Supplemental Digital Content is available in the text
Keywords: cognitive function, dexmedetomidine, general anesthesia, meta-analysis, postoperative cognitive dysfunction, randomized controlled trial
Abstract
Background:
Neuroprotective effects of dexmedetomidine are reported in preclinical and clinical studies but evidence regarding the postoperative neurocognitive function is still unclear. This study performed a meta-analysis on outcomes of studies which examined neurocognitive performance and inflammatory factors to investigate the effects of dexmedetomidine on postoperative cognitive dysfunction (POCD) and inflammation in patients after general anaesthesia.
Methods:
Literatures were searched in several electronic databases and studies were selected by following precise inclusion criteria. We searched PubMed, EMBASE, the Cochrane Library, China Academic Journals full-text database (CNKI), and Google Scholar to find randomized controlled trials (RCTs) of the influence of dexmedetomidine on POCD and inflammation in patients who had undergone general anaesthesia. Two researchers independently screened the literature, extracted data, and evaluated quality of methodology against inclusion and exclusion criteria. Meta-analyses of pooled ORs of POCD incidences and mean differences in neurocognitive assessment scores and inflammation levels were carried out and subgroup analyses were performed. Stata 12.0 was used to conduct our meta-analysis.
Results:
Twenty-six RCTs were included. Compared with controls, perioperative dexmedetomidine treatment significantly reduced the incidence of POCD (pooled ORs = 0.59, 95% confidence interval (CI) 0.45–2.95) and improved Mini-Mental State Examination (MMSE) score (standardized mean difference (SMD) = 1.74, 95% CI 0.43–3.05) on the first postoperative day. Furthermore, perioperative dexmedetomidine treatment significantly decreased IL-6 (SMD = −1.31, 95% CI −1.87–0.75, P < .001) and TNF-α (SMD = −2.14, 95% CI −3.14–1.14, P < .001) compared to saline/comparators treatment. In the stratified analysis by surgical type, age, type of control, and study region, the differences were also significant between dexmedetomidine- and saline-treated patients.
Conclusion:
Perioperative dexmedetomidine treatment is associated with significantly reduced incidence of POCD and inflammation and better neurocognitive function postoperatively in comparison with both saline controls and comparator anaesthetics.
1. Introduction
Postoperative cognitive dysfunction (POCD) which is characterized by short-term damage to cognitive abilities and involves memory, mood, confusion, and sleep disorders is one of the most common complications affecting the central nervous system after general anaesthesia, especially in elderly patients.[1] Its clinical manifestations include cognitive function disorder, personality change, and memory impairment.[2] POCD often leads to increased mortality, delayed recovery period, unexpected complications, and prolonged hospitalization, and leads to increased medical costs.[3] Even more importantly, this short-term dysfunction can develop into permanent cognitive impairment, such as Alzheimer disease (AD), can result in the loss of an independent life for patients, and causes serious physical and psychological harm post-operation.[4,5] The Mini Mental State Examination (MMSE) is commonly used to evaluate cognitive function postoperatively.[6] The incidence of POCD is approximately 10% to 12% and varies with clinical, demographic, and surgical variables, as well as the interval between surgery and assessment.[7] Consequently, POCD has become a crucial concern for aesthesiologists. However, the mechanism by which anaesthesia might provoke POCD and the influence of different modes of anaesthesia on the incidence of POCD remain unclear.[8]
Dexmedetomidine is a potent and highly selective transmembrane G protein coupled central α2-receptor agonist which decreases central nervous system sympathetic outflow and provides sedation and analgesia besides reducing blood pressure and heart rate without respiratory depression.[9] Sleep electroencephalographic studies have revealed that dexmedetomidine sedation resembles S2 sleep in humans.[10] Moreover, opioid-sparing effects of dexmedetomidine are well known. These properties make it a suitable option for sedation in the intensive care unit (ICU) and in perioperative conditions.
Preclinical studies with animal models have indicated that dexmedetomidine provide neuroprotective effects and improve cognitive function after surgery.[11,12] In clinical studies, many have reported beneficial effects of dexmedetomidine in reducing neurocognitive side effects while others could not observe the similar results. In order to gain refined evidence, the present study systematically reviews and meta-analyzes the outcomes of RCTs which utilized dexmedetomidine with general anaesthesia perioperatively.
2. Materials and methods
2.1. Inclusion and exclusion criteria
We identified RCTs of patients undergoing surgery under general anaesthesia. The intervention in the experimental group was a single or continuously-administered intravenous dose of dexmedetomidine before and during anaesthesia; the control groups received an intravenous injection of placebo or comparator. The main outcome indicators in eligible studies were the incidence of cognitive dysfunction on the first, third and seventh postoperative day, MMSE score, and inflammation levels (IL-6 and TNF-α) on the first postoperative day. We excluded studies that lacked specific exclusion data or with only figures and tables, duplicate publications, studies that used formulations of dexmedetomidine that are not widely available, studies with incomplete information or data, and articles for which we could not obtain the full text. Furthermore, stratified analyses by study region and type of control were also performed to obtain an accurate result.
2.2. Search strategy
The present study was designed based on Cochrane guidelines for systematic reviews of interventional studies. The present meta-analysis includes only human subjects. Therefore an extensive search was performed in the PubMed, Embase, the Cochrane Library, Google Scholar, and China Academic Journals full-text database (CNKI) up to April 2018 with the use of appropriate combination of keywords related to dexmedetomidine and POCD. An example of a search strategy that was used in PubMed is as follows: ((“dexmedetomidine” or “Dex”) AND (“postoperative cognitive dysfunction” OR “POCD”)).
2.3. Literature screening, data extraction, and quality assessment
Two authors (Y.W and K.LS) independently searched the international literature for relevant studies to include in this review. Authors attempted to search for both published and unpublished data in order to exclude publication bias. Unpublished data were not found and thus not included in this study. Titles and abstracts were reviewed, and the studies that were potentially relevant were each downloaded in full text form. Once the manuscripts were downloaded, they were independently reviewed by at least 2 co-authors. Any discrepancy as to whether or not to include a study was resolved by the senior author (L.Y). Data were recorded in a checklist designed on the basis of instructions in the PRISMA statement. The collected data included the name of first writer, publication year, country, sample size, age, surgical setting, surgical site, administrations for patients, incidences of POCD, MMSE, and inflammation levels. In some cases, the articles’ authors were contacted for supplementary data or further elucidation. Information from articles by 2 participants who worked independently was considered, and their findings and results were compared later. Disagreements about study eligibility were resolved by group discussion. The information and data were entered in a standard data extraction form and finally into Microsoft Excel.
2.4. Statistical analysis
We used Stata 12.0 to conduct the meta-analysis. For dichotomous data, odds ratios (ORs) with 95% confidence intervals (CIs) were used to express effect-size, while standardized mean difference (SMD) and 95% CIs were used for continuous data. First, we conducted a heterogeneity test (significance level α = 0.05) on included studies using the χ2 test, and judged the extent of heterogeneity in combination with the I2 test. A fixed effects model was used to conduct the meta-analysis if no heterogeneity (P > .05 and I2 < 50.0%) was found among the studies. If significant heterogeneity (P≤.05 or I2≥50.0%) was identified, we sought its source. For studies with significant clinical heterogeneity, subgroup or sensitivity analysis was employed, while for studies without distinct clinical heterogeneity, a random effects model was carefully applied for the meta-analysis. Bias or publication bias was evaluated as quality using funnel plots, Egger regressions, and the Begg–Mazumdar correlation test. Values of P < .05 were considered as valid for heterogeneity tests. For statistical analyses, Stata (version 12.0) software was used. All statistical tests were 2 sided.
3. Results
The meta-analysis and report of the results were based on the PRISMA checklist and the details are shown in Table S1. This report included all of the items in the PRISMA checklist.
3.1. Selected articles
In the initial electronic search, 1317 potential articles were identified. A manual search of the bibliographies and reference lists of these articles identified 42 additional articles. Altogether, 1359 articles were identified through the literature search. After the initial screening of abstracts and titles, 1295 articles were excluded based on the inclusion criteria and 64 articles remained for full text review. In a secondary screening and after a full-text review, another 36 articles were excluded; 14 studies were not related to POCD, 19 studies did not demonstrate the data on dexmedetomidine, 3 studies reported in meta-analysis. However, 2 studies did not contain clear data. Twenty six studies were selected for the final analysis after these exclusions.[13–38] A flowchart of the study screening and selection process was given in Figure 1.
Figure 1.
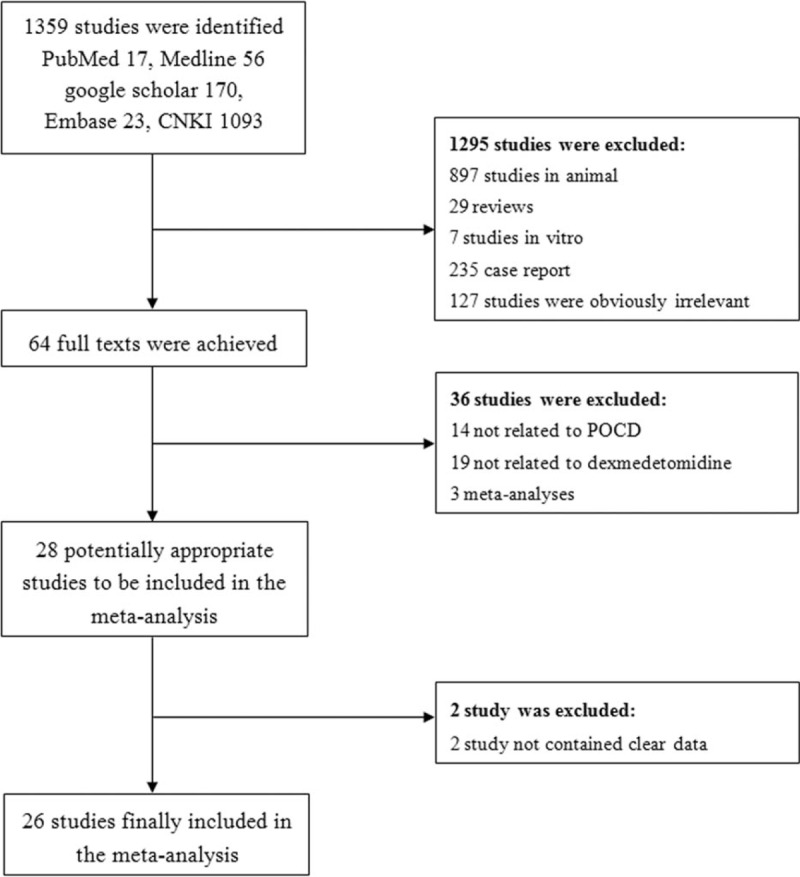
Flowchart of the literature search.
3.2. Characteristics of Included Studies
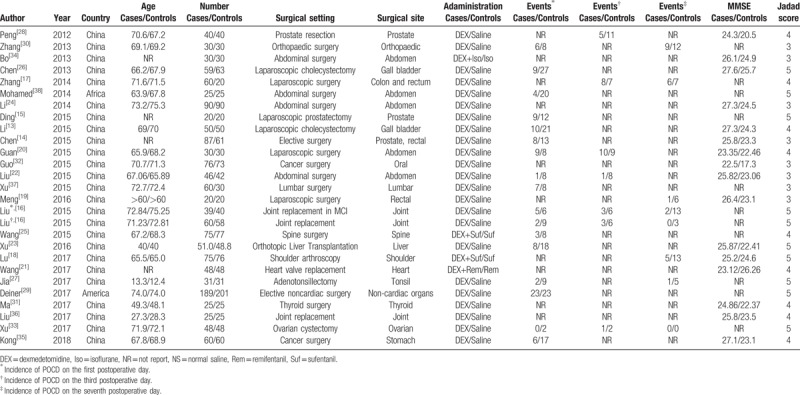
All included studies were RCTs. In total, these studies involved 1438 participants treated with dexmedetomidine and 580 cases treated with saline/comparator. The earliest study was published in 2012 and the latest in 2018, and all of the studies were published within the past 6 years. One study focused on the association of dexmedetomidine levels and POCD in young people (<18 years),[27] while 19 studies investigated the association in old patients (>60 years). Dosage of dexmedetomidine was in the range of 0.5 to 1.5 μg/kg body weight loading followed by continuous infusion at a rate of 0.15 to 0.80 μg/kg/h. Table 1 shows detailed information of each of the included studies, incorporating countries or districts, sample size, number of cases, surgical setting, surgical site, administrations for patients, incidence of POCD, and MMSE. Quality of the included studies was generally moderate to good.
Table 1.
Characteristics of the studies included in the meta-analysis.
3.3. Postoperative MMSE score
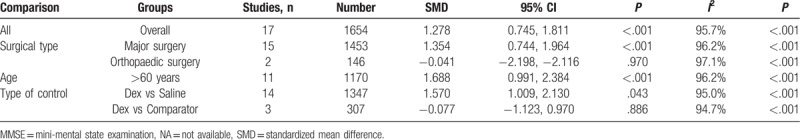
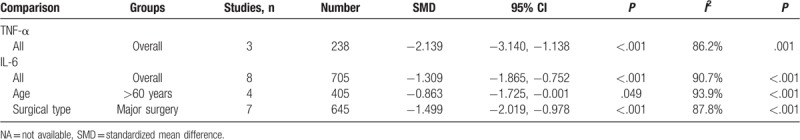
Seventeen RCTs including 1654 patients reported MMSE score on the first post-operative day. A random effects model was employed for meta-analysis, and the results suggested that MMSE was significantly higher on the first postoperative day in the dexmedetomidine group than the control group (SMD = 2.73, 95% CI 1.33–4.12, P < .001) (Fig. 2). In subgroup-analyses, submeta-analyses of patients over 60 years of age led to significant difference between dexmedetomidine group and control group (SMD = 1.69, 95% CI 0.99–2.38, P < .001). Submeta-analyses of patients undergo major surgery suggested significant difference between dexmedetomidine group and control group (SMD = 1.35, 95% CI 0.74–1.96, P < .001). However, no difference of MMSE score was observed between dexmedetomidine group and control group in patients with orthopaedic surgery. Distinctly, dexmedetomidine treatment was associated with better cognitive performance in comparison with saline treated controls. But submeta-analyses did not show significant difference in MMSE score between dexmedetomidine treatment and comparators treated group (Table 2).
Figure 2.
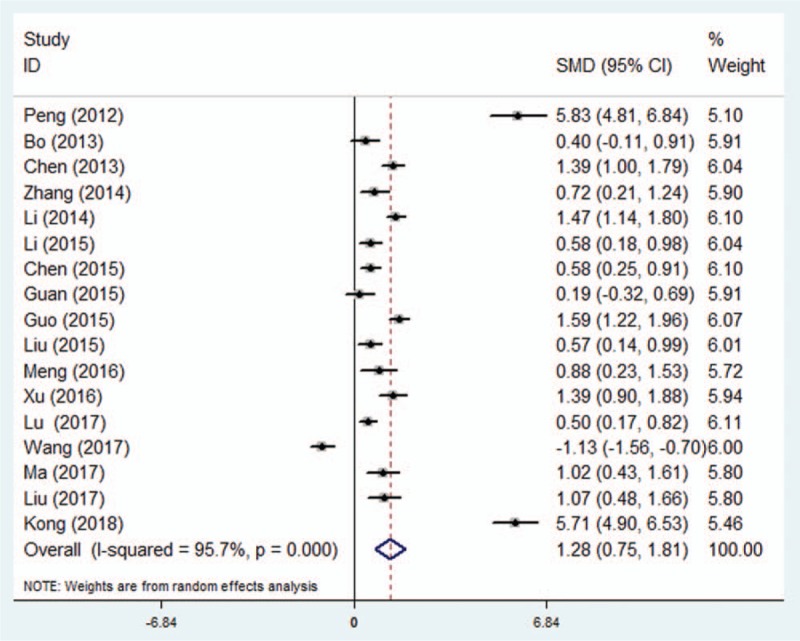
Effect of dexmedetomidine treatment on MMSE score in patients with general anaesthesia. Forest plot of mean difference analyzed in random-effect model at 95% confidence intervals (CI). MMSE = Mini-Mental State Examination.
Table 2.
Meta-analysis of the association between dexmedetomidine and MMSE score.
3.4. Incidence of POCD
Eighteen RCTs including 1935 patients reported the incidence of POCD on the first postoperative day. There was no substantial heterogeneity between the studies (P = .39, I2 = 5.4%) (Fig. 3a). A fixed effect model was therefore adopted for meta-analysis, and the results showed that the incidence of POCD in dexmedetomidine group was significantly lower than controls (OR = 0.49, 95% CI 0.39–0.63, P < .001). In subgroup-analyses by surgical type, age, type of control and study region, lower incidence of POCD in dexmedetomidine group compared to control group was reported distinctly (Table 3).
Figure 3.
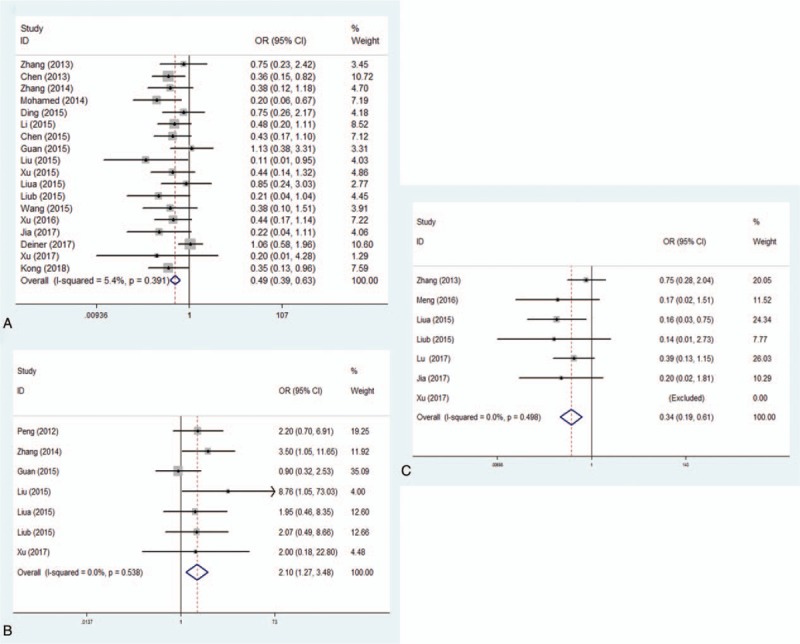
Prevention of POCD by dexmedetomidine in patients compared to controls on first, third, and seventh day. Forest plot of odds ratio, analyzed by Mantel–Haenszel statistics in the random-effect model. Meta-analysis of the relationship on first day (Fig. 3a), third day (Fig. 3b) and seventh day (Fig. 3c), respectively. POCD = postoperative cognitive dysfunction.
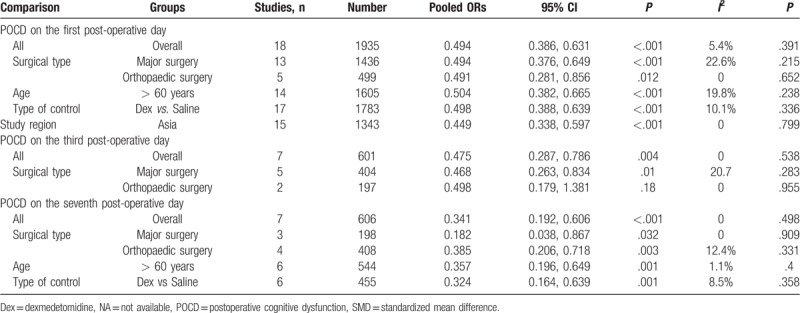
Table 3.
Meta-analysis of the association between dexmedetomidine and POCD.
Seven RCTs including 601 patients reported the incidence of POCD on the third postoperative day. There was no substantial heterogeneity between the studies (P = .54, I2 = 0%) (Fig. 3b). A fixed effect model was therefore adopted for meta-analysis, and the results suggested that the incidence of POCD in dexmedetomidine group was significantly lower than controls (OR = 0.48, 95% CI 0.29–0.79, P = .004). In subgroup-analyses by surgical type, significantly lower incidence of POCD in dexmedetomidine group compared to control group was showed in patients undergone major surgery, but similar results were not found in patients undergone orthopaedic surgery (Table 3).
Seven RCTs including 606 patients reported the incidence of POCD on the seventh postoperative day. There was no substantial heterogeneity between the studies (P = .50, I2 = 0%) (Fig. 3c). Again, a fixed effect model was adopted for meta-analysis, and the results showed that the incidence of POCD in dexmedetomidine group was significantly lower than controls (OR = 0.34, 95% CI 0.19–0.61, P < .001). In subgroup-analyses by surgical type, age, type of control, and study region, lower incidence of POCD in dexmedetomidine group was reported compared to control group (Table 3).
3.5. Inflammation levels
A total of 3 studies (n = 238 subjects, 12 patients and 206 controls) and 8 studies (n = 705 subjects, 367 patients and 338 controls) which measured the level of TNF-α and IL-6, respectively in controls and in patients before treatment, were included in our meta-analysis. Using the random-effects model, we found statistically significant effects of antidepressant treatment on TNF-α (SMD = −2.14, 95% CI −3.14–1.14, P < .001) and IL-6 levels (SMD = −1.31, 95% CI −1.87–0.75, P < .001) (Fig. 4). There was high heterogeneity in the studies that measured changes in TNF-α and IL-6 levels. In subgroup-analyses by surgical type and age, reduced inflammation levels in dexmedetomidine group compared to control group were reported distinctly (Table 4).
Figure 4.
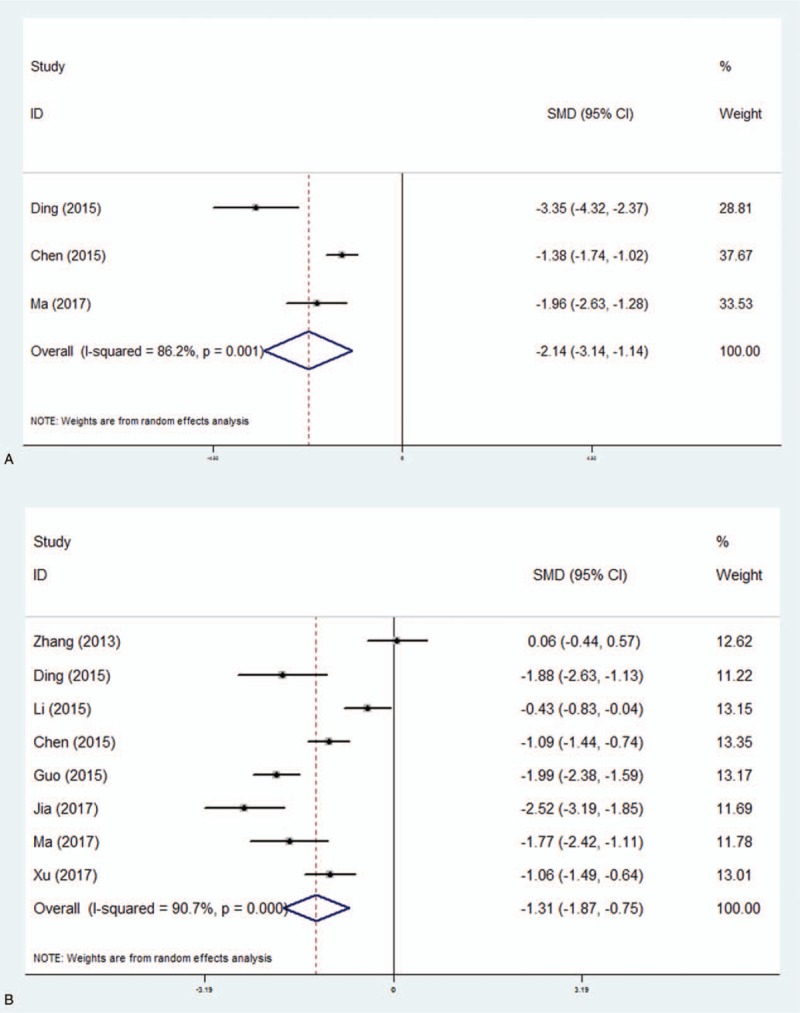
Reduction of inflammation by dexmedetomidine in patients compared to controls. Forest plot of odds ratio, analyzed by Mantel–Haenszel statistics in the random-effect model. Meta-analysis of the effect on TNF-α (Fig. 4a) and IL-6 (Fig. 4b), respectively.
Table 4.
Meta-analysis of the association between dexmedetomidine and inflammation.
3.6. Analysis of publication bias
The funnel plot was symmetrical, suggesting that there was no publication bias (Fig. 5). Further subgroup analyses, on the basis of the types of surgery and surgical sites, had little effect on the pooled results.
Figure 5.
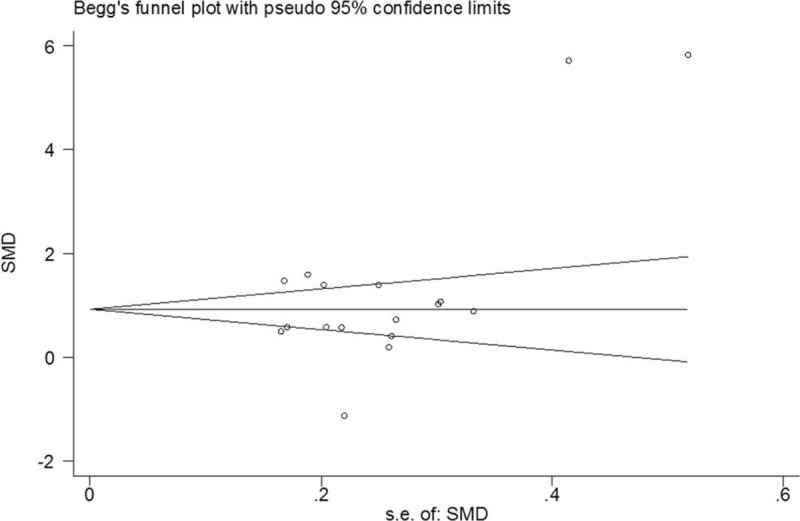
Funnel plot of studies that examined the association between dexmedetomidine treatment and MMSE score. MMSE = Mini-Mental State Examination.
4. Discussion
As the trend of aging population aggravates, more and more elderly patients are undergoing surgery, and are therefore exposed to the risk of POCD. The incidence of POCD in elderly patients is high, and there is increasing interest in identifying strategies to prevent POCD, given its consequences. The cause of POCD is still uncertain. Some investigators have reported that the main symptoms of POCD include decreased memory and degeneration of intelligence.[39,40] Mild POCD may prolong hospitalization and increase the costs of care along with changes in memory, intelligence, verbal ability, personality, and sociability which may lead to patients’ inability to engage with work and life activities.[41] Severe POCD may cause patients to lose the ability to speak, bring about personality change and AD, and impair their capacity for self-care. Although POCD is common, its pathophysiologic mechanism is poorly understood.
Our meta-analysis suggested that dexmedetomidine can significantly reduce the incidence of early POCD in patients and improve postoperative MMSE score. It acts at the locus coeruleus in the brain stem, which contains the highest concentration of α2 adrenoceptors.[42] By regulating neurotransmitter release, the locus coeruleus is critical for coordination of waking and sleeping, and is the source of noradrenergic pathways from the medulla oblongata to the spinal cord.[43,44] By acting on α2 adrenoceptors in the brain and spinal cord, dexmedetomidine inhibits neuronal discharge, thus inhibiting the effects of sympathetic nervous system activity.[27] An association between POCD and the inflammatory response has also been reported. Studies examined whether systemic inflammation in response to surgical trauma gives rise to subsequent memory impairment and hippocampal inflammation in a mouse model of orthopaedic surgery, and found that inflammation played a critical role in the pathogenesis of POCD and could be reversed by nonspecific inhibitor of inflammation.[45,46] The trauma of surgery stimulates the immune cascade and the release of inflammatory mediators, which may then provoke POCD. A number of animal experiments have shown that dexmedetomidine can reduce inflammation, which may also explain how it reduces the incidence of POCD.[47,48] An important finding of the current study is that dexmedetomidine treatment was associated with significantly better neurocognitive performance in patients over 60 years of age. Furthermore, meta-regression analyses could also find significant association between age and neurocognitive performance in dexmedetomidine treated patients. Previous studies have suggested that intraoperative dexmedetomidine is associated with significant reductions proinflammatory cytokines and significant stress alleviating (cortisol reducing) properties.[31,33] Both the effects can be more beneficial in the elderly. Whether these results suggest that dexmedetomidine is more effective in elderly patients is subject to further investigations with larger datasets.
Overall, our results are similar to meta-analyses of risk ratios carried out by Pasin et al and Zhou et al who found that dexmedetomidine treatment was associated with reduced incidence of delirium, agitation and confusion, and increased MMSE score in comparison with both saline treated or comparator anesthetic treated patients.[49,50] Pasin et al used studies with incidence (binary) data and Zhou et al used studies with MMSE score (continuous) data, whereas, we have meta-analyzed studies with both continuous and binary data. Neurocognitive assessment was carried out mainly with CAM-ICU in their eligible studies. In our meta-analysis, POCD incidences and MMSE scores were the most utilized indicators, besides inflammatory factors were also analyzed in current meta-analysis.
POCD is a complicated entity with subjective variability and numerous contributing factors. The best treatment for POCD is prevention, with early recognition and management of potential perioperative risk factors. Preventive strategies should involve close collaboration between surgeons, anesthesiologists, and geriatricians in order to reduce the total hospital stay by choosing the optimum surgical technique of short duration as a mean to decrease inflammatory response. The causes and current preventive strategies are grouped into patient-, surgical-, and anesthesia-related factors. Age, educational level, mental health, and comorbidities are contributory patient factors. The duration and complexity of surgery increase the incidence and severity of POCD. Research is in progress to investigate the biomarkers of neural injury in POCD. Biomarkers such as inflammatory cytokines, amyloid-β, and tau deposition in the cerebrospinal fluid have gained attention. Pharmacological strategies developed for symptomatic treatment of Alzheimer disease could be of importance in treating POCD. Cholinergic neurons are strongly impaired in Alzheimer disease, and 4 cholinesterase inhibitors (tacrine, rivastigmine, galantamine, and donepezil) have been licensed to slow down the breakdown of acetylcholine in the synaptic clefts, thereby improving cognitive processes. These agents are effective in reducing cognitive impairment and improving activities of daily living; however, their effectiveness in POCD is yet to be proved.
Our study had several limitations. The number of studies and the combined sample size were relatively small, and the doses and methods of administration of dexmedetomidine given to patients varied substantially. The included studies’ inclusion and exclusion criteria, body weight, anesthetic doses, test methods of inflammation levels, and consequently the characteristics of the patient cohorts, were also varied, which might have resulted in heterogeneity. Clinical heterogeneity of the sample population can also impact the results of a meta-analysis examining the dexmedetomidine's efficacy in overcoming post-anaesthesia neurocognitive dysfunction. Besides age, duration of delirium, severity and type of surgery, preoperative benzodiazepines use, trauma, duration of surgery, preoperative delirium or coma, dementia, hypertension, mechanical ventilation, and low education level are also recognized as risk factors for postoperative neurocognitive dysfunction. High statistical heterogeneity in some comparisons of the present study may underlie above-mentioned factors of clinical and methodological heterogeneity. Diagnosis of POCD was confirmed by performing baseline cognitive performance tests prior to surgery and comparing the cognitive status following surgery. Different studies had different definition of POCD, which might lead to high heterogeneity in current study.
In conclusion, dexmedetomidine can ameliorate the postoperative cognitive functions of patients who received surgical operations under general anesthesia, and effectively decrease the incidence of POCD and inflammation level without any obvious or severe adverse reaction. Thus, it can serve as a kind of adjuvant drug for general anesthesia in clinical practice.
Author contributions
Y.W and K.LS conducted the analysis, analyzed the data, and wrote the manuscript; W.RX, Z.XX, C.LR, and L.Y provided key information and critical comments.
Conceptualization: Wan Yang, Ying Liu, Lan-Ren Chen.
Data curation: Wan Yang.
Formal analysis: Wan Yang.
Methodology: Wan Yang, Ling-Suo Kong, Rui-Xiang Wang, Ying Liu.
Software: Ling-Suo Kong, Xing-Xing Zhu, Ying Liu.
Supervision: Xing-Xing Zhu.
Validation: Xing-Xing Zhu.
Writing – original draft: Wan Yang.
Writing – review & editing: Rui-Xiang Wang, Lan-Ren Chen.
Supplementary Material
Footnotes
Abbreviations: AD = Alzheimer disease, CI = confidence interval, MMSE = Mini-Mental State Examination, ORs = odds ratios, POCD = postoperative cognitive dysfunction, RCTs = randomized controlled trials, SMD = standardized mean difference.
This work was supported by the Science Research Fund of Anhui Province (090413265X) and the Foundation for Young Scholars of Anhui Provincial Cancer Hospital (2018YJQN006).
The authors declare that they have no competing interests.
Supplemental Digital Content is available for this article.
References
- [1].Hood R, Budd A, Sorond FA, et al. Peri-operative neurological complications. Anaesthesia 2018;73Suppl 1:67–75. [DOI] [PubMed] [Google Scholar]
- [2].Hermanides J, Qeva E, Preckel B, et al. Perioperative hyperglycaemia and neurocognitive outcome after surgery: a systematic review. Minerva Anestesiol 2018;84:1178–88. [DOI] [PubMed] [Google Scholar]
- [3].Wang B, Li S, Cao X, et al. Blood-brain barrier disruption leads to postoperative cognitive dysfunction. Curr Neurovasc Res 2017;14:359–67. [DOI] [PubMed] [Google Scholar]
- [4].Steinthorsdottir KJ, Kehlet H, Aasvang EK. Surgical stress response and the potential role of preoperative glucocorticoids on post-anesthesia care unit recovery. Minerva Anestesiol 2017;83:1324–31. [DOI] [PubMed] [Google Scholar]
- [5].Feinkohl I, Winterer G, Spies CD, et al. Cognitive reserve and the risk of postoperative cognitive dysfunction. Dtsch Arztebl Int 2017;114:110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vide S, Gambús PL. Tools to screen and measure cognitive impairment after surgery and anesthesia. Presse Med 2018;47(4 Pt 2):e65–72. [DOI] [PubMed] [Google Scholar]
- [7].Winterer G, Androsova G, Bender O, et al. Personalized risk prediction of postoperative cognitive impairment - rationale for the EU-funded BioCog project. Eur Psychiatry 2018;50:34–9. [DOI] [PubMed] [Google Scholar]
- [8].Benhamou D, Brouquet A. Postoperative cerebral dysfunction in the elderly: diagnosis and prophylaxis. J Visc Surg 2016;153(6S):S27–32. [DOI] [PubMed] [Google Scholar]
- [9].Shutes BL, Gee SW, Sargel CL, et al. Dexmedetomidine as single continuous sedative during noninvasive ventilation: typical usage, hemodynamic effects, and withdrawal. Pediatr Crit Care Med 2018;19:287–97. [DOI] [PubMed] [Google Scholar]
- [10].Ray T, Tobias JD. Dexmedetomidine for sedation during electroencephalographic analysis in children with autism, pervasive developmental disorders, and seizure disorders. J Clin Anesth 2008;20:364–8. [DOI] [PubMed] [Google Scholar]
- [11].Wu J, Vogel T, Gao X, et al. Neuroprotective effect of dexmedetomidine in a murine model of traumatic brain injury. Sci Rep 2018;8:4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hu J, Vacas S, Feng X, et al. Dexmedetomidine prevents cognitive decline by enhancing resolution of high mobility group box 1 protein-induced inflammation through a vagomimetic action in mice. Anesthesiology 2018;128:921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li Y, He R, Chen S, et al. Effect of dexmedetomidine on early postoperative cognitive dysfunction and peri-operative inflammation in elderly patients undergoing laparoscopic cholecystectomy. Exp Ther Med 2015;10:1635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen W, Liu B, Zhang F, et al. The effects of dexmedetomidine on post-operative cognitive dysfunction and inflammatory factors in senile patients. Int J Clin Exp Med 2015;8:4601–5. [PMC free article] [PubMed] [Google Scholar]
- [15].Ding L, Zhang H, Mi W, et al. Effects of dexmedetomidine on anesthesia recovery period and postoperative cognitive function of patients after robot-assisted laparoscopic radical cystectomy. Int J Clin Exp Med 2015;8:11388–95. [PMC free article] [PubMed] [Google Scholar]
- [16].Liu Y, Ma L, Gao M, et al. Dexmedetomidine reduces postoperative delirium after joint replacement in elderly patients with mild cognitive impairment. Aging Clin Exp Res 2016;28:729–36. [DOI] [PubMed] [Google Scholar]
- [17].Zhang Y, Xing Z, Xu Y, et al. Effects of different doses of dexmedetomidine on cognitive dysfunction in elderly patients early after laparoscopic surgery for colorectal cancer. Nan Fang Yi Ke Da Xue Xue Bao 2014;34:743–6. [PubMed] [Google Scholar]
- [18].Lu J, Chen G, Zhou H, et al. Effect of parecoxib sodium pretreatment combined with dexmedetomidine on early postoperative cognitive dysfunction in elderly patients after shoulder arthroscopy: a randomized double blinded controlled trial. J Clin Anesth 2017;41:30–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Meng Y, Zhang H, Yu H. Effect of dexmedetomidine on cerebral oxygen saturation and cognitive function in elder patients afterlaparoscopic surgery. J Tianjin Med Univ 2016;22:66–8. [Google Scholar]
- [20].Guan Y, Chen Y, Su S, et al. Influence of subanesthetic dose ketamine and loading dose dexmedetomidineon postoperative cognitive dysfunction in the elderly patients. China Med Herald 2015;12:61–3. [Google Scholar]
- [21].Wang Z, Chen Q, Guo H, et al. Effects of dexmedetomidine on H-FABP, CK-MB, cTnI levels, neurological function and near-term prognosis in patients undergoing heart valve replacement. Exp Ther Med 2017;14:5851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu Y, Wan X, Liu M, et al. Influence of dexmedetomidineon postoperative cognitive dysfunction in the elderly patients. Herald Med 2015;34:214–7. [Google Scholar]
- [23].Xu G, Li LL, Sun ZT, et al. Effects of dexmedetomidine on postoperative cognitive dysfunction and serum levels of b-amyloid and neuronal microtubule-associated protein in orthotopic liver transplantation patients. Ann Transplant 2016;21:508–15. [DOI] [PubMed] [Google Scholar]
- [24].Li Y, Dai H. Effect of dexmedetomidineon postoperative cognitive dysfunction in the elderly patients receiving abdominal operation in general anesthesia. J Clin Anesthesiol 2014;30:964–7. [Google Scholar]
- [25].Wang K, Li C, Shi J, et al. Effects of patient-controlled intravenous analgesia with dexmedetomidine and sufentanil on postoperative cognition in elderly patients after spine surgery. Zhonghua Yi Xue Za Zhi 2015;95:2437–41. [PubMed] [Google Scholar]
- [26].Chen J, Yan J, Han X. Dexmedetomidine may benefit cognitive function after laparoscopic cholecystectomy in elderly patients. Exp Ther Med 2013;5:489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jia ZM, Hao HN, Huang ML, et al. Influence of dexmedetomidine to cognitive function during recovery period for children with general anesthesia. Eur Rev Med Pharmacol Sci 2017;21:1106–11. [PubMed] [Google Scholar]
- [28].Peng Z, Zhang W, Chu Q. Effect of dexmedetomidineon early postoperative cognitive function after transurethrue resection of prostate in elderly patients. J Clin Anesthesiol 2013;29:945–7. [Google Scholar]
- [29].Deiner S, Luo X, Lin HM, et al. Intraoperative infusion of dexmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. JAMA Surg 2017;152:e171505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang X, Piao M, Wang Y, et al. Influence of sub-anesthetic dose of ketamine and dexmedetomidine on early postoperative cognitive function in elderly orthopedic patients under total intravenous anesthesia. J Jilin Univ 2013;39:133–7. [Google Scholar]
- [31].Ma XD, Li BP, Wang DL, et al. Postoperative benefits of dexmedetomidine combined with flurbiprofenaxetil after thyroid surgery. Exp Ther Med 2017;14:2148–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Guo Y, Sun L, Zhang J, et al. Preventive effects of low-dose dexmedetomidine on postoperative cognitive function and recovery quality in elderly oral cancer patients. Int J Clin Exp Med 2015;8:16183–90. [PMC free article] [PubMed] [Google Scholar]
- [33].Xu HY, Fu GH, Wu GS. Effect of dexmedetomidine-induced anesthesia on the postoperative cognitive function of elder patients after laparoscopic ovarian cystectomy. Saudi J Biol Sci 2017;24:1771–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bo P, Yan D, Shui C. Influence of dexmedetomidine on the postoperative cognitive function under surgery in elder patients. Chongqing Yixue 2013;42:2107–9. [Google Scholar]
- [35].Kong L, Zhang Y, Lu X. Effect of dexmedetomidineon perioperative cerebral oxygen metabolism and postoperative cognitive function in elderly patients undergoing stomach cancer surgery. China J Modern Med 2018;28:52–6. [Google Scholar]
- [36].Liu J, Wang J, Zhang R, et al. Effect of dexmedetomidineon postoperative cognitive function in patients with obstructive sleep apnea. J Pract Med 2017;33:1479–82. [Google Scholar]
- [37].Xu Y, Song Y, Xu F. Dexmedetomidine effects on hemodynamic changes and cognitive ability of elderly patients undergoing lumbar surgery. Chin J Tissue Eng Res 2015;19:1788–92. [Google Scholar]
- [38].Mohamed S, Shaaban A. The effect of dexmedetomidineon the incidence of postoperative cognitive dysfunction in elderly patients after prolonged abdominal surgery. Am J Transl Res 2017;9:5040–7.29218102 [Google Scholar]
- [39].Bilotta F, Qeva E, Matot I. Anesthesia and cognitive disorders: a systematic review of the clinical evidence. Expert Rev Neurother 2016;16:1311–20. [DOI] [PubMed] [Google Scholar]
- [40].Brioni JD, Varughese S, Ahmed R, et al. A clinical review of inhalation anesthesia with sevoflurane: from early research to emerging topics. J Anesth 2017;31:764–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Müller A, Lachmann G, Wolf A, et al. Peri- and postoperative cognitive and consecutive functional problems of elderly patients. Curr Opin Crit Care 2016;22:406–11. [DOI] [PubMed] [Google Scholar]
- [42].Cheon SY, Kim JM, Kam EH, et al. Cell-penetrating interactomic inhibition of nuclear factor-kappa B in a mouse model of postoperative cognitive dysfunction. Sci Rep 2017;7:13482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ma G, Chen C, Jiang H, et al. Ribonuclease attenuates hepatic ischemia reperfusion induced cognitive impairment through the inhibition of inflammatory cytokines in aged mice. Biomed Pharmacother 2017;90:62–8. [DOI] [PubMed] [Google Scholar]
- [44].Qian XL, Zhang W, Liu MZ, et al. Dexmedetomidine improves early postoperative cognitive dysfunction in aged mice. Eur J Pharmacol 2015;746:206–12. [DOI] [PubMed] [Google Scholar]
- [45].Zhu YJ, Peng K, Meng XW, et al. Attenuation of neuroinflammation by dexmedetomidine is associated with activation of a cholinergic anti-inflammatory pathway in a rat tibial fracture model. Brain Res 2016;1644:1–8. [DOI] [PubMed] [Google Scholar]
- [46].Su ZY, Ye Q, Liu XB, et al. Dexmedetomidine mitigates isoflurane-induced neurodegeneration in fetal rats during the second trimester of pregnancy. Neural Regen Res 2017;12:1329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Martin-Flores M, Sakai DM, Honkavaara J, et al. Hemodynamic effects of low-dose atipamezole in isoflurane-anesthetized cats receiving an infusion of dexmedetomidine. J Feline Med Surg 2017;1098612X17722265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Han XR, Wen X, Wang YJ, et al. MicroRNA-140-5p elevates cerebral protection of dexmedetomidine against hypoxic-ischaemic brain damage via the Wnt/β-catenin signalling pathway. J Cell Mol Med 2018;22:3167–82. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [49].Zhou C, Zhu Y, Liu Z, et al. Effect of dexmedetomidine on postoperative cognitive dysfunction in elderly patients after general anaesthesia: a meta-analysis. J Int Med Res 2016;44:1182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pasin L, Landoni G, Nardelli P, et al. Dexmedetomidine reduces the risk of delirium, agitation and confusion in critically Ill patients: a meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth 2014;28:1459–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


