Abstract
Background:
The primary objective of the study was to evaluate the efficacy of 300 milligrams (mg) and 600 mg of pregabalin compared to placebo in the reduction of pain in patients with noncritical partial and full thickness burn injuries.
Methods:
A prospective, randomized, double-blinded, single center, placebo-controlled trial was conducted. Simple randomization method was used in this trial. After subjects met all the inclusion and none of the exclusion criteria, they were randomized and assigned to 1 of the 3 18-day treatments groups: Pregabalin 300 group, Pregabalin 600 group, or Placebo group. Demographics and clinical characteristics were recorded. The severity of pain was assessed by using the visual analog scale for pain intensity at baseline on day 3, day 9 ± 3, day 25 ± 7, day 90 ± 6, and day 180 ± 12.
Results:
A total of 54 subjects were randomly assigned, and 51 were included in the data analysis. Demographics and clinical characteristics did not differ significantly between the 3 groups. There was a statistically significant difference in pain between the Pregabalin 300 and Pregabalin 600 groups (P-value = .0260). The Pregabalin 300 group had 17.93 units (95% confidence interval: 1.83–34.04) higher pain scores on average than the Pregabalin 600 group, regardless of time. The adjusted P-value comparing 0 to 300 was .1618, while the adjusted P-value for 0 versus 600 was .5304. There was an overall difference in pain across time regardless of study group (P-value = <.0001). An overall difference in opioid consumption (P-value = .0003) and BSHS (P-value = .0013) across time regardless of study group was noted.
Conclusions:
Pregabalin could be part of a promising multimodal analgesic regimen in noncritical burn population. Future placebo-controlled studies assessing the use of pregabalin in burn victim patients may further endorse our findings.
Keywords: burn, full thickness, opioid consumption, partial thickness, pregabalin
1. Introduction
Burns are a common injury requiring medical attention in the US. Of 500,000 burn cases per year, 40,000 require hospitalization.[1] One of the major challenges of burn injuries is managing pain, which presents itself in different forms: a background nociceptive pain, procedural pain, and neuropathic pain.[2,3] The type of pain experienced by burn survivors changes after the healing process, and this residual pain is akin to the neuropathic pain described by patients with diabetes or postherpetic neuralgia.[4] While neuropathic pain is produced by a disease or injury of the peripheral somatosensory nervous system, nociceptive pain is caused by the stimulation of nociceptors due to non-neural tissue harm.[5] The incidence of chronic neuropathic pain in burn patients reported by the literature is as high as 52%, and is mostly described in patients that have experienced considerably higher intensities of acute pain, extensive body surface area burns, and those requiring skin grafting.[3,6,7] There are several mechanisms by which burn scarring could lead to neuropathic pain, but the most relevant of these are nerve entrapment by nearby scar tissue and neuroma formation.[3] Neuropathic pain is usually reported 4.3 ± 0.5 months following the burn injuries and can persist for years.[4] Patients commonly describe the pain as a stabbing, itching, burning, electric shock-like, pins and needles, and/or shooting sensation.[2,4,7,8] Managing burn-related neuropathic pain is challenging, and it usually continues after traditional or scar modulation therapies.[3] The pain experienced by burn patients is typically treated on a daily basis with a multimodal regimen of narcotics, antipruritics, anticonvulsants, and/or antidepressants that often compromise patients’ quality of life due to side effects of such a regimen.[3,9] Additionally, opioids are currently the most frequently used medications for the treatment of intense pain in burn patients. One of the most notable challenges burn injuries carry is a pain-related decrease in functional ability and quality of life.[7] Consequently, post-burn heat sensitivity hinders an individual's ability to participate in outdoor activities, and this lack of physical movement and/or exercise can indirectly lead to further decline on quality of life, including the onset of symptoms of depression.[10]
Pregabalin is an analog of gamma aminobutyric acid.[9,11] Pregabalin decreases the synaptic release of multiple neurotransmitters (glutamate, noradrenaline, serotonin, dopamine, and Substance P) by binding to the α2δ-subunits of the presynaptic voltage-dependent calcium channels.[9,12] As a result, the drug inhibits neuronal excitability, especially in the central nervous system.[12–14] Pregabalin has been shown in multiple studies to be an effective analgesic agent in both peripheral and central neuropathic pain, and it is particularly effective in reducing post-burn pain.[4,9,13,15–17]
We hypothesized that pregabalin is effective in reducing pain in noncritical recovering burn patients. Secondarily, pregabalin for pain management will improve patient quality of life with a significant reduction of opioid consumption.
2. Methods
This study has been described according to the consolidated standards of reporting trials (CONSORT) guidelines for the presentation of clinical trials.[18] A prospective, randomized, double-blinded, single center, placebo-controlled trial was conducted. After obtaining institutional review board (Office of Responsible Research Practices-The Ohio State University) approval (Protocol Number 2009H167, approved on January 22, 2010), subjects with noncritical partial and full thickness burn injuries that required hospitalization to manage pain control, social issues or support with personal daily activities at The Ohio State University Wexner Medical Center were approached and provided their written informed consent and completed essential study procedures required to analyze the data between April 2010 and November 2014. This trial was not registered in ClinicalTrials.gov or any other publicly accessible registry because it was not a requirement from institutional guidelines at the moment of IRB approval and enrollment period.
Study inclusion criteria consisted of burn patients with non-critical partial and full thickness burn injuries whose ages ranged from 18 to 85 years, referring moderate-to-severe pain requiring pain management lasting a minimum of 18 days and able to receive oral medication. Subjects with clinically significant renal impairment (defined as a creatinine clearance of ≤60 ml/min), history of allergy to any anti-epileptic medication, pregabalin or gabapentin, history of cognitive impairment condition, prior consumption of pregabalin or gabapentin (7 days prior of enrollment), current pregnant or breastfeeding, or history of seizure were excluded from the study.
Demographics including age, height, weight, body mass index, race, and gender were collected before randomization. Additionally, clinical characteristics such as total body surface area (TBSA), duration of hospital stay, mean number of treatment days, mean number of total doses administered, and mean numbers of hours from admission time to first investigational product (IP) of the 3 groups were recorded.
After subjects met all of the inclusion and none of the exclusion criteria, a subject identification number from the randomization list was assigned, and consequently the unblinded pharmacist prepared and dispensed IP. Study medication administration started usually right after subjects were assessed as clinically stable and transferred to the floor through the emergency department.
The 18-day oral pregabalin administration regimen consisted of a tapering-up period (day 1–2), maintenance period (day 3–16), and tapering-down period (day 17–18) in accordance with the following schedule:
Pregabalin 300 group received 150 mg bi-daily (BID) during the maintenance period, with a dose of 50 mg BID on day 1 and 18, and 100 mg BID for day 2 and 17 during initial and last tapering periods respectively.
Pregabalin 600 group received 300 mg BID during the maintenance period, with a dose of 75 mg BID on day 1 and 18, and 150 mg BID for day 2 and 17 during initial and last tapering periods respectively.
The placebo group received matching placebo tablets BID for the duration of the treatment period.
Our institutional standard regimen for pain control consisted of either intravenous morphine preservative-free 2 mg every 2 hours as needed or hydromorphone injection 0.5 mg every 2 hours as needed for severe pain and oxycodone (immediate release) tablet 5 mg every 6 hours as needed for moderate pain. Subjects received intravenous and/oral opioid medication as pain rescue during hospitalization and subsequently, opioid consumption during hospitalization was collected and converted to oral morphine units for data analysis purposes.
Subjects were discharged home with the remaining drug regimen pertinent to this study. In addition to the IP, subjects received a prescription for standard burn oral pain medication (oral opioids) for use during the time between discharge from the hospital and their next scheduled follow-up visit.
2.1. Outcome measurement
The primary objective of the study was to evaluate the efficacy of 300 mg and 600 mg of pregabalin compared to placebo in the reduction of pain in patients with noncritical partial and full thickness burn injuries. The severity of pain was assessed by using the visual analog scale (VAS) for pain intensity; it consisted of a 100 mm long line with 2 descriptors at each end representing pain intensities (no pain and extreme pain). Subjects were asked to mark their pain intensity somewhere on the line, then the VAS score was assessed by measuring the distance from the “no pain” end to the mark placed by the subject. VAS pain assessments were performed at baseline, on day 3, day 9 ± 3, day 25 ± 7, day 90 ± 6, and day 180 ± 12.
As secondary outcomes, opioid consumption measurement and quality of life assessments were made at different time points throughout the study for comparison. Opioid consumption (oral morphine per day) before first dose of IP until hospital discharge or day 3 (whichever occurs first) was recorded. A Quality of Life questionnaire – the Burn Specific Health Scale (BSHS)[19] – was administered to all study groups at baseline, on day 25 ± 7, day 90 ± 6, and day 180 ± 12.
Side effects, if reported, were collected immediately after first dose of IP and during each study visit. Complete blood count with differential, electrolytes, and liver function tests at baseline day 25 ± 7 were collected and assessed by a licensed physician acting as a sub-investigator
2.2. Sample size
A sample size of 33 per group, with an 80% power to detect significant differences in pain levels of at least 17 mm on the VAS for pain between the treatment groups and placebo group at any of the evaluated time points was calculated. A conservative standard deviation of 20 was assumed for this calculation. Also, we had 80% power to detect at least an effect size of 0.85 in opioid consumption between the 2 treatment groups and control groups at discharge. A 1 sided 2-sample t test with an alpha of 0.006 (0.05/8) was assumed in this calculation to account for 2 comparisons at each of the 3-time points for pain score, and 2 comparisons at discharge for opioid consumption. A total of 40 patients per group was planned to be enrolled into the study to account for drop-outs or lost to follow-up patients. However, due to a high incidence of loss of follow up during the last period of the study and an unanticipated change of institutional standard of care for burn patients (use the application of long-acting Silver dressing for 7 days for partial thickness burns and early discharge [24–36 hours after its application] instead of topical antibiotics and daily dressing changes), the investigators decided to close study enrollment and conduct the data analysis with the available 51 subjects in order to be proceed with this publication as a preliminary study.
The randomization method utilized in this study was simple randomization; before enrollment activities, an unblinded research pharmacist generated a randomization list (using an online random list generator http://randomization.com) and allocated each listed subject to 1 of the 3 treatments groups: Pregabalin 300 group, Pregabalin 600 group, or Placebo group.
The blinding process was maintained by using placebo tablets identical to the active tablets with different doses. Subjects, health care providers, and research personnel were blinded throughout the study. Only the research pharmacist, who prepared the IP following randomization allocation, was aware of the specifics of each patient's drug regimen.
2.3. Statistical methods
Continuous demographic and clinical variables were compared between treatment groups using analysis of variance or nonparametric Kruskal–Wallis tests where relevant. Categorical variables were compared using chi-square or Fisher exact tests where relevant. A linear mixed effects model was fit to estimate and compare the primary pain longitudinal outcome and included main effects and an interaction term for study group and time. The model also included random intercepts to account for repeated measures within patient. Multiple hypotheses testing between study groups were adjusted for using the Bonferroni–Holm step down method to control the overall type 1 error rate at 5%. A similar mixed effect model was fit to assess the BSHS secondary endpoint. Kruskal–Wallis nonparametric tests were used to test differences in opioid consumption between study groups during hospitalization before and after IP administration, respectively. The data were analyzed using SAS, version 9.4 (SAS Institute Inc, Cary, NC). Adjusted P-values <.05 were considered to be statistically significant.
3. Results
The flow diagram of this clinical trial according to the CONSORT 2010 is shown in Figure 1.[18] Over a 55-month period (April 2010 to November 2014), a total of 316 subjects with acute burn injury were assessed as potential candidates for this study after admission. After the eligibility assessment of each subject, 262 were considered ineligible or refused to participate in the initial phase of this trial. The remaining 54 subjects underwent screening procedures and were consequently enrolled into the study. However, 3 subjects were considered early termination after randomization; 2 subjects withdrew from the study after receiving the first dose due to adverse events that they related to IP, including nausea and dizziness, and 1 subject withdrew from the study before receiving the study medication was started. After removal of the blind, 19 subjects were allocated to the Placebo group, 18 to the Pregabalin group and 14 to the Pregabalin 600 group. Therefore, data analysis of 51 subjects was conducted using an intention-to-treat manner.
Figure 1.
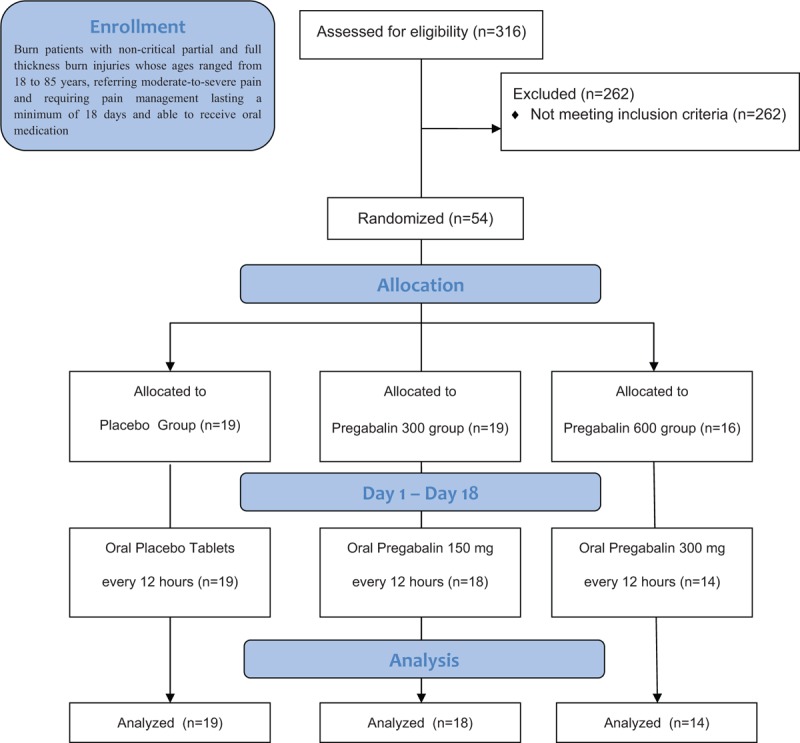
Trial profile according to CONSORT guidelines. CONSORT = consolidated standards of reporting trials.
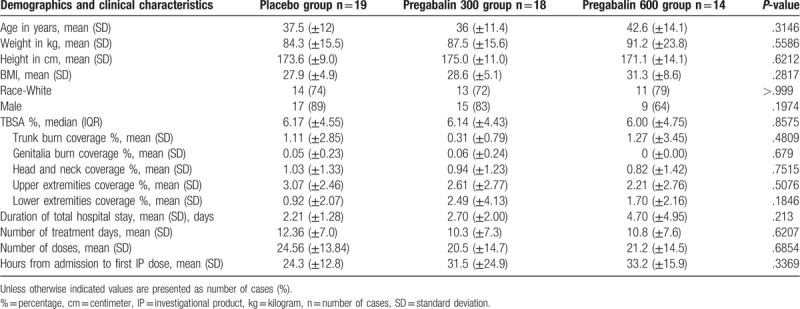
There were no significant differences in demographics and clinical characteristics, duration of hospital stay, mean number of treatment days, mean number of total doses administered, mean number of hours from admission time to first IP among the 3 groups) among the 3 groups (Table 1).
Table 1.
Demographics and clinical characteristics.
The primary outcome measured pain scores over day 9, day 22, day 90, and day 180. There were no statistically significant differences in median pain scores between the 3 groups at any of the time points (Table 2). Additionally, the rate of change of pain scores did not differ between study groups (interaction term P-value = .9060) (Fig. 2).
Table 2.
Pain throughout study time points.
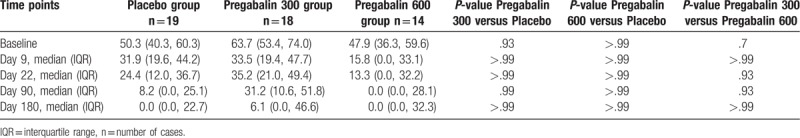
Figure 2.
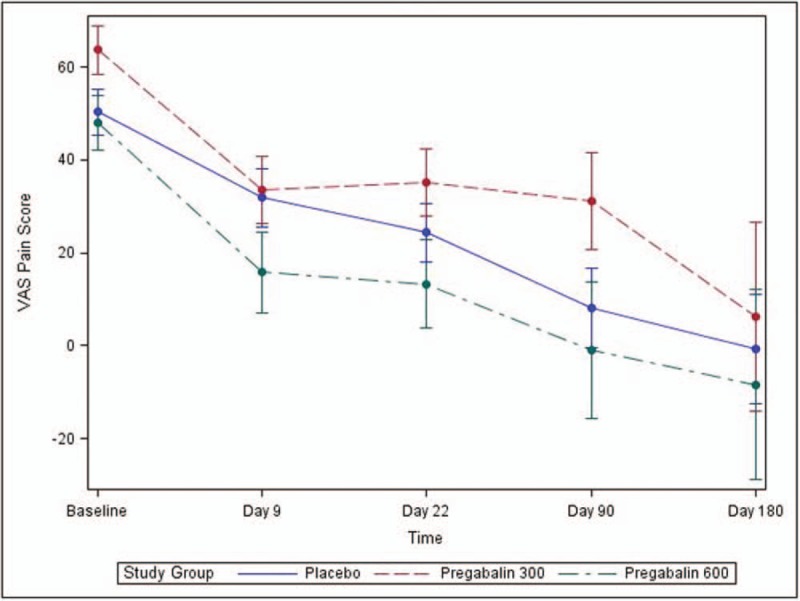
Pain throughout study time points (VAS for pain intensity). VAS = visual analog scale.
In addition, there was no statistically significant difference in opioid consumption before IP administration (oral morphine, milligrams per day [mg/d])) among all the groups (P-value = .8789). In addition, there was no statistically significant difference in opioid consumption before discharge (oral morphine, mg/d) among all the groups after IP initiation (P-value = .3064). (Table 3 and Fig. 3)
Table 3.
Opioid consumption, prior and after investigational product administration (oral morphine, mg/day).
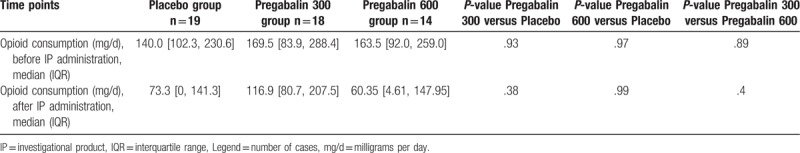
Figure 3.
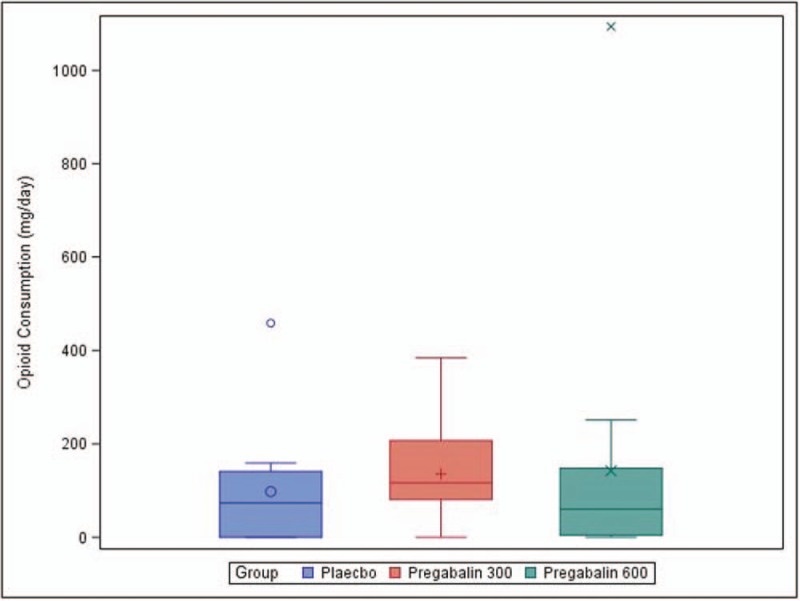
Opioid consumption throughout study time points (oral morphine, mg/day).
Pro re nata opioid medication most commonly used during hospitalization were as follows: intravenous fentanyl, intravenous morphine, intravenous hydromorphone, oral oxycodone, oral methadone, oral hydrocodone, and oral tramadol.
There was no statistically significant difference in BSHS between the groups (P-value = .6411); however, an overall difference in BSHS across time regardless of study group (P-value = .0013) was noted.
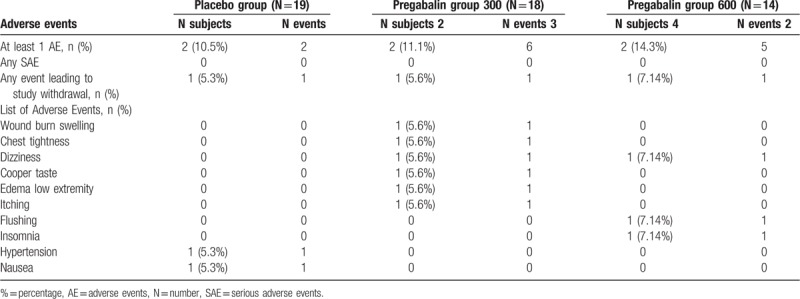
Adverse events related to study drug were uncommon with no difference among all groups and no serious adverse events were reported throughout the study (Table 4).
Table 4.
Adverse events.
4. Discussion
A few limitations during the conduction of the study are important to mention. The first limitation involves an attrition bias; the investigators experienced a high incidence of lack of follow-ups once subjects were discharged from the hospital. The second limitation was the lack of post-hospital discharge opioid consumption data collection due to the aforementioned follow-up gapping and accuracy of information provided by the subjects. A third limitation that disrupted the enrollment rate and the completion of the study was the aforementioned unanticipated change of institutional standard of care of burn patients, the implementation of conservative management of burn wounds led to a reduction of hospital admissions or length of stay in the burn population at our institution. As a result of these important limitations, the investigators decided to close study enrollment early and run an unplanned data analysis with the available data of 51 subjects of the 120 planned subjects, to be presented in this publication.
This prospective, randomized, double-blinded, single center, placebo-controlled trial demonstrated that dosing 600 mg of oral pregabalin per day provides superior pain control in patients with both noncritical partial and full thickness burn injuries as compared to dosing oral 300 mg pregabalin per day. A substantial reduction of pain relief was observed in the Pregabalin 600 group when compared to the Pregabalin 300 group (P < .260). In addition, no statistically significant degree of pain relief was revealed in the Pregabalin 300 group and Pregabalin 600 group when compared to the placebo group (P < .5304). However, there was an overall difference in pain and opioid consumption across time regardless of study group (P-value = <.0001); indicating that subjects had lower pain values and consume less opioid medication on average as the treatment period progressed. These facts could suggest that the dosage amount of pregabalin could be essential for adequate pain management in burn patients. Finally, there was an overall difference in BSHS across time regardless of study group (P-value = .0013); this indicated that people had higher average BSHS scores as recovery time progressed, but this did not differ by study group. Therefore, quality of life did not differ significantly between study groups but improved on average over time regardless of study group. These facts could suggest that the dosage amount of pregabalin could be essential for adequate pain management in burn patients. Previous to our study, only 3 other published clinical trials have assessed the efficacy of pregabalin in the management of post-burn pain.[2,4]
Pregabalin is an anti-epileptic drug, as well as a gabapentinoid, with a chemical structure similar to gabapentin.[9,14] Commonly described side effects of pregabalin include dizziness, somnolence, peripheral edema, weight gain, and asthenia.[13] The recommended dose of pregabalin for pain management is 100 mg 3 times per day (300 mg/d), with an initial titration period of 50 mg 3 times per day (150 mg/d) during the first week.[20] Both pregabalin and gabapentin have been shown to be a well-tolerated and effective for neuropathic pain disorders; however, pregabalin has demonstrated equivalent efficacy in comparison to gabapentin while requiring lower doses.[4,20,21] Pregabalin has linear pharmacokinetics, undergoes minimal metabolism, and 98% of the absorbed dose is renally excreted unaffected.[20] Thus, its mean elimination half-life of 5.5 to 6.7 hours.[20]
A retrospective review study published in 2009 by Wong et al reported their experience administering pregabalin (up to 600 mg) to 13 subjects that referred subjective symptoms of post-burn neuropathic pain in an outpatient setting.[4] This study showed a pain score reduction on 69% of the treated subjects and a proficient tolerability profile of pregabalin.[4]
In 2010, a randomized, double-blinded, placebo-controlled trial with 90 inpatients with 5% or greater TBSA burn injury and treated with pregabalin for 28 days; Gray et al demonstrated that hot (P = .01) and sharp (P = .04) pain could be significantly reduced following a pregabalin regimen of progressively increasing doses starting at 75 mg twice per day to a maximum of 300 mg twice per day.[2] There was no statistical difference in adverse events, opioid consumption, length of stay, or pain at 6 months between the treatment groups.[2] In addition, secondary outcome measures of itch, unpleasantness, surface pain, and procedural pain were significantly lower (P < .05) in the pregabalin group compared to the placebo group.[2] In Gray's study, the greatest improvement in pain scores was seen in the cohorts of patients with greater TBSA.[2] However, Gray et al discussed their exclusion subjects with ≤5% TBSA as a limitation of their study and suggested that future studies include patients with burn areas of less than 5%.[2] We consider that our study addressed this limitation, since our study population had a median TBSA of 5.0% (3.0%–9.00%) with suitable pain reduction after pregabalin regimens.
Another retrospective study conducted in 2017 assessed the efficacy of gabapentin versus pregabalin and gabapentin in the treatment of post-burn neuropathic pain and itching in 136 subjects under 20 years of age.[9] Out of these 136 subjects, only 24 received both pregabalin and gabapentin; where 88.2% of the subjects expressed a satisfactory response for management of pain and pruritus.[9]
In conclusion, this is another prospective, randomized, double-blinded, single center, placebo-controlled trial study that assessed the use of pregabalin on post-burn pain in an acute setting. The primary goal of this 3-armed study was to compare the efficacy of 300 mg pregabalin versus 600 mg pregabalin versus a placebo for post-burn pain management. The different doses of pregabalin in this study were well tolerated. However, our results in conjunction with other published clinical trials demonstrated that future placebo-controlled studies assessing the use of pregabalin in non-critical burn population may be needed in order to have a better understanding of the role of pregabalin as part of a multimodal analgesia approach and other related post-burn clinical outcomes. A search for funding to support the implementation of future placebo-controlled studies assessing the use of pregabalin in noncritical burn victims as part of a multimodal analgesia approach for the management of post-burn neuropathic pain and other related post-burn clinical outcomes will be conducted to further endorse our findings.
Acknowledgments
The authors gratefully acknowledge Nicolas Kumar, Jonah Stavsky and Nicoleta Stoicea, MD, PhD for their writing and editing collaboration (all of them provided authorization to be named on this publication) and to the deceased Dr Sidney Miller (†) for his initial collaboration and guidance on this project.
Author contributions
LJ participated in methodology, data collection, and manuscript editing. SB, AU, EP, and RC participated in literature search, study design, methodology, data collection, data analysis, and manuscript editing. MA participated in data analysis and manuscript editing. CM participated in study design, methodology, data collection, and manuscript editing
Conceptualization: Rebecca Coffey, Erika G. Puente, Sergio D. Bergese.
Data curation: Alberto Uribe, Rebecca Coffey, Erika G. Puente, Sergio D. Bergese.
Formal analysis: Alberto Uribe, Larry M. Jones, Rebecca Coffey, Mahmoud Abdel-Rasoul, Sergio D. Bergese.
Funding acquisition: Alberto Uribe, Larry M. Jones, Rebecca Coffey, Erika G. Puente, Sergio D. Bergese.
Investigation: Alberto Uribe, Larry M. Jones, Rebecca Coffey, Erika G. Puente.
Methodology: Alberto Uribe, Rebecca Coffey, Erika G. Puente, Sergio D. Bergese, Claire V. Murphy.
Project administration: Alberto Uribe, Larry M. Jones, Rebecca Coffey, Sergio D. Bergese.
Supervision: Alberto Uribe, Larry M. Jones, Rebecca Coffey.
Validation: Larry M. Jones, Rebecca Coffey.
Writing – original draft: Alberto Uribe.
Writing – review and editing: Alberto Uribe, Larry M. Jones, Rebecca Coffey, Erika G. Puente, Mahmoud Abdel-Rasoul, Claire V. Murphy, Sergio D. Bergese.
Alberto Uribe orcid: 0000-0001-7897-8322.
Footnotes
Abbreviations: BID = bi-daily, BSHS = burn-specific health scale, CONSORT = consolidated standards of reporting trials, IP = investigational product, IR = immediate release, mg = milligrams, TBSA = total body surface area, VAS = visual analog scale.
This study was supported by Pfizer Inc.
The authors declare no potential conflict of interest.
References
- [1].American Burn Association. Burn Incidence and Treatment in the US: 2000 Fact Sheet. Chicago: ABA; 2000. [Google Scholar]
- [2].Gray P, Kirby J, Smith MT, et al. Pregabalin in severe burn injury pain: a double-blind, randomised placebo-controlled trial. Pain 2011;152:1279–88. [DOI] [PubMed] [Google Scholar]
- [3].Fredman R, Edkins RE, Hultman CS. Fat grafting for neuropathic pain after severe burns. Ann Plast Surg 2016;76Suppl 4:S298–303. [DOI] [PubMed] [Google Scholar]
- [4].Wong L, Turner L. Treatment of post-burn neuropathic pain: evaluation of pregablin. Burns 2010;36:769–72. [DOI] [PubMed] [Google Scholar]
- [5].Cappelleri JC, Koduru V, Bienen J, et al. Mapping painDETECT, a neuropathic pain screening tool, to the EuroQol (EQ-5D-3L). Qual Life Res 2016;26:467–77. [DOI] [PubMed] [Google Scholar]
- [6].Taverner T, Prince J. Acute neuropathic pain assessment in burn injured patients: a retrospective review. J Wound Ostomy Continence Nurs 2016;43:51–5. [DOI] [PubMed] [Google Scholar]
- [7].Nedelec B, Calva V, Chouinard A, et al. Somatosensory rehabilitation for neuropathic pain in burn survivors: a case series. J Burn Care Res 2016;37:e37–46. [DOI] [PubMed] [Google Scholar]
- [8].Portilla AS, Bravo GL, Miraval F, et al. A feasibility study assessing cortical plasticity in chronic neuropathic pain following burn injury. J Burn Care Res 2013;34:e48–52. [DOI] [PubMed] [Google Scholar]
- [9].Kaul I, Amin A, Rosenberg M, et al. Use of gabapentin and pregabalin for pruritus and neuropathic pain associated with major burn injury: a retrospective chart review. Burns 2017. [DOI] [PubMed] [Google Scholar]
- [10].Cooney GM, Dwan K, Greig CA, et al. Exercise for Depression. The Cochrane Library 2017;44:414–22. [Google Scholar]
- [11].Foroutan N, Nikvarz N. Role of pregabalin in management of pruritus: a literature review. J Pharm Pharm Sci 2016;19:465–74. [DOI] [PubMed] [Google Scholar]
- [12].Taylor CP, Angelotti T, Fauman E. Pharmacology and mechanism of action of pregabalin: the calcium channel (2–( (alpha 2–delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res 2007;73:137–50. [DOI] [PubMed] [Google Scholar]
- [13].Singh RK, Sinha VK, Pal US, et al. Pregabalin in post traumatic neuropathic pain: case studies. Nat J Maxillofac Surg 2012;3:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Toth C. Pregabalin: latest safety evidence and clinical implications for the management of neuropathic pain. Ther Adv Drug Saf 2013;5:38–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Finnerup NB, Jensen TS. Clinical use of pregabalin in the management of central neuropathic pain. Neuropsychiatr Dis Treat 2007;3:885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Finnerup NB, Sindrup SH, Jensen TS. Management of painful neuropathies. Handb Clin Neurol 2013;115:279–90. [DOI] [PubMed] [Google Scholar]
- [17].Sadosky A, Parsons B, Birol A, et al. Pain relief and functional improvement in patients with neuropathic pain associated with spinal cord injury: an exploratory analysis of pregabalin clinical trials. J Pain Res 2016;9:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Begg C, Cho M, Eastwood S, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276:637–9. [DOI] [PubMed] [Google Scholar]
- [19].Blades B, Mellis N, Munster AM. A burn specific health scale. J Trauma Acute Care Surg 1982;22:872–5. [DOI] [PubMed] [Google Scholar]
- [20].Gajraj NM. Pregabalin: its pharmacology and use in pain management. Anesth Analg 2007;105:1805–15. [DOI] [PubMed] [Google Scholar]
- [21].Ahuja RB, Gupta GK. A four arm, double blind, randomized and placebo controlled study of pregabalin in the management of post-burn pruritus. Burns 2013;39:24–9. [DOI] [PubMed] [Google Scholar]


