Abstract
There have been few studies comparing percutaneous transhepatic variceal embolization (PTVE) and transjugular intrahepatic portosystemic shunt (TIPS) for the prevention of recurrent gastric variceal bleeding (GVB).
Compare the outcomes of these 2 procedures in patients with GVB.
A total of 74 cirrhosis patients with GVB who underwent TIPS and modified PTVE were enrolled. The rebleeding and mortality rates, portal vein pressure (PVP) variation, and rates of hepatic encephalopathy (HE) were compared between the 2 groups.
A total of 43 PTVE and 31 TIPS patients were enrolled in this study. The difference of rebleeding rate in the 2 groups was not statistically significant (P = .190). The difference of early rebleeding rates and cumulative rebleeding-free rates were all not statistically significant (P = .256, P = .200). The difference of mortality rates in the 2 groups was not statistically significant (χ2 = 1.206, P = .272). The rate of HE in TIPS group was statistically higher than that in PTVE group (P < .0001).
Both PTVE and TIPS were effective for preventing rebleeding of GVs. There were no significant differences in rebleeding and mortality rates. The incidence of HE after TIPS was higher than PTVE.
Keywords: gastric varices, liver cirrhosis, prognosis, PTVE, TIPS
1. Introduction
Gastric variceal bleeding is a serious complication of portal hypertension, which may lead to death in patients with liver cirrhosis patients. Although the occurrence rate of gastric variceal bleeding is lower than esophageal variceal bleeding,[1] when it occurs, the result can be serious and have a high mortality rate.[2,3] Without intervention for gastric variceal bleeding the rebleeding rate is high, which increases the mortality rate. Thus, the prevention of recurrent bleeding is important. However, treatment of ruptured gastric varices (GVs), especially varices located in the gastric fundus, requires special therapeutic methods because of the location and rapid blood flow.[4,5] The treatment of GVs is complicated especially when a gastrorenal shunt is present. Although endoscopic therapy, balloon retrograde transvenous obliteration (BRTO) and transjugular intrahepatic portosystemic shunt (TIPS) are currently available, there is no universally accepted standard for the treatment of GVs.
Percutaneous transhepatic variceal embolization (PTVE) was developed in the 1970 s for the treatment of esophageal and GVs.[6] However, it has not become widely adopted because of the high rebleeding rate. With the introduction of cyanoacrylate as a modification of the PTVE technique, it has become a more effective and safe method for preventing rebleeding of GV.[7–9] We have used a modified PTVE technique to obliterate all lower esophageal and peri- or para-esophageal varices, and the adventitial plexus of the cardia and fundus for treating esophageal variceal bleeding and achieved good therapeutic effects.[10] Using this same technique, we have also achieved good results in the treatment of GVs. However, for GV with a gastrorenal shunt, BRTO was preferred. However, the operation time is longer and the risk is higher than for PTVE.[11] Therefore, it is necessary to further evaluate the significance of PTVE in the treatment of gastric varices.
The TIPS has emerged as an effective measure for preventing variceal bleeding.[12] Although TIPS is more effective than endoscopic therapy for preventing variceal bleeding, TIPS can increase the risk of hepatic encephalopathy (HE).[13–15] Furthermore, the high operation cost may limit the access to the procedure.
There have been few studies comparing between PTVE and TIPS for the treatment of GVs.[14,15] Accordingly, we aimed to compare changes in portal vein pressure (PVP), and long-term clinical outcomes in patients treated with PTVE and TIPS in this retrospective study.
2. Materials and methods
2.1. Patients
The medical records of patients with liver cirrhosis with a history of gastric variceal bleeding who underwent either PTVE or TIPS in our hospital from January 2010 to June 2015 were reviewed (Fig. 1). All patients underwent diagnostic endoscopy and computed tomography (CT) and/or portal imaging before the procedures to confirm GVs as the bleeding cause. The above–mentioned examinations confirmed the severity of GVs, the diameter of GVs, the anatomical relationship of blood vessels, the existence of a gastrorenal shunt, the presence of ascites, and portal vein thrombosis. Along with those assessments we measured liver function, blood biochemistry, blood routine, and blood coagulation.
Figure 1.

A schematic diagram of the study and numbers of cases per group.
The inclusion criteria were: First, diagnosis of liver cirrhosis by clinical examination and imaging, including ultrasounds, CT, or magnetic resonance (MR) imaging. Second, history of upper gastrointestinal hemorrhage including hematemesis, melena with no other potential source of bleeding (such as ulcer bleeding). Third, preoperative gastroscopy endoscopy showing bleeding from mild to severe GVs. Fourth, variceal bleeding not controlled by endoscopic therapy and pharmacological treatment or recurrent episodes of bleeding from varices after endoscopic therapy as per the Baveno VI definitions.[16]Fifth, ages between 18 and 80 years.
The exclusion criteria were: First, concomitant hepatocellular carcinoma or other malignancies. Second, no bleeding history after treatment including surgical operation, endoscopic treatment, interventional treatment. Third, concomitant widespread portal vein thrombosis. Fourth, severe hypertension, coronary heart disease or cardiopulmonary insufficiency.
Choice of treatment method was based on patient decisions after providing sufficient explanation of the two treatment methods. Informed written consent was obtained from each patient according to the guidelines of local ethics committee that approved our study.
2.2. Treatment
2.2.1. PTVE procedure
The PTVE was performed alone or combined with left renal vein obstruction with a balloon when a large gastrorenal shunt was found. Patients were placed in the supine position, and after transhepatic puncture of an intrahepatic portal vein branch with ultrasonography or fluoroscopy guidance, a 5F Cobra catheter was introduced into the portal venous system. Digital subtraction angiography (DSA) radiography was performed to evaluate the GVs, the feeding vessels, draining veins, and the possible presence of a gastrorenal shunt. The main feeding vessels were selected and injected with cyanoacrylate to block the blood flow. The above procedure was repeated until blood flow towards the varices were totally obstructed. As in our previous reports, all lower esophageal and peri- or para-esophageal varices, and the adventitial plexus of the cardia and fundus were completely obliterated with cyanoacrylate.[10] Finally, a 5F sheath system was withdrawn after all the GVs and other varicosed veins were obliterated with cyanoacrylate, and the puncture tract was embolized with microcoils.
2.2.2. TIPS procedure
The TIPS technique to establish a portosystemic shunt has been described previously.[10] The right hepatic vein was cannulated by a transjugular approach. Under fluoroscopic guidance, a needle was then passed through the cannula to puncture the liver, aiming at the intrahepatic portion of a main branch of the portal vein. Once the needle was in the portal vein, the cannula was advanced over the needle. A guidewire was then passed into the portal vein, and the parenchymatous tract was dilated with an angioplasty catheter. After balloon dilation, a polytetrafluoroethylene-covered stent was deployed. When there was no gastrorenal shunt, balloon-occluded antegrade transvenous obliteration (BATO) was used as an alternative route to access GVs through an existing transjugular intrahepatic portosystemic shunt.[11,17] Then, the GVs and other varices were obliterated with cyanoacrylate. When there was large gastrorenal shunts, BRTO might be the 1st choice for the treatment of.
2.2.3. Patient follow-up
All patients were followed until death or loss to follow-up. Telephone follow-up, clinical follow-up, medical histories and records were used to collect the information. Rebleeding, survival, and complications were recorded. Follow-up endoscopy was performed for both groups at intervals of 1, 3, and 6 months after the procedures, and then every 6 to 12 months or whenever it was considered necessary. Endoscopy was performed to identify the causes of rebleeding. Imaging was performed to detect portal vein thrombosis, shunt patency, variceal recanalization, and the presence of collateral vessels.
Recurrent bleeding was defined as the presence of hematemesis, melena, 20 g/L decrease in hemoglobin levels or clinical evidence of hypovolemic shock, and demonstration of the source of bleeding by endoscopy. Early rebleeding was defined as recurrent bleeding from 5 days to 6 weeks after the procedure. Survival time was defined as the period beginning with the 1st procedure (PTVE or TIPS) in our hospital and ending with death or at the study endpoint.
2.3. Statistical analysis
All results were expressed as mean ± SD, or as a percentage. Quantitative variables were compared by 2-tailed Student t test, and qualitative variables were compared by the Fisher exact test or the chi-squared test (with Yates correction) where appropriate. The comparisons of the PVPs before and after the procedure were analyzed by paired-sample t tests. The Kaplan–Meier estimation was used to examine recurrence and rebleeding of gastric varices, and rates of survival. Comparisons were performed using the log-rank test. A P value less than .05 was considered significant. Statistical computation was performed using SPSS 19.0 software.
3. Results
3.1. Demographics
A total of 74 patients were enrolled during the study period. In this, 47 males (63.5%) and 27 females (36.5%) were enrolled, a ratio of 1.74:1. The ages ranged from 23 to 77 with the mean of 50.3 ± 12.05. The etiologies of cirrhosis included hepatitis B and hepatitis C (41 patients, 5.4%), alcohol (8 patients, 10.8%), idiopathic cirrhosis and other (25 patients, 33.8%) including autoimmune cirrhosis, Wilson's disease, cholestasis liver cirrhosis (Table 1). The diagnosis of cirrhosis was made by abdominal CT or MR portal imaging. Forty three received PTVE, and 31 underwent TIPS. The clinical characteristics of the patients in the 2 groups, including sex, age, Child-Pugh classification, etiologies of liver cirrhosis, degree of esophageal varices (EVs) and red-color sign of GVs were not significant different from each other (Table 1). There were 9 patients who lost to follow-up overall (6 in PTVE group and 3 in TIPS group). The mean follow-up period was 29.9 ± 16.8 months (PTVE group, 33.4 ± 18.0 months and TIPS group, 25.3 ± 13.9 months).
Table 1.
Clinical characteristics of patients included in the study.

3.2. Technique results
There were 9 patients who lost to follow-up overall. The clinical characteristics such as sex, age, Child-Pugh classification, etiologies of liver cirrhosis, degree of EVs and red-color sign of GVs were not significantly different (Table 2).
Table 2.
Clinical characteristics of patients with follow-up and patients lost to follow-up.

Two patients in the PTVE group were treatment failures. One had severe abdominal infection due to injury of bile ducts during the operation, and recurrent bleeding accompanied with hepatic failure 4 weeks after the operation. The 2nd patient died of hepatic failure. The TIPS group had 2 treatment failures. One had recurrent bleeding after 3 days due to occlusion of the stents. The 2nd had hepatic artery bleeding after the operation and died of hepatic failure after hemostasis was achieved. The success rates between 2 groups were 95.3% and 93.5%, but there was no statistical difference (P = .560) between them.
The changes in PVP before and after operation are shown in Table 3. Before operation, there was no significant difference between the PVPs of the 2 groups. The PVP after PTVE procedure was slightly lower than before, but the difference was not statistically significant (P > .05). The PVP after TIPS decreased significantly (P < .0001).
Table 3.
PVP before and after operation.

3.3. Early recurrent bleeding
There were 67 patients who had early follow-up (6 weeks after operation), 39 in PTVE group, and 28 in TIPS group. The rate of early recurrent bleeding in PTVE group was 15.4%, 6 patients. Three patients received endoscopic treatment, and others received pharmacological therapy.[16] One of the 6 patients developed hematemesis after 1 month which could not be controlled and resulted in death. The rate of early recurrent bleeding in TIPS group was 7.1%, 2 of those patients had occlusion of the TIPS after several days and received endoscopic therapy.
The early recurrent bleeding rates were 15.4% in the PTVE group, and 7.1% in the TIPS group. The difference was not statistically significant (P = .256).
3.4. Cumulative recurrent bleeding
The number of patients with follow-up was 65 in total, with the average follow-up period of 29.9 ± 16.8 months (PTVE group, 33.4 ± 18.0 months and TIPS group, 25.3 ± 13.9 months). The total cumulative rebleeding rate was 35.4% (23 patients). The cumulative rebleeding-free rates in PTVE group at 1, 2, and 3 years were 72.1%, 66.1%, 50.7%, respectively, each of which were lower than that in TIPS group 85.0%, 75.7%, 75.7%, respectively. However, the differences were not statistically significant (χ2 = 1.645, P = .200) (Fig. 2).
Figure 2.

Kaplan–Meier curve analysis of the probability of rebleeding.
The causes of recurrent bleeding after PTVE included portal hypertensive gastropathy and gastric ulcer (4 cases), recurrent GVs (9 cases) and unknown (3 cases). In most of the rebleeding cases, hemorrhage was controlled. Six of these cases received endoscopic therapy while the others received pharmacological therapy. Three of the rebleeding patients died of uncontrolled re-hemorrhage.
The cause of recurrent bleeding in TIPS group included obstruction of TIPS (2 cases), gastric ulcers, and unknown. One stopped bleeding spontaneously, 2 received pharmaceutical therapy, 1 had removal of the TIPS obstruction, and had a repeat TIPS. One patient died from an uncontrolled massive hemorrhage.
3.5. Survival
Eight PTVE patients and 3 TIPS patients died during the course of follow-up. Liver dysfunction and variceal rebleeding were the 2 main causes of death. In PTVE group, 3 died of recurrent EGVB, 2 of hepatic failure, and the rest died from severe infection (1 patient), and myocardial infraction (1 patient), and unknown causes. Death in the TIPS group was caused by liver dysfunction (1 patient), variceal rebleeding (1 patient) and hypovolemic shock caused by liver artery injury during operation.
The mortality rates in PTVE and TIPS were 21.1% and 10.7%, respectively, but the difference was not statistically significant (χ2 = 0.333, P = .564) (Fig. 3).
Figure 3.

Kaplan–Meier curve analysis of the probability of survival.
3.6. Hepatic encephalopathy and other complications
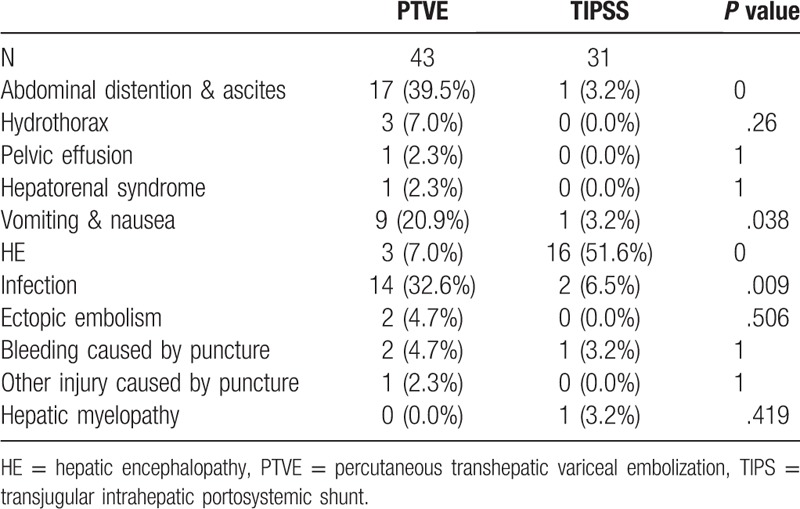
As shown in Table 4, the main complications in the 2 groups included hepatic encephalopathy (HE), abdominal distention and ascites, hydrothorax, pelvic effusion, hepatorenal syndrome, nausea and vomiting, infection, ectopic embolism, injuries caused by puncture, hepatic myelopathy. According to the analysis, the incidence rate of hepatic encephalopathy in the TIPS group was much higher than that in the PTVE group (P < .0001). In the PTVE group, the incidence rates of abdominal distention and ascites, infection, vomit and nausea were higher than those in the TIPS group (P < .05). As for other complications, no significant inter-group differences were observed (P > .05).
Table 4.
Procedural complications.
4. Discussion
Standard endoscopic therapies used for EVs, such as sclerotherapy and band ligation, are less effective for GVs and have been shown to be associated with high complication rates.[18] This leads to the need for more aggressive and costly interventions, such as TIPS and PTVE. However, these treatments also have limitations. The current methods for the treatment of GVs are far from ideal.
The PTVE was introduced in the 1970 s for the management of EGVB.[6] Because of the high rebleeding rate, the procedure has not attained widespread clinical acceptance. The theoretical background for using modified PTVE to prevent rebleeding is based on previous reports. The PTVE with cyanoacrylate is considered to be a modification of the standard percutaneous transhepatic procedure for obliteration of varices.[6,19] Although this technique was used in a small number of GV patients without gastrorenal shunts, it has become more widely performed for this purpose. A modified PTVE using 2-octyl cyanoacrylate (2-OCA) in the treatment of EV has been reported in our previous studies on treatment of gastric varices.[8,9]
The technique of placing a TIPS to establish a portosystemic shunt has been described previously.[10] In recent years, TIPS has been regarded as a standard therapy for portal hypertension accompanied with complications, including EVs, GVs, ascites, portal vein thrombosis. There have been many studies on TIPS in the treatment of EVs, but few on the treatment of GVs.[11,20–24]
In this retrospective study, we compared modified PTVE with TIPS in the treatment of gastric varices. In the current study, there was no significant difference in PVP before or after PTVE procedure. This result is different from other reports in which PTVE worsened portal hypertension.[25] However, TIPS procedure can significantly decrease the PVP, which can reduce the degree of GVs and lower the risk of recurrent bleeding. However, Sanyal et al found TIPS to be ineffective for GVs associated with large gastrorenal shunts, even when the portosystemic pressure gradient (PSG) was decreased to below a critical bleeding threshold (12 mm Hg).[26] Therefore, BRTO might be the 1st choice for the treatment of large gastrorenal shunts.
The cumulative rebleeding rate in our study was 35.4%. The cumulative rebleeding-free rates at 1, 2 and 3 years in PTVE group were lower than that in TIPS group, but the result was not statistically significant. The present study showed that modified PTVE has similar efficacy as TIPS with regard to prevention of GVs rebleeding. This is different than some previous reports which found that patients undergoing TIPS had higher rates of rebleeding.[14,15] Some studies have shown that PTVE can increase portal hypertension, and result in portal hypertensive complications, including worsening non-gastric varices[13,27] including bleeding from EVs.[14] In contrast, in the present study, the portal vein pressure of patients in PTVE group decreased and not increased. The cause of the portal hypertension had not been eliminated. Recurrent GVs was the main cause of rebleeding in the PTVE group, which means that although PTVE disconnected the GVs in cirrhosis in a certain period of time, recurrent GVs still would occur because of the existence of portal hypertension. As for TIPS group, re-obstruction of TIPS stents was the main cause of rebleeding in our study. The re-obstruction of stents increased portal hypertension, which made the recurrence or deterioration of GVs. As a result, maintaining stents patency is an important factor in maintaining the effectiveness of a TIPS. This has also been reported previously.[28]
The mortality rates in PTVE and TIPS are 21.1% and 10.7% respectively. The PTVE group was higher than the TIPS group, without statistical difference. The HE is a common complication that occurs after TIPS. In the present study, 16/31 patients (51.6%) in the TIPS group developed HE, but only 3/43 (7.0%) patients in the PTVE group did. The distribution of HE patients among these 2 groups was statistically significant. This represents a possible advantage of PTVE compared with TIPS and is consistent with the findings of other studies.[14] The rate of infection in PTVE group is higher than TIPS group, by the reason that PTVE was sometimes operated with PSE constantly. Most of the patients with infection were underwent PTVE combined with PSE. The incidence of abdominal distention and ascites in PTVE group is more than TIPS group. One study by Lee et al reported that PTVE increased the risk of worsening ascites.[14]
Specific limitations of this study must be acknowledged. First, due to its retrospective design, there was a potential for selection bias in each treatment approach. Second, the patients were from just 1 center. There might be technique bias and experience bias. This might account for differences between our results and those of others. Third, data on the outcome of gastric varices after procedures were not fully available because a lack of complete follow-up endoscopic examination. The TIPS and modified PTVE were not analyzed for differences in Chinese patients with gastric variceal bleeding (GVB). In fact, there have only been a few such studies.[14,15] We plan to address this question with a multi-center prospective, randomized, and controlled trial.
In conclusion, the results indicate that in patients with gastric varices, PTVE offers similar survival and rebleeding-free rate compared to TIPS. Both PTVE and TIPS are considered effective treatments for GVB. Attention should be paid to the development of hepatic encephalopathy after TIPS.
Author contributions
Conceptualization: Kai Zhang, Mingyan Zhang.
Data curation: Kai Zhang, Xiangguo Tian.
Formal analysis: Xiaoyan Sun, Mingyan Zhang, Zhe Wu, Xiangguo Tian.
Funding acquisition: Guangchuan Wang, Mingyan Zhang, Zhe Wu, Xiangguo Tian.
Investigation: Guangchuan Wang, Xiangguo Tian, Chunqing Zhang.
Methodology: Xiaoyan Sun, Guangchuan Wang, Xiangguo Tian, Chunqing Zhang.
Project administration: Mingyan Zhang.
Resources: Guangchuan Wang, Zhe Wu.
Software: Kai Zhang, Xiaoyan Sun, Mingyan Zhang.
Supervision: Guangchuan Wang.
Validation: Kai Zhang.
Visualization: Xiaoyan Sun.
Writing – original draft: Xiaoyan Sun.
Footnotes
Abbreviations: BATO = balloon-occluded antegrade transvenous obliteration, BRTO = balloon retrograde transvenous obliteration, CT = computed tomography, DSA = digital subtraction angiography, GVB = gastric variceal bleeding, HE = hepatic encephalopathy, MR = magnetic resonance, PTVE = percutaneous transhepatic variceal embolization, PVP = portal vein pressure, TIPS = transjugular intrahepatic portosystemic shunt.
KZ and XS contributed equally.
This work was supported by Focus on Research and Development Plan in Shandong Province, China (Project No. 2015GSF118012, 2017GSF218026 and 2018GSF118100) and the Health and Family Planning Commission of Shandong Province, China (Project No.2017WS199).
The authors have no conflicts of interest to disclose.
References
- [1].Orloff MJ, Hye RJ, Wheeler HO, et al. Randomized trials of endoscopic therapy and transjugular intrahepatic portosystemic shunt versus portacaval shunt for emergency and elective treatment of bleeding gastric varices in cirrhosis. Surgery 2015;157:1028–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Henry Z, Uppal D, Saad W, et al. Gastric and ectopic varices. Clin Liver Dis 2014;18:371–88. [DOI] [PubMed] [Google Scholar]
- [3].Sarin SK, Lahoti D, Saxena SP, et al. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology 1992;16:1343–9. [DOI] [PubMed] [Google Scholar]
- [4].Gaba RC, Couture PM, Lakhoo J. Gastroesophageal variceal filling and drainage pathways: an angiographic description of afferent and efferent venous anatomic patterns. J Clin Imaging Sci 2015;5:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schubert TT, Schnell GA, Walden JM. Bleeding from varices in the gastric fundus complicating sclerotherapy. Gastrointest Endosc 1989;35:268–9. [DOI] [PubMed] [Google Scholar]
- [6].Lunderquist A, Vang J. Transhepatic catheterization and obliteration of the coronary vein in patients with portal hypertension and esophageal varices. N Engl J Med 1974;291:646–9. [DOI] [PubMed] [Google Scholar]
- [7].Bian S, Tian XG, Hu JH, et al. Percutaneous transhepatic variceal embolization combined with endoscopic ligation for the prevention of variceal rebleeding. J Dig Dis 2013;14:388–95. [DOI] [PubMed] [Google Scholar]
- [8].Zhang CQ, Liu FL, Liang B, et al. A modified percutaneous transhepatic variceal embolization with 2-octyl cyanoacrylate versus endoscopic ligation in esophageal variceal bleeding management: randomized controlled trial. Dig Dis Sci 2008;53:2258–67. [DOI] [PubMed] [Google Scholar]
- [9].Zhang CQ, Liu FL, Liang B, et al. A modified percutaneous transhepatic varices embolization with 2-octyl cyanoacrylate in the treatment of bleeding esophageal varices. J Clin Gastroenterol 2009;43:463–9. [DOI] [PubMed] [Google Scholar]
- [10].Tian X, Shi Y, Hu J, et al. Percutaneous transhepatic variceal embolization with cyanoacrylate vs transjugular intrahepatic portal systematic shunt for esophageal variceal bleeding. Hepatol Int 2013;7:636–44. [DOI] [PubMed] [Google Scholar]
- [11].Kim DJ, Darcy MD, Mani NB, et al. Modified balloon-occluded retrograde transvenous obliteration (BRTO) techniques for the treatment of gastric varices: vascular plug-assisted retrograde transvenous obliteration (PARTO)/coil-assisted retrograde transvenous obliteration (CARTO)/balloon-occluded antegrade transvenous obliteration (BATO). Cardiovasc Intervent Radiol 2018;41:835–47. [DOI] [PubMed] [Google Scholar]
- [12].Brunner F, Berzigotti A, Bosch J. Prevention and treatment of variceal haemorrhage in 2017. Liver Int 2017;37Suppl 1:104–15. [DOI] [PubMed] [Google Scholar]
- [13].Lee SJ, Kim SU, Kim MD, et al. Comparison of treatment outcomes between balloon-occluded retrograde transvenous obliteration and transjugular intrahepatic portosystemic shunt for gastric variceal bleeding hemostasis. J Gastroenterol Hepatol 2017;32:1487–94. [DOI] [PubMed] [Google Scholar]
- [14].Ninoi T, Nakamura K, Kaminou T, et al. TIPS versus transcatheter sclerotherapy for gastric varices. AJR Am J Roentgenol 2004;183:369–76. [DOI] [PubMed] [Google Scholar]
- [15].Sabri SS, Abi-Jaoudeh N, Swee W, et al. Short-term rebleeding rates for isolated gastric varices managed by transjugular intrahepatic portosystemic shunt versus balloon-occluded retrograde transvenous obliteration. J Vasc Interv Radiol 2014;25:355–61. [DOI] [PubMed] [Google Scholar]
- [16].de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2005;43:167–76. [DOI] [PubMed] [Google Scholar]
- [17].Saad WE, Sze DY. Variations of balloon-occluded retrograde transvenous obliteration (BRTO): balloon-occluded antegrade transvenous obliteration (BATO) and alternative/adjunctive routes for BRTO. Semin Intervent Radiol 2011;28:314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cat TB, Liu-DeRyke X. Medical management of variceal hemorrhage. Crit Care Nurs Clin North Am 2010;22:381–93. [DOI] [PubMed] [Google Scholar]
- [19].Lunderquist A, Borjesson B, Owman T, et al. Isobutyl 2-cyanoacrylate (bucrylate) in obliteration of gastric coronary vein and esophageal varices. AJR Am J Roentgenol 1978;130:1–6. [DOI] [PubMed] [Google Scholar]
- [20].Gaba RC. Retrograde-antegrade accelerated trap obliteration: a modified approach to transvenous eradication of gastric varices. J Vasc Interv Radiol 2017;28:291–4. [DOI] [PubMed] [Google Scholar]
- [21].Gwon DI, Ko GY, Kwon YB, et al. Plug-assisted retrograde transvenous obliteration for the treatment of gastric varices: the role of intra-procedural cone-beam computed tomography. Korean J Radiol 2018;19:223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hirota S, Kobayashi K, Kako Y, et al. Balloon-occluded retrograde transvenous obliteration of varices: focusing on the portal hemodynamics and the recent techniques. Hepatol Int 2018;12:102–11. [DOI] [PubMed] [Google Scholar]
- [23].Kim SK, Lee KA, Sauk S, et al. Comparison of transjugular intrahepatic portosystemic shunt with covered stent and balloon-occluded retrograde transvenous obliteration in managing isolated gastric varices. Korean J Radiol 2017;18:345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wu Q, Jiang H, Linghu E, et al. BRTO assisted endoscopic Histoacryl injection in treating gastric varices with gastrorenal shunt. Minim Invasive Ther Allied Technol 2016;25:337–44. [DOI] [PubMed] [Google Scholar]
- [25].Ninoi T, Nishida N, Kaminou T, et al. Balloon-occluded retrograde transvenous obliteration of gastric varices with gastrorenal shunt: long-term follow-up in 78 patients. AJR Am J Roentgenol 2005;184:1340–6. [DOI] [PubMed] [Google Scholar]
- [26].Sanyal AJ, Freedman AM, Luketic VA, et al. The natural history of portal hypertension after transjugular intrahepatic portosystemic shunts. Gastroenterology 1997;112:889–98. [DOI] [PubMed] [Google Scholar]
- [27].Cho SK, Shin SW, Lee IH, et al. Balloon-occluded retrograde transvenous obliteration of gastric varices: outcomes and complications in 49 patients. AJR Am J Roentgenol 2007;189:W365–372. [DOI] [PubMed] [Google Scholar]
- [28].Rees CJ, Nylander DL, Thompson NP, et al. Do gastric and oesophageal varices bleed at different portal pressures and is TIPS an effective treatment? Liver 2000;20:253–6. [DOI] [PubMed] [Google Scholar]


