The expression of β-myosin heavy chain (β-MHC) in the guinea pig heart increases during postnatal development. Reda et al. show that this increase in β-MHC enhances length-mediated increases in myofilament Ca2+ sensitivity and sarcomere length–dependent changes in contractile function.
Abstract
Shifts in myosin heavy chain (MHC) isoforms in cardiac myocytes have been shown to alter cardiac muscle function not only in healthy developing hearts but also in diseased hearts. In guinea pig hearts, there is a large age-dependent shift in MHC isoforms from 80% α-MHC/20% β-MHC at 3 wk to 14% α-MHC/86% β-MHC at 11 wk. Because kinetic differences in α- and β-MHC cross-bridges (XBs) are known to impart different cooperative effects on thin filaments, we hypothesize here that differences in α- and β-MHC expression in guinea pig cardiac muscle impact sarcomere length (SL)–dependent contractile function. We therefore measure steady state and dynamic contractile parameters in detergent-skinned cardiac muscle preparations isolated from the left ventricles of young (3 wk old) or adult (11 wk old) guinea pigs at two different SLs: short (1.9 µm) and long (2.3 µm). Our data show that SL-dependent effects on contractile parameters are augmented in adult guinea pig cardiac muscle preparations. Notably, the SL-mediated increase in myofilament Ca2+ sensitivity (ΔpCa50) is twofold greater in adult guinea pig muscle preparations (ΔpCa50 being 0.11 units in adult preparations but only 0.05 units in young preparations). Furthermore, adult guinea pig cardiac muscle preparations display greater SL-dependent changes than young muscle preparations in (1) the magnitude of length-mediated increase in the recruitment of new force-bearing XBs, (2) XB detachment rate, (3) XB strain-mediated effects on other force-bearing XBs, and (4) the rate constant of force redevelopment. Our findings suggest that increased β-MHC expression enhances length-dependent activation in the adult guinea pig cardiac myocardium.
Introduction
Shifts in myosin heavy chain (MHC) isoforms in cardiac myocytes have been shown to alter cardiac muscle function not only in healthy developing hearts (Everett et al., 1983; Cappelli et al., 1989; Reiser et al., 2001) but also in diseased hearts (Lowes et al., 1997; Nakao et al., 1997; Miyata et al., 2000). Of particular importance to our study is the large shift in MHC isoform in developing healthy guinea pig hearts because it offers insight into the relationship between functional adaptation at the molecular and whole organ levels. A consequence of the age-related increase in the size of the animal is that the load on the heart increases; thus, the heart must be able to increase its stroke volume in response to bigger changes in end-diastolic volume to maintain physiological cardiac output. This fundamental property of the heart is known as the Frank–Starling mechanism (Plotnick et al., 1986; Moss and Fitzsimons, 2002) and plays a significant role in development and disease. The molecular mechanism underpinning the Frank–Starling mechanism is length-dependent activation (Nowak et al., 2007; Abraham et al., 2016), whereby an increase in cardiac muscle sarcomere length (SL) enhances myofilament Ca2+ sensitivity and force production (Allen and Kentish, 1985; Wang and Fuchs, 1994; Konhilas et al., 2002). A large shift from α- to β-MHC in the developing heart has relevance to length-dependent activation because β-MHC may contain features that enhance length-dependent activation (Korte and McDonald, 2007; Ford and Chandra, 2013).
Although α- and β-MHC isoforms share a high degree of sequence homology (Kurabayashi et al., 1988; McNally et al., 1989), they exhibit strikingly different enzymatic properties, which is reflected in two- to threefold differences in the rate of ATPase activity between α- and β-MHC isoforms (van der Velden et al., 1998; Krenz et al., 2003; Rundell et al., 2005; Tschirgi et al., 2006; Ford and Chandra, 2013). Compatible with this large difference in enzymatic activity is the notion that developmentally related shifts in α- and β-MHC isoforms are correlated with functional adaptations of the heart as it increases in size with age. There is ample evidence to suggest that α- and β-MHC isoforms impart different effects on thin filaments in accordance with their intrinsic kinetic properties (Ford et al., 2012; Chandra et al., 2015; Gollapudi et al., 2015; Gollapudi and Chandra, 2016). The longer dwell time of β-MHC cross-bridges (XBs), which is ascribed to their slower XB cycling kinetics, may enhance XB-based cooperative feedback effect on thin filaments (Ford and Chandra, 2013; Mamidi and Chandra, 2013; Michael et al., 2014; Chandra et al., 2015; Reda et al., 2016). Because XB-mediated activation is known to play a role in cardiac length-dependent activation (Allen and Kentish, 1985; Fitzsimons and Moss, 1998; Moss and Fitzsimons, 2002; Smith et al., 2009), it is reasonable to posit that β-MHC may respond differently to SL changes when compared with α-MHC. Other supporting evidence comes from structural studies that suggest important differences in the organization of α- and β-MHC isoforms in the myofilament. For example, x-ray diffraction studies show that myosin head orientation is sensitive to SL (Farman et al., 2011). Electron microscopy and optical diffraction studies demonstrate differences in radial/azimuthal flexibility of XBs in myofilaments containing α- and β-MHC isoform (Weisberg and Winegrad, 1998). Furthermore, interventions that modify XB activity are known to modulate SL-dependent function in cardiac muscle (Fitzsimons and Moss, 1998; Gollapudi et al., 2017). Collectively, these observations suggest that α- and β-MHC may differently sense the effect of changes in SL. Thus, a large shift from α- to β-MHC may alter length-dependent activation in guinea pig cardiac muscle.
To test our hypothesis that age-related increase in β-MHC expression enhances length-dependent activation, we measured steady state and dynamic contractile function in detergent-skinned cardiac muscle preparations from young (3 wk old) and adult (11 wk old) guinea pigs at two different SLs: short SL (1.9 µm) and long SL (2.3 µm). Assessment of MHC isoform composition demonstrated that the total percentage of β-MHC was 20% in young (3 wk old) guinea pig hearts and 86% in adult (11 wk old) guinea pig hearts. Our data demonstrate that the SL-mediated increase in myofilament Ca2+ sensitivity was twofold greater in adult cardiac muscle preparations. Furthermore, the magnitude of length-mediated increase in the recruitment of new force-bearing XBs, the SL-dependent attenuation of XB detachment rate, and the SL-dependent attenuation of XB strain-mediated effect on other force-bearing XBs were greater in adult cardiac muscle preparations. We discuss the relevance of our findings to the correlation between increased expression of β-MHC and length-dependent activation in the young vs. adult guinea pig hearts.
Materials and methods
Animal protocols
Young (3 wk old; 21–22 d) and adult (11 wk old; 79–80 d) Dunkin–Hartley female guinea pigs (Cavia porcellus) were used in this study (acquired from Charles River). All animals were housed in environmentally controlled rooms of an Association for Assessment and Accreditation of Laboratory Animal Care–accredited facility under 12-h light and dark cycles. All animals received proper care and treatment in accordance with the guidelines set by the Washington State University Institutional Animal Care and Use Committee. The procedures for euthanizing guinea pigs conform to the recommendations of the American Veterinary Medical Association, as outlined in the Guidelines for the Euthanasia of Animals.
Measurement of steady state tension and ATPase activity in detergent-skinned cardiac multicellular preparations
Steady state isometric tension and ATPase activity (de Tombe and Stienen, 1995; Stienen et al., 1995; Gollapudi and Chandra, 2012) were measured in detergent-skinned cardiac multicellular preparations (see supplemental material). In brief, T-shaped aluminum clips were used to attach muscle preparations between a motor arm (322C; Aurora Scientific Inc.) and a force transducer (AE 801; Sensor One Technologies). The SL of muscle preparations was adjusted to 1.9 or 2.3 µm in relaxing solution using the He-Ne laser diffraction technique (Lieber et al., 1984). Muscle preparations were then subjected to two cycles of maximal Ca2+ activation (pCa 4.3) and relaxation (pCa 9.0), and the SL was readjusted to the desired value if necessary. The length and cross section of the muscle preparation were measured, and the muscle preparation was then exposed to various solutions with pCa ranging from 4.3 to 9.0 in a constantly stirred chamber. The composition of pCa 4.3 and pCa 9.0 solutions can be found in the supplemental material. All measurements were made at 20°C. Steady state isometric ATPase activity was measured using an enzymatically coupled assay that estimates changes in ATPase activity from changes in NADH (de Tombe and Stienen, 1995; Stienen et al., 1995; Gollapudi and Chandra, 2012). Details on the measurement of ATPase activity can be found in the supplemental material.
Measurement of contractile dynamic parameters
A family of various amplitude quick stretches/releases (±0.5, ±1.0, ±1.5, and ±2.0% of the initial muscle length [ML]) was imposed on muscle preparations in the steady state of maximal Ca2+ activation (pCa 4.3), and the corresponding force responses were recorded, as described previously (Ford et al., 2010). A nonlinear recruitment-distortion (NLRD) model was fitted to this family of force responses to estimate five NLRD model parameters: the magnitude of instantaneous increase in force caused by a sudden change in ML (ED); the rate by which force dissipates due to detachment of strongly bound XBs (c); the nonlinear interaction parameter representing the negative impact of strained XBs on other force-bearing XBs (γ); the rate of delayed force rise as XBs are recruited into the force-bearing state at the increased ML (b); and the magnitude of ML-mediated increase in steady state force due to recruitment of additional force-bearing XBs (ER). The characteristic features of ML-mediated force responses and the physiological significance of the NLRD model can be found in the supplemental material. Fig. S1 shows a length protocol of 2% sudden stretch (Fig. S1 A) and the corresponding force response (Fig. S1 B) from a representative muscle preparation.
Rate constant of tension redevelopment (ktr)
ktr was estimated using a modified version of the large slack/restretch maneuver originally designed by Brenner and Eisenberg (1986) and is described in detail in the supplemental material.
Statistical analysis
Our experimental model involved two factors, MHC (young and adult guinea pig cardiac muscle preparations) and SL (1.9 and 2.3 µm). Therefore, two-way ANOVA was used to determine if there was a significant SL–MHC interaction effect on a given parameter. A significant SL–MHC interaction effect suggested that the effect of SL on a given contractile parameter was dissimilar in young (20% β-MHC) and adult (86% β-MHC) guinea pig cardiac muscle preparations. When the SL–MHC interaction effect was not significant, we assessed the main effect of SL and main effect of MHC on a given parameter. To probe the underlying cause for a significant interaction or main effect of SL on a given parameter, Holm–Sidak post hoc multiple comparison analysis was performed. The criterion for statistical significance was set to P ≤ 0.05. Data are presented as means ± SEM. For all groups, the number of cardiac muscle preparations measured was 10 from two hearts.
Online supplemental material
Details regarding determination of MHC composition, stoichiometry, and phosphorylation status of sarcomeric proteins in guinea pig cardiac muscle preparations, preparation of guinea pig cardiac muscle preparations, composition of pCa solutions, measurement of ATPase activity, NLRD model parameters, and measurement of ktr can be found in the supplemental Materials and methods. Also included are Fig. S1, depicting a representative force response to a sudden 2% stretch in ML; Fig. S2, showing SDS-PAGE analysis of MHC composition, stoichiometry, and phosphorylation status of sarcomeric proteins; and Table S1, showing the SL dependency of contractile parameters in young and adult guinea pig cardiac muscle preparations.
Results
Analysis of MHC composition, stoichiometry, and phosphorylation status of sarcomeric proteins in young and adult guinea pig cardiac muscle preparations
The relative composition of MHC isoforms was determined by analyzing the SDS-solubilized muscle protein samples from young and adult guinea pig ventricular tissue on a large 6% SDS gel. Fig. S2 A shows a representative gel demonstrating the MHC composition in young and adult guinea pig ventricles. Densitometric analysis showed that β-MHC expression was 20 ± 3% in young guinea pig hearts and 86 ± 4% in adult guinea pig hearts; therefore, as guinea pigs aged from 3 to 11 wk, β-MHC expression increased by 66%. Significant age-dependent shifts from α- to β-MHC isoforms have been observed in several other rodent species (Everett et al., 1983; Lompré et al., 1984; Schuyler and Yarbrough, 1990; Schwartz et al., 1992; Fitzsimons et al., 1999). With regard to guinea pigs, our findings are in disagreement with a previous study (van der Velden et al., 1998), which showed only a 13% increase in β-MHC expression as guinea pigs aged from 1–8 wk old (young; 17% α-MHC/83% β-MHC) to 9–26 wk old (adult; 4% α-MHC/96% β-MHC). Such discrepancy is likely related to the observation that 1–8-wk-old guinea pigs were considered a single group in the aforementioned study (van der Velden et al., 1998), whereas our study compared two specific age groups: 3 wk and 11 wk. Collectively, these observations demonstrate a major shift in MHC isoform during the 3–11-wk period; therefore, for a meaningful interpretation of the impact of MHC isoforms on cardiac function, it is important to consider the specific age of the animal.
Fig. S2 B shows a representative 12.5% SDS gel of the stoichiometry of sarcomeric proteins in young and adult guinea pig hearts. The total amount of each sarcomeric protein (MHC, MyBP-C, Desmin, Actin, TnT, TnI, MLC-1, and MLC-2) is similar in young and adult guinea pig hearts. In regard to developmental changes in titin isoform expression, a previous study found no significant difference in titin expression between neonatal and adult guinea pig hearts (Krüger et al., 2006). Fig. S2 C shows a representative Pro-Q phospho-stained gel demonstrating that the phosphorylation levels of various proteins were not different in young and adult guinea pig hearts. These observations showed that an age-dependent increase in β-MHC (66%) occurred in the absence of any changes in stoichiometry or phosphorylation levels of sarcomeric proteins. For MHC composition, values are reported as means ± SEM from three gels.
SL dependency of Ca2+-activated maximal tension and ED in young and adult guinea pig cardiac muscle preparations
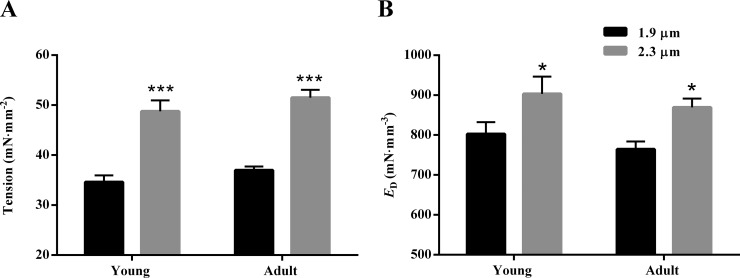
Steady state tension at maximal Ca2+ activation (pCa 4.3) was measured at short (1.9 µm) and long (2.3 µm) SLs to assess the impact of SL on maximal tension in guinea pig young and adult cardiac muscle preparations (Table S1). Two-way ANOVA did not demonstrate a significant SL–MHC interaction effect on maximal tension (P = 0.91) or a significant main effect of MHC (P = 0.12); however, the main effect of SL was significant (P < 0.001). Post hoc analysis showed that an increase in SL from 1.9 to 2.3 µm significantly increased maximal tension by 41% (P < 0.001; Fig. 1 A) in young and 39% (P < 0.001; Fig. 1 A) in adult muscle preparations. Our observations on maximal tension were corroborated by ED. We have previously shown that ED is correlated to maximal tension, and it is an approximate measure of the number of force-bearing XBs (Campbell et al., 2004; Ford et al., 2010; Mamidi et al., 2013; Chandra et al., 2015). Two-way ANOVA did not demonstrate a significant SL–MHC interaction effect (P = 0.94) or main effect of MHC (P = 0.24) on ED, but the main effect of SL was significant (P = 0.002). Post hoc analysis revealed that an increase in SL significantly increased ED in young and adult muscle preparations by 13% (P = 0.036; Fig. 1 B) and 14% (P = 0.036; Fig. 1 B), respectively. Collectively, our findings on maximal tension and ED suggest that the SL-mediated increase in the number of strongly bound XBs is similar in young and adult muscle preparations.
Figure 1.
SL dependency of maximal tension and ED in young and adult guinea pig cardiac muscle preparations. Maximal tension was measured by exposing muscle preparations to saturating Ca2+ concentrations (pCa 4.3) in a constantly stirred chamber. ED was estimated as the slope of the linear relationship between F1–Fss and the corresponding ML changes (see Fig. S1) and thus provides an approximate measure of the number of strongly bound XBs. (A and B) Bar graphs showing the SL-dependent effect on (A) maximal tension and (B) ED in young and adult muscle preparations. Two-way ANOVA revealed that the main effect of SL was significant for both maximal tension (P < 0.001) and ED (P = 0.002). The main effect of MHC was not significant for both maximal tension (P = 0.91) and ED (P = 0.24). Post hoc multiple comparisons (Holm–Sidak method) were used to determine significant differences between groups. Asterisks and hash marks indicate a significant difference when compared with data within each group (*, P < 0.05 and ***, P < 0.001 for 1.9 vs. 2.3 µm). The number of preparations measured for all groups was 10. Data are expressed as means ± SEM.
SL dependency of the pCa–tension relationship in young and adult guinea pig cardiac muscle preparations
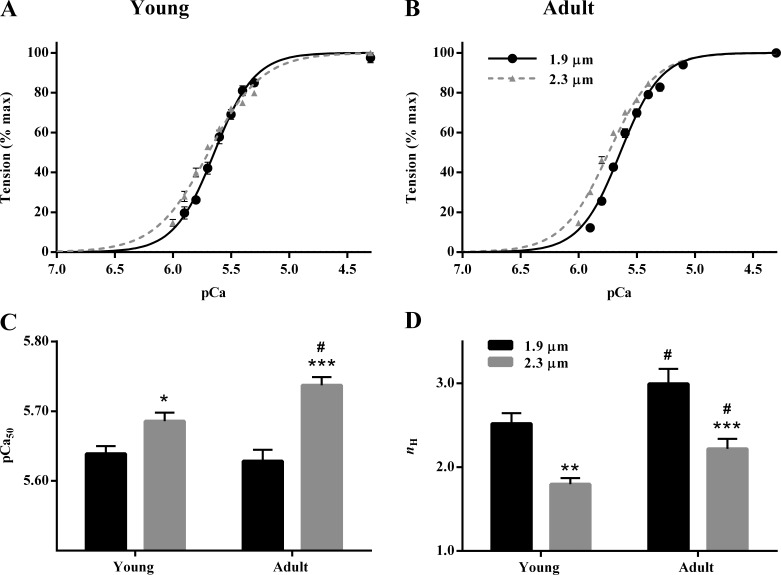
Fig. 2, A and B, demonstrates that an increase in SL resulted in a greater leftward shift in the pCa–tension relationship of adult than in young muscle preparations. This observation suggested that myofilament Ca2+ sensitivity was augmented to a greater extent in response to an increase in SL in adult than in young muscle preparations. Fig. 2, A and B, also demonstrates that an increase in SL reduced the steepness of the pCa–tension relationship in both young and adult muscle preparations. To quantify the SL-dependent effect on pCa–tension relationship in young and adult guinea pigs, we used Hill model–derived parameters, pCa50 and nH (Table S1). Two-way ANOVA showed a significant SL–MHC interaction effect on pCa50 (P = 0.02), suggesting that the SL-dependent effect on pCa50 was dissimilar in young and adult muscle preparations. Post hoc multiple comparison analysis confirmed that the increase in pCa50 associated with an increase in SL (ΔpCa50) was more than twofold greater in adult than in young muscle preparations; for example, it was 0.11 pCa units (P < 0.001; Fig. 2 C) in adult muscle preparations but only 0.05 pCa units (P = 0.032; Fig. 2 C) in young muscle preparations. We also sought to quantify the effect of MHC on pCa50 at short and long SL. At short SL, pCa50 was not different between young and adult muscle preparations (P = 0.57; Fig. 2 C); however, at long SL, pCa50 was 0.05 units greater in adult than in young muscle preparations (P = 0.02; Fig. 2 C).
Figure 2.
SL dependency of the pCa–tension relation in young and adult guinea pig cardiac muscle preparations. Normalized steady state tensions at various pCa were plotted against pCa to construct the pCa–tension relationships. The Hill model was fitted to pCa–tension relationships to derive myofilament Ca2+ sensitivity (pCa50) and myofilament cooperativity (nH). (A and B) Comparisons of pCa–tension relationships at short and long SL in young (A) and adult (B) muscle preparations. The traces connecting the experimental data points are the Hill model fits. Error bars are obscured by the symbols in some cases. (C and D) Bar graphs showing the SL-dependent effect on pCa50 (C) and nH (D) in young and adult muscle preparations. Two-way ANOVA revealed a significant SL–MHC interaction effect (P = 0.02) on pCa50. For nH, the main effects of both SL (P < 0.001) and MHC (P = 0.0013) were significant. Post hoc multiple comparisons (Holm–Sidak method) were used to determine significant differences between groups. Asterisks and hash marks indicate significant difference when compared with data within each group (*, P < 0.05; **, P < 0.01; and ***, P < 0.001 for 1.9 vs. 2.3 µm; #, P < 0.05 for young vs. adult). The number of preparations measured for all groups was 10. Data are expressed as means ± SEM.
As for nH, two-way ANOVA did not show a significant SL–MHC interaction effect (P = 0.84), but the main effects of SL (P < 0.001) and MHC (P = 0.0013) were significant. Post hoc multiple comparisons showed that an increase in SL significantly decreased nH by 29% (P = 0.002; Fig. 2 D) and 26% (P < 0.001; Fig. 2 D) in young and adult muscle preparations, respectively. Furthermore, we found that nH was significantly greater in adult than in young muscle preparations at both short SL (P = 0.042; Fig. 2 D) and long SL (P = 0.049; Fig. 2 D).
SL dependency of the XB detachment rate (g) in young and adult guinea pig cardiac muscle preparations
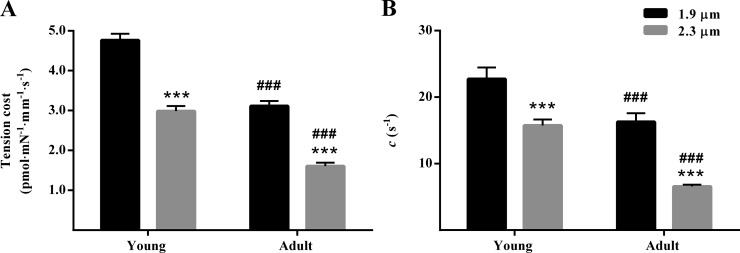
Previous studies have shown that an increase in SL leads to a decrease in XB detachment rate (g; Stelzer and Moss, 2006; Ford and Chandra, 2013; Reda and Chandra, 2018). To determine whether the age-related increase in β-MHC expression altered the SL dependency of g in guinea pig myocardium, we assessed tension cost and c. Tension cost was derived as the slope of the linear relationship between steady state tension and ATPase activity at various pCa (de Tombe and Stienen, 1995; Stienen et al., 1995). Parameter c governs the rate of force decay following a step-like ML change and is estimated by fitting the NLRD model to the fiber-elicited force responses to various amplitude stretch/release perturbations (Fig. S1; Ford et al., 2010). We have previously shown that tension cost and c are approximate measures of g (Campbell et al., 2004; Gollapudi et al., 2015; Reda et al., 2016). Two-way ANOVA of tension cost did not show a significant SL–MHC interaction effect (P = 0.29), but it demonstrated a significant main effect of SL (P < 0.001) and MHC (P < 0.001). Post hoc analysis showed that an increase in SL significantly decreased tension cost by 49% (P < 0.001; Fig. 3 A) in adult muscle preparations and by 37% (P < 0.001; Fig. 3 A) in young muscle preparations (Table S1). Our analysis also showed that tension cost was significantly lower in adult than in young muscle preparations at both short (P < 0.001; Fig. 3 A) and long SLs (P < 0.001; Fig. 3 A). Our observations on tension cost were corroborated by c. Two-way ANOVA did not reveal a significant SL–MHC interaction effect (P = 0.24) on c, but it indicated a significant main effect of SL (P < 0.001) and MHC (P < 0.001). Post hoc analysis showed that an increase in SL decreased c by 59% (P < 0.001; Fig. 3 B) in adult muscle preparations but only by 31% (P < 0.001; Fig. 3 B) in young muscle preparations (Table S1). Moreover, c was significantly lower in adult than in young guinea pig preparations at both short (P < 0.001; Fig. 3 B) and long SLs (P < 0.001; Fig. 3 B). Consistent findings on the SL dependency of both tension cost and c in adult muscle preparations suggested that the age-related increase in β-MHC expression enhances the SL-dependent attenuation of g in the guinea pig myocardium.
Figure 3.
SL dependency of tension cost and c in young and adult guinea pig cardiac muscle preparations. Tension cost was derived as the slope of the linear relationship between steady state tension and ATPase measurements at various pCa (de Tombe and Stienen, 1995; Stienen et al., 1995). The value of c was estimated by fitting the NLRD model to the family of muscle-elicited force responses to various amplitude step-like ML perturbations at pCa 4.3 (Ford et al., 2010). (A and B) Bar graphs showing the SL-dependent effect on tension cost (A) and c (B) in young and adult muscle preparations. Two-way ANOVA revealed that the main effect of SL was significant for both tension cost (P < 0.001) and c (P < 0.001). The main effect of MHC was significant for both tension cost (P < 0.001) and c (P < 0.001). Post hoc multiple comparisons (Holm–Sidak method) were used to determine significant differences between groups. Asterisks and hash marks indicate a significant difference when compared with data within each group (***, P < 0.001 for 1.9 vs. 2.3 µm; ###, P < 0.001 for young vs. adult). The number of preparations measured for all groups was 10. Data are expressed as means ± SEM.
SL dependency of γ in young and adult guinea pig cardiac muscle preparations
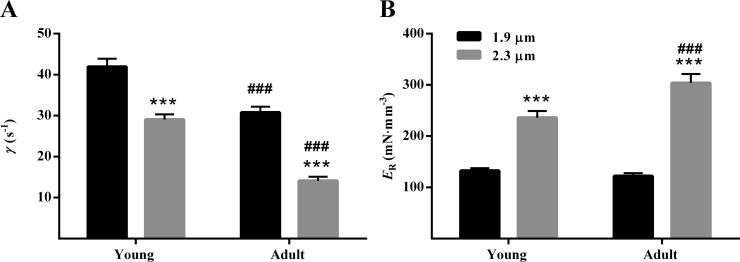
In our NLRD model, parameter γ represents the negative effect of strained XBs on other force-bearing XBs and is estimated by fitting the NLRD model to a family of force responses to various amplitude length perturbations (Fig. S1; Ford et al., 2010). When the negative impact of strained XBs on other force-bearing XBs is less pronounced, force declines to a lesser extent (less prominent nadir), and thus γ is lower. Because such XB-based interactions are mediated by cooperative interactions within the thick and thin filaments, γ is thought to be influenced by allosteric/cooperative mechanisms in the myofilaments. For instance, an increase in cooperativity associated with an increase in SL has been shown to attenuate γ at long SL (Reda and Chandra, 2018). To determine whether the age-related increase in β-MHC expression altered myofilament allosteric/cooperative mechanisms in an SL-dependent manner, we assessed γ (Table S1). Two-way ANOVA did not reveal a significant SL–MHC interaction effect (P = 0.19) on γ, but it confirmed a significant main effect of SL (P < 0.001) and MHC (P < 0.001). Post hoc analysis revealed that the SL-dependent decrease in γ was 54% (P < 0.001; Fig. 4 A) in adult muscle preparations but only 31% (P < 0.001; Fig. 4 A) in young muscle preparations, suggesting that the SL-dependent effect on cooperative mechanisms is greater in adult muscle preparations (higher expression of β-MHC). Furthermore, our analysis showed that γ was significantly lower in adult than in young muscle preparations at both short (P < 0.001; Fig. 4 A) and long (P < 0.001; Fig. 4 A) SL.
Figure 4.
SL dependency of γ and ER in young and adult guinea pig cardiac preparations. The values of γ and ER were estimated by fitting the NLRD model to the family of muscle-elicited force responses to various amplitude step-like ML perturbations (Ford et al., 2010). γ represents the negative impact of strained XBs on the recruitment of other force-bearing XBs. ER is estimated from the slope of the linear relationship between Fnss–Fss and ML perturbation (see Fig. S1) and thus represents the magnitude of force rise in response to an increase in ML. (A and B) Bar graphs showing the SL-dependent effect on γ (A) and ER (B) in young and adult muscle preparations. Two-way ANOVA revealed that the main effects of SL (P < 0.001) and MHC (P < 0.001) on γ were significant. For ER, two-way ANOVA revealed a significant SL–MHC interaction effect (P = 0.003). Post hoc multiple comparisons (Holm–Sidak method) were used to determine significant differences between groups. Asterisks and hash marks indicate significant difference when compared with data within each group (***, P < 0.001 for 1.9 vs. 2.3 µm; ###, P < 0.001 for young vs. adult). The number of preparations measured for all groups was 10. Data are expressed as means ± SEM.
SL dependency of stretch activation parameters, b and ER, in young and adult guinea pig cardiac muscle preparations
To determine whether the age-related increase in β-MHC expression altered stretch activation in a SL-dependent manner, we assessed estimates of b and ER (Table S1). Parameters b and ER are estimated by fitting the NLRD model to a family of force responses to various amplitude ML perturbations (Fig. S1; Ford et al., 2010). b represents the rate constant of delayed force rise in response to an increase in ML. Two-way ANOVA of b did not show a significant SL–MHC interaction effect (P = 0.64) or a significant main effect of SL (P = 0.26). This is because an increase in SL did not significantly alter b in both young and adult muscle preparations. In young muscle preparations, b was 7.18 ± 0.32 s−1 at short SL and 7.37 ± 0.45 s−1 at long SL; in adult muscle preparations, b was 3.59 ± 0.10 s−1 at short SL and 4.04 ± 0.15 s−1 at long SL. As expected, the main effect of MHC (P < 0.001) on b was significant; post hoc analysis showed that b was significantly slower in adult than in young muscle preparations at both short (P < 0.001) and long (P < 0.001) SL.
ER represents the magnitude of the delayed force rise in response to an increase in ML, an effect that is mediated by XB-based cooperative mechanisms (Campbell et al., 2004; Campbell and Chandra, 2006; Stelzer et al., 2006). Two-way ANOVA revealed a significant SL–MHC interaction effect (P = 0.003) on ER, indicating that the SL-dependent effect on ER was dissimilar in young and adult muscle preparations. Post hoc analysis confirmed that the SL-dependent increase in ER was 149% (P < 0.001; Fig. 4 B) in adult muscle preparations but only 79% (P < 0.001; Fig. 4 B) in young muscle preparations. Interestingly, the effect of MHC on ER was similar to the effect observed on pCa50; at short SL, ER was not different between young and adult muscle preparations (P = 0.56; Fig. 4 B); however, at long SL, ER was significantly greater in adult than in young muscle preparations (P < 0.001; Fig. 4 B). Our observations here suggest that that the age-related increase in β-MHC expression enhances SL-dependent effects on cooperative mechanisms governing ER in the guinea pig myocardium.
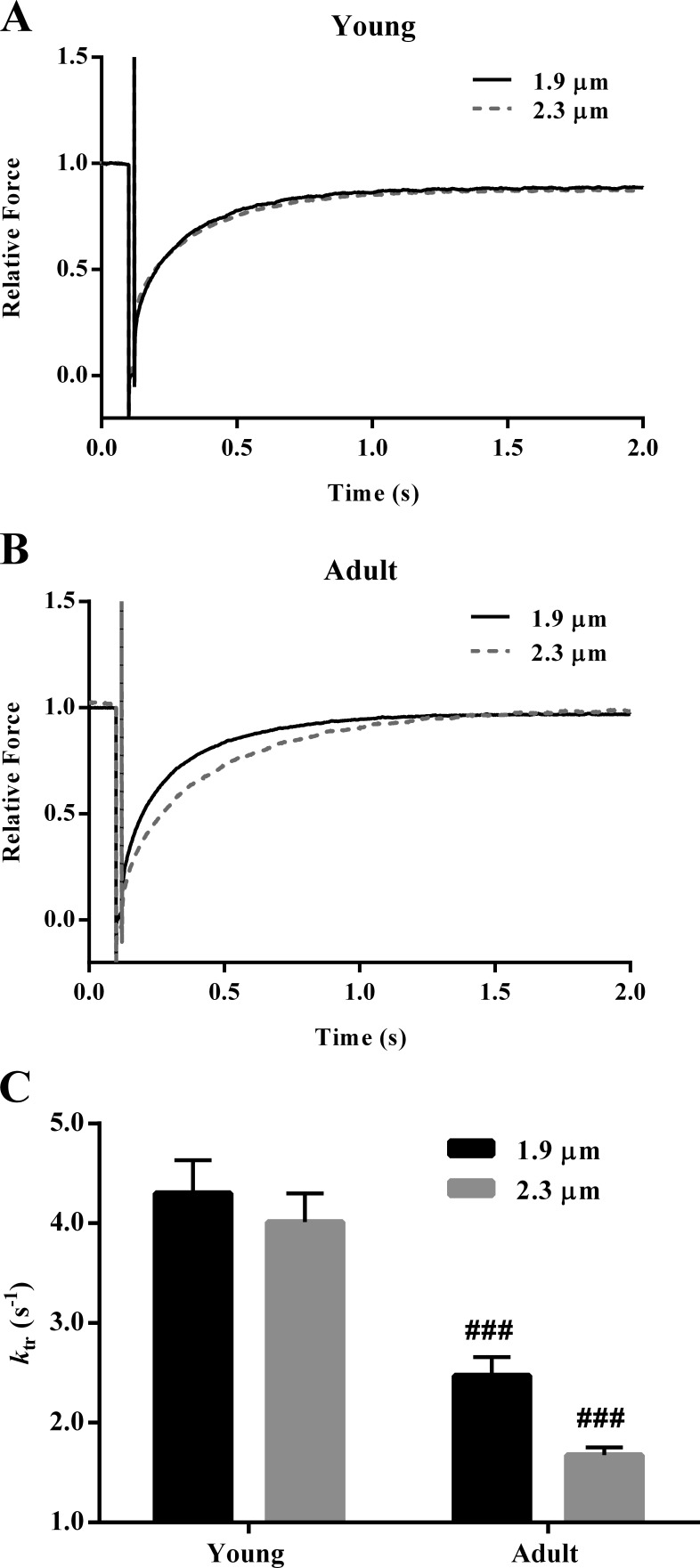
SL dependency of ktr in young and adult guinea pig cardiac muscle preparations
To determine whether the age-related increase in β-MHC expression altered the SL dependency of XB turnover rate in guinea pig myocardium, we assessed changes in ktr (Table S1). ktr is estimated by fitting a monoexponential function to the rising phase of the force response to a large slack/restretch perturbation. An increase in SL did not affect the rising phase of the force response in young muscle preparations (Fig. 5 A), while it resulted in a rightward shift in the rising phase of the force response in adult muscle preparations (Fig. 5 B), suggesting slower ktr. Two-way ANOVA of ktr did not demonstrate a significant SL–MHC interaction effect (P = 0.31), but it showed a significant main effect of SL (P = 0.033) and MHC (P < 0.001). Post hoc analysis showed that an increase in SL decreased ktr by 7% (P = 0.40; Fig. 5 C) in young muscle preparations. An increase in SL decreased ktr by 32% in adult muscle preparations, but it was only marginally significant (P = 0.057; Fig. 5 C). Moreover, ktr was significantly lower in adult than in young muscle preparations at both short (P < 0.001; Fig. 5 C) and long (P < 0.001; Fig. 5 C) SL, suggesting augmented cooperativity in adult muscle.
Figure 5.
SL dependency of ktr in young and adult guinea pig cardiac muscle preparations. ktr is estimated by fitting a monoexponential function to the rising phase of the force response to a large release/restretch perturbation. (A and B) Comparisons of force responses at short and long SL from young (A) and adult (B) guinea pig muscle preparations in response to a large release/restretch perturbation. Forces were normalized by the isometric steady state before ML perturbation. (C) Bar graph showing the SL-dependent effect on ktr in young and adult muscle pig preparations. Two-way ANOVA revealed that the main effect of SL (P = 0.033) and MHC (P < 0.001) on ktr were significant. Post hoc multiple comparisons (Holm–Sidak method) were used to determine significant differences between groups. Asterisks and hash marks indicate significant difference when compared with data within each group (###, P < 0.001 for young vs. adult). The number of preparations measured for all groups was 10. Data are expressed as means ± SEM.
Discussion
A significant change in myofilament Ca2+ sensitivity—associated with an increase in SL—is the molecular basis for length-dependent activation in cardiac muscle preparations (Allen and Kentish, 1985; Wang and Fuchs, 1994; Konhilas et al., 2002). Our study demonstrates that the SL-mediated increase in myofilament Ca2+ sensitivity is greater in adult (86% β-MHC) than in young (20% β-MHC) guinea pig cardiac muscle preparations, indicating enhancement of length-dependent activation in adult guinea pig myocardium. Given that (1) α- and β-MHC isoforms differentially impact cardiac contractile dynamics and (2) length-dependent activation of cardiac muscle is modulated by XB-based cooperative mechanisms (Allen and Kentish, 1985; Fitzsimons and Moss, 1998; Moss and Fitzsimons, 2002; Smith et al., 2009), mechanisms underlying such enhanced length-dependent activation may result from greater expression of β-MHC in adult guinea pig myocardium. Furthermore, adult muscle preparations exhibit greater SL-dependent changes in ER, g, ktr, and γ. Such greater changes in contractile parameters are expected to increase SL-dependent function in hearts containing β-MHC.
It has been previously shown that XB detachment kinetics, g, is more sensitive to changes in SL in β-MHC than in α-MHC expressing cardiac muscle from propylthiouracil-treated mouse hearts (Ford and Chandra, 2013). Another study investigating the impact of the SL-dependent effect on cardiac muscle shortening found that a decrease in SL attenuated peak power output to a greater extent in β-MHC myocytes (propylthiouracil-treated rat hearts) than in α-MHC myocytes from normal hearts (Korte and McDonald, 2007). These observations may have an important bearing on the dynamics of heart function in mammals because age-related changes in heart development coincide with significant shifts in MHC isoforms. Our data demonstrate that a 66% increase in total percentage of β-MHC augments SL-dependent changes in contractile parameters in guinea pig cardiac muscle preparations. A novel finding in our study is that an increase in SL from 1.9 to 2.3 µm results in a twofold greater increase in pCa50 (ΔpCa50) in adult muscle preparations than in young muscle preparations (Fig. 2 C). Such SL-mediated increase in myofilament Ca2+ sensitivity (enhanced length-dependent activation) has been attributed to greater contribution from XB-based cooperative mechanisms that feedback to enhance thin filament Ca2+ sensitivity (Wang and Fuchs, 1994; Fitzsimons and Moss, 1998; Smith et al., 2009). It is well documented that β-MHC XBs impart greater cooperative effects on myofilaments when compared with α-MHC XBs (Rundell et al., 2005; Ford and Chandra, 2013; Mamidi and Chandra, 2013; Michael et al., 2014; Chandra et al., 2015; Reda et al., 2016). This is presumably due to the slower cycling kinetics of β-MHC (VanBuren et al., 1995), which increases XB dwell time in the strongly bound state, thereby allowing for allosteric length-sensing mechanisms to induce greater cooperative effects on contractile function. Collectively, pCa–tension experiments suggest that higher amounts of β-MHC in adult muscle preparations (86% β-MHC) and augmented XB-based cooperativity lead to greater SL-dependent changes in pCa50 when compared with young muscle preparations (20% β-MHC). These observations are suggestive of a strong correlation between enhanced length-dependent activation and higher percentage of β-MHC in adult guinea pig cardiac muscle preparations.
Differences in SL-mediated effects on contractile parameters between adult and young guinea pig muscle preparations appear to be independent of the number of strongly bound XBs that are recruited in response to an increase in SL. For instance, the SL-dependent increase in steady state maximal tension and ED is similar in both adult and young muscle preparations (Fig. 1), but higher amounts of β-MHC in adult muscle preparations lead to greater SL-dependent changes in contractile dynamic parameters. This is demonstrated by a greater SL dependency of ER, c, tension cost, ktr, and γ in adult muscle preparations. Higher amounts of β-MHC in adult muscle preparations and enhanced XB-based cooperativity are correlated with an increase in ER because an increase in XB-based cooperativity augments ER and a decrease in XB-based cooperativity attenuates ER (Campbell et al., 2004; Campbell and Chandra, 2006; Stelzer and Moss, 2006). Data shown in Fig. 4 B demonstrate that the magnitude of SL-dependent increase in ER is significantly greater in adult muscle preparations (149%) than young muscle preparations (79%), substantiating the notion that XB-based cooperativity is enhanced in adult muscle preparations at long SL. Furthermore, our observations on ER follow the same trend as pCa50; ER is similar in young and adult muscle preparations at short SL but substantially greater in adult muscle preparations at long SL.
As expected, two independent measures of XB detachment rate, tension cost and c (Fig. 3), demonstrate that higher amounts of β-MHC attenuate g in adult guinea pig muscle preparations. However, the interesting observation on g is that an increase in SL (from 1.9 to 2.3 µm) results in a greater attenuation of g in adult than in young muscle preparations. For example, the magnitude of SL-dependent attenuation of c is 31% in young muscle preparations, while it is 56% in adult muscle preparations (Fig. 3 B). Slower g at long SL (longer XB dwell time) presumably augments XB-based cooperative feedback effects on thin filaments, increases XB-mediated activation of neighboring regulatory units, and enhances pCa50 in adult muscle preparations (Fig. 2 C). Enhanced XB-based cooperativity in adult muscle preparations may be supported by our observations on ktr because enhanced XB-based cooperativity attenuates ktr (Campbell, 1997; Razumova et al., 2000). ktr is significantly lower in adult than in young at both short and long SL (Fig. 5 C; P < 0.001). Although we did not measure g at intermediate SL, studies from other laboratories show a similar trend. For example, a previous mouse study showed that an increase in SL from 1.9 to 2.1 µm significantly decreased g (Mamidi et al., 2016). One limitation of our study is that our contractile measurements are performed at 20°C. A previous study has suggested that myofilament Ca2+ sensitivity and XB cycling kinetics are temperature dependent (de Tombe and Stienen, 2007). How such changes in XB activity affect length-dependent activation differentially at 37°C and how such changes are differently altered by MHC isoform remain to be determined.
Additional insights on how greater XB-based cooperative effects are modified by changes in SL may be gleaned from comparisons of γ in adult and young guinea pig muscle preparations. γ represents one kind of cooperativity by which strained XBs negatively impact other force-bearing XBs through allosteric/cooperative mechanisms within myofilaments (Ford et al., 2010). Previously, we have shown that an increase in XB-based cooperativity associated with an increase in SL attenuates γ (Reda and Chandra, 2018). We suggested that increased XB-based cooperativity counteracts the negative impact of strained XBs on force-bearing XBs, leading to a decrease in γ. Our data demonstrate that γ is greater at short than at long SL in both young and adult muscle preparations (Fig. 4 A); however, a key observation is that the SL-dependent attenuation of γ is greater in adult (54%) than in young (30%) muscle preparations. This greater attenuation of γ is suggestive of increased XB-based cooperativity in adult muscle preparations, which most likely results from increased levels of β-MHC as suggested above.
Significance of our findings to whole heart function
Our observations demonstrate that developmental up-regulation of β-MHC in guinea pig myocardium enhances the SL-dependent increase in myofilament Ca2+ sensitivity, a hallmark of cardiac length-dependent activation. Because β-MHC is known to be up-regulated in several species during development, our findings have important implications regarding the significance of MHC composition on contractile function. Enhancement of the SL-mediated increase in myofilament Ca2+ sensitivity suggests that an increase in end-diastolic volume may enhance systolic force generation to a greater extent in adult guinea pig hearts, leading to a greater stroke volume. This is important because as the size of the animal increases during development, the heart must be able to increase cardiac output in response to an increase in preload. Indeed, a study investigating the relationship between ventricular volumes and body weight in mammals has shown that end-diastolic volume and stroke volume increase with body weight (Holt et al., 1968). As the size of the animal increases with age, heart rate decreases (Holt et al., 1968; Jones et al., 2004; Rhodes et al., 2012; Milani-Nejad and Janssen, 2014), and such a decrease in heart rate has been correlated with an increase in β-MHC (Hamilton and Ianuzzo, 1991). Given that cardiac output is a product of stroke volume and heart rate, lower heart rates in larger animals demand that stroke volume must increase such that cardiac output is not only maintained but increases in relation to the animal’s size/age. Therefore, an increase in the amount of β-MHC in larger animals is expected to enhance length-dependent activation and pressure development for a given increase in end-diastolic volume (SL).
The extrapolation of our results from guinea pig studies to other large animals may be speculative because the magnitude of isoform shift varies in different species (Lompre et al., 1981; Everett et al., 1983; Lompré et al., 1984; Cappelli et al., 1989; van der Velden et al., 1998; Reiser et al., 2001; Carnes et al., 2004); however, it must be pointed out that even small shifts in MHC isoforms lead to significant changes in cardiac contractile function (Herron and McDonald, 2002; Korte et al., 2005; Narolska et al., 2005; Rundell et al., 2005). In failing human hearts, there is a small but significant increase in β-MHC (Miyata et al., 2000; Reiser et al., 2001), which may impact length-dependent activation. In this regard, an increase in β-MHC may be a compensatory mechanism to enhance length-dependent activation. Augmentation of dynamic contractile parameters such as ER—paired with lengthened duty ratio of XBs (due to slower g)—may improve stroke volume by prolonging the duration of systole (Stelzer et al., 2006; Stelzer and Moss, 2006).
Supplementary Material
Acknowledgments
This manuscript does not contain clinical studies of patient data.
This work was supported, in part, by the intramural grant from College of Veterinary Medicine (MC) and Poncin Scholarship (SR).
The authors declare no competing financial interests.
Author contributions: S.M. Reda: study conception, design, data acquisition, data analysis, data interpretation, and drafting of the manuscript. S.K. Gollapudi: study conception, design, data acquisition, data analysis, data interpretation, and drafting of the manuscript. M. Chandra: study conception, design, data interpretation, and drafting of the manuscript.
Henk L. Granzier served as editor.
Footnotes
This work is part of a special collection on myofilament function.
References
- Abraham D.M., Davis R.T. III, Warren C.M., Mao L., Wolska B.M., Solaro R.J., and Rockman H.A.. 2016. beta-Arrestin mediates the Frank-Starling mechanism of cardiac contractility. Proc. Natl. Acad. Sci. USA. 113:14426–14431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D.G., and Kentish J.C.. 1985. The cellular basis of the length-tension relation in cardiac muscle. J. Mol. Cell. Cardiol. 17:821–840. 10.1016/S0022-2828(85)80097-3 [DOI] [PubMed] [Google Scholar]
- Brenner B., and Eisenberg E.. 1986. Rate of force generation in muscle: Correlation with actomyosin ATPase activity in solution. Proc. Natl. Acad. Sci. USA. 83:3542–3546. 10.1073/pnas.83.10.3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. 1997. Rate constant of muscle force redevelopment reflects cooperative activation as well as cross-bridge kinetics. Biophys. J. 72:254–262. 10.1016/S0006-3495(97)78664-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K.B., and Chandra M.. 2006. Functions of stretch activation in heart muscle. J. Gen. Physiol. 127:89–94. 10.1085/jgp.200509483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K.B., Chandra M., Kirkpatrick R.D., Slinker B.K., and Hunter W.C.. 2004. Interpreting cardiac muscle force-length dynamics using a novel functional model. Am. J. Physiol. Heart Circ. Physiol. 286:H1535–H1545. 10.1152/ajpheart.01029.2003 [DOI] [PubMed] [Google Scholar]
- Cappelli V., Bottinelli R., Poggesi C., Moggio R., and Reggiani C.. 1989. Shortening velocity and myosin and myofibrillar ATPase activity related to myosin isoenzyme composition during postnatal development in rat myocardium. Circ. Res. 65:446–457. 10.1161/01.RES.65.2.446 [DOI] [PubMed] [Google Scholar]
- Carnes C.A., Geisbuhler T.P., and Reiser P.J.. 2004. Age-dependent changes in contraction and regional myocardial myosin heavy chain isoform expression in rats. J. Appl. Physiol. 97:446–453. 10.1152/japplphysiol.00439.2003 [DOI] [PubMed] [Google Scholar]
- Chandra V., Gollapudi S.K., and Chandra M.. 2015. Rat cardiac troponin T mutation (F72L)-mediated impact on thin filament cooperativity is divergently modulated by alpha- and beta-myosin heavy chain isoforms. Am. J. Physiol. Heart Circ. Physiol. 309:H1260–H1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Tombe P.P., and Stienen G.J.. 1995. Protein kinase A does not alter economy of force maintenance in skinned rat cardiac trabeculae. Circ. Res. 76:734–741. 10.1161/01.RES.76.5.734 [DOI] [PubMed] [Google Scholar]
- de Tombe P.P., and Stienen G.J.. 2007. Impact of temperature on cross-bridge cycling kinetics in rat myocardium. J. Physiol. 584:591–600. 10.1113/jphysiol.2007.138693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett A.W., Clark W.A., Chizzonite R.A., and Zak R.. 1983. Change in synthesis rates of alpha- and beta-myosin heavy chains in rabbit heart after treatment with thyroid hormone. J. Biol. Chem. 258:2421–2425. [PubMed] [Google Scholar]
- Farman G.P., Gore D., Allen E., Schoenfelt K., Irving T.C., and de Tombe P.P.. 2011. Myosin head orientation: A structural determinant for the Frank-Starling relationship. Am. J. Physiol. Heart Circ. Physiol. 300:H2155–H2160. 10.1152/ajpheart.01221.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons D.P., and Moss R.L.. 1998. Strong binding of myosin modulates length-dependent Ca2+ activation of rat ventricular myocytes. Circ. Res. 83:602–607. 10.1161/01.RES.83.6.602 [DOI] [PubMed] [Google Scholar]
- Fitzsimons D.P., Patel J.R., and Moss R.L.. 1999. Aging-dependent depression in the kinetics of force development in rat skinned myocardium. Am. J. Physiol. 276:H1511–H1519. [DOI] [PubMed] [Google Scholar]
- Ford S.J., and Chandra M.. 2013. Length-dependent effects on cardiac contractile dynamics are different in cardiac muscle containing α- or β-myosin heavy chain. Arch. Biochem. Biophys. 535:3–13. 10.1016/j.abb.2012.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford S.J., Chandra M., Mamidi R., Dong W., and Campbell K.B.. 2010. Model representation of the nonlinear step response in cardiac muscle. J. Gen. Physiol. 136:159–177. 10.1085/jgp.201010467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford S.J., Mamidi R., Jimenez J., Tardiff J.C., and Chandra M.. 2012. Effects of R92 mutations in mouse cardiac troponin T are influenced by changes in myosin heavy chain isoform. J. Mol. Cell. Cardiol. 53:542–551. 10.1016/j.yjmcc.2012.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollapudi S.K., and Chandra M.. 2012. Cardiomyopathy-related mutations in cardiac troponin C, L29Q and G159D, have divergent effects on rat cardiac myofiber contractile dynamics. Biochem. Res. Int. 2012:824068 10.1155/2012/824068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollapudi S.K., and Chandra M.. 2016. Dilated cardiomyopathy mutation (R134W) in mouse cardiac troponin T induces greater contractile deficits against α-myosin heavy chain than against β-myosin heavy chain. Front. Physiol. 7:443 10.3389/fphys.2016.00443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollapudi S.K., Tardiff J.C., and Chandra M.. 2015. The functional effect of dilated cardiomyopathy mutation (R144W) in mouse cardiac troponin T is differently affected by alpha- and beta-myosin heavy chain isoforms. Am. J. Physiol. Heart Circ. Physiol. 308:H884–H893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollapudi S.K., Reda S.M., and Chandra M.. 2017. Omecamtiv mecarbil abolishes length-mediated increase in guinea pig cardiac myofiber Ca2+ sensitivity. Biophys. J. 113:880–888. 10.1016/j.bpj.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton N., and Ianuzzo C.D.. 1991. Contractile and calcium regulating capacities of myocardia of different sized mammals scale with resting heart rate. Mol. Cell. Biochem. 106:133–141. 10.1007/BF00230179 [DOI] [PubMed] [Google Scholar]
- Herron T.J., and McDonald K.S.. 2002. Small amounts of alpha-myosin heavy chain isoform expression significantly increase power output of rat cardiac myocyte fragments. Circ. Res. 90:1150–1152. 10.1161/01.RES.0000022879.57270.11 [DOI] [PubMed] [Google Scholar]
- Holt J.P., Rhode E.A., and Kines H.. 1968. Ventricular volumes and body weight in mammals. Am. J. Physiol. 215:704–715. [DOI] [PubMed] [Google Scholar]
- Jones S.A., Lancaster M.K., and Boyett M.R.. 2004. Ageing-related changes of connexins and conduction within the sinoatrial node. J. Physiol. 560:429–437. 10.1113/jphysiol.2004.072108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konhilas J.P., Irving T.C., and de Tombe P.P.. 2002. Length-dependent activation in three striated muscle types of the rat. J. Physiol. 544:225–236. 10.1113/jphysiol.2002.024505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte F.S., and McDonald K.S.. 2007. Sarcomere length dependence of rat skinned cardiac myocyte mechanical properties: Dependence on myosin heavy chain. J. Physiol. 581:725–739. 10.1113/jphysiol.2007.128199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte F.S., Herron T.J., Rovetto M.J., and McDonald K.S.. 2005. Power output is linearly related to MyHC content in rat skinned myocytes and isolated working hearts. Am. J. Physiol. Heart Circ. Physiol. 289:H801–H812. 10.1152/ajpheart.01227.2004 [DOI] [PubMed] [Google Scholar]
- Krenz M., Sanbe A., Bouyer-Dalloz F., Gulick J., Klevitsky R., Hewett T.E., Osinska H.E., Lorenz J.N., Brosseau C., Federico A., et al. 2003. Analysis of myosin heavy chain functionality in the heart. J. Biol. Chem. 278:17466–17474. 10.1074/jbc.M210804200 [DOI] [PubMed] [Google Scholar]
- Krüger M., Kohl T., and Linke W.A.. 2006. Developmental changes in passive stiffness and myofilament Ca2+ sensitivity due to titin and troponin-I isoform switching are not critically triggered by birth. Am. J. Physiol. Heart Circ. Physiol. 291:H496–H506. 10.1152/ajpheart.00114.2006 [DOI] [PubMed] [Google Scholar]
- Kurabayashi M., Tsuchimochi H., Komuro I., Takaku F., and Yazaki Y.. 1988. Molecular cloning and characterization of human cardiac alpha- and beta-form myosin heavy chain complementary DNA clones. Regulation of expression during development and pressure overload in human atrium. J. Clin. Invest. 82:524–531. 10.1172/JCI113627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber R.L., Yeh Y., and Baskin R.J.. 1984. Sarcomere length determination using laser diffraction. Effect of beam and fiber diameter. Biophys. J. 45:1007–1016. 10.1016/S0006-3495(84)84246-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lompre A.M., Mercadier J.J., Wisnewsky C., Bouveret P., Pantaloni C., D’Albis A., and Schwartz K.. 1981. Species- and age-dependent changes in the relative amounts of cardiac myosin isoenzymes in mammals. Dev. Biol. 84:286–290. 10.1016/0012-1606(81)90396-1 [DOI] [PubMed] [Google Scholar]
- Lompré A.M., Nadal-Ginard B., and Mahdavi V.. 1984. Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J. Biol. Chem. 259:6437–6446. [PubMed] [Google Scholar]
- Lowes B.D., Minobe W., Abraham W.T., Rizeq M.N., Bohlmeyer T.J., Quaife R.A., Roden R.L., Dutcher D.L., Robertson A.D., Voelkel N.F., et al. 1997. Changes in gene expression in the intact human heart. Downregulation of alpha-myosin heavy chain in hypertrophied, failing ventricular myocardium. J. Clin. Invest. 100:2315–2324. 10.1172/JCI119770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamidi R., and Chandra M.. 2013. Divergent effects of α- and β-myosin heavy chain isoforms on the N terminus of rat cardiac troponin T. J. Gen. Physiol. 142:413–423. 10.1085/jgp.201310971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamidi R., Mallampalli S.L., Wieczorek D.F., and Chandra M.. 2013. Identification of two new regions in the N-terminus of cardiac troponin T that have divergent effects on cardiac contractile function. J. Physiol. 591:1217–1234. 10.1113/jphysiol.2012.243394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamidi R., Gresham K.S., Verma S., and Stelzer J.E.. 2016. Cardiac myosin binding protein-C phosphorylation modulates myofilament length-dependent activation. Front. Physiol. 7:38 10.3389/fphys.2016.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally E.M., Kraft R., Bravo-Zehnder M., Taylor D.A., and Leinwand L.A.. 1989. Full-length rat alpha and beta cardiac myosin heavy chain sequences: Comparisons suggest a molecular basis for functional differences. J. Mol. Biol. 210:665–671. 10.1016/0022-2836(89)90141-1 [DOI] [PubMed] [Google Scholar]
- Michael J.J., Gollapudi S.K., and Chandra M.. 2014. Effects of pseudo-phosphorylated rat cardiac troponin T are differently modulated by α- and β-myosin heavy chain isoforms. Basic Res. Cardiol. 109:442 10.1007/s00395-014-0442-9 [DOI] [PubMed] [Google Scholar]
- Milani-Nejad N., and Janssen P.M.. 2014. Small and large animal models in cardiac contraction research: Advantages and disadvantages. Pharmacol. Ther. 141:235–249. 10.1016/j.pharmthera.2013.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S., Minobe W., Bristow M.R., and Leinwand L.A.. 2000. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ. Res. 86:386–390. 10.1161/01.RES.86.4.386 [DOI] [PubMed] [Google Scholar]
- Moss R.L., and Fitzsimons D.P.. 2002. Frank-Starling relationship: Long on importance, short on mechanism. Circ. Res. 90:11–13. 10.1161/res.90.1.11 [DOI] [PubMed] [Google Scholar]
- Nakao K., Minobe W., Roden R., Bristow M.R., and Leinwand L.A.. 1997. Myosin heavy chain gene expression in human heart failure. J. Clin. Invest. 100:2362–2370. 10.1172/JCI119776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narolska N.A., Eiras S., van Loon R.B., Boontje N.M., Zaremba R., Spiegelen Berg S.R., Stooker W., Huybregts M.A., Visser F.C., van der Velden J., and Stienen G.J.. 2005. Myosin heavy chain composition and the economy of contraction in healthy and diseased human myocardium. J. Muscle Res. Cell Motil. 26:39–48. 10.1007/s10974-005-9005-x [DOI] [PubMed] [Google Scholar]
- Nowak G., Peña J.R., Urboniene D., Geenen D.L., Solaro R.J., and Wolska B.M.. 2007. Correlations between alterations in length-dependent Ca2+ activation of cardiac myofilaments and the end-systolic pressure-volume relation. J. Muscle Res. Cell Motil. 28:415–419. 10.1007/s10974-008-9136-y [DOI] [PubMed] [Google Scholar]
- Plotnick G.D., Becker L.C., Fisher M.L., Gerstenblith G., Renlund D.G., Fleg J.L., Weisfeldt M.L., and Lakatta E.G.. 1986. Use of the Frank-Starling mechanism during submaximal versus maximal upright exercise. Am. J. Physiol. 251:H1101–H1105. [DOI] [PubMed] [Google Scholar]
- Razumova M.V., Bukatina A.E., and Campbell K.B.. 2000. Different myofilament nearest-neighbor interactions have distinctive effects on contractile behavior. Biophys. J. 78:3120–3137. 10.1016/S0006-3495(00)76849-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda S.M., and Chandra M.. 2018. Cardiomyopathy mutation (F88L) in troponin T abolishes length dependency of myofilament Ca2+ sensitivity. J. Gen. Physiol. 150:809–819. 10.1085/jgp.201711974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda S.M., Gollapudi S.K., and Chandra M.. 2016. L71F mutation in rat cardiac troponin T augments crossbridge recruitment and detachment dynamics against α-myosin heavy chain, but not against β-myosin heavy chain. J. Muscle Res. Cell Motil. 37:215–223. 10.1007/s10974-016-9460-6 [DOI] [PubMed] [Google Scholar]
- Reiser P.J., Portman M.A., Ning X.H., and Schomisch Moravec C.. 2001. Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. Am. J. Physiol. Heart Circ. Physiol. 280:H1814–H1820. 10.1152/ajpheart.2001.280.4.H1814 [DOI] [PubMed] [Google Scholar]
- Rhodes S.S., Camara A.K., Heisner J.S., Riess M.L., Aldakkak M., and Stowe D.F.. 2012. Reduced mitochondrial Ca2+ loading and improved functional recovery after ischemia-reperfusion injury in old vs. young guinea pig hearts. Am. J. Physiol. Heart Circ. Physiol. 302:H855–H863. 10.1152/ajpheart.00533.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell V.L., Manaves V., Martin A.F., and de Tombe P.P.. 2005. Impact of beta-myosin heavy chain isoform expression on cross-bridge cycling kinetics. Am. J. Physiol. Heart Circ. Physiol. 288:H896–H903. 10.1152/ajpheart.00407.2004 [DOI] [PubMed] [Google Scholar]
- Schuyler G.T., and Yarbrough L.R.. 1990. Effects of age on myosin and creatine kinase isoforms in left ventricles of Fischer 344 rats. Mech. Ageing Dev. 56:23–38. 10.1016/0047-6374(90)90112-S [DOI] [PubMed] [Google Scholar]
- Schwartz K., Carrier L., Chassagne C., Wisnewsky C., and Boheler K.R.. 1992. Regulation of myosin heavy chain and actin isogenes during cardiac growth and hypertrophy. Symp. Soc. Exp. Biol. 46:265–272. [PubMed] [Google Scholar]
- Smith L., Tainter C., Regnier M., and Martyn D.A.. 2009. Cooperative cross-bridge activation of thin filaments contributes to the Frank-Starling mechanism in cardiac muscle. Biophys. J. 96:3692–3702. 10.1016/j.bpj.2009.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer J.E., and Moss R.L.. 2006. Contributions of stretch activation to length-dependent contraction in murine myocardium. J. Gen. Physiol. 128:461–471. 10.1085/jgp.200609634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer J.E., Larsson L., Fitzsimons D.P., and Moss R.L.. 2006. Activation dependence of stretch activation in mouse skinned myocardium: Implications for ventricular function. J. Gen. Physiol. 127:95–107. 10.1085/jgp.200509432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienen G.J., Zaremba R., and Elzinga G.. 1995. ATP utilization for calcium uptake and force production in skinned muscle fibres of Xenopus laevis. J. Physiol. 482:109–122. 10.1113/jphysiol.1995.sp020503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschirgi M.L., Rajapakse I., and Chandra M.. 2006. Functional consequence of mutation in rat cardiac troponin T is affected differently by myosin heavy chain isoforms. J. Physiol. 574:263–273. 10.1113/jphysiol.2006.107417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBuren P., Harris D.E., Alpert N.R., and Warshaw D.M.. 1995. Cardiac V1 and V3 myosins differ in their hydrolytic and mechanical activities in vitro. Circ. Res. 77:439–444. 10.1161/01.RES.77.2.439 [DOI] [PubMed] [Google Scholar]
- van der Velden J., Moorman A.F., and Stienen G.J.. 1998. Age-dependent changes in myosin composition correlate with enhanced economy of contraction in guinea-pig hearts. J. Physiol. 507:497–510. 10.1111/j.1469-7793.1998.497bt.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.P., and Fuchs F.. 1994. Length, force, and Ca(2+)-troponin C affinity in cardiac and slow skeletal muscle. Am. J. Physiol. 266:C1077–C1082. 10.1152/ajpcell.1994.266.4.C1077 [DOI] [PubMed] [Google Scholar]
- Weisberg A., and Winegrad S.. 1998. Relation between crossbridge structure and actomyosin ATPase activity in rat heart. Circ. Res. 83:60–72. 10.1161/01.RES.83.1.60 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.