Abstract
To evaluate the effect of hemodialysis on choroidal thickness and the choroidal vascularity index (CVI) in patients with end-stage renal disease (ESRD) by using swept-source optical coherence tomography.
Thirty-two eyes of 32 patients with ESRD undergoing hemodialysis were recruited prospectively. Detailed ophthalmologic examinations and swept-source optical coherence tomography were performed immediately before and after hemodialysis. Choroidal thickness maps were generated automatically by using built-in software. The CVI was calculated using binarized choroidal optical coherence tomography images. Systemic parameters such as body weight and blood pressure were also measured. The changes in systemic and ocular parameters during hemodialysis were evaluated. Subjects were divided into 2 groups (diabetes mellitus [DM] vs non-diabetes mellitus) for subgroup analysis.
Total choroidal thickness showed a significant overall decrease after hemodialysis (−10.9 ± 14.0, P <.001). In the subgroup analysis, total choroidal thickness significantly decreased in both patients with DM (−11.3 ± 13.6, P = .004) and those without (−10.6 ± 14.9, P = .020), but the reduction of choroidal thickness was observed in more subfields in patients with DM than in those without. The CVI did not significantly change after hemodialysis (P = .717). No significant systemic and ocular factors affected the changes in total choroidal thicknesses.
Choroidal thickness significantly decreased after hemodialysis in most subfields regardless of the presence of DM. Peri-hemodialysis choroidal changes could be considered in the management of patients with ESRD. Swept-source optical coherence tomography can provide ample and reliable quantitative data for monitoring ocular hemodynamic changes.
Keywords: choroidal thickness, choroidal vascular index, hemodialysis, swept-source optical coherence tomography
1. Introduction
End-stage renal disease (ESRD) is a common cause of systemic accumulation of body fluids. Hemodialysis can remove excessive water and uremic substances and correct the composition and volume of body fluids. Patients with ESRD undergoing hemodialysis exhibit a wide range of ocular effects, including refractive changes, dry eye, increased tear osmolarity, corneal endothelial changes, and intraocular pressure (IOP) changes.[1–8] Among the ocular structures, the choroid is a highly vascularized and cavernous tissue, which plays a role in the transport of oxygen and nutrients to the outer retina. Sudden changes in body fluids can affect choroidal volume and thickness during hemodialysis. Therefore, most previous studies have focused on the changes in choroidal thickness after hemodialysis.[1,2,7,9–11] These studies measured the choroidal thickness of a limited area by using an enhanced depth imaging (EDI) technique of spectral-domain optical coherence tomography (SD-OCT). However, it could not reflect the choroidal thickness of the entire macula because the choroid is composed of a highly anastomosed network of choriocapillaries. Moreover, the choroidal vascularity index (CVI) has recently been used instead of choroidal thickness as a novel OCT marker for assessing the choroid.[12–14]
Recently, swept-source OCT (SS-OCT) has been introduced, and it allows for better resolution imaging when studying chorioretinal diseases because of the longer wavelength employed (around 1050 nm), higher number of scans that can be averaged, higher image capture rate, and uniform image quality over depth. Copete et al suggested that SS-OCT is a superior modality for measuring the choroidal thickness of normal eyes.[15] Therefore, we aimed to investigate choroidal thickness maps by using SS-OCT before and after hemodialysis in patients with ESRD caused by diabetes mellitus (DM) and non-DM. In addition, we measured the CVI to determine the changes in choroidal vasculature and stroma.
2. Methods
2.1. Subjects
Thirty-two eyes of 32 patients with ESRD undergoing hemodialysis at the Dialysis Center of Hanyang Guri Hospital were recruited prospectively from September 4, 2017 to September 7, 2017 for this study, which was performed according to the tenets of the Declaration of Helsinki. The institutional review board approved the study protocol (IRB 2016-05-005). The inclusion criteria were a best-corrected visual acuity (BCVA) exceeding 6/60 and availability of OCT images with unremarkable media opacity. When both eyes met the inclusion criteria, the eye with better BCVA was selected. On the basis of the hemodialysis method, we included patients who underwent hemodialysis sessions 3 times a week (on alternate days) for more than 1 year. The exclusion criteria were as follows: the presence of any retinal disease except diabetic retinopathy, such as macular hole, neovascular age-related macular degeneration, or macular edema of any origin; a history of glaucoma; recent history (within 3 months) of intraocular surgery or intravitreal injection; too long or short axial length (AXL; less than 21 mm or more than 27 mm); and low-quality OCT images.
The patients underwent 3- to 4-h hemodialysis sessions 3 times a week, using a high-performance dialyzer at a blood flow rate of 250 mL/min. The standard dialysate flow rate was 500 mL/min. The causes of ESRD were DM (n = 17); hypertensive nephrosclerosis (n = 4); polycystic kidney disease (n = 1); and chronic glomerulonephritis, for example, IgA nephropathy and focal segmental glomerulosclerosis (n = 5). Five patients had ESRD of unknown origin. Patients were divided into 2 groups: DM group (18 eyes of 18 patients) and non-DM group (14 eyes of 14 patients). Participants in the DM group has no history of retinal diseases, with the exception of mild non-proliferative diabetic retinopathy.
2.2. Measurements
All measurements were performed near the dialysis center. Measurements were performed on Monday (for patients who underwent dialysis sessions on Monday, Wednesday, and Friday) or Tuesday (for patients who underwent dialysis sessions on Tuesday, Thursday, and Saturday). Detailed ophthalmologic examinations, including BCVA, IOP, AXL, and SS-OCT, were performed on each subject within 10 minutes before and after a single hemodialysis session by using the same time intervals. IOP was measured using Tonopen (Reichert, NY). The AXL was measured using IOLMaster500 (Carl Zeiss, Germany). The body weight, ultrafiltration volume, and systolic/diastolic blood pressure were measured before and after hemodialysis. The ultrafiltration volume represented the expected amount of fluid removed during hemodialysis.
2.3. SS-OCT scan protocol
Choroidal thickness maps were obtained using SS-OCT (DRI OCT Triton, Topcon, Tokyo). A well-trained technician performed all OCT examinations, without pupil dilation. We selected the 3D combination volume wide scan protocol that provided 10 high-averaged raster scans (5 horizontal and 5 vertical) centered on the fovea as well as a volumetric scan consisting of 512 A-scans and 256 B-scans, which covered the entire macular area (12 × 9 mm2). The choroidal thickness topographic maps were generated automatically by the built-in automated segmentation software in the Early Treatment Diabetic Retinopathy Study (ETDRS) style.[16] Representative points within the ETDRS grid were determined as the central (fovea), and 1 and 2.25 mm temporal, superior, nasal, and inferior to the macula. Two retinal specialists (YUS and HC) corrected all segmentation errors. Averaged values from the 2 readers were used for analysis.
2.4. Choroidal vascularity index measurement
For calculating the CVI, the SS-OCT images were binarized using public domain software, Image J (version 1.47; http://imagej.nih.gov/ij). We selected each horizontal and vertical high-averaged scan centered on the fovea. Bruch's membrane and the chorio-scleral interface were delineated using the machine's built-in automatic segmentation software, and manual fine adjustments were made when necessary; the subfoveal choroidal thickness (SFCT) was automatically measured using the built-in caliper. Horizontal and vertical scans were binarized using the Niblack autolocal threshold tool described by Sonoda et al.[17] Dark pixels represented the lumen of blood vessels, and light pixels represented the stromal tissue between the blood vessels. The area with dark pixels was called the luminal area (LA), and the area with light pixels was called the stromal area (SA). The total choroidal area (TCA) of interest was calculated by multiplying the standard width of 150 μm (75 μm on either side of the fovea) by the SFCT (between Bruch's membrane and the chorio-scleral interface; Fig. 1). The average values of LA, SA, and TCA from a horizontal and vertical scan were used. The CVI was calculated as the ratio of the LA to the TCA.
Figure 1.
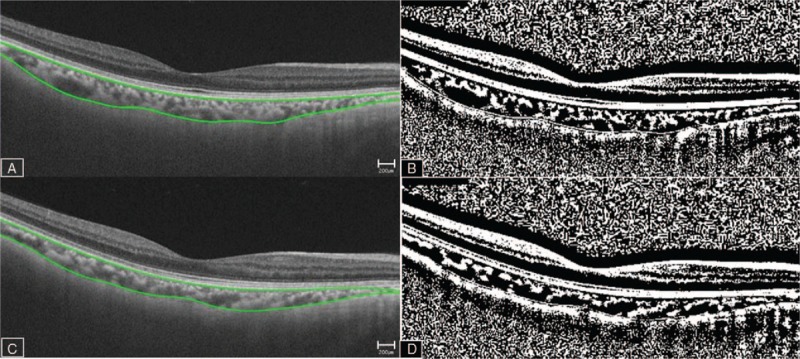
Image binarization for the choroid. Swept-source optical coherence tomography image acquired before (A, B) and after (C, D) hemodialysis. Each scan is binarized using the ImageJ software (B, D). The LA is defined as the area with dark pixels. The SA is defined as the area with light pixels. The TCA of interest is calculated by multiplying the standard width of 150 μm (75 μm on either side of the fovea) by (SFCT; between Bruch's membrane and the chorio-scleral interface). The average of the LA, SA, and SFCT of the horizontal and vertical scans are used. The choroidal vascularity index is calculated as the ratio of the LA to the TCA. LA = luminal area, SA = stromal area, SFCT = subfoveal choroidal thickness, TCA = total choroidal area.
2.5. Statistical analysis
All values are expressed as the mean ± standard deviation. The Mann−Whitney test was used to compare characteristics between the DM and non-DM groups. The Wilcoxon signed-rank test was used to compare the IOP, AXL, and choroidal thickness before and after hemodialysis. The repeated-measures ANOVA test was used to compare the change in choroidal thickness after hemodialysis according to the subgroups (DM vs non-DM). A regression analysis was performed to identify factors related to the changes in choroidal thickness. IBM SPSS Statistics for Windows/Macintosh, Version 22.0 (IBM Corp., Armonk, NY) was used for all statistical analyses. P less than .05 was considered significant.
3. Results
3.1. Baseline characteristics
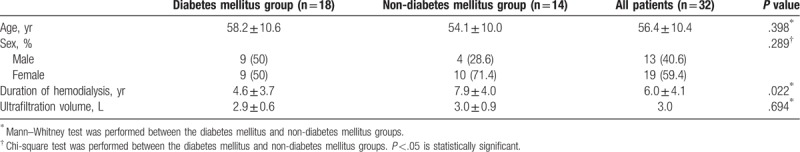
We enrolled 32 eyes of 32 patients (13 men and 19 women; mean age, 56.4 ± 10.4 years) who met the study criteria. Five patients were excluded because of macular edema (2 eyes), low-quality SS-OCT images (2 eyes), or too long AXL (1 eye). Eighteen patients had diabetes and 24 patients had hypertension. Ten patients had both diabetes and hypertension. At the time of the study, all the patients were taking medications for hypertension and/or diabetes. The baseline clinical characteristics are shown in Table 1. The patients were divided into 2 groups: DM group (18 eyes of 18 patients) and non-DM group (14 eyes of 14 patients). The mean age of the patients with ESRD in the DM and non-DM groups was 58.2 ± 10.6 and 54.1 ± 10.0 years, respectively. The mean age did not differ significantly between the 2 groups (P = .268). The average duration of DM was 15.7 years. We did not measure blood glucose levels before and after the hemodialysis, but the mean of HbA1c values measured 3 months prior this study was 6.5%. At that time, all patients in the DM group had mild non-proliferative diabetic retinopathy, without macular edema.
Table 1.
Demographic characteristics of each study group before hemodialysis.
3.2. Effect of hemodialysis on choroidal thickness
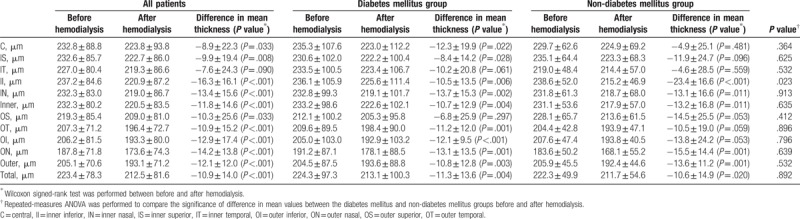
The mean total choroidal thickness significantly decreased in almost all subfields (except the inner temporal subfield), and total choroidal thickness change was significantly low in all patients (−10.9 ± 14.0, P <.001). In the subgroup analysis, total choroidal thickness significantly decreased in both the DM (-11.3 ± 13.6, P = .004) and non-DM groups (−10.6 ± 14.9, P = .020). Choroidal thickness decreased in more subfields in the DM group than in the non-DM group. In the DM group, choroidal thickness in all subfields decreased, except in the inner temporal and outer superior subfields. However, in the non-DM group, no change in choroidal thickness was observed in 6 of the 9 subfields (central, inner superior, inner temporal, outer superior, outer temporal, and outer inferior). No significant difference was observed in the values of overall choroidal thickness change (Δ) between 2 groups (P = .892), but the decrease in choroidal thickness in the inner inferior subfields of the DM group was significantly higher than that in the non-DM group (P = .023). The detailed values of choroidal thickness in each subfield are presented in Figure 2 and Table 2.
Figure 2.
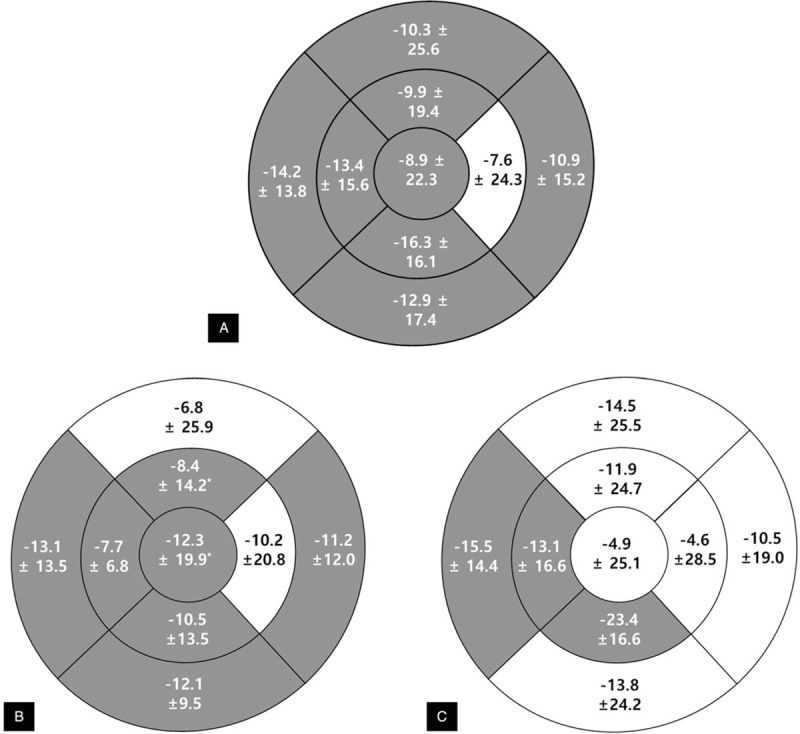
Choroidal thickness changes (Δ, μm) after hemodialysis in all patients and subgroups (diabetes mellitus vs. non-diabetes mellitus). In all patients (A), choroidal thickness significantly decreases in most subfields except in the inner temporal subfield after hemodialysis. Total choroidal thickness change significantly decreases after hemodialysis (−10.9 ± 14.0 μm, P <.001). In subgroup analysis, choroidal thickness changes (Δ) decrease in more subfields in the diabetes mellitus group (B) than in the non-diabetes mellitus group (C). Gray color indicates statistically significant differences before and after hemodialysis (P <.05).
Table 2.
Effect of hemodialysis on each subfield of choroidal thickness in patients with end-stage renal disease.
3.3. Changes in the CVI
The LA, SA, TCA, and SFCT significantly decreased after hemodialysis (LA: −0.009 ± 0.01, P = .001; SA: −0.005 ± 0.010, P = .004; TCA: −0.02 ± 0.04, P = .002; SFCT: −14.1 ± 23.5, P = .002). However, the CVI did not change significantly after hemodialysis (0.6 ± 4.2, P = .393). No significant differences were observed between the DM and non-DM groups in the LA, SA, TCA, and CVI before and after hemodialysis.
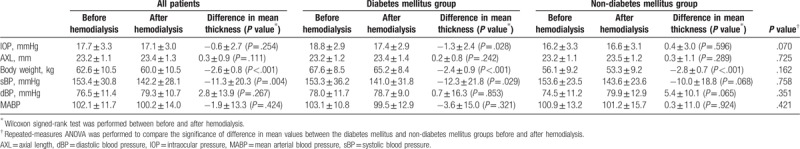
3.4. Changes in other ocular and systemic parameters before and after hemodialysis
IOP decreased in all patients after hemodialysis (−0.6 ± 2.7), but it was significant only in the DM group (−1.3 ± 2.4, P <.05). AXL changes were not significant after hemodialysis. The body weight decreased significantly in all patients (−2.6 ± 0.8, P <.05; DM: −2.4 ± 0.9; non-DM: −2.8 ± 0.7). Systolic blood pressure decreased in all patients (−11.3 ± 20.3, P <.05), but it was significant only in the DM group (−12.3 ± 21.8, P <.05). Diastolic blood pressure and mean arterial blood pressure changes were not significant after hemodialysis. No significant difference was observed in the mean values between the DM and non-DM groups. The detailed values are presented in Table 3.
Table 3.
Ocular and systemic parameter changes before and after hemodialysis.
3.5. Associations between the changes in choroidal thickness and other parameters before and after hemodialysis
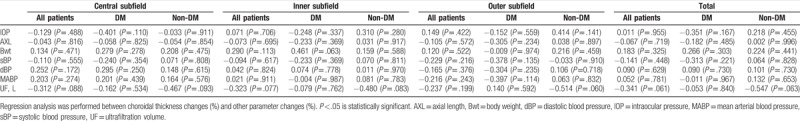
Univariate regression analysis revealed no significant association between choroidal thickness and other parameters, such as changes in IOP, body weight, systolic, and diastolic blood pressure, mean arterial blood pressure, and ultrafiltration rate. The detailed values are presented in Table 4.
Table 4.
Correlation between choroidal thickness changes (%) and changes (%) in other parameters of each subfield.
4. Discussion
The present study using SS-OCT showed that choroidal thickness was significantly decreased in most subfields after hemodialysis in patients with ESRD and that the change in choroidal thickness was lower in more subfields in the DM group than in the non-DM group. The LA, SA, TCA, and SFCT significantly decreased after hemodialysis; however, the CVI showed no significant difference after hemodialysis. Moreover, these findings showed no significant differences between the DM and non-DM groups.
Although previous studies have examined the effect of hemodialysis on choroidal thickness, their results have been controversial. Akihiro et al who measured the horizontal and vertical sections of SFCT, as the perpendicular distance from the center of the fovea by using SD-OCT, found that SFCT decreased in all eyes after hemodialysis. They also found that SFCT in the DM group was significantly lower than that in the non-DM group.[18] Ulas et al included patients without DM but with ESRD and measured choroidal thicknesses at 3 points, the central fovea and 1500 μm nasal and temporal from the center of the fovea, by using EDI-OCT. They also showed that choroidal thickness of these points significantly decreased.[9] These results are consistent with those of our study. Another study performed by Jung et al showed that SFCT increased after hemodialysis.[11] However, they measured SFCT manually by using SD-OCT without using the EDI mode.
The similarity between these previous studies was in the use of SD-OCT for measuring choroidal thickness. SD-OCT using the EDI technique can identify the chorio-scleral interface, but it cannot obtain data covering the entire macular area because it analyzes a small number of scans. These limitations of SD-OCT do not allow the measurement of choroidal thickness of the entire macular area. Because the choroid is a fully vascularized and cavernous structure, choroidal thickness measurement in only a limited area would not reflect the accurate choroidal status. Therefore, we used SS-OCT in this study to overcome these limitations. Recent advances in SS-OCT imaging have enabled us to visualize deeper layers and determine the full choroidal length, and automated segmentation has enabled the analysis of the entire macular area. To our knowledge, no previous studies have investigated choroidal thickness changes after hemodialysis by using SS-OCT. In this study, we aimed to obtain more reliable and accurate data regarding choroidal thickness than those obtained in previous studies using SD-OCT.
Unlike previous studies, we assessed the change in the CVI after hemodialysis. Currently, the choroidal components that contribute to variations in choroidal thickness remain unknown. Because the choroid is composed of various tissues such as blood vessels, nerves, and connective tissue, measuring choroidal thickness alone does not provide us information on which tissues in the choroid change.[12] The changes in choroidal thickness may be due to a blood vessel, stromal tissue, or both. Therefore, choroidal thickness might not be an accurate tool in clinical study, because many ocular and systemic factors affect choroidal thickness.[19] No investigation has been performed on the choroidal structures that contribute to the change in choroidal thickness in patients with ESRD after hemodialysis. Agrawal et al introduced the CVI as a novel marker for assessing the choroidal status.[12] They found that the CVI showed lesser variability and was influenced by fewer physiologic factors than was choroidal thickness; this indicated that the CVI was a relatively stable index for studying choroidal changes.[12] This index has been applied as a disease monitoring tool in patients with DM[14] and in those with Vogt-Koyanagi-Harada disease.[13] In this study, we also applied the CVI to assess choroidal changes after hemodialysis. Our study showed that both the LA and SA significantly decreased after hemodialysis. However, the CVI did not show a significant change after hemodialysis. Moreover, no significant difference was observed between the DM and non-DM groups in the LA, SA, and CVI before and after hemodialysis.
Choroidal changes after hemodialysis in this study may be caused by the change in the amount of body fluids during hemodialysis. Almost all the patients with ESRD who underwent hemodialysis gained weight over their dry weight because of fluid accumulation in the intra/extravascular compartments before hemodialysis. Hemodialysis leads to an increased plasma colloid osmotic pressure and transcapillary colloid osmotic gradient and results in a parallel decrease in plasma volume and interstitial fluid volume.[20] In the choroid, the vasculature is well fenestrated and the extravascular (interstitial) compartment has very high permeability for fluids and small molecules.[21,22] Systemic fluid accumulation before hemodialysis may be associated with fluid accumulation in the intravascular and interstitial compartments of the choroid. After hemodialysis, the decreased choroidal thickness, LA, and SA might be associated with the removal of the intravascular and interstitial fluids because of the increased transcapillary colloid osmotic gradient. The CVI was defined as the proportion of LA to TCA. Therefore, in cases in which both the LA and SA have decreased, the CVI before and after hemodialysis will not be significantly changed.
Our study showed that the change in choroidal thickness after hemodialysis was more prominent in the DM group than in the non-DM group. A histopathologic study on diabetic choroidopathy reported that the choriocapillaries and other choroidal vessels showed marked basement membrane thickening and luminal narrowing of the capillaries, capillary dropout, and focal scarring, which may lead to choroidal compromise.[23] Another recent study suggested that patients with diabetic neuropathy showed increased choroidal thickness than did normal subjects, possibly because of a dysregulated autonomic nervous system.[24] We hypothesized that histologic alterations and an impaired autonomic system in the diabetic choroid might lead to the dysregulation of both osmotic and hydrostatic pressures and that more choroidal changes might be expected after hemodialysis in the DM group. In this study, we did not classify the DM group in detail. However, if the study sample were larger, the association between the degree of diabetic retinopathy or neuropathy and choroidal changes after hemodialysis might have been clearer.
We investigated the changes in ocular and non-ocular parameters during hemodialysis to identify significant factors associated with choroidal thickness. Previous studies have reported various results regarding the association between choroidal thickness and other factors. Ulas et al reported no significant correlations between the change in SFCT and the change in IOP, plasma colloid osmotic pressure, plasma osmolality, and ultrafiltration volume after hemodialysis; these results were similar to our results.[9] However, Yang et al showed a significant correlation between total weight loss after hemodialysis and choroidal thickness change.[2] A recent study that divided patients into the DM and non-DM groups suggested that only the non-DM group showed a significant relationship between the changes in SFCT and body fluid removal.[18] This difference may be attributed to limited sample sizes, inhomogeneous selection of patients with ESRD, and differences in OCT devices used.
Our study has some strength over previous studies. First, our data may be more reliable than those obtained in previous hemodialysis-related OCT studies because we estimated choroidal thickness map changes by using automated segmentation of SS-OCT, which enabled us to visualize deeper layers and to minimize subjective errors in delineating the chorio-scleral interface. Second, our measurements were performed on Mondays (for patients who underwent dialysis on Monday, Wednesday, and Friday) and Tuesdays (for patients who underwent dialysis on Tuesday, Thursday, and Saturday) because we intended to maximize the change in choroidal thickness caused by hemodialysis through the accumulation of body fluids over the weekends (2 days without hemodialysis). Third, all measurements were performed near the dialysis center. This immediate measurement could reduce the error caused by the time lag between dialysis and ophthalmic examinations and decrease patient discomfort.
However, our study has some limitations. First, although the measurements performed on Mondays and Tuesdays were strength of our study, it was also a shortcoming because the hemodialysis sessions performed on these days after the weekend were not part of a regular hemodialysis cycle like the sessions on other weekdays. Therefore, our results should be interpreted with caution. Second, in measuring the CVI, it is necessary to assume that dark areas on the choroid indicate vascular regions of the choroid and the light areas indicate stromal regions. However, no study has proven this assumption. In addition, we used only 2 high-averaged 2-dimensional scans to obtain the CVI because volume scan images acquired using SS-OCT were too numerous to analyze and their image quality was lower than that of a single high-averaged scan. Further high-quality volume scans and the use of automated algorithms for measuring the CVI may provide more reliable data. Finally, choroidal thickness may be affected by systemic conditions such as diabetes or hypertension.[25,26] Long term use of anti-hypertensive and/or anti-diabetic drugs may potentially affect choroidal vascular flow. However, in this study, we did not investigate the effect of systemic medications on choroid thickness.
In conclusion, SS-OCT enabled us to analyze more accurate and reliable data regarding choroidal thickness maps in patients with ESRD. Choroidal thickness significantly decreased after hemodialysis in most subfields. This alteration was observed in more subfields in patients with DM. When choroidal thickness decreased, both the LA and SA of the choroid also decreased simultaneously. When evaluating patients with ESRD, who are undergoing hemodialysis, ophthalmologists should consider these factors in assessing the choroidal status.
Acknowledgments
We would like to thank Eunwoo, Nam, PhD (Biostatistical Consulting and Research Lab, Hanyang University, Seoul, Korea) for assistance with statistical analysis.
Author contributions
Conceptualization: Sang-Woong Han, Joo-Hark Yi, Heeyoon Cho.
Data curation: Sang Eun Lee, Sang-Woong Han, Joo-Hark Yi, Heeyoon Cho.
Formal analysis: Yong Un Shin, Sang Eun Lee, Min Ho Kang, Sang-Woong Han, Heeyoon Cho.
Funding acquisition: Yong Un Shin, Heeyoon Cho.
Methodology: Yong Un Shin, Sang Eun Lee, Sang-Woong Han, Joo-Hark Yi, Heeyoon Cho.
Resources: Sang-Woong Han, Joo-Hark Yi.
Supervision: Sang-Woong Han, Joo-Hark Yi, Heeyoon Cho.
Validation: Joo-Hark Yi, Heeyoon Cho.
Writing – original draft: Yong Un Shin, Sang Eun Lee.
Writing – review & editing: Yong Un Shin, Sang Eun Lee, Min Ho Kang, Joo-Hark Yi, Heeyoon Cho.
Footnotes
Abbreviations: AXL = axial length, BCVA = best-corrected visual acuity, CVI = choroidal vascularity index, DM = diabetes mellitus, EDI = enhanced depth imaging, ESRD = End-stage renal disease, IOP = intraocular pressure, LA = luminal area, SA = stromal area, SD-OCT = spectral-domain optical coherence tomography, SFCT = subfoveal choroidal thickness, SS-OCT = swept-source OCT, TCA = total choroidal area.
YUS and SEL contributed equally to this article.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (NRF-2017R1D1A1B03030905 and NRF-2017R1D1A1B03031934).
The authors have no conflicts of interest to disclose.
References
- [1].Chelala E, Dirani A, Fadlallah A, et al. Effect of hemodialysis on visual acuity, intraocular pressure, and macular thickness in patients with chronic kidney disease. Clin Ophthalmol 2015;9:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yang SJ, Han YH, Song GI, et al. Changes of choroidal thickness, intraocular pressure and other optical coherence tomographic parameters after haemodialysis. Clin Exp Optom 2013;96:494–9. [DOI] [PubMed] [Google Scholar]
- [3].Levy J, Tovbin D, Lifshitz T, et al. Intraocular pressure during haemodialysis: a review. Eye (Lond) 2005;19:1249–56. [DOI] [PubMed] [Google Scholar]
- [4].Hu J, Bui KM, Patel KH, et al. Effect of hemodialysis on intraocular pressure and ocular perfusion pressure. JAMA Ophthalmol 2013;131:1525–31. [DOI] [PubMed] [Google Scholar]
- [5].Samsudin A, Mimiwati Z, Soong T, et al. Effect of haemodialysis on intraocular pressure. Eye (Lond) 2010;24:70–3. [DOI] [PubMed] [Google Scholar]
- [6].Doshiro A, Ban Y, Kobayashi L, et al. Intraocular pressure change during hemodialysis. Am J Ophthalmol 2006;142:337–9. [DOI] [PubMed] [Google Scholar]
- [7].Jung JW, Yoon MH, Lee SW, et al. Effect of hemodialysis (HD) on intraocular pressure, ocular surface, and macular change in patients with chronic renal failure. Effect of hemodialysis on the ophthalmologic findings. Graefes Arch Clin Exp Ophthalmol 2013;251:153–62. [DOI] [PubMed] [Google Scholar]
- [8].Dinc UA, Ozdek S, Aktas Z, et al. Changes in intraocular pressure, and corneal and retinal nerve fiber layer thickness during hemodialysis. Int Ophthalmol 2010;30:337–40. [DOI] [PubMed] [Google Scholar]
- [9].Ulas F, Dogan U, Keles A, et al. Evaluation of choroidal and retinal thickness measurements using optical coherence tomography in non-diabetic haemodialysis patients. Int Ophthalmol 2013;33:533–9. [DOI] [PubMed] [Google Scholar]
- [10].Azem N, Spierer O, Shaked M, et al. Effect of hemodialysis on retinal thickness in patients with diabetic retinopathy, with and without macular edema, using optical coherence tomography. J Ophthalmol 2014;2014:709862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jung JW, Chin HS, Lee DH, et al. Changes in subfoveal choroidal thickness and choroidal extravascular density by spectral domain optical coherence tomography after haemodialysis: a pilot study. Br J Ophthalmol 2014;98:207–12. [DOI] [PubMed] [Google Scholar]
- [12].Agrawal R, Gupta P, Tan KA, et al. Choroidal vascularity index as a measure of vascular status of the choroid: measurements in healthy eyes from a population-based study. Sci Rep 2016;6:21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Agrawal R, Li LK, Nakhate V, et al. Choroidal vascularity index in Vogt-Koyanagi-Harada disease: an EDI-OCT derived tool for monitoring disease progression. Transl Vis Sci Technol 2016;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tan KA, Laude A, Yip V, et al. Choroidal vascularity index - a novel optical coherence tomography parameter for disease monitoring in diabetes mellitus. Acta Ophthalmol 2016;94:e612–6. [DOI] [PubMed] [Google Scholar]
- [15].Copete S, Flores-Moreno I, Montero JA, et al. Direct comparison of spectral-domain and swept-source OCT in the measurement of choroidal thickness in normal eyes. Br J Ophthalmol 2014;98:334–8. [DOI] [PubMed] [Google Scholar]
- [16].Michalewski J, Michalewska Z, Nawrocka Z, et al. Correlation of choroidal thickness and volume measurements with axial length and age using swept source optical coherence tomography and optical low-coherence reflectometry. Biomed Res Int 2014;2014:639160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sonoda S, Sakamoto T, Yamashita T, et al. Luminal and stromal areas of choroid determined by binarization method of optical coherence tomographic images. Am J Ophthalmol 2015;159: 1123-1131 e1121. [DOI] [PubMed] [Google Scholar]
- [18].Ishibazawa A, Nagaoka T, Minami Y, et al. Choroidal thickness evaluation before and after hemodialysis in patients with and without diabetes. Invest Ophthalmol Vis Sci 2015;56:6534–41. [DOI] [PubMed] [Google Scholar]
- [19].Gupta P, Jing T, Marziliano P, et al. Distribution and determinants of choroidal thickness and volume using automated segmentation software in a population-based study. Am J Ophthalmol 2015;159:293–301. [DOI] [PubMed] [Google Scholar]
- [20].Fauchald P. Transcapillary colloid osmotic gradient and body fluid volumes in renal failure. Kidney Int 1986;29:895–900. [DOI] [PubMed] [Google Scholar]
- [21].Bill A, Sperber G, Ujiie K. Physiology of the choroidal vascular bed. Int Ophthalmol 1983;6:101–7. [DOI] [PubMed] [Google Scholar]
- [22].Bill A, Tornquist P, Alm A. Permeability of the intraocular blood vessels. Trans Ophthalmol Soc U K 1980;100:332–6. [PubMed] [Google Scholar]
- [23].Hidayat AA, Fine BS. Diabetic choroidopathy. Light and electron microscopic observations of seven cases. Ophthalmology 1985;92:512–22. [PubMed] [Google Scholar]
- [24].Yazici A, Sogutlu Sari E, Koc R, et al. Alterations of choroidal thickness with diabetic neuropathy. Invest Ophthalmol Vis Sci 2016;57:1518–22. [DOI] [PubMed] [Google Scholar]
- [25].Kase S, Endo H, Yokoi M, et al. Choroidal thickness in diabetic retinopathy in relation to long-term systemic treatments for diabetes mellitus. Eur J Ophthalmol 2016;26:158–62. [DOI] [PubMed] [Google Scholar]
- [26].Akay F, Gundogan FC, Yolcu U, et al. Choroidal thickness in systemic arterial hypertension. Eur J Ophthalmol 2016;26:152–7. [DOI] [PubMed] [Google Scholar]


