Abstract
Background:
To evaluate the impact of neutrophil-to-lymphocyte ratios (NLR) as a prognostic factor in predicting treatment outcomes after radiotherapy (RT) for solid tumors.
Methods:
PubMed and Embase databases were used to search for articles published by February 2019 based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline. Hazard ratios (HR) with 95% confidence intervals (CI) were used to evaluate the association between NLR levels and treatment outcomes after RT. The primary endpoint was overall survival (OS) rates. Secondary endpoints included progression-free survival, disease-free survival, and disease-specific survival rates.
Results:
Thirty-eight datasets with a total of 7065 patients were included in the meta-analysis. Patients with high pretreatment NLR demonstrated significantly worse OS with a pooled HR of 1.90 (95% CI 1.66–2.17, P < .001). In patients receiving RT alone, the pooled HR for OS was 1.71 (95% CI 1.44–2.04, P < .001) with no between-study heterogeneity (I2 = 0%, P = .46).
Conclusion:
Elevated pretreatment NLR is associated with poorer survival in cancer patients undergoing RT. Elevated pretreatment NLR prior to RT initiation may be a useful biomarker to predict treatment outcomes and select a subgroup of patients in need of a more aggressive treatment approach.
Keywords: neutrophil-to-lymphocyte ratio, NLR, prognosis, radiotherapy, solid tumor
1. Introduction
Regardless of the advances that have been achieved in modern radiotherapy (RT) techniques, many solid tumors continue to demonstrate unsatisfactory treatment outcomes. Predictive markers for identifying patients with higher risk of unfavorable prognosis are increasingly being emphasized. Several reports have demonstrated that the presence of inflammatory responses in the tumor microenvironment influences cancer development and progression.[1,2] The relationship of inflammation and cancer involve both chronic inflammatory processes leading to carcinogenesis and intrinsic oncogene mutations that lead to recruitment of inflammatory cells.[3–5]
Various blood sample parameters of systemic inflammation have been investigated for prognostic significance.[2,6,7] The neutrophil-to-lymphocyte ratio (NLR) is a relatively simple and economical alternative for quantifying subclinical inflammation compared to emerging disease-specific biomarkers that are expensive and take time to obtain results. Increased levels of NLR have shown to be associated with poorer survival in a variety of solid tumors such as colorectal carcinoma, esophageal carcinoma, and non-small cell lung cancer.[6–9] In terms of its relationship with treatment outcomes in patients that receive RT, however, only a limited number of studies have reported on the potential role of NLR as a predictive factor. Suggestions that high levels of NLR advocate poorer survival have been reported, but the degree of its significance is unclear and data are inconsistent.[10] We thus aimed to evaluate the prognostic value of high levels of NLR in predicting adverse clinical outcomes in patients treated with RT alone or concurrent chemoradiotherapy (CCRT). This systematic review focused on[1]: the general prognostic value of NLR after RT in several solid tumor types and[2] NLR in patients that received RT alone.
2. Materials and methods
2.1. Search strategy
A systematic review of previously documented literature assessing the correlation of NLR and treatment outcomes after RT was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline.[11] PubMed, Embase, and Cochrane Library databases were used to search for articles published by February 28, 2019 using the following combination of keywords: “NLR OR neutrophil-to-lymphocyte ratio” AND “cancer OR malignancy” AND “prognosis OR survival OR response” AND “radiotherapy OR radiation therapy.”
2.2. Study selection
Initial assessment was solely based on the title and abstract of each reference. Full articles of relevant references were then reviewed for eligibility using the following criteria: studies that report outcomes of[1] patients with solid tumors treated with RT with or without concurrent chemotherapy,[2] analyses for prognostic significance of NLR on overall survival (OS),[3] baseline levels of NLR before initiation of RT, and[4] hazard ratios (HR) or informative data that could be used to estimate the HR. Both lab-based and clinical observational studies were included, and prospective studies and randomized controlled trials were excluded because such reports have not yet been presented. Data published only as an abstract, letter, editorial, commentary, review article, or case reports were excluded. Exclusion criteria also included documentations that were not written in English, were not human-based studies, were duplicates, or had overlapping data.
2.3. Data extraction
Preliminary database searches and subsequent screening and selection of references were done independently by 2 authors (HCK and NC). Data from eligible studies were extracted using a preset data extraction spreadsheet. The same 2 reviewers assessed all full articles for eligibility. In the case of inter-reviewer disagreement, consensus was reached after appropriate review. OS was the primary end-point and progression-free survival (PFS), disease-free survival (DFS), distant metastasis-free survival (DMFS), disease-specific survival (DSS), and complete response (CR) were secondary outcomes of interest. Information summarized in the preset included: author, year, and journal of publication; primary site of malignancy; sample size; aim and method of treatment; NLR cut-off value; use of receiver operating characteristic (ROC) curves; HR and 95% confidence interval (CI) for OS, PFS, DFS, DMFS, and DSS; and odd ratio (OR) and 95% CI for CR. In the case of any discrepancy during the process of data extraction and evaluation, a cross-check was performed by a third author (JG) with expertise in biostatistics.
2.4. Data synthesis and statistical analysis
This study is a pooled analysis of patients with various cancers of heterogeneous clinical features, inclusion criteria, NLR cut-off values, and study quality. Thus, to reflect the diversity of the selected studies, estimates of the pooled logarithms of the HR (logHR) and its 95% CI were combined using the random-effects model with inverse-variance weighting. I2 was used to identify and quantify the degree of heterogeneity between the eligible studies included for analysis.[12,13] Heterogeneity was not considered to be statistically significant if I2 was <50%. The funnel plot and Egger's linear regression method were used to detect and evaluate publication bias. Survival outcomes were expressed as estimated logHR, using either the reported 95% CI or indirect calculation based on available data.[14,15] All statistical analysis was performed using RevMan version 5.3 (Cochrane Collaboration, Copenhagen, Denmark).
3. Results
3.1. Study selection
A total of 227 articles were identified through initial database searching. After screening titles and abstracts of identified articles, 38 duplicated articles and 101 articles that were of nonrelevant topics were excluded. Eighty-eight full-text articles were assessed for eligibility. A final of 37 articles were selected, among which 1 article comparing oropharyngeal and nonoropharyngeal head and neck cancers was separated as 2 individual datasets.[16] Thus, a final of 38 datasets with 7065 patients were included for meta-analytic comparison. A schematic flow diagram of the study selection process is depicted in Figure 1.
Figure 1.
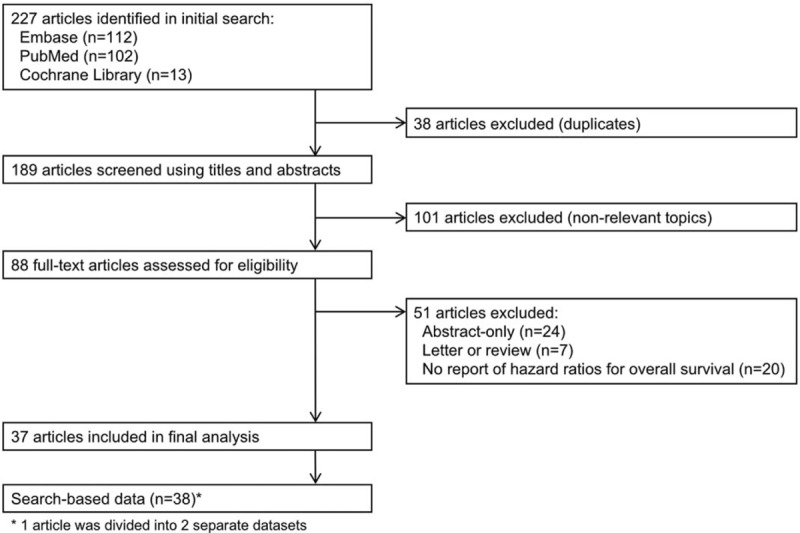
Flow chart of study selection.
3.2. Characteristics of included studies
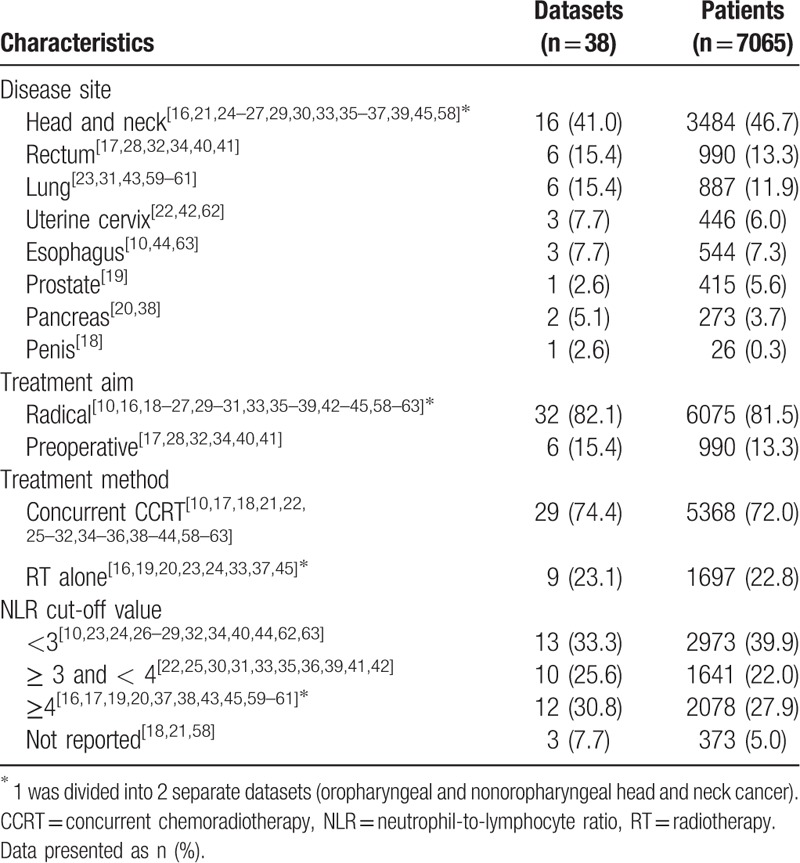
Table 1 summarizes the characteristics of patients included in the selected studies. All articles were published after 2012 and analyzed OS as the primary endpoint. Analyses included PFS in 15 studies,[10,17–30] DFS in 11 studies,[16,17,31–37] DMFS in 8 studies,[19,23,27,30,38,39] DSS in 1 study,[23] and pathologic CR in 2 studies on preoperative CCRT.[25,40] The median NLR cut-off value was 3.1 (range 1.9–5.0). ROC curves were used to estimate the optimal NLR cut-off value in 14 studies.[10,22–24,27–29,32,35,39,41–44] Majority included all or locally advanced stages, while 2 studies focused only on early stage cancer.[23,44]
Table 1.
Characteristics of included datasets.
3.3. Impact of NLR on OS, PFS, and DFS
The HR for OS was statistically significant on univariate analysis in 34 studies (89.5%), ranging from 1.1 to 7.7 with a median of 2.1. NLR was included in multivariate analyses in 35 studies, among which high NLR retained independently significant prognostic value in 30 studies (85.7%). A forest plot of the pooled analysis including all studies is given in Fig. 2A. Patients with pretreatment NLR greater than the cut-off value demonstrated significantly worse OS (HR 1.90, 95% CI 1.66–2.17, P < .001) with high heterogeneity (I2 = 74%, P < .001). When including only studies that provided data from multivariate analysis using NLR cut-off values determined by ROC curves (Fig. 2B), interstudy heterogeneity substantially decreased (I2 = 18%, P = .25) with no remarkable change of the pooled HR (HR 1.93, 95% CI 1.67–2.24, P < .001).
Figure 2.
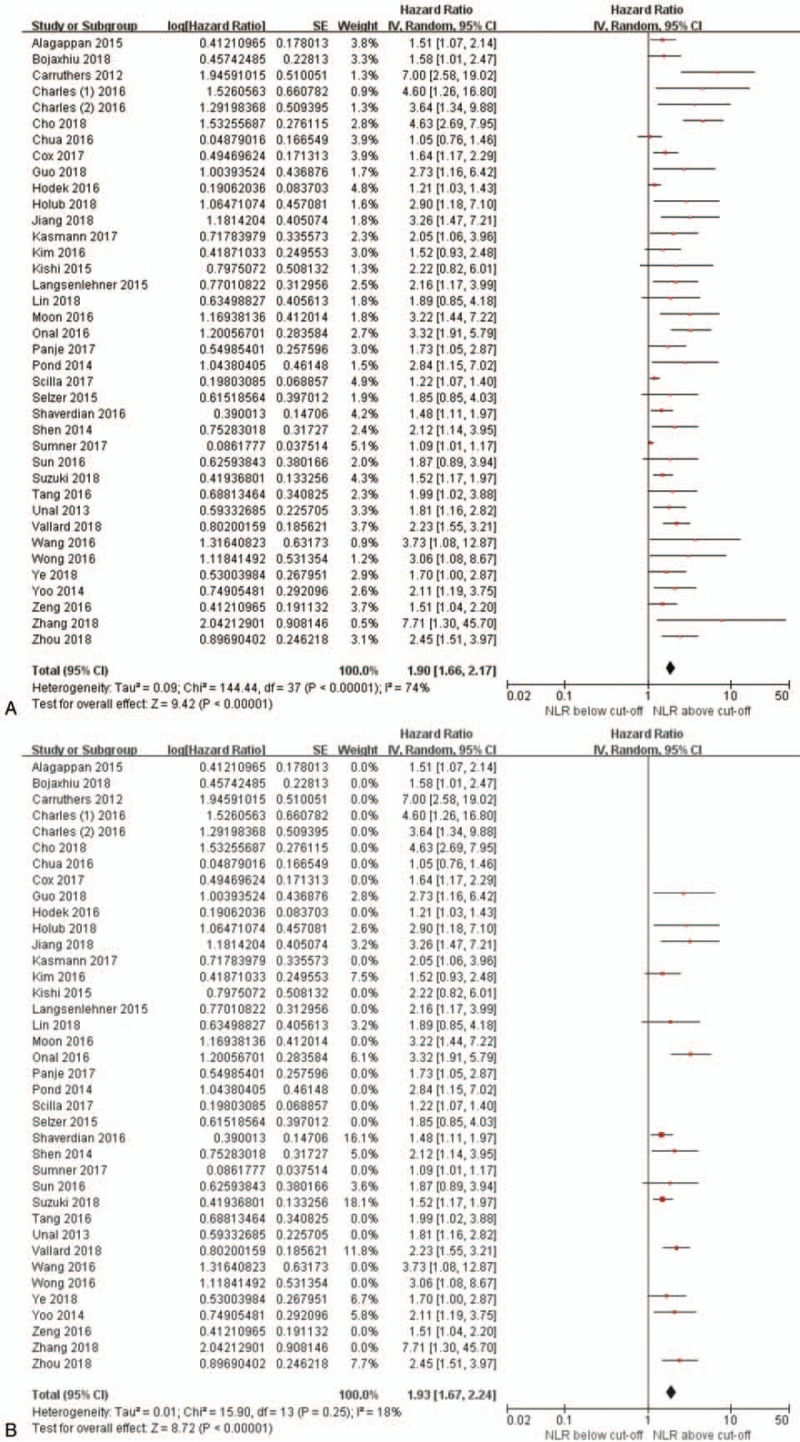
Forest plots showing hazard ratios for overall survival between pretreatment NLR greater than or less than the cut-off value in (A) all studies and (B) studies based on data from multivariate analysis and receiver operating characteristic curves.
HRs for PFS was reported in 15 studies with a total of 3537 patients. A pooled analysis for PFS demonstrated a HR of 2.12 (95% CI 1.64–2.75, P < .001) with moderately high heterogeneity (I2 = 67%, P < .001). DFS was reported in 11 studies for 1750 patients and resulted in a pooled HR of 1.54 (95% CI 1.23–1.94, P < .001), also with moderately high heterogeneity (I2 = 64%, P < .001).
3.4. NLR and RT alone
Figure 3 provides a forest plot for studies that investigated the effect of NLR in patients receiving RT without the addition of systemic cytotoxic therapy. This included 1697 patients in 9 datasets, consisting of 6 datasets on head and neck cancer[16,24,33,37,45] and 1 dataset each on prostate cancer, non-small cell lung cancer, and pancreatic cancer.[19,20,23] The pooled HR for OS was 1.71 (95% CI 1.44–2.04, P < .001) with no inter-study heterogeneity (I2 = 0%, P = .46) (Fig. 4).
Figure 3.
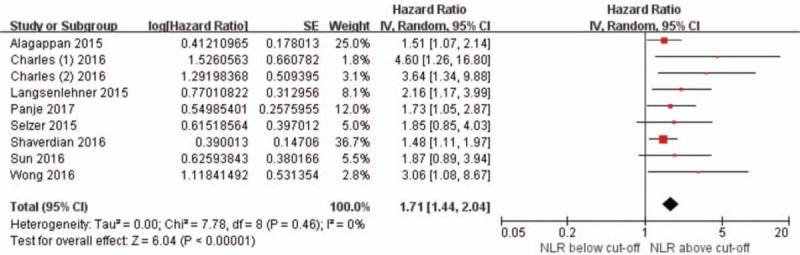
Forest plots showing hazard ratios for overall survival between pretreatment NLR greater than or less than the cut-off value in studies pertaining to radiation therapy alone.
Figure 4.
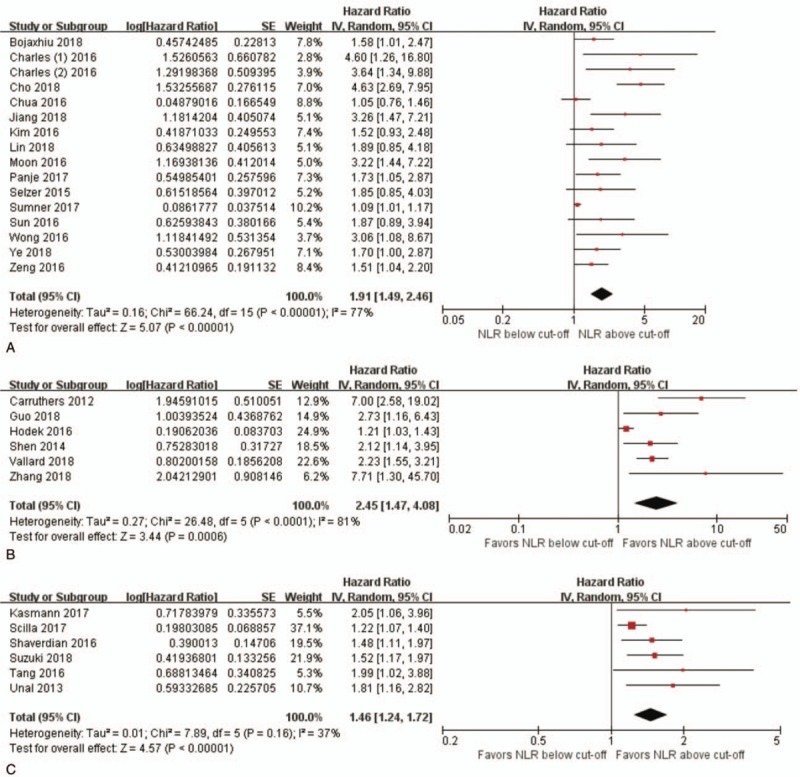
Forest plots showing hazard ratios for overall survival between pretreatment NLR greater than or less than the cut-off value in studies on (A) head and neck cancer, (B) rectal cancer, and (C) lung cancer.
3.5. NLR by disease subsite
Subgroup analyses was done for subsites with the largest number of patients and studies: head and neck (3484 patients in 16 studies), rectum (990 patients in 6 studies), and lung (887 patients in 6 studies). High pretreatment NLR significantly influenced OS by roughtly 2-fold in head and neck cancers (HR 1.91, 95% CI 1.49–2.46, P < .001), 2.5-fold in rectal cancers (HR 2.45, 95% CI 1.47–4.08, P < .001), and 1.5-fold in lung cancers (HR 1.46, 95% CI 1.24–1.72, P < .001). Heterogeneity was low for subgroup analysis of studies on lung cancer (I2 = 37%, P = .16).
3.6. Publication bias
Results of Egger's linear regression test suggested potential publication bias for OS (P < .001). The trim and fill method was used for estimation and adjustment of hypothetically missing studies. The pooled analysis of adjusted data were recalculated, with high NLR demonstrating significantly poorer OS (HR 1.46, 95% CI 1.24–1.71, P < .001). Egger's linear regression test after adjusting with the trim and fill method demonstrated insignificant between-study heterogeneity (P = .461). The funnel plot is given in Figure 5.
Figure 5.
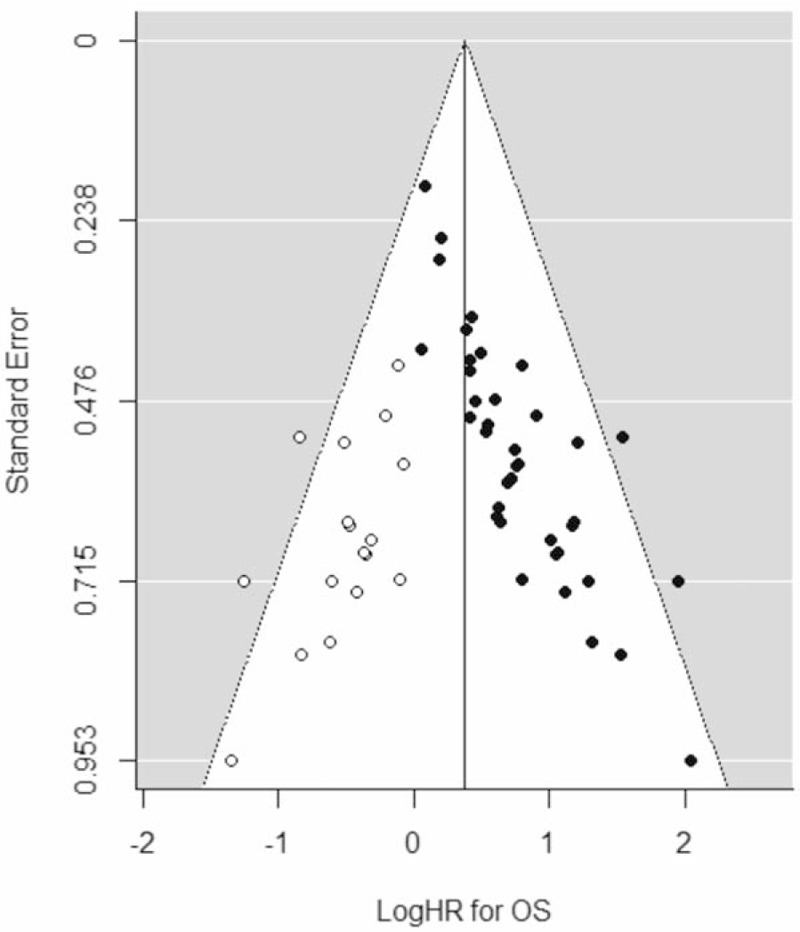
Funnel plot for OS. Funnel plot with trim and fill adjustment of logHRs for OS (x-axis) and corresponding standard errors (y-axis). Closed circles represent all included studies, open circles represent hypothetical studies obtained by the trim and fill method, and the vertical line is the estimated logHR of the pooled effect. logHR = logarithms of the hazard ratio, OS = overall survival.
4. Discussion
This meta-analysis of 38 datasets including 7065 patients demonstrated that high levels of pretreatment NLR is a statistically significant predictor of worse survival outcomes in patients receiving RT alone or in combination with systemic cytotoxic chemotherapy. All studies included in our study were retrospective observational analyses of various solid tumors and stages. Treatment modality was limited to preoperative or definitive RT alone or in combination with concurrent chemotherapy.
Because studies reporting on the relationship between pretreatment NLR and outcomes after RT have been documented in small numbers, this study is limited by the inevitable heterogeneity between studies. However, a pooled analysis of studies reporting OS outcomes based on multivariate analyses and NLR cut-off values determined by ROC curves demonstrated low between-study heterogeneity (I2 = 18%, P = .25), suggesting meta-analytic stability. Compared to studies focusing on NLR generally in relation to cancer progression, documentation of its impact on clinical outcomes after RT has been reported in relatively low numbers. Our results show that NLR could be of potential prognostic value in predicting worse treatment outcomes in patients receiving either definitive CCRT or RT. Particularly for patients treated by RT alone, the pooled HR for OS was 1.71 (95% CI 1.44–2.04, P < .001) with no between-study heterogeneity (I2 = 0%, P = .46), suggesting potential clinical significance of NLR as a pretreatment prognostic marker.
The relationship between inflammation and cancer has been widely studied in terms of factors such as pro-inflammatory cytokines and reactive oxygen species that activate transcription factors, which in turn promote the expression of genes that lead to carcinogenesis and cancer progression.[46–48] Various blood sample parameters have been investigated for prognostic significance in the treatment of several cancers, including high levels of C-reactive protein, interleukins, hypoalbuminemia, anemia, and thrombocytosis.[49–51] The advantage of ratios such as NLR and platelet-to-lymphocyte ratio is that these values are readily available without the need to perform additional laboratory testing. The use of NLR as a prognostic factor has been studied by several studies in relation to various disease entities and treatment modalities. A meta-analysis by Qi et al[52] demonstrated that low baseline levels of NLR were significantly associated with better survival in patients diagnosed with hepatocellular carcinoma. Furthermore, the authors showed that low NLR levels after treatment completion were also significantly correlated with improved clinical outcomes.
Inflammatory processes, as seen in cancer progression, may have a potentially significant role in prognosis after RT. The results of our series demonstrate that the presence of high NLR measurements prior to irradiation correlate with poorer treatment outcomes. A possible explanation for this relationship is hypoxia. Hypoxia is not only associated with tumor proliferation, differentiation, and resistance to radiation to radiation and chemotherapy, it also initiates alterations in cytokine expression that cause suppression of immune response, and possibly encouraging an inflammatory response.[53–56] As solid tumors grow, the supply of oxygen and nutrients eventually become inadequate, leading to tumor necrosis and release of pro-inflammatory mediates that recruit more inflammatory cells.[1] This cascade toward hypoxia may thus lead to the reduced therapeutic response in patients with high NLR.
A commonly referenced example for inflammation-associated carcinogenesis is the correlation of chronic inflammatory bowel disease and colon cancer. In a review by Terzić et al,[57] a prevalence of more than 20% of patients with inflammatory bowel disease were observed to develop colitis-associated cancer. Studies of sporadic colorectal cancer at molecular levels have also shown activation of transcription factors that act as important inflammatory pathways, suggesting the potential similarity of extrinsic inflammatory response seen in colitis-associated cancer and intrinsic cytokine recruitment seen in sporadic colorectal cancer.[47]
NLR has been suggested to have prognostic value in various solid tumors, but with inconsistent findings. Results of analyses by subsite revealed that high NLR increased the risk of poor OS most in rectal cancers, roughly by 2.5-fold (HR 2.45, 95% CI 1.47[57]4.08, P < .001). Elevated baseline NLR demonstrated to have prognostic value for OS and tumor response after neoadjuvant chemoradiation in a retrospective series analyzing 199 locally advanced rectal cancer patients.[32] In this study, multivariate analysis identified that a cut-off value of NLR greater than or equal to 2.8 was an independent factor for poorer OS with a HR of 2.1 (P = .018). This group of patients also showed worse DFS in univariate, but not in multivariate analyses. Hodek et al[34] evaluated 173 local advanced rectal adenocarcinoma patients that underwent neoadjuvant chemoradiation and compared NLR at various threshold values. The authors reported that NLR ranging between 2.2 and 2.8 had significantly better OS and tumor response, but the rate of pathologic CR was not significantly dependent on the pretreatment value of NLR.
Though large amounts of studies emphasizing the potential role of inflammatory parameters are emerging, results continue to be inconsistent across studies due to heterogeneity. With basis on prior documentation on various solid tumors and inflammatory carcinogenesis, NLR may be a useful tool for predicting treatment response especially in disease entities where complete response to treatment can be precisely evaluated. Future studies with larger, homogeneous populations are mandatory for improved assessment of the true role of NLR for predicting clinical outcomes in patients receiving RT.
In conclusion, elevated pretreatment NLR in cancer patients is associated with poorer survival outcomes after RT. The evaluation of NLR prior to oncologic therapy may be a useful and easy-to-obtain parameter for predicting treatment outcomes and selecting a subgroup of patients in need of a more aggressive treatment approach. Further assessment of the basic biologic mechanism behind the prognostic significance of NLR will be needed. Classifying patients with high risks of poor prognosis based on NLR or other similar biologic parameters could play as a stepping stone towards the introduction of targeted therapy to repair or inhibit high NLR, potentially opening a new paradigm for cancer treatment. Our results cannot be conclusive on the magnitude of its usefulness, but could become an elemental basis for future clinical trials for appropriately individualizing RT according to the degree of risks.
Author contributions
Preliminary database searches and subsequent screening and selection of references were done independently by Kang and Choi. Kang and Choi performed meta-analytic and statistical analyses, which was cross-checked by Gim. All authors read and approved the final manuscript.
Conceptualization: Noorie Choi, Hyun-Cheol Kang.
Data curation: Noorie Choi, Hyun-Cheol Kang.
Formal analysis: Noorie Choi, Hyun-Cheol Kang.
Funding acquisition: Eui Kyu Chie.
Investigation: Noorie Choi, Hyun-Cheol Kang.
Methodology: Noorie Choi, Hyun-Cheol Kang.
Project administration: Hyun-Cheol Kang.
Supervision: Jin Ho Kim, Eui Kyu Chie, Jungsoo Gim, Hyun-Cheol Kang.
Validation: Jin Ho Kim, Eui Kyu Chie, Jungsoo Gim, Hyun-Cheol Kang.
Visualization: Noorie Choi.
Writing – original draft: Noorie Choi.
Writing – review & editing: Noorie Choi, Hyun-Cheol Kang.
Footnotes
Abbreviations: CI = confidence interval, CR = complete response, DFS = disease-free survival, DMFS = distant metastasis-free survival, DSS = disease-specific survival, HR = hazard ratio, LogHR = logarithms of the hazard ratio, NLR = neutrophil-to-lymphocyte ratio, OR = odds ratio, OS = overall survival, PFS = progression-free survival, ROC = receiver operating characteristic, RT = radiotherapy.
This research was supported by National R&D Program through the Dong-Nam Institute of Radiological & Medical Sciences (DIRAMS) funded by the Ministry of Science, ICT & Future Planning (DIRAMS grant number 50590-2017, received by Chie.
The authors have no conflicts of interest to disclose.
References
- [1].Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218–30. [DOI] [PubMed] [Google Scholar]
- [3].Aggarwal BB, Shishodia S, Sandur SK, et al. Inflammation and cancer: how hot is the link? Biochem Pharmacol 2006;72:1605–21. [DOI] [PubMed] [Google Scholar]
- [4].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- [5].Colotta F, Allavena P, Sica A, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009;30:1073–81. [DOI] [PubMed] [Google Scholar]
- [6].Yodying H, Matsuda A, Miyashita M, et al. Prognostic significance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: a systematic review and meta-analysis. Ann Surg Oncol 2016;23:646–54. [DOI] [PubMed] [Google Scholar]
- [7].Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:1–1. [DOI] [PubMed] [Google Scholar]
- [8].Kumar R, Geuna E, Michalarea V, et al. The neutrophil-lymphocyte ratio and its utilisation for the management of cancer patients in early clinical trials. Br J Cancer 2015;112:1157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Malietzis G, Giacometti M, Kennedy RH, et al. The emerging role of neutrophil to lymphocyte ratio in determining colorectal cancer treatment outcomes: a systematic review and meta-analysis. Ann Surg Oncol 2014;21:3938–46. [DOI] [PubMed] [Google Scholar]
- [10].Yoo EJ, Park JC, Kim EH, et al. Prognostic value of neutrophil-to-lymphocyte ratio in patients treated with concurrent chemoradiotherapy for locally advanced oesophageal cancer. Dig Liver Dis 2014;46:846–53. [DOI] [PubMed] [Google Scholar]
- [11].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Higgins J, Green S. (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Updated March 2011. The Cochrane Collaboration. 2011. Available at: www.handbook.cochrane.org Accessed 1 Mar 2017. [Google Scholar]
- [13].Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815–34. [DOI] [PubMed] [Google Scholar]
- [15].Williamson PR, Smith CT, Hutton JL, et al. Aggregate data meta-analysis with time-to-event outcomes. Stat Med 2002;21:3337–51. [DOI] [PubMed] [Google Scholar]
- [16].Charles KA, Harris BD, Haddad CR, et al. Systemic inflammation is an independent predictive marker of clinical outcomes in mucosal squamous cell carcinoma of the head and neck in oropharyngeal and non-oropharyngeal patients. BMC Cancer 2016;16:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Carruthers R, Tho LM, Brown J, et al. Systemic inflammatory response is a predictor of outcome in patients undergoing preoperative chemoradiation for locally advanced rectal cancer. Colorectal Dis 2012;14:e701–707. [DOI] [PubMed] [Google Scholar]
- [18].Pond GR, Milowsky MI, Kolinsky MP, et al. Concurrent chemoradiotherapy for men with locally advanced penile squamous cell carcinoma. Clin Genitourin Cancer 2014;12:440–6. [DOI] [PubMed] [Google Scholar]
- [19].Langsenlehner T, Thurner EM, Krenn-Pilko S, et al. Validation of the neutrophil-to-lymphocyte ratio as a prognostic factor in a cohort of European prostate cancer patients. World J Urol 2015;33:1661–7. [DOI] [PubMed] [Google Scholar]
- [20].Alagappan M, Pollom EL, von Eyben R, et al. Albumin and neutrophil-lymphocyte ratio (NLR) predict survival in patients with pancreatic adenocarcinoma treated with SBRT. Am J Clin Oncol 2016;41:242–7. [DOI] [PubMed] [Google Scholar]
- [21].Moon H, Roh JL, Lee SW, et al. Prognostic value of nutritional and hematologic markers in head and neck squamous cell carcinoma treated by chemoradiotherapy. Radiother Oncol 2016;118:330–4. [DOI] [PubMed] [Google Scholar]
- [22].Onal C, Guler OC, Yildirim BA. Prognostic use of pretreatment hematologic parameters in patients receiving definitive chemoradiotherapy for cervical cancer. Int J Gynecol Cancer 2016;26:1169–75. [DOI] [PubMed] [Google Scholar]
- [23].Shaverdian N, Veruttipong D, Wang J, et al. Pretreatment immune parameters predict for overall survival and toxicity in early-stage non-small-cell lung cancer patients treated with stereotactic body radiation therapy. Clin Lung Cancer 2016;17:39–46. [DOI] [PubMed] [Google Scholar]
- [24].Sun W, Zhang L, Luo M, et al. Pretreatment hematologic markers as prognostic factors in patients with nasopharyngeal carcinoma: Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Head Neck 2016;38suppl 1:E1332–1340. [DOI] [PubMed] [Google Scholar]
- [25].Zeng YC, Chi F, Xing R, et al. Pre-treatment neutrophil-to-lymphocyte ratio predicts prognosis in patients with locoregionally advanced laryngeal carcinoma treated with chemoradiotherapy. Jpn J Clin Oncol 2016;46:126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cho Y, Kim JW, Yoon HI, et al. The prognostic significance of neutrophil-to-lymphocyte ratio in head and neck cancer patients treated with radiotherapy. J Clin Medicine 2018;7:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jiang Y, Qu S, Pan X, et al. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in intensity modulated radiation therapy for nasopharyngeal carcinoma. Oncotarget 2018;9:9992–10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vallard A, Garcia MA, Diao P, et al. Outcomes prediction in pre-operative radiotherapy locally advanced rectal cancer: Leucocyte assessment as immune biomarker. Oncotarget 2018;9:22368–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ye L, Oei RW, Kong F, et al. Prognostic values of hematological biomarkers in nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. Eur Arch Otorhinolaryngol 2018;275:1309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bojaxhiu B, Templeton AJ, Elicin O, et al. Relation of baseline neutrophil-to-lymphocyte ratio to survival and toxicity in head and neck cancer patients treated with (chemo-) radiation. Radiat Oncol 2018;13:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Unal D, Eroglu C, Kurtul N, et al. Are neutrophil/lymphocyte and platelet/lymphocyte rates in patients with non-small cell lung cancer associated with treatment response and prognosis? Asian Pac J Cancer Prev 2013;14:5237–42. [DOI] [PubMed] [Google Scholar]
- [32].Shen L, Zhang H, Liang L, et al. Baseline neutrophil-lymphocyte ratio (≥2.8) as a prognostic factor for patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiation. Radiat Oncol 2014;9:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wong BY, Stafford ND, Green VL, et al. Prognostic value of the neutrophil-to-lymphocyte ratio in patients with laryngeal squamous cell carcinoma. Head Neck 2016;38suppl 1:E1903–1908. [DOI] [PubMed] [Google Scholar]
- [34].Hodek M, Sirák I, Ferko A, et al. Neoadjuvant chemoradiotherapy of rectal carcinoma: baseline hematologic parameters influencing outcomes. Strahlenther Onkol 2016;192:632–40. [DOI] [PubMed] [Google Scholar]
- [35].Kim DY, Kim IS, Park SG, et al. Prognostic value of posttreatment neutrophil-lymphocyte ratio in head and neck squamous cell carcinoma treated by chemoradiotherapy. Auris Nasus Larynx 2017;44:199–204. [DOI] [PubMed] [Google Scholar]
- [36].Chua ML, Tan SH, Kusumawidjaja G, et al. Neutrophil-to-lymphocyte ratio as a prognostic marker in locally advanced nasopharyngeal carcinoma: A pooled analysis of two randomised controlled trials. Euro J Cancer 2016;67:119–29. [DOI] [PubMed] [Google Scholar]
- [37].Panje C, Riesterer O, Glanzmann C, et al. Neutrophil-lymphocyte ratio complements volumetric staging as prognostic factor in patients treated with definitive radiotherapy for oropharyngeal cancer. BMC Cancer 2017;17:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kishi T, Nakamura A, Itasaka S, et al. Pretreatment C-reactive protein level predicts outcome and patterns of failure after chemoradiotherapy for locally advanced pancreatic cancer. Pancreatology 2015;15:694–700. [DOI] [PubMed] [Google Scholar]
- [39].Lin AJ, Rao YJ, Chin RI, et al. Post-operative radiation effects on lymphopenia, neutrophil to lymphocyte ratio, and clinical outcomes in palatine tonsil cancers. Oral Oncol 2018;86:1–7. [DOI] [PubMed] [Google Scholar]
- [40].Zhang X, Li J, Peng Q, et al. Association of markers of systemic and local inflammation with prognosis of patients with rectal cancer who received neoadjuvant radiotherapy. Cancer Manag Research 2018;11:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Guo D, Han A, Jing W, et al. Preoperative to postoperative change in neutrophil-to-lymphocyte ratio predict survival in colorectal cancer patients. Future Oncol 2018;14:1187–96. [DOI] [PubMed] [Google Scholar]
- [42].Holub K, Biete A. Impact of systemic inflammation biomarkers on the survival outcomes of cervical cancer patients. Clin Transl Oncol doi: 10.1007/s12094-018-1991-4. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [43].Suzuki R, Lin SH, Wei X, et al. Prognostic significance of pretreatment total lymphocyte count and neutrophil-to-lymphocyte ratio in extensive-stage small-cell lung cancer. Radiother Oncol 2018;126:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhou YC, Chen LL, Xu HB, et al. Aging-related prognosis analysis of definitive radiotherapy for very elderly esophageal cancer. Cancer Med 2018;7:1837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Selzer E, Grah A, Heiduschka G, et al. Primary radiotherapy or postoperative radiotherapy in patients with head and neck cancer: comparative analysis of inflammation-based prognostic scoring systems. Strahlenther Onkol 2015;191:486–94. [DOI] [PubMed] [Google Scholar]
- [46].Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 2009;6:149–63. [DOI] [PubMed] [Google Scholar]
- [47].Meira LB, Bugni JM, Green SL, et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest 2008;118:2516–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Akbay EA, Koyama S, Liu Y, et al. Interleukin-17A promotes lung tumor progression through neutrophil attraction to tumor sites and mediating resistance to PD-1 blockade. J Thorac Oncol 2017;12:1268–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tao CJ, Chen YY, Jiang F, et al. The C-reactive protein/albumin ratio is an independent prognostic factor for overall survival in patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy. J Cancer 2016;7:2005–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Thomsen M, Kersten C, Sorbye H, et al. Interleukin-6 and C-reactive protein as prognostic biomarkers in metastatic colorectal cancer. Oncotarget 2016;7:75013–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kawano M, Mabuchi S, Matsumoto Y, et al. Prognostic significance of pretreatment thrombocytosis in cervical cancer patients treated with definitive radiotherapy. Int J Gyn Cancer 2015;25:1656–62. [DOI] [PubMed] [Google Scholar]
- [52].Qi X, Li J, Deng H, et al. Neutrophil-to-lymphocyte ratio for the prognostic assessment of hepatocellular carcinoma: a systematic review and meta-analysis of observational studies. Oncotarget 2016;7:45283–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mamlouk S, Wielockx B. Hypoxia-inducible factors as key regulators of tumor inflammation. Int J Cancer 2013;132:2721–9. [DOI] [PubMed] [Google Scholar]
- [54].Nordsmark M, Bentzen SM, Rudat V, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol 2005;77:18–24. [DOI] [PubMed] [Google Scholar]
- [55].Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer 2008;8:967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev 2007;26:225–39. [DOI] [PubMed] [Google Scholar]
- [57].Terzić J, Grivennikov S, Karin E, et al. Inflammation and colon cancer. Gastroenterology 2010;138:2101–14. [DOI] [PubMed] [Google Scholar]
- [58].Sumner WA, Stokes WA, Oweida A, et al. Survival impact of pre-treatment neutrophils on oropharyngeal and laryngeal cancer patients undergoing definitive radiotherapy. J Transl Med 2017;15:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tang H, Ma H, Peng F, et al. Prognostic performance of inflammation-based prognostic indices in locally advanced non-small-lung cancer treated with endostar and concurrent chemoradiotherapy. Mol Clin Oncol 2016;4:801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kasmann L, Bolm L, Schild SE, et al. Neutrophil-to-lymphocyte ratio predicts outcome in limited disease small-cell lung cancer. Lung 2017;195:217–24. [DOI] [PubMed] [Google Scholar]
- [61].Scilla KA, Bentzen SM, Lam VK, et al. Neutrophil-lymphocyte ratio is a prognostic marker in patients with locally advanced (stage IIIa and IIIb) non-small cell lung cancer treated with combined modality therapy. Oncologist 2017;22:737–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wang YY, Bai ZL, He JL, et al. Prognostic value of neutrophil-related factors in locally advanced cervical squamous cell carcinoma patients treated with cisplatin-based concurrent chemoradiotherapy. Dis Markers 2016;2016:3740794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cox S, Hurt C, Grenader T, et al. The prognostic value of derived neutrophil to lymphocyte ratio in oesophageal cancer treated with definitive chemoradiotherapy. Radiother Oncol 2017;125:154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


