Supplemental Digital Content is available in the text
Keywords: carcinomas, Krüppel-like factor 8(KLF8), meta-analysis, metastasis, prognosis, TNM stage
Abstract
Background and objective:
Krüppel-like factor 8 (KLF8), a transcription factor, belongs to the KLF8 family. Currently, studies have shown that KLF8 is highly expressed in some tumors. However, the prognostic value and metastasis of KLF8 in cancers remain unclear. For the first time, we conducted meta-analysis to explore the relationship between KLF8 expression with prognosis and metastasis in various carcinomas patients.
Methods:
Web of Science, PubMed, Embase, and Cochrane Library were systematically searched for eligible articles. Pooled hazard ratios (HRs) and their 95% confidence intervals (95% CIs) were calculated to evaluate the prognostic value and metastasis of KLF8 expression in human cancer patients.
Results:
The result revealed that highly expression level of KLF8 was significantly associated with poor overall survival (OS) (HR = 1.56, 95% CI: 1.26–1.87). Meanwhile, this significant correlation was also observed in subgroup analysis stratified by cancer types, source of HR, sample size, follow-up (months). In addition, highly expression of KLF8 was also closely associated with metastasis (HR = 1.37, 95% CI: 0.57–2.17) and tumor node metastasis stage (HR = 1.58, 95% CI: 0.90–2.25) in carcinomas.
Conclusion:
In summary, our meta-analysis indicates that overexpression of KLF8 may be associated with poor prognosis and higher incidence of metastasis in various carcinomas, and KLF8 may be used as a prognostic and metastatic indicator in human cancers.
1. Introduction
The malignancy is one of the biggest culprits affecting human health, its high incidence and high mortality have posed a significant threat to the health of all humanity,[1] and the morbidity and mortality have been increasing year by year. Tumor metastasis is one of the most prominent biological characteristics of malignant tumors, and is the main cause of death in cancer patients. In recent decades, great progress has been made in the diagnosis of cancer and new treatment methods. However, the prognosis of many cancers is still not very satisfactory.[2] Therefore, the identification of new tumor biomarkers is crucial for diagnosis, treatment, and prognosis of cancers.
As a member of the Krüppel-like factor (KLF) transcription factor family, KLF8 consists of 359 amino acids. It was originally isolated from leukemia cell lines and is too lowly expressed to detect in most normal cells and tissues.[3] Like other members of the KLF family, KLF8 contains 3 conserved C2H2 zinc finger DNA-binding domains at its C-terminus, but has unique sequences in the N-terminal domain that determine its functional specificity through the recruitment of proteins.[4,5] KLF8 is originally identified as a broad transcriptional repressor.[6] Some studies have shown that KLF8 plays an important role in cell proliferation,[3] differentiation, tumor metastasis, and epithelial-to-mesenchymal transition (EMT) process.[7,8] In addition, it is also reported that KLF8 is highly expressed in various cancers, such as gastric cancer,[9] lung cancer,[10] pancreatic cancer,[11] breast cancer,[12] colorectal cancer, and liver cancer,[7,13] and high level of KLF8 is associated with prognosis.[7,10]
So far, systematic meta-analysis has not been found to elucidate the relationship between KLF8 and the prognosis and metastasis of human cancers. Many studies only assess the role of KLF8 in tumor prognosis or metastasis. Here, we first conducted a systematic meta-analysis to assess the prognostic value and metastasis of KLF8 in tumors to further confirm whether KLF8 can be a reliable cancer biomarker.
2. Materials and methods
2.1. Ethics committee and institutional review board
This is a meta-analysis. Ethical approval was not necessary.
2.2. Literature search strategy
This systematic meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.[14] Potentially qualified studies were comprehensively searched by 2 independently authors (J.L. and Y.D.) in multiple electronic databases, Web of Science, PubMed, Embase, and Cochrane Library. The following search terms and their possible combinations were used: (“KLF8” or “Krüppel-Like Factor 8”) and (“prognosis” or “outcome” or “survival” or “mortality” or “recurrence” or “progression” or “metastasis”) and (“cancer” or “tumor” or “carcinoma” or “neoplasm”). References language in relevant articles was limited to English and the search ended on July 2018. Additionally, we also screened the references for articles to ensure that additional relevant articles were not missed.
2.3. Inclusion and exclusion criteria
All eligible studies had to meet the following criteria: the studies evaluated the correlation between KLF8 expression and the prognosis of cancer patients; the levels of KLF8 expression were detected by immunohistochemistry (IHC) or Western blotting (WB); the studies reported clinicopathological features and survival data, such as overall survival (OS) or cancer-specific survival (CSS); the studies have definite cut-off value to classify KLF8 expression as “positive” and “negative” or “high” and “low”; the studies provided hazard ratios (HRs) with 95% confidence intervals (CIs) directly or Kaplan–Meier survival curves at different KLF8 expression level; the studies published in English.
The exclusion criteria were as follows: duplicated studies; lack of sufficient data; the studies only studied the structure and functions of KLF8; review articles, case reports, animal studies, letters, and non-English papers.
2.4. Data extraction and quality assessment
Two reviewers (J.L. and Y.D.) independently summarized and extracted data from every eligible study in accordance with the inclusion and exclusion criteria. Any disagreements were discussed by the 2 investigators. The following information was obtained from the qualified studies: first author's name, year of publication, country, tumor type, sample size, disease stage, treatment method, detection method, outcome, follow-up duration, KLF8 level (high level and low level), cut-off value of KLF8, HR estimate, score of KLF8 assessment. When survival information was provided only with the Kaplan–Meier curves in some studies, the GetData Graph Digitizer software (http://getdata-graphdigitizer.com/) was then utilized to acquire the survival data, and Tierney's method to calculate the HRs with 95% (95% CIs.[15] The quality of each selected studies was assessed by Newcastle-Ottawa Scale (NOS).[16] The NOS included 3 quality items: selection (4 points), comparability (2 points), and outcome assessment (3 points). According to the NOS criteria, all of studies with an NOS score ≥6 are considered as high-quality articles. Details about NOS score of the quality articles were revealed in Supplemental Table 1.
2.5. Statistical strategy
With the data extracted for each eligible study, we performed the systematic meta-analysis to evaluate the connection between the level of KLF8 expression and cancer patients’ prognosis. The statistical analysis was performed by STATA12.0 (Stata Corporation, College Station, TX). The heterogeneity between studies was calculated by the Chi-square-based Q test and I2 statistics. The Cochran Q test: P heterogeneity<0.10 or I2≥50% was defined as significant heterogeneity and the fixed-effects model was used to test the pooled HRs, otherwise the random effects model was used.[17] Pooled HRs and 95% CIs for OS were calculated through a fixed effects model. Publication bias was assessed with Begg's funnel plot and Egger's test. P values were determined by 2-sided tests and P < .05 was considered as statistically significant. The pooled results were also examined the stability via sensitivity analysis.
3. Results
3.1. Characteristics of eligible studies
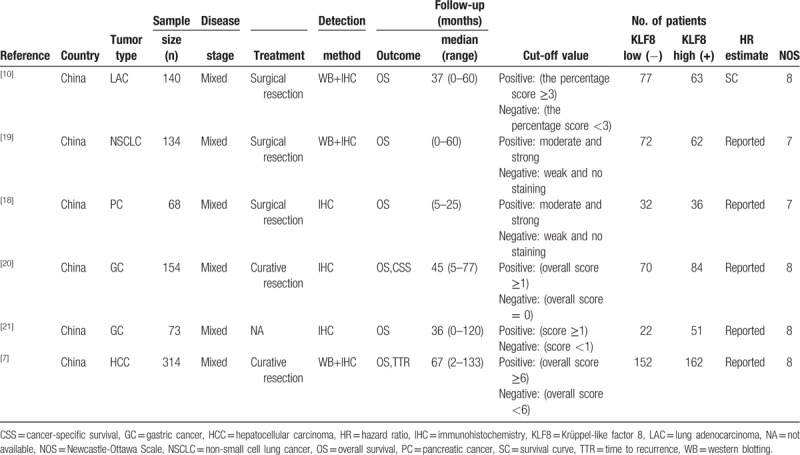
The search and selection strategy is shown in the flowchart (flow diagram). A total of 280 articles were initially identified through abovementioned searching strategies. Twenty-five studies were excluded due to duplicated studies. After the titles and abstracts were reviewed, and 248 irrelevant studies were excluded. Moreover, after reading the full article carefully, excluded 1 article with useless data. Finally, in according to the inclusion and exclusion criteria, 6 studies and a total number of 883 cases were eligible in the meta-analysis.[10,18–21,7] The 6 included articles were analyzed relationship between the level of KLF8 expression with tumor prognosis and metastasis. The specific characteristics of these eligible studies were revealed in Table 1. All 6 studies were conducted in China and foreign articles were not retrieved in the abovementioned electronic database. These studies were published between 2010 and 2018. Among the 6 studies, the sample sizes varied from 68 to 314, with a median of 137 patients. As for tumor types, these studies were distributed in the respiratory system and digestive system, which successively were lung adenocarcinoma, non-small cell lung cancer (NSCLC),pancreatic cancer, gastric cancer (n = 2) and hepatocellular carcinoma (HCC). All articles used IHC to assess the level of KLF8 expression, 3 of which also used WB. All 6 studies described the relationship between KLF8 expression and OS, 2 of which were also mentioned CSS and time to recurrence. In all studies, the cut-off value of KLF8 was defined by scoring the positive and negative of KLF8 on the basis of the intensity of immunohistochemical staining and the proportion of positively stained cells. In data analysis, the HR values of 5 articles were reported directly and 1 was not mentioned and was derived from the survival curve analysis.
Table 1.
The characteristics of all included eligible studies.
3.2. Association between the KLF8 expression level and OS
As shown in Table 2, 6 studies reported the OS based on a total of 883 patients’ different expression levels of KLF8. As shown in Figure 1, the estimated pooled HR was 1.56 (95% CI = 1.26–1.87, P = .000) and the heterogeneity of these included studies was not significant (I2 = 0.0%, P heterogeneity = 0.628). Therefore, the fixed effects model was adopted to reckon the HRs with 95% CIs. Our data analysis suggested that high level of KLF8 had a positive association with poor OS in cancer patients.
Table 2.
Results of this meta-analysis according to OS.
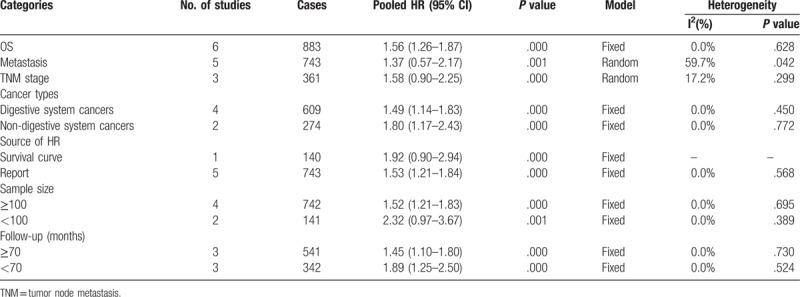
Figure 1.
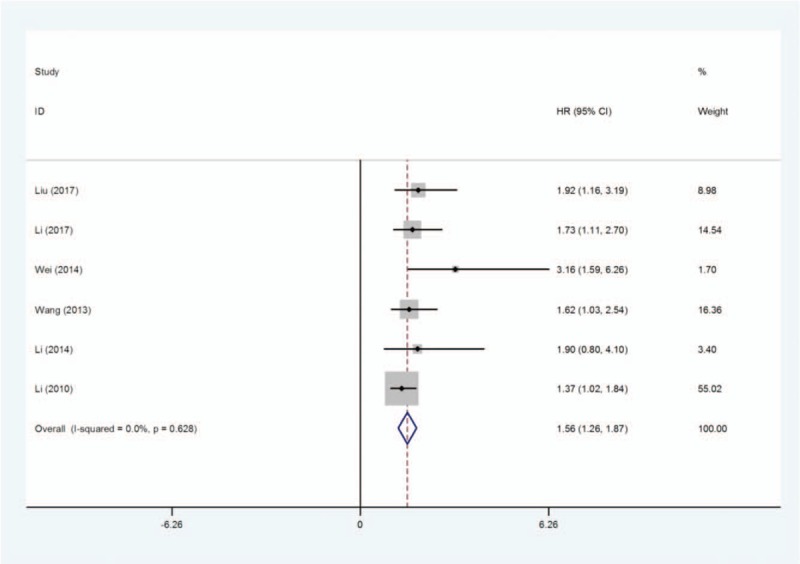
Forest plot of HRs for the association between KLF8 overexpression and the overall survival (OS) of patients with human cancers. HR = hazard ratio, KLF = Krüppel-like factor.
3.3. Subgroup analysis
Furthermore, we also performed a subgroup analysis utilizing the fixed effects model in the light of article publication year, cancer types, source of HR, sample size, and follow-up (months) (Table 2). As shown in Figure 2 , for cancer types, the pooled HRs of increased KLF8 expression on OS in patients with non-digestive system cancers and digestive system cancers, respectively, were 1.80 (95% CI = 1.17–2.43; P = .000) and 1.49 (95% CI = 1.14–1.83; P = .000) (Fig. 2 A). The above results also showed the effects of increased KLF8 expression on OS.
Figure 2.
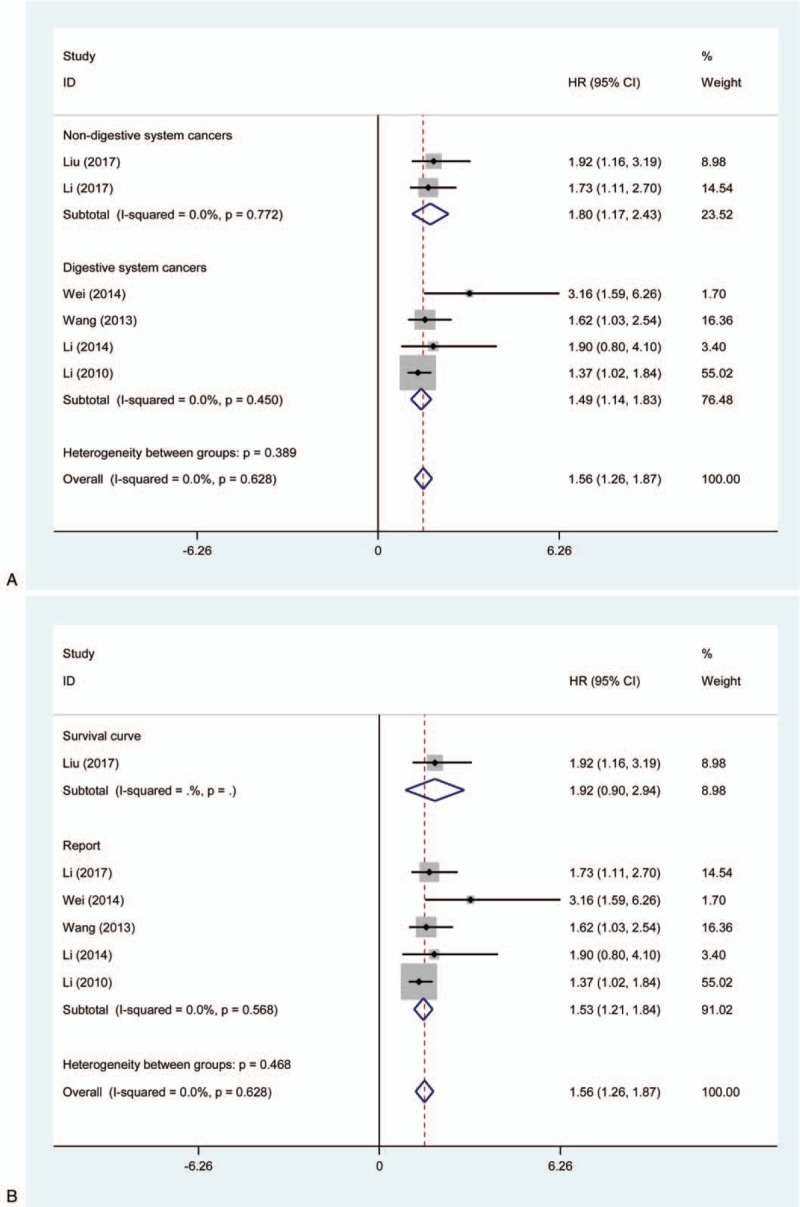
Forest plot of HRs for the association between high levels of KLF8 and subgroups in cancer patients.(A) Cancer types; (B) source of HR; (C) sample size; (D) follow-up (months). HR = hazard ratio, KLF = Krüppel-like factor.
Similarly, we further stratified subgroup analysis about source of HR, sample size, and follow-up (months), and revealed similar results in concern of the effects of high level of KLF8 on OS (Fig. 2 B–D).
3.4. Association between the KLF8 expression level and metastasis
As shown in Table 2, 5 studies and a total number of 743 patients directly reported HRs and assess the effect of overexpression of KLF8 on metastasis. The 5 studies included NSCLC, pancreatic cancer, gastric cancer (n = 2), and HCC. We used the random effects model and the pooled HRs with 95% CI showed significant association in metastasis incidence between KLF8 expression 1.37 (95% CI = 0.57–2.17; P = .001, random effects model) with heterogeneity (I2 = 59.7%, P heterogeneity = 0.042) (Fig. 3). Considering heterogeneity, we performed a sensitivity analysis and excluding the Li[19] study, the observed heterogeneity disappeared and the results were the same as before. The results suggested that highly expression of KLF8 significantly predicted a higher incidence of metastasis in patients with human cancer.
Figure 2 (Continued).
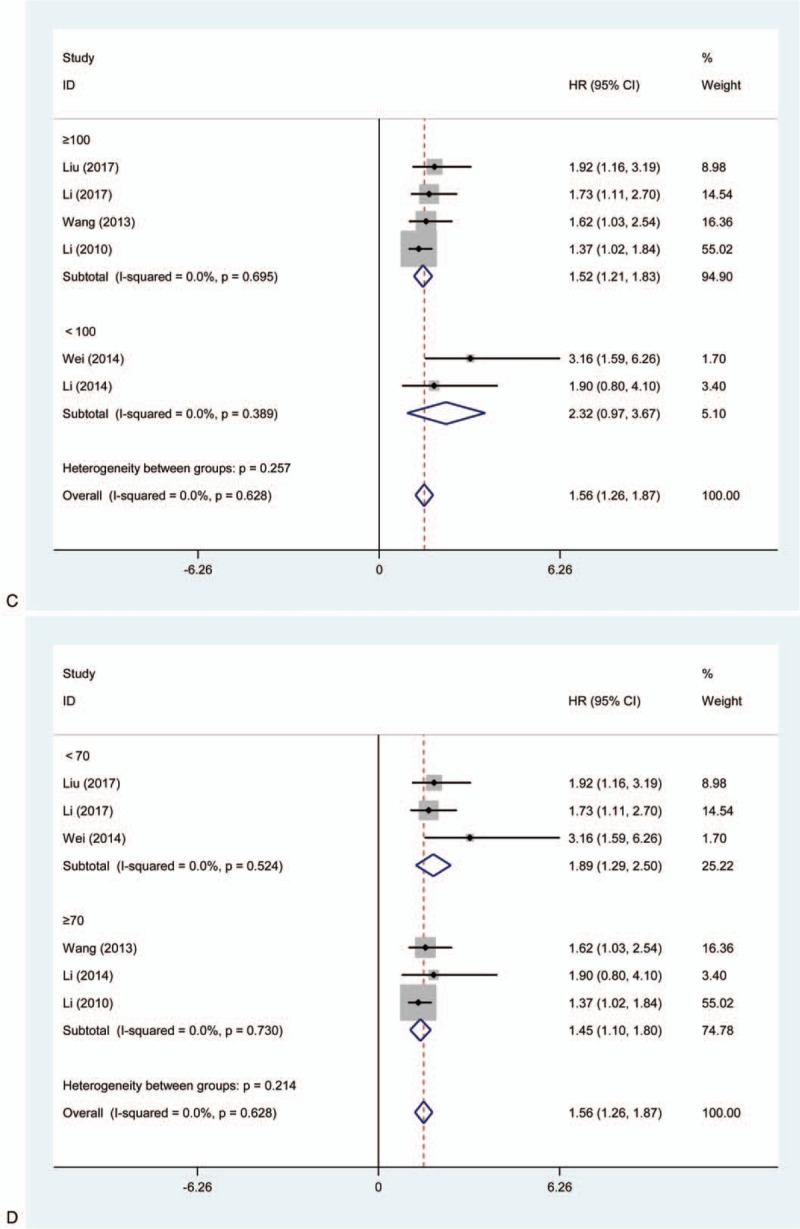
Forest plot of HRs for the association between high levels of KLF8 and subgroups in cancer patients.(A) Cancer types; (B) source of HR; (C) sample size; (D) follow-up (months). HR = hazard ratio, KLF = Krüppel-like factor.
3.5. Association between the KLF8 expression level and TNM stage
Among the 6 studies, 3 studies and a total number of 361 cases directly reported HRs and evaluate the effect of highly expression KLF8 on tumor node metastasis (TNM) stage. The 3 studies included NSCLC and gastric cancer (n = 2). Similarly, we also used the random effects model. The pooled HRs with 95% CI showed significant relationship between KLF8 expression and TNM stage 1.58 (95% CI = 0.90–2.25; P = .000, random effects model) with small heterogeneity (I2 = 17.2%, P heterogeneity = 0.299) (Fig. 4). The results showed that overexpression of KLF8 and TNM stage were closely related in patients with carcinomas.
Figure 3.
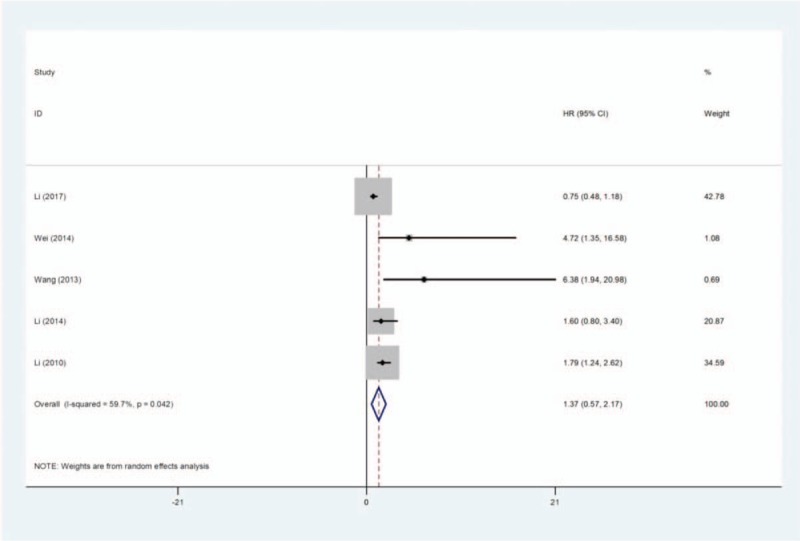
Forest plot of HRs for the association between KLF8 overexpression and metastasis of patients with human cancers. HR = hazard ratio, KLF = Krüppel-like factor.
3.6. Publication bias and sensitivity analysis
We evaluated the publication bias of included articles through Begg's tests and Egger's tests. In the Begg's funnel plots, as shown in Figure 5A, it showed that there was no obvious asymmetry. In addition, comprehensive analysis the results of Begg's test (P = .133) and Egger's tests (P = .039) demonstrated the possibility of partial publication bias. Therefore, we conducted trim and fill method and acquired the pooled HR of 1.504 (95% CI = 1.275–1.774). As shown in Figure 5B, after using the trim and fill method of map, it was still statistically significant. It showed that our results were still credible and not subjected to publication bias. Due to the number of included studies was little (n = 5), publication bias was not analyzed in metastasis.
Figure 4.
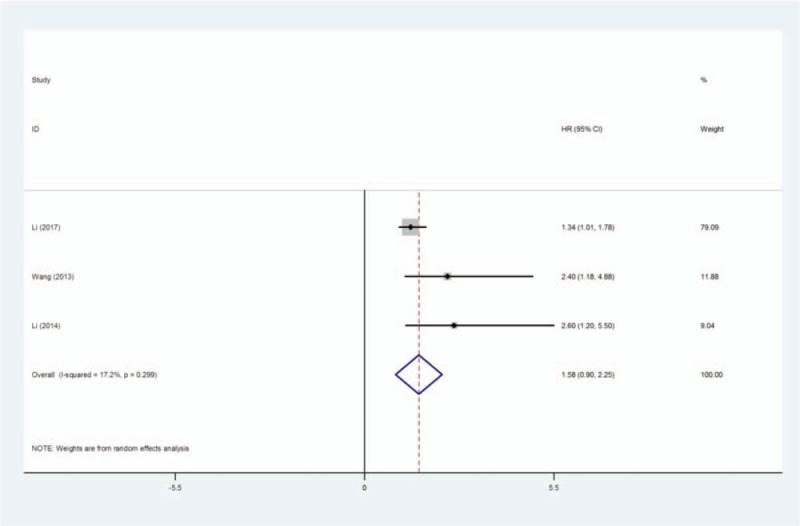
Forest plot of HRs for the association between KLF8 overexpression and TNM stage of patients with human cancers. HR = hazard ratio, KLF = Krüppel-like factor, TNM = tumor node metastasis.
Furthermore, the sensitivity analysis was performed by randomly deleting each study from the pooled analysis to assess the stability of the study. As we could see in Figure 6, indicating that the results of our meta-analysis were reliable and stable.
Figure 5.
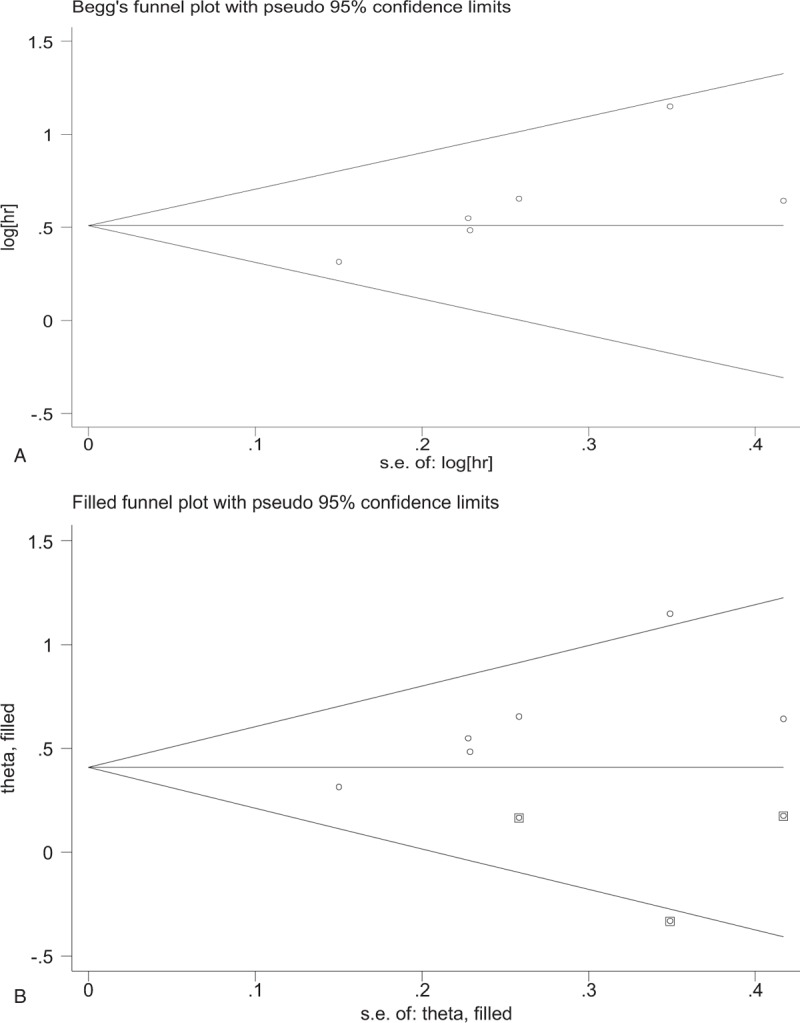
Funnel plot for publication bias assessment. (A) Begg's funnel plot; (B) funnel plot with trim and fill. Circle mean included studies, square mean estimated missing studies after modified publication bias.
Figure 6.
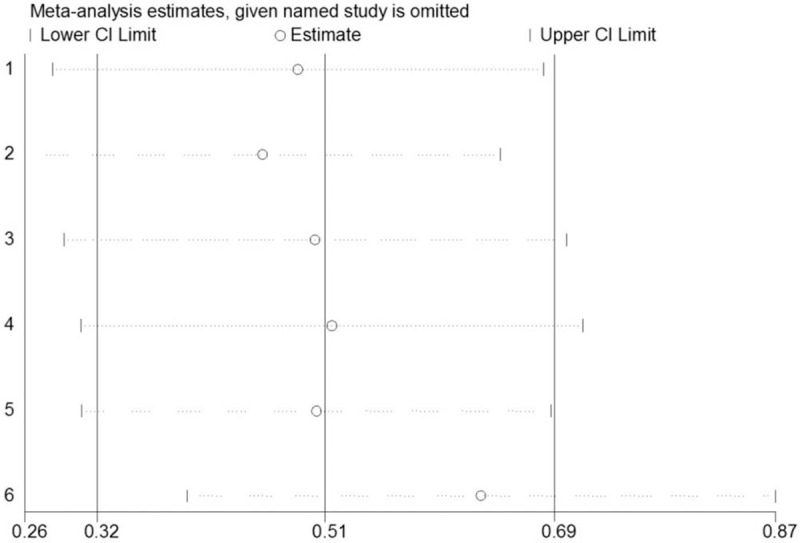
Sensitivity analysis of the meta-analysis of KLF8 overexpression. KLF = Krüppel-like factor.
4. Discussion
Malignant tumor is 1 of the major diseases which threat people's lives and social economic development with an increasing incidence and mortality rate.[22] Its characteristic includes progress rapidly, poor prognosis, metastasis and high fatality rate, and so on. Development and progression of tumor is related to many factors. At the molecular level, there are genetic mutations and the regulatory factors expression disorder, such as transcription factors and so on. Therefore, it is very important that the level of expression of proved tumor-associated factors or molecular markers, which could be monitored for the early prediction and treatment of tumors. At present, transcription factor as a molecular marker is also 1 of the hot spots.
KLF8 is a class of basic zinc finger transcription factor and belongs to a member of the KLFs family. It can be combined with the DNA of GC box or related CACCC element, through the regulation of gene expression rich in GC promoter, it is widely involved in the physiological and pathological processes such as cell proliferation, apoptosis, differentiation and embryonic development, cancer development, and angiogenesis. [23] KLF8 was originally identified from drosophila, a large number of literature studies found that like other members of this family, it plays a key role in cell cycle progression,[3] cell invasion and EMT,[12] oncogenic transformation of cells[24] and oncogenesis. Some researches have shown that KLF8 is the target of focal adhesion kinase (FAK). FAK can induce the expression of KLF8. Activated KLF8 acts on the cyclin D1 promoter GC box and activates cyclin D1 to exert its role in regulating the cell cycle.[3] In terms of invasion and transformation, other study found that KLF8 is a strong EMT and invasion inducing protein in breast cancer. There are also some studies found that in renal cell carcinoma, KLF8 protein and messenger RNA levels were significantly higher than adjacent nontumor tissue.[11] Moreover, there are many studies on the relationship between the expression level of KLF8 and prognosis. This is also a problem that we are concerned about. For example, all qualified studies in my article, these articles imply that overexpression KLF8 is closely related to tumor development and prognosis, and KLF8 may become a tumor biomarker. Therefore, through the meta-analysis of KLF8 research, we hope to illuminate the relationship between KLF8 and the prognosis of human tumors and to better guide clinical practice in the future.
Our study included 6 studies with 883 patients and analyzed whether overexpression KLF8 is a significant prognostic and metastatic risk factor in human cancers. We obtained the pooled HRs (1.56; 95% CI = 1.26–1.87), and the result is no heterogeneity. It showed that high expression KLF8 significantly predicted poor OS. In addition, highly expression of KLF8 was also closely associated with metastasis and TNM stage in carcinomas. Similarly, subgroup analyzes also yielded similar results. For the detection method, KLF8 is mainly detected by IHC and can also be detected by WB. The KLF8 antibodies used were uniform in the 6 articles, but considering the different manufacturers who purchased the antibodies, the evaluation criteria of the results were not uniform and there were many interference factors, so we did not make further analysis. In addition, our meta-analysis is the first to analyze the relationship between the level of KLF8 expression and tumor prognosis and metastasis. According to the Begg's funnel plots, the Egger's tests and the result of trim and fill method, we can also indicate that our study has a high degree of credibility.
4.1. Limitations
Of course, our study also has several limitations. First, the included studies in the meta-analysis were all from China. If incorporated into some other region studies, the results will be more persuasive. Second, according to Egger's tests, the possibility of partial publication bias was showed. Because of qualified studies relatively little, this may be resulted in a small publication bias. Analysis of association between the KLF8 expression with metastasis and TNM stage, there were too few articles that meet the conditions and the results were somewhat heterogeneous. In the future, the article volume should be expanded to analyze again to increase credibility. Third, due to the lack of uniform IHC assessment criteria and cut-off points now, the evaluation of KLF8 expression and the ascertainment of cut-off value usually depend on personal judgment. Finally, the follow-up time from the included articles was incomplete, the subgroup analysis was not deep enough, and the tumor types currently only had from 2 systems. Therefore, we require more other types of cancer in different systems to confirm our results. From the above, in regard to these limitations mentioned above, a larger sample size, more tumor types, and modulated particular data are required to realize a more convincing conclusion.
5. Conclusion
In conclusion, our meta-analysis demonstrates that the high expression level of KLF8 is relevant to poor OS and higher incidence of metastasis in various malignancies, which suggests that KLF8 may be served as a potential prognostic and metastasis biomarker and a promising therapeutic target for human cancers. Nevertheless, the number of eligible articles in this study is comparatively less, and more qualified studies are remained to confirm our results in the future.
Author contributions
Conceptualization: Jingrong Li.
Data curation: Jiaojiao Guo, Linhui Hu.
Funding acquisition: Shudao Xiong.
Investigation: Yangyang Ding.
Methodology: Manman Li.
Project administration: Lianfang Pu.
Resources: Huimin Zheng.
Software: Linhui Hu.
Writing – original draft: Jun Liu.
Writing – review & editing: Jun Liu.
Supplementary Material
Footnotes
Abbreviations: CSS = cancer-specific survival, FAK = focal adhesion kinase, GC = gastric cancer, HCC = hepatocellular carcinoma, HR = hazard ratio, IHC = immunohistochemistry, KLF8 = Krüppel-like factor 8, LAC = lung adenocarcinoma, NA = not available, NOS = Newcastle-Ottawa Scale, NSCLC = non-small cell lung cancer, OS = overall survival, PC = pancreatic cancer, SC = survival curve, TNM = tumor node metastasis, TTR = time to recurrence, WB = western blotting.
This work was partly supported by Key Research and Development Plan of Anhui Province, China (201904a07020058), Key Natural Science Research Project in Higher School of Anhui Province, China (KJ2018A0198), Scientific Research Foundation of the Institute for Translational Medicine (SRFITMAP, 2017zhyx13), National Science Foundation of China (81272259) and the Foundation of Anhui Medical University (2015xkj115).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Wu S, Powers S, Zhu W, et al. Substantial contribution of extrinsic risk factors to cancer development. Nature 2016;529:43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [3].Zhao J, Bian ZC, Yee K, et al. Identification of transcription factor KLF8 as a downstream target of focal adhesion kinase in its regulation of cyclinD1 and cell cycle progression. Mol Cell 2003;11:1503–15. [DOI] [PubMed] [Google Scholar]
- [4].Bieker JJ. Krüppel-like factors: three fingers in many pies. J Biol Chem 2001;276:34355–8. [DOI] [PubMed] [Google Scholar]
- [5].Pearson R, Fleetwood J, Eaton S, et al. Krüppellike transcription factors: a functional family. Int J Biochem Cell Biol 2008;40:1996–2001. [DOI] [PubMed] [Google Scholar]
- [6].Van Vliet J, Turner J, Crossley M. Human Krüppel-like factor 8: a CACCC-box binding protein that associates with CtBP and represses transcription. Nucleic Acids Res 2000;28:1955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li JC, Yang XR, Sun HX, et al. Upregulation of Kruppel-like factor 8 promotes tumor invasion and indicates poor prognosis for hepatocellular carcinoma. Gastroenterology 2010;139:2146–57. [DOI] [PubMed] [Google Scholar]
- [8].Lee HM, Hwang KA, Choi KC. Diverse pathways of epithelial mesenchymal transition related with cancer progression and metastasis and potential effects of endocrine disrupting chemicals on epithelial mesenchymal transition process. Mol Cell Endocrinol 2017;457:103–13. [DOI] [PubMed] [Google Scholar]
- [9].Liu N, Wang Y, Zhou Y, et al. Krüppel-like factor 8 involved in hypoxia promotes the invasion and metastasis of gastric cancer via epithelial to mesenchymal transition. Oncol Rep 2014;32:2397–404. [DOI] [PubMed] [Google Scholar]
- [10].Liu YF, Yao XF, Zhang Q. Expression of Krüppel–like factor 8 and Ki67 in lung adenocarcinoma and prognosis. Exp Ther Med 2017;14:1351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yi X, Zai H, Long X, et al. Krüppel-like factor 8 induces epithelial-to-mesenchymal transition and promotes invasion of pancreatic cancer cells through transcriptional activation of four and a half LIM-only protein 2. Oncol Lett 2017;14:4883–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mukherjee D, Lu H, Yu L, et al. Krüppel-like factor 8 activates the transcription of C-X-C cytokine receptor type 4 to promote breast cancer cell invasion, transendothelial migration and metastasis. Oncotarget 2016;7:23552–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yan Q, Zhang W, Wu Y, et al. KLF8 promotes tumorigenesis, invasion and metastasis of colorectal cancer cells by transcriptional activation of FHL2. Oncotarget 2015;6:25402–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- [15].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 2015;8:2–10. [DOI] [PubMed] [Google Scholar]
- [17].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [18].Wei YX, Chen G, You L, et al. Krüppel-like factor 8 is a potential prognostic factor for pancreatic cancer. Chin Med J 2014;127:856–9. [PubMed] [Google Scholar]
- [19].Li JC, Liu YF, Xue JH, et al. Krüppel-like factor 8 overexpression correlates with poor prognosis in non-small cell lung cancer. Pathol Oncol Res 2019;25:115–21. [DOI] [PubMed] [Google Scholar]
- [20].Wang WF, Li J, Du LT, et al. Krüppel-like factor 8 overexpression is correlated with angiogenesis and poor prognosis in gastric cancer. World J Gastroenterol 2013;19:4309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li SH, Wu PR, Yeh KT, et al. Positive nuclear expression of KLF8 might be correlated with shorter survival in gastric adenocarcinoma. Ann Diagn Pathol 2014;18:74–7. [DOI] [PubMed] [Google Scholar]
- [22].Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin 2016;66:271–89. [DOI] [PubMed] [Google Scholar]
- [23].Lee H, Kim HJ, Lee YJ, et al. Krüppel-like factor KLF8 plays a critical role in adipocyte differentiation. PLoS One 2012;7:e52474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lin F, Sheen Z, Tang LN, et al. KLF8 knockdown suppresses proliferation and invasion in human osteosarcoma cells. Mol Med Rep 2014;9:1613–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


