Abstract
Colson discusses a recent investigation of the functional effect of slow myosin binding protein-C in slow-twitch skeletal muscle fibers.
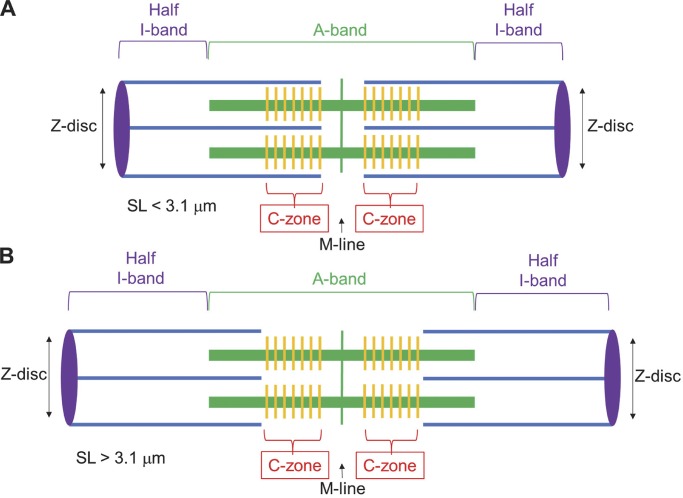
Myosin binding protein-C (MyBP-C) is a multidomain protein that resides in the thick filaments of striated muscle sarcomeres. Separate genes (MYBPC1, MYBPC2, and MYBPC3) encode the three isoforms present in slow skeletal (s-), fast skeletal (f-), and cardiac (c-) muscle, respectively. All these isoforms are thought to function as major regulators of muscle contractility. Evidence for MyBP-C function has arisen largely from studies of cardiac muscle, motivated by the fact that mutations in cMyBP-C are a leading cause of hypertrophic cardiomyopathy. Although sMyBP-C is implicated in muscle disorders such as Duchenne muscular dystrophy and arthrogryposis myopathy, its role in modulating force development and shortening in skeletal muscle has remained unclear due to the lack of studies on this isoform. In all striated muscle types, MyBP-C is localized to the cross-bridge-bearing C-zone (Fig. 1), in the center half of each A-band in the sarcomere. However, the reason for this unique placement of MyBP-C is not well understood. In part, this is because of the challenge of resolving mechanisms of the intact lattice of the muscle sarcomere while differentiating between structures, forces, and contractile properties that occur when thin filaments are inside (Fig. 1 A) versus outside (Fig. 1 B) the MyBP-C-containing C-zone. Interpreting studies of MyBP-C structure and function in muscle are further complicated by its promiscuous interaction with either myosin or actin. In this issue of JGP, Robinett et al. present elegant and striking insights into the functional effects of sMyBP-C in slow-twitch skeletal muscle fibers. Their results suggest that sMyBP-C modifies myosin cross-bridge recruitment to force-generating states, contractile kinetics of cross-bridges, and drag forces within the C-zone (Fig. 1), all of which is further modified by phosphorylation. The basis for these conclusions is a series of mechanical muscle fiber approaches to monitor skeletal muscle force, shortening under load, and thin filament sliding into the C-zone.
Figure 1.
Skeletal muscle sarcomere. (A and B) Sarcomere lengths (SL), measured from Z-disc to Z-disc, are shown for the case with the C-zone overlapping with thin filaments (A) and with the C-zone having no thin filament overlap (B). Thick filaments containing myosin are shown as thick green lines and interdigitating thin filaments containing actin are shown as blue lines. Skeletal MyBP-C molecules are shown in sets of seven orange stripes on the thick filaments. Note that only the I-band region increases in length as SL is extended beyond 3.1 µm. In Robinett et al. (2019), thin filaments are assumed to be ∼1.05 µm in length (Greaser and Pleitner, 2014).
Regulation of MyBP-C by protein kinase A phosphorylation in skeletal muscle
Robinett et al. (2019) take a major step forward in deciphering the effects of skeletal MyBP-C on slow- and fast-twitch muscle contractile properties. In slow fibers they observe clear regulatory effects of sMyBP-C phosphorylation by PKA. Physiological conditions of stress, such as the fight-or-flight response, increase β-adrenergic signaling and elicit PKA-catalyzed phosphorylation of sMyBP-C and cMyBP-C. No such phosphorylation has been observed in fMyBP-C, presumably due to its lack of PKA target sequences. Slow-twitch skeletal muscle offers a simple system for studying PKA regulation as sMyBP-C is the only sarcomeric protein phosphorylated by this kinase. In heart muscle, the situation is more complicated as the cardiac isoforms of troponin I and titin are phosphorylated by PKA in addition to cMyBP-C.
Measures of contractile properties in Ca2+-activated skinned muscle fibers
To determine the role that MyBP-C plays in the regulation of skeletal muscle mechanics, Robinett et al. (2019) activated slow- and fast-twitch muscles with a range of Ca2+ concentrations to induce increasing amounts of force. The authors used rat soleus (slow) and psoas (fast) muscles and separated the dissected skeletal muscles into small bundles of fibers. After the cell membrane (sarcolemma) was permeabilized by a detergent (“skinning” procedure), the levels of intracellular Ca2+ could be precisely controlled by the extracellular buffer. For mechanical measurements of force development, ∼1-mm-long (∼80-µm-wide) sections of single muscle fibers were mounted between a force transducer and motor while in a bath of physiological buffer (relaxing or activating). The authors secured each end of the fiber to the mechanical apparatus by a suture tie-in procedure (Moss, 1979). This minimizes muscle segment end compliance, distortion of the fiber at segment ends, and slippage from under the suture pin at the muscle ends during Ca2+-activated force generation. Thus, the approximate number of sarcomeres generating force in the bath remained constant during repeated activations of muscle force. Increasing Ca2+ concentrations between 63 nM and 32 µM (i.e., pCa 6.2–4.5) were used in the buffer to activate the muscle to produce increasing amounts of force. Important contractile properties of (a) cross-bridge recruitment, (b) cross-bridge cycling kinetics, and (c) internal drag forces of the sarcomere were then measured.
Transient force overshoot and cross-bridge recruitment in skinned fibers
Transient force overshoot measurements resulted in the surprising finding that sMyBP-C facilitates greater cross-bridge recruitment in response to mechanical perturbations than fMyBP-C. Transient force overshoot is a phenomenon that occurs in skinned muscle fibers following a slack-and-restretch maneuver. In the present study, the muscle was placed in a Ca2+-activating solution (e.g., pCa 6.0). Next, the muscle was slackened by ∼15% of its length to release all bound cross-bridges and allow force to drop to near zero while the muscle remained in the activating Ca2+ solution in the bath. After slackening the muscle for a brief 20 ms, the length was rapidly reset to the original length, and force was recorded by the force transducer as cross-bridges rebound and cycled to produce Ca2+-activated force. This rate of force redevelopment is known as the ktr. The force following this ktr measurement “transiently overshoots” the original force for a period of time before returning to the original level. It is likely that this overshoot is due to an increase in the cooperative activation process resulting in additional cross-bridges binding during this time period, as opposed to more force generation per cross-bridge. The cooperative activation process includes cross-bridge-induced sensitization of thin filaments, as initial myosin head binding perturbs tropomyosin strand position to favor additional myosin binding (Lehrer and Morris, 1982; Gordon et al., 2000). Changes in the thick filament structure itself have also been proposed, such that the changes brought about by the binding of one myosin head to actin alter the dynamics of neighboring heads, recruiting them to form additional cross-bridges (Lehrer, 1994; Moss et al., 2004). Studies of cardiac muscle suggest that both thin and thick filaments transition from OFF to ON states during force generation and that cMyBP-C may play a role in regulating these states (Kampourakis et al., 2014). These states are consistent with “compliant realignment” of the thick and thin filaments, from less ordered states to more ordered states in the force-generating process. In other words, mechanical compliance of the myofilaments is predicted to lead to additional myosin binding sites on actin being newly aligned and available for additional myosin cross-bridge binding following the initiation of force redevelopment (Campbell, 2006).
While details of transient force overshoot remain to be firmly established, Robinett et al. (2019) have found a major difference in this property in slow- and fast-twitch fibers. As fast-twitch skeletal muscle is more cooperative than slow-twitch skeletal muscle, i.e., exhibits a steeper force–pCa relationship, it was reasoned that fast fibers would have a greater overshoot than slow fibers (McDonald, 2000). That slow fibers were observed to have a greater transient overshoot than fast fibers suggests that slow-twitch skeletal muscle actually has a greater capacity for cross-bridge recruitment in response to mechanical perturbations than fast-twitch skeletal muscle. This finding suggests that the transient force overshoot in skinned striated muscle preparations is not entirely due to cooperative processes inherent to the myofilament upon activation. Robinett et al. (2019) revealed that one missing piece to the transient force overshoot puzzle is MyBP-C. In slow fibers, PKA phosphorylation of sMyBP-C induced a doubling of the already high transient overshoot, primarily at low Ca2+ activation levels (∼25% of maximum force). Phosphorylation of sMyBP-C appears to release a reserve of myosin heads that are normally constrained by unphosphorylated sMyBP-C. Myosin heads can then move in closer proximity to actin, which increases the likelihood of entering into the cycling pool of cross-bridges that generate force. It is expected that phosphorylation of Ser-59 (of the Pro/Ala-rich linker) and Ser-204 (in the M-domain between Ig-like domains C1 and C2) at the sMyBP-C N terminus disrupts the putative interaction with the myosin subfragment-2 (S2) portion of the myosin molecule due to the introduction of negative charge. This idea is consistent with structural and functional effects of PKA-mediated phosphorylation of cMyBP-C observed in cardiac muscle (Levine et al., 2001; Stelzer et al., 2006; Colson et al., 2008; Kensler et al., 2017). A difference here is that the PKA sites of cMyBP-C all reside in the M-domain, whereas sMyBP-C sites are additionally found in the Pro/Ala linker (Ackermann and Kontrogianni-Konstantopoulos, 2011). N-terminal cMyBP-C also undergoes a conformational change upon phosphorylation, giving rise to new protein surface contacts that could make or break binding interactions (Colson et al., 2016). It remains unknown how the structure of N-terminal sMyBP-C is altered upon phosphorylation.
Phosphorylation of sMyBP-C accelerates cross-bridge cycling kinetics
Fitting of force redevelopment curves by a double exponential reveals that phosphorylation of sMyBP-C accelerates cross-bridge cycling kinetics. The slack-and-restretch maneuver that elicits the force overshoot includes the preceding rising phase of the force redevelopment. Force is near zero at the onset of the restretch and increases exponentially until developed force recrosses the threshold of the initial force (before the slack). At this onset of the overshoot, a second exponential phase encompasses the transient increase in force. Because the ktr (sec−1) rate constant is the sum of both the forward (fapp) and backward (gapp) apparent rates of cross-bridge cycling, an increase in this rate suggests that cross-bridges are cycling faster. However, from this measurement alone, one cannot distinguish between faster attachments versus faster detachments versus changes in both aspects of cycling kinetics. Fast-twitch muscle fibers exhibit faster rates of cross-bridge cycling than slow-twitch fibers at all levels of activation. These differences in rates of force redevelopment are set, in part, by the 2A, 2B, or 2X myosin heavy chain isoform present in the muscle fiber. The relative contributions of fMyBP-C and sMyBP-C to these cycling kinetics remain to be elucidated. In the slow skeletal fibers used by Robinett et al. (2019), PKA treatment of sMyBP-C caused an increase in ktr at all levels of Ca2+-activation and rates of both exponentials (k1 and k2) were increased. This suggests that phosphorylation of sMyBP-C accelerates the kinetics of cross-bridge cycling. These effects were also reversed by lambda phosphatase treatment to dephosphorylate basally phosphorylated serines of sMyBP-C in slow-twitch fibers. The release of constrained heads by sMyBP-C phosphorylation would be expected to increase the probability of cross-bridge interactions with myosin that is in closer proximity to the binding sites on actin. With cross-bridges in closer proximity to actin in phosphorylated slow fibers, the apparent cooperativity of activation, which is not instantaneous but takes time to occur, is decreased as cross-bridges are “primed” for more immediate force production. Thus, phosphorylation-mediated modulation of cross-bridge cycling kinetics is a common feature of both cMyBP-C and sMyBP-C. For recent studies by this group on phosphorylation in cardiac muscle, see Hanft et al. (2016) and Hanft and McDonald (2009). Interestingly, cMyBP-C phosphorylation decreased cross-bridge cycling kinetics at submaximal Ca2+ activations in rat myocardium (Hanft and McDonald, 2009), whereas the rates of cross-bridge cycling increased in mouse myocardium following PKA treatment (Stelzer et al., 2006). The mechanistic details of these divergent species-specific effects warrants further investigation. It also remains to be uncovered whether there are posttranslational modifications of fMyBP-C that influence cycling in fast fibers. sMyBP-C exists with numerous alternatively spliced sites (Ackermann and Kontrogianni-Konstantopoulos, 2013), including PKA sites, so future studies will inspect the roles of these splice variants.
Dephosphorylation of sMyBP-C by phosphatase inhibits loaded shortening
Results from experiments performed at varying sarcomere lengths (SL) suggest that dephosphorylated sMyBP-C can interact with the thin filament and act as an internal load capable of opposing myofilament sliding. This interaction is alleviated by PKA phosphorylation of sMyBP-C. The disposition of MyBP-C’s N terminus in the striated muscle sarcomere is not yet known. While the C terminus is anchored to the thick filament with submicromolar affinity (<1 µM; Okagaki et al., 1993), the N terminus extends away to bind myosin S2 and/or actin. These interactions have more moderate affinities (∼10 µM) and binding is reduced upon PKA treatment (Gruen et al., 1999; Shaffer et al., 2009). In the case of sMyBP-C interactions with actin, this mechanism would provide a physical link between the thick and thin filaments. In skeletal muscle, the SL can be increased from normal resting lengths of ∼2.4 µm, with full overlap between the thin filament and the MyBP-C-containing C-zone of the thick filament, to sarcomere lengths beyond ∼3.1 µm in which the thick filament no longer overlaps with the C-zone (Fig. 1). Here, any links between sMyBP-C and actin would be absent. When the muscle is shortened back to below ∼3.1 µm, then sMyBP-C could again interact with actin and reestablish thick–thin filament connections. To investigate this experimentally, Robinett et al. (2019) determined the effects of PKA treatment on slow skeletal fibers under conditions of lightly loaded shortening, starting from SLs in which thin filaments were situated either inside or outside the C-zone. In one protocol, thin filaments were initially positioned outside the C-zone (SL ∼3.15 µm); then, the fiber was activated by Ca2+ to ∼30–40% maximal force before thin filaments were driven into the C-zone (SL ∼3.06 µm) by force clamps in which the force applied was less than steady-state force for ∼700 ms. The fiber was then slackened to reduce force to near zero to allow for estimation of the relative load sustained during isotonic shortening. Finally, the fiber was reextended to its original length. During this loaded shortening, the change in SL was greater in PKA-treated fibers than basally phosphorylated fibers. This is consistent with less deceleration and faster loaded shortening during force clamps in PKA-treated fibers with phosphorylated sMyBP-C, compared with untreated or phosphatase-treated fibers. The change in SL was smallest in dephosphorylated fibers that also exhibited a prominent “bump” during lightly loaded shortening. This recoil occurred at the same SL ∼3.06 where the thin filaments would be sliding into overlap with the C-zone. It is tempting to conclude that this bump in sarcomere length coincides with establishment of thick−thin filament scaffolding via dephosphorylated sMyBP-C.
In another protocol of the present study, skinned skeletal fiber preparations were lengthened by light loads greater than steady-state force (supra-isometric load clamps), starting with thin filaments either inside or outside the C-zone. For thin filaments starting outside the C-zone (SL >3.08 µm), supra-isometric force clamps exhibited linear lengthening in one phase, whereas fibers starting at SLs within the C-zone (∼3.03 µm) exhibited a biphasic rate of lengthening when exiting the C-zone (∼3.05–3.08 µm). A major advance from this work is the observation of biphasic behavior in the C-zone, sensitive to the level of MyBP-C phosphorylation, in skinned fibers during changes in SL under loaded shortening/lengthening (Robinett et al., 2019). It should be noted that thin filaments were estimated to be ∼1.05 µm in length, following measurements in fast skeletal muscle (Greaser and Pleitner, 2014); however, exact thin filament lengths from slow skeletal muscle were not reported. Conceivably, if slow-twitch thin filaments were slightly longer, e.g., 1.15–1.2 µm, then the possibility remains that the effects on sarcomere shortening could arise due to other myofilament proteins, including titin. Electron micrographs of MyBP-C antibody-labeled sarcomeres revealed the seven to nine stripes on each half of the A-band (Fig. 1) in fast skeletal muscle; however, these stripes were not visualized in slow skeletal muscle (Bennett et al., 1986). While sMyBP-C presumably exists in these same stripes, uncertainty remains in the exact C-zone widths. Electron microscopy studies of soleus muscle using antibodies specific for sMyBP-C may confirm this. Future studies are needed to determine if these links are indeed between sMyBP-C and actin or whether other myofilament proteins are involved. While it appears that these inhibitory effects on shortening are dependent upon thick-thin overlap in the C-zone, it remains to be elucidated whether sMyBP-C effects in the C-zone propagate into the D-zone (region of the A-band outside of the C-zone where MyBP-C is absent) or if effects are completely localized to the C-zone.
New insights in the role of skeletal MyBP-C as a regulator of skeletal contractility
The results of the transient overshoot, cycling kinetics, and loaded shortening experiments suggest a model for sMyBP-C whereby unphosphorylated sMyBP-C can interact with both myosin and actin to slow cross-bridge cycling kinetics, dampen force, and inhibit filament shortening. Upon phosphorylation of sMyBP-C, these interactions are alleviated, enhancing the rate and magnitude of force and speeding up filament sliding. This work extends our understanding of the mechanism by which MyBP-C operates in striated muscle and emphasizes remaining mechanistic questions that promise to fuel skeletal MyBP-C research for years to come. Fine tuning of contraction permits precise timing and strength of force development. The marked changes in force, kinetics, and sliding with sMyBP-C phosphorylation, and at variable levels of Ca2+ activation, speak to the dynamic range of muscle performance. The potential mechanism of sMyBP-C and its phosphorylation warrants testing in skinned and intact skeletal muscle using low angle x-ray diffraction, site-directed spectroscopy, and other structural approaches. Mouse models targeting skeletal MyBP-C and experimental tools for using biophysical, biochemical and physiological methods will be key to this understanding. Additional mechanistic insights like those provided in Robinett et al. (2019), will pave the way for developing new therapies for contractile dysfunction in muscle disorders. This is evident from work showing that mutations in MYBPC1 result in distal arthrogryposis myopathy (Gurnett et al., 2010; Ackermann et al., 2013) and muscle tremors and that phosphorylation of MYBPC1 is reduced in Duchenne muscular dystrophy (Ackermann et al., 2015). Mechanistic discoveries in skeletal MyBP-C will additionally provide insight and spark new ideas for studying cardiac MyBP-C. This includes investigations of its role in stretch activation properties in heart muscle, as well as thick–thin filament drag forces on sarcomeric shortening. These drag forces would be expected to increase with dephosphorylated cMyBP-C in end-stage heart failure and decrease with cMyBP-C mutation in hypertrophic cardiomyopathy.
Acknowledgments
This work was supported by National Institute of Health grants R00 HL122397 and R01 HL141564.
The author declares no competing financial interests.
Henk L. Granzier served as editor.
References
- Ackermann M.A., and Kontrogianni-Konstantopoulos A.. 2011. Myosin binding protein-C slow is a novel substrate for protein kinase A (PKA) and C (PKC) in skeletal muscle. J. Proteome Res. 10:4547–4555. 10.1021/pr200355w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann M.A., and Kontrogianni-Konstantopoulos A.. 2013. Myosin binding protein-C slow: a multifaceted family of proteins with a complex expression profile in fast and slow twitch skeletal muscles. Front. Physiol. 4:391 10.3389/fphys.2013.00391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann M.A., Patel P.D., Valenti J., Takagi Y., Homsher E., Sellers J.R., and Kontrogianni-Konstantopoulos A.. 2013. Loss of actomyosin regulation in distal arthrogryposis myopathy due to mutant myosin binding protein-C slow. FASEB J. 27:3217–3228. 10.1096/fj.13-228882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann M.A., Ward C.W., Gurnett C., and Kontrogianni-Konstantopoulos A.. 2015. Myosin Binding Protein-C Slow Phosphorylation is Altered in Duchenne Dystrophy and Arthrogryposis Myopathy in Fast-Twitch Skeletal Muscles. Sci. Rep. 5:13235 10.1038/srep13235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P., Craig R., Starr R., and Offer G.. 1986. The ultrastructural location of C-protein, X-protein and H-protein in rabbit muscle. J. Muscle Res. Cell Motil. 7:550–567. 10.1007/BF01753571 [DOI] [PubMed] [Google Scholar]
- Campbell K.S. 2006. Filament compliance effects can explain tension overshoots during force development. Biophys. J. 91:4102–4109. 10.1529/biophysj.106.087312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson B.A., Bekyarova T., Locher M.R., Fitzsimons D.P., Irving T.C., and Moss R.L.. 2008. Protein kinase A-mediated phosphorylation of cMyBP-C increases proximity of myosin heads to actin in resting myocardium. Circ. Res. 103:244–251. 10.1161/CIRCRESAHA.108.178996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson B.A., Thompson A.R., Espinoza-Fonseca L.M., and Thomas D.D.. 2016. Site-directed spectroscopy of cardiac myosin-binding protein C reveals effects of phosphorylation on protein structural dynamics. Proc. Natl. Acad. Sci. USA. 113:3233–3238. 10.1073/pnas.1521281113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A.M., Homsher E., and Regnier M.. 2000. Regulation of contraction in striated muscle. Physiol. Rev. 80:853–924. 10.1152/physrev.2000.80.2.853 [DOI] [PubMed] [Google Scholar]
- Greaser M.L., and Pleitner J.M.. 2014. Titin isoform size is not correlated with thin filament length in rat skeletal muscle. Front. Physiol. 5:35 10.3389/fphys.2014.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruen M., Prinz H., and Gautel M.. 1999. cAPK-phosphorylation controls the interaction of the regulatory domain of cardiac myosin binding protein C with myosin-S2 in an on-off fashion. FEBS Lett. 453:254–259. 10.1016/S0014-5793(99)00727-9 [DOI] [PubMed] [Google Scholar]
- Gurnett C.A., Desruisseau D.M., McCall K., Choi R., Meyer Z.I., Talerico M., Miller S.E., Ju J.S., Pestronk A., Connolly A.M., et al. 2010. Myosin binding protein C1: a novel gene for autosomal dominant distal arthrogryposis type 1. Hum. Mol. Genet. 19:1165–1173. 10.1093/hmg/ddp587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanft L.M., and McDonald K.S.. 2009. Sarcomere length dependence of power output is increased after PKA treatment in rat cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 296:H1524–H1531. 10.1152/ajpheart.00864.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanft L.M., Cornell T.D., McDonald C.A., Rovetto M.J., Emter C.A., and McDonald K.S.. 2016. Molecule specific effects of PKA-mediated phosphorylation on rat isolated heart and cardiac myofibrillar function. Arch. Biochem. Biophys. 601:22–31. 10.1016/j.abb.2016.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampourakis T., Yan Z., Gautel M., Sun Y.B., and Irving M.. 2014. Myosin binding protein-C activates thin filaments and inhibits thick filaments in heart muscle cells. Proc. Natl. Acad. Sci. USA. 111:18763–18768. 10.1073/pnas.1413922112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler R.W., Craig R., and Moss R.L.. 2017. Phosphorylation of cardiac myosin binding protein C releases myosin heads from the surface of cardiac thick filaments. Proc. Natl. Acad. Sci. USA. 114:E1355–E1364. 10.1073/pnas.1614020114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer S.S. 1994. The regulatory switch of the muscle thin filament: Ca2+ or myosin heads? J. Muscle Res. Cell Motil. 15:232–236. 10.1007/BF00123476 [DOI] [PubMed] [Google Scholar]
- Lehrer S.S., and Morris E.P.. 1982. Dual effects of tropomyosin and troponin-tropomyosin on actomyosin subfragment 1 ATPase. J. Biol. Chem. 257:8073–8080. [PubMed] [Google Scholar]
- Levine R., Weisberg A., Kulikovskaya I., McClellan G., and Winegrad S.. 2001. Multiple structures of thick filaments in resting cardiac muscle and their influence on cross-bridge interactions. Biophys. J. 81:1070–1082. 10.1016/S0006-3495(01)75764-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald K.S. 2000. Ca2+ dependence of loaded shortening in rat skinned cardiac myocytes and skeletal muscle fibres. J. Physiol. 525:169–181. 10.1111/j.1469-7793.2000.00169.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R.L. 1979. Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J. Physiol. 292:177–192. 10.1113/jphysiol.1979.sp012845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R.L., Razumova M., and Fitzsimons D.P.. 2004. Myosin crossbridge activation of cardiac thin filaments: implications for myocardial function in health and disease. Circ. Res. 94:1290–1300. 10.1161/01.RES.0000127125.61647.4F [DOI] [PubMed] [Google Scholar]
- Okagaki T., Weber F.E., Fischman D.A., Vaughan K.T., Mikawa T., and Reinach F.C.. 1993. The major myosin-binding domain of skeletal muscle MyBP-C (C protein) resides in the COOH-terminal, immunoglobulin C2 motif. J. Cell Biol. 123:619–626. 10.1083/jcb.123.3.619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinett J.C., Hanft L.M., Geist J., Kontrogianni-Konstantopoulos A., and McDonald K.S.. 2019. Regulation of myofilament force and loaded shortening by skeletal myosin binding protein C. J. Gen. Physiol. 10.1074/jgp.201812200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer J.F., Kensler R.W., and Harris S.P.. 2009. The myosin-binding protein C motif binds to F-actin in a phosphorylation-sensitive manner. J. Biol. Chem. 284:12318–12327. 10.1074/jbc.M808850200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer J.E., Patel J.R., and Moss R.L.. 2006. Protein kinase A-mediated acceleration of the stretch activation response in murine skinned myocardium is eliminated by ablation of cMyBP-C. Circ. Res. 99:884–890. 10.1161/01.RES.0000245191.34690.66 [DOI] [PubMed] [Google Scholar]