Abstract
Background:
Thoracotomy is one of the most painful surgeries; therefore, video-assisted thoracoscopic surgery (VATS) was developed to reduce the surgical stress of thoracotomy. Although VATS results in reduced postoperative pain compared with thoracotomy, it is still painful. Serratus plane block (SPB) is a novel technique that provides lateral chest wall analgesia by blocking the lateral branch of the intercostal nerve.
Methods:
We conducted a prospective study in 50 patients, aged 20 to 75 years, undergoing three-port VATS lobectomy. Group G (n = 25) received conventional general anesthesia and Group S (n = 25) received SPB before induction of general anesthesia. In Group S, 20 ml of 0.375% ropivacaine was injected between the serratus anterior and latissimus dorsi muscles. During surgery, anesthesia was maintained by adjusting the propofol dose to maintain a bispectral index of 40 to 60 and the remifentanil dose to maintain blood pressure and heart rate within 70 to 130% of baseline.
Results:
Intraoperative remifentanil consumption was significantly lower in Group S compared to that in Group G (519.9 μg vs 1047.7 μg, P < .001). Moreover, emergence time was significantly shorter in Group S compared to Group G (10.8 minutes vs 14.9 minutes, P = .01). However, there were no significant differences in systolic blood pressure and heart rate (HR) between the groups at each time point. The doses of rescue drugs for the control of blood pressure and HR were not significantly different between the 2 groups.
Conclusions:
Ultrasound-guided SPB could be a safe and effective regional anesthesia technique for VATS
Keywords: anesthesia, opioid, pain management, perioperative care, vital signs
1. Introduction
Thoracotomy is known as one of the most painful surgeries.[1] Thus, video-assisted thoracoscopic surgery (VATS) has been developed to reduce surgical stress in terms of operation technique. Although it has reduced postoperative pain and complications compared with thoracotomy, VATS is still quite a painful operation.[2,3]
Opioids are essential part of balanced general and total intravenous anesthesia. However, reducing opioid consumption has become important because its side effects, such as delayed recovery from general anesthesia, opioid-induced hyperalgesia, sedation, nausea, and respiratory depression.[4–6] Several modalities to reduce intraoperative opioid use have been suggested, including medications and nerve blocks.[7–10]
Serratus plane block (SPB) is a novel technique which provide analgesic effect against lateral chest wall pain by blocking the lateral branch of the intercostal nerve.[11] It has been reported to be used for pain management, including the pain of rib fractures and herpes zoster, and postoperative pain.[12–14] The block has become a popular analgesic modality because it is easy to perform and relatively safe.
However, the effects of SPB during intraoperative period have not yet been studied. Therefore, we investigated the effects of SPB on intraoperative opioid consumption, emergence time, and hemodynamic stability in patients undergoing VATS.
2. Methods
The present study was approved by hospital ethics committee (KNUH 2018–06–006–002) and informed written consent for participating in the study was obtained from all patients. This study was registered at https://clinicaltrials.gov (NCT03718377).
Fifty patients with American Society of Anesthesiologists physical status class I or II, aged 20 to 75 years, undergoing three-port VATS lobectomy under general anesthesia were enrolled in this study. Exclusion criteria were: a history of allergic reaction to local anesthetics, coagulopathy, local infection at the injection site, and systemic infection.
Enrolled patients were divided into 2 groups in a 1:1 allocation ratio by computer-generated block randomization using block sizes of 2, 4, and 6. Opaque sealed envelopes were used to conceal the allocation sequence by unrelated assistant to this study. Group G (n = 25) received conventional general anesthesia care, and Group S (n = 25) received SPB in the regional-anesthesia unit outside operation room before induction of general anesthesia. Data were collected by the attending anesthesiologist, who was blind to the group assignment.
All SPBs were performed by a single practitioner. After sterilization of the injection site on the lateral chest wall, a high-frequency linear transducer was used to identify the fifth rib at the mid-axillar line. The serratus anterior and latissimus dorsi muscles were then easily identified above the rib. A 20-gauge Tuohy needle was introduced in the interfascial space between the serratus anterior and latissimus dorsi muscles using an in-plane technique. After confirming the interfascial space using hydrodissection with normal saline, 20 ml of 0.375% ropivacaine was injected. Loss of pinprick sensation was checked using a blunt needle for confirming success of the block.
No premedication was administered. Using standard monitoring protocols in the operating room, anesthesia was induced with propofol (2 mg/kg), remifentanil (0.3–1.0 μg/kg/minutes), and rocuronium (0.8 mg/kg). Intubation was performed using a double-lumen tube. A radial artery catheter was used for continuous blood pressure monitoring.
During surgery, anesthesia was maintained by adjusting propofol concentration for a bispectral index of 40 to 60 and remifentanil concentration for blood pressure and heart rate within 70% to 130% of the baselines. Rocuronium (0.2 mg/kg) was administered every 30 minutes. If abrupt hypotension, hypertension, bradycardia, or tachycardia developed beyond 70% to 130% of the baseline and was hard to control by adjustment of remifentanil and fluid therapy, appropriate medication including ephedrine, phenylephrine, nicardipine, esmolol, or glycopyrrolate was administered. At the completion of surgery, sugammadex 4 mg/kg was administered to all patients. Extubation was conducted after confirmation of bispectral index above 80, opening of the eyes, sufficient spontaneous respiration, and full recovery of motor functions.
The primary outcome of the present study was intraoperative remifentanil consumption. Secondary outcomes were emergence time, systolic blood pressure (SBP), heart rate (HR), and doses of rescue drugs used to control blood pressure and HR. The recording time of SBP and HR was set for 1 hour because mean surgery time (107.2 minutes) was shorter than 2 hours during a pilot study. The SBP and HR were recorded before induction, immediately after incision, and subsequently, at 5, 15, 30, and 60 minutes during the surgery.
The sample size was calculated based on a pilot study comparing remifentanil consumption in 8 patients: group receiving general anesthesia care, 869.8 ± 117.7 μg; and group receiving SPB before general anesthesia, 693.3 ± 245.0 μg. The present study required 22 patients in each group for alpha = 0.05 and statistical power = 0.9. Considering a 10% dropout rate, 25 patients would be required per group.
The IBM SPSS software (version 20; IBM Corp., Armonk, NY, USA) was used for statistical analysis. For continuous variables, after assessment of normality using Shapiro–Wilk test, Student's t test and Mann–Whitney U test were used to compare normally and non-normally distributed continuous data, respectively. Categorical variables were compared by using Fisher's exact test. P values < .05 were considered statistically significant.
3. Results
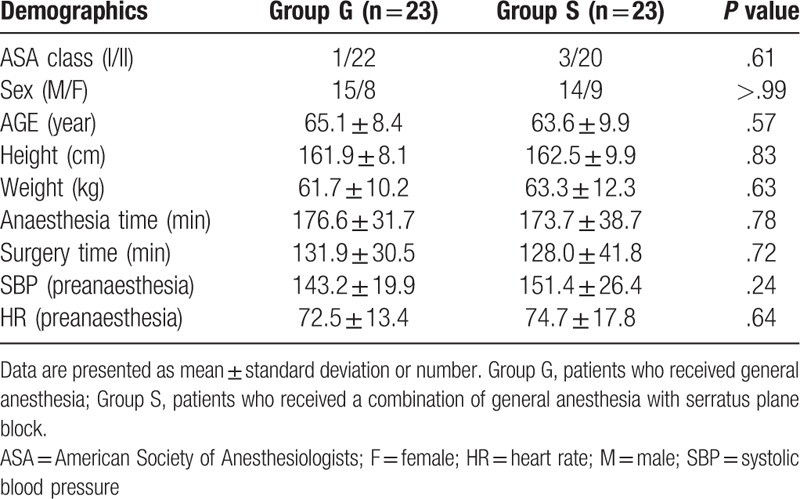
Fifty patients were included in the present trial. Among them, 4 patients were excluded because of conversion to open thoracotomy (2 from Group G and 1 from Group S) and closure without performing surgery due to unexpected pleural metastasis (1 from Group S). Therefore, 46 patients were analyzed (Fig. 1). There were no differences between the groups in terms of demographics, duration of anesthesia and surgery, and initial SBP and HR (Table 1).
Figure 1.
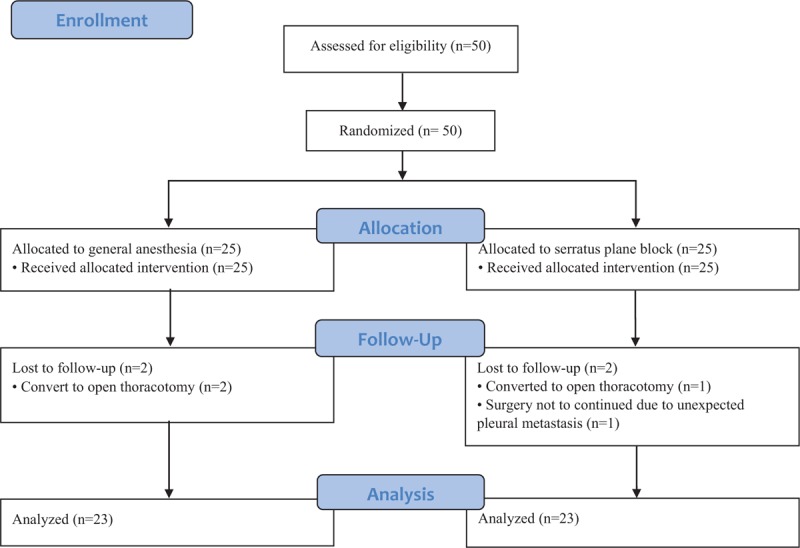
Flow diagram of the study design.
Table 1.
Demographics of the study patients.
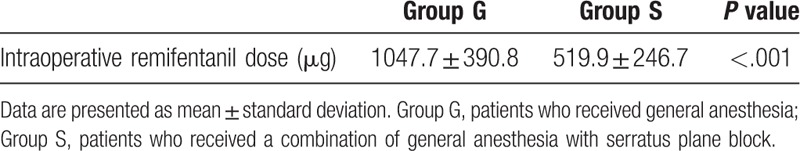
Intraoperative remifentanil consumption was significantly lower in Group S (519.9 ± 246.7 μg) compared to Group G (1047.7 ± 390.8 μg) (P < .001) (Table 2).
Table 2.
Intraoperative remifentanil consumption.
Emergence time was significantly shorter in Group S (10.8 ± 4.6 minutes) compared to Group G (14.9 ± 6.1 minutes) (P = .01) (Table 3). There were no significant differences in SBP and HR between the groups at each time point (Figs. 2 and 3). The doses of rescue drugs used for controlling blood pressure and HR were not significantly different between the 2 groups.
Table 3.
Emergence time.

Figure 2.
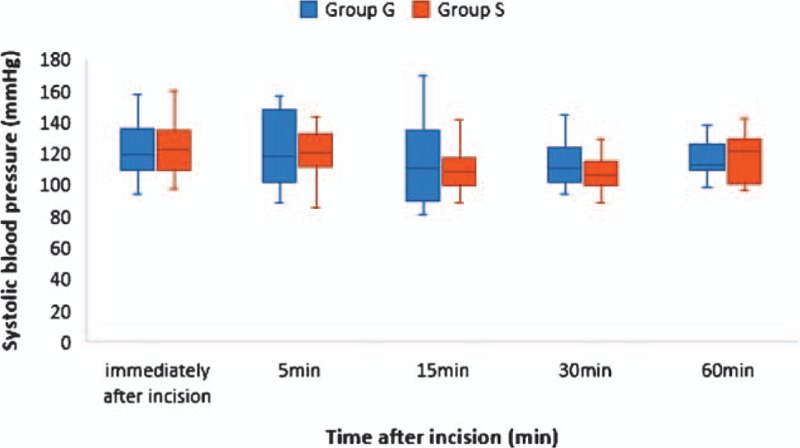
Time course of systolic blood pressure. The box-and-whisker plot shows the values immediately and 5, 15, 30, and 60 minutes after incision. The middle line in the box represents median value, the upper, and lower margins of the box represent the third and first quartiles, respectively, and the whiskers represent the maximum and minimum values. There was no significant difference between Group G and Group S. Group G, patients who received general anesthesia alone; Group S, patients who received a combination of general anesthesia and serratus plane block.
Figure 3.
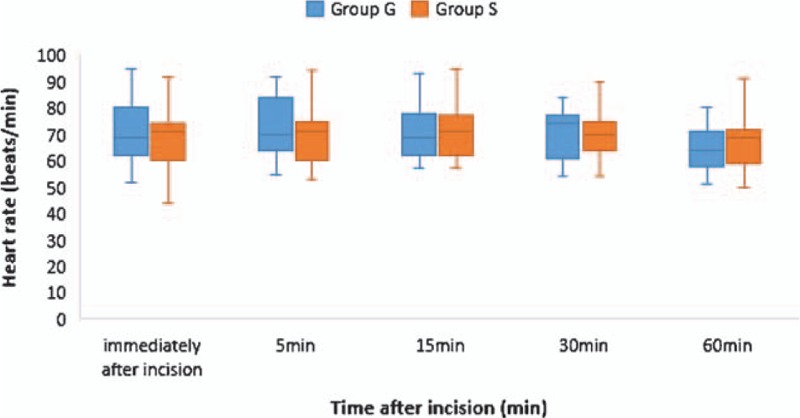
Time course of heart rate. The box-and-whisker plot shows the values immediately and 5, 15, 30, and 60 minutes after incision. The middle line in the box represents median value, the upper and lower margins of the box represent first and third quartiles, respectively, and the whiskers represent the minimum and maximum values. There was no significant difference between Group G and Group S. Group G, patients who received general anesthesia alone; Group S, patients who received a combination of general anesthesia and serratus plane block.
There were no block-related complications, such as local anesthetic toxicity, pneumothorax, bleeding, and infection.
4. Discussion
In the present study, we demonstrated that ultrasound-guided SPB, in combination with general anesthesia, significantly reduced intraoperative remifentanil consumption and shortened emergence time compared with general anesthesia alone in patients who underwent VATS lobectomy. Intraoperative hemodynamic parameters were maintained stably in both groups, and there were no block-related complications, such as local anesthetic toxicity, bleeding, and infection.
Over the past 2 decades, VATS has been widely used for lobectomy as an alternative to thoracotomy. Numerous studies have reported advantages of VATS including less postoperative pain and morbidity, shorter duration of hospitalization, and improved quality of life.[3,15–18] Although VATS reduces postopertive pain, it is still a painful surgery.[2,3] A recent randomized study reported that the proportion of patients with clinically relevant pain was significantly lower in the VATS group than the thoracotomy group during the first 24 hours; however, 38% of patients in the VATS group still had moderate-to-severe pain.[3]
While conventional VATS lobectomy has been performed with multiport approaches (our institution uses a standard three-port VATS technique) uniportal VATS lobectomy has recently emerged. Some studies have reported that the uniportal approach has several benefits compared with multiportal VATS, including shorter duration of hospitalization, reduction in the duration of postoperative drainage, and morbidity.[19,20] However, uniportal VATS has not been widely performed and its efficacy is still under debate. [21,22]
Preemptive analgesia is the notion that pain control is more effective if administered before the surgical incision.[23] The goal of preemptive analgesia is to prevent sensitization of the peripheral and central nervous systems, thereby decreasing perioperative opioid consumption and postoperative pain. Therefore, techniques of regional anesthesia techniques have been suggested as one of the means to achieve this goal by blocking the conduction of surgical stimuli.[24–27]
Epidural, paravertebral, and intercostal blocks are some of the conventional techniques of regional anesthesia for thoracic surgery. However, each of these blocks has some drawbacks. Epidural block has been considered the gold standard for managing thoracotomy pain.[23] However, it involves unnecessary bilateral block, including the sympathetic nervous system, and several complications, such as hypotension, epidural hematoma, abscess, and dural puncture.[28,29] Hence, some clinicians have suggested paravertebral block as the new gold standard because it has similar efficacy with regard to pain control; less hypotension; and lower risk of epidural hematoma and abscess, and dural puncture compared with epidural block.[1,30,31] However, it has also some drawbacks, such as pneumothorax and difficult technique despite the use of ultrasound.[32–34] Intercostal nerve block is easy to perform using ultrasound and does not contain the sympathetic block; however, it also has some shortcomings, such as pneumothorax, short duration, high plasma absorption of local anesthetics, and need to block multiple nerve levels.[32,34] Thus, regional anesthesia for thoracic surgery is a challenging task for many anesthesiologists despite its considerable advantages.
SPB was introduced as a novel regional block technique for unilateral thoracic wall pain.[11] In SPB, local anesthetics are injected into the serratus plane at the fifth rib level at the mid-axilla line under ultrasound guidance. The local anesthetics spread along the plane where the lateral branches of the intercostal nerves pass through, and thus, the lateral chest wall is blocked.
In the present study, intraoperative remifentanil consumption was significantly lower in patients administered SPB than in those who received general anesthesia alone. This could be attributed to the analgesic effect of the SPB, which probably acted as a substitute for opioids with regard to managing hemodynamic stability. The SPB blocks peripheral nerves, that is, the lateral cutaneous branches of the intercostal nerves.[11] Since nociceptive afferent transmission is blocked, the sympathetic response of increasing blood pressure and HR is reduced. Therefore, SPB could reduce the required dose of remifentanil without causing hemodynamic instability. This result suggests that SPB is effective in maintaining hemodynamic stability intraoperatively during VATS lobectomy.
In this study, the doses of rescue drugs were not statistically significantly different between the 2 groups. This study was designed to compare the required dose of opioids between SPB with general anesthesia and general anesthesia alone. Thus, hemodynamic changes during surgery were preferentially managed using remifentanil to reduce the effect of other variables. Rescue drugs for hemodynamic stability were administered only when the vital signs were not controlled using remifentanil and fluid administration.
In the present study, emergence time was significantly shorter in patients administered SPB than those administered general anesthesia alone. Side effects of opioids, such as opioid-induced hyperalgesia, sedation, nausea, and respiratory depression, could delay recovery from general anesthesia.[4–6,35] Uncontrolled pain is associated with insufficient spontaneous respiration and could delay extubation.[36] We speculate that the emergence time was short in patients administered SPB because there was adequate pain control and reduced opioid consumption.[37,38]
To our knowledge, this is the first study to describe the effects of SPB during the intraoperative period as regional anesthesia technique for VATS. However, our study has several limitations. First, our sample size was relatively small, although we calculated the minimal sample size based on a pilot study to achieve an adequate power. Second, patients in the study were from a single center. Therefore, the results may not be generalizable. Further, multicenter studies in various populations are needed to validate our findings. Finally, the effect of SPB on postoperative opioid consumption and pain were not evaluated. However, previous reports have already shown that SPB is effective in reducing postoperative opioid consumption and pain.[13,39,40]
In conclusion, we demonstrated that ultrasound-guided SPB decreases intraoperative opioid consumption in patients undergoing VATS. In addition, emergence time was reduced and hemodynamic stability was maintained without block-related complications. Therefore, ultrasound-guided SPB could be a safe and effective technique for achieving regional anesthesia technique for VATS.
Author contributions
Conceptualization: Saeyoung Kim.
Data curation: Jungwon Lee.
Formal analysis: Saeyoung Kim, Jungwon Lee.
Investigation: Jungwon Lee.
Methodology: Saeyoung Kim.
Supervision: Saeyoung Kim.
Writing – original draft: Jungwon Lee.
Writing – review & editing: Saeyoung Kim, Jungwon Lee.
Jungwon Lee orcid: 0000-0003-3510-315X.
Saeyoung Kim orcid: 0000-0003-1650-3385.
Footnotes
Abbreviations: HR = Heart rate, SBP = Systolic blood pressure, SPB = Serratus plane block, VATS = Video-assisted thoracic surgery.
The authors have no conflicts of interests to disclose.
References
- [1].Elmore B, Nguyen V, Blank R, et al. Pain management following thoracic surgery. Thorac Surg Clin 2015;25:393–409. [DOI] [PubMed] [Google Scholar]
- [2].Kaplowitz J, Papadakos PJ. Acute pain management for video-assisted thoracoscopic surgery: an update. J Cardiothorac Vasc Anesth 2012;26:312–21. [DOI] [PubMed] [Google Scholar]
- [3].Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836–44. [DOI] [PubMed] [Google Scholar]
- [4].Clarke H, Soneji N, Ko DT, et al. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ 2014;348:g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fletcher D, Martinez V. Opioid-induced hyperalgesia in patients after surgery: a systematic review and a meta-analysis. Br J Anaesth 2014;112:991–1004. [DOI] [PubMed] [Google Scholar]
- [6].Campbell WI. Analgesic side effects and minor surgery:which anlagesic for minor and day-case surgery? BJA 1990;64:617–20. [DOI] [PubMed] [Google Scholar]
- [7].Kumar K, Kirksey MA, Duong S, et al. A review of opioid-sparing modalities in perioperative pain management: methods to decrease opioid use postoperatively. Anesth Analg 2017;125:1749–60. [DOI] [PubMed] [Google Scholar]
- [8].Erdogan MA, Ozgul U, Uçar M, et al. Effect of transversus abdominis plane block in combination with general anesthesia on perioperative opioid consumption, hemodynamics, and recovery in living liver donors: the prospective, double-blinded, randomized study. Clini Transplant 2017;31:e12931. [DOI] [PubMed] [Google Scholar]
- [9].Trabulsi EJ, Patel J, Viscusi ER, et al. Preemptive multimodal pain regimen reduces opioid analgesia for patients undergoing robotic-assisted laparoscopic radical prostatectomy. Urology 2010;76:1122–4. [DOI] [PubMed] [Google Scholar]
- [10].Ziemann-Gimmel P, Goldfarb A, Koppman J, et al. Opioid-free total intravenous anaesthesia reduces postoperative nausea and vomiting in bariatric surgery beyond triple prophylaxis. Br J Anaesth 2014;112:906–11. [DOI] [PubMed] [Google Scholar]
- [11].Blanco R, Parras T, McDonnell JG, et al. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia 2013;68:1107–13. [DOI] [PubMed] [Google Scholar]
- [12].Kunhabdulla NP, Agarwal A, Gaur A, et al. Serratus anterior plane block for multiple rib fractures. Pain Physician 2014;17:E553–5. [PubMed] [Google Scholar]
- [13].Zocca JA, Chen GH, Puttanniah VG, et al. Ultrasound-guided serratus plane block for treatment of postmastectomy pain syndromes in breast cancer patients: a case series. Pain Pract 2017;17:141–6. [DOI] [PubMed] [Google Scholar]
- [14].Ahiskalioglu A, Alici HA, Yayik AM, et al. Ultrasound guided serratus plane block for management of acute thoracic herpes zoster. Anaesth Crit Care Pain Med 2017;36:323–4. [DOI] [PubMed] [Google Scholar]
- [15].Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362–5. [DOI] [PubMed] [Google Scholar]
- [16].Ang SC, Cao C, Yan TD, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small cell lung cancer: a meta-analysis of propensity score-matched patients. Interact Cardiovasc Thorac Surg 2012;16:244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11–8. [DOI] [PubMed] [Google Scholar]
- [18].Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366–78. [DOI] [PubMed] [Google Scholar]
- [19].Louis SG, Gibson WJ, King CL, et al. Uniportal video-assisted thoracoscopic surgery (VATS) technique is associated with decreased narcotic usage over traditional VATS lobectomy. J Vis Surg 2017;3:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg 2016;5:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sihoe AD. Reasons not to perform uniportal VATS lobectomy. J Thorac Dis 2016;8Suppl 3:S333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Berfield KS, Farjah F, Mulligan MS. Video assisted thoracoscopic lobectomy for lung cancer. Ann Thorac Surg 2019;107:603–9. [DOI] [PubMed] [Google Scholar]
- [23].Erturk E, Aydogdu Kaya F, Kutanis D, et al. The effectiveness of preemptive thoracic epidural analgesia in thoracic surgery. BioMed Res Int 2014;2014:673682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sentürk M, Özcan PE, Talu GK, et al. The effects of three different analgesia techniques on long-term postthoracotomy pain. Anesth Analg 2002;94:11–5. [DOI] [PubMed] [Google Scholar]
- [25].Katz J, Kavanagh BP, Sandler AN, et al. Preemptive analgesia. Clinical evidence of neuroplasticity contributing to postoperative pain. Anesthesiology 1992;77:439–46. [DOI] [PubMed] [Google Scholar]
- [26].Obata H, Saito S, Fujita N, et al. Epidural block with mepivacaine before surgery reduces long-term post-thoracotomy pain. Can J Anesth 1999;46:1127. [DOI] [PubMed] [Google Scholar]
- [27].Richardson J, Sabanathan S, Mearns A, et al. Efficacy of pre-emptive analgesia and continuous extrapleural intercostal nerve block on post-thoracotomy pain and pulmonary mechanics. J Cardiovasc Surg 1994;35:219–28. [PubMed] [Google Scholar]
- [28].Freise H, Van Aken H. Risks and benefits of thoracic epidural anaesthesia. Br J Anaesth 2011;107:859–68. [DOI] [PubMed] [Google Scholar]
- [29].Jeon DG, Song JG, Kim S-K, et al. Epidural hematoma after thoracic epidural analgesia in a patient treated with ketorolac, mefenamic acid, and naftazone: a case report. Korean J Anesthesiol 2014;66:240–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Baidya DK, Khanna P, Maitra S. Analgesic efficacy and safety of thoracic paravertebral and epidural analgesia for thoracic surgery: a systematic review and meta-analysis. Interac Cardiovasc Thorac Surg 2014;18:626–35. [DOI] [PubMed] [Google Scholar]
- [31].Peter Slinger MDF. Principles and Practice of Anesthesia for Thoracic Surgery. New York: Springer; 2011. [Google Scholar]
- [32].Gerner P. Postthoracotomy pain management problems. Anesthesiol Clin 2008;26:355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Krediet AC, Moayeri N, van Geffen G-J, et al. Different approaches to ultrasound-guided thoracic paravertebral block: an illustrated review. Anesthes J Am Soc Anesthesiol 2015;123:459–74. [DOI] [PubMed] [Google Scholar]
- [34].Yakşi E, Yakşi O. Current treatment options for post-thoracotomy pain syndrome: a review. Curr Thorac Surg 2017;2:103–10. [Google Scholar]
- [35].McGrath B, Chung F. Postoperative recovery and discharge. Anesthes Clin North Am 2003;21:367–86. [DOI] [PubMed] [Google Scholar]
- [36].Ortega R, Connor C, Rodriguez G, et al. Endotracheal extubation. N Engl J Med 2014;370:e4. [DOI] [PubMed] [Google Scholar]
- [37].Memis D, Inal MT, Kavalci G, et al. Intravenous paracetamol reduced the use of opioids, extubation time, and opioid-related adverse effects after major surgery in intensive care unit. J Crit Care 2010;25:458–62. [DOI] [PubMed] [Google Scholar]
- [38].Bonnet F, Marret E. Influence of anaesthetic and analgesic techniques on outcome after surgery. Br J Anaesth 2005;95:52–8. [DOI] [PubMed] [Google Scholar]
- [39].Kim D-H, Oh YJ, Lee JG, et al. Efficacy of ultrasound-guided serratus plane block on postoperative quality of recovery and analgesia after video-assisted thoracic surgery: a randomized, triple-blind, placebo-controlled study. Anesth Analg 2018;126:1353–61. [DOI] [PubMed] [Google Scholar]
- [40].Broseta A, Errando C, De Andrés J, et al. Serratus plane block: the regional analgesia technique for thoracoscopy? Anaesthesia 2015;70:1329–30. [DOI] [PubMed] [Google Scholar]


