Abstract
Purpose:
The aim of the present study is to evaluate the effects of deliberate hypotensive anesthesia on intraocular pressure (IOP) and ocular perfusion pressure (OPP) and compare the effects of propofol total intravenous anesthesia (TIVA) and desflurane anesthesia on IOP and OPP.
Methods:
A total of 50 patients undergoing arthroscopic shoulder surgery in the lateral decubitus position were randomized to receive desflurane or propofol anesthesia. Mean arterial pressure (MAP) was maintained in the range of 60–75 mm Hg during hypotensive anesthesia. IOP was measured using a handheld tonometer at 7 time points: before induction (T1, baseline); immediately after endotracheal intubation (T2); 10 minutes after position change to lateral decubitus (T3); 10, 30, and 50 minutes after the start of hypotensive anesthesia (T4–T6); and at the end of surgery (T7).
Results:
MAP decreased about 35% to 38% during hypotensive anesthesia. Compared to baseline values, the IOP at T6 in dependent and non-dependent eyes decreased by 0.43 and 2.74 mm Hg, respectively in desflurane group; 3.61 and 6.05 mm Hg, respectively in the propofol group. IOP of both eyes in the propofol group was significantly lower than in the desflurane group from T2 to T7. OPP of both eyes in both groups was significantly lower than at baseline, except at T2 in the desflurane group. OPP of both eyes in the propofol group was significantly higher than that in the desflurane group at T5 and T6.
Conclusions:
Hypotensive anesthesia reduced IOP and OPP, but propofol TIVA maintained higher OPP than desflurane anesthesia. These findings suggest that propofol TIVA can help mitigate the decrease of OPP during hypotensive anesthesia.
Keywords: controlled hypotension, desflurane, intraocular pressure, ocular perfusion pressure, propofol
1. Introduction
Perioperative visual loss (POVL) is a serious complication without known treatment.[1] Causes of POVL include central retinal artery occlusion, cortical blindness due to occipital area infarct and ischemic optic neuropathy.[1] Proper maintenance of ocular perfusion pressure (OPP) can help prevent these conditions.[2] However, ophthalmic circulatory autoregulation does not work properly under general anesthesia; as a result, ocular perfusion pressure may decrease continuously.[2] Ocular perfusion pressure can be defined as mean arterial pressure minus intraocular pressure (IOP). Therefore, measurement and reduction of IOP during surgeries that are at high risk for a decrease in OPP can be helpful.[2]
Deliberate hypotension is an important anesthetic technique for procedures such as orthognathic, arthroscopic, facial, or spine surgeries.[3,4] Mean arterial blood pressure (MAP) is controlled as 50 to 65 mm Hg for reduced blood loss, better visualization, and shorter operative time in hypotensive anesthesia. However, during hypotensive anesthesia, OPP may decrease because of low MAP. A case report previously described four patients who developed ischemic optic neuropathy after prolonged lumbar spine surgery under deliberate hypotensive anesthesia maintained between 85 to 100 mm Hg systolic and 45 to 65 mm Hg diastolic to reduce bleeding.[5] Although animal experiments have demonstrated that hypotension does not lower IOP and that neither hypotension nor hypertension causes glaucoma or ocular complications,[6,7] there has been no clinical study to evaluate IOP and OPP changes in patients undergoing deliberate hypotension. The effects of anesthetics on IOP have been studied, and propofol is more effective than inhalation anesthetics in attenuating an IOP increase during anesthesia.[8,9] The hypothesis of this study was that propofol total intravenous anesthesia (TIVA) may be beneficial for maintaining OPP during hypotensive anesthesia because propofol may be more effective at lowering IOP.
Therefore, we aimed to evaluate IOP and OPP changes during hypotensive anesthesia and compare the effects of desflurane and propofol TIVA on IOP and OPP changes in patients undergoing arthroscopic shoulder surgery.
2. Materials and methods
2.1. Study population and ethical approval
The study protocol was approved by the Institutional Review Board of St. Vincent Hospital, The Catholic University of Korea (VC18MESI0033) and was registered on the Clinical Research Information Service of the Korea National Institute of Health (CRIS, http://cris.nih.go.kr, identification number: KCT0002795). Written informed consent was obtained from all patients.
Fifty patients aged 30 to 75 years with an American Society of Anesthesiologists (ASA) physical status I or II who were scheduled for elective arthroscopic shoulder surgery between March and August 2018 in Catholic University St. Vincent Hospital were enrolled in this prospective randomized and controlled study. Patients with previous eye surgery, preexisting eye disease including glaucoma, uncontrolled cardiovascular disease or lung disease, history of allergic reaction to the study drugs, and baseline IOP ≥ 30 mm Hg were excluded. Patients with uncontrolled blood pressure (MAP was higher than 75 mm Hg even though nicardipine was injected twice) were withdrawn.
2.2. Study protocol
All patients were fasted for 8 hours before the induction of anesthesia and administered intravenous lactated Ringer solution at a rate of 1.5 to 2 ml/kg/h during preoperative fasting. None of the patient was premedicated. On arrival to the operation theater, patients were allocated to either the desflurane or propofol group using a computer-generated randomization table with an allocation ratio of 1:1. The randomization scheme was generated using the website http://www.randomization.com. The randomization was conducted by a nurse who was not involved in the anesthetic management or the data collection. Basic monitoring, including electrocardiography, noninvasive blood pressure, peripheral oxygen saturation (SpO2), and bispectral index (BIS), was applied and an IOP measuring device was prepared. In the desflurane group, anesthesia was induced with intravenous thiopental sodium 5 to 6 mg/kg, remifentanil continuous infusion and rocuronium 1 mg/kg. After endotracheal intubation, mechanical ventilation was started and adjusted to maintain an end tidal CO2 (ETCO2) 30 to 35 mm Hg in all patients. Anesthesia was maintained with 5 to 8 vol% desflurane inhalation with 50% oxygen in air and continuous infusion of remifentanil. The desflurane concentration and effect site concentration of remifentanil were titrated to maintain BIS values within a target range of 40 to 60 and target MAP 60 to 75 mm Hg during the operation. MAP was calculated using an automatic noninvasive blood pressure machine. Remifentanil was administered via a target-controlled infusion (TCI) system using a TCI device (Orchestra Base Primea, Fresenius Kabi, Austria) and the target effect site concentration of remifentanil was 3 to 6 ng/ml. In the propofol group, anesthesia was induced with intravenous 1% propofol 1.5 to 2.5 mg/kg, remifentanil continuous infusion and rocuronium 1 mg/kg. After endotracheal intubation, anesthesia was maintained with continuous infusion of 2% propofol and remifentanil with 50% oxygen in air. Propofol was administered via a TCI system using a TCI device (target effect site concentration 2.5–5 μg/ml). The effect site concentration of propofol and remifentanil were titrated to maintain BIS values within a target range 40 to 60 and target MAP 60 to 75 mm Hg during surgery. Ringer lactate solution (4–6 ml/kg/h) was used for fluid maintenance during anesthesia. After induction of general anesthesia, the position of all patients was changed to lateral decubitus and then restored to the supine position after the end of surgery. In the lateral decubitus position, the dorsal aspect of the patient's head was aligned with the back. The height of the pillow was adjusted to keep the angle of the neck parallel to the operation table. Doughnut-shaped pillow with towels padding was used in order to prevent any extraocular pressure in the dependent eye. After the position change to lateral decubitus, blood pressure was measured at the lower arm and controlled hypotensive anesthesia was initiated in all patients. Considering the location of the lower arm below the heart, MAP was targeted 60 to 75 mm Hg. If MAP was higher than 75 mm Hg after more than 2 successive measurements, intravenous nicardipine 1 mg was administered. Hypotension (MAP < 50 mm Hg) was treated with intravenous ephedrine 10 mg and bradycardia (HR < 50 bpm) was treated with intravenous atropine 0.5 mg. Hypotensive anesthesia was maintained until removal of the arthroscope.
Before anesthesia induction, IOP was measured with a hand-held tonometer (Tono-Pen AVIA, Reichert Technologies, Depew, NY, USA) after application of 2 drops of 0.5% Alcaine (proparacaine HCl 5 mg, Alcon-Couvreur N.V., Puurs, Belgium) for topical anesthesia. After topical anesthesia, the tonometer tip was placed perpendicular to the patient's cornea and gently contacted on the center of the cornea. Indentation or additional pressure was avoided. Seven IOP measurements were as follows: before anesthetic induction in the supine position (T1, baseline); immediately after endotracheal intubation (T2); 10 minutes after position change to lateral decubitus (T3); 10 minutes after the start of controlled hypotensive anesthesia (T4); 30 minutes after the start of controlled hypotensive anesthesia (T5); 50 minutes after the start of controlled hypotensive anesthesia (T6); at the end of surgery (T7).
The tonometer averaged readings from 6 successful measurements and displayed the mean value along with a statistical confidence indicator. If the statistical confidence indicator was less than 95%, the value was discarded, and measurements were repeated. At the time of each IOP measurement, MAP, heart rate, ETCO2, BIS, and peak inspiratory pressure (PIP) were recorded. The OPP was calculated as MAP minus IOP [MAP = diastolic blood pressure + 1/3 (systolic blood pressure – diastolic blood pressure)]. Duration of the controlled hypotensive anesthesia, total volume of arthroscopic irrigation fluids and intravenous fluids, and hemoglobin values before and after the surgery were recorded. After emergence from anesthesia, all patients were asked about ophthalmic complications, such as visual disturbance, in the recovery room.
All anesthetic management was performed and recorded by 1 experienced anesthesiologist who was not involved in the analysis of study data. The IOP was measured by another anesthesiologist who had experience in measuring IOP during the previous study and did not participate in the data analysis. All operations were performed by a single experienced surgeon. No patient received supplementary regional nerve blockade in the perioperative period.
2.3. Statistical analysis
Sample size calculation was performed with a power analysis based on data from a pilot study and previous study,[9] in which the mean IOP of the dependent eye in the lateral decubitus position in the propofol anesthesia group was 19.5 mm Hg compared with 23 mm Hg in the sevoflurane group. Power estimation analysis suggested that 21 patients per group would be required to detect a mean (± SD) difference in IOP of 3.5 ± 4 mm Hg with a power of 80%, considering a type I error of 0.05. To compensate for unexpected losses, recruitment was increased by 20%.
Statistical analyses were performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA) for Windows (Microsoft Corporation, Redmond, WA, USA). Demographic data were analyzed using the χ2 test and t test. Repeated-measures ANOVA was performed to compare IOP, OPP, MAP, PIP, and ETCO2 between the 2 groups, with ‘group’ and ‘time point’ as independent variables, after confirming normal distribution using the Shapiro–Wilk test (P > .05). Differences between the 2 groups were then computed using the t test followed by Bonferroni post hoc test (adjusted P value for significance P < .007 for IOP, OPP and MAP; P < .008 for PIP and ETCO2). The Student t test was used to compare initial IOP and IOP at the remainder of the time points. The relationships between PIP and IOP, ETCO2, and IOP, and MAP and IOP of the dependent eye in both groups were analyzed using Pearson correlation test. A P value < .05 was considered to be statistically significant.
3. Results
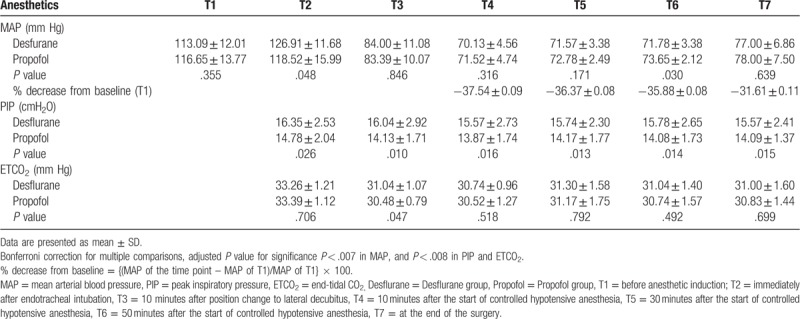
In total, 50 patients were enrolled in this study. Of these, 4 patients (2 in the desflurane group and2 in the propofol group) were excluded because MAP was maintained over 75 mm Hg even after administration of nicardipine. Therefore, 46 patients completed the study: 23 in the desflurane group and 23 in the propofol group (Fig. 1). Demographic data and perioperative data are shown in Table 1. No patient complained of any visual disturbance after surgery in the recovery room. There were no significant differences in MAP, PIP, or ETCO2 between the 2 groups (Table 2). MAP decreased by 35% to 38% from the baseline value during hypotensive anesthesia (Table 2). Two patients in the propofol group and 1 patient in the desflurane group received atropine because of bradycardia. One patient with transient hypotension in the desflurane group received ephedrine.
Figure 1.
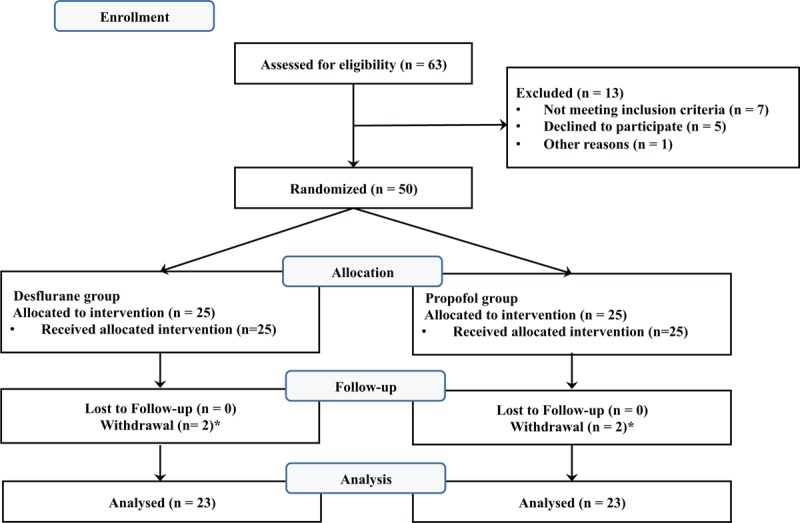
Consort flow diagram to illustrate the study design. ∗Two patients each in the propofol and desflurane groups dropped out because of uncontrolled blood pressure during surgery.
Table 1.
Demographic data and perioperative outcomes.
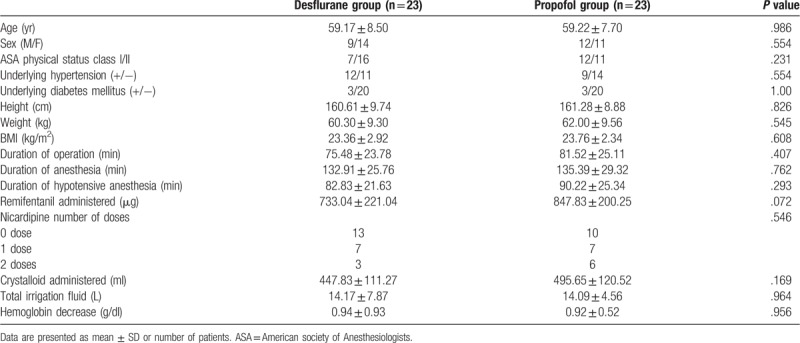
Table 2.
The changes of mean arterial pressure, peak inspiratory pressure and end-tidal CO2.
3.1. Changes in IOP
There was no significant difference in the baseline IOP of both eyes between groups (Fig. 2). As shown in Table 3, IOP of both eyes in patients in the propofol group was significantly lower than in patients in the desflurane group from immediately after intubation to the end of surgery.
Figure 2.
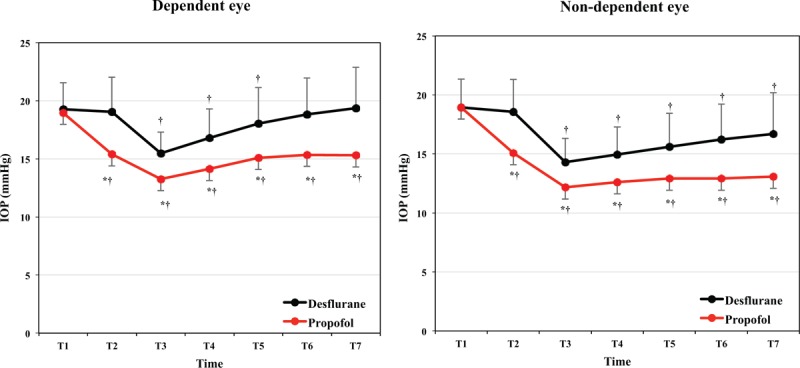
Comparison of intraocular pressure (IOP) between groups in dependent and non-dependent eyes. ∗Bonferroni correction for multiple comparisons, adjusted P value for significance P < .007. ∗P < .007 vs desflurane group, †P < .05 vs baseline value (T1) in each group. T1: before anesthesia induction, T2: immediately after endotracheal intubation, T3: 10 minutes after position change to lateral decubitus, T4: 10 minutes after the start of controlled hypotensive anesthesia, T5: 30 minutes after the start of controlled hypotensive anesthesia, T6: 50 minutes after the start of controlled hypotensive anesthesia, T7: at the end of surgery. Desflurane: desflurane group, Propofol: propofol group.
Table 3.
Comparisons of intraocular pressure (IOP) and ocular perfusion pressure (OPP) values.
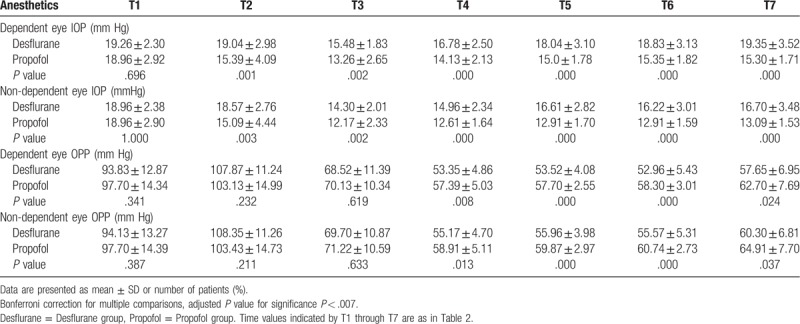
In the desflurane group, compared with baseline, IOP of the dependent eye was significantly lower starting 10 minutes after the position change to lateral decubitus and continuing to 30 minutes after the start of controlled hypotensive anesthesia. IOP of the non-dependent eye was significantly lower starting 10 minutes after the position change to lateral decubitus until the end of surgery. In the propofol group, IOP of both eyes was significantly lower from immediately after intubation to the end of surgery compared to baseline (Fig. 2). The IOP values at 50 minutes after the start of controlled hypotensive anesthesia in the dependent and non-dependent eye were approximately 0.43 and 2.74 mm Hg, respectively, lower than baseline values in the desflurane group, and 3.61 and 6.05 mm Hg, respectively, lower than baseline values in the propofol group (Table 3).
3.2. Changes in OPP
OPP of both eyes was markedly lower during hypotensive anesthesia. Initial OPP was approximately 90 to 100 mm Hg and OPP decreased to 50 to 60 mm Hg during hypotensive anesthesia in both groups (Table 3). In the comparison of OPP at baseline vs the remainder time points, OPP of both eyes in the desflurane group rose significantly after intubation but decreased significantly at other time points. In the propofol group, OPP of both eyes was significantly lower after the position change to lateral decubitus compared to baseline (Fig. 3). Between the 2 groups, OPP of both eyes was significantly higher in the propofol group than in the desflurane group at 30 minutes and 50 minutes after the start of controlled hypotensive anesthesia (Table 3).
Figure 3.

Comparison of ocular perfusion pressure (OPP) between groups in dependent and non-dependent eyes. ∗Bonferroni correction for multiple comparisons, adjusted P value for significance P < .007. ∗P < .007 vs desflurane group, †P < .05 vs baseline value (T1) in each group. Time values indicated by T1 through T7 are as in Figure 2. Desflurane: desflurane group, Propofol: propofol group.
3.3. Correlation of IOP or OPP with other variables
During anesthesia, IOP of the dependent eye was weakly correlated with PIP (r = 0.402, P < .001), MBP (r = 0.234, P < .001), and ETCO2 (r = 0.124, P = .039). OPP of the dependent eye was weakly negatively correlated with PIP (r = −0.398, P < .001).
4. Discussion
The aim of the present study was to evaluate changes in IOP and OPP during hypotensive anesthesia with desflurane or propofol in patients undergoing arthroscopic shoulder surgery. IOP was lower during hypotensive anesthesia in both the propofol and desflurane groups but was more significantly reduced in patients undergoing propofol TIVA compared to desflurane anesthesia. During hypotensive anesthesia, OPP also decreased regardless of anesthetic and was significantly higher in patients undergoing propofol TIVA than in patients undergoing desflurane anesthesia.
Hypotensive anesthesia is a safe technique that decreases MAP about 30% from baseline with a limit of 50 mm Hg while maintaining perfusion of vital organs and peripheral circulation in patients without a vascular anomaly.[10] Hypotensive anesthesia is effective for minimizing surgical blood loss and improving the quality of surgical field visualization.[3] In arthroscopic surgery of the subacromial space of the shoulder, a tourniquet cannot be used and there is no synovial lining to restrict fluid extravasation in the subacromial space.[4] Good visualization requires a decrease in the difference of blood pressure and subacromial pressure during arthroscopy,[4] and hypotensive anesthesia is usually applied. The safety of hypotensive anesthesia has been proven; patients can tolerate a 30% to 40% decrease in mean arterial pressure without side effects during shoulder arthroscopy in the beach-chair position.[3]
POVL is a devastating complication of anesthesia, and various pathologies caused by decreased OPP can result in POVL.[11] Although the incidence of POVL is very rare during hypotensive anesthesia,[11] blindness related to ischemic optic neuropathy can occur.[5] Decreased blood pressure may be a risk factor of anterior ischemic optic neuropathy, leading to vascular insufficiency in the optic nerve head.[12] Cerebral and optic nerve blood flow are controlled by different mechanisms; as a result, the optic nerve may be placed at risk of ischemia due to decreased OPP during hypotensive anesthesia to maintain perfusion of the brain.[11]
In addition to blood pressure, OPP can be determined by IOP. In the eyes, the intraocular veins are compressed by the IOP and will collapse unless venous transmural pressure exceeds the IOP. Mean OPP can be calculated by MAP minus IOP. Therefore, low OPP may be caused by a decrease in MAP and/or an increase in IOP. If MAP is sustained at a fixed level, control of IOP can be critical for OPP maintenance. In addition, optic nerve head blood flow does not only depend on OPP, but also on the MAP and IOP.[13] However, IOP can be increased by uncontrollable factors such as surgical technique, underlying disease, the position of the patient and the duration of surgery during anesthesia. Therefore, IOP monitoring and selection of adequate anesthetics that can reduce IOP can be helpful in patients with risk factors for decreased OPP, such as hypotensive anesthesia administration.
During anesthesia, the patient's position is one of the main factors that can change IOP because of the effect of gravity.[8] A few studies have evaluated IOP changes in patients undergoing general anesthesia in a lateral decubitus position.[9,14] Hwang et al reported that IOP of the dependent eye increased about 5 mm Hg from baseline by the end of the surgery in patients undergoing lung surgery under sevoflurane anesthesia.[14] Yamada et al compared the effects of sevoflurane and propofol on IOP and documented that IOP of the dependent eye increased approximately 8 mm Hg and 3.6 mm Hg from baseline with sevoflurane and propofol anesthesia, respectively, 1 hour after the position change to lateral decubitus.[9] Our current findings differ from these 2 previous studies. We found that the IOP of both eyes in patients undergoing propofol TIVA decreased even after intubation and was maintained at a lower level than baseline values until the end of surgery. In the desflurane group, IOP in the non-dependent eye decreased starting after the position change to lateral decubitus to the end of surgery. The IOP of the dependent eye in the desflurane group decreased after the position change to lateral decubitus but was elevated to baseline level at 50 minutes after the start of hypotensive anesthesia. In addition, although the correlation coefficient (r) value was low, the correlation of IOP and MAP was statistically significant. Therefore, an IOP decrease may be caused by a decrease of MAP and the effect of anesthetics.
Previous studies have suggested a proportional relationship between IOP and blood pressure. The Egna–Neumarkt Glaucoma Study found that elevated systemic blood pressure is associated with a slight increase in IOP; 10 mm Hg increases in systolic and diastolic blood pressures were associated with a 0.24 mm Hg and 0.4 mm Hg increase in IOP, respectively.[15] Using univariate mixed effects models Blecha et al showed that MAP can be a significant predictor for IOP elevation during propofol anesthesia.[16] Most clinical trials have reported the relationship of hypertension and IOP, but the relationship between induced hypotension and IOP has been only elucidated in a few animal experiments. An anesthetized porcine model for deliberate hypotension showed that adenosine induced hyperdynamic hypotension and increased IOP through uveal vessel dilatation, while isoflurane induced hypodynamic hypotension and did not significantly affect IOP.[6] In the present study, we confirmed that controlled hypotension maintaining 30% to 40% lower MAP than baseline values can induce IOP reduction despite use of the lateral decubitus position.
Anesthetics can also cause a decrease in IOP. Because the choice of anesthetics is a manageable factor that can prevent IOP elevation, many studies have evaluated the effects of various anesthetics on IOP. Desflurane is one of the most widely used inhalation anesthetics because it has a faster recovery profile than other potent inhalation anesthetics.[17] A few studies have compared the effects of desflurane and propofol on IOP. Desflurane has an effect on IOP comparable to propofol in the reverse Trendelenburg position,[18] but is less effective than propofol in alleviating the elevation of IOP in that position.[8,19] Propofol reduces IOP by decreasing the rate of aqueous humor formation to a greater extent than it decreases trabecular outflow facility.[20] Inhalation anesthetics, including desflurane, also reduce IOP in proportion to anesthetic depth by reducing choroidal volume, relaxing extraocular muscles, and facilitating aqueous humor outflow.[21] However, desflurane is less effective than propofol in reducing IOP in conditions that can increase IOP, such as head down positions or pneumoperitoneum.[8,9,19] In the present study, IOP was significantly lower in patients receiving propofol TIVA than in patients receiving desflurane anesthesia. Therefore, we suggest that propofol can decrease IOP more effectively than desflurane during hypotensive anesthesia.
With respect to IOP differences between the 2 eyes, we found that the difference in IOP between dependent and non-dependent eyes was between 2 and 3 mm Hg regardless of group allocation. Previous studies have reported a difference between 3 and 4 mm Hg and 5 mm Hg under sevoflurane and propofol anesthesia, respectively, in the lateral decubitus position.[9] Declines in IOP due to hypotensive anesthesia may have reduced the difference in the IOP values of both eyes in the present study.
Both PIP and ETCO2 are known to contribute to IOP elevation. Increases in intrathoracic pressure may elevate central venous pressure, decreasing the outflow of aqueous humor through the episcleral venous system; therefore, high PIP may increase IOP.[22] An attributable mechanism for a relationship between ETCO2 and IOP is that ETCO2 reflects arterial CO2 (PaCO2) and an increase in PaCO2 can lead to choroidal blood flow increase, consequently causing IOP elevation.[23] In the present study, both PIP and ETCO2 were weakly correlated with IOP in the dependent eye. Most studies have found that high PIP relates to IOP elevation under general anesthesia.[16,19] However, the relationship between ETCO2 and IOP during anesthesia has been controversial in previous studies,[22,24] which were conducted in patients under pneumoperitoneum or in the Trendelenburg position, both of which increase IOP. We found that PIP and ETCO2 were positively correlated with IOP when IOP decreased. Therefore, control of PIP and ETCO2 may be helpful for IOP reduction during hypotensive anesthesia.
The critical mean OPP value at which retinal or optic nerve function is damaged has not yet been defined, and the recommended target physiological mean OPP range is 45 to 55 mm Hg in patients with ocular ischemic risk factors.[2] In the present study, OPP in both eyes in the 2 groups dropped below baseline at all time points except immediately after tracheal intubation. Although OPP during hypotensive anesthesia was maintained at 50 to 60 mm Hg regardless of group allocation, OPP was about 4 to 5 mm Hg higher in the propofol group than in the desflurane group at 30 minutes and 50 minutes after the start of hypotensive anesthesia. The difference may be because MAP was maintained at a similar level, but IOP was significantly lower in the propofol group than in the desflurane group. Although the effects of propofol and desflurane on OPP can be clinically comparable in patients without any specific risk factors, as in the present study, OPP can decrease markedly in patients with risk factors such as impaired retinal perfusion, glaucoma and high IOP during hypotensive anesthesia. Further study of patients at high risk for OPP decrease is still needed, but propofol anesthesia may alleviate the significant OPP reduction in such patients. Several studies have suggested that low OPP is also related to an increased prevalence of glaucoma.[25–27] Zheng et al reported that low diastolic BP and low mean and diastolic perfusion pressure were independent risk factors for open-angle glaucoma.[27] The Baltimore Eye Survey indicated that individuals with diastolic ocular perfusion pressures <30 mm Hg had a six-fold higher risk of developing primary open-angle glaucoma than individuals with diastolic ocular perfusion pressures >50 mm Hg.[26] Therefore, a patient's ocular ischemic risk factor profile should be reviewed preoperatively, and proper anesthetics that can reduce IOP and mitigate OPP decrease should be selected for hypotensive anesthesia.
There are few limitations in this study. First, half of the patients received nicardipine. Although the number of patients receiving nicardipine was not significantly different between the 2 groups, nicardipine may act as a confounding factor on the effects of anesthetics on IOP or OPP. However, Yatsuka et al demonstrated that nicardipine increases the blood flow velocity of the ophthalmic artery but has no significant effect on IOP.[28] Second, we did not measure ocular blood flow at the optic nerve head. A nonhuman primates study reported that optic nerve head blood flow is more susceptible to a decrease in OPP induced by low BP compared with that induced by an IOP increase.[29] Measurement of ocular blood flow may be helpful to elucidate the mechanism underlying the effects of hypotensive anesthesia on OPP and IOP. Finally, the duration of hypotensive anesthesia was relatively short in the present study. POVL mostly occurs after lengthy procedures in patients undergoing hypotensive anesthesia.[5] In the present study, the duration of hypotensive anesthesia was approximately 80 minutes; thus, the clinical effect of hypotensive anesthesia in patients undergoing prolonged procedures was not evaluated. Further studies are needed to clarify the effects of long procedures under hypotensive anesthesia on IOP and OPP.
5. Conclusion
Hypotensive anesthesia reduced IOP and OPP. Propofol TIVA more effectively reduced IOP and maintained higher OPP than desflurane anesthesia during hypotensive anesthesia. Although further studies of patients at risk for a decrease in OPP are needed, our results suggest that propofol TIVA may be more helpful in mitigating decreased OPP during hypotensive anesthesia than desflurane.
Author contributions
Conceptualization: Yong-Shin Kim, Kwon Hui Seo.
Data curation: Na-Re Han.
Formal analysis: Kwon Hui Seo.
Investigation: Yong-Shin Kim.
Methodology: Yong-Shin Kim.
Validation: Kwon Hui Seo.
Writing – original draft: Yong-Shin Kim.
Writing – review & editing: Kwon Hui Seo.
Kwon Hui Seo orcid: 0000-0003-4397-9207.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, BIS = bispectral index, ETCO2 = end-tidal carbon dioxide, IOP = intraocular pressure, MAP = mean arterial blood pressure, OPP = ocular perfusion pressure, PIP = peak inspiratory pressure, POVL = perioperative visual loss, TCI = target-controlled infusion, TIVA= total intravenous anesthesia.
The authors report no conflicts of interest in this work.
References
- [1].Lee LA. Perioperative visual loss and anesthetic management. Curr Opin Anaesthesiol 2013;26:375–81. [DOI] [PubMed] [Google Scholar]
- [2].Kelly DJ, Farrell SM. Physiology and role of intraocular pressure in contemporary anesthesia. Anesth Analg 2018;126:1551–62. [DOI] [PubMed] [Google Scholar]
- [3].Gillespie R, Shishani Y, Streit J, et al. The safety of controlled hypotension for shoulder arthroscopy in the beach-chair position. J Bone Joint Surg Am 2012;94:1284–90. [DOI] [PubMed] [Google Scholar]
- [4].Morrison DS, Schaefer RK, Friedman RL. The relationship between subacromial space pressure, blood pressure, and visual clarity during arthroscopic subacromial decompression. Arthroscopy 1995;11:557–60. [DOI] [PubMed] [Google Scholar]
- [5].Katz DM, Trobe JD, Cornblath WT, et al. Ischemic optic neuropathy after lumbar spine surgery. Arch Ophthalmol 1994;112:925–31. [DOI] [PubMed] [Google Scholar]
- [6].Jantzen JP, Hennes HJ, Rochels R, et al. Deliberate arterial hypotension does not reduce intraocular pressure in pigs. Anesthesiology 1992;77:536–40. [DOI] [PubMed] [Google Scholar]
- [7].Tani T, Nagaoka T, Nakabayashi S, et al. Autoregulation of retinal blood flow in response to decreased ocular perfusion pressure in cats: comparison of the effects of increased intraocular pressure and systemic hypotension. Invest Ophthalmol Vis Sci 2014;55:360–7. [DOI] [PubMed] [Google Scholar]
- [8].Hwang JW, Oh AY, Hwang DW, et al. Does intraocular pressure increase during laparoscopic surgeries? It depends on anesthetic drugs and the surgical position. Surg Laparosc Endosc Percutan Tech 2013;23:229–32. [DOI] [PubMed] [Google Scholar]
- [9].Yamada MH, Takazawa T, Iriuchijima N, et al. Changes in intraocular pressure during surgery in the lateral decubitus position under sevoflurane and propofol anesthesia. J Clin Monit Comput 2016;30:869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rodrigo C. Induced hypotension during anesthesia with special reference to orthognathic surgery. Anesth Prog 1995;42:41–58. [PMC free article] [PubMed] [Google Scholar]
- [11].Kitaba A, Martin DP, Gopalakrishnan S, et al. Perioperative visual loss after nonocular surgery. J Anesth 2013;27:919–26. [DOI] [PubMed] [Google Scholar]
- [12].Bulboaca A, Nicula C. Arterial hypotension-risk factor in nonarteritic anterior ischemic optic neuropathy. Oftalmologia 2002;53:52–5. [PubMed] [Google Scholar]
- [13].Boltz A, Schmidl D, Werkmeister RM, et al. Regulation of optic nerve head blood flow during combined changes in intraocular pressure and arterial blood pressure. J Cereb Blood Flow Metab 2013;33:1850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hwang JW, Jeon YT, Kim JH, et al. The effect of the lateral decubitus position on the intraocular pressure in anesthetized patients undergoing lung surgery. Acta Anaesthesiol Scand 2006;50:988–92. [DOI] [PubMed] [Google Scholar]
- [15].Bonomi L, Marchini G, Marraffa M, et al. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology 2000;107:1287–93. [DOI] [PubMed] [Google Scholar]
- [16].Blecha S, Harth M, Schlachetzki F, et al. Changes in intraocular pressure and optic nerve sheath diameter in patients undergoing robotic-assisted laparoscopic prostatectomy in steep 45 degrees Trendelenburg position. BMC Anesthesiol 2017;17:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kaygusuz K, Yildirim A, Kol IO, et al. Hypotensive anaesthesia with remifentanil combined with desflurane or isoflurane in tympanoplasty or endoscopic sinus surgery: a randomised, controlled trial. J Laryngol Otol 2008;122:691–5. [DOI] [PubMed] [Google Scholar]
- [18].Asuman AO, Baris A, Bilge K, et al. Changes in intraocular pressures during laparoscopy: a comparison of propofol total intravenous anesthesia to desflurane-thiopental anesthesia. Middle East J Anaesthesiol 2013;22:47–52. [PubMed] [Google Scholar]
- [19].Seo KH, Kim YS, Joo J, et al. Variation in intraocular pressure caused by repetitive positional changes during laparoscopic colorectal surgery: a prospective, randomized, controlled study comparing propofol and desflurane anesthesia. J Clin Monit Comput 2018;32:1101–9. [DOI] [PubMed] [Google Scholar]
- [20].Artru AA. Trabecular outflow facility and formation rate of aqueous humor during propofol, nitrous oxide, and halothane anesthesia in rabbits. Anesth Analg 1993;77:564–9. [DOI] [PubMed] [Google Scholar]
- [21].Mc-Graw-Hill Education, Butterworth JF, Mackey DC, Wasnick JD. Chap.36 Anesthesia for Ophthalmic surgery. In: Morgan and Mikhail's Clinical Anesthesiology. 2018;773-785. [Google Scholar]
- [22].Awad H, Santilli S, Ohr M, et al. The effects of steep trendelenburg positioning on intraocular pressure during robotic radical prostatectomy. Anesth analg 2009;109:473–8. [DOI] [PubMed] [Google Scholar]
- [23].Pournaras CJ, Rungger-Brandle E, Riva CE, et al. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res 2008;27:284–330. [DOI] [PubMed] [Google Scholar]
- [24].Yoo YC, Shin S, Choi EK, et al. Increase in intraocular pressure is less with propofol than with sevoflurane during laparoscopic surgery in the steep Trendelenburg position. Can J Anaesth 2014;61:322–9. [DOI] [PubMed] [Google Scholar]
- [25].Leske MC, Wu SY, Hennis A, et al. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology 2008;115:85–93. [DOI] [PubMed] [Google Scholar]
- [26].Tielsch JM, Katz J, Sommer A, et al. Hypertension, perfusion pressure, and primary open-angle glaucoma. A population-based assessment. Arch Ophthalmol 1995;113:216–21. [DOI] [PubMed] [Google Scholar]
- [27].Zheng Y, Wong TY, Mitchell P, et al. Distribution of ocular perfusion pressure and its relationship with open-angle glaucoma: the singapore malay eye study. Invest Ophthalmol Vis Sci 2010;51:3399–404. [DOI] [PubMed] [Google Scholar]
- [28].Yatsuka YI, Matsukubo S, Tsutsumi K, et al. Short-term effects of nicardipine and propranolol on ocular and systemic hemodynamics in healthy Japanese subjects. J Clin Pharmacol 1998;38:68–73. [DOI] [PubMed] [Google Scholar]
- [29].Wang L, Cull GA, Fortune B. Optic nerve head blood flow response to reduced ocular perfusion pressure by alteration of either the blood pressure or intraocular pressure. Curr Eye Res 2015;40:359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]


