Abstract
Despite its 5-year event-free survival rate increasing to 60–65% due to surgery and chemotherapy, osteosarcoma (OS) remains one of the most threatening malignant human tumors, especially in young patients. Therefore, a new approach that combines early diagnosis with efficient tumor eradication and bioimaging is urgently needed. Here, a new type of mesoporous silica–coated bismuth sulfide nanoparticles (Bi2S3@MSN NPs) is developed. The well distributed mesoporous pores and large surface areas hold great promise for drug protection and encapsulation (doxorubicin (DOX), 99.85%). Moreover, the high photothermal efficiency of Bi2S3@MSNs (36.62%) offers great possibility for cancer synergistic treatment and highly near-infrared-triggered drug release (even at an ultralow power density of 0.3 W cm–2). After covalently conjugated to arginine-glycine-aspartic acid (RGD) peptide [c(RGDyC)], the NPs exhibit a high specificity for osteosarcoma and finally accumulate in the tumor cells (tenfold more than peritumoral tissues) for computed tomography (CT) imaging and tumor ablation. Importantly, the synergistic photothermal therapy–chemotherapy of the RGD-Bi2S3@MSN/DOX significantly ablates the highly malignant OS. It is further proved that the superior combined killing effect is achieved by activating the mitochondrial apoptosis pathway. Hence, the smart RGD–Bi2S3@MSN/DOX theranostic platform is a promising candidate for future applications in CT monitoring and synergistic treatment of malignant tumors.
Keywords: Bi2S3@MSN, mitochondrial apoptosis pathway, osteosarcoma, photothermal therapy–chemotherapy, X-ray computed tomography
1. Introduction
With a high annual incidence rate of 3.1 cases per million people, osteosarcoma (OS) is one of the most common primary bone tumors, accounting for 20% of all malignant primary tumors of bone affecting both children and adults. OS seriously threatens the health and lives of patients, especially teenagers.[1] The considerable efforts made to improve OS patient survival during the past few decades have helped increase the current 5-year eventfree survival rate to 60–65%, with a combination of chemotherapy with surgical resection as the preferred approach.[2] However, despite the advances in the current treatment, one-third of OS patients experience local recurrence or develop metastasis, after which they on average survive less than 1 year. Thus, new therapeutic strategies are urgently needed.[3]
Near infrared (NIR)-induced photothermal therapy (PTT) has received considerable interest as a cancer treatment because NIR light (700–1300 nm) can penetrate more deeply into soft tissues than UV or visible light, thereby enabling more accurate therapy at the tumor site.[4] In this regard, various NIR light-absorbing nanoparticles (NPs), such as noble metal nanostructures,[5] semiconductors,[6] carbon composites,[7] and organic NPs,[8] have been developed for PTT. Among them, low-cost bismuth sulfide (Bi2S3) NPs are particular promising because their narrow bandgap (≈1.3 eV), inducing outstanding absorbance in the NIR region.[9] Moreover, due to their high atomic number, bismuth-doped nanomaterials also hold substantial promise as novel X-ray computed tomography (CT) contrast agents.[10] Overall, imaging-guided PTT based on bismuth-doped NPs provides a versatile new theranostic platform.
To date, different forms of bismuth-doped nanoparticles have been developed for CT imaging and PTT, including Bi2S3 nanorods,[11] Cu3BiS3 nanodots,[12] and hexahedron Cu3BiS3 NPs.[13] The hyperthermia resulting from these bismuth-doped agents can induce cell death in a certain content and exert a defined therapeutic effect. However, the only use of PTT in tumor treatment remains limited, especially in highly malignant cancer therapy, which may require a theranostic platform combining diagnosis and synergistic treatment.[14] In order to increase the therapeutic effect, Cu3BiS3,t[15] reduced graphene oxide (rGO)/Bi2S3 NPs,[16] and our previously constructed Bi2S3@mesporous silica (mPS)[17] have introduced for combined photothermal therapy–chemotherapy, but their drug loading efficiency and/or tumor targeting effect was limited. Hence, the aim of this study is to develop an active targeting Bi2S3 NPs with ultrahigh drug loading ability.
In addition, the possible causes of the enhanced tumor killing effect by the combined therapy are still unclear. Mitochondria play a crucial role in cell energy metabolism and the programed cell death occurs mostly via mitochondrial apoptosis pathways. The Bcl-2 family members alter and permeabilize the mitochondrial outer membrane, then stimulate mitochondrial proteins releasing that activate downstream caspases.[18] However, tumors undergo limitless replication and the overexpressed antiapoptotic Bcl-2 protein confers a favorable progress in tumorigenesis. Malignant tumor can inhibit mitochondrial apoptosis by upregulating the antiapoptotic Bcl-2 protein to prevent caspase activation.[19] Hence, another goal of this study is to explore whether the photothermal therapy–chemotherapy can alter Bcl-2-dependent mitochondrial apoptosis pathway.
Toward these goals, we proposed the unique actively targeted mesoporous silica–coated bismuth sulfide nanoparticles (Bi2S3@ MSNs) for highly malignant OS theranostics. The internalized arginine-glycine-aspartic acid (RGD) peptide (CRGDKGPDC), which can target tumor vasculature and tumor cells via αvβ3 and αvβ5 integrin receptors, and then penetrate deep tumor tissue, was introduced as targeted peptide for OS. The Bi2S3@ MSNs were then conjugated with RGD through N-hydroxysuccinimide (NHS) ester of polyethylene glycol maleimide (NHS–PEG–Mal) linker to improve biocompatibility, decrease reticuloendothelial system (RES) clearance,[20] and increase targeted effect. The as-synthesized Bi2S3@MSNs exhibited an outstanding photothermal effect and superb drug encapsulation efficiency (99.85%) owing to their well-distributed porous structures and large surface area. Importantly, the doxorubicin (DOX)-loaded Bi2S3@MSNs (Bi2S3@MSN/DOX) are highly NIR-sensitive (even with an ultralow power density of 0.3 W cm–2) that reduces drug release and side effects in normal tissues. Moreover, because of their well-controlled size, spherical structure, and RGD modification, the RGD–Bi2S3@MSN/DOX exhibited an excellent active targeting ability for accurate OS ablation and CT-guided localization (Scheme 1). The combined photothermal therapy–chemotherapeutic effect was enhanced due to the decreased Bcl-2 protein and increased caspase-3 expression through mitochondrial apoptotic pathway. Hence, the as-synthesized NPs are excellent candidates for both OS diagnosis and therapy.
Scheme 1.

The smart RGD-Bi2S3@MSN/DOX nanoplatform for OS real-time X-ray CT imaging and NIR-responsive photothermal therapy-chemotherapy.
2. Results and Discussions
2.1. Characterization
Bi2S3@MSNs with a size of ≈100 nm were readily synthesized via a modified template method. First, X-ray diffraction (XRD) spectroscopic analysis was conducted to study the crystallinity and phase structure of the as-synthesized Bi2S3@MSNs. As shown in Figure 1a, all peaks in the Bi2S3@ MSN samples could be readily indexed to the orthorhombic Bi2S3 crystal (JCPDS 65–2431). Moreover, there was no impurity peak corresponding to the standard orthorhombic Bi2S3 crystal phase, demonstrating that high-quality Bi2S3 was synthesized. Figure 1b shows transmission electron microscopy (TEM) images of typical Bi2S3@MSN with a diameter of ≈100 nm. In this work, the cationic surfactant cetyltrimethylammonium chloride (CTAC) functions as both a stabilizer and template agent for the successful synthesis of Bi2S3@MSN.[21]
Figure 1.
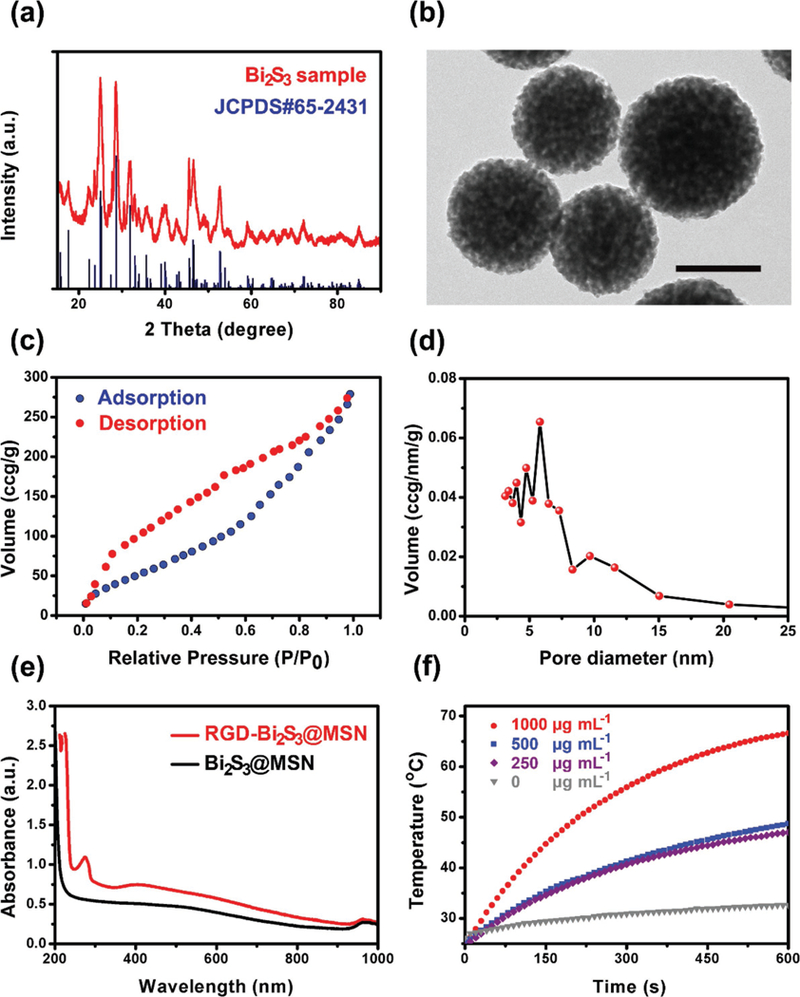
Characterization of the Bi2S3@MSN. a) XRD spectra of the Bi2S3 NPs and the JCPDS standard for Bi2S3. b) TEM image of the Bi2S3@MSN. Scale bar = 100 nm. c) Nitrogen adsorption/desorption isotherms. d) Pore size distribution. e) UV–vis–NIR absorption spectrum of the Bi2S3@MSN and RGD–Bi2S3@MSN, the RGD–Bi2S3@MSNs exhibit a new peak in 270 nm, indicating the successful conjugation of RGD. f) Quantitative temperature change of Bi2S3@MSN different concentrations under 1 W cm–2 808 nm laser irradiation.
To determine the importance of mesoporous framework of the NPs used in this study, N2 adsorption/desorption experiments were employed to examine the specific surface area and pore size distribution of the Bi2S3@MSN. A typical mesoporous structure was observed in the Bi2S3@MSN, as shown in Figure 1c,d, with a specific surface area of 273.89 m2 g–1 and a pore size of 6 nm. The relatively large surface area and appropriate pore size of the Bi2S3@MSN are highly favorable for drug delivery applications. Furthermore, Bi2S3@MSN dispersed in aqueous media has a mean hydrodynamic diameter of 120 nm with a uniform distribution, as measured by dynamic light scattering (DLS) analysis (Figure S1, Supporting Information). Meanwhile, both the ideal size and shape of the Bi2S3@MSN are crucial and beneficial for further applications, e.g., the photothermal effect and tumor cell internalization.
The NIR absorption ability of the Bi2S3@MSN was measured via UV–vis–NIR spectroscopic analysis. As shown in Figure 1e, the NPs exhibited a broad and intense absorption from the visible to the NIR region, which is characteristic of bismuth sulfide, suggesting the potential for converting photons into heat under NIR laser irradiation. After conjugated with RGD, the samples showed a new strong absorption peak in 250–300 nm (RGD peak). Furthermore, the zeta potential (Figure S2, Supporting Information) of Bi2S3@MSN and Bi2S3@MSN–NH2 was –10.7 and 23 mV, respectively. The final RGD–Bi2S3@MSN was –13.2 mV, indicating the successful conjugation of RGD with Bi2S3@MSN–NH2. To investigate the stability of the RGD-Bi2S3@MSN nanosystem, the zeta potential and hydrophilic diameter of the NPs in different solutions including purified water, phosphate buffer (PBS) solution, and cell medium (H-DMEM with 10% FBS high glucose Dulbecco’s Modified Eagle Medium (H-DMEM) with 10% fetal bovine serum (FBS)) over 3 days were tested. As shown in Figure S3 (Supporting Information), the zeta potential fluctuated between –15 and –10 mV during 3 days of incubation in different solutions at 37 °C, indicating the good stability of the NPs. Furthermore, the sizes of the NPs were stable in all solutions in the first day with a diameter between 122 and 132 nm. The particle size increased from ≈120 to 280 nm in the next 2 days in PBS and purified water, which may due to the slight aggregation. The diameter of the NPs in cultural medium was ranged from 120 to 200 nm in 3 days, which may be due to the swelling and protein adsorption effect.
2.2. Photothermal Effect In Vitro
The interesting features (broad NIR absorption) of the as-synthesized Bi2S3@MSNs motivated us to investigate their NIR photothermal efficiency. First, the temperature changes in Bi2S3@MSN aqueous solutions with varied concentrations under an 808 nm NIR laser (1 W cm–2) were carefully investigated. As shown in Figure 1f, 10 min of irradiation (808 nm, 1 W cm–2) increased the temperature of the Bi2S3@MSN solution (1 mg mL–1) up to 66 °C compared to only 28 °C in the pure water group. Moreover, The photothermal effect showed concentration and time dependence. When the concentration was 250 μg mL–1, the temperature rose from 25 to 45 °C after 10 min NIR irradiation, which can efficiently induce cell apoptosis/necrosis.[4] The high and efficient light–heat conversion effect was remarkable. Next, the photothermal conversion (PTC) efficiency was carefully measured (shown in Figure 2b). The PTC efficiency of the Bi2S3@MSN reached 36.62%, which was even higher than that of the Bi2S3 NPs (28.1%).[22] We also compared the PTC efficiency previously reported Bi2S3 agents in Table S1 (Supporting Information) and the PTC efficiency of the Bi2S3@MSN is relative high among these agents.
Figure 2.

NIR-triggered release of DOX from Bi2S3@MSN at varied power densities. a) Illustration of drug release behavior with or without NIR laser irradiation. Burst drug release occurred after applying NIR laser irradiation. b) Photothermal conversion efficiency (η) of the NPs. Changes in drug release and temperature under c) 0.3 W cm–2, d) 0.5 W cm–2, e) 1 W cm–2, f) and 0 W cm–2 NIR irradiation.
2.3. Drug Loading and Release
The unique MSN structure provides protection for drugs and offers many attractive features, including improved drug delivery, better drug stability, and controllable drug release. Here, the commonly used OS chemotherapeutic drug DOX was chosen as a model drug to evaluate the encapsulation and release characteristics of the Bi2S3@MSN. Excitingly, as determined by the UV–vis absorbance in 482 nm (Figure S4, Supporting Information), the drug encapsulation efficiency was 99.85% and the corresponding loading degree was 49.93%.
The outstanding PTC capacity of the Bi2S3@MSN should help to trigger drug release. The NIR-responsive drug release ability of the NPs was carefully investigated. As shown in Figure 2, after the Bi2S3@MSN/DOX NPs were dispersed in aqueous solution, the NIR laser at different power densities was employed to stimulate drug release. Excitingly, even with a very low power density (0.3 W cm–2), burst drug release can be observed after 10 min continuous irradiation (12%). As the power density increased to 1 W cm–2, almost 30% of the DOX was released from the NPs. Notably, compared to previously reported Cu3BiS3 NPs,[15] core–shell CuS@MSN,[23] and Bi2S3@mPS NPs,[17] the drug release efficiency of the Bi2S3@ MSN in response to NIR light is significantly improved, even with an ultralow power density. These NIR-responsive features are highly desirable in tumor therapy because the NIR laser irradiation only applies at the tumor site during treatment and the controllable release of DOX can efficiently reduce side effects to normal tissues.
2.4. In Vitro Osteosarcoma Targeting Effect
To investigate the actively targeted effect of RGD modification, the fluorescein isothiocyanate (FITC) labeled NPs (FITC–RGD–Bi2S3@MSN or FITC–Bi2S3@MSN) and free FITC were cocultured with UMR-106 cells in dark for 24 h. After rigorous washing, all noninternalized NPs were removed and the cells were subjected to fluorescence imaging and flow cytometry analysis. As shown in Figure 3a, the strong green fluorescence was observed in the UMR-106 cells cocultured with RGD–Bi2S3@MSNs, while cells in the other groups presented a very weak fluorescence under the same condition. The flow cytometry results (Figure 3b,c) further confirmed that the mean fluorescence intensity of cells in the RGD–Bi2S3@MSN group was distinguished enhanced. These results indicated the actively osteosarcoma cells targeting capability of RGD-modified NPs.
Figure 3.

In vitro active targeting effect of the RGD–Bi2S3@MSN. a) Fluorescence images ofUMR-106 cells cocultured with free FITC, FITC–Bi2S3@MSN, and FITC–RGD–Bi2S3@MSN. b) Flow cytometry analysis of UMR-106 cells stained by the free FITC, FITC–Bi2S3@MSN, and FITC–RGD–Bi2S3@MSN. c) Quantitative analysis of fluorescence intensity of different groups in (b). Scale bar = 50 μ.m. Each value is the mean ± standard deviation (n = 3 in each group); *p < 0.05, **p < 0.01, ***p < 0.001 compared to the control group.
2.5. Biocompatibility and In Vitro Tumor Ablation Effect
The excellent drug delivery, NIR-responsive abilities, and actively targeted effect encouraged us to investigate the biocompatibility and therapeutic effect of the NPs. First, the cytotoxicity of Bi2S3@MSN was evaluated by the standard 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) method. Even at concentrations up 1 mg mL–1, the Bi2S3@MSN had no influence on cell proliferation, indicating the good biocompatibility of Bi2S3@MSN (Figure 4a). The influence of the as-synthesized Bi2S3@MSN on the hemolytic activity of red blood cells (RBCs) was investigated (Figure 4b). There was negligible hemolysis of RBCs in the PBS and Bi2S3@ MSN groups, even at a concentration of 1 mg mL–1. In contrast, the pure water group turned red because of the hemoglobin from the RBCs. Therefore, both the cell biocompatibility and hemocompatibility make the Bi2S3@MSN a promising candidate for further exploration.
Figure 4.

Biocompatibility and tumor cells killing effect in vitro. a) Cell viability of UMR-106 cells cocultured with different Bi2S3@MSN concentrations for 24 h. b) Hemolysis test of human RBCs exposed to the different concentrations of Bi2S3@MSN. The inset figure shows the picture of the centrifuge tubes after centrifugation. (+) represents the purified water, (–) represents the PBS solution. c) Tumor cells killing effect of different groups. The RGD–Bi2S3@MSN/DOX+NIR exhibited the most significantly killing effect. d) Fluorescence microscopy images of live/dead dye-stained UMR-106 cells after co-cultured with different NPs and treatment with or without 808 nm (1 W cm–2) NIR irradiation. Scale bar = 50 μ.m. e) Effects of different treatments on the express levels of Bcl-2 and caspase-3 proteins in UMR-106 cells after cocultured for 24 h using western blot. Each value is the mean ± standard deviation (n = 3 in each group); *p < 0.05, **p < 0.01, ***p < 0.001 compared to the control group.
The in vitro photothermal therapy–chemotherapy effect was studied (Figure 4c). Cell viabilities (CVs) decreased when DOX was loaded on the NPs. Under 1 W cm–2 NIR irradiation, the NPs exhibited strong photothermal and/or chemo effect on the UMR-106 cells but the CVs were still more than 20%. Impressively, cell viability in the RGD–Bi2S3@MSN/DOX+NIR group was lower than 5% due to the synergistic effect of photothermal therapy–chemotherapy. Live–dead staining was employed to prove this effect. As shown in Figure 4d, the cells in the control, NIR, and Bi2S3@MSN groups were in good condition, as most of them were stained green and in normal spindle shapes. Similarly, most tumor cells were alive in Bi2S3@ MSN+NIR group due to the lack of targeting effect. In the DOX, DOX-loaded NPs, and RGD–Bi2S3@MSN+NIR groups, the cell margin became vague, but some tumor cells remained alive. In contrast, in the RGD–Bi2S3@MSN/DOX+NIR group, all cells were red (dead) due to the targeted synergistic therapy effect. These results further confirmed the limited killing efficiency of only chemotherapy or PTT in vitro and the enhanced therapeutic effect by targeted photothermal therapy–chemotherapy.
To further understanding the mechanism of strengthened tumor cells killing effect by the combined therapy, we evaluate the Bcl-2 and caspase-3 expression in tumor cells with different treatments by western blot. Generally, DOX triggers cell apoptosis through mitochondria-dependent caspase activation and internucleosomal DNA damage. However, the overexpressed antiapoptosis protein Bcl-2 in osteosarcoma inhibits mitochondrial apoptosis and is associated with tumor chemotherapy resistance.[24] As shown in Figure 4e, the Bcl-2 protein slightly downregulated in RGD–Bi2S3@MSN/DOX and PTT groups. In the combined group, Bcl-2 significantly reduced and resulted in increased apoptosis and improved chemosensitivity. Caspase-3 is the final activate effecter in mitochondrial apoptosis pathway that leads to intracellular proteins cleavage of specific and cell death.[25] We found the combined treatment group exhibited the highest caspase-3 expression and induced more severe cell damage. Therefore, our results suggested that the synergistic photothermal therapy–chemotherapy can enhance tumor cells eradication effect through the mitochondrial apoptosis pathway.
2.6. In Vitro and In Vivo CT Imaging
In addition to the good biocompatibility and synergistic photothermal therapy–chemotherapeutic effect in vitro, the Bi2S3@MSNs address the issue of CT imaging owing to the high X-ray attenuation of Bi. To verify the CT monitoring hypothesis, different concentrations of aqueous Bi2S3@MSN were evaluated for in vitro CT imaging. As shown in Figure 5a, the CT signal intensity improves with the increase in NPs. The Hounsfield units (HUs) increased linearly ith the gradient concentrations. The slope of the HU values was ≈32.83 HU L g–1, which was higher than those of the iobitridol (25.63) and iopromide (16.38).[26]
Figure 5.

CT imaging performance of the NPs. a) In vitro CT value (HU) of Bi2S3@MSN and iobitridol. Inset: CT images of the Bi2S3@MSN and iobitridol suspensions with different concentrations. b) In vivo CT images of UMR-106 tumor-bearing nude mice recorded at 2 and 24 h after i.v. injection of the RGD–Bi2S3@MSN and Bi2S3@MSN (dosage: 200 of Bi2S3@MSN (5 mg kg–1)). The tumor site is highlighted by the red circle.
We further evaluated the CT performance in vivo. All animal studies were approved by Institutional Animal Care and Use Committee (IACUC) of Guangzhou General Hospital of Guangzhou Military Command of PLA. The aqueous RGD–Bi2S3@MSN and Bi2S3@MSN solution (5 mg kg–1) was injected into OS-bearing mice intravenously (i.v.), respectively. Figure 5b shows the CT images of the two groups. The tumor site was clearly presented and could be distinguished from the surrounding tissues in the RGD–Bi2S3@MSN group due to the active targeting effect. Moreover, the CT contrast at the tumor site was strengthened from 2 to 24 h. In the Bi2S3@MSN group, there was negligible enhancement at the tumor site. Thus, the as-synthesized RGD–Bi2S3@MSNs could serve as an ideal CT contrast agent and their long blood circulation is beneficial to tumor diagnosis and therapy.
2.7. In Vivo Tumor Ablation Effect
The temperature change in the tumor sites was carefully studied after i.v. injection of the RGD–Bi2S3@MSN, Bi2S3@MSN, and NaCl with or without 1 0 min of NIR (1 W cm–2) treatment. As shown in Figure 6a,b, the temperature in the RGD–Bi2S3@ MSN group increased to 49 °C after 10 min of continuous NIR irradiation, indicating the outstanding active targeting and photo thermal effect in vivo. The tumor temperature change also raised about 15 °C in the Bi2S3@MSN group due to the enhanced permeability and retention (EPR) effect. By contrast, in the NaCl group, the temperature change was less than 8 °C after 10 min irradiation, suggesting that the negligible effect of NIR. These results were in accordance with the in vitro results. We also measured the temperature changes under lower power densities (0.3 and 0.5 W cm–2) after i.v. injection of RGD–Bi2S3@ MSN. Under 0.3 and 0.5 W cm−2 irradiation, the temperature of the tumor site achieved 36.4 and 39.4 °C after 10 min, respectively. As expected, the temperature in tumor site was increased with power densities. Previously, Del Rosal et al.[27] have investigated the in vivo photothermal therapy effect by treating quantum dots under different power densities in tumor bearing mice. They found that intratumoral temperature increments between 35 and 50 °C and surface temperature increment near 25 °C can lead to efficient thermal therapy. In order to guarantee the therapy effect and reduce the irradiation damage to normal tissues, 1 W cm–2 was chosen for the next in vivo tumor therapy.
Figure 6.

In vivo therapy effect of different treatment groups. a) NIR photothermal images of UMR-106 bearing nude mice after i.v. injection of RGD–Bi2S3@MSN and Bi2S3@MSN. b) Quantitative temperature change of the tumor site from (a). c) Representative images of tumor bearing mice from different treatment groups. d) Images of tumor collected from tumor bearing mice with different treatment. e) Tumor volume growth curves of different treatment groups. f) Body weight changes in different treatment groups. Each value is the mean ± standard deviation (n = 4 in each group); *p < 0.05, **p < 0.01, ***p < 0.001 compared to the control group.
The highly malignant OS UMR-106 cells were injected into the back of nude mice, which led to the growth of a 5–7 mm lump after 7 days. Then, the UMR-106 tumor-bearing mice were divided into 5 groups (with 4 mice in each group). After various treatments, the tumor volume and weight change of the mice were carefully recorded every other day over the next 2 weeks. Because of the high malignancy of the OS, the tumor volume in the control group increased quickly. The tumor volume changes in the DOX+NIR, RGD-Bi2S3@MSN/DOX, and Bi2S3@MSN/ DOX+NIR groups were slightly smaller compared with the control group unfortunately. In the RGD–Bi2S3@MSN+NIR group, although a significant decrease in tumor size appeared in the first 7 days, it began to increase gradually over time. Hence, only the hyperthermia from the photothermal effect was not enough to eradicate the malignant tumor totally, allowing the residual tumor to quickly enter the logarithmic phase. Notably, when the RGD–Bi2S3@MSN/DOX was applied under NIR irradiation, the active targeting effect, burst drug release, and hyperthermia together could efficiently kill the tumors, leading to efficient suppression on the malignant sarcoma. These results indicate that the hyperthermia will enhance DOX toxicity and the combined photothermal therapy–chemotherapy will provide a significantly improved therapeutic effect. Furthermore, the changes in body weight of all groups were recorded. There were no obvious changes in body weight among all groups, whereas the mice became weaker as the tumor volume increased in the unsatisfied treatment groups.
In addition, as shown in Figure 7a, hematoxylin and eosin (H&E) staining of the tumor tissues was conducted. The cells in the control, DOX+NIR, Bi2S3@MSN/DOX+NIR, RGD–Bi2S3@ MSN/DOX groups appeared to be typical OS cancer cells, i.e., spindle shaped and a clear nucleus. Although tumor cells were damaged in the RGD–Bi2S3@MSN+NIR group, but OS recurrence can be found. In marked contrast, the cells in the Bi2S3@ MSN/DOX+NIR group exhibited serious deformation and shrinkage. Moreover, the tumor edge was also serious damaged, and no metastases were observed in the peritumoral tissues, which indicates efficient prevention of recurrence.
Figure 7.

Histological study and western blot. a) H&E staining of tumors collected from different treatments. b) Effects of RGD–Bi2S3@MSN/DOX, RGD–Bi2S3@MSN+NIR, and RGD–Bi2S3@MSN/DOX+NIR on Bcl-2 and caspase-3 protein expression in tumor tissues using western blot. c) H&E staining of main organs in different treatment groups.
In vivo Bcl-2 and caspase-3 expression in tumor tissue was also studied (Figure 7b). Obviously, the expression of Bcl-2 decreased and casepase-3 increased in the RGD–Bi2S3@MSN/DOX+NIR group, whereas these proteins only changed a little in the RGD– Bi2S3@MSN+NIR and RGD–Bi2S3@MSN/DOX groups. These results demonstrate that under NIR illumination, the RGD–Bi2S3@MSN/DOX caused intensified tumor tissue damage, which was consistent with the abovementioned findings. Therefore, based on their excellent active targeting ability, drug delivery property, and photothermal conversion effect, these NPs have great potential as antitumor nanoagents for clinical applications.
2.8. Biodistribution and H&E Staining
To further investigate the metabolism and biosafety of the as-synthesized NPs, biodistribution studies and H&E staining of the main mouse organs were employed. First, the accumulation of the Bi2S3@MSN in the main organs of the tumorbearing nude mice was determined after i.v. injection. As shown in Figure S6 (Supporting Information), the Bi2S3@ MSN mostly accumulated in the reticuloendothelial organs, such as the liver and spleen, which was consistent with previous studies.[28] In contrast, the RGD–Bi2S3@MSN accumulation was decreased in most organs, including heart, spleen, and kidney, but the Bi content in tumor was significantly increased about tenfold more than peritumoral tissues due to the active targeting effect. H&E staining (Figure 7c) showed that the main organs were in normal shapes and no obvious damages were observed in all groups. The results suggested that the NPs and the treatment approaches were in good biocompatibility. However, besides the promising advantages of Bi2S3@MSN theranostic here, new category of biodegradable Bi2S3@MSNs is still needed to further explore. In addition, the detailed signaling mechanism and a molecular level understanding involved in photothermal therapy–chemotherapy remain to be studied.
3. Conclusions
In conclusion, we synthesized for the first time uniform and well-dispersed spherical RGD–Bi2S3@MSN with active targeting effect, ultrahigh drug encapsulation efficiency (99.85%), and excellent PTT efficiency (36.62%) for both highly malignant OS diagnosis via real-time CT imaging and synergistic therapy. The DOX-loaded NPs exhibited NIR-sensitive drug release, even under an ultralow power density (0.3 W cm–2). Furthermore, the RGD–Bi2S3@MSN could function as an ideal CT contrast agent (32.83 HU L g–1) at a lower dose than the clinically used agent (iobitridol, 25.63 HU L g–1) and showed distinct CT contrast imaging at the tumor site. Significantly, the RGD–Bi2S3@MSN/ DOX combined with NIR irradiation efficiently ablate malignant OS and prevent its recurrence because the actively targeted photothermal therapy–chemotherapy can activate mitochondrial apoptosis pathway. Above all, the as-synthesized Bi2S3 NPs could be developed as promising candidates for malignant tumor diagnosis and combined photothermal therapy–chemotherapy.
Supplementary Material
Acknowledgements
Y.L. and L.H.L. contributed equally to this work. The authors gratefully acknowledge financial support from the National Key Research Program of China (Grant No. 2017YFB0702604), the National Natural Science Foundation of China (Grant No. 31771038, 81402409), the Fundamental Research Funds for the Central Universities, the Hundred, Thousand and Ten Thousand Leading Talent Project in Guangdong Program for Special Support of Eminent Professionals, the Natural Science Foundation of Guangdong Province, China (Grant No. 2015A030312004), the Scientific and Technological Projects of Guangzhou, China (Grant No. 201604020110), as well as the National Institutes of Health (Grant Nos. R01MH103133, R21GM126532). All animal experiments were approved and performed in compliance with the local ethics committee and Guangzhou General Hospital of Guangzhou Military Command institutional guidelines.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Dr Yao Lu, Department of Orthopedics, Zhujiang Hospital, Southern Medical University, 253 Gongye Road, Guangzhou 510282, China; Department of Orthopedics, Guangdong Key Lab of Orthopedic Technology and Implant Key Laboratory of Trauma & Tissue Repair of Tropical Area of PLA Guangzhou General Hospital of Guangzhou Military Command of PLA 111 Liuhua Road, Guangzhou, Guangdong 510010, China gzxiahong2@126.com.
Dr Lihua Li, Department of Orthopedics, Guangdong Key Lab of Orthopedic Technology and Implant Key Laboratory of Trauma & Tissue Repair of Tropical Area of PLA Guangzhou General Hospital of Guangzhou Military Command of PLA 111 Liuhua Road, Guangzhou, Guangdong 510010, China gzxiahong2@126.com; China–Germany Research Center for Photonic Materials and Device the State Key Laboratory ofLuminescent Materials and Devices School of Materials Science and Engineering South China University of Technology 381 Wushan Road, Guangzhou 510641, China pengmingying@scut.edu.cn.
Dr Zefeng Lin, Department of Orthopedics, Guangdong Key Lab of Orthopedic Technology and Implant Key Laboratory of Trauma & Tissue Repair of Tropical Area of PLA Guangzhou General Hospital of Guangzhou Military Command of PLA 111 Liuhua Road, Guangzhou, Guangdong 510010, China gzxiahong2@126.com.
Dr Mei Li, Department of Orthopedics, Guangdong Key Lab of Orthopedic Technology and Implant Key Laboratory of Trauma & Tissue Repair of Tropical Area of PLA Guangzhou General Hospital of Guangzhou Military Command of PLA 111 Liuhua Road, Guangzhou, Guangdong 510010, China gzxiahong2@126.com.
Dr Xiaoming Hu, Department of Orthopedics, Guangdong Key Lab of Orthopedic Technology and Implant Key Laboratory of Trauma & Tissue Repair of Tropical Area of PLA Guangzhou General Hospital of Guangzhou Military Command of PLA 111 Liuhua Road, Guangzhou, Guangdong 510010, China gzxiahong2@126.com.
Yu Zhang, Department of Orthopedics, Guangdong Key Lab of Orthopedic Technology and Implant Key Laboratory of Trauma & Tissue Repair of Tropical Area of PLA Guangzhou General Hospital of Guangzhou Military Command of PLA 111 Liuhua Road, Guangzhou, Guangdong 510010, China gzxiahong2@126.com
Mingying Peng, China–Germany Research Center for Photonic Materials and Device the State Key Laboratory ofLuminescent Materials and Devices School of Materials Science and Engineering South China University of Technology 381 Wushan Road, Guangzhou 510641, China pengmingying@scut.edu.cn
Hong Xia, Department of Orthopedics, Guangdong Key Lab of Orthopedic Technology and Implant Key Laboratory of Trauma & Tissue Repair of Tropical Area of PLA Guangzhou General Hospital of Guangzhou Military Command of PLA 111 Liuhua Road, Guangzhou, Guangdong 510010, China gzxiahong2@126.com
Gang Han, Department of Biochemistry and Molecular Pharmacology University of Massachusetts Medical School Worcester, MA 01605, USA gang.Han@umassmed.edu
References
- [1].Kansara M, Teng MW, Smyth MJ, Thomas DM, Nat. Rev Cancer 2014, 14, 722. [DOI] [PubMed] [Google Scholar]
- [2].Fujiwara T, Ozaki T, Stem Cells Int. 2016, 2016, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ma H, Jiang C, Zhai D, Luo Y, Chen Y, Lv F, Yi Z, Deng Y, Wang J, Chang J, Adv. Funct. Mater. 2016, 26, 1197. [Google Scholar]
- [4].Jaque D, Martinez Maestro L, del Rosal B, Haro-Gonzalez P, Benayas A, Plaza JL, Martin Rodriguez E, Garcia Sole J, Nanoscale 2014, 6, 9494. [DOI] [PubMed] [Google Scholar]
- [5].a) Kuo W-S, Chang C-N, Chang Y-T, Yang M-H, Chien Y-H, Chen S-J, Yeh C-S, Angew. Chem. 2010, 122, 2771; [DOI] [PubMed] [Google Scholar]; b) Ren F, Bhana S, Norman DD, Johnson J, Xu L, Baker DL, Parrill AL, Huang X, Bioconjugate Chem. 2013, 24, 376. [DOI] [PubMed] [Google Scholar]
- [6].a) Li L, Yang X, Hu X, Lu Y, Wang L, Peng M, Xia H, Yin Q, Zhang Y, Han G, J. Mater. Chem. B 2017, 5, 6740; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Li L, Rashidi LH, Yao M, Lun M, Chen L, Zhang J, Yu Z, Wei C, Photodiagn. Photodyn. Ther. 2017, 19, 5; [DOI] [PubMed] [Google Scholar]; c) Lu Y, Li L, Lin Z, Wang L, Lin L, Li M, Zhang Y, Yin Q, Li Q, Xia H, Adv. Healthcare Mater. 2018, 7, 1800013. [DOI] [PubMed] [Google Scholar]
- [7].a) Nair LV, Nagaoka Y, T. Maekawa, Sakthikumar D, Jayasree RS, Small 2014, 10, 2771; [DOI] [PubMed] [Google Scholar]; b) Ge J Jia Q, Liu W, Lan M, Zhou B, Guo L, Zhou H, Zhang H, Wang Y, Gu Y, Meng X, Wang P, Adv. Healthcare Mater. 2016, 5, 665. [DOI] [PubMed] [Google Scholar]
- [8].a) Jian WH, Yu TW, Chen CJ, Huang WC, Chiu HC, Chiang WH, Langmuir 2015, 31, 6202; [DOI] [PubMed] [Google Scholar]; b) Hu D, Liu C, Song L, Cui H, Gao G, Liu P, Sheng Z, Cai L, Nanoscale 2016, 8, 17150. [DOI] [PubMed] [Google Scholar]
- [9].a) Thomson JW, Lawson G, O’Brien P, Klenkler R, Helander MG, Petrov S, Lu ZH, Kherani NP, Adronov A, Ozin G, Adv. Mater. 2010, 22, 4395; [DOI] [PubMed] [Google Scholar]; b) Martinez L, Bernechea M, Arquer FPGD, Konstantatos G, Adv. Energy Mater. 2011, 1, 1029. [Google Scholar]
- [10].a) Liu Y, Ai K, Lu L, Acc. Chem. Res. 2012, 45, 1817; [DOI] [PubMed] [Google Scholar]; b) Lusic H, Grinstaff MW, Chem. Rev. 2013, 113, 1641; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wei B, Zhang X, Zhang C, Jiang Y, Fu YY, Yu C, Sun SK, Yan XP, ACS Appl. Mater. Interfaces 2016, 8, 12720. [DOI] [PubMed] [Google Scholar]
- [11].Liu J, Zheng X, Yan L, Zhou L, Tian G, Yin W, Wang L, Liu Y, Hu Z, Gu Z, ACS Nano 2015, 12, 696. [DOI] [PubMed] [Google Scholar]
- [12].Liu J, Wang P, Zhang X, Wang L, Wang D, Gu Z, Tang J, Guo M, Cao M, Zhou H, Liu Y, Chen C, ACS Nano 2016, 10, 4587. [DOI] [PubMed] [Google Scholar]
- [13].Li B, Ye K, Zhang Y, Qin J, Zou R, Xu K, Huang X, Xiao Z, Zhang W, Lu X, Hu J, Adv. Mater. 2015, 27, 1339. [DOI] [PubMed] [Google Scholar]
- [14].Aerts A, Impens NR, Gijs M, D’Huyvetter M, Vanmarcke H, Ponsard B, Lahoutte T, Luxen A, Baatout S, Curr. Pharm. Des. 2014, 20, 5218. [DOI] [PubMed] [Google Scholar]
- [15].Zhou SM, Ma DK, Zhang SH, Wang W, Chen W, Huang SM, Yu K, Nanoscale 2016, 8, 1374. [DOI] [PubMed] [Google Scholar]
- [16].Dou R, Du Z, Bao T, Dong X, Zheng X, Yu M, Yin W, Dong B, Yan L, Gu Z, Nanoscale 2016, 8, 11531. [DOI] [PubMed] [Google Scholar]
- [17].Li L, Lu Y, Jiang C, Zhu Y, Yang X, Hu X, Lin Z, Zhang Y, Peng M, Xia H, Adv. Funct. Mater. 2018, 28, 1704623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ichim G, Lopez J, Ahmed SU, Muthalagu N, Giampazolias E, Delgado ME, Haller M, Riley JS, Mason SM, Athineos D, Parsons MJ, van de Kooij B, Bouchier-Hayes L, Chalmers AJ, Rooswinkel RW, Oberst A, Blyth K, Rehm M, Murphy DJ, Tait SWG, Mol. Cell 2015, 57, 860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Delbridge ARD, Valente LJ, Strasser A, Cold Spring Harbor Perspect. Biol 2012, 4, 843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Roberts MJ, Bentley MD, Harris JM, Adv. Drug Delivery Rev 2012, 64, 116. [DOI] [PubMed] [Google Scholar]
- [21].Gorelikov I, Matsuura N, Nano Lett. 2008, 8, 369. [DOI] [PubMed] [Google Scholar]
- [22].Liu J, Zheng X, Yan L, Zhou L, Tian G, Yin W, Wang L, Liu Y, Hu Z, Gu Z, ACS Nano 2015, 9, 696. [DOI] [PubMed] [Google Scholar]
- [23].Chen F, Hong H, Goel S, Graves SA, Orbay H, Ehlerding EB, Shi S, Theuer CP, Nickles RJ, Cai W, ACS Nano 2015, 9, 3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].a) Nedelcu T, Kubista B, Koller A, Sulzbacher I, Mosberger I, Arrich F, Trieb K, Kotz R, Toma CD, J. Cancer Res. Clin. Oncol. 2008, 134, 237; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Huigsloot M, Tijdens IB, Mulder GJ,van de Water B, J. Biol. Chem. 2002, 277, 35869. [DOI] [PubMed] [Google Scholar]
- [25].Strasser A, O’Connor L, Dixit VM, Annu. Rev. Biochem. 2003, 69, 217. [DOI] [PubMed] [Google Scholar]
- [26].a) Barreto JA, O’Malley W, Kubeil M, Graham B, Stephan H, Spiccia L, Adv. Mater. 2011, 23, 18; [DOI] [PubMed] [Google Scholar]; b) Krause W, Schönborn A, Rupp K,J. Liposome Res. 2011, 21, 229. [DOI] [PubMed] [Google Scholar]
- [27].Del Rosal B, Carrasco E, Ren F, Benayas A, Vetrone F, Sanz-Rodriguez F, Ma D, Juarranz A, Jaque D, Adv. Funct. Mater. 2016, 26, 6060. [Google Scholar]
- [28].a) Wang Y, Wu Y, Liu Y, Shen J, Lv L, Li L, Yang L, Zeng J, Wang Y, Zhang LW, Li Z, Gao M, Chai Z, Adv. Funct. Mater. 2016, 26, 5335; [Google Scholar]; b) Xiao T, Wang Z, Sun X, Song J, Jacobson O, Gang N, Kiesewetter DO, Chen X, Theranostics 2016, 6, 2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


