retains the ability to interact with the opposite cytosine and extrude 8-oxoG from the DNA helix (Bravard et al., 2006; Morreall et al., 2015; Pan, Zhu, et al., 2016). This interaction of OGG1 results in a sharp (~70°) bending of the DNA duplex and architectural changes in adjacent sequences (Banerjee, Yang, Karplus, & Verdine, 2005; Bruner, Norman, & Verdine, 2000). Such conformational changes in the DNA helix have been shown to facilitate protein binding in chromatin (Ghosh & Karin, 2002; Ghosh, Wang, Huang, & Fusco, 2012; Moore, Kruchten, Toomire, & Strauss, 2016). Characteristically, upon oxidative stress or during inflammation, increased levels of ROS inhibit OGG1 enzymatic activity transiently, and transform OGG1 into a potent chromatin recruiter of transcription factors, such as NF-κB (Choudhary, Boldogh, & Brasier, 2016; Pan et al., 2017; Pan, Zhu, et al., 2016). Conversely, in mouse lung, OGG1 depletion decreased ROS-induced gene expression substantially (Ba, Bacsi, et al., 2014). This suggests that OGG1 functions initially as a ROS-induced facilitator of transcription factor binding, and upon reestablished cellular redox state, gradually advances to facilitate the DNA repair process (Ba & Boldogh, 2018).
Therefore, paradoxically a DNA repair enzyme functions also as an inducer of inflammation. This apparent contradiction can be explained by the need of tissues, especially the lung, to operate with an adjustable sensor that induces inflammation once ROS exceed a critical threshold, before the process of DNA repair resumes. This way, cells that have accumulated DNA damage will induce an immune response to limit cancer development. Genomic 8-oxoG-bound OGG1 facilitates recruitment not only of NF-κB, but also other transacting factors including specificity protein 1, transcription initiation factor II-D, and RNA polymerase II on their target gene promoters, resulting in the rapid expression of cytokines/chemokines and inflammatory cell accumulation into mammalian airways (Ba, Bacsi, et al., 2014). In particular relevance to the inflammatory response, OGG1 at 8-oxoG facilitates the expression of cytokines that include CCL20, IL-1β, TNFα, CXCL1, and CXCL2 (Ba, Bacsi, et al., 2014; Pan, Zhu, et al., 2016). CXCL1 and CXCL2 are two of the chemokines expressed in mice that could provide chemotactic activity similar to the human 1L-8 (Haurogne, Pavlovic, Rogniaux, Bach, & Lieubeau, 2015). Cells involved in inflammation express varying proportions of chemokine receptors, which cause distinct patterns of cell migration toward chemokine gradients. For example 1L-8 receptors CXCR1 and CXCR2 mediate successive waves of chemotaxis of neutrophils, cytotoxic T-cells, and regulatory T-cells, during the acute phases, and the resolution of inflammation (Ha, Debnath, & Neamati, 2017; Hosoki, Itazawa, Boldogh, & Sur, 2016). In human tumor xenografts in mice, CXCL1 activates signaling pathways of STAT3, NF-κB and H1F-1, inhibits apoptosis, and enables tumor growth (Han et al., 2015). CXCL1 also recruits neutrophils that inhibit proliferation of CD3 + CD4+ and CD3 + CD8+ T cells (Yuan et al., 2016).
On the genomic chromatin, TNFα-induced ROS cause enrichment of OGG1 primarily at regulatory regions of specific genes. The time course of OGG1 enrichment is limited to the scale required for immediate cellular responses to ROS. Depletion of OGG1 modulated NF-κB binding and differential gene expression, especially affecting several key inflammatory mediators (Hao et al., 2018). OGG1 therefore functions as a link between the intracellular rise of ROS and the expression of inflammatory cytokines, including chemokines.
4.1. Interdependence between OGG1 and ROS-induced activity of the RAS GTPases
OGG1 binds the free 8-oxoG base with a high affinity, forming an OGG1•8-oxoG complex. Remarkably, this complex interacts with the RAS family GTPases, and catalyzes replacement of GDP with GTP. OGG1•8-oxoG, thus functions as a guanine nucleotide exchange factor (GEF) (Boldogh et al., 2012). OGG1GEF activates K-Ras, neuroblastoma RAS viral oncogene homolog (N)-Ras, and Harvey (H)-Ras. In turn, Ras-GTP activates mitogen-activated protein kinase (MAPK) kinase (MEK1/2), extracellular signal-regulated kinase (ERK1/2), and phosphatidylinositol-4,5-bisphosphate 3-kinases (PI3K) (Aguilera-Aguirre et al., 2014; Boldogh et al., 2012; Choudhary et al., 2016; German et al., 2013). This cascade of events leads to phosphorylation and nuclear translocation of transcription factors such as NF-κB, a key regulator of inflammation (Brasier, 2006). Accordingly, an increase in free 8-oxoG levels in airways may cause rapid K-Ras GDP → GTP exchange, leading to pro-inflammatory gene expression, and recruitment of inflammatory cells (Aguilera-Aguirre et al., 2014; Choudhary et al., 2016). ROS also induce formation of 8-oxoG in the redox-sensitive guanine quadruplexes in the KRAS promoter itself, and the ensuing recruitment of OGG1, which provides an essential amplification of KRAS gene expression (Cogoi, Ferino, Miglietta, Pedersen, & Xodo, 2017; Fleming et al., 2017).
On the other hand, activated K-Ras can also render cells dependent on OGG1 DNA repair activity. In the case of neoplasia OGG1 influences RAS-dependent cell transformation on multiple levels. In fact, RAS transformation requires OGG1 and CUX1-dependent repair of oxidative DNA damage to prevent cell senescence (Ramdzan et al., 2014). Additionally, the OGG1•8-oxoG complex activates the NF-κB arm of the innate immune response (IIR). IIR of airway epithelial cells is induced after contact with environmental pollutants, or viruses, or bacterial pathogens. These agents interact with the pattern recognition receptors (PRRs) located on cell membranes and endosomal cellular compartments to trigger a cascade of events needed for activation and nuclear import of transcriptional activators (Choudhary et al., 2016). NF-κB and its intracellular activator TNF receptor-associated factor 6 (TRAF6) are well-characterized mediators of RAS-induced carcinogenesis (Finco et al., 1997; Starczynowski et al., 2011). Moreover, the NF-κB subunit p65 RelA is essential to suppress antitumor immunity in the mouse during Ras-induced oncogenic transformation, by inhibiting surveillance of both innate and adaptive immune cells (Wang, Ratnam, et al., 2014).
In respect to the IIR, the signal pathways activated via OGG1•8-oxoG complex are accompanied by increased expression of inflammatory chemokines and cytokines, as well as the induction of an IIR in the airways. Downstream of OGG1•8-oxoG, K-Ras-GTP activates PI3K, MEK1,2, ERK1,2, p38, mitogen-stress related kinase-1 (MSK-1) and the IκB kinase (IKK) enzyme complex (Aguilera-Aguirre et al., 2014; Floyd et al., 2005). IKK activates the nuclear translocation of RelA and thereby the NF-κB pathway, a central mediator of airway mucosal inflammation, while MSK-1 phosphorylates RelA at Ser 276 and enables expression of inflammatory genes (Aguilera-Aguirre et al., 2014). All of the above events converge on the full activation of NF-κB-dependent inflammation via transcriptional activation of immediate early pro- inflammatory gene subnetworks (Fig. 2). However, these events also activate mechanisms that form the basis of a cancer niche during lung tumorigenesis (Brichkina et al., 2016).
Fig. 2.
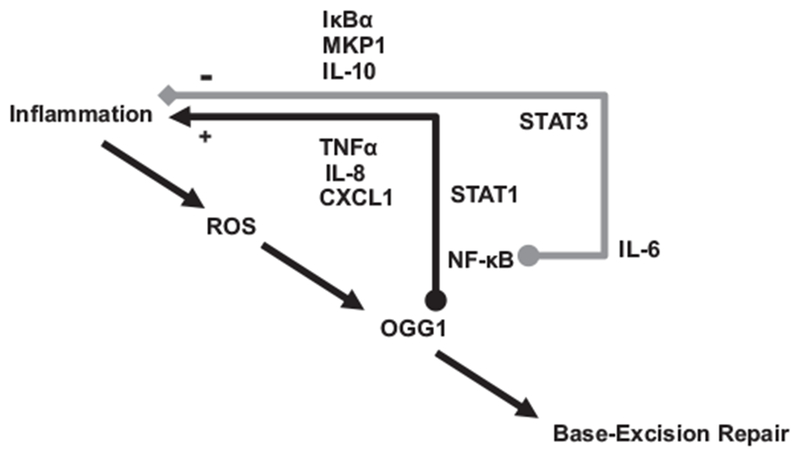
Roles of OGG1-regulated genes in NSCLC pathology, and dynamics of ROS impact on OGG1 and inflammation. Expression of immediate early genes increases during the acute phase, to become repressed during resolution of inflammation, due to negative feedback from anti-inflammatory genes that give products which gradually accumulate. Genes such as IL-10 ultimately contribute to termination of inflammation. In malignant cells feedback to NF-κB-mediated signaling is defective, disrupting tissue homeostasis, and permitting cancer progression.
4.2. ROS and OGG1 modulate NF-κB - driven gene expression and lung tissue physiology
Epidemiological studies and basic research show that a balance between OGG1 DNA repair activity and the OGG1-mediated enhancement of inflammatory gene expression is essential for the protection and recovery of the mammalian host from inflammation and neoplastic disease (Janik et al., 2011; Peng et al., 2014). A specific range of ROS is critical in tissue physiology and immunity, and therefore it is necessary to define the exact mediator of ROS-induced chemokine expression in a given tissue (Battisti et al., 2017; Chougnet et al., 2015). ROS-triggered antigen presentation by dendritic cells is essential in T-cell driven immunity (Peng et al., 2012). ROS induction of the NF-κB pathway is linked to the DNA damage response, via both the enzymes “ataxia telangiectasia mutated (ATM)” and OGG1. ATM mediates TNF-induced activation of PKAc, via an ATM/PKAc interaction in the cytoplasm, to induce phosphorylation on RelA at Ser276 (Fang et al., 2014). And OGG1, as already mentioned, mediates both the effects of RAS-induced signal cascades, as well as the recruitment of transcription factors.
Consequently, whole transcriptome analysis reveals an OGG1-driven gene expression linked to essential biological processes that include macrophage activation and airway remodeling. OGG1-driven transcripts include mRNAs of chemokines, cytokines, gonadotropin-releasing hormone receptor, integrin, and interleukin signaling pathways. Upon chronic stimulation, the resulting gene expression led to modulation of the actin family cytoskeleton, extracellular matrix, cell adhesion, cadherin, and cell junctions, regulation of liquid-surface tension, affecting biological processes such as tissue development, cell-to-cell adhesion, cell communication, homeostatic response, and the immune system (Aguilera-Aguirre et al., 2015a; Aguilera-Aguirre et al., 2015b; Luo et al., 2014).
The role of OGG1 in the activation and DNA occupancy of NF-κB explains the following intriguing finding that is relevant to the control of lung immunity: Ogg1-knockout mice exhibited decreased inflammatory cell infiltration and oxidative stress in the lungs after ovalbumin challenge, when compared to wild-type mice. At the molecular level, the absence of OGG1 caused decreased NF-κB phosphorylation after ovalbumin challenge (Li et al., 2012). Consequently in comparison to wild-type controls, Ogg1 knockout mice were resistant to lipopolysaccharide (LPS)-induced organ dysfunction, diabetes, and contact hypersensitivity reactions as well as oxidative stress (Mabley et al., 2005). At the level of tissue homeostasis, OGG1 regulates expression of genes that mediate cellular movement and interactions during the immune response. NF-κB activation and its DNA recruitment by OGG1 is a key point during inflammatory signaling that affects function of both the cell and its microenvironment. On the other hand OGG1 protects against several types of lung injury that correlate with genomic 8-oxoG abundance (Deslee et al., 2010; Yang et al., 2014; Zhang, Li, et al., 2017).
After allergen challenge in the mouse alveolar epithelium, OGG1 mediates inflammation with expression of T helper 2 (Th2) cytokines, including interleukins (ILs) IL-4, IL-5, IL-13, IL-6, IL-10, and IL-17. These cytokines facilitate the accumulation of eosinophils, airway metaplasia and mucus production (Bacsi et al., 2013; Li et al., 2012). NF-κB activity in epithelial cells is already known to elicit activation of lung resident and recruitment of monocyte-derived macrophages, cells that can persist in a tissue over the life span (Cai, Batra, Lira, Kolls, & Jeyaseelan, 2010; Misharin et al., 2017; Saxon et al., 2016). This suggests that NF-κB activity renders lung tissue particularly sensitive to factors that control epithelium and immune cell phenotypes (Choudhary et al., 2016). Activation of Ras via OGG1•8-oxoG complex activates expression of numerous cytokines and chemokines, including TNFα, IL-1a, IL-1b, IL-10, CCL3, CXCL1, CXCL5, and transiently CCL2, IL-3, IL-4, IL-13, and several others, and causes recruitment of neutrophils at a magnitude similar to TNF itself (Aguilera-Aguirre et al., 2014; Ba et al., 2014). A similar observation was made with ovalbumin challenge that elicits allergic response in the lungs. Knockout of the Ogg1 gene decreases inflammatory cell infiltration, RelA phosphorylation at Ser276, and expression of IL-4, IL-6, IL-10, and IL-17 in lung tissues (Li et al., 2012).
To map the binding sites of the TNF-induced OGG1 synergy with RelA on chromatin in epithelial cells, we treated an epithelial cell line with TNFα and precipitated chromatin with antibodies against OGG1 and RelA (Fig. 3). Analysis of genes with the highest enrichment for both OGG1 and NF-κB binding by gene ontology indicated that the IIR process manifests in the generation and secretion of soluble mediators. The most significantly modulated processes involve the positive regulation of cytokine production (P = 1.81E-10), including TNFα (P = 3.18E-06), IL-6 (P = 3.62E-07), C-X-C motif chemokine ligand 8 (IL-8) (P = 1.39E-07), interleukin-1 (P = 3.22E-06), the heterodimeric cytokine interleukin 23 (IL-23, 3.34E-04) and IL-17 (P = 1.4E-04). Therefore, it is evident that not only the release of NF-κB from inhibitory complexes in the cytoplasm and its nuclear translocation but also access of NF-κB to specific classes of binding motifs in the chromatin are also actively controlled (Ghosh et al., 2012; Pan, Zhu, et al., 2016). These DNA repair-associated changes within the chromatin are considered epigenetic regulatory and are utilized by cellular transcriptional machineries as a mechanism of gene expression enhancement under inflammation-generated oxidative stress. Thus, it may be concluded that oxidative modification of guanine to 8-oxoG has an epigenetic role in promoters that is driven by oxidative stress.
Fig. 3.
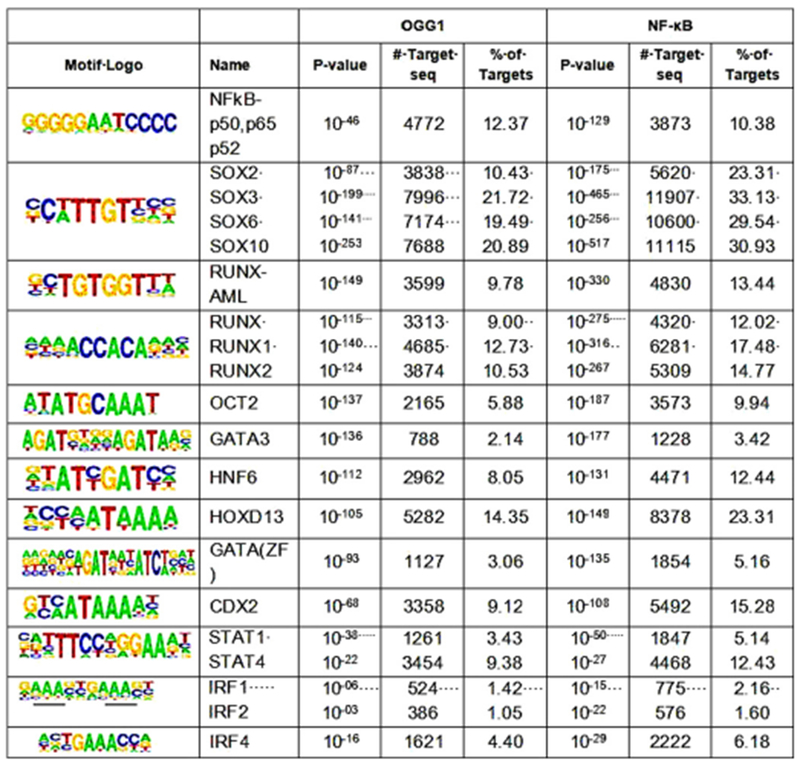
The OGG1 and NF-κB proteins bind to characterized DNA recognition sites of a number of transcription factors, on the chromatin. Cells from the human embryonic kidney cell line HEK293 were exposed to 20 ng/ml TNF for 30 min. Chromatin was precipitated with antibodies either against OGG1, or against the NF-κB subunit p65 (RelA), and the bound DNA was amplified, and sequenced. DNA binding motifs of transcription factors were identified as previously described (Pan et al., 2016).
However, inflammation can also provide feedback regulation on Ogg1 itself and affect tissue function. Impaired regulation of the Ogg1 gene, as well as repair activity of OGG1 are associated with pathology in the lung and other tissues. In NSCLC, promoter methylation of Ogg1 can decrease expression, depending on the position of the DNA sequence that is methylated. In particular, increased promoter methylation of Ogg1 in NSCLC is likely due to hyperactivity of the NF-κB target gene DNA-methyltransferase 1 (DNMT1) in bronchial epithelial cells (Qin et al., 2017; Zhou, Lei, & Wang, 2012). DNMT1 activity can decrease Nfkbia, (IκBα) expression, and thereby enhance expression of antiapoptotic and inflammatory genes (O’Gorman, Colleran, Ryan, Mann, & Egan, 2010). As opposed to the activation of inflammatory genes, RelA phosphorylation at Ser276 induces RelA-DNMT1 interactions, chromatin loading of DNMT1, and subsequent promoter methylation and transcriptional repression of the tumor suppressor gene “breast cancer metastasis suppressor 1” in NSCLC cells (Liu et al., 2012). This links RelA activation, and promoter methylation to cancer. In breast cancer, higher methylation of the Ogg1 gene promoter was significantly associated to lower gene expression (Fleischer et al., 2014). The same inverse correlation between methylation and Ogg1 expression was noted in mouse brain tissue during aging, to explain decreased BER activity (Langie et al., 2017). Increased DNMT1 activity in a tissue causes cellular senescence, which can be prevented by OGG1, thus constituting irrefutable evidence of the need for sufficient OGG1 expression and activity for optimum tissue function (Grasso et al., 2015; Ramdzan et al., 2014). Consequently, increased all-cancer incidence was found in study participants with higher baseline Ogg1 genetic locus methylation in a single promoter CpG site, which suggests an essential role of OGG1 in tissue homeostasis (Gao et al., 2016).
5. Innate immunity and OGG1 influence lung carcinogenesis and prognosis
Lung cancer is one of the main causes of death from malignancy in the world (Formisano, Jansen, Marciano, & Bianco, 2017; Singh, Williams, Siahpush, & Mulhollen, 2011; Syriopoulou, Bower, Andersson, Lambert, & Rutherford, 2017). The majority of lung cancer cases belong to the NSCLC type (Chan & Hughes, 2015; Schallenberg, Merkelbach-Bruse, & Buettner, 2017). Systemic inflammation is more pronounced in patients with lung cancer than in patients with other cancers (Wieshammer & Dreyhaupt, 2017). Lung cancer lobes show increased TNFα and decreased antitumor capacity in macrophages from bronchoalveolar lavage (Schoenhals et al., 2017). Inflammatory mediators have an established prognostic value in lung cancer. For example, increased concentration of circulating interleukin-6 (IL-6) and C-reactive protein (CRP) were significantly associated with poor prognosis especially in patients with unresectable NSCLC (Liao et al., 2014). In resected NSCLC cases, high preoperative neutrophil-to- lymphocyte ratio and concentration of CRP predict poor survival (Tomita, Shimizu, Ayabe, Nakamura, & Onitsuka, 2012). Furthermore, baseline neutrophil-lymphocyte ratio is a significant prognostic indicator in locally advanced NSCLC patients who receive chemoradiation with or without surgery (Scilla et al., 2017). Statistical evidence shows that in the lung, pathologies caused by a severe imbalance in ROS turnover such as COPD are associated with an increased risk for cancer (Durham & Adcock, 2015; Spyratos, Papadaki, Lampaki, & Kontakiotis, 2017).
Epidemiologically, cytokines IL-6 and IL-8, which are encoded by OGG1-responsive genes, are associated with the risk for developing lung cancer, both in blood samples taken close to diagnosis, as well as in those taken >15 years post-diagnosis (Brenner et al., 2017). In lung cancer, KRAS mutant cell subclones are frequent and have the potential to cause resistance to EGFR inhibitors (Myers, McKim, Meng, & Parsons, 2015). Consequently, in a KRAS mutant mouse model COPD-like airway inflammation elicits NF-κB-induced cytokines and chemokines, and thereby promotes lung cancer (Caetano et al., 2016). In particular IL-8 is a chemokine that is encoded by a characteristic gene expressed through TNF-induced, ROS-dependent NF-κB transcriptional activity (Vlahopoulos et al., 1999). In lung cancer patients treated with anti-PD-1 antibodies nivolumab or pembrolizumab, increased IL-8 predicts lack of response (Sanmamed et al., 2017).
High systemic IL-6 is associated with worse prognosis in patients with NSCLC (Silva et al., 2017). Excess of IL-6 inhibits differentiation of dendritic cells that are essential in antigen presentation (Yu et al., 2007). Moreover, IL-6 generates a microenvironment with increased regulatory T (Treg) cells and immunosuppressive neutrophils that secrete IL-1, and do not respond to PD-1 inhibiting antibodies (Kitajima et al., 2016; Koyama et al., 2016). This leads to the hypothesis that signaling from IL-6 and the STAT3 activators, Janus kinases (JAK), is involved in immunosuppression. Actually JAK/STAT3 readily induce PD-L1 expression in cultured NSCLC cells (Zhang et al., 2016; Zhang et al., 2017). Preclinical research suggests that combination of inhibitors that target MEK/ERK kinases and the JAK/STAT3/IL-6/PD-L1 pathway can be more effective than either approach alone in killing K-Ras-driven cancer cells (Kitajima et al., 2016).
Decreased OGG1 BER activity is a significant variable in the epidemiological characterization of lung cancer (Leitner-Dagan et al., 2012; Sevilya et al., 2014). In particular, OGG1 enzyme activity in peripheral blood mononuclear cells from lung cancer patients has been found to be significantly lower than in control subjects (Leitner-Dagan et al., 2012). Analysis of DNA repair activities in peripheral blood mononuclear cells suggests that the integrated score of three enzyme activities that include OGG1, methylpurine DNA glycosylase (MPG), and APE1, is an important protective factor against lung cancer (Sevilya et al., 2014). In advanced inoperable NSCLC patients who received treatment with platinum-based chemotherapy, Ogg1 genetic allele variant carrying a C-to-G substitution at codon 326 was associated with poor progression-free survival (Peng et al., 2014). This allelic variant gives rise to OGG1 with a cysteine in place of serine residue 326, which does not recover within an hour from oxidant stress after stimulation of cells with physiological concentration of TNFα (Morreall et al., 2015). Thus, the polymorphism giving rise to the protein product with the Ser326Cys substitution generates an inefficient enzyme (Kaur et al., 2014). Consequently, repair of oxidative DNA damage is delayed by several hours in cells that contain only the Ser326Cys variant of Ogg1 (Kershaw & Hodges, 2012). The DNA repair activity of OGG1 offers significant protection from lung cancer. This is evident both in peripheral blood mononuclear cells and lung tissue of human study volunteers in comparison to NSCLC patients, as well as by development of lung cancer in mice lacking a functional Ogg1 gene (Paz-Elizur et al., 2003; Sakumi et al., 2003).
Some studies have shown association between the OGG1 Ser326Cys polymorphism and lung cancer susceptibility, while other studies show no effect of this genetic variant (Geng, Yao, & Zhu, 2014; Xu, Yu, & Zhang, 2013). A potential explanation is that the wildtype enzyme and its variant differ substantially only under an excessive burden of oxidant stress, as is evident during conditions that involve chronic inflammation. Furthermore, the association of the Ser326Cys gene polymorphism and lung cancer becomes significant when including only studies that are consistent with the Hardy-Weinberg equilibrium principle, which could provide a further explanation for at least a portion of the divergent results (Kang, Kim, Park, Chung, & Ban, 2017). The odds for developing lung cancer with the Ser326Cys polymorphism increase in smokers, as suggested by a study in Taiwan, and a subsequent meta-analysis for Asian volunteers (Chang et al., 2009; Yin et al., 2014). The effect of smoking can be linked to ROS-induced effects on OGG1, and to ROS-associated lung pathology. The alveolar wall of COPD patients shows increased nucleic acid damage, including genomic 8-oxoG, which is modeled both in study using mice and in cultured human alveolar fibroblasts by exposure to cigarette smoke that induces ROS (Deslee et al., 2010). The imbalance between the Ser326Cys OGG1-enhanced inflammation and the delayed Ser326Cys OGG1 repair activity would be expected to impact lung pathology, especially in neoplastic tissue. Indeed, Ser326Cys Ogg1 was linked to shorter progression -free survival in inoperable NSCLC (Peng et al., 2014).
6. OGG1 as a criterion in the selection of intervention targets
All of the above suggest that ROS regulate cytokine gene expression through OGG1, and therefore defective OGG1 derails control of ROS and coordination between innate and adaptive immunity in the lung. Coordination is crucial because targeting inducers of oncogenic pathways such as RAS has not yet led to an effective treatment, due to aberrant expression of cytokines (S. A. Vlahopoulos et al., 2015). In the lung OGG1 mediates ROS-induced cytokine expression on the one hand (by recruiting RelA on DNA and inducing RelA S276 phosphorylation) and on the other hand DNA BER, helping restore tissue homeostasis (Aguilera-Aguirre et al., 2015a; Deslee et al., 2010; Lee et al., 2017).
RelA phosphorylated on S276 recruits BRD4 and expression of RelA/BRD4-responsive genes such as CXCL8 can be blocked by BRD4 inhibitors. For example, the abundance of the CXCL8 transcript decreases by BRD4 inhibition in airway inflammation and COPD (Khan, Kirkham, Barnes, & Adcock, 2014). Consequently, clinical trials for bromodomain and extra-terminal motif (BET) inhibitors include NSCLC (Andrieu, Belkina, & Denis, 2016) (Table 1). In vitro, BET inhibitors sensitize KRAS-mutated NSCLC to pro-apoptotic agents such as cisplatin or to tyrosine kinase inhibitor dasatinib (Klingbeil, Lesche, Gelato, Haendler, & Lejeune, 2016; Xu et al., 2015).
Table 1.
Clinical studies of BET inhibitors that include lung cancer patients, registered with the database of the United States National Library of Medicine at the National Institutes of Health.
| Registration Identifier | Recruitment Status as of 8/2018 | Aim | Responsible Party |
|---|---|---|---|
| 2,259,114 | Completed | A Dose-Finding Study of OTX105/MK-8628, a Small Molecule Inhibitor of the Bromodomain and Extra-Terminal (BET) Proteins, in Adults With Selected Advanced Solid Tumors (MK-8628-003) | Oncoethix GmbH |
| 3,266,159 | Withdrawn | A Dose Escalation Study to Investigate the Safety, Pharmacokinetics (PK), Pharmacodynamics (PD), and Clinical Activity of GSK525762 Plus Trametinib in Subjects With Solid Tumors | GlaxoSmithKline |
| 2,698,176 | Terminated | A Dose Exploration Study With MK-8628 in Participants With Selected Advanced Solid Tumors (MK-8628-006) | Merck Sharp & Dohme Corp. |
| 2,959,437 | Active, not recruiting | Azacitidine Combined With Pembrolizumab and Epacadostat in Subjects With Advanced Solid Tumors (ECHO-206) | Incyte Corporation |
| 2,391,480 | Recruiting | A Study Evaluating the Safety and Pharmacokinetics of ABBV-075 in Subjects With Cancer | AbbVie |
| 1,587,703 | Active, not recruiting | A Study to Investigate the Safety, Pharmacokinetics, Pharmacodynamics, and Clinical Activity of GSK525762 in Subjects With NUT Midline Carcinoma (NMC) and Other Cancers | GlaxoSmithKline |
| 2,431,260 | Completed | An Open-Label, Dose-Escalation Study of INCB054329 in Patients With Advanced Malignancies | Incyte Corporation |
| 2,711,137 | Active, not recruiting | Open-Label Safety and Tolerability Study of INCB057643 in Subjects With Advanced Malignancies | Incyte Corporation |
Tumors that grow independently of ROS and RelA-276-phosphorylation can be targeted by inhibition of proteins that interact with RelA directly. Such a downstream cofactor is poly(ADP-ribose) polymerase (PARP)-1. Prevention of PARP-1 activation and RelA-536-phosphorylation protects against both ROS-induced airway epithelial cell injury in vitro and airway inflammation in vivo (Beck, Robert, Reina-San-Martin, Schreiber, & Dantzer, 2014). OGG1, PARP-1 and RelA have the capacity to interact physically, share NF-κB gene targets as in the case of CXCL2 in asthma, and show complementary patterns of activity in cancer cells as well (Dziaman et al., 2014; Ghonim et al., 2015; Tempka et al., 2018; Wang et al., 2018). The cancer drug olaparib can induce cell death by suppressing PAR-associated NF-κB signaling (Kwon, Jang, Kim, & Roh, 2016).
In spite of the difference in enzymatic activities and interactions between OGG1 and PARP-1, it would be interesting to determine if during lung cancer, in phases with low OGG1 expression, PARP-1 acquires a stronger role as a RelA cofactor (Kim, Naura, Errami, Ju, & Boulares, 2011; Sethi, Dharwal, & Naura, 2017; Zerfaoui et al., 2008). In fact, Ogg1(−/−) cells are more sensitive to PARP inhibitors alone or in combination with a DNA-damaging agent (Noren Hooten, Kompaniez, Barnes, Lohani, & Evans, 2011). This observation can be encouraging in light of the discovery that in cells from several cancer types, inhibition of BET activity has been shown to sensitize homologous recombination-proficient cancers to PARP inhibition (Yang et al., 2017). This sensitization can be achieved with BRD4 inhibitors, which decrease expression of enzymes essential to homologous recombination. Thus PARP/RelA and OGG1/RelA/BRD4 are two complementary targets for cancer, as they induce alternative mechanisms of RelA-mediated inflammation.
In chromatin RelA can bind to cognate sites of other transcription factors through protein-protein interactions (Fig. 3). This enables a cell to induce expression of diverse genes and a multiplicity of biochemical programs in response to OGG1-driven RelA activation (Aguilera-Aguirre et al., 2015a). Moreover, acting through an alternative signaling pathway in the tumor microenvironment, myeloid cell RelA also promotes lung cancer cell proliferation through activation of Wingless-type MMTV integration site family protein (Wnt)/β-catenin signaling (D. Li et al., 2014). β-Catenin selectively represses subsets of NF-κB target genes involved in apoptosis, while increasing uncontrolled expression of other genes, such as IL-6 (Du & Geller, 2010; Koopmans, Eilers, Menzen, Halayko, & Gosens, 2017). However, endogenous agents exist that can overcome the cytokine-induced immunosuppression in the lung. One example is melatonin, which is decreased in advanced lung cancer, and shows promising oncosuppressive effects in lung cancer study models (Ma et al., 2016; Mazzoccoli, Carughi, De Cata, La Viola, & Vendemiale, 2005). In a normal tissue melatonin functions as an antioxidant and decreases the burden of 8-oxoG (Karbownik, Reiter, Cabrera, & Garcia, 2001). It is possible that in cancerous tissue melatonin generates cytotoxic metabolites (Patterson, Gonzalez, & Idle, 2010). Even though melatonin is an endogenous substance, when administered exogenously it inhibits several mechanisms of NSCLC progression, including both intracellular signal pathways, as well as the immunosuppressive microenvironment; a few clinical trials indicate that melatonin can be used in combination with other drugs in NSCLC (Ma et al., 2016). However, although melatonin decreases the 8-oxoG burden in NSCLC patients, it does not improve survival in combination with chemotherapy (Sookprasert et al., 2014). Therefore melatonin still needs effective tissue targeting and improved drug combinations, possibly with immunotherapeutic agents. Immunotherapeutic agents that directly inhibit checkpoints downstream of NF-κB include PD-L1 antibodies, and have shown the capacity to induce tumor responses across adenocarcinoma, squamous-cell carcinoma, and SCLC (Tan et al., 2016). This is clear evidence of the ability of the immune system to eradicate cancer cells. However, as innate immunity has a pivotal impact in the expression of checkpoint molecules, it is imperative that controlling the innate immune response will help streamline combinatorial schemes.
7. Conclusion
Lung cancer elicits detectable changes in key mediators of immunity. OGG1 is a mediator of the homeostatic response of a tissue exposed to oxidant stress, which regulates inflammation. Optimum tissue function requires a timely change in OGG1 activity from a chromatin recruitment factor for NF-κB to an enzyme that contributes to DNA repair. Cytokine-induced ROS temporarily transform OGG1 into an epigenetic regulator of inflammatory gene expression with substantial impact on the cytokines that control lung cancer. In contrast to any attempt for a systemic intervention on ROS signaling with antioxidants, the disruption of NF-κB recruitment by stalled OGG1 is selective for the inflammatory genes. This selective effect will enable a precise intervention on the immunopathology of lung cancer. Therefore activity of OGG1, and the level of its substrate 8-oxoG, constitute candidate markers for lung cancer progression.
Acknowledgements
This work was in part supported by Grants from the United States National Institute of Environmental Health Sciences (Grant number: RO1 ES018948 to IB); P30 ES006676 (IB); and United States National Institute of Allergy and Infectious Diseases (Grant number: AI062885, to IB). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- AP
apurinic/apyrimidinic
- BER
base excision repair
- BET
bromodomain and extraterminal domain
- BRD
bromodomain
- CCL
CC-motif ligand
- CDK
cyclin-dependent kinase
- CHD
chromodomain helicase DNA-binding protein
- COPD
chronic obstructive pulmonary disease
- CXCL
CXC-motif ligand
- CXCR
CXC chemokine receptor
- CUX
Cut homeobox
- DNMT
DNA (Cytosine-5-)-Methyltransferase
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal-regulated kinase
- IIR
Innate Immune Response
- IKK
IκB kinase
- IL
interleukin
- JAK
janus kinase
- KRAS
Kirsten-Ras
- MMP
matrix met alloprotease
- NIK
NF-κB-inducing kinase
- NF-κB
Nuclear Factor kappa B
- NSCLC
non-small cell lung cancer
- 8-oxoG
8-oxoguanine
- OGG1
8-oxoguanine glycosylase 1
- PARP
poly(ADP-ribose) polymerase
- PD-L
programmed death ligand
- RelA
v-rel avian reticuloendotheliosis viral oncogene homolog A
- RIG-I
retinoic acid-inducible gene I
- ROS
reactive oxygen species
- STAT
signal transducer and activator of transcription
- TGFβ
Transforming growth factor beta
- Th
T-helper cell type
- TNFα
tumor necrosis factor alpha
- Treg
regulatory T cell
Footnotes
Conflict of interest statement
The authors have no financial and personal relationships to disclose.
References
- Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, … Massagué J (2012). A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 150(1), 165–178. 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera-Aguirre L, Bacsi A, Radak Z, Hazra TK, Mitra S, Sur S, … Boldogh I. (2014). Innate inflammation induced by the 8-oxoguanine DNA glycosylase-1-KRAS-NF-κB pathway. Journal of Immunology (Baltimore, Md.: 1950) 193(9), 4643–4653. 10.4049/jimmunol.1401625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera-Aguirre L, Hosoki K, Bacsi A, Radák Z, Sur S, Hegde ML, … Boldogh I. (2015a). Whole transcriptome analysis reveals a role for OGG1-initiated DNA repair signaling in airway remodeling. Free Radical Biology & Medicine 89, 20–33. 10.1016/j.freeradbiomed.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera-Aguirre L, Hosoki K, Bacsi A, Radák Z, Wood TG, Widen SG, … Boldogh I (2015b). Whole transcriptome analysis reveals an 8-oxoguanine DNA glycosylase-1-driven DNA repair-dependent gene expression linked to essential biological processes. Free Radical Biology & Medicine 81, 107–118. 10.1016/j.freeradbiomed.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbay EA, Koyama S, Liu Y, Dries R, Bufe LE, Silkes M, … Wong K-K (2017). Interleukin-17A Promotes Lung Tumor Progression through Neutrophil Attraction to Tumor Sites and Mediating Resistance to PD-1 Blockade. Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer 12(8), 1268–1279. 10.1016/j.jtho.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alegre E, Fusco JP, Restituto P, Salas-Benito D, Rodríguez-Ruiz ME, Andueza MP, … Gonzalez A (2016). Total and mutated EGFR quantification in cell-free DNA from non-small cell lung cancer patients detects tumor heterogeneity and presents prognostic value. Tumour Biology 37(10), 13687–13694. 10.1007/s13277-016-5282-9. [DOI] [PubMed] [Google Scholar]
- Andrieu G, Belkina AC, & Denis GV (2016). Clinical trials for BET inhibitors run ahead of the science. Drug Discovery Today. Technologies 19, 45–50. 10.1016/j.ddtec.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba X, & Boldogh L (2018). 8-Oxoguanine DNA glycosylase 1: Beyond repair of the oxidatively modified base lesions. Redox Biology 14, 669–678. 10.1016/j.redox.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba X, Aguilera-Aguirre L, Rashid QTAN, Bacsi A, Radak Z, Sur S, … Boldogh I (2014). The role of 8-oxoguanine DNA glycosylase-1 in inflammation. International Journal of Molecular Sciences 15(9), 16975–16997. 10.3390/ijms150916975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba X, Aguilera-Aguirre L, Sur S, & Boldogh I (2015). 8-Oxoguanine DNA glycosylase-1-driven DNA base excision repair: Role in asthma pathogenesis. Current Opinion in Allergy and Clinical Immunology 15(1), 89–97. 10.1097/ACI.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba X, Bacsi A, Luo J, Aguilera-Aguirre L, Zeng X, Radak Z, & Boldogh I (2014). 8-oxoguanine DNA glycosylase-1 augments proinflammatory gene expression by facilitating the recruitment of site-specific transcription factors. Journal of Immunology (Baltimore, Md.: 1950) 192(5), 2384–2394. 10.4049/jimmunol.1302472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacsi A, Aguilera-Aguirre L, Szczesny B, Radak Z, Hazra TK, Sur S, … Boldogh I (2013). Down-regulation of 8-oxoguanine DNA glycosylase 1 expression in the airway epithelium ameliorates allergic lung inflammation. DNA Repair 12(1), 18–26. 10.1016/j.dnarep.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F, & Joffroy C(2010). TNF: A tumor-suppressing factor or a tumor-promoting factor? Future Oncology (London, England) 6(12), 1833–1836. 10.2217/fon.10.155. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Yang W, Karplus M, & Verdine GL (2005). Structure of a repair enzyme interrogating undamaged DNA elucidates recognition of damaged DNA. Nature 434 (7033), 612–618. 10.1038/nature03458. [DOI] [PubMed] [Google Scholar]
- Barnes PJ (2016). Kinases as Novel Therapeutic Targets in Asthma and Chronic Obstructive Pulmonary Disease. Pharmacological Reviews 68(3), 788–815. 10.1124/pr.116.012518. [DOI] [PubMed] [Google Scholar]
- Bassères DS, Ebbs A, Cogswell PC, & Baldwin AS (2014). IKK is a therapeutic target in KRAS-Induced lung cancer with disrupted p53 activity. Genes & Cancer 5(1-2), 41–55. 10.18632/genesandcancer.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti F , Napoletano C , Rahimi Koshkaki H, Belleudi F, Zizzari IG, Ruscito I, … Rughetti A (2017). Tumor-Derived Microvesicles Modulate Antigen Cross-Processing via Reactive Oxygen Species-Mediated Alkalinization of Phagosomal Compartment in Dendritic Cells. Frontiers in Immunology 8, 1179 10.3389/fimmu.2017.01179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C, Robert I, Reina-San-Martin B, Schreiber V, & Dantzer F (2014). Poly(ADP-ribose) polymerases in double-strand break repair: Focus on PARP1, PARP2 and PARP3. Experimental Cell Research 329(1), 18–25. 10.1016/j.yexcr.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Blackwell TS, Hipps AN, Yamamoto Y, Han W, Barham WJ, Ostrowski MC, … Prince LS (2011). NF-κB signaling in fet al lung macrophages disrupts airway morphogenesis. Journal of Immunology (Baltimore, Md.: 1950) 187(5), 2740–2747. 10.4049/jimmunol.1101495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr VA (2002). Repair of oxidative DNA damage in nuclear and mitochondrial DNA, and some changes with aging in mammalian cells. Free Radical Biology & Medicine 32(9), 804–812. [DOI] [PubMed] [Google Scholar]
- Boldogh I, Hajas G, Aguilera-Aguirre L, Hegde ML, Radak Z, Bacsi A, … Mitra S (2012). Activation of ras signaling pathway by 8-oxoguanine DNA glycosylase bound to its excision product, 8-oxoguanine. The Journal of Biological Chemistry 287 (25), 20769–20773. 10.1074/jbc.C112.364620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham CA, & Vacratsis PO (2009). Redox regulation of the human dual specificity phosphatase YVH1 through disulfide bond formation. The Journal of Biological Chemistry 284(34), 22853–22864. 10.1074/jbc.M109.038612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier AR (2006). The NF-kappaB regulatory network. Cardiovascular Toxicology 6(2), 111–130. [DOI] [PubMed] [Google Scholar]
- Brasier AR, Tian B, Jamaluddin M, Kalita MK, Garofalo RP, & Lu M (2011). RelA Ser276 phosphorylation-coupled Lys310 acetylation controls transcriptional elongation of inflammatory cytokines in respiratory syncytial virus infection. Journal of Virology 85(22), 11752–11769. 10.1128/JVI.05360-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravard A, Vacher M, Gouget B, Coutant A, de Boisferon FH, Marsin S, … Radicella JP (2006). Redox regulation of human OGG1 activity in response to cellular oxidative stress. Molecular and Cellular Biology 26(20), 7430–7436. 10.1128/MCB.00624-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner DR, Fanidi A, Grankvist K, Muller DC, Brennan P, Manjer J, … Johansson M (2017). Inflammatory Cytokines and Lung Cancer Risk in 3 prospective Studies. American Journal of Epidemiology 185(2), 86–95. 10.1093/aje/kww159. [DOI] [PubMed] [Google Scholar]
- Brichkina A, Bertero T, Loh HM, Nguyen NTM, Emelyanov A, Rigade S, … Bulavin DV (2016). p38MAPK builds a hyaluronan cancer niche to drive lung tumorigenesis. Genes & Development 30(23), 2623–2636. 10.1101/gad.290346.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner SD, Norman DP, & Verdine GL (2000). Structural basis for recognition and repair of the endogenous mutagen 8-oxoguanine in DNA. Nature 403(6772), 859–866. 10.1038/35002510. [DOI] [PubMed] [Google Scholar]
- Caetano MS, Zhang H, Cumpian AM, Gong L, Unver N, Ostrin EJ, … Moghaddam SJ (2016). IL6 Blockade Reprograms the Lung Tumor Microenvironment to Limit the Development and Progression of K-ras-Mutant Lung Cancer. Cancer Research 76(11), 3189–3199. 10.1158/0008-5472.CAN-15-2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Li R, Xu X, Zhang L, Wu S, Yang T, … Huang Y (2015). URGCP promotes non-small cell lung cancer invasiveness by activating the NF-κB-MMP-9 pathway. Oncotarget 6(34), 36489–36504. 10.18632/oncotarget.5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Batra S, Lira SA, Kolls JK, & Jeyaseelan S (2010). CXCL1 regulates pulmonary host defense to Klebsiella Infection via CXCL2, CXCL5, NF-kappaB, and MAPKs. Journal of Immunology (Baltimore, Md.: 1950) 185(10), 6214–6225. 10.4049/jimmunol.0903843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campalans A, Amouroux R, Bravard A, Epe B, & Radicella JP (2007). UVA irradiation induces relocalisation of the DNA repair protein hOGG1 to nuclear speckles. Journal of Cell Science 120(Pt 1), 23–32. 10.1242/jcs.03312. [DOI] [PubMed] [Google Scholar]
- Chan BA, & Hughes BGM (2015). Targeted therapy for non-small cell lung cancer: Current standards and the promise of the future. Translational Lung Cancer Research 4(1), 36–54. 10.3978/j.issn.2218-6751.2014.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-H, Hsiao C-F, Chang G-C, Tsai Y-H, Chen Y-M, Huang M-S, … Hsiung CA (2009). Interactive effect of cigarette smoking with human 8-oxoguanine DNA N-glycosylase 1 (hOGG1) polymorphisms on the risk of lung cancer: A case-control study in Taiwan. American Journal of Epidemiology 170(6), 695–702. 10.1093/aje/kwp019. [DOI] [PubMed] [Google Scholar]
- Chatterjee B, Banoth B, Mukherjee T, Taye N, Vijayaragavan B, Chattopadhyay S, … Basak S (2016). Late-phase synthesis of IκBα insulates the TLR4-activated canonical NF-κB pathway from noncanonical NF-κB signaling in macrophages. Science Signaling 9(457), ra120 10.1126/scisignal.aaf1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Jiang CC, Jin L, & Zhang XD (2016). Regulation of PD-L1: A novel role of prosurvival signalling in cancer. Annals of Oncology: Official Journal of the European Society for Medical Oncology 27(3), 409–416. 10.1093/annonc/mdv615. [DOI] [PubMed] [Google Scholar]
- Chen X, Song M, Zhang B, & Zhang Y (2016). Reactive Oxygen Species Regulate T Cell Immune Response in the Tumor Microenvironment. Oxidative Medicine and Cellular Longevity 2016. 10.1155/2016/1580967 1580967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary S, Boldogh I, & Brasier AR (2016). Inside-out Signaling Pathways from Nuclear Reactive Oxygen Species Control Pulmonary Innate Immunity. Journal of Innate Immunity 8(2), 143–155. 10.1159/000442254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chougnet CA, Thacker RI, Shehata HM, Hennies CM, Lehn MA, Lages CS, & Janssen EM (2015). Loss of Phagocytic and Antigen Cross-Presenting Capacity in Aging Dendritic Cells is Associated with Mitochondrial Dysfunction. Journal of Immunology (Baltimore, Md.: 1950) 195(6), 2624–2632. 10.4049/jimmunol.1501006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian F, Smith EL, & Carmody RJ (2016). The Regulation of NF-κB Subunits by Phosphorylation. Cell 5(1). 10.3390/cells5010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogoi S, Ferino A, Miglietta G, Pedersen EB, & Xodo LE (2017). The regulatory G4 motif of the Kirsten ras (KRAS) gene is sensitive to guanine oxidation: Implications on transcription. Nucleic Acids Research.. 10.1093/nar/gkx1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha-Benavente F, Srivastava RM, Trivedi S, Lei Y, Chandran U, Seethala RR, … Ferris RL (2016). Identification of the Cell-Intrinsic and -Extrinsic Pathways Downstream of EGFR and IFNγ that Induce PD-L1 Expression in Head and Neck Cancer. Cancer Research 76(5), 1031–1043. 10.1158/0008-5472.CAN-15-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damjanovic D, Divangahi M, Kugathasan K, Small C-L, Zganiacz A, Brown EG, … Xing Z (2011). Negative regulation of lung inflammation and immunopathology by TNF-α during acute influenza infection. The American Journal of Pathology 179 (6), 2963–2976. 10.1016/j.ajpath.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decotiis C, Hu Y, Greenberg AK, Huie M, Tsay J-CJ, Pass H, … Rom WN (2016). Inflammatory cytokines and non-small cell lung cancer in a CT-scan screening cohort: Background review of the literature. Cancer Biomarkers: Section A of Disease Markers 16(2), 219–233. 10.3233/CBM-150559. [DOI] [PubMed] [Google Scholar]
- Deslee G, Adair-Kirk TL, Betsuyaku T, Woods JC, Moosre CH, Gierada DS, … Pierce RA (2010). Cigarette smoke induces nucleic-acid oxidation in lung fibroblasts. American Journal of Respiratory Cell and Molecular Biology 43(5), 576–584. 10.1165/rcmb.2009-0221OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devadas S, Zaritskaya L, Rhee SG, Oberley L, & Williams MS (2002). Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: Selective regulation of mitogen-activated protein kinase activation and fas ligand expression. The Journal of Experimental Medicine 195(1), 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishop MK, & Kuruvilla S (2008). Primary and metastatic lung tumors in the pediatric population: A review and 25-year experience at a large children’s hospital. Archives of Pathology & Laboratory Medicine 132(7), 1079–1103. 10.1043/1543-2165. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M (1985). Formation of an 8-hydroxyguanine moiety in deoxyribonucleic acid on gamma-irradiation in aqueous solution. Biochemistry 24(16), 4476–4481. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M (2005). Base-excision repair of oxidative DNA damage by DNA glycosylases. Mutation Research 591(1-2), 45–59. 10.1016/j.mrfmmm.2005.01.033. [DOI] [PubMed] [Google Scholar]
- Du Q, & Geller DA (2010). Cross-Regulation between Wnt and NF-κB Signaling Pathways. Forum on Immunopathological Diseases and Therapeutics 1(3), 155–181. 10.1615/ForumImmunDisTher.v1.i3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan M-C, Han W, Jin P-W, Wei Y-P, Wei Q, Zhang L-M, & Li J-C (2015). Disturbed Th17/Treg Balance in patients with Non-small Cell Lung Cancer. Inflammation 38(6), 2156–2165. 10.1007/s10753-015-0198-x. [DOI] [PubMed] [Google Scholar]
- Durham AL, & Adcock IM (2015). The relationship between COPD and lung cancer. Lung Cancer (Amsterdam, Netherlands) 90(2), 121–127. 10.1016/j.lungcan.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziaman T, Ludwiczak H, Ciesla JM, Banaszkiewicz Z, Winczura A, Chmielarczyk M, … Olinski R (2014). PARP-1 expression is increased in colon adenoma and carcinoma and correlates with OGG1. PLoS One 9(12), e115558 10.1371/journal.pone.0115558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide HA, Halvorsen AR, Sandhu V, Fåne A, Berg J, Haakensen VD, … Helland Å (2016). Non-small cell lung cancer is characterised by a distinct inflammatory signature in serum compared with chronic obstructive pulmonary disease. Clinical & Translational Immunology 5(11), e109 10.1038/cti.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikawa S, Ohue Y, Kitaoka K, Aji T, Uenaka A, Oka M, & Nakayama E (2010). Enrichment of Foxp3+ CD4 regulatory T cells in migrated T cells to IL-6- and IL-8-expressing tumors through predominant induction of CXCR1 by IL-6. Journal of Immunology (Baltimore, Md.: 1950) 185(11), 6734–6740. 10.4049/jimmunol.1000225. [DOI] [PubMed] [Google Scholar]
- Fang L, Choudhary S, Zhao Y, Edeh CB, Yang C, Boldogh I, & Brasier AR (2014). ATM regulates NF-κB-dependent immediate-early genes via RelA Ser 276 phosphorylation coupled to CDK9 promoter recruitment. Nucleic Acids Research 42(13), 8416–8432. 10.1093/nar/gku529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finco TS, Westwick JK, Norris JL, Beg AA, Der CJ, & Baldwin AS (1997). Oncogenic Ha-Ras-induced signaling activates NF-kappaB transcriptional activity, which is required for cellular transformation. The Journal of Biological Chemistry 272(39), 24113–24116. [DOI] [PubMed] [Google Scholar]
- Fleischer T, Edvardsen H, Solvang HK, Daviaud C, Naume B, Borresen-Dale A-L, … Tost J (2014). Integrated analysis of high-resolution DNA methylation profiles, gene expression, germline genotypes and clinical end points in breast cancer patients. International Journal of Cancer 134(11), 2615–2625. 10.1002/ijc.28606. [DOI] [PubMed] [Google Scholar]
- Fleming AM, Ding Y, & Burrows CJ (2017). Oxidative DNA damage is epigenetic by regulating gene transcription via base excision repair. Proceedings of the National Academy of Sciences of the United States of America 114(10), 2604–2609. 10.1073/pnas.1619809114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd HS, Farnsworth CL, Kock ND, Mizesko MC, Little JL, Dance ST, … Miller MS (2005). Conditional expression of the mutant Ki-rasG12C allele results in formation of benign lung adenomas: Development of a novel mouse lung tumor model. Carcinogenesis 26(12), 2196–2206. 10.1093/carcin/bgi190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formisano L, Jansen VM, Marciano R, & Bianco R (2017). From biology to therapy: Improvements of therapeutic options in Lung cancer. Anti-Cancer Agents in Medicinal Chemistry.. 10.2174/1871520617666170912123416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Yagishita S, Hagiwara K, Yoshioka Y, Kosaka N, Takeshita F, … Ochiya T. (2015). The clinical relevance of the miR-197/CKS1B/STAT3-mediated PD-L1 network in chemoresistant non-small-cell lung cancer. Molecular Therapy: The Journal of the American Society of Gene Therapy 23(4), 717–727. 10.1038/mt.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallelli L, Falcone D, Scaramuzzino M, Pelaia G, D’Agostino B, Mesuraca M, … Savino R (2014). Effects of simvastatin on cell viability and proinflammatory pathways in lung adenocarcinoma cells exposed to hydrogen peroxide. BMC Pharmacology & Toxicology 15, 67 10.1186/2050-6511-15-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan WQ, Man SFP, Senthilselvan A, & Sin DD (2004). Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax 59(7), 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T, Joyce BT, Liu L, Zheng Y, Dai Q, Zhang Z, … Hou L (2016). DNA methylation of oxidative stress genes and cancer risk in the Normative Aging Study. American Journal of Cancer Research 6(2), 553–561. [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Cornfield DN, Stenmark KR, Thebaud B, Abman SH, & Raj JU (2016). Unique aspects of the developing lung circulation: Structural development and regulation of vasomotor tone. Pulmonary Circulation 6(4), 407–425. 10.1086/688890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng P, Yao J, & Zhu Y (2014). hOGG1 Ser326Cys polymorphism and lung cancer susceptibility: A meta-analysis. Molecular Biology Reports 41 (4), 2299–2306. 10.1007/s11033-014-3083-z. [DOI] [PubMed] [Google Scholar]
- German P, Szaniszlo P, Hajas G, Radak Z, Bacsi A, Hazra TK, … Boldogh I (2013). Activation of cellular signaling by 8-oxoguanine DNA glycosylase-1-initiated DNA base excision repair. DNA Repair 12(10), 856–863. 10.1016/j.dnarep.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghonim MA, Pyakurel K, Ibba SV, Wang J, Rodriguez P, Al-Khami AA, … Boulares AH (2015). PARP is activated in human asthma and its inhibition by olaparib blocks house dust mite-induced disease in mice. Clinical Science (London, England: 1979) 129(11), 951–962. 10.1042/CS20150122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh G, Wang VY-F, Huang D-B, & Fusco A (2012). NF-κB regulation: Lessons from structures. Immunological Reviews 246(1), 36–58. 10.1111/j.1600-065X.2012.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, & Karin M (2002). Missing pieces in the NF-κB puzzle. Cell 109(2 SUPPL 1), S81–S96. 10.1016/S0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Giopanou I, Lilis I, Papaleonidopoulos V, Marazioti A, Spella M, Vreka M, … Stathopoulos GT (2015). Comprehensive Evaluation of Nuclear factor-κB Expression patterns in Non-Small Cell Lung Cancer. PLoS One 10(7), e0132527 10.1371/journal.pone.0132527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L, da Silva Caetano M, Cumpian AM, Daliri S, Garza Flores A, Chang SH, … Moghaddam SJ (2016). Tumor necrosis factor links chronic obstructive pulmonary disease and K-ras mutant lung cancer through induction of an immunosuppressive pro-tumor microenvironment. Oncoimmunology 5(10), e1229724 10.1080/2162402X.2016.1229724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso D, Bintz J, Lomberk G, Molejon MI, Loncle C, Garcia MN, … Iovanna JL (2015). Pivotal Role of the Chromatin Protein Nupr1 in Kras-Induced Senescence and Transformation. Scientific Reports 5, 17549 10.1038/srep17549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Jin D, & Chen X (2014). Lipocalin 2 is a regulator of macrophage polarization and NF-κB/STAT3 pathway activation. Molecular Endocrinology (Baltimore, Md.) 28 (10), 1616–1628. 10.1210/me.2014-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha H, Debnath B, & Neamati N (2017). Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics 7(6), 1543–1588. 10.7150/thno.15625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K-Q, He X-Q, Ma M-Y, Guo X-D, Zhang X-M, Chen J, … Zhao W-Z (2015). Targeted silencing of CXCL1 by siRNA inhibits tumor growth and apoptosis in hepatocellular carcinoma. International Journal of Oncology 47(6), 2131–2140 10.3892/ijo.2015.3203. [DOI] [PubMed] [Google Scholar]
- Hao W, Qi T, Pan L, Wang R, Zhu B, Aguilera-Aguirre L, … Boldogh I (2018). Effects of the stimuli-dependent enrichment of 8-oxoguanine DNA glycosylase1 on chromatinized DNA. Redox Biology 18, 43–53. 10.1016/j.redox.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haurogné K, Pavlovic M, Rogniaux H, Bach J-M, & Lieubeau B (2015). Type 1 Diabetes Prone NOD mice have Diminished Cxcr1 mRNA Expression in Polymorphonuclear Neutrophils and CD4+ T Lymphocytes. PLoS One 10(7), e0134365 10.1371/journal.pone.0134365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazra TK, Hill JW, Izumi T, & Mitra S (2001). Multiple DNA glycosylases for repair of 8-oxoguanine and their potential in vivo functions. Progress in Nucleic Acid Research and Molecular Biology 68, 193–205. [DOI] [PubMed] [Google Scholar]
- He Z, Huang C, Lin G, & Ye Y (2016). siRNA-induced TRAF6 knockdown promotes the apoptosis and inhibits the invasion of human lung cancer SPC-A1 cells. Oncology Reports 35(4), 1933–1940. 10.3892/or.2016.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiley CT, Le Quesne J, Santis G, Sharpe R, de Castro DG, Middleton G, & Swanton C (2016). Challenges in molecular testing in non-small-cell lung cancer patients with advanced disease. Lancet (London, England) 388(10048), 1002–1011. 10.1016/S0140-6736(16)31340-X. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, … Shibuya M (2002). MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2(4), 289–300. [DOI] [PubMed] [Google Scholar]
- Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ, Wu Y-L, & Paz-Ares L (2017). Lung cancer: Current therapies and new targeted treatments. Lancet (London, England) 389(10066), 299–311. 10.1016/S0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- Hoegl S, Ehrentraut H, Brodsky KS, Victorino F, Golden-Mason L, Eltzschig HK, & McNamee EN (2017). NK cells regulate CXCR2+ neutrophil recruitment during acute lung injury. Journal of Leukocyte Biology 101(2), 471–480. 10.1189/jlb.3A0516-227R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoki K, Itazawa T, Boldogh I, & Sur S (2016). Neutrophil recruitment by allergens contribute to allergic sensitization and allergic inflammation. Current Opinion in Allergy and Clinical Immunology 16(1), 45–50. 10.1097/ACI.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Shen F, Huang H, Ling C, & Zhang G (2017). Th1high in tumor microenvironment is an indicator of poor prognosis for patients with NSCLC. Oncotarget 8(8), 13116–13125. 10.18632/oncotarget.14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani K, Herzlinger S, & Finkel T (1994). Ras proteins regulate multiple mitogenic pathways in A10 vascular smooth muscle cells. Biochemical and Biophysical Research Communications 202(3), 1252–1258. 10.1006/bbrc.1994.2065. [DOI] [PubMed] [Google Scholar]
- Jamaluddin M, Tian B, Boldogh I, Garofalo RP, & Brasier AR (2009). Respiratory syncytial virus infection induces a reactive oxygen species-MSK1-phospho-Ser-276 RelA pathway required for cytokine expression. Journal of Virology 83(20), 10605–10615. 10.1128/JVI.01090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamaluddin M, Wang S, Boldogh I, Tian B, & Brasier AR (2007). TNF-alpha-induced NF-kappaB/RelA Ser(276) phosphorylation and enhanceosome formation is mediated by an ROS-dependent PKAC pathway. Cellular Signalling 19(7), 1419–1433. https://doi.org/m1016/j.cellsig.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Janik J, Swoboda M,Janowska B, Ciesla JM, Gackowski D, Kowalewski J, … Speina E (2011). 8-Oxoguanine incision activity is impaired in lung tissues of NSCLC patients with the polymorphism of OGG1 and XRCC1 genes. Mutation Research 709-710, 21–31. 10.1016/j.mrfmmm.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Jinesh GG, Sambandam V, Vijayaraghavan S, Balaji K, & Mukherjee S (2017). Molecular genetics and cellular events of K-Ras-driven tumorigenesis. Oncogene. 10.1038/onc.2017.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger M, Finn SP, Cuffe S, Byrne AT, & Gray SG (2016). The IL-17-Th1/Th17 pathway: An attractive target for lung cancer therapy? Expert Opinion on Therapeutic Targets 20(11), 1339–1356. 10.1080/14728222.2016.1206891. [DOI] [PubMed] [Google Scholar]
- Kang SW, Kim SK, Park HJ, Chung J-H, & Ban JY. (2017). Human 8-oxoguanine DNA glycosylase gene polymorphism (Ser326Cys) and cancer risk: Updated meta-analysis. Oncotarget 8(27), 44761–44775. 10.18632/oncotarget.16226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbownik M, Reiter RJ, Cabrera J, & Garcia JJ (2001). Comparison of the protective effect of melatonin with other antioxidants in the hamster kidney model of estradiol-induced DNA damage. Mutation Research 474(1-2), 87–92. [DOI] [PubMed] [Google Scholar]
- Kaur MP, Guggenheim EJ, Pulisciano C, Akbar S, Kershaw RM, & Hodges NJ (2014). Cellular accumulation of Cys326-OGG1 protein complexes under conditions of oxidative stress. Biochemical and Biophysical Research Communications 447(1), 12–18. 10.1016/j.bbrc.2014.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw RM, & Hodges NJ (2012). Repair of oxidative DNA damage is delayed in the Ser326Cys polymorphic variant of the base excision repair protein OGG1. Mutagenesis 27(4), 501–510. 10.1093/mutage/ges012. [DOI] [PubMed] [Google Scholar]
- Khan YM, Kirkham P, Barnes PJ, & Adcock IM (2014). Brd4 is essential for IL-1β-induced inflammation in human airway epithelial cells. PLoS One 9(4), e95051. 10.1371/journal.pone.0095051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kietzmann T, Petry A, Shvetsova A, Gerhold JM, & Görlach A (2017). The epigenetic landscape related to reactive oxygen species formation in the cardiovascular system. British Journal of Pharmacology 174(12), 1533–1554. 10.1111/bph.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgoz HO, Bender G, Scandura JM, Viale A, & Taneri B (2016). KRAS and the reality of Personalized Medicine in Non-small Cell Lung Cancer. Molecular Medicine (Cambridge, Mass.) 22 10.2119/molmed.2016.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Kim W, Lee S, Chun J, Kang J, Park G, … Youn B (2017). TRAF4 promotes lung cancer aggressiveness by modulating tumor microenvironment in normal fibroblasts. Scientific Reports 7(1), 8923 10.1038/s41598-017-09447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Naura AS, Errami Y,Ju J, & Boulares AH (2011). Cordycepin blocks lung injury-associated inflammation and promotes BRCA1 -deficient breast cancer cell killing by effectively inhibiting PARP. Molecular Medicine (Cambridge, Mass.) 17(9-10), 893–900. 10.2119/molmed.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, McMillan E, Kim HS, Venkateswaran N, Makkar G, Rodriguez-Canales J, … White MA (2016). XPO1-dependent nuclear export is a druggable vulnerability in KRAS-mutant lung cancer. Nature 538(7623), 114–117. 10.1038/nature19771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima S, Thummalapalli R, & Barbie DA (2016). Inflammation as a driver and vulnerability of KRAS mediated oncogenesis. Seminars in Cell & Developmental Biology 58, 127–135. 10.1016/j.semcdb.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingbeil O, Lesche R, Gelato KA, Haendler B, & Lejeune P (2016). Inhibition of BET bromodomain-dependent XIAP and FLIP expression sensitizes KRAS-mutated NSCLC to pro-apoptotic agents. Cell Death & Disease 7(9), e2365 10.1038/cddis.2016.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans T, Eilers R, Menzen M, Halayko A, & Gosens R (2017). β-Catenin Directs Nuclear factor-KB p65 output via CREB-Binding Protein/p300 in Human Airway Smooth Muscle. Frontiers in Immunology 8, 1086 10.3389/fimmu.2017.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Akbay EA, Li YY, Aref AR, Skoulidis F, Herter-Sprie GS, … Wong K-K (2016). STK11/LKB1 Deficiency Promotes Neutrophil Recruitment and Proinflammatory Cytokine Production to Suppress T-cell activity in the Lung Tumor Microenvironment. Cancer Research 76(5), 999–1008. 10.1158/0008-5472.CAN-15-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiecien I, Stelmaszczyk-Emmel A, Polubiec-Kownacka M, Dziedzic D, & Domagala-Kulawik J (2017). Elevated regulatory T cells, surface and intracellular CTLA-4 expression and interleukin-17 in the lung cancer microenvironment in humans. Cancer Immunology, Immunotherapy: CII 66(2), 161–170. 10.1007/s00262-016-1930-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon M, Jang H, Kim EH, & Roh J-L (2016). Efficacy of poly (ADP-ribose) polymerase inhibitor olaparib against head and neck cancer cells: Predictions of drug sensitivity based on PAR-p53-NF-κB interactions. Cell Cycle (Georgetown, Tex.) 15(22), 3105–3114. 10.1080/15384101.2016.1235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langie SAS, Cameron KM, Ficz G, Oxley D, Tomaszewski B, Gorniak JP, … Mathers JC (2017). The Ageing Brain: Effects on DNA Repair and DNA Methylation in mice. Genes 8(2). 10.3390/genes8020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH (2017). Treatments for EGFR-mutant non-small cell lung cancer (NSCLC): The road to a success, paved with failures. Pharmacology & Therapeutics 174, 1–21. 10.1016/j.pharmthera.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Lee Y-L, Obiako B, Gorodnya OM, Ruchko MV, Kuck JL, Pastukh VM, … Gillespie MN (2017). Mitochondrial DNA damage Initiates Acute Lung Injury and Multi-Organ System failure Evoked in Rats by Intra-Tracheal Pseudomonas Aeruginosa. Shock (Augusta, Ga.) 48(1), 54–60. 10.1097/SHK.0000000000000838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner-Dagan Y, Sevilya Z, Pinchev M, Kramer R, Elinger D, Roisman LC, … Paz-Elizur T (2012). N-methylpurine DNA glycosylase and OGG1 DNA repair activities: Opposite associations with lung cancer risk. Journal of the National Cancer Institute 104(22), 1765–1769. 10.1093/jnci/djs445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Beisswenger C, Herr C, Hellberg J, Han G, Zakharkina T, … Bals R (2014). Myeloid cell RelA/p65 promotes lung cancer proliferation through Wnt/β-catenin sigaling in murine and human tumor cells. Oncogene 33(10), 1239–1248. 10.1038/onc.2013.75. [DOI] [PubMed] [Google Scholar]
- Li F, Du X, Zhang H, Ju T, Chen C, Qu Q, … Lizee G (2017). Next-generation sequencing of Chinese stage IV lung cancer patients reveals an association between EGFR mutation status and survival outcome. Clinical Genetics 91(3), 488–493. 10.1111/cge.12809. [DOI] [PubMed] [Google Scholar]
- Li G, Yuan K, Yan C, Fox J, Gaid M, Breitwieser W, … Wu M (2012). 8-Oxoguanine-DNA glycosylase 1 deficiency modifies allergic airway inflammation by regulating STAT6 and IL-4 in cells and in mice. Free Radical Biology & Medicine 52(2), 392–401. 10.1016/j.freeradbiomed.2011.10.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G-G, Guo Z-Z, Ma X-F, Cao N, Geng S-N, Zheng Y-Q, … Du G-J (2016). The M2 macrophages induce autophagic vascular disorder and promote mouse sensitivity to urethane-related lung carcinogenesis. Developmental and Comparative Immunology 59, 89–98. 10.1016/j.dci.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Li R, Fang F, Jiang M, Wang C, Ma J, Kang W, … Xiao W (2017). STAT3 and NF-κB are simultaneously Suppressed in Dendritic Cells in Lung Cancer. Scientific Reports 7, 45395 10.1038/srep45395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C, Yu Z, Guo W, Liu Q, Wu Y, Li Y, & Bai L (2014). Prognostic value of circulating inflammatory factors in non-small cell lung cancer: A systematic review and metaanalysis. Cancer Biomarkers: Section A of Disease Markers 14(6), 469–481. 10.3233/CBM-140423. [DOI] [PubMed] [Google Scholar]
- Liao C, Yu Z-B, Meng G, Wang L, Liu Q-Y, Chen L-T, … Bai L (2015). Association between Th17-related cytokines and risk of non-small cell lung cancer among patients with or without chronic obstructive pulmonary disease. Cancer 121 (Suppl. 17), 3122–3129. 10.1002/cncr.29369. [DOI] [PubMed] [Google Scholar]
- Lin K, Cheng J, Yang T, Li Y, & Zhu B (2015). EGFR-TKI down-regulates PD-L1 in EGFR mutant NSCLC through inhibiting NF-κB. Biochemical and Biophysical Research Communications 463(1-2), 95–101. 10.1016/j.bbrc.2015.05.030. [DOI] [PubMed] [Google Scholar]
- Liu P, Li K, Garofalo RP, & Brasier AR (2008). Respiratory syncytial virus induces RelA release from cytoplasmic 100-kDa NF-kappa B2 complexes via a novel retinoic acid-inducible gene-I{middle dot}NF-kappa B-inducing kinase signaling pathway. The Journal of Biological Chemistry 283(34), 23169–23178. 10.1074/jbc.M802729200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T, … Wu K (2016). The CXCL8-CXCR1/2 pathways in cancer. Cytokine & Growth Factor Reviews 31, 61–71. 10.1016/j.cytogfr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ranganathan R, Jiang S, Fang C, Sun J, Kim S, … Moon EK (2016). A Chimeric Switch-Receptor Targeting PD1 Augments the Efficacy of Second-Generation CAR T Cells in Advanced Solid Tumors. Cancer Research 76(6), 1578–1590. 10.1158/0008-5472.CAN-15-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y,Mayo MW, Nagji AS, Smith PW, Ramsey CS, Li D, & Jones DR (2012). Phosphorylation of RelA/p65 promotes DNMT-1 recruitment to chromatin and represses transcription of the tumor metastasis suppressor gene BRMS1., Phosphorylation of RelA/p65 promotes DNMT-1 recruitment to chromatin and represses transcription of the tumor metastasis suppressor gene BRMS1. Oncogene, 31(91143–1154. 10.1038/onc.2011.308, 10.1038/onc.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y-N, Wang X-B, Wang T, Zhang C, Zhang K-P, Zhi X-Y, … Sun K-L (2016). Macrophage Inhibitory Cytokine-1 as a Novel Diagnostic and Prognostic Biomarker in stage I and II Nonsmall Cell Lung Cancer. Chinese Medical Journal 129(17), 2026–2032. 10.4103/0366-6999.189052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wei J, Guo, & Zhou (2015). Norepinephrine-induced myeloid-derived suppressor cells block T-cell responses via generation of reactive oxygen species. Immunopharmacology and Immunotoxicology 37(4), 359–365. 10.3109/08923973.2015.1059442. [DOI] [PubMed] [Google Scholar]
- Lu J-H, Wei H-J, Peng B-Y, Chou H-H, Chen W-H, Liu H-Y, & Deng W-P (2016). Adipose-Derived Stem Cells Enhance Cancer Stem Cell Property and Tumor Formation Capacity in Lewis Lung Carcinoma Cells through an Interleukin-6 Paracrine Circuit. Stem Cells and Development 25(23), 1833–1842. 10.1089/scd.2016.0163. [DOI] [PubMed] [Google Scholar]
- Luo J, Hosoki K, Bacsi A, Radak Z, Hegde ML, Sur S, … Boldogh I (2014). 8-Oxoguanine DNA glycosylase-1-mediated DNA repair is associated with Rho GTPase activation and α-smooth muscle actin polymerization. Free Radical Biology & Medicine 73, 430–438. 10.1016/j.freeradbiomed.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv M, Xu Y, Tang R, Ren J, Shen S, Chen Y, … Wang T (2014). miR141-CXCL1-CXCR2 signaling-induced Treg recruitment regulates metastases and survival of non-small cell lung cancer. Molecular Cancer Therapeutics 13(12), 3152–3162. 10.1158/1535-7163.MCT-14-0448. [DOI] [PubMed] [Google Scholar]
- Ma X, Song Y, Zhang K, Shang L, Gao Y, Zhang W, … Li M (2015). Recombinant mutated human TNF in combination with chemotherapy for stage IIIB/IV non-small cell lung cancer: A randomized, phase III study. Scientific Reports 4, 9918 10.1038/srep09918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Yang Y, Fan C, Han J, Wang D, Di S, … Yan X (2016). Melatonin as a potential anticarcinogen for non-small-cell lung cancer. Oncotarget 7(29), 46768–46784. 10.18632/oncotarget.8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabley JG, Pacher P, Deb A, Wallace R, Elder RH, & Szabo C (2005). Potential role for 8-oxoguanine DNA glycosylase in regulating inflammation. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology 19 (2), 290–292. 10.1096/fj.04-2278fje. [DOI] [PubMed] [Google Scholar]
- Manning L, & Nemunaitis J (2017). Harnessing the immune response to target tumors. F1000Research 6, 710 10.12688/f1000research.10795.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martincuks A, Andryka K, Kuster A, Schmitz-Van De Leur H, Komorowski M, & Muller-Newen G (2017). Nuclear translocation of STAT3 and NF-κB are independent of each other but NF-κB supports expression and activation of STAT3. Cellular Signalling 32, 36–47. 10.1016/j.cellsig.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Mazzoccoli G, Carughi S, De Cata A, La Viola M, & Vendemiale G (2005). Melatonin and cortisol serum levels in lung cancer patients at different stages of disease. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research 11(6), CR284–288. [PubMed] [Google Scholar]
- Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, … Perlman H (2017). Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. The Journal of Experimental Medicine 214(8), 2387–2404. 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S, Izumi T, Boldogh I, Bhakat KK, Hill JW, & Hazra TK (2002). Choreography of oxidative damage repair in mammalian genomes. Free Radical Biology & Medicine 33(1), 15–28. [DOI] [PubMed] [Google Scholar]
- Moore SPG, Kruchten J, Toomire KJ, & Strauss PR (2016). Transcription factors and DNA repair enzymes compete for damaged promoter sites. Journal of Biological Chemistry 291(11), 5452–5460. 10.1074/jbc.M115.672733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosavi SM, Prabhala P, & Ammit AJ (2017). Role and regulation of MKP-1 in airway inflammation. Respiratory Research 18(1), 154 10.1186/s12931-017-0637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morreall J, Limpose K, Sheppard C, Kow YW, Werner E, & Doetsch PW (2015). Inactivation of a common OGG1 variant by TNF-alpha in mammalian cells. DNA Repair 26, 15–22. 10.1016/j.dnarep.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MB, McKim KL, Meng F, & Parsons BL (2015). Low-frequency KRAS mutations are prevalent in lung adenocarcinomas. Personalized Medicine 12(2), 83–98. 10.2217/pme.14.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AH, Berim IG, & Agrawal DK (2014). Cellular and molecular immunology of lung cancer: Therapeutic implications. Expert Review of Clinical Immunology 10(12), 1711–1730. 10.1586/1744666X.2014.975692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka K, Ohtsubo T, Oda H, Fujiwara T, Kang D, Sugimachi K, & Nakabeppu Y (1999). Expression and differential intracellular localization of two major forms of human 8-oxoguanine DNA glycosylase encoded by alternatively spliced OGG1 mRNAs. Molecular Biology of the Cell 10(5), 1637–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren Hooten N, Kompaniez K, Barnes J, Lohani A, & Evans MK (2011). Poly(ADP-ribose) polymerase 1 (PARP-1) binds to 8-oxoguanine-DNA glycosylase (OGG1). The Journal of Biological Chemistry 286(52), 44679–44690. 10.1074/jbc.M111.255869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak DE, Tian B, Jamaluddin M, Boldogh I, Vergara LA, Choudhary S, & Brasier AR (2008). RelA Ser276 phosphorylation is required for activation of a subset of NF-kappaB-dependent genes by recruiting cyclin-dependent kinase 9/cyclin T1 complexes. Molecular and Cellular Biology 28(11), 3623–3638. 10.1128/MCB.01152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuovo GJ, Folcik VA, & Magro C (2017). Viral-induced Modulation of Multiple Checkpoint Proteins in Cancers. Applied Immunohistochemistry & Molecular Morphology: AIMM 25(6), 407–414. 10.1097/PAI.0000000000000429. [DOI] [PubMed] [Google Scholar]
- Ogawa A, Watanabe T, Shoji S, & Furihata C (2015). Enzyme kinetics of an alternative splicing isoform of mitochondrial 8-oxoguanine DNA glycosylase, ogg1–1b, and compared with the nuclear ogg1–1a. Journal of Biochemical and Molecular Toxicology 29 (2), 49–56. 10.1002/jbt.21605. [DOI] [PubMed] [Google Scholar]
- O’Gorman A, Colleran A, Ryan A, Mann J, & Egan LJ (2010). Regulation of NF-kappaB responses by epigenetic suppression of IkappaBalpha expression in HCT116 intestinal epithelial cells. American Journal of Physiology Gastrointestinal and Liver Physiology 299 (1), G96–G105. 10.1152/ajpgi.00460.2009. [DOI] [PubMed] [Google Scholar]
- Pan L, Hao W, Zheng X, Zeng X, Ahmed Abbasi A, Boldogh I, & Ba X (2017). OGG1-DNA interactions facilitate NF-κB binding to DNA targets. Scientific Reports 7, 43297 10.1038/srep43297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Zhu B, Hao W, Zeng X, Vlahopoulos SA, Hazra TK, … Boldogh I (2016). Oxidized Guanine Base Lesions Function in 8-Oxoguanine DNA Glycosylase1-Mediated Epigenetic Regulation of Nuclear factor kappaB-driven Gene Expression. The Journal of Biological Chemistry. 10.1074/jbc.M116.751453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YW, Zhou ZG, Wang M, Dong JQ, Du KP, Li S, … Gao JB (2016). Combination of IL-6, IL-10, and MCP-1 with traditional serum tumor markers in lung cancer diagnosis and prognosis. Genetics and Molecular Research: GMR 15(4). 10.4238/gmr15048949. [DOI] [PubMed] [Google Scholar]
- Park M-T, Kim M-J, Suh Y, Kim R-K, Kim H, Lim E-J, … Lee S-J (2014). Novel signaling axis for ROS generation during K-Ras-induced cellular transformation. Cell Death and Differentiation 21(8), 1185–1197. 10.1038/cdd.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AD, Gonzalez FJ, & Idle JR (2010). Xenobiotic metabolism: A view through the metabolometer. Chemical Research in Toxicology 23(5), 851–860. 10.1021/tx100020p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Elizur T, Krupsky M, Blumenstein S, Elinger D, Schechtman E, & Livneh Z (2003). DNA repair activity for oxidative damage and risk of lung cancer. Journal of the National Cancer Institute 95(17), 1312–1319. [DOI] [PubMed] [Google Scholar]
- Peng H, Guerau-De-Arellano M, Mehta VB, Yang Y, Huss DJ, Papenfuss TL, … Racke MK (2012). Dimethyl fumarate inhibits dendritic cell maturation via nuclear factor κB (NF-κB) and extracellular signal-regulated kinase 1 and 2 (ERK1/2) and mitogen stress-activated kinase 1 (MSK1) signaling. The Journal of Biological Chemistry 287 (33), 28017–28026. 10.1074/jbc.M112.383380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Li Z, Zhang S, Xiong Y, Cun Y, Qian C, … Wang D (2014). Association of DNA base excision repair genes (OGG1, APE1 and XRCC1) polymorphisms with outcome to platinum-based chemotherapy in advanced nonsmall-cell lung cancer patients. International Journal of Cancer 135(11), 2687–2696. 10.1002/ijc.28892. [DOI] [PubMed] [Google Scholar]
- Qiao X, Zhai X, Wang J, Zhao X, Yang X, Lv J, … Yue W (2016). Sequential measurements of serum matrix met alloproteinase 9 to monitor chemotherapy responses in patients with advanced non-small-cell lung cancer. OncoTargets and Therapy 9, 3299–3305. 10.2147/OTT.S102351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Zhu J, Zeng Y, Du W, Shen D, Lei Z, … Liu Z (2017). Aberrant promoter methylation of hOGG1 may be associated with increased risk of non-small cell lung cancer. Oncotarget 8(5), 8330–8341. 10.18632/oncotarget.14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn KM, Kan W-T, Watson KA, Liddicoat BJ, Swan NG, McQuilten H, … La Gruta NL (2017). Extrinsically derived TNF is primarily responsible for limiting antiviral CD8+ T cell response magnitude. PLoS One 12(9), e0184732 10.1371/journal.pone.0184732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan H, Ilm K, Walther W, Shirasawa S, Sasazuki T, Daniel PT, … Stein U (2017). MACC1 regulates Fas mediated apoptosis through STAT1/3 - Mcl-1 signaling in solid cancers. Cancer Letters 403, 231–245. 10.1016/j.canlet.2017.06.020. [DOI] [PubMed] [Google Scholar]
- Ramdzan ZM, Pal R, Kaur S, Leduy L, Berube G, Davoudi S, … Nepveu A (2015). The function of CUX1 in oxidative DNA damage repair is needed to prevent premature senescence of mouse embryo fibroblasts. Oncotarget 6(6), 3613–3626. 10.18632/oncotarget.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdzan ZM, Vadnais C, Pal R, Vandal G, Cadieux C, Leduy L, … Nepveu A (2014). RAS transformation requires CUX1-dependent repair of oxidative DNA damage. PLoS Biology 12(3), e1001807 10.1371/journal.pbio.1001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remon J, & Besse B (2016). Unravelling signal escape through maintained EGFR activation in advanced non-small cell lung cancer (NSCLC): New treatment options. ESMO Open 1(4), e000081 10.1136/esmoopen-2016-000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakumi K, Tominaga Y, Furuichi M, Xu P, Tsuzuki T, Sekiguchi M, & Nakabeppu Y (2003). Ogg1 knockout-associated lung tumorigenesis and its suppression by Mth1 gene disruption. Cancer Research 63(5), 902–905. [PubMed] [Google Scholar]
- Sanmamed MF, Perez-Gracia JL, Schalper KA, Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, … Melero I (2017). Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Annals of Oncology: Official Journal of the European Society for Medical Oncology 28(8), 1988–1995. 10.1093/annonc/mdx190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon JA, Sherrill TP, Polosukhin VV, Sai J, Zaynagetdinov R, McLoed AG, … Blackwell TS (2016). Epithelial NF-κB signaling promotes EGFR-driven lung carcinogenesis via macrophage recruitment. Oncoimmunology 5(6), e1168549 10.1080/2162402X.2016.1168549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallenberg S, Merkelbach-Bruse S, & Buettner R (2017). Lung cancer as a paradigm for precision oncology in solid tumours. Virchows Archiv: An International Journal of Pathology. 10.1007/s00428-017-2183-2. [DOI] [PubMed] [Google Scholar]
- Schoenhals JE, Seyedin SN, Anderson C, Brooks ED, Li YR, Younes AI, … Welsh JW (2017). Uncovering the immune tumor microenvironment in non-small cell lung cancer to understand response rates to checkpoint blockade and radiation. Translational Lung Cancer Research 6(2), 148–158. 10.21037/tlcr.2017.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scilla KA, Bentzen SM, Lam VK, Mohindra P, Nichols EM, Vyfhuis MA, … Feliciano JL (2017). Neutrophil-Lymphocyte Ratio is a Prognostic Marker in patients with locally Advanced (Stage IIIA and IIIB) Non-Small Cell Lung Cancer Treated with combined Modality Therapy. The Oncologist 22(6), 737–742. 10.1634/theoncologist.2016-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi GS, Dharwal V, & Naura AS (2017). Poly(ADP-Ribose)Polymerase-1 in Lung Inflammatory Disorders: A Review. Frontiers in Immunology 8, 1172 10.3389/fimmu.2017.01172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilya Z, Leitner-Dagan Y, Pinchev M, Kremer R, Elinger D, Rennert HS, … Livneh Z (2014). Low integrated DNA repair score and lung cancer risk. Cancer Prevention Research (Philadelphia, Pa.) 7(4), 398–406. 10.1158/1940-6207.CAPR-13-0318. [DOI] [PubMed] [Google Scholar]
- Shahriary A, Panahi Y, Shirali S, & Rahmani H (2017). Relationship of serum levels of interleukin 6, interleukin 8, and C-reactive protein with forced expiratory volume in first second in patients with mustard lung and chronic obstructive pulmonary diseases: Systematic review and meta-analysis. Postepy Dermatologii I Alergologii 34(3), 192–198. 10.5114/ada.2017.67841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzad T, Radajewski S, Chao C-M, Bellusci S, & Ehrhardt H (2016). Pathogenesis of bronchopulmonary dysplasia: When inflammation meets organ development. Molecular and Cellular Pediatrics 3(1), 23 10.1186/s40348-016-0051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva EM, Mariano VS, Pastrez PRA, Pinto MC, Castro AG, Syrjanen KJ, & Longatto-Filho A (2017). High systemic IL-6 is associated with worse prognosis in patients with non-small cell lung cancer. PLoS One 12(7), e0181125 10.1371/journal.pone.0181125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanshu DK, Nissley DV, & McCormick F (2017). RAS Proteins and their regulators in Human Disease. Cell 170(1), 17–33. 10.1016/j.cell.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh GK, Williams SD, Siahpush M, & Mulhollen A (2011). Socioeconomic, Rural-Urban, and racial Inequalities in US Cancer Mortality: Part I-All Cancers and Lung Cancer and Part II-Colorectal, Prostate, Breast, and Cervical Cancers. Journal of Cancer Epidemiology 2011, 107497 10.1155/2011/107497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sookprasert A, Johns NP, Phunmanee A, Pongthai P, Cheawchanwattana A, Johns J, …Jitpimolmard S (2014). Melatonin in patients with cancer receiving chemotherapy: A randomized, double-blind, placebo-controlled trial. Anticancer Research 34 (12), 7327–7337. [PubMed] [Google Scholar]
- Spaks A(2017). Role of CXC group chemokines in lung cancer development and progression. Journal of Thoracic Disease 9(Suppl. 3), S164–S171. 10.21037/jtd.2017.03.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyratos D, Papadaki E, Lampaki S, & Kontakiotis T (2017). Chronic obstructive pulmonary disease in patients with lung cancer: Prevalence, impact and management challenges. Lung Cancer (Auckland, N.Z.) 8, 101–107. 10.2147/LCTr.S117178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starczynowski DT, Lockwood WW, Delehouzee S, Chari R, Wegrzyn J, Fuller M, … Karsan A (2011). TRAF6 is an amplified oncogene bridging the RAS and NF-κB pathways in human lung cancer. The Journal of Clinical Investigation 121(10), 4095–4105. 10.1172/JCI58818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenken S, & Jovanovic SV (1997). How Easily Oxidizable is DNA? One-Electron Reduction Potentials of Adenosine and Guanosine Radicals in Aqueous solution. Journal of the American Chemical Society 119(3), 617–618. 10.1021/ja962255b. [DOI] [Google Scholar]
- Sumimoto H, Takano A, Teramoto K, & Daigo Y (2016). RAS-Mitogen-Activated Protein Kinase Signal is Required for Enhanced PD-L1 Expression in Human Lung Cancers. PLoS One 11(11), e0166626 10.1371/journal.pone.0166626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, Irani K, & Finkel T (1995). Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science (New York, N.Y.) 270(5234), 296–299. [DOI] [PubMed] [Google Scholar]
- Syriopoulou E, Bower H, Andersson TM-L, Lambert PC, & Rutherford MJ (2017). Estimating the impact of a cancer diagnosis on life expectancy by socio-economic group for a range of cancer types in England. British Journal of Cancer 117(9), 1419–1426. 10.1038/bjc.2017.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szebeni GJ, Vizler C, Kitajka K, & Puskas LG (2017). Inflammation and Cancer: Extra- and Intracellular Determinants of Tumor-Associated Macrophages as Tumor Promoters. Mediators of Inflammation 2017, 9294018 10.1155/2017/9294018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W-L, Jain A, Takano A, Newell EW, Iyer NG, Lim W-T, … Tan DSW (2016). Novel therapeutic targets on the horizon for lung cancer. The Lancet. Oncology 17(8), e347–e362. 10.1016/S1470-2045(16)30123-1. [DOI] [PubMed] [Google Scholar]
- Tang X, Liu D, Shishodia S, Ozburn N, Behrens C, Lee JJ, … Wistuba II (2006). Nuclear factor-kappaB (NF-kappaB) is frequently expressed in lung cancer and preneoplastic lesions. Cancer 107(11), 2637–2646. 10.1002/cncr.22315. [DOI] [PubMed] [Google Scholar]
- Tempka D, Tokarz P, Chmielewska K, Kluska M, Pietrzak J, Rygielska Z, … Robaszkiewicz A (2018). Downregulation of PARP1 transcription by CDK4/6 inhibitors sensitizes human lung cancer cells to anticancer drug-induced death by impairing OGG1-dependent base excision repair. Redox Biology 15, 316–326. 10.1016/j.redox.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Zhao Y, Kalita M, Edeh CB, Paessler S, Casola A, … Brasier AR (2013). CDK9-dependent transcriptional elongation in the innate interferon-stimulated gene response to respiratory syncytial virus infection in airway epithelial cells. Journal of Virology 87(12), 7075–7092. 10.1128/JVI.03399-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M, Shimizu T, Ayabe T, Nakamura K, & Onitsuka T (2012). Elevated preoperative inflammatory markers based on neutrophil-to-lymphocyte ratio and C-reactive protein predict poor survival in resected non-small cell lung cancer. Anticancer Research 32(8), 3535–3538. [PubMed] [Google Scholar]
- Tran PT, Bendapudi PK, Lin HJ, Choi P, Koh S, Chen J, … Felsher DW (2011). Survival and death signals can predict tumor response to therapy after oncogene inactivation. Science Translational Medicine 3(103), 103ra99 10.1126/scitranslmed.3002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahopoulos S, Boldogh I, Casola A, & Brasier AR (1999). Nuclear factor-kappaB-dependent induction of interleukin-8 gene expression by tumor necrosis factor alpha: Evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood 94(6), 1878–1889. [PubMed] [Google Scholar]
- Vlahopoulos SA (2017). Aberrant control of NF-κB in cancer permits transcriptional and phenotypic plasticity, to curtail dependence on host tissue: Molecular mode. Cancer Biology & Medicine 14(3), 254–270. 10.20892/j.issn.2095-3941.2017.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahopoulos SA, Cen O, Hengen N, Agan J, Moschovi M, Critselis E, … Chrousos GP (2015). Dynamic aberrant NF-κB spurs tumorigenesis: A new model encompassing the microenvironment. Cytokine & Growth Factor Reviews 26(4), 389–403 10.1016/j.cytogfr.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Song N, Yu T, Zhou L, Zhang H, Duan L, … Zhu M (2014). Expression of tumor necrosis factor-alpha-mediated genes predicts recurrence-free survival in lung cancer. PLoS One 9(12), e115945 10.1371/journal.pone.0115945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DJ, Ratnam NM, Byrd JC, & Guttridge DC (2014). NF-κB functions in tumor initiation by suppressing the surveillance of both innate and adaptive immune cells. Cell Reports 9(1), 90–103. 10.1016/j.celrep.2014.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Li C, Qiao P, Xue Y, Zheng X, Chen H, … Ba X (2018). OGG1-initiated base excision repair exacerbates oxidative stress-induced parthanatos. Cell Death & Disease 9(6), 628 10.1038/s41419-018-0680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yang L, Huang F, Zhang Q, Liu S, Ma L, &You Z (2017). Inflammatory cytokines IL-17 and TNF-α up-regulate PD-L1 expression in human prostate and colon cancer cells. Immunology Letters 184, 7–14. 10.1016/j.imlet.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-F, Zhu Y-T, Wang J-J, Zeng D-X, Mu C-Y, Chen Y-B, … Huang J-A (2017). The prognostic value of interleukin-17 in lung cancer: A systematic review with meta-analysis based on Chinese patients. PLoS One 12(9), e0185168 10.1371/journal.pone.0185168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waqar SN, & Morgensztern D (2017). Treatment advances in small cell lung cancer (SCLC). Pharmacology & Therapeutics 180, 16–23. 10.1016/j.pharmthera.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Whitaker AM, Schaich MA, Smith MR, Flynn TS, & Freudenthal BD (2017). Base excision repair of oxidative DNA damage: From mechanism to disease. Frontiers in Bioscience (Landmark Edition) 22, 1493–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieshammer S, & Dreyhaupt J (2017). Smoking exposure, loss of forced expiratory volume in one second and the risk of lung cancer among patients with malignant disease who present with cardiac or pulmonary symptoms: A cross-sectional study. Tobacco Induced Diseases 15, 16 10.1186/s12971-017-0122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Liang L, Huang M, Qiu Q, Zeng S, Shi M, … Xu H (2016). Bromodomain and extra-terminal domain bromodomain inhibition prevents synovial inflammation via blocking IκB kinase-dependent NF-κB activation in rheumatoid fibroblast-like synoviocytes. Rheumatology (Oxford, England) 55(1), 173–184. 10.1093/rheumatology/kev312. [DOI] [PubMed] [Google Scholar]
- Xiong H-Y, Ma T-T, Wu B-T, Lin Y, & Tu Z-G (2014). IL-12 regulates B7-H1 expression in ovarian cancer-associated macrophages by effects on NF-κB signalling. Asian Pacific Journal of Cancer Prevention: APJCP 15(14), 5767–5772. [DOI] [PubMed] [Google Scholar]
- Xu CH, Yu L, Zhan P, & Zhang Y (2014). Elevated pleural effusion IL-17 is a diagnostic marker and outcome predictor in lung cancer patients. European Journal of Medical Research 19, 23 10.1186/2047-783X-19-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Buczkowski KA, Zhang Y, Asahina H, Beauchamp EM, Terai H, … Hammerman PS (2015). NSCLC Driven by DDR2 Mutation is Sensitive to Dasatinib and JQ1 Combination Therapy. Molecular Cancer Therapeutics 14(10), 2382–2389. 10.1158/1535-7163.MCT-15-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Yu L, & Zhang X (2013). Association between the hOGG1 Ser326Cys polymorphism and lung cancer susceptibility: A meta-analysis based on 22,475 subjects. Diagnostic Pathology 8, 144 10.1186/1746-1596-8-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhang Y, Shan W, Hu Z, Yuan J, Pi J, … Zhang L (2017). Repression of BET activity sensitizes homologous recombination-proficient cancers to PARP inhibition. Science Translational Medicine 9(400). 10.1126/scitranslmed.aal1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Wu H, Zhao J, Wu X, Zhao J, Ning Q, … Xie J (2014). Feasibility of 8-OHdG formation and hOGG1 induction in PBMCs for assessing oxidative DNA damage in the lung of COPD patients. Respirology (Carlton, Vic.) 19(8), 1183–1190. 10.1111/resp.12378. [DOI] [PubMed] [Google Scholar]
- Yew-Booth L, Birrell MA, Lau MS, Baker K, Jones V, Kilty I, & Belvisi MG (2015). JAK-STAT pathway activation in COPD. The European Respiratory Journal 46(3), 843–845. 10.1183/09031936.00228414. [DOI] [PubMed] [Google Scholar]
- Yin Z-B, Hua R-X, Li J-H, Sun C, Zhu J-H, Su X., … Hua Z-M (2014). Smoking and hOGG1 Ser326Cys polymorphism contribute to lung cancer risk: Evidence from a meta-analysis. Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine 35(2), 1609–1618. 10.1007/s13277-013-1222-0. [DOI] [PubMed] [Google Scholar]
- Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, … Zhang H-G (2007). Tumor exosomes inhibit differentiation of bone marrow dendritic cells. Journal of Immunology (Baltimore, Md.: 1950) 178(11), 6867–6875. [DOI] [PubMed] [Google Scholar]
- Yuan M , Zhu H, Xu J, Zheng Y, Cao X, & Liu Q (2016). Tumor-Derived CXCL1 Promotes Lung Cancer Growth via Recruitment of Tumor-Associated Neutrophils. Journal of Immunology Research 2016, 6530410 10.1155/2016/6530410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaynagetdinov R, Sherrill TP, Gleaves LA, Hunt P, Han W, McLoed AG, … Blackwell TS (2016). Chronic NF-κB activation links COPD and lung cancer through generation of an immunosuppressive microenvironment in the lungs. Oncotarget 7(5), 5470–5482. 10.18632/oncotarget.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Zhang Q, Wang H, Lu M, Kong H, Zhang Y, & Shi H (2015). Prognostic significance of interleukin-17 in solid tumors: A meta-analysis. International Journal of Clinical and Experimental Medicine 8(7), 10515–10536. [PMC free article] [PubMed] [Google Scholar]
- Zerfaoui M, Suzuki Y, Naura AS, Hans CP, Nichols C, & Boulares AH (2008). Nuclear translocation of p65 NF-kappaB is sufficient for VCAM-1, but not ICAM-1, expression in TNF-stimulated smooth muscle cells: Differential requirement for PARP-1 expression and interaction. Cellular Signalling 20(1), 186–194. 10.1016/j.cellsig.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G-Q, Han F, Fang X-Z, & Ma X-M (2012). CD4+, IL17 and Foxp3 expression in different pTNM stages of operable non-small cell lung cancer and effects on disease prognosis. Asian Pacific Journal of Cancer Prevention: APJCP 13(8), 3955–3960. [DOI] [PubMed] [Google Scholar]
- Zhang N, Zeng Y, Du W, Zhu J, Shen D, Liu Z, & Huang J-A (2016). The EGFR pathway is involved in the regulation of PD-L1 expression via the IL-6/JAK/STAT3 signaling pathway in EGFR-mutated non-small cell lung cancer. International Journal of Oncology 49(4), 1360–1368. 10.3892/ijo.2016.3632. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li J, Li Y, Liu Y, Guo H, & Xu X (2017). Nitric Oxide Synthase activity Correlates with OGG1 in Ozone-Induced Lung Injury Animal Models. Frontiers in Physiology 8, 249 10.3389/fphys.2017.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zeng Y, Qu Q, Zhu J, Liu Z, Ning W, … Huang J-A (2017). PD-L1 induced by IFN-γ from tumor-associated macrophages via the JAK/STAT3 and PI3K/AKT signaling pathways promoted progression of lung cancer. International Journal of Clinical Oncology 22(6), 1026–1033. 10.1007/s10147-017-1161-7. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Lei Y, & Wang C (2012). Analysis of aberrant methylation in DNA repair genes during malignant transformation of human bronchial epithelial cells induced by cadmium. Toxicological Sciences: An Official Journal of the Society of Toxicology 125(2), 412–417. 10.1093/toxsci/kfr320. [DOI] [PubMed] [Google Scholar]
- Zou Z, Huang B, Wu X, Zhang H, Qi J, Bradner J, … Chen L-F (2014). Brd4 maintains constitutively active NF-κB in cancer cells by binding to acetylated RelA. Oncogene 33 (18), 2395–2404. 10.1038/onc.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]


