Abstract
Purpose
This study investigated whether filtering blebs can be evaluated using optical coherence tomography angiography (OCT-A) and compared vascularity parameters with conventional bleb grading systems.
Methods
A total of 92 patients with glaucoma, who underwent mitomycin C–augmented trabeculectomy, were enrolled in this study, and 92 eyes were assessed in total. The participants underwent OCT-A in external mode and anterior segment photography for bleb evaluation. For evaluation of bleb vascularity, a blinded observer carefully drew the bleb area on the original OCT-A image using a semiautomated program that calculated the color and brightness densities of the selected area. A blinded observer also classified the grades of the bleb vessels using the Indiana Bleb Appearance Grading Scale (IBAGS) and Moorfields Bleb Grading System (MBGS). The vascularity parameters using OCT-A were compared with the IBAGS and MBGS results. In addition, the correlation between intraocular pressure (IOP) and the bleb vascularity parameters was assessed.
Results
Vessel density measured by OCT-A demonstrated excellent inter- and intraobserver reproducibility. The color and brightness densities were positively correlated with the IBAGS and MBGS vascularity scores. There was no difference in accuracy when predicting IOP risk using vascularity scores from the IBAGS and MBGS or when estimating IOP risk using the color and brightness densities on the net reclassification index.
Conclusions
Bleb evaluation using OCT-A can evaluate vessel vascularity and showed correlation to the IBAGS and MBGS vascularity grading.
Translational Relevance
Bleb vascularity measurements using OCT-A could potentially provide objective and quantitative vessel parameters for bleb evaluation following trabeculectomy.
Keywords: bleb, Moorfields Bleb Grading System, optical coherence tomography angiography, trabeculectomy
Introduction
Trabeculectomy remains the standard surgical procedure for glaucoma when maximal pressure-lowering medical treatment or laser treatments fail to achieve the target intraocular pressure (IOP).1 The maintenance of a functional filtering bleb is an important aspect of the delicate care required after a successful trabeculectomy.2 Hence, the evaluation of bleb morphology is mandatory, not only for the glaucoma specialist but also for the general ophthalmologist. When signs that the bleb may be failing manifest, additional antiglaucoma medication, procedures such as needling with or without 5-fluorouracil (5-FU) or mitomycin C (MMC), or bleb revision are warranted to maintain the target IOP.3,4 Several studies have reported on the structural and morphological features of a successful bleb, which have led to the development of bleb grading systems.5–7 Two commonly used clinical grading systems are the Indiana Bleb Appearance Grading Scale (IBAGS)5 and Moorfields Bleb Grading System (MBGS).6,8 These grading systems include measurements of the bleb area, height, and vascularity, which are known as indicators of surgical success. In addition to these parameters, corkscrew vessels, dragged vessels and conjunctival suture line contraction, wall thickness, subconjunctival blood, microcysts, and bleb leakage are also associated with bleb prognosis.2,5–8
Recently, several studies have evaluated the microstructure of filtering blebs using imaging techniques such as ultrasound biomicroscopy9,10 and anterior segment optical coherence tomography (AS-OCT),11–22 which showed good objective results for evaluating bleb morphology, such as the bleb wall thickness, internal cavity, microcysts, subflap space, and internal ostium. These studies provided details on the internal structure of successful blebs.12–15 The increase in bleb vascularity and tortuous vessels on the bleb during external evaluation are signs of bleb failure, as fibrosis is related to wound remodeling.2,5,6,23 Hence, measuring bleb vascularity is an important index and early parameter to assess failure. Both IBAGS and MBGS present vessel-grading systems that are based on comparing standard photographs. Thus, as these methods are quite subjective and use categorical variables, a new method for more objective and quantitative analysis was required. The recently developed OCT-angiography (OCT-A) technique could be applied to address this17,24–26 and provide a basis for bleb evaluation to assess successful glaucoma surgery. Thus, the aim of the current study was, we believe for the first time, to analyze bleb vessels and to compare this quantitative bleb analysis using OCT-A with the vascularity scores from the conventional bleb grading systems.
Methods
This study was conducted on patients with glaucoma who attended the Pusan National University Yangsan Hospital Glaucoma Clinic from December 2016 to August 2017 and who satisfied the inclusion criteria. The study was approved by the Pusan National University Yangsan Hospital Institutional Review Board and conformed to the Declaration of Helsinki (IRB No. 05-2017-089).
Prior to the study, patients with glaucoma underwent a complete ophthalmological examination, including refraction, Goldmann applanation tonometry, gonioscopy, central corneal thickness measurement (Pachmate; DGH Technology Inc., Exton, PA), stereoscopic disc photography, red-free retinal nerve fiber layer (RNFL) photography, standard automated perimetry (Humphrey Field Analyzer 750 and Swedish Interactive Threshold Algorithm Standard; Carl Zeiss Meditec, Dublin, CA), and peripapillary RNFL thickness measurement by spectral domain OCT (Cirrus; Carl Zeiss Meditec). Axial lengths were measured (IOLMaster 500; Carl Zeiss Meditec AG, Jena, Germany).
The patients with glaucoma who were included in this study underwent swept-source OCT-A (DRI OCT-A Atlantis upgraded version [equivalent to Triton]; Topcon, Tokyo, Japan) using external mode and anterior segment photography (SL-D7 and FD-21; Topcon) for bleb evaluation from December 2016 to August 2017. These patients had also previously undergone trabeculectomy with adjunctive MMC at the Pusan National University Yangsan Hospital, which was applied on patients who were observed for more than 6 months after trabeculectomy. The reason for fixing the timeframe at 6 months at minimum was that wound healing required 2 to 3 months after trabeculectomy.27 The patients were diagnosed with primary glaucoma or pseudoexfoliation glaucoma, uveitic glaucoma, or steroid-induced glaucoma. Indications for surgery were based on the following situations: (1) IOP not adequately controlled with maximal tolerated medical or laser therapy or both and (2) allergy or intolerance to current medication.
Exclusion criteria included (1) patients who were not willing to undergo OCT-A for bleb evaluation, (2) poor OCT-A image quality that did not meet analysis due to eye movements or tight eyelids, and (3) patients who needed antiglaucoma medication or additional trabeculectomy due to bleb failure.
Trabeculectomy
An experienced glaucoma specialist (J.H.S.) performed all of the surgeries using the following method. A superior fornix-based conjunctiva flap was dissected from Tenon's tissue and sclera in the superotemporal or superonasal quadrant. A 4.0 × 3.0-mm-sized, partial-thickness, rectangular scleral flap was dissected at the 11 o'clock or 1 o'clock positions. MMC (0.4 mg/mL) was applied below the scleral and conjunctival flap for 2 to 3.5 minutes. The area exposed to MMC was irrigated with 10 mL balanced salt solution. The trabeculectomy was performed using Descemet's membrane punch (Katena Products Inc., Denville, NJ), followed by peripheral iridectomy. The scleral flap was closed using two fixed, watertight sutures with gentle tension, followed by closure of the Tenon's tissue and conjunctiva. Postoperative medication included prednisolone acetate 1% eye drops and moxifloxacin hydrochloride 0.5% eye drops four times a day for 1 month and twice a day for 2 months. Furthermore, 1% atropine sulfate hydrate eye drops were applied twice a day for 1 week. Laser suture lysis was performed in case of inadequate IOP control (target IOP) or a poor filtering bleb within the first 2 postoperative weeks. Our protocol for postoperative management in the event of IOP elevation over the target IOP involved the use of 5-FU injections or needlings with 5-FU initially. If three consecutive needlings with 5-FU failed or the patient did not want to undergo needling, we administered, in order, beta blockers, fixed combination dorzolamide/timolol therapy, and prostaglandin analogues. Patients receiving topical glaucoma eye drops were excluded from this study.
Bleb Evaluation Using OCT-A
The bleb was acquired by swept-source OCT-A using the external mode with 4.5 × 4.5-mm cube scan. Of the three scan sizes, that is, 3.0 × 3.0 mm, 4.5 × 4.5 mm, and 6.0 × 6.0 mm, the 3 × 3-mm cube scan was too small as the scleral flap size could be anywhere between 3 and 4 mm, and the 6 × 6-mm cube scan was too wide, leading to potential eye lid blocking issues; thus the 4.5 × 4.5-mm cube scan was most appropriate for this study. Swept-source OCT utilized 1050-nm wavelength, which enhanced visualization of deep structures with a scanning speed of 100,000 A-scans/s. As the OCT-A program (IMAGEnet 6; Topcon, Tokyo, Japan) did not provide vascularity density of the conjunctiva, we developed a custom Windows software with Microsoft Visual Studio 2012 (Microsoft, Redmond, WA) and C# language (Microsoft, Redmond, WA) with a dot net library to analyze new vessel density parameters, that is, color and brightness density. The color density was calculated from the density map (0 (blue) to 100 (red), according to IMAGEnet). The brightness density ranged from black to white based on the superficial vascular layer image. These vessel density parameters were unitless values that ranged from 0 to 100. The clinical implication of the color density was weighted mean vascular density, whereas brightness density measured vascular density on gray scale.
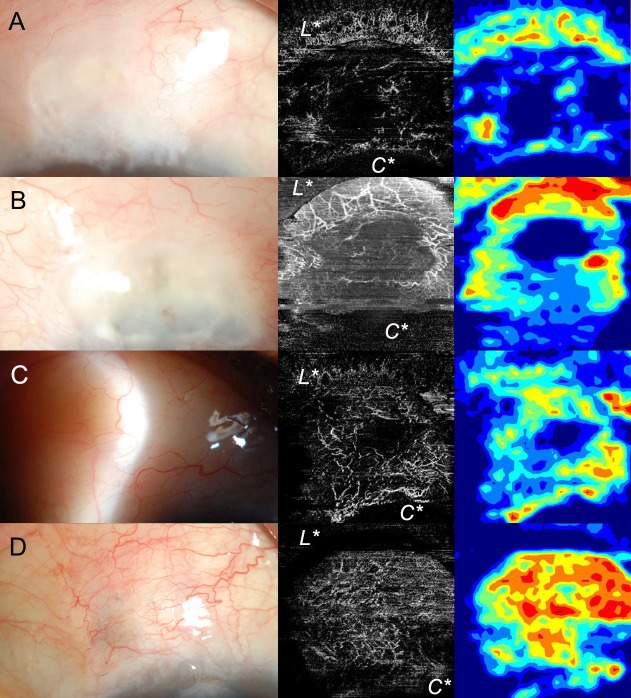
It required two image files, the superficial vascular layer image and color vessel density map, exported from the OCT-A instrument. After the superficial vascular layer image and density map (with “transparent box” checked off) were exported as JPEG images, the two images were used for analysis using a semiautomated program (Fig. 1). A blinded observer drew the bleb area in our program watching the overlay map and checking the delineation line on the IMAGEnet program (Fig. 1B, C). Next, our program was applied to calculate the number of pixels, color density, and brightness density of the selected area. The examiner drew up to six bleb areas, and of these, one was chosen as the area-major. The area-major was defined as the area that most accurately represented the shape of the bleb. Figure 2 shows representative cases with OCT-A images and matched anterior segment photographs of blebs.
Figure 1.
Measurement of vascularity parameters using OCT-A images. (A) Scanning in rectangular, external mode, OCT-A was used to focus on the bleb area. (B) The OCT-A shows an infrared image (see cross line–horizontal light-blue line and vertical pink line), whereas (C) the B-scan image depicts the scan layer (light-blue area, right-to-left horizontal scan of B). (D) Using OCT-A grayscale images and (E) density maps, a blinded observer drew the bleb area, following which, (F) the program calculated the color and brightness densities. The observer drew the bleb by checking the image using the reference lines in (B) and reliable segmentation area in (C). A skilled observer drew up to six bleb areas, one of which was chosen as the area-major and used for data analysis. In this figure, two bleb areas are drawn.
Figure 2.
Cases showing anterior segment photographs and images obtained using OCT-A for comparison with conventional grading systems. (A) V1 (avascular cystic) in IBAGS, grade 1 (avascular) in MBGS, 12.96 in color density, 8.26 in brightness density. (B) V1 (avascular cystic) in IBAGS, grade 1 (avascular) in MBGS, 17.07 in color density, 14.00 in brightness density. (C) Posteriorly located diffuse bleb, V2 (mild vascularity) in IBAGS, grade 2 (normal) in MBGS, 30.80 in color density, 13.33 in brightness density. (D) V3 (moderate vascularity) in IBAGS, grade 4 (moderate) in MBGS, 52.80 in color density, 19.32 in brightness density. C*, cornea; L*, lid.
Reproducibility of OCT Vascularity Parameters
Measurements of bleb vascularity indices (i.e., color and brightness density) were performed by an observer (J.H.S.), who was blinded to the clinical information of patients, including IOP and anterior segment photographs. To evaluate the interobserver reproducibility of our method, 30 randomly selected OCT-A datasets were evaluated by three independent examiners (J.H.S., K.H.P., and Y.A.K.). In order to calculate the intraobserver reproducibility of our software, 30 randomly selected subjects were evaluated repeatedly by the same observer (J.H.S.) at 1-month intervals. Both of the intraclass correlation coefficients (ICCs) were calculated.
Grading Using Conventional Bleb Grading Systems
Anterior segment photographs were taken with the patient gazing downward after bleb location was confirmed by a review of the surgical record. Photographs were taken on the same day to compare the bleb evaluation method of OCT-A with conventional vascular score grading systems, that is, IBAGS and MBGS. The photographs were independently graded by blinded observers (J.H.S. and Y.A.K.) using the vascularity classifications of IBAGS and MBGS. In IBAGS, the vascularity scores were classified as follows: V0, avascular white; V1, avascular cystic; V2, mild vascularity; V3, moderate vascularity; and V4, extensive vascularity.5 According to MBGS, the vascularity gradings were classified as follows: 1, avascular; 2, normal; 3, mild; 4, moderate; and 5, severe.6 It should be noted that this study did not utilize the full grading systems of IBAGS or MBGS and only compared the vascularity scores of these systems.
Comparison of the New Vessel Parameters and Conventional Bleb Grading Systems
In order to compare the new vessel parameters with the existing bleb grading methods, the agreement was analyzed and a cross table prepared. Agreement analysis of IBAGS and MBGS in the study was performed using weighted κ. Comparison of color and brightness density with grading according to the IBAGS and MBGS was analyzed using the Kendall rank correlation coefficient. A 1-way analysis of variance (ANOVA) with a post hoc test was conducted to compare vessel density parameters according to the IBAGS and MBGS scores.
The relationship between IOP and bleb vessel parameters measured by OCT-A was analyzed using Pearson's correlation coefficient, whereas the relationship between IOP and anterior segment photograph grading by IBAGS and MBGS was analyzed by the Kendall τ correlation coefficient. In addition, a linear regression analysis was performed to draw graphs. To compare the two correlation coefficients, the Kendall τ coefficient was converted into Pearson's correlation coefficient,28 following which, Steiger's Z-test was applied to compare the correlation coefficients of conventional bleb grading scores with IOP and new vascularity parameter values with IOP. In addition, the net reclassification index (NRI) was calculated, assuming an IOP cutoff value of 15 mm Hg, to compare the accuracy of the new vessel parameters and conventional bleb grading scores. Although an IOP > 15 mm Hg was an arbitrary criteria for NRI, some studies reporting on low-teen and high-teen IOP in glaucoma suggest this to be an possible cutoff.29–31 Using the decision tree classification based on IBAGS and MBGS, we predicted the appropriate cutoff values for color and brightness density.
Subgroup Analysis According to Anteriorly Located Bleb and Posteriorly Located Bleb
Since bleb location could affect OCT-A quality, we classified blebs into subgroups according to their location, that is, anteriorly located bleb and posteriorly located bleb. The anteriorly located bleb was defined as one for which the highest point of the bleb was within 4 mm of the limbus, whereas the posteriorly located bleb had its highest point 4 mm outside the limbus. This classification was related to the OCT cube scan area (4.5 × 4.5 mm). According to a previous study on the relationship between the location of filtration opening using AS-OCT and laser suture lysis,32 group analysis was performed to determine whether these bleb location differences would have an impact. Comparison of correlation between color density, and brightness density with grades from the IBAGS and MBGS were performed using a Fisher z test.
Data Analyses
All data analyses were performed by a statistical expert (Y.L.), using software (Statistical Package for Social Sciences, version 18.0; SPSS Inc., Chicago, IL, and R Statistical Package, version 3.4.1; R Foundation for Statistical Computing, Vienna, Austria), using the cocor, irr, PredictABEL, and party packages. Statistical significance was set at P < 0.05.
Results
The study initially involved 105 eyes from 105 participants who met the inclusion criteria. Of these, three participants were excluded because of poor-quality OCT-A images due to eye movement. A further six participants were excluded due to low image quality that prevented clear delineation, and four were excluded due to the use of topical glaucoma medication, leaving a final sample of 92 eyes from 92 participants. The study included 63 men and 29 women, with a mean age of 62.67 ± 13.65 years (Table 1). The mean best corrected visual acuity (BCVA) was 0.62 ± 0.76 logarithm of the minimum angle of resolution (logMAR), and mean spherical equivalent was −1.15 ± 1.90 diopters (D). The IOP decreased from 27.72 ± 8.92 mm Hg to 11.21 ± 2.77 mm Hg after the trabeculectomy. The visual field mean deviation was −20.11 ± 8.72 dB.
Table 1.
Demographic and Baseline Characteristics of Study Subjects
| Variables |
n = 92 |
| Age, y | 62.67 ± 13.65 |
| Male/female | 63/29 |
| BCVA, logMAR | 0.62 ± 0.76 |
| Diabetic mellitus, n (%) | 21 (22.88) |
| Systemic hypertension, n (%) | 32 (34.78) |
| Diagnosis, n (%) | |
| POAG/secondary glaucoma | 74 (80.43)/18 (19.57) |
| Period at the time of OCT and anterior segment photography after surgery, mo | 12.87 ± 6.19 |
| Preoperative IOP, mm Hg | 27.72 ± 8.92 |
| Postoperative IOP, mm Hga | 11.21 ± 2.77 |
| Spherical equivalents, diopters | −1.15 ± 1.90 |
| Central corneal thickness, μm | 542.41 ± 44.08 |
| Axial length, mm | 24.08 ± 1.38 |
| Visual field, MD, dB | −20.11 ± 8.72 |
| Visual field, VFI, % | 41.34 ± 32.20 |
BCVA, best corrected visual acuity; MD, mean deviation; POAG, primary open angle glaucoma; VFI, visual field index.
IOP at the time of OCT-A bleb scanning and anterior segment photography.
The ICC values of the color and brightness densities were 0.951 and 0.907, respectively, for the interobserver reproducibility and 0.961 and 0.934, respectively, for the intraobserver reproducibility of our method. These ICC values indicate excellent inter- and intraobserver reproducibilities. In this study, the mean color density was 30.67 ± 12.75 and brightness density was 13.78 ± 4.75 (Table 2). Comparison between IBAGS and MBGS scores of the participants demonstrated a weighted κ value of 0.752 with P < 0.0001 (Table 2).
Table 2.
Measurement of Bleb Vascularity Parameters Using OCT-A and Cross Tables of the IBAGS and MBGS of Study Subjects
| Vascularity Parameters | N = 92 | ||||
| Color Density, (Range) | 30.67 ± 12.75 (3.59–69.43) | ||||
| Brightness Density, (Range) |
13.78 ± 4.75 (5.63–28.54) |
||||
| IBAGS |
MBGS |
||||
| 1 |
2 |
3 |
4 |
5 |
|
| V0 | 2 | 1 | 0 | 0 | 0 |
| V1 | 25 | 2 | 0 | 0 | 0 |
| V2 | 0 | 27 | 20 | 1 | 0 |
| V3 | 0 | 0 | 0 | 12 | 0 |
| V4 | 0 | 0 | 0 | 0 | 2 |
Comparison between IBAGS and MBGS of subjects showed that the weighted κ value was 0.752 with P < 0.0001.
Comparison of the New Parameters With the Conventional Bleb Grading Systems
The color and brightness densities were significantly positively correlated with IBAGS and MBGS scores (Table 3; Fig. 3). A 1-way ANOVA showed that IBAGS and MBGS presented different color and brightness density values (Table 4), with a post hoc test revealing three and four different groups, respectively.
Table 3.
Correlation Between the IBAGS and MBGS of Study Subjects
| Color Density |
Brightness Density |
IBAGS |
MBGS |
IOP |
|
| Color density | 1.000 | 0.801b | 0.494a | 0.528a | 0.152b |
| Brightness density | 1.000 | 0.408a | 0.437a | 0.116b | |
| IBAGS | 1.000 | 0.850a | 0.040a | ||
| MBGS | 1.000 | 0.062a | |||
| IOP | 1.000 |
Coefficients written in bold, if P < 0.05.
Coefficients using Kendall τ correlation test.
Coefficients using Pearson correlation test.
Figure 3.
Comparison of vascularity parameters using OCT-A and vascular grading results from the conventional bleb grading systems, IBAGS and MBGS.
Table 4.
Comparison of Vessel Density Parameters With IBAGS and MBGS
|
n |
Color Density |
Brightness Density |
|
| IBAGS | |||
| V0 | 3 T | 24.39 ± 4.47 B, C | 11.54 ± 1.91 B, C |
| V1 | 27 T | 20.02 ± 8.59 C | 11.16 ± 4.15 C |
| V2 | 48 T | 33.66 ± 11.05 A, B | 14.12 ± 4.34 B |
| V3 | 12 T | 40.38 ± 10.19 A, B | 16.79 ± 2.90 B |
| V4 | 2 T | 53.87 ± 11.33 A | 26.07 ± 0.30 A |
| P value | <0.0001 | <0.0001 | |
| MBGS | |||
| 1 | 27 T | 19.88 ± 8.56 C | 11.34 ± 4.13 D |
| 2 | 30 T | 30.47 ± 10.13 B | 12.70 ± 4.18 C, D |
| 3 | 20 T | 35.45 ± 8.35 A, B | 14.90 ± 2.87 B, C |
| 4 | 13 T | 42.62 ± 12.65 A | 17.69 ± 4.28 B |
| 5 | 2 T | 53.87 ± 11.33 A | 26.07 ± 0.30 A |
| P value | <0.0001 | <0.0001 | |
T: The same letters indicate insignificant differences between groups based on Tukey's multiple comparisons test.
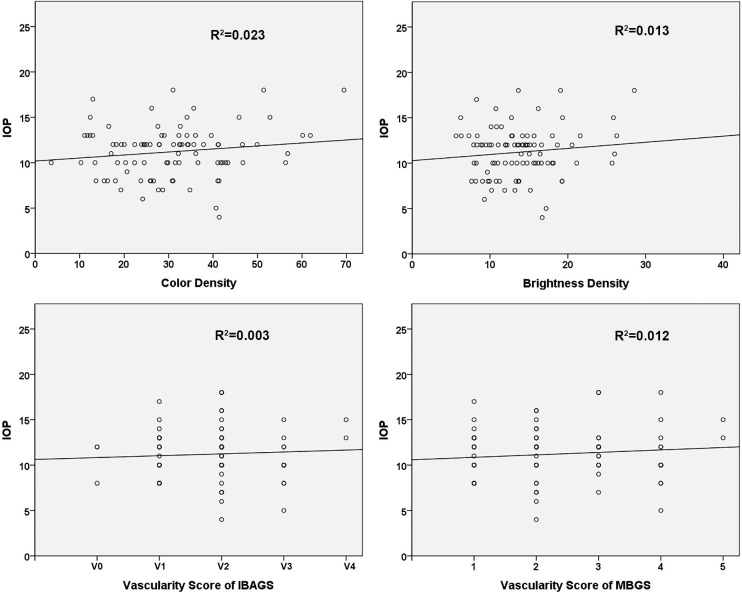
Postoperative IOP (IOP on the day of OCT-A scan and photography) was not significantly correlated with color density (R2 = 0.023; Table 3; Fig. 4) or brightness density (R2 = 0.013). In addition, the IOP was not related to the IBAGS (R2 = 0.003) or MBGS (R2 = 0.012) scores. Hence, postoperative IOP may reflect that our new parameters produce vascularity scores similar to those from the conventional grading systems. Steiger's Z-test revealed that the correlation coefficient between IOP and color density was the same as the correlation coefficient between IOP and IBAGS grading (Table 5). In addition, other correlation coefficients did not show any differences. There was also no difference in accuracy when predicting IOP risk using vascularity scores from the IBAGS and MBGS methods and when estimating IOP risk using the new parameters—color and brightness densities—on NRI, assuming an IOP cutoff value of 15 mm Hg (Table 5).
Figure 4.
Linear regression of IOP and vascularity parameters measured using OCT-A and of IOP and vascularity scores using conventional grading systems, IBAGS and MBGS.
Table 5.
Comparison of Correlation of IOP With New Vessel Parameters and IOP With Conventional Bleb Vascularity Scores Using IBAGS and MBGS
| Parameters |
IBAGS |
MBGS |
|
| Color density | Pa = 0.2607 | Pa = 0.4560 | |
| Brightness density | Pa = 0.5680 | Pa = 0.8320 | |
| Gold Standard |
New Parametersb |
NRI Value (95% CI) |
P Valueb |
| IBAGS | Color density | −0.2049 (−0.8113, 0.4016) | 0.5079 |
| Brightness density | −0.2537 (−0.8607, 0.3534) | 0.4128 | |
| MBGS | Color density | 0.1463 (−0.5094, 0.8021) | 0.6618 |
| Brightness density | −0.0537 (−0.6976, 0.5903) | 0.8703 |
CI, confidence interval.
P values by Steiger's Z-test. Alternative hypothesis: correlation coefficients between IOP and new vessel parameters are not the same as the correlation coefficients between IOP and IBAGS or MBGS.
P values by NRI.
We drew decision trees based on the IBAGS and MBGS scores (Fig. 5). Based on the IBAGS, the cutoff value was 18.23 for color density with two nodes and 10.14 for brightness density with two nodes (Fig. 5A, B). Conversely, based on the MBGS, the cutoff value was 18.23 for color density with two nodes and 10.14 for brightness density with two nodes (Fig. 5C, D).
Figure 5.
Decision tree based on classification of the IBAGS and MBGS. The decision tree shows two nodes: (A) color density based on IBAGS, (B) brightness density based on IBAGS, (C) color density based on MBGS, and (D) brightness density based on MBGS. Horizontal numbers (x-axis) in node graphs indicate the vascularity score using the IBAGS grades in (A) and (B), whereas those in (C) and (D) indicate the vascularity score using the MBGS grades.
Subgroup Analysis According Anteriorly Located Bleb and Posteriorly Located Bleb
In this study, the bleb was located anteriorly in 70 participants and posteriorly in 22 participants. The color and brightness densities of the posteriorly located bleb were higher than those of the anteriorly located bleb (Table 6). The correlation coefficient between the color and brightness densities and the vascularity scores from the IBAGS and MBGS grading in the anteriorly located bleb group was not different from the correlation coefficient in the posteriorly located bleb group (Table 6).
Table 6.
Subgroup Analysis of Bleb Locations
| Anteriorly Located Bleb, n = 70 |
Posteriorly Located Bleb, n = 22 |
P Valuea |
|
| Color density | 27.39 ± 11.52 | 41.12 ± 10.86 | <0.0001 |
| Brightness density | 12.84 ± 4.52 | 16.75 ± 4.29 | 0.0007 |
| IBAGS |
MBGS |
||
| Color density | Pb = 0.0627 | Pb = 0.2123 | |
| Brightness density | Pb = 0.1280 | Pb = 0.5060 |
P value using independent t-test for comparison between the anteriorly located bleb and posteriorly located bleb.
P value using Fisher z test for comparison of correlation coefficients of new vessel parameters and gradings of IBAGS and MBGS conversion from Kendall τ correlation coefficient to Pearson correlation coefficient. Alternative hypothesis: the correlation coefficient of the anteriorly located bleb group is different from that of the posteriorly located bleb group.
Discussion
We demonstrated that bleb evaluations using the new vascularity parameters, that is, the color and brightness densities, were related to the IBAGS and MBGS vascularity grading. In addition, these parameters provided objective and quantitative scores. Thus, these novel parameters can potentially be used as biomarkers for assessing bleb vascularity after trabeculectomy. To our knowledge, this is the first study to analyze blebs with new vascularity parameters using OCT-A in external mode. Although it is a primitive index that requires validation, anterior segment OCT-A could provide objective and quantitative data in the near future. Since we were more interested in the vascularity factors of the bleb using OCT-A, we studied the vascularity of the bleb itself. Images obtained using OCT-A could also provide both the height and extent of the bleb, similar to previous grading from the IBAGS and MBGS; however, we did not include this in the study as there was the possibility of distortion given that our OCT was not specialized for anterior evaluation. Upon developing anterior segment–oriented OCT-A, further studies comparing the complete grading system using OCT-A combined with AS-OCT (bleb structure and vascularity) are warranted, as IBAGS and MBGS included various factors, such as bleb area, height, vessel grade location, etc.
Several studies have previously classified blebs using OCT approaches, such as AS-OCT, based on bleb height, bleb extent, and internal reflectivity.17–20,22 Recently, Wen et al.22 suggested that novel AS-OCT–based bleb grades were related to MBGS variables throughout the 1-year postoperative period. In their study, AS-OCT grading was correlated with postoperative IOP, which corroborated our results. Herein, we have also presented an objective and quantitative method of measuring bleb vascularity using OCT-A.
Our study excluded patients using antiglaucoma medications as medication could affect the conjunctiva, and this would have been a bias in the study. With our protocol of management, we performed needle revision with 5-FU rather than administering additional glaucoma medications. In reviewing previous studies, bleb functionality may relate with not only vascularity but also bleb height, bleb wall thickness, etc.22,33 This may explain the insignificant correlation of IOP with vascularity densities (color and brightness densities, vascularity scores of IBAGS and MBGS). Hence, additional structural information would be helpful for predicting IOP. Our bleb vascularity index was based on a continuous scale and had wide-ranged values (color density: 3.59–69.43; brightness density: 5.63–28.54); however, in contrast, the vascular grade of the IBAGS and MBGS are four and five discontinuous numbers, respectively, which may suggest an advantage in using our parameters. In addition, a previous study classified bleb functionality according to IOP levels, with cutoff values of 14 and 18 mm Hg.34 These values were consistent with our study, in which we set the IOP level for NRI analysis at 15 mm Hg. Although this criterion is arbitrary, it is only provisional to present a tendency rather than a clear cutoff. These studies attempted to develop an objective and quantitative method for bleb evaluation.
The recently developed OCT-A is a noninvasive method of blood vessel visualization based on the detection of motion contrast from perfused blood vessels without the use of exogenous dyes. When compared with B-scans, changes detected by OCT-A are largely attributed to erythrocyte movement in the perfused vasculature.35 There are several studies on glaucoma optic nerve head and peripapillary vessel density using OCT-A.24,36–42 It is noteworthy that these studies were conducted to develop surrogate markers to diagnose glaucoma and detect its progression. In contrast, our study was intended to assess bleb vascularity using OCT-A in external mode to monitor the success of glaucoma surgery. A recent advance in the OCT technique, polarization-sensitive (PS)-OCT, enabled the more quantitative evaluation of filtering blebs.34,43,44 The authors expected that PS-OCT would be helpful in resolving image quality.
In a recent paper with the same study period as the current study, Yin et al.45 performed OCT-A–based (Optovue, Fremont, CA) measurements of the blood vessel area of the conjunctiva and analyzed postoperative changes. It is worth noting the differences between the method used in their study and that used in our study. In their study, bleb vessel area measurements at 1-month post trabeculectomy could predict the IOP 6 months post trabeculectomy, whereas in our study, we compared IOP with the scores from the conventional grading system and vascularity density with OCT-A, in terms of vessel density.
We must note several limitations in the current study. First, the OCT-A in this study was developed for the posterior pole, although it had an external eye mode. It only allowed the evaluation of a limited area and depth compared to AS-OCT, which was designed for the external eye. Thus, to overcome this challenge, AS-OCT with angiography technology needs to be developed. In this way, AS-OCT with angiography may help diagnose and assess other diseases, such as episcleritis, scleritis, and corneal neovascularization after corneal transplantation. Second, we were more focused on the vascularity factors of blebs. As such, we did not assess other factors, such as the height and extent of the bleb, which could be measured using an OCT-A scan. Third, since we enrolled various types of glaucoma patients in this study (primary glaucoma, pseudoexfoliation glaucoma, uveitic glaucoma, and steroid-induced glaucoma), we acknowledge that this could have a minor impact on the outcome. Finally, as preoperative conjunctival status was not measured, the relationship with postoperative conjunctival status could not be assessed. Future studies will include this preoperative data. Given the cross-sectional design of the current study, whether vascularity parameters are predictive factors for bleb encapsulation remains to be investigated with a randomized longitudinal study. We excluded patients who needed additional trabeculectomy due to bleb failure, as the method for measurement of conjunctival vascularity using OCT-A is not the standard method but rather is still an experimental approach. In addition, needling, 5-FU, and topical medications could change conjunctival vascularity (although we excluded the topical medication group), and as the measurement of the OCT-A and slit-lamp vascularity gradings were not necessarily performed before further needling or addition of glaucoma drops, the OCT-A measurements with IOP could have been confounded. Further prospective studies will be required.
In conclusion, bleb evaluation using OCT-A could be used to evaluate bleb vascularity and showed positive correlation with vessel grading using conventional bleb grading systems. It could potentially provide objective vessel parameters of bleb evaluation after successful trabeculectomy. Further study is warranted to predict bleb failure in advance using OCT-A, as fibrosis was related to vessel density. Additionally, a new classification system for blebs, which combines bleb structure and angiography, using OCT, would help better predict the prognosis of glaucoma surgery.
Acknowledgments
Disclosure: J.H. Seo, None; Y.A. Kim, None; K.H. Park, None; Y. Lee, None
References
- 1.Cairns JE. Trabeculectomy. Preliminary report of a new method. Am J Ophthalmol. 1968;66:673–679. [PubMed] [Google Scholar]
- 2.Picht G, Grehn F. Classification of filtering blebs in trabeculectomy: biomicroscopy and functionality. Curr Opin Ophthalmol. 1998;9:2–8. doi: 10.1097/00055735-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Broadway DC, Bloom PA, Bunce C, Thiagarajan M, Khaw PT. Needle revision of failing and failed trabeculectomy blebs with adjunctive 5-fluorouracil: survival analysis. Ophthalmology. 2004;111:665–673. doi: 10.1016/j.ophtha.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Feyi-Waboso A, Ejere HO. Needling for encapsulated trabeculectomy filtering blebs. Cochrane Database Syst Rev. 2004. CD003658. [DOI] [PubMed]
- 5.Cantor LB, Mantravadi A, WuDunn D, Swamynathan K, Cortes A. Morphologic classification of filtering blebs after glaucoma filtration surgery: the Indiana Bleb Appearance Grading Scale. J Glaucoma. 2003;12:266–271. doi: 10.1097/00061198-200306000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Wells AP, Crowston JG, Marks J, et al. A pilot study of a system for grading of drainage blebs after glaucoma surgery. J Glaucoma. 2004;13:454–460. doi: 10.1097/00061198-200412000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Wells AP, Ashraff NN, Hall RC, Purdie G. Comparison of two clinical bleb grading systems. Ophthalmology. 2006;113:77–83. doi: 10.1016/j.ophtha.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 8.Crowston JG, Kirwan JF, Wells A, Kennedy C, Murdoch IE. Evaluating clinical signs in trabeculectomized eyes. Eye (Lond) 2004;18:299–303. doi: 10.1038/sj.eye.6700638. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto T, Sakuma T, Kitazawa Y. An ultrasound biomicroscopic study of filtering blebs after mitomycin C trabeculectomy. Ophthalmology. 1995;102:1770–1776. doi: 10.1016/s0161-6420(95)30795-6. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa H, Liebmann JM, Ritch R. Quantitative assessment of the anterior segment using ultrasound biomicroscopy. Curr Opin Ophthalmol. 2000;11:133–139. doi: 10.1097/00055735-200004000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Leung CK, Yick DW, Kwong YY, et al. Analysis of bleb morphology after trabeculectomy with Visante anterior segment optical coherence tomography. Br J Ophthalmol. 2007;91:340–344. doi: 10.1136/bjo.2006.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh M, Aung T, Friedman DS, et al. Anterior segment optical coherence tomography imaging of trabeculectomy blebs before and after laser suture lysis. Am J Ophthalmol. 2007;143:873–875. doi: 10.1016/j.ajo.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Singh M, Chew PT, Friedman DS, et al. Imaging of trabeculectomy blebs using anterior segment optical coherence tomography. Ophthalmology. 2007;114:47–53. doi: 10.1016/j.ophtha.2006.05.078. [DOI] [PubMed] [Google Scholar]
- 14.Kawana K, Kiuchi T, Yasuno Y, Oshika T. Evaluation of trabeculectomy blebs using 3-dimensional cornea and anterior segment optical coherence tomography. Ophthalmology. 2009;116:848–855. doi: 10.1016/j.ophtha.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Singh M, Aung T, Aquino MC, Chew PT. Utility of bleb imaging with anterior segment optical coherence tomography in clinical decision-making after trabeculectomy. J Glaucoma. 2009;18:492–495. doi: 10.1097/IJG.0b013e31818d38ab. [DOI] [PubMed] [Google Scholar]
- 16.Golez E, III, Latina M. The use of anterior segment imaging after trabeculectomy. Semin Ophthalmol. 2012;27:155–159. doi: 10.3109/08820538.2012.707275. [DOI] [PubMed] [Google Scholar]
- 17.Mastropasqua R, Fasanella V, Agnifili L, Curcio C, Ciancaglini M, Mastropasqua L. Anterior segment optical coherence tomography imaging of conjunctival filtering blebs after glaucoma surgery. Biomed Res Int. 2014;2014:610623. doi: 10.1155/2014/610623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Napoli PE, Zucca I, Fossarello M. Qualitative and quantitative analysis of filtering blebs with optical coherence tomography. Can J Ophthalmol. 2014;49:210–216. doi: 10.1016/j.jcjo.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Kojima S, Inoue T, Nakashima K, Fukushima A, Tanihara H. Filtering blebs using 3-dimensional anterior-segment optical coherence tomography: a prospective investigation. JAMA Ophthalmol. 2015;133:148–156. doi: 10.1001/jamaophthalmol.2014.4489. [DOI] [PubMed] [Google Scholar]
- 20.Kokubun T, Kunikata H, Tsuda S, Himori N, Maruyama K, Nakazawa T. Quantification of the filtering bleb's structure with anterior segment optical coherence tomography. Clin Exp Ophthalmol. 2016;44:446–454. doi: 10.1111/ceo.12689. [DOI] [PubMed] [Google Scholar]
- 21.Narita A, Morizane Y, Miyake T, Seguchi J, Baba T, Shiraga F. Characteristics of successful filtering blebs at 1 year after trabeculectomy using swept-source three-dimensional anterior segment optical coherence tomography. Jpn J Ophthalmol. 2017;61:253–259. doi: 10.1007/s10384-017-0504-2. [DOI] [PubMed] [Google Scholar]
- 22.Wen JC, Stinnett SS, Asrani S. Comparison of anterior segment optical coherence tomography bleb grading, Moorfields Bleb Grading System, and intraocular pressure after trabeculectomy. J Glaucoma. 2017;26:403–408. doi: 10.1097/IJG.0000000000000636. [DOI] [PubMed] [Google Scholar]
- 23.Sacu S, Rainer G, Findl O, Georgopoulos M, Vass C. Correlation between the early morphological appearance of filtering blebs and outcome of trabeculectomy with mitomycin C. J Glaucoma. 2003;12:430–435. doi: 10.1097/00061198-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Jia Y, Wei E, Wang X, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology. 2014;121:1322–1332. doi: 10.1016/j.ophtha.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson P, Romano A. The impact of new methods of investigation and treatment on the understanding of the pathology of scleral inflammation. Eye (Lond) 2014;28:915–930. doi: 10.1038/eye.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi WJ, Pepple KL, Zhi Z, Wang RK. Optical coherence tomography based microangiography for quantitative monitoring of structural and vascular changes in a rat model of acute uveitis in vivo: a preliminary study. J Biomed Opt. 2015;20:016015. doi: 10.1117/1.JBO.20.1.016015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller MH, Grierson I, Unger WI, Hitchings RA. Wound healing in an animal model of glaucoma fistulizing surgery in the rabbit. Ophthalmic Surg. 1989;20:350–357. [PubMed] [Google Scholar]
- 28.Walker DA. JMASM9: converting Kendall's tau for correlational or meta-analytic analyses. J Mod Appl Stat Methods. 2003;2:525–530. [Google Scholar]
- 29.Kim DM, Seo JH, Kim SH, Hwang SS. Comparison of localized retinal nerve fiber layer defects between a low-teen intraocular pressure group and a high-teen intraocular pressure group in normal-tension glaucoma patients. J Glaucoma. 2007;16:293–296. doi: 10.1097/IJG.0b013e31803bda3d. [DOI] [PubMed] [Google Scholar]
- 30.Aoyama Y, Murata H, Aihara M. Targeting a low-teen intraocular pressure by trabeculectomy with a fornix-based conjunctival flap: continuous Japanese case series by a single surgeon. J Glaucoma. 2015;24:225–232. doi: 10.1097/IJG.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 31.Lee SH, Kim GA, Lee W, Bae HW, Seong GJ, Kim CY. Vascular and metabolic comorbidities in open-angle glaucoma with low- and high-teen intraocular pressure: a cross-sectional study from South Korea. Acta Ophthalmol. 2017;95:e564–e574. doi: 10.1111/aos.13487. [DOI] [PubMed] [Google Scholar]
- 32.Cho HK, Kojima S, Inoue T, Fukushima A, Kee C, Tanihara H. Effect of laser suture lysis on filtration openings: a prospective three-dimensional anterior segment optical coherence tomography study. Eye (Lond) 2015;29:1220–1225. doi: 10.1038/eye.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh LJ, Wong E, Lam J, Clement CI. Comparison of bleb morphology between trabeculectomy and deep sclerectomy using a clinical grading scale and anterior segment optical coherence tomography. Clin Exp Ophthalmol. 2017;45:701–707. doi: 10.1111/ceo.12953. [DOI] [PubMed] [Google Scholar]
- 34.Fukuda S, Fujita A, Kasaragod D, et al. Quantitative evaluation of phase retardation in filtering blebs using polarization-sensitive optical coherence tomography. Invest Ophthalmol Vis Sci. 2016;57:5919–5925. doi: 10.1167/iovs.16-19548. [DOI] [PubMed] [Google Scholar]
- 35.Wan KH, Leung CKS. Optical coherence tomography angiography in glaucoma: a mini-review. F1000Res. 2017;6:1686. doi: 10.12688/f1000research.11691.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, Jia Y, Takusagawa HL, et al. Optical coherence tomography angiography of the peripapillary retina in glaucoma. JAMA Ophthalmol. 2015;133:1045–1052. doi: 10.1001/jamaophthalmol.2015.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suh MH, Zangwill LM, Manalastas PI, et al. Deep retinal layer microvasculature dropout detected by the optical coherence tomography angiography in glaucoma. Ophthalmology. 2016;123:2509–2518. doi: 10.1016/j.ophtha.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Relationship between optical coherence tomography angiography vessel density and severity of visual field loss in glaucoma. Ophthalmology. 2016;123:2498–2508. doi: 10.1016/j.ophtha.2016.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung JK, Hwang YH, Wi JM, Kim M, Jung JJ. Glaucoma diagnostic ability of the optical coherence tomography angiography vessel density parameters. Curr Eye Res. 2017;42:1458–1467. doi: 10.1080/02713683.2017.1337157. [DOI] [PubMed] [Google Scholar]
- 40.Lee EJ, Lee SH, Kim JA, Kim TW. Parapapillary deep-layer microvasculature dropout in glaucoma: topographic association with glaucomatous damage. Invest Ophthalmol Vis Sci. 2017;58:3004–3010. doi: 10.1167/iovs.17-21918. [DOI] [PubMed] [Google Scholar]
- 41.Rao HL, Kadambi SV, Weinreb RN, et al. Diagnostic ability of peripapillary vessel density measurements of optical coherence tomography angiography in primary open-angle and angle-closure glaucoma. Br J Ophthalmol. 2017;101:1066–1070. doi: 10.1136/bjophthalmol-2016-309377. [DOI] [PubMed] [Google Scholar]
- 42.Rao HL, Pradhan ZS, Weinreb RN, et al. A comparison of the diagnostic ability of vessel density and structural measurements of optical coherence tomography in primary open angle glaucoma. PLoS One. 2017;12:e0173930. doi: 10.1371/journal.pone.0173930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukuda S, Beheregaray S, Kasaragod D, et al. Noninvasive evaluation of phase retardation in blebs after glaucoma surgery using anterior segment polarization-sensitive optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55:5200–5206. doi: 10.1167/iovs.14-14474. [DOI] [PubMed] [Google Scholar]
- 44.Kasaragod D, Fukuda S, Ueno Y, Hoshi S, Oshika T, Yasuno Y. Objective evaluation of functionality of filtering bleb based on polarization-sensitive optical coherence tomography. Invest Ophthalmol Vis Sci. 2016;57:2305–2310. doi: 10.1167/iovs.15-18178. [DOI] [PubMed] [Google Scholar]
- 45.Yin X, Cai Q, Song R, He X, Lu P. Relationship between filtering bleb vascularization and surgical outcomes after trabeculectomy: an optical coherence tomography angiography study. Graefes Arch Clin Exp Ophthalmol. 2018;256:2399–2405. doi: 10.1007/s00417-018-4136-0. [DOI] [PubMed] [Google Scholar]