Abstract
Little evidence is available about the correlation between diabetes mellitus and herpes zoster in Taiwan. This study aimed to investigate the correlation between diabetes mellitus and herpes zoster in Taiwan.
A population-based cohort study was conducted using the database of Taiwan National Health Insurance Program. There were 27,369 subjects aged 20 to 84 years with newly diagnosed diabetes mellitus from 2000 to 2012 as the diabetes mellitus group and 107,705 sex- and age-matched subjects without diabetes mellitus as the nondiabetes mellitus group. The incidence of herpes zoster at the end of 2013 was estimated. The multivariable Cox proportional hazards regression model was used to calculate the hazard ratio (HR) and 95% confidence interval (CI) of herpes zoster associated with diabetes mellitus.
The overall incidence of herpes zoster was 1.16-fold higher in the diabetes mellitus group than the nondiabetes mellitus group (7.85 vs 6.75 per 1000 person-years, 95% CI 1.12–1.20). After adjustment for co-variables, the adjusted HR of herpes zoster was 1.17 for subjects with diabetes mellitus (95% CI 1.10–1.23), compared with subjects without diabetes mellitus.
Patients with diabetes mellitus are associated with 1.17-fold increased risk for developing herpes zoster.
Keywords: cohort study, diabetes mellitus, herpes zoster, Taiwan National Health Insurance Program
1. Introduction
In 2016, there were approximately 2082,8000 new cases of diabetes mellitus and 383,453,000 cases with diabetes mellitus in the world.[1] In 2017, diabetes mellitus and its complications were the fifth leading cause of death in Taiwan.[2] There were 9845 deaths related to diabetes mellitus, which accounted for 5.73% of 171,857 total deaths in Taiwan in 2017.[2] In addition to its microvascular and macrovascular complications, diabetes mellitus has been found to be associated with immunologic dysfunctions,[3,4] which have let diabetic patients more susceptible to developing infections, including lower respiratory tract infection, pulmonary tuberculosis, pleural empyema, urinary tract infection, and skin infection.[5–7]
Herpes zoster is caused by the reactivation of latent varicella-zoster virus in sensory ganglia when an individual's specific cell-mediated immunity to varicella-zoster virus is waning, which is caused by the underlying etiologies.[8,9] Based on the theory of decreased specific cell-mediated immunity to varicella-zoster virus found in diabetic patients,[10] previous epidemiological studies have demonstrated that diabetic patients have an increased risk for development of herpes zoster,[11–14] but little evidence is available about the correlation between diabetes mellitus and herpes zoster in Taiwan.
Given diabetes mellitus being a public health concern in Taiwan due to its high prevalence, incidence, and mortality,[2,15] a population-based cohort study was conducted using the database of the Taiwan National Health Insurance Program to investigate the correlation between diabetes mellitus and herpes zoster in Taiwan.
2. Methods
2.1. Study design and data source
A population-based cohort study was conducted using the database of Taiwan National Health Insurance Program. The program was launched in March 1, 1995, and it has covered about 99.6% of 23 million residents living in the independent country of Taiwan.[16,17] The database of Taiwan National Health Insurance Program contains reimbursement claim data of each insured individual, including sex, birth date, and all medical service records. The details of the program can be found in previous studies.[18–20]
2.2. Study subjects
Subjects aged 20 to 84 years with newly diagnosed diabetes mellitus from 2000 to 2012 were selected as the diabetes mellitus group (International Classification of Diseases, Ninth Revision, Clinical Modification, ICD-9 code 250). The date for diagnosing diabetes mellitus was defined as the index date. For every subject with diabetes mellitus, nearly 4 subjects without diabetes mellitus were selected from the same database as the nondiabetes mellitus group. The diabetes mellitus and the nondiabetes mellitus groups were matched for sex, age (in 5-year span), comorbidities, and the year of index date (Fig. 1).
Figure 1.
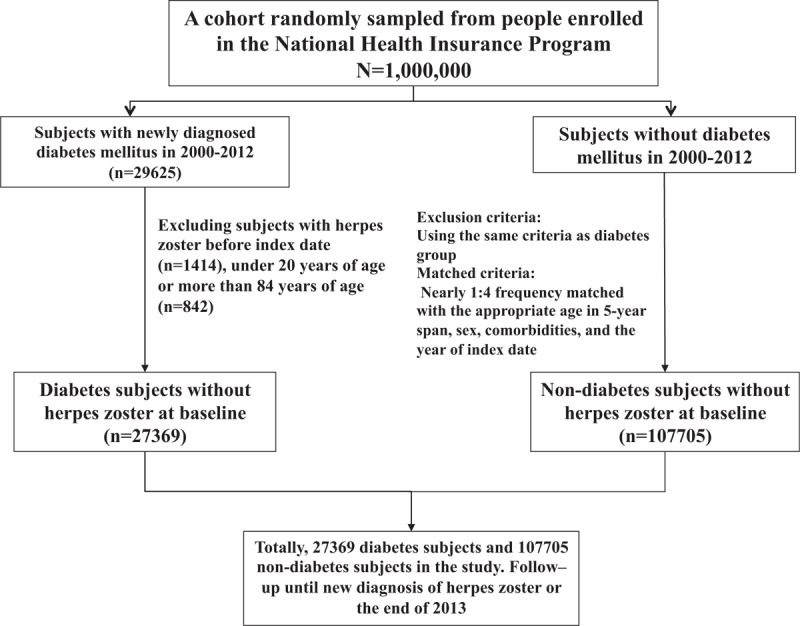
Flowchart of study subjects’ selection.
2.3. Major outcome
The main outcome was a new diagnosis of herpes zoster during the follow-up period. Each subject was followed from the index date until the subject being diagnosed with herpes zoster, or until the end of 2013.
2.4. Comorbidities
Comorbidities before the index date were included as follows: alcohol-related disease, cancer, chronic kidney disease, chronic liver disease including cirrhosis, hepatitis B, hepatitis C, and other chronic hepatitis, chronic obstructive pulmonary disease, hyperlipidemia, hypertension, as well as cardiovascular disease including coronary artery disease, heart failure, cerebrovascular disease, and peripheral atherosclerosis. All comorbidities were diagnosed based on ICD-9 codes, which have been well assessed in previous studies.[21–24]
2.5. Statistical analysis
The distributions of sex, age, and comorbidities between the diabetes mellitus group and the nondiabetes mellitus group were analyzed by using the Chi-square test for categorical variables and the t test for continuous variables. The incidence of herpes zoster was estimated as the event number of herpes zoster found during the follow-up period, divided by the total follow-up person-years for each group. The incidence rate ratio with 95% confidence interval (CI) of herpes zoster for diabetes mellitus group versus non-diabetes mellitus group was estimated by using Poisson regression, stratified by sex and age. All variables were included in a univariable model. Those variables found to be statistically significant in a univariable model were further included in a multivariable model. The multivariable Cox proportional hazards regression model was used to calculate the hazard ratio (HR) and 95% CI of herpes zoster associated with diabetes mellitus and comorbidities. All statistical analyses were performed by using the SAS 9.2 version (SAS Institute, Cary, NC). Two-tailed P < .05 was considered statistically significant.
3. Results
3.1. Baseline characteristics of the study population
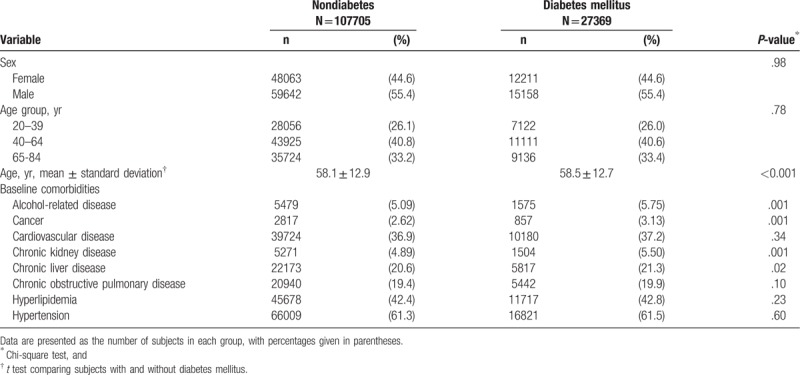
Table 1 disclosed the distributions of sex, age, and comorbidities between the diabetes mellitus group and the nondiabetes mellitus group. There were 27,369 subjects with diabetes mellitus and 107,705 subjects without diabetes mellitus, with a similar distribution of sex. Males constituted a higher proportion of the study population (55.4%). The mean ages (standard deviation) of the study subjects were 58.5 (12.7) years in the diabetes mellitus group and 58.1 (12.9) years in the nondiabetes mellitus group (t test, P < .001). The proportions of alcohol-related disease, cancer, chronic kidney disease, and chronic liver disease were significantly higher in the diabetes mellitus group than the non-diabetes mellitus group (Chi-square test, P < .05).
Table 1.
Baseline information between diabetes mellitus group and nondiabetes mellitus group.
3.2. Incidences of herpes zoster stratified by sex and age
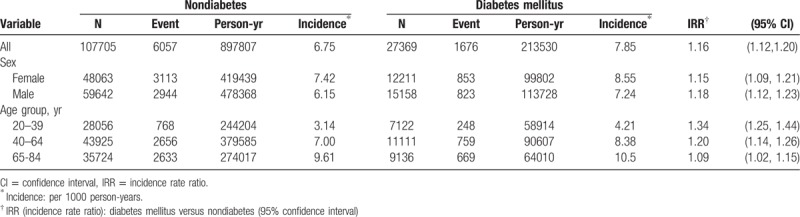
Table 2 disclosed the incidences of herpes zoster stratified by sex and age. At the end of the cohort study, the overall incidence of herpes zoster was 1.16-fold higher in the diabetes mellitus group than the nondiabetes mellitus group (7.85 vs 6.75 per 1000 person-years, 95% CI 1.12–1.20). The incidences of herpes zoster, as stratified by sex and age, were all higher in the diabetes mellitus group than the non-diabetes mellitus group. The diabetes mellitus group aged 65 to 84 years had the highest incidence of herpes zoster (10.5 per 1000 person-years). During the first 3 years of follow-up, the incidence of herpes zoster was 1.29-fold higher in the diabetes mellitus group than that in the nondiabetes mellitus group (7.38 vs 5.73 per 1000 person-years, 95% CI 1.24–1.34). After 3 years of follow-up, the incidence of herpes zoster was 1.12-fold higher in the diabetes mellitus group than the nondiabetes mellitus group (8.11 vs 7.27 per 1000 person-years, 95% CI 1.07–1.16).
Table 2.
Incidences of herpes zoster stratified by sex and age between diabetes mellitus group and nondiabetes group.
The Kaplan–Meier model disclosed that the diabetes mellitus group had a higher cumulative incidence of herpes zoster than the nondiabetes mellitus group at the end of follow-up (9.36% vs 8.08%, P < .001, Fig. 2).
Figure 2.
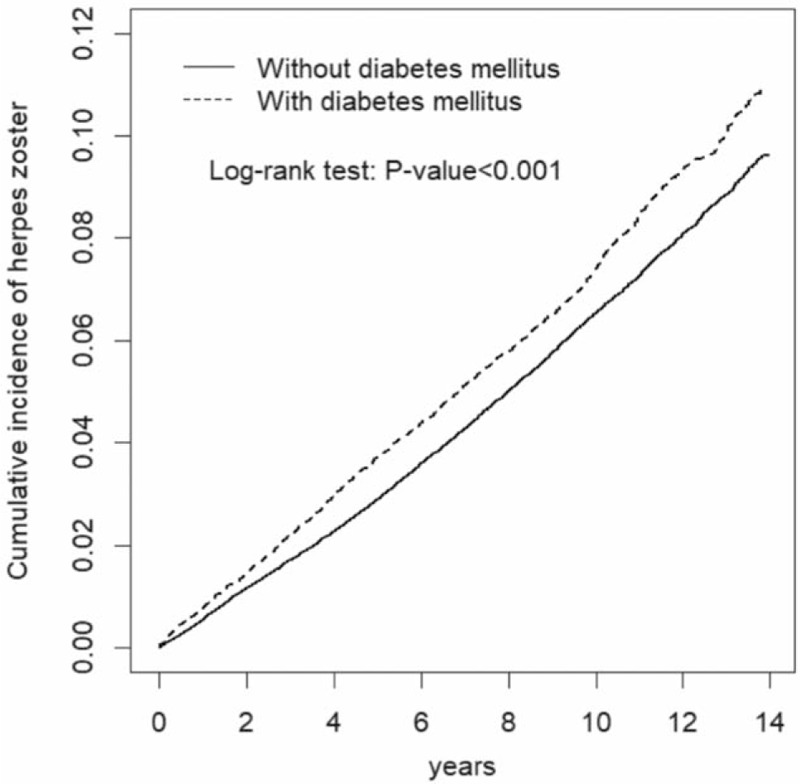
The Kaplan–Meier model disclosed that the diabetes mellitus group had a higher cumulative incidence of herpes zoster than the non-diabetes group at the end of follow-up (P < .001).
3.3. HR of herpes zoster associated with diabetes mellitus and comorbidities
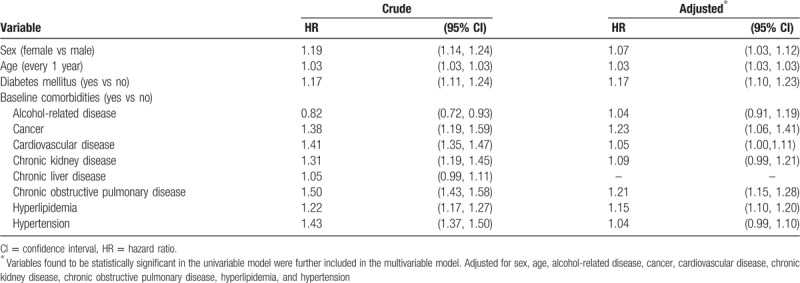
After adjustment for co-variables, the adjusted HR of herpes zoster was 1.17 for subjects with diabetes mellitus (95% CI 1.10–1.23), compared with subjects without diabetes mellitus (Table 3). In addition, female (adjusted HR 1.07, 95% CI 1.03–1.12), age (every 1 year, adjusted HR 1.03, 95% CI 1.03–1.03), cancer (adjusted HR 1.23, 95% CI 1.06–1.41), cardiovascular disease (adjusted HR 1.05, 95% CI 1.00–1.11), chronic obstructive pulmonary disease (adjusted HR 1.21, 95% CI 1.15–1.28), and hyperlipidemia (adjusted HR 1.15, 95% CI 1.10–1.20), were statistically associated with herpes zoster.
Table 3.
Hazard ratio and 95% confidence interval of herpes zoster associated with diabetes mellitus and comorbidities.
In further analysis in subjects with diabetes mellitus, the adjusted HR of herpes zoster was 0.31 for subjects with use of any antidiabetic drugs (95% CI 0.25–0.38, Table not shown), compared with no use of antidiabetic drugs.
4. Discussion
Although not showing the new knowledge, we observed that the incidence of herpes zoster was higher in patients with diabetes mellitus than patients without diabetes mellitus (7.85 vs 6.75 per 1000 person-years), even after 3 years of follow-up. The incidence of herpes zoster in diabetic patients in Taiwan seemed to be lower than those in Spain and in USA (7.85, 9.3, and 7.96 per 1000 person-years, respectively).[12,13] We observed that the diabetes mellitus group had a higher cumulative incidence of herpes zoster than the nondiabetes mellitus group at the end of follow-up shown by the Kaplan–Meier model. Based on the above findings, we highlight that the risk of herpes zoster remains to be high over time in diabetic patients, even after 3 years of follow-up.
After adjustment for co-variables, we observed that diabetic patients were associated with a 1.17-fold increased risk of herpes zoster, which was compatible with previous epidemiological studies.[11–14] Because the live herpes zoster vaccine effectively increases specific cell-mediated immunity to varicella-zoster virus,[25] from a point of primary prevention, we suggest diabetic patients should receive the live herpes zoster vaccine, particularly for older adults with diabetes mellitus due to their higher incidence of herpes zoster (10.5 per 1000 person-years, Table 2).
As well known, the hemoglobin A1c levels indicate the status of glycemic control. One cohort study in Denmark disclosed that patients with high hemoglobin A1c levels were at increased risk of infections.[26] This could partially explain our findings that patients taking any antidiabetic drugs were associated with a reduced risk of herpes zoster (adjusted HR 0.31), compared with those not taking antidiabetic drugs. That is, patients taking antidiabetic drugs could have low hemoglobin A1c levels. Thus, the risk of infections including herpes zoster further reduced. Moreover, due to the natural limitation of the database, the hemoglobin A1c levels were not obtained in the database. We were unable to confirm whether an optimal glycemic control could reduce the development of herpes zoster. It indicates a future research direction on the correlation between the hemoglobin A1c levels and herpes zoster.
We conclude that patients with diabetes mellitus are at increased risk for developing herpes zoster. Vaccination of herpes zoster should be suggested among patients with diabetes mellitus.
Author contributions
Specific author contributions: Shih-Wei Lai contributed to the conception of the article, initiated the draft of the article, and has approved the final draft submitted.
Conceptualization: Shih-Wei Lai.
Formal analysis: Cheng-Li Lin, Kuan-Fu Liao.
Writing – original draft: Shih-Wei Lai.
Writing – review and editing: Shih-Wei Lai.
Footnotes
Abbreviation: ICD-9 code = International Classification of Diseases, Ninth Revision, Clinical Modification.
C-LL and K-FL conducted data analysis.
Insurance reimbursement claims data used in this study were available for public access. Patient identification numbers were scrambled to ensure confidentiality. Patient informed consent was not required. This study was approved by the Research Ethics Committee of China Medical University and Hospital in Taiwan (CMUH-104-REC2-115).
This study was supported in part by the Clinical Trial Center, Ministry of Health and Welfare in Taiwan (MOHW108-TDU-B-212-133004) and China Medical University Hospital in Taiwan (DMR-107-192). These funding agencies did not influence the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors disclose no conflicts of interest.
References
- [1].GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ministry of Health and Welfare Taiwan. 2017 Statistics of Causes of Death. Available at: http://www.mohw.gov.tw/EN/Ministry/Index.aspx. [Accessed on July 1, 2018], English version. [Google Scholar]
- [3].Pizzul MG, Betterle C, De Campo C, et al. Changes in the immunologic profile of newly-diagnosed diabetic patients during the first year of the disease. Pediatr Med Chir 1988;10:481–5. [PubMed] [Google Scholar]
- [4].Khan S, Raghuram GV, Bhargava A, et al. Role and clinical significance of lymphocyte mitochondrial dysfunction in type 2 diabetes mellitus. Transl Res 2011;158:344–59. [DOI] [PubMed] [Google Scholar]
- [5].Muller LM, Gorter KJ, Hak E, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis 2005;41:281–8. [DOI] [PubMed] [Google Scholar]
- [6].Lin HF, Liao KF, Chang CM, et al. Anti-diabetic medication reduces risk of pulmonary tuberculosis in diabetic patients: a population-based cohort study in Taiwan. Kuwait Med J 2017;49:22–8. [Google Scholar]
- [7].Lai SW, Lin CL, Liao KF. Population-based cohort study investigating the correlation of diabetes mellitus with pleural empyema in adults in Taiwan. Medicine 2017;96:e7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis 2004;4:26–33. [DOI] [PubMed] [Google Scholar]
- [9].Tyring SK. Natural history of varicella zoster virus. Semin Dermatol 1992;11:211–7. [PubMed] [Google Scholar]
- [10].Okamoto S, Hata A, Sadaoka K, et al. Comparison of varicella-zoster virus-specific immunity of patients with diabetes mellitus and healthy individuals. J Infect Dis 2009;200:1606–10. [DOI] [PubMed] [Google Scholar]
- [11].Queenan JA, Farahani P, Ehsani-Moghadam B, et al. The prevalence and risk for herpes zoster infection in adult patients with diabetes mellitus in the Canadian primary care sentinel surveillance network. Can J Diabetes 2018;42:465–9. [DOI] [PubMed] [Google Scholar]
- [12].Munoz-Quiles C, Lopez-Lacort M, Ampudia-Blasco FJ, et al. Risk and impact of herpes zoster on patients with diabetes: a population-based study, 2009–2014. Hum Vaccin Immunother 2017;13:2606–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Suaya JA, Chen SY, Li Q, et al. Incidence of herpes zoster and persistent post-zoster pain in adults with or without diabetes in the United States. Open Forum Infect Dis 2014;1:ofu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Heymann AD, Chodick G, Karpati T, et al. Diabetes as a risk factor for herpes zoster infection: results of a population-based study in Israel. Infection 2008;36:226–30. [DOI] [PubMed] [Google Scholar]
- [15].Jiang YD, Chang CH, Tai TY, et al. Incidence and prevalence rates of diabetes mellitus in Taiwan: analysis of the 2000–2009 Nationwide Health Insurance database. J Formos Med Assoc 2012;111:599–604. [DOI] [PubMed] [Google Scholar]
- [16].Ministry of Health and Welfare Taiwan. 2016 Taiwan Health and Welfare Report. Available at: http://www.mohw.gov.tw. [Accessed on March 1, 2019], English version. [Google Scholar]
- [17].Lai SW, Lin CL, Liao KF. Weight loss might be an early clinical manifestation of undiagnosed cancer: a nation-based cohort study. BioMedicine (Taiwan) 2018;8:23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lai SW, Liao KF, Liao CC, et al. Polypharmacy correlates with increased risk for hip fracture in the elderly: a population-based study. Medicine 2010;89:295–9. [DOI] [PubMed] [Google Scholar]
- [19].Wang IK, Lai SW, Lai HC, et al. Risk of and fatality from acute pancreatitis in long-term hemodialysis and peritoneal dialysis patients. Perit Dial Int 2018;38:30–6. [DOI] [PubMed] [Google Scholar]
- [20].Lin SY, Lin CL, Sung FC, et al. Risk of subsequent health disorders among living kidney donors. Medicine (Baltimore) 2019;98:e14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liao KF, Huang PT, Lin CC, et al. Fluvastatin use and risk of acute pancreatitis:a population-based case-control study in Taiwan. BioMedicine (Taiwan) 2017;7:24–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lai SW, Lin CH, Lin HF, et al. Herpes zoster correlates with increased risk of Parkinson's disease in older people: a population-based cohort study in Taiwan. Medicine 2017;96:e6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lin HF, Liao KF, Chang CM, et al. Tamoxifen usage correlates with increased risk of Parkinson's disease in older women with breast cancer: a case-control study in Taiwan. Eur J Clin Pharmacol 2018;74:99–107. [DOI] [PubMed] [Google Scholar]
- [24].Hung SC, Liao KF, Hung HC, et al. Using proton pump inhibitors correlates with an increased risk of chronic kidney disease: a nationwide database-derived case-controlled study. Fam Pract 2018;35:166–71. [DOI] [PubMed] [Google Scholar]
- [25].Hata A, Inoue F, Yamasaki M, et al. Safety, humoral and cell-mediated immune responses to herpes zoster vaccine in subjects with diabetes mellitus. J Infect 2013;67:215–9. [DOI] [PubMed] [Google Scholar]
- [26].Mor A, Dekkers OM, Nielsen JS, et al. Impact of glycemic control on risk of infections in patients with type 2 diabetes: a population-based cohort study. Am J Epidemiol 2017;186:227–36. [DOI] [PubMed] [Google Scholar]


