Abstract
Chase examines a study using the MUSICO model of striated muscle to evaluate the function of giant elastic proteins titin and nebulin.
The contractile proteins in myofibers, myofilaments, are arranged into repeating units called sarcomeres. As well as thick and thin filaments, each sarcomere contains the elastic proteins nebulin and titin, which are central to the theme of the 2018 Madison Myofilament Meeting, “Elastic Domains in Proteins of the Sarcomere: Stressors, Regulators or Rulers?” This meeting is a biennial gathering in Madison, Wisconsin, in which the muscle community present and learn about the latest research on myofilament function and dysfunction. In this issue, which contains publications based on work presented at that meeting, Mijailovich et al. present computational modeling work that directly addresses the central question of the 2018 meeting.
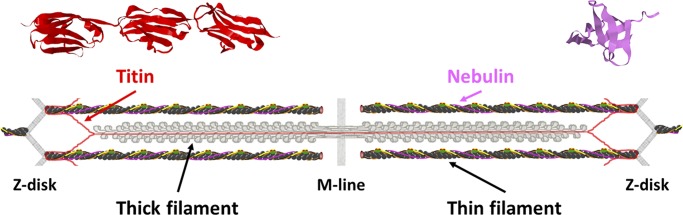
Titin (connectin) and nebulin are large proteins (Fig. 1) whose elastic nature derives in part from small domains that are arranged in series within the larger protein. The core structure of many of these domains is β-sheet (Fig. 1), which can be disrupted under strain and refold after strain is relieved. Titin is the largest protein in humans. Each titin molecule in striated muscle stretches from the Z-disk to the M-line, associating with the thick filament in one half of the A-band. Because a portion of titin extends from the end of a thick filament to the Z-disk—i.e., the portion of titin in the I-band—titin is sometimes referred to as the third filament system of muscle. Titin’s β-sheet-containing domains are immunoglobulin-like (Ig) and fibronectin-like (type III, FnIII) domains. Titin’s I-band portion also contains a region rich in proline, glutamate, valine, and lysine residues (PEVK domain), and cardiac titin’s I-band portion has an additional sequence (N2B region). Both the PEVK domain and N2B region contribute to titin’s properties as an elastic molecule (Watanabe et al., 2002), but these two regions are distinct from the rest of titin because neither has β-sheet at its core. Nebulin, while not quite as large as titin, extends along the length of the thin filament in skeletal muscle. Nebulin’s β-sheet-containing domain is an SRC homology 3 (SH3) domain (Fig. 1). Titin and nebulin were identified by the careful and patient work of Koscak Maruyama (Maruyama et al., 1977, 1981), Kuan Wang (Wang et al., 1979; Wang and Williamson, 1980) and their respective colleagues. For a comprehensive history of the discovery of titin and nebulin—proteins that were largely ignored because they were too big to enter standard SDS-PAGE matrices—along with relevant background arguments that such elastic proteins must be present in muscle, see Rall (2014).
Figure 1.
Molecular view of a sarcomere in vertebrate skeletal muscle. The sarcomere, adapted (with permission) from Mijailovich et al. (2019), highlights structures of a few of the β-sheet-containing domains within titin (red) and nebulin (violet). Top left: X-ray diffraction crystal structure of three contiguous Ig domains (I9-I11), a small portion of the I-band region of human titin (PDB accession no. 5JDD; Bogomolovas et al., 2016). Top right: Solution NMR structure of the SH3 domain from human nebulin (PDB accession no. 1NEB; Politou et al., 1998).
The study by Mijailovich et al. (2019) used structural and functional data from the Granzier laboratory to evaluate and adjust parameters in their computational model. The typical thoroughness of work from the Granzier laboratory has generated a substantial body of knowledge about the structural and physiological roles of titin and nebulin in striated muscles. They developed a nebulin knock-out (Neb-KO) mouse that, despite the severely shortened life span of homozygous animals, allowed a clear demonstration of nebulin’s role as a ruler for regulating thin filament length in skeletal muscle (Witt et al., 2006). Subsequently, this Neb-KO mouse model has been extensively characterized. They also identified titin as a primary contributor to passive tension of the cardiac myofibril (Granzier and Irving, 1995), followed by a number of clever approaches to extensively characterize titin in this role. However, the hypotheses developed from the careful physiological and structural measurements by the Granzier laboratory, and others, need independent validation (or refutation). Thus, to explore possible molecular mechanisms that may underlie the roles of nebulin and titin in striated muscles, the authors of the present study used the comprehensive modeling program MUSICO developed by Mijailovich and colleagues (Mijailovich et al., 2016; Mijailovich, 2017). MUSICO is a multiscale model that utilizes modules to accommodate structural, kinetic, and biomechanical differences in various muscle types (Mijailovich et al., 2016, 2017). Modeling is an important component of testing hypotheses about titin and nebulin because it is challenging to design unambiguous experimental tests involving manipulation of such large proteins and genes. This is because protein biochemistry becomes difficult, as does targeted genetic manipulation, even if the result does not shorten life span as noted for Neb-KO mice; mechanical manipulations may influence multiple parameters at the same time (e.g., sarcomere length and filament lattice spacing).
The nebulin studies in the present work examined experimentally determined changes in thin filament length distribution and the Ca2+-dependence of steady-state isometric force in skeletal muscle. The modular nature of MUSICO allowed the authors to begin by incorporating the increased variability of thin filament lengths in skeletal muscle of Neb-KO mice (Witt et al., 2006). For investigations on cardiac muscle, they could alternatively incorporate the nonuniformity of thin filament lengths of normal cardiac muscle (Robinson and Winegrad, 1977) due to the presence of nebulette instead of nebulin. Nebulette cannot serve as a ruler for thin filament length in the same way as nebulin because nebulette in cardiac muscle is much shorter than nebulin in skeletal muscle (Moncman and Wang, 1995). They were also able to incorporate the increased compliance of thin filaments in Neb-KO skeletal muscle (Kawai et al., 2018; Kiss et al., 2018); this is important because earlier modeling investigations suggested that changes in thin filament compliance can alter both maximum isometric force (Daniel et al., 1998) and Ca2+ dependence of isometric force (Chase et al., 2004; Kataoka et al., 2007; Tanner et al., 2012). MUSICO’s output indicates that prior predictions were pointing in the right direction but that neither changes in thin filament length distribution nor changes in thin filament compliance can, alone or together, fully explain altered contractile function in Neb-KO skeletal muscle. Specifically, the altered functions that needed further explanation were: reductions in isometric force at physiologically relevant sarcomere lengths (Ottenheijm et al., 2009); reduced slope (Hill coefficient, nH) of the isometric force-pCa relationship (that effectively increased the apparent Ca2+-sensitivity) at sarcomere length (SL) 2.5 µm (Witt et al., 2006); and decreased Ca2+-sensitivity (rightward shifted pCa50) and nH for isometric force generation at SL 2.0 µm (Chandra et al., 2009).
The titin studies examined experimentally determined changes in the Ca2+ dependence of steady-state isometric force along with the effects of sarcomere length and filament lattice spacing in cardiac muscle. MUSICO’s adherence to the 3-D structure of striated muscle allowed the authors to test the effects of titin’s contribution to passive tension at different sarcomere lengths, along with correlated changes in filament lattice spacing. Passive tension is well known to increase as a relaxed muscle is stretched, thus increasing SL. MUSICO could incorporate molecular stress-strain information from single titin molecules that had previously been correlated with SL-dependent passive tension of individual cardiomyocytes (Helmes et al., 1999). Experimentally, passive tension was varied in mouse cardiomyocytes by varying SL, conducting a prestretch/release maneuver which temporarily reduces the stiffness of titin due to the molecule’s viscoelastic nature (hysteresis) during unfolding and refolding of elastic domains, or by trypsin proteolysis (Cazorla et al., 2001). Reducing passive tension by any of these means was associated with lower maximum Ca2+-activated tension and decreased Ca2+ sensitivity (rightward-shifted pCa50; Cazorla et al., 2001). Filament lattice compression was achieved in the same study by adding high molecular weight dextrans (∼2.5% wt/vol dextran T-500) to solutions. Osmotic compression of the filament lattice with dextran increased maximum Ca2+-activated tension and also increased Ca2+ sensitivity (leftward-shifted pCa50), although the changes associated with reduced passive tension in the presence of dextran were in the same direction as observed in the absence of dextran (Cazorla et al., 2001). As with the modeling of Neb-KO muscle, it was not possible to simulate all of the data simply by taking into account the changes in lattice spacing associated with varying SL (and passive tension) during the titin modeling.
The modeling results indicate that there may be unexpected changes in kinetic parameters for actomyosin and thin filament regulatory units. To explain reductions in maximum Ca2+-activated tension over and above the contributions of the known structural changes in both studies, Mijailovich et al. (2019) found it necessary to reduce the model’s affinity of myosin for the thin filament by reducing the rate of myosin binding to actin (and, conversely, increasing the rate of myosin binding to actin to explain increased tension). Similarly, it was not possible to simulate all of the changes in Ca2+ sensitivity of isometric tension simply from known structural changes. For example, to effect a further decrease in Ca2+ sensitivity, Ca2+ affinity for TnC was reduced by increasing the rate of Ca2+ dissociation (or, equivalently for computation, decreasing the rate of Ca2+ binding to TnC, although this rate is generally thought to be diffusion limited in most physiologically relevant circumstances), which can be equated to a decrease in the rate at which TnI dissociates from actin. While the modeling implies that common kinetic pathways may be affected, it seems likely that different mechanisms are at work when nebulin or titin are altered.
What molecular mechanisms might explain the modeling predictions? Nebulin on thin filaments could influence the function of tropomyosin and perhaps also troponin, and could also influence the structure of actin. More subtly, nebulin could affect the strain dependence of these thin filament properties. Furthermore, nebulin could alter the electrostatic field on the thin filament, which would be expected to influence the electrostatic guidance of myosin toward actin, i.e., the initial, weak cross-bridge formation that is thought to precede force generation (Brenner et al., 1982). Changes in the electrostatic field on the thin filament could also influence Ca2+ binding to TnC and/or actin binding of the C terminus of TnI, a region of TnI that contains a high density of charged residues. The absence of nebulin in Neb-KO muscle could of course alter any or all of these possibilities. Titin’s contribution to passive tension through its association with thick filaments may affect myosin structure with increased passive tension reducing the number of myosins that are in a super-relaxed configuration, thus enhancing the number of myosin available to form cross-bridges in activated regulatory units on thin filaments (Linari et al., 2015; Fusi et al., 2016; Reconditi et al., 2017; Zhang et al., 2017; Piazzesi et al., 2018). The presence of titin on thick filaments could also affect the compliance of those filaments, which in turn could affect contractile kinetics (Martyn et al., 2002; Piazzesi et al., 2014).
Mijailovich et al. (2019) have tackled the formidable job of incorporating into MUSICO known changes, such as the distribution of thin filament lengths in sarcomeres without nebulin and detailed perturbations used in experiments such as trypsin digestion of titin and variations in SL and myofilament lattice spacing. The model is clearly serving its purpose: evaluating hypotheses using experimental data, and in turn prompting ideas for new experiments and measurements. Among those measurements are the kinetics of force generation, which could help to place additional constraints on parameters in the model. This modeling study is undoubtedly a valuable addition to evaluating our understanding of giant elastic proteins and the function of elastic domains in muscle. We can look forward to new results prompted by this study and further use of MUSICO to address many other problems in muscle biology.
Acknowledgments
The author declares no competing financial interests.
Richard L. Moss served as guest editor.
References
- Bogomolovas J., Fleming J.R., Anderson B.R., Williams R., Lange S., Simon B., Khan M.M., Rudolf R., Franke B., Bullard B., et al. . 2016. Exploration of pathomechanisms triggered by a single-nucleotide polymorphism in titin’s I-band: the cardiomyopathy-linked mutation T2580I. Open Biol. 6:160114 10.1098/rsob.160114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Schoenberg M., Chalovich J.M., Greene L.E., and Eisenberg E.. 1982. Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc. Natl. Acad. Sci. USA. 79:7288–7291. 10.1073/pnas.79.23.7288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazorla O., Wu Y., Irving T.C., and Granzier H.. 2001. Titin-based modulation of calcium sensitivity of active tension in mouse skinned cardiac myocytes. Circ. Res. 88:1028–1035. 10.1161/hh1001.090876 [DOI] [PubMed] [Google Scholar]
- Chandra M., Mamidi R., Ford S., Hidalgo C., Witt C., Ottenheijm C., Labeit S., and Granzier H.. 2009. Nebulin alters cross-bridge cycling kinetics and increases thin filament activation: a novel mechanism for increasing tension and reducing tension cost. J. Biol. Chem. 284:30889–30896. 10.1074/jbc.M109.049718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase P.B., Macpherson J.M., and Daniel T.L.. 2004. A spatially explicit nanomechanical model of the half-sarcomere: myofilament compliance affects Ca2+-activation. Ann. Biomed. Eng. 32:1559–1568. 10.1114/B:ABME.0000049039.89173.08 [DOI] [PubMed] [Google Scholar]
- Daniel T.L., Trimble A.C., and Chase P.B.. 1998. Compliant realignment of binding sites in muscle: transient behavior and mechanical tuning. Biophys. J. 74:1611–1621. 10.1016/S0006-3495(98)77875-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusi L., Brunello E., Yan Z., and Irving M.. 2016. Thick filament mechano-sensing is a calcium-independent regulatory mechanism in skeletal muscle. Nat. Commun. 7:13281 10.1038/ncomms13281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier H.L., and Irving T.C.. 1995. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys. J. 68:1027–1044. 10.1016/S0006-3495(95)80278-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmes M., Trombitás K., Centner T., Kellermayer M., Labeit S., Linke W.A., and Granzier H.. 1999. Mechanically driven contour-length adjustment in rat cardiac titin’s unique N2B sequence: titin is an adjustable spring. Circ. Res. 84:1339–1352. 10.1161/01.RES.84.11.1339 [DOI] [PubMed] [Google Scholar]
- Kataoka A., Hemmer C., and Chase P.B.. 2007. Computational simulation of hypertrophic cardiomyopathy mutations in troponin I: influence of increased myofilament calcium sensitivity on isometric force, ATPase and [Ca2+]i. J. Biomech. 40:2044–2052. 10.1016/j.jbiomech.2006.09.026 [DOI] [PubMed] [Google Scholar]
- Kawai M., Karam T.S., Kolb J., Wang L., and Granzier H.L.. 2018. Nebulin increases thin filament stiffness and force per cross-bridge in slow-twitch soleus muscle fibers. J. Gen. Physiol. 150:1510–1522. 10.1085/jgp.201812104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss B., Lee E.-J., Ma W., Li F.W., Tonino P., Mijailovich S.M., Irving T.C., and Granzier H.L.. 2018. Nebulin stiffens the thin filament and augments cross-bridge interaction in skeletal muscle. Proc. Natl. Acad. Sci. USA. 115:10369–10374. 10.1073/pnas.1804726115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linari M., Brunello E., Reconditi M., Fusi L., Caremani M., Narayanan T., Piazzesi G., Lombardi V., and Irving M.. 2015. Force generation by skeletal muscle is controlled by mechanosensing in myosin filaments. Nature. 528:276–279. 10.1038/nature15727 [DOI] [PubMed] [Google Scholar]
- Martyn D.A., Chase P.B., Regnier M., and Gordon A.M.. 2002. A simple model with myofilament compliance predicts activation-dependent crossbridge kinetics in skinned skeletal fibers. Biophys. J. 83:3425–3434. 10.1016/S0006-3495(02)75342-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., Matsubara S., Natori R., Nonomura Y., and Kimura S.. 1977. Connectin, an elastic protein of muscle. Characterization and Function. J. Biochem. 82:317–337. [PubMed] [Google Scholar]
- Maruyama K., Kimura S., Ohashi K., and Kuwano Y.. 1981. Connectin, an elastic protein of muscle. Identification of “titin” with connectin. J. Biochem. 89:701–709. 10.1093/oxfordjournals.jbchem.a133249 [DOI] [PubMed] [Google Scholar]
- Mijailovich S.M.2017. MUSICO Computational Platform. https://www.mijailovichlab.org/musico
- Mijailovich S.M., Kayser-Herold O., Stojanovic B., Nedic D., Irving T.C., and Geeves M.A.. 2016. Three-dimensional stochastic model of actin-myosin binding in the sarcomere lattice. J. Gen. Physiol. 148:459–488. 10.1085/jgp.201611608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijailovich S.M., Nedic D., Svicevic M., Stojanovic B., Walklate J., Ujfalusi Z., and Geeves M.A.. 2017. Modeling the actin.myosin ATPase cross-bridge cycle for skeletal and cardiac muscle myosin isoforms. Biophys. J. 112:984–996. 10.1016/j.bpj.2017.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijailovich S.M., Stojanovic B., Nedic D., Svicevic M., Geeves M.A., Irving T.C., and Granzier H.. 2019. Nebulin and titin modulate cross-bridge cycling and length-dependent calcium sensitivity. J. Gen. Physiol. 10.1085/jgp.201812165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncman C.L., and Wang K.. 1995. Nebulette: a 107 kD nebulin-like protein in cardiac muscle. Cell Motil. Cytoskeleton. 32:205–225. 10.1002/cm.970320305 [DOI] [PubMed] [Google Scholar]
- Ottenheijm C.A.C., Witt C.C., Stienen G.J., Labeit S., Beggs A.H., and Granzier H.. 2009. Thin filament length dysregulation contributes to muscle weakness in nemaline myopathy patients with nebulin deficiency. Hum. Mol. Genet. 18:2359–2369. 10.1093/hmg/ddp168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazzesi G., Dolfi M., Brunello E., Fusi L., Reconditi M., Bianco P., Linari M., and Lombardi V.. 2014. The myofilament elasticity and its effect on kinetics of force generation by the myosin motor. Arch. Biochem. Biophys. 552-553:108–116. 10.1016/j.abb.2014.02.017 [DOI] [PubMed] [Google Scholar]
- Piazzesi G., Caremani M., Linari M., Reconditi M., and Lombardi V.. 2018. Thick filament mechano-sensing in skeletal and cardiac muscles: a common mechanism able to adapt the energetic cost of the contraction to the task. Front. Physiol. 9:736 10.3389/fphys.2018.00736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politou A.S., Millevoi S., Gautel M., Kolmerer B., and Pastore A.. 1998. SH3 in muscles: solution structure of the SH3 domain from nebulin. J. Mol. Biol. 276:189–202. 10.1006/jmbi.1997.1521 [DOI] [PubMed] [Google Scholar]
- Rall J.A. 2014. Mechanism of Muscle Contraction. Springer, New York. 471 pp. [Google Scholar]
- Reconditi M., Caremani M., Pinzauti F., Powers J.D., Narayanan T., Stienen G.J.M., Linari M., Lombardi V., and Piazzesi G.. 2017. Myosin filament activation in the heart is tuned to the mechanical task. Proc. Natl. Acad. Sci. USA. 114:3240–3245. 10.1073/pnas.1619484114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T.F., and Winegrad S.. 1977. Variation of thin filament length in heart muscles. Nature. 267:74–75. 10.1038/267074a0 [DOI] [PubMed] [Google Scholar]
- Tanner B.C., Daniel T.L., and Regnier M.. 2012. Filament compliance influences cooperative activation of thin filaments and the dynamics of force production in skeletal muscle. PLOS Comput. Biol. 8:e1002506 10.1371/journal.pcbi.1002506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., and Williamson C.L.. 1980. Identification of an N2 line protein of striated muscle. Proc. Natl. Acad. Sci. USA. 77:3254–3258. 10.1073/pnas.77.6.3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., McClure J., and Tu A.. 1979. Titin: major myofibrillar components of striated muscle. Proc. Natl. Acad. Sci. USA. 76:3698–3702. 10.1073/pnas.76.8.3698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Nair P., Labeit D., Kellermayer M.S.Z., Greaser M., Labeit S., and Granzier H.. 2002. Molecular mechanics of cardiac titin’s PEVK and N2B spring elements. J. Biol. Chem. 277:11549–11558. 10.1074/jbc.M200356200 [DOI] [PubMed] [Google Scholar]
- Witt C.C., Burkart C., Labeit D., McNabb M., Wu Y., Granzier H., and Labeit S.. 2006. Nebulin regulates thin filament length, contractility, and Z-disk structure in vivo. EMBO J. 25:3843–3855. 10.1038/sj.emboj.7601242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Kampourakis T., Yan Z., Sevrieva I., Irving M., and Sun Y.-B.. 2017. Distinct contributions of the thin and thick filaments to length-dependent activation in heart muscle. eLife. 6:e24081 10.7554/eLife.24081 [DOI] [PMC free article] [PubMed] [Google Scholar]