Abstract
Exercise is regarded as a potent stimulus in modulation of glucose utility and mitochondrial adaptations in skeletal muscle, leading to enhanced metabolic health. As mitochondria play a crucial role in sustaining metabolic homeostasis, and disturbances in mitochondrial function are highly linked with development of metabolic diseases, a comprehensive understanding of exercise-mediated mitochondrial remodeling under the pathophysiological condition of type 2 diabetes is warranted to develop an efficient therapeutic strategy. Although it is evident that the primary etiology of type 2 diabetes is insulin resistance, there is accumulating evidence linking abnormal mitochondrial functional and morphological properties to development of type 2 diabetes. Despite this, the precise molecular and cellular events that underline these phenomena remain uncertain. Mitochondria are highly dynamic subcellular organelles that can change mass and shape as necessary via coordinated processes such as mitochondrial fusion, fission, and biogenesis. Mitochondrial fusion is controlled by proteins, including mitofusin-1, mitofusin-2, and optic atrophy protein 1, while the fission process is mainly modulated by control of fission protein 1 and dynamin-related protein 1. Peroxisome proliferator-activated receptor gamma coactivator-1α acts as a master controller of mitochondrial biogenesis. The present review’s primary aims were to briefly discuss the cellular mechanisms of muscle fiber type-dependent glucose uptake and to highlight emerging evidence linking disturbances in mitochondrial dynamics to development of insulin resistance and type 2 diabetes. The potential for exercise to normalize type 2 diabetes-induced aberrant mitochondrial integrity is also addressed.
Keywords: Mitochondrial dynamics, Mitochondrial biogenesis, Exercise, Type 2 diabetes mellitus
INTRODUCTION
Although direct causality between mitochondria and insulin resistance is still under investigation, a wide spectrum of evidence indicates that mitochondrial dysfunction is associated with increased insulin resistance in skeletal muscle.1,2 Muscle insulin resistance is the manifestation of type 2 diabetes, as skeletal muscle is the largest human organ and is involved in approximately one third of whole body energy metabolism at rest and up to 90% during active exercise.3 As the mitochondrion is an energy powerhouse responsible for complete oxidation of glucose- and fat-derived metabolites to generate adenosine triphosphate (ATP)4, skeletal muscle bioenergetics is considered to be a major factor in metabolic health. Skeletal muscle mitochondria are highly interconnected as a form of reticulum that promotes the relocation of substrates and metabolites to bioenergetically active areas within mitochondria.5 Thus, it is believed that, along with increases in mitochondrial mass, changes in mitochondrial morphology via fusion and fission are significantly related with development of metabolism-related diseases.
Exercise is a potent and nonpharmaceutical intervention for management and treatment of a wide spectrum of lifestyle-related diseases.6,7 While the therapeutic effects of exercise are unequivocal, the exercise-mediated molecular responses that modulate mitochondrial dynamics under type 2 diabetes remain relatively less known. In this miniature review, we will briefly introduce how plasma glucose is absorbed into skeletal muscle. We will also emphasize major molecular mechanisms between mitochondrial biogenesis, fusion, and fission, with respect to how mitochondria dynamics are dysregulated under type 2 diabetes and how exercise may orchestrate the mitochondrial reticulum.
INSULIN-DEPENDENT GLUCOSE UPTAKE IN SKELETAL MUSCLE
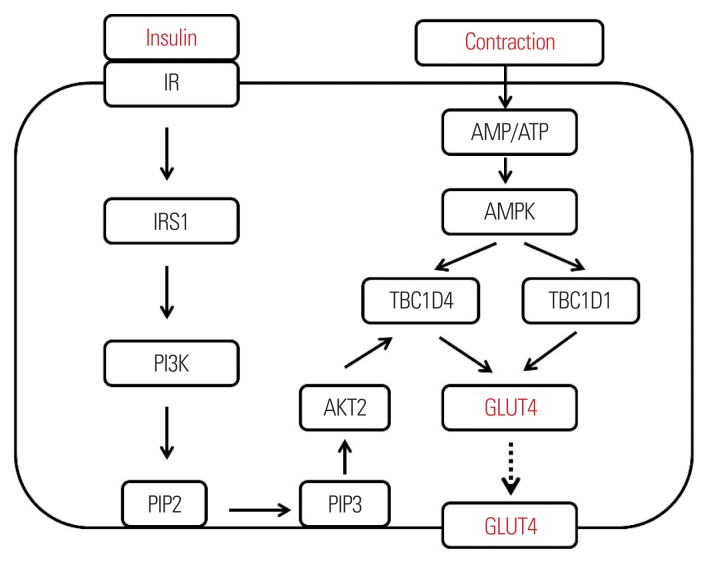
It has been well documented that pancreas-secreted insulin initiates the transportation of plasma glucose into myofibers via a series of molecular signaling cascades.8 This cellular process is accomplished by relocating glucose transporter type 4 (GLUT4) to the plasma membrane (Fig. 1). Briefly, upon insulin binding to the insulin receptor located on the periphery of skeletal muscle cells, the insulin receptor is autophosphorylated to induce subsequent activation of insulin receptor substrate 1 (IRS1) and PI3 kinase.8 This promotes the conversion of phosphatidylinositol 4,5-bisphosphate to phosphatidylinositol 3,4,5-triphosphate at the plasma membrane, which ultimately induces phosphorylation and conformational changes of protein kinase B2 (AKT2; a master controller of GLUT4).9 Once AKT2 is activated, cytosolic GLUT4 is translocalized to the plasma membrane with the help of signal-relaying molecules to induce intake of plasma glucose into myofibers.10,11
Figure 1.
Independent modulation of insulin- and muscle contraction-induced glucose uptake into myofibers. The line arrows indicate activation of a series of signaling pathways. The dotted arrow indicates translocalization of GLUT4. IR, insulin receptor; IRS1, insulin receptor substrate 1; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; PIP2, phosphatidylinositol 4,5-bisphosphate; AMP, 5′ adenosine monophosphate; ATP, adenosine triphosphate; AMPK, AMP-activated protein kinase; TBC1D4 and 1, tre-2/USP6, BUB2, cdc16 domain family members 4 and 1; AKT2, protein kinase B2; GLUT4, glucose transporter type 4; PIP3, phosphatidylinositol 3,4,5-bisphosphate.
MUSCLE CONTRACTION-INDUCED GLUCOSE UPTAKE IN SKELETAL MUSCLE
Skeletal muscle contraction has been identified as a potent independent stimulus to induce glucose uptake into myofibers, which is also mainly modulated by the expression and translocation of GLUT4.12 Interestingly, although it is evident that insulin sensitivity is improved by exercise and muscle contraction13, the major mechanism(s) by which muscle contraction increases the rate of glucose uptake is somewhat independent of insulin action. This notion has been documented by several lines of evidence indicating that deletion of major insulin signaling markers (IRS1 and AKT2) does not diminish muscle contraction-induced glucose uptake14,15 and insulin combined with muscle contraction stimulates glucose uptake into myofibers synergistically.16,17 There are potent molecular markers initiating insulin-independent glucose uptake in skeletal muscle. Among them, 5′ adenosine monophosphate-activated protein kinase (AMPK) has garnered significant attention in the past few decades. AMPK consists of an α catalytic subunit and two types of regulatory subunits (β and γ).18 All three types of subunits work in coordination for maximal activation of the enzyme.19 The enzymatic activation of this kinase is primarily induced by energy-demanding conditions such as skeletal muscle contraction and orchestrates an energy-related network via minimizing the ATP-consuming pathway and maximizing the ATP-generating pathway (i.e., glucose uptake, mitochondrial biogenesis, and remodeling).8 In regard to glucose uptake, the critical downstream molecules of AMPK are tre-2/USP6, BUB2, cdc16 domain family members 1 and 4 (TBC1D1 and TBC1D4).20,21 AMPK-induced phosphorylation of specific sites of TBC1D1 and TBC1D4 leads to translocation of glucose transporter (GLUT4) to the myofiber membrane.22,23 Once GLUT4 is incorporated into the membrane, plasma glucose is funneled into myofiber through GLUT4 by passive diffusion. When myofibers are under an oxygen-rich condition, glucose-derived metabolites are further processed in mitochondria for complete oxidation.24 Therefore, the enhanced quality of mitochondria is considered to be linked with muscle glucose uptake and metabolism in a coordinated manner.
SKELETAL MUSCLE FIBER TYPE AND GLUCOSE UTILIZATION
Although skeletal muscle, the largest organ of the body, is considered to be a significant contributor to modulation of glucose uptake and metabolism25, individual muscles may contribute differently based on fiber type composition.26 Type I myofibers embedded with abundant mitochondria have a higher glucose-processing capacity, mainly due to a greater mitochondrial oxidative capacity for substrate utilization.27 On the contrary, type II muscle fibers embedded with less mitochondria are considered to be less insulin-sensitive and contribute less to substrate oxidation.28 This notion has been further supported by a human biopsy study of greater insulin responsiveness in slow-twitch fiber.29 Aerobic exercise has been consistently reported as a potential strategy to increase mitochondrial oxidative capacity in skeletal muscle and induce the transition of myofiber to more oxidative traits.30 In that regard, a physically active lifestyle and regular aerobic exercise are warranted to prevent or manage type 2 diabetes.
MITOCHONDRIAL BIOGENESIS AND TYPE 2 DIABETES
Skeletal muscle is the dominant site of both insulin-dependent and -independent glucose utilization in the body. While glucose could be metabolized in cytoplasm as a rapid form of glycolysis, a majority of glucose-derived metabolites is funneled into the mitochondrial matrix for oxidative metabolism through a series of enzymatic processes to generate ATP.31 Therefore, disrupted mitochondrial biogenesis is considered to diminish the ability of skeletal muscle to oxidize glucose-derived substrates and can have negative consequences on glucose uptake in skeletal muscle.32
A wide variety of signaling molecules serve as a platform for orchestrating the coordinated assembly of mitochondrial reticulum. In particular, the peroxisome proliferator-activated receptor-gamma coactivator-1 (PGC-1) family has garnered significant attention. Among them, PGC-1α has been extensively studied due to its versatile and dynamic ability to induce the expression of mitochondrial biogenesis- and substrate oxidation-related genes in both nuclear and mitochondrial genomes.33 This complex process is initiated via deacetylation of PGC-1α, followed by its individual binding to multiple transcription factors. When combined with certain nuclear transcription factors (i.e., NRF-1 and NRF-2), PGC-1α guides them to translocate from the cytoplasmic region to specific promoter sites of nuclear DNA, allowing for the expression of numerous mitochondrial components.34 Although a vast majority of mitochondrial genes (~1,300) are transcriptionally expressed in nuclear DNA, a separate set of 13 mitochondrial genes is encoded in a circular form of the mitochondrial genome.35 When PGC-1α is connected to mitochondrial transcription factor A in cytoplasm, they localize to mitochondrial DNA, increasing the number of mitochondrial DNA copies.36 Therefore, PGC-1α is considered as a master regulator of mitochondrial biogenesis.
There are a series of studies reporting that deficiencies in mitochondrial content, oxidative phosphorylation, and substrate oxidation are observed in skeletal muscles of subjects with types 2 diabetes and metabolic syndrome.37–39 The reduced mitochondrial contents and function are retained even following biopsy-derived cultures of myocytes obtained from individuals with type 2 diabetes.40 These changes were linked with reduced mitochondrial mass and density, suggesting that PGC-1α may be an early biomarker in metabolic diseases.34,41 This notion is further supported by a study indicating that rodents abundant with PGC-1α tend to have not only higher mitochondrial capacity, but also greater glucose utilization41, suggesting the importance and implication of exercise-induced PGC-1α in skeletal muscles.
MITOCHONDRIAL DYNAMICS AND TYPE 2 DIABETES
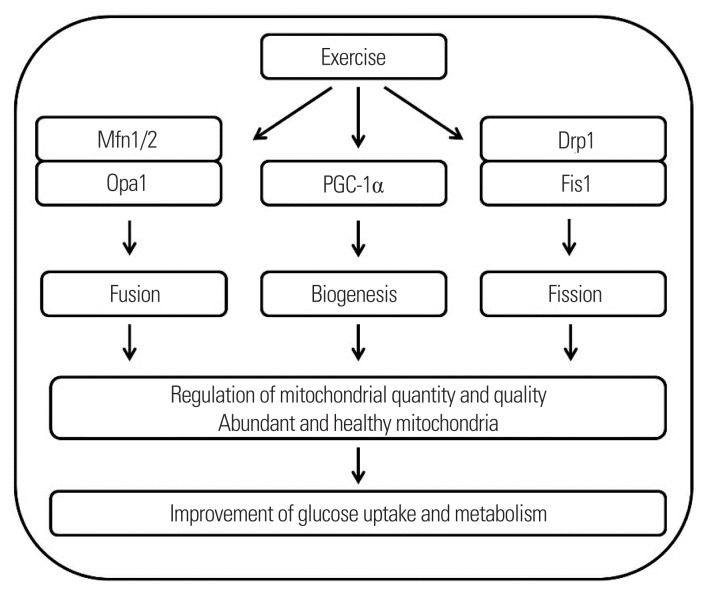
Mitochondrion, a cellular energy power house, changes its shape, size, and location to adapt to fluctuating energetic demands.42 These changes are accomplished through the coordinated cycles of mitochondrial biogenesis, fission, and fusion.43 Mitochondrial biogenesis is defined as the addition of new mitochondria. Mitochondrial fusion produces large interconnected reticulum of mitochondria, whereas small fragmented mitochondria are created via a process called fission. Balanced coordination of these complex networks is essential for maximizing mitochondrial efficiency, suggesting that mitochondrial quality control is as important as mitochondrial quantity for substrate oxidation (Fig. 2). Briefly, mitochondrial fusion is mainly modulated by a group of GTPases identified as mitofusins (Mfns) and optic atrophy protein 1 (Opa1).44 Mfn1/2 are required to fuse the outer mitochondrial membrane, while Opa1 induces fusion of the mitochondrial inner membrane.44 Dynamin-related protein 1 and fission protein 1 (Fis1) are recruited to the outer mitochondrial membrane to induce mitochondrial division.45 Cellular events of fission and fusion are tightly monitored and modulated under normal physiological conditions, as imbalances in mitochondrial dynamics lead to the development of various types of diseases including metabolic diseases.46,47 It has been reported that the morphological structure of mitochondria is different in patients with type 2 diabetes versus in healthy individuals.48 This suggests that aberrant mitochondrial morphology is related with key remodeling proteins. In line with this idea, the protein expression of mitochondrial fusion (Mfn2 and Opa1) has been reported to be reduced in skeletal muscles of patients with type 2 diabetes.48,49 Additionally, animal models of dysfunctional Mfn2 demonstrate reduced substrate metabolism50, whereas overexpression of Mfn2 and Opa1 restores mitochondrial respiration efficiency, glucose oxidation, and insulin resistance.51,52 Although it seems to be evident that aberrant mitochondrial dynamics are associated with glucose metabolism and insulin resistance, several studies have shown that aberrant mitochondrial abnormity is not observed in skeletal muscles with type 2 diabetes53,54, inducing an active discussion of whether mitochondrial abnormality is a consequence or cause of type 2 diabetes. Therefore, future studies with an integrative approach (cell to human) are warranted to delineate the specific mechanisms of mitochondrial dynamics in type 2 diabetes.
Figure 2.
Schematic diagram of exercise-mediated mitochondrial quality and quantity control in skeletal muscle and its implications in the improvement of glucose uptake and metabolism. The arrows indicate molecular and physiological processes. Mfn, mitofusin; Opa1, optic atrophy protein 1; PGC-1α, proliferator-activated receptor-gamma coactivator-1α; Drp1, dynamin-related protein 1; Fis1, fission protein 1.
TARGETING MITOCHONDRIAL DYNAMICS THROUGH EXERCISE
Although exercise-induced mitochondrial biogenesis has been well documented, the effects of exercise on mitochondrial dynamics in skeletal muscle have been less extensively explored. Several recent studies have indicated that skeletal muscle contraction appears to have a capacity to modulate both mitochondrial biogenesis and dynamics in a coordinated manner, suggesting that exercise and physical activity modulate not only mitochondrial quantity, but also quality in skeletal muscle in a synergistic manner. For example, high-intensity aerobic exercise has been demonstrated to induce the protein expression of mitochondrial fusion (Mfn1) and fission (Fis1).55 In agreement with this, a single bout of aerobic exercise increased the messenger RNA expression of Mfn1 and Mfn2 in the skeletal muscles of both rodents and humans.56,57 However, the specific upstream mechanism regulating these processes during exercise remains largely unknown. In line with this, although it was tested in an in vitro condition, a novel study showed that mitochondrial fusion is regulated by PGC-1α via estrogen-related receptor α.56 Given that exercise-induced expression of PGC-1α precedes induction of Mfn1 and Mfn256, it is highly likely that not only might mitochondrial biogenesis, but also the dynamics of mitochondrial fusion and fission be regulated by PGC-1α.58 Based on previous research indicating that aberrant mitochondrial integrity is associated with development of type 2 diabetes and that increased expression of PGC-1α in skeletal muscle is a central dogma in exercise physiology, identifying the optimal exercise volume and intensity to maximize induction of PGC-1α in skeletal muscle is warranted to provide a potential nonpharmaceutical strategy for patients with type 2 diabetes.
CONCLUSION
The regulation of glucose uptake and metabolism in skeletal muscle is significantly controlled in a mitochondrial health-dependent manner, and aberrant mitochondrial functional properties in skeletal muscle are linked with development of type 2 diabetes. The quantity and quality of mitochondria are modulated via a series of coordinated cellular processes of mitochondrial biogenesis, fusion, and fission. Multiple studies discussed in this review suggest that the major signaling proteins controlling mitochondrial remodeling are dysregulated in the skeletal muscle of type 2 diabetes, and that exercise has a great potential to regulate not only mitochondrial biogenesis, but also dynamics, improving the overall quality of these organelles in diabetic skeletal muscle. While the therapeutic effects of exercise are unequivocal, the exercise mode-, intensity-, and volume-mediated molecular responses modulating mitochondrial dynamics in patients with type 2 diabetes remain relatively less identified. In that regard, it will be crucial that future research be devoted to elucidating the interplay between specific exercise protocols, mitochondrial dynamics, and type 2 diabetes to provide a stepping stone for development of a novel therapeutic exercise strategy.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning (NRF-2013M3A9B6046417, Korea Mouse Phenotyping Project NRF-2013M3A9D5072550, 2013M3A9D5072560, 2017M3A9D5A01052447, and MEST 2011-030135).
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Martin SD, McGee SL. The role of mitochondria in the aetiology of insulin resistance and type 2 diabetes. Biochim Biophys Acta. 2014;1840:1303–12. doi: 10.1016/j.bbagen.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2011;8:92–103. doi: 10.1038/nrendo.2011.138. [DOI] [PubMed] [Google Scholar]
- 3.Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest. 1990;86:1423–7. doi: 10.1172/JCI114857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szendroedi J, Schmid AI, Chmelik M, Toth C, Brehm A, Krssak M, et al. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med. 2007;4:e154. doi: 10.1371/journal.pmed.0040154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glancy B, Hartnell LM, Malide D, Yu ZX, Combs CA, Connelly PS, et al. Mitochondrial reticulum for cellular energy distribution in muscle. Nature. 2015;523:617–20. doi: 10.1038/nature14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care. 2010;33:2692–6. doi: 10.2337/dc10-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1433–8. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- 8.O’Neill HM. AMPK and exercise: glucose uptake and insulin sensitivity. Diabetes Metab J. 2013;37:1–21. doi: 10.4093/dmj.2013.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–51. doi: 10.1002/j.1460-2075.1996.tb01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peck GR, Chavez JA, Roach WG, Budnik BA, Lane WS, Karlsson HK, et al. Insulin-stimulated phosphorylation of the Rab GTPase-activating protein TBC1D1 regulates GLUT4 translocation. J Biol Chem. 2009;284:30016–23. doi: 10.1074/jbc.M109.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sano H, Kane S, Sano E, Mîinea CP, Asara JM, Lane WS, et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278:14599–602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 12.Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93:993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- 13.Holloszy JO. Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol (1985) 2005;99:338–43. doi: 10.1152/japplphysiol.00123.2005. [DOI] [PubMed] [Google Scholar]
- 14.Dumke CL, Wetter AC, Arias EB, Kahn CR, Cartee GD. Absence of insulin receptor substrate-1 expression does not alter GLUT1 or GLUT4 abundance or contraction-stimulated glucose uptake by mouse skeletal muscle. Horm Metab Res. 2001;33:696–700. doi: 10.1055/s-2001-19141. [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto K, Arnolds DE, Fujii N, Kramer HF, Hirshman MF, Goodyear LJ. Role of Akt2 in contraction-stimulated cell signaling and glucose uptake in skeletal muscle. Am J Physiol Endocrinol Metab. 2006;291:E1031–7. doi: 10.1152/ajpendo.00204.2006. [DOI] [PubMed] [Google Scholar]
- 16.King PA, Betts JJ, Horton ED, Horton ES. Exercise, unlike insulin, promotes glucose transporter translocation in obese Zucker rat muscle. Am J Physiol. 1993;265(2 Pt 2):R447–52. doi: 10.1152/ajpregu.1993.265.2.R447. [DOI] [PubMed] [Google Scholar]
- 17.Brozinick JT, Jr, Etgen GJ, Jr, Yaspelkis BB, 3rd, Ivy JL. Contraction-activated glucose uptake is normal in insulin-resistant muscle of the obese Zucker rat. J Appl Physiol (1985) 1992;73:382–7. doi: 10.1152/jappl.1992.73.1.382. [DOI] [PubMed] [Google Scholar]
- 18.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dyck JR, Gao G, Widmer J, Stapleton D, Fernandez CS, Kemp BE, et al. Regulation of 5′-AMP-activated protein kinase activity by the noncatalytic beta and gamma subunits. J Biol Chem. 1996;271:17798–803. doi: 10.1074/jbc.271.30.17798. [DOI] [PubMed] [Google Scholar]
- 20.Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem. 2006;281:31478–85. doi: 10.1074/jbc.M605461200. [DOI] [PubMed] [Google Scholar]
- 21.Frøsig C, Pehmøller C, Birk JB, Richter EA, Wojtaszewski JF. Exercise-induced TBC1D1 Ser237 phosphorylation and 14-3-3 protein binding capacity in human skeletal muscle. J Physiol. 2010;588(Pt 22):4539–48. doi: 10.1113/jphysiol.2010.194811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, et al. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes. 2006;55:2067–76. doi: 10.2337/db06-0150. [DOI] [PubMed] [Google Scholar]
- 23.An D, Toyoda T, Taylor EB, Yu H, Fujii N, Hirshman MF, et al. TBC1D1 regulates insulin- and contraction-induced glucose transport in mouse skeletal muscle. Diabetes. 2010;59:1358–65. doi: 10.2337/db09-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montgomery MK, Turner N. Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect. 2015;4:R1–15. doi: 10.1530/EC-14-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng JM, Azuma K, Kelley C, Pencek R, Radikova Z, Laymon C, et al. PET imaging reveals distinctive roles for different regional adipose tissue depots in systemic glucose metabolism in nonobese humans. Am J Physiol Endocrinol Metab. 2012;303:E1134–41. doi: 10.1152/ajpendo.00282.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner N, Cooney GJ, Kraegen EW, Bruce CR. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J Endocrinol. 2014;220:T61–79. doi: 10.1530/JOE-13-0397. [DOI] [PubMed] [Google Scholar]
- 27.Albers PH, Pedersen AJ, Birk JB, Kristensen DE, Vind BF, Baba O, et al. Human muscle fiber type-specific insulin signaling: impact of obesity and type 2 diabetes. Diabetes. 2015;64:485–97. doi: 10.2337/db14-0590. [DOI] [PubMed] [Google Scholar]
- 28.Nyholm B, Qu Z, Kaal A, Pedersen SB, Gravholt CH, Andersen JL, et al. Evidence of an increased number of type IIb muscle fibers in insulin-resistant first-degree relatives of patients with NIDDM. Diabetes. 1997;46:1822–8. doi: 10.2337/diab.46.11.1822. [DOI] [PubMed] [Google Scholar]
- 29.Stuart CA, McCurry MP, Marino A, South MA, Howell ME, Layne AS, et al. Slow-twitch fiber proportion in skeletal muscle correlates with insulin responsiveness. J Clin Endocrinol Metab. 2013;98:2027–36. doi: 10.1210/jc.2012-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan Z, Okutsu M, Akhtar YN, Lira VA. Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J Appl Physiol (1985) 2011;110:264–74. doi: 10.1152/japplphysiol.00993.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Picard M, Hepple RT, Burelle Y. Mitochondrial functional specialization in glycolytic and oxidative muscle fibers: tailoring the organelle for optimal function. Am J Physiol Cell Physiol. 2012;302:C629–41. doi: 10.1152/ajpcell.00368.2011. [DOI] [PubMed] [Google Scholar]
- 32.Joseph AM, Hood DA. Relationships between exercise, mitochondrial biogenesis and type 2 diabetes. Med Sport Sci. 2014;60:48–61. doi: 10.1159/000357335. [DOI] [PubMed] [Google Scholar]
- 33.Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab. 2012;23:459–66. doi: 10.1016/j.tem.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonen A. PGC-1alpha-induced improvements in skeletal muscle metabolism and insulin sensitivity. Appl Physiol Nutr Metab. 2009;34:307–14. doi: 10.1139/H09-008. [DOI] [PubMed] [Google Scholar]
- 35.Alston CL, Rocha MC, Lax NZ, Turnbull DM, Taylor RW. The genetics and pathology of mitochondrial disease. J Pathol. 2017;241:236–50. doi: 10.1002/path.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collu-Marchese M, Shuen M, Pauly M, Saleem A, Hood DA. The regulation of mitochondrial transcription factor A (Tfam) expression during skeletal muscle cell differentiation. Biosci Rep. 2015;35:e00221. doi: 10.1042/BSR20150073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277(6 Pt 1):E1130–41. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 38.Sreekumar R, Nair KS. Skeletal muscle mitochondrial dysfunction & diabetes. Indian J Med Res. 2007;125:399–410. [PubMed] [Google Scholar]
- 39.Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1039–44. doi: 10.1152/ajpendo.2000.279.5.E1039. [DOI] [PubMed] [Google Scholar]
- 40.Gaster M, Rustan AC, Aas V, Beck-Nielsen H. Reduced lipid oxidation in skeletal muscle from type 2 diabetic subjects may be of genetic origin: evidence from cultured myotubes. Diabetes. 2004;53:542–8. doi: 10.2337/diabetes.53.3.542. [DOI] [PubMed] [Google Scholar]
- 41.Summermatter S, Shui G, Maag D, Santos G, Wenk MR, Handschin C. PGC-1α improves glucose homeostasis in skeletal muscle in an activity-dependent manner. Diabetes. 2013;62:85–95. doi: 10.2337/db12-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wai T, Langer T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol Metab. 2016;27:105–17. doi: 10.1016/j.tem.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Ni HM, Williams JA, Ding WX. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015;4:6–13. doi: 10.1016/j.redox.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anesti V, Scorrano L. The relationship between mitochondrial shape and function and the cytoskeleton. Biochim Biophys Acta. 2006;1757:692–9. doi: 10.1016/j.bbabio.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 45.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–56. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Züchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36:449–51. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- 47.Waterham HR, Koster J, van Roermund CW, Mooyer PA, Wanders RJ, Leonard JV. A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med. 2007;356:1736–41. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- 48.Bach D, Naon D, Pich S, Soriano FX, Vega N, Rieusset J, et al. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes. 2005;54:2685–93. doi: 10.2337/diabetes.54.9.2685. [DOI] [PubMed] [Google Scholar]
- 49.Joseph AM, Adhihetty PJ, Buford TW, Wohlgemuth SE, Lees HA, Nguyen LM, et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell. 2012;11:801–9. doi: 10.1111/j.1474-9726.2012.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–92. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 51.Pich S, Bach D, Briones P, Liesa M, Camps M, Testar X, et al. The Charcot-Marie-Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Hum Mol Genet. 2005;14:1405–15. doi: 10.1093/hmg/ddi149. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Z, Wakabayashi N, Wakabayashi J, Tamura Y, Song WJ, Sereda S, et al. The dynamin-related GTPase Opa1 is required for glucose-stimulated ATP production in pancreatic beta cells. Mol Biol Cell. 2011;22:2235–45. doi: 10.1091/mbc.e10-12-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsøe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia. 2007;50:790–6. doi: 10.1007/s00125-007-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rabøl R, Højberg PM, Almdal T, Boushel R, Haugaard SB, Madsbad S, et al. Effect of hyperglycemia on mitochondrial respiration in type 2 diabetes. J Clin Endocrinol Metab. 2009;94:1372–8. doi: 10.1210/jc.2008-1475. [DOI] [PubMed] [Google Scholar]
- 55.Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol. 2010;588(Pt 23):4795–810. doi: 10.1113/jphysiol.2010.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cartoni R, Léger B, Hock MB, Praz M, Crettenand A, Pich S, et al. Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J Physiol. 2005;567(Pt 1):349–58. doi: 10.1113/jphysiol.2005.092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ding H, Jiang N, Liu H, Liu X, Liu D, Zhao F, et al. Response of mitochondrial fusion and fission protein gene expression to exercise in rat skeletal muscle. Biochim Biophys Acta. 2010;1800:250–6. doi: 10.1016/j.bbagen.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 58.Yan Z, Lira VA, Greene NP. Exercise training-induced regulation of mitochondrial quality. Exerc Sport Sci Rev. 2012;40:159–64. doi: 10.1097/JES.0b013e3182575599. [DOI] [PMC free article] [PubMed] [Google Scholar]