Abstract
Although it is recommended to initiate postoperative chemotherapy for colon cancer within 8 weeks after surgery, the feasibility and impact of initiating chemotherapy before discharge after surgical resection has not been investigated.
Patients with stage II–IV colon cancer who received postoperative chemotherapy were dichotomized into early (chemotherapy initiation before discharge) and control (chemotherapy initiation after discharge) groups. A multivariable logistic regression model was used to determine factors associated with delayed chemotherapy, defined as more than 6 or 8 weeks after surgery.
From January 2004 to December 2012, of 729 patients with stage II–IV colon adenocarcinoma, 555 patients (76.1%) underwent postoperative chemotherapy. Of them, 181 (32.6%) patients were included in the early group. Time to initiation of chemotherapy was significantly shorter in the early group than in the control group (14.9 days vs 31.5 days, P < . 001). Multivariate analysis revealed that tumor stage and chemotherapy initiation strategy (odds ratio 8.4; 95% confidence interval, 1–66, P = .041) were independent predictors of delayed initiation of chemotherapy at more than 8 weeks. There was no difference in the completion rate of planned chemotherapy cycles between the 2 groups (P > .05).
The strategy of initiating chemotherapy before discharge after surgery is safe and feasible and might reduce the potential delay in chemotherapy initiation in patients with colon cancer.
Keywords: chemotherapy, colon cancer, survival
1. Introduction
Adjuvant chemotherapy after surgical resection could improve survival outcomes, especially in patients with stage III colon cancer. 5-fluorouracil/leucovorin with oxaliplatin has been recommended as a standard chemotherapy regimen.[1] Although still under debate, adjuvant chemotherapy is recommended for patients with stage II colon cancer with high-risk features such as T4, poorly differentiation, perforation or obstruction, and an insufficient number of harvested lymph nodes.
Deciding when to initiate chemotherapy is dependent on the patient's condition, and the Eastern Cooperative Oncology Group performance status scale is used as the main primary indicator. Various factors are associated with a delay in the initiation of chemotherapy.[2] It is well known that delaying the initiation of adjuvant chemotherapy until more than 8 weeks after surgery for colorectal cancer adversely affects overall survival (OS).[3,4] Because of these results, many clinicians have attempted to initiate postoperative chemotherapy as soon as possible. However, there have been concerns that patients who receive postoperative chemotherapy earlier may experience more side effects due to insufficient postoperative recovery.
In our center, we use 2 different strategies for initiating chemotherapy after colorectal cancer surgery. The first is a discussion between the physician and patient regarding the date of initiating chemotherapy at the first out-patient clinic visit after discharge. The second is commencement of chemotherapy by the surgical oncologist before discharge after surgery. However, to our knowledge, few studies have examined the efficacy or feasibility of initiating chemotherapy before discharge for patients with colon cancer.[5]
Therefore, the aim of this study was to determine the feasibility and impact of initiating chemotherapy earlier after colon cancer surgery.
2. Materials and methods
2.1. Patient selection
We identified 729 patients who were diagnosed with and underwent surgery for stage II, III, or IV colon cancer between January 2004 and December 2012. All patients included in this study were pathologically confirmed with colon cancer based on surgically resected specimens. Patients who did not receive postoperative chemotherapy (n = 174) were excluded. A total of 555 patients were selected for this analysis. This study was based on a retrospective review from a single institution and was approved by the Gangnam Severance Hospital's Institutional Review Board (3-2017-0256). Informed consent was waived for this retrospective study.
2.2. Surgery and chemotherapy initiation
Every patient diagnosed with colon cancer underwent surgical procedures including right hemicolectomy, transverse colectomy, left colectomy, anterior resection, subtotal colectomy, and total colectomy. Principles of surgical resection included complete mesocolic excision or total mesorectal excision. Detailed surgical procedures were described in our previous report.[6]
Chemotherapy regimens used in our hospital included oral 5FU, intravenous 5FU/LV, FOLFOX (folinic acid, 5-fluorouracil, oxaliplatin), and FOLFIRI (folinic acid, 5-fluorouracil, irinotecan). Some patients received 5FU-based chemotherapy because oxaliplatin was not covered by the national insurance reimbursement during the earlier periods of our study. Cetuximab or bevacizumab was relatively recently added, especially for stage IV patients. The chemotherapy agent for each patient was discussed between the physician and patient with consideration of patient recovery status and recommended guidelines for postoperative chemotherapy.
In our hospital, in most cases, chemotherapy after colorectal cancer resection was administered by the attending surgical oncologists. The decision of when to start chemotherapy was based on the surgical oncologist's assessment of fitness to start chemotherapy, and the ECOG performance status scale was used as a major tool. Although multidisciplinary team (MDT) meeting was available in our hospital, not all of the patients were discussed as the MDT meeting mainly focused on stage IV patients or patients with recurrent disease.
2.3. Definition of “early group” and “delayed chemotherapy”
During the study period, 2 different strategies were implemented for initiating chemotherapy after surgery. In the first, patients were encouraged to be discharged as soon as possible when they recovered sufficiently to consume a soft diet and the physicians assessed the patients’ recovery status and discussed the date of initiating chemotherapy in the out-patient clinic. In the second, the surgical oncologist initiated chemotherapy before discharge after surgery. In this study, patients were categorized into the early group when they received chemotherapy before discharge and the control group when they received chemotherapy after discharge (Fig. 1). Although the decision of chemotherapy initiating was dependent on the surgical oncologist's preference, the early chemotherapy initiation strategy was mainly driven by specific surgical oncologists in our hospital.
Figure 1.
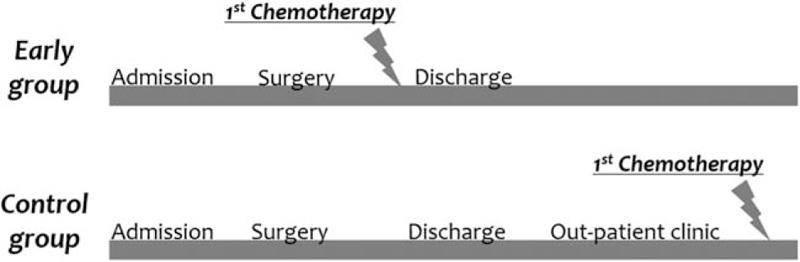
Definition of early chemotherapy initiation group (early group).
We calculated the interval from the date of surgery to the date of initiation of chemotherapy and defined “delayed chemotherapy” as patients for whom that interval was either >6 or >8 weeks. Univariate and multivariate analyses were performed to identify the factors that contributed to the difference in time to initiation of chemotherapy.
2.4. Measured outcomes
Demographic data including gender, age, body mass index (BMI), cancer stage, and American Society of Anesthesiologists (ASA) grade were collected. Clinicopathologic variables including tumor location (proximal colon, distal colon, synchronous), surgery time, estimated blood loss, and complications were assessed. Follow-up-related outcomes included resumption of soft diet (days), length of hospital stay (LOH) (days), time to initiation of adjuvant chemotherapy (days), and survival time. As most previous studies defined LOH as the time gap from surgery to the discharge date, the LOH in the “early group” comprised the routine recovery time after surgery and additional days for first-line postoperative chemotherapy, while the LOH in the “control group” included only recovery days.
Patients were also assessed separately as subgroups according to the presence of complications during the recovery period. Complete chemotherapy was defined as completing the planned cycles of chemotherapy. Incomplete chemotherapy was defined as discontinuing or changing the chemotherapy regimen for any reason before completing the planned schedule.
2.5. Statistical analysis
All statistical analyses were performed using IBM SPSS version 23.0 (IBM Corp, Armonk, NY) and R version 3.5.1 (R-project, Institute for Statistics and Mathematics). Differences in clinicopathologic features between the early and control groups were analyzed using the chi-square test or Fisher exact test for categorical variables and using the Student t test or Mann–Whitney U test for continuous variables. OS was defined as the time from the date of surgery to the date of death or last follow-up. The Kaplan–Meier method was used for survival analysis. OS was categorized by patients who received adjuvant chemotherapy within 6 and after 6 weeks and patients who received adjuvant chemotherapy before and after discharge. The log-rank test was used to compare survival outcomes between the 2 groups. Univariate and multivariate analyses were performed using logistic regression analysis. A P value <.05 was considered to indicate significance.
3. Results
3.1. Patient demographics
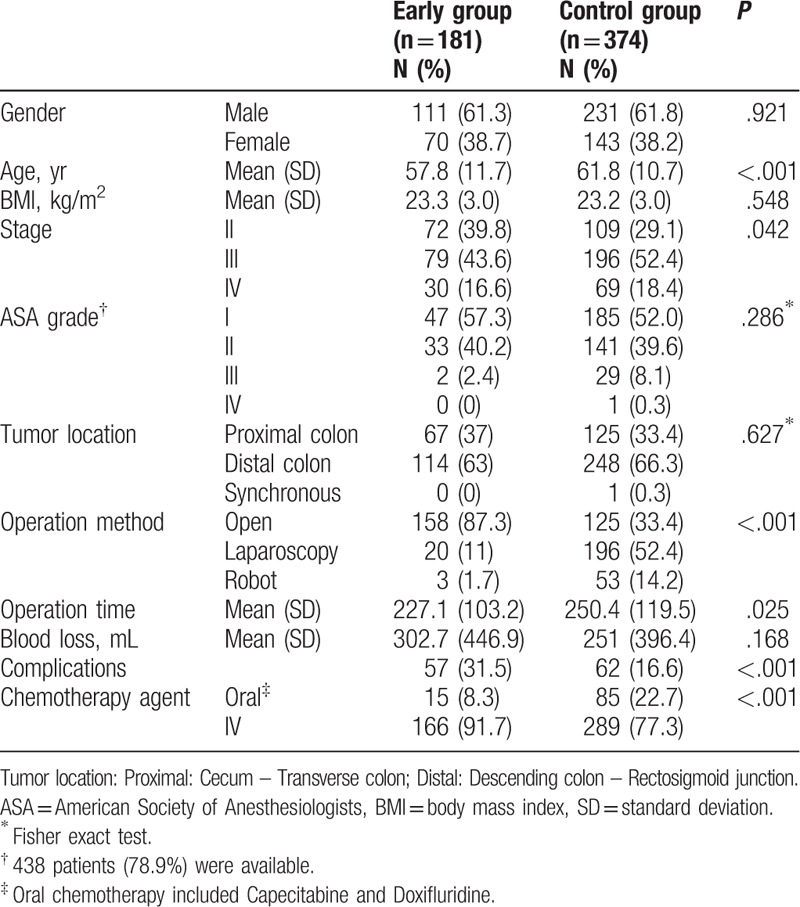
Of the 555 patients who received adjuvant chemotherapy, 181 (32.6%) initiated treatment before discharge (early group) and 374 (67.4%) initiated treatment after discharge (control group). Open, laparoscopic, and robotic surgeries were performed in 158 (87.3%), 20 (11%), and 3 (1.7%) of the 181 patients in the early group and in 125 (33.4%), 196 (52.4%), and 53 (14.2%) of the 374 patients in the control group, respectively.
Although there was no difference in gender, BMI, ASA grade, and tumor location between the 2 groups, there were some significant differences in group characteristics. The mean age was significantly lower in the early group than in the control group (57.8 years in the early group vs 61.8 years in the control group, P < .001). The proportion of patients with stage III was slightly higher in the control group (P = .042). The surgery time was shorter in the early group than in the control group (227 minutes in the early group vs 250 minutes in the control group, P = .025). The complication rate was higher in the early group than in the control group (31.5% in the early group vs 16.6% in the control group, P < .001). Oral chemotherapy was more frequently administered in the control group (8.3% in the early group vs 22.7% in the control group, P < .001) (Table 1).
Table 1.
Comparison of patient demographics and perioperative outcomes between the 2 groups.
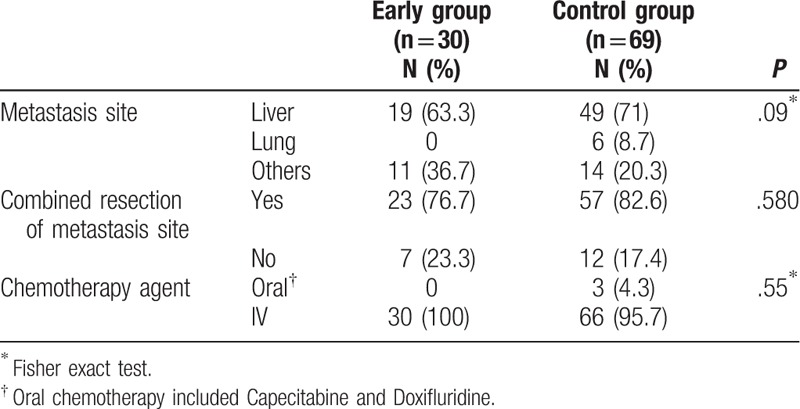
Detailed demographic characteristics of patients with stage IV cancer are described in Table 2. All patients who were staged as stage IV in our study underwent primary colon resection with or without combined resection of the metastatic site. The distribution of metastasis sites did not differ between the 2 subgroups, and the liver was the main metastatic site in both subgroups (63.3% in the early group vs 71% in the control group, P = .09). Combined resection of the metastatic site, so-called metastasectomy, was performed in 76.7% of patients with stage IV cancer in the early group and 82.6% of patients with stage IV cancer in the control group, which did not differ between the 2 subgroups (P = .580).
Table 2.
Demographics of Stage IV patients between the 2 groups (N = 99).
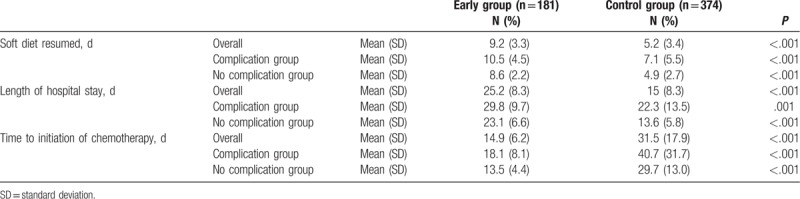
3.2. Comparison of time to initiation of chemotherapy
Time to resumption of a soft diet was significantly longer in the early group than in the control group (9.2 days vs 5.2 days, P < .001). This tendency was maintained when the analysis was repeated according to complication and no-complication groups. The LOH was significantly longer in the early group than in the control group (25.2 days vs 14 days, P < .001). The time to initiation of chemotherapy was significantly shorter in the early group than in the control group (14.9 days vs 31.5 days, P < .001). In subgroup analysis, time to initiation of chemotherapy was significantly shorter in the early group than in the control group in both the complication and no-complication groups (Table 3).
Table 3.
Postoperative recovery outcomes and time to initiation of adjuvant chemotherapy according to presence of complication between the 2 groups.
3.3. Factors associated with delayed chemotherapy
Factors associated with delayed chemotherapy of more than 6 or 8 weeks are shown in Table 4. In univariate analysis, stage and chemotherapy initiation strategy were identified as significant predictors of delayed chemotherapy for more than 8 weeks. Multivariate analysis revealed that stage and chemotherapy initiation strategy (odds ratio [OR], 8.4; 95% confidence interval [CI], 1–66, P = .041) were independent predictors of delayed initiation of chemotherapy for more than 8 weeks.
Table 4.
Factors associated with delayed chemotherapy according to the delayed time either 6 wk or 8 wk.
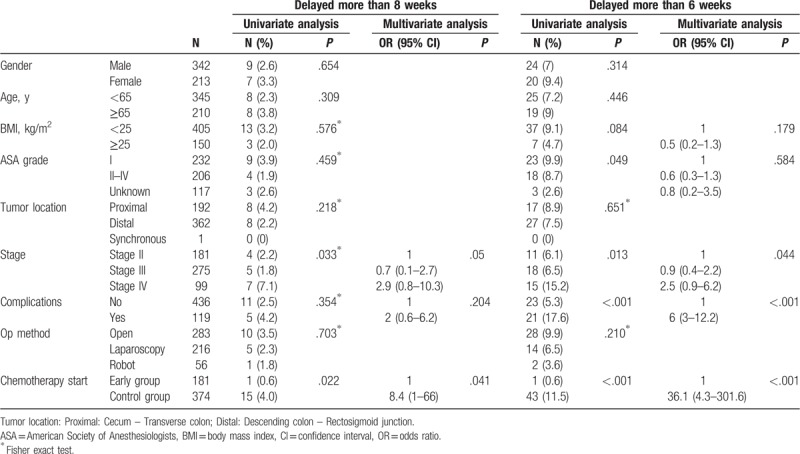
ASA grade, stage, complications, and chemotherapy initiation strategy were associated with delayed chemotherapy for more than 6 weeks in univariate analysis. Stage, postoperative complications, and chemotherapy initiation strategy (OR, 36.1; 95% CI, 4.3–301.6, P < .001) were significantly associated with delayed initiation of chemotherapy for more than 6 weeks (Table 4).
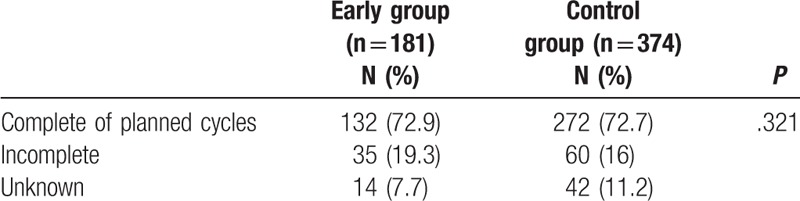
3.4. Comparison of completion rate of planned chemotherapy cycles between the 2 groups
There was no difference in the rate of completion of the planned cycles between the 2 groups (72.9% in the early group vs 72.7% in the control group, P = .321) (Table 5).
Table 5.
Comparison of completion rate of planned chemotherapy cycles between the 2 groups.
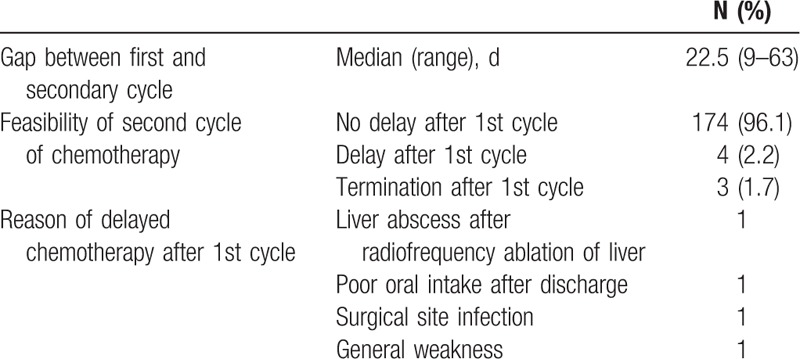
3.5. Characteristics of secondary cycle chemotherapy in the early group
Most of the second cycles of chemotherapy in the early group were performed at the scheduled time (96.1%). There were 4 delays (2.2%) due to liver abscess development after radiofrequency ablation, poor oral intake, general weakness, and surgical site infection detected after discharge. Three patients (1.7%) did not undergo further chemotherapy because of patient refusal (Table 6).
Table 6.
Characteristics of secondary cycle chemotherapy in the early group.
3.6. OS according to chemotherapy initiation time or chemotherapy initiation strategy
Figure 2 presents the Kaplan–Meier curve for OS in patients with stage II–IV colon cancer who received adjuvant chemotherapy within and after 6 weeks following surgery. Among patients in all stages, the 5-year OS was significantly better in patients who initiated adjuvant chemotherapy within 6 weeks than in patients who delayed chemotherapy until after 6 weeks (5-year OS: 78.8% vs 60.9%, P < .001) (Fig. 2A). In subgroup analysis, patients with delayed chemotherapy for >6 weeks had a much worse prognosis of stage III colon cancer (82.8% vs 60%, P = .001) (Fig. 2C), although there was no difference in OS between the stage II and IV groups (Fig. 2B and D). Figure 3 shows the OS in patients who received adjuvant chemotherapy before and after discharge. Early initiation of postoperative chemotherapy was not associated with worse OS for all stages.
Figure 2.
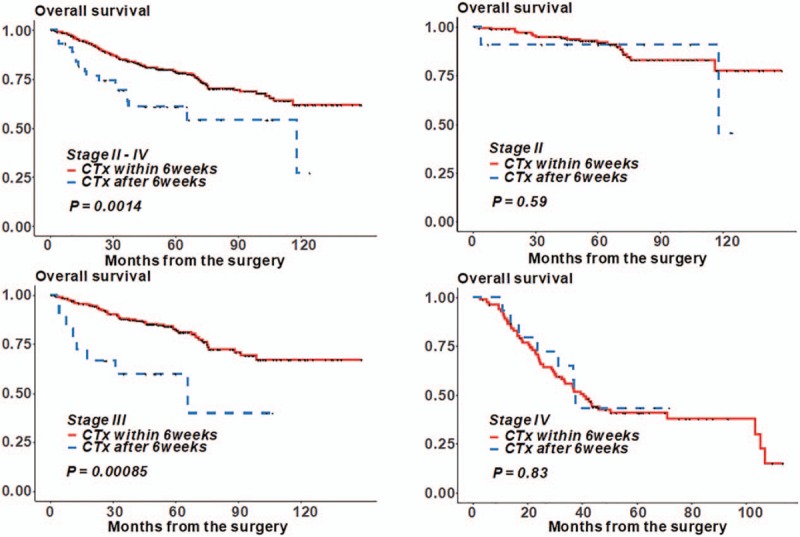
Overall survival according to the time interval from surgery to the initiation of chemotherapy.
Figure 3.
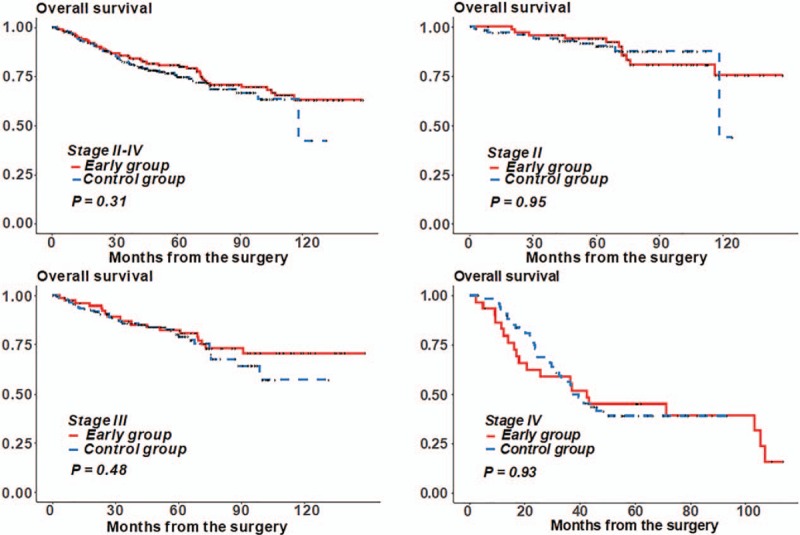
Overall survival according to the early group and the control group.
4. Discussion
This study demonstrated that early chemotherapy initiation before discharge could be performed safely without delaying the second cycle of chemotherapy or deteriorating the completion of planned chemotherapy cycles in patients with colon cancer who have undergone surgical resection. Early chemotherapy initiation before discharge may be one possible option to reduce the potential delay in chemotherapy initiation in patients with colon cancer, although this should be confirmed in a further prospective study.
A recent meta-analysis demonstrated that increased age, single marital status, low socioeconomic status, poor comorbidity status, low tumor grade, prolonged length of stay, and readmission are associated with chemotherapy delays in colorectal cancer.[2] Laparoscopy or robotic surgery compared with the open approach is known to be a predictor of earlier initiation of adjuvant chemotherapy, and this is regarded as one of the benefits of minimally invasive surgery.[6,7] In addition, systematic delay within or out of the hospital might be one of the most significant factors for chemotherapy delay. It has been reported that delayed time to adjuvant chemotherapy is more process-related than patient-related, with significant prolongation of intervals such as referral and consultation.[8] Bayraktar et al also reported that 30% of the delays in chemotherapy after surgical resection of colon cancer are caused by the hospital system, such as referrals or insurance identification.[9] These factors reflect the different medical environments in each country. Thus, to reduce the time interval between surgery and chemotherapy it is better to focus on correctable causes such as reducing the readmission rate or length of stay, performing laparoscopic surgeries, and eliminating the time gap caused by systematic barriers. In our hospital, surgical oncologists are mainly responsible for deciding chemotherapy initiation for patients with colorectal cancer. Although the benefit of surgical oncologist-driven chemotherapy has not been investigated, the surgeon can predict patient recovery in more detail because they already know the patients’ condition with respect to fitness to initiate chemotherapy. In this regard, the surgical oncologist might be more comfortable in deciding the initiation of early chemotherapy. Initiation of chemotherapy before discharge could potentially eliminate the time gap caused by systematic delays.
Previous studies have demonstrated that postoperative complications significantly delay the time to initiation of adjuvant chemotherapy in patients with colon cancer.[9,10] Longer hospital stay and delayed consumption of adequate nutrition in patients with complications might cause delayed initiation of chemotherapy. However, in our analysis, the presence of complications did not affect the rate of delayed chemotherapy >8 weeks (2.5% in the no-complication group vs 4.2% in the complication group, P = .354). In contrast, when we defined delayed chemotherapy as commencement of chemotherapy later than 6 weeks, postoperative complications were an independent predictor of delayed chemotherapy. One reason for this discordance might be a type II error related to the insufficient sample size of patients with delayed chemotherapy >8 weeks.
Most studies accept the definition of delayed chemotherapy as >8 weeks (56 days). The reported proportion of patients who receive delayed chemotherapy range from 26% to 54%.[8,11–13] In contrast, our study showed a significantly lower proportion of patients in the delayed chemotherapy group (2.9% in the >8 weeks and 7.9% for >6 weeks). Interestingly, another study conducted in South Korea demonstrated a low proportion of patients receiving delayed chemotherapy for colorectal cancer.[5] It remains to be determined whether the difference in chemotherapy delay is derived from ethnic differences or different medical systems.
This study has several limitations. An important limitation of our study was the potential selection bias, as this study was conducted in a single center. Because of the retrospective study design, definite inclusion criteria of early chemotherapy initiation could not be confirmed, and it is difficult to describe how many patients were not able to receive the intended early chemotherapy, although earlier chemotherapy was the principal strategy of specific surgical oncologists in our hospital. In our study, different chemotherapeutic agents were used for patients with stage II–IV cancer. Surgery type and resection extent were diverse between the groups especially for patients with stage IV cancer. Nevertheless, our patients with stage IV cancer showed similar metastasis distributions, and all of them underwent primary colon resection with similar rates of metastasectomy, which demonstrates that the surgical burden imposed on patients with stage IV cancer might be comparable between the 2 groups. Another concern of early chemotherapy initiation is increasing hospital stay. Longer hospital stays may increase the incidence of hospital-acquired infections, although this concern could not be evaluated using our retrospective data. In our nation's specific socioeconomic status, chemotherapy maintenance during the hospital stay after surgery was not strongly prohibited because of the relatively low hospital stay charge. Although we believe that surgical oncologist-driven chemotherapy might reduce the interval between surgeon referral and medical oncologist consultation, the medical oncologist is still in charge of postoperative chemotherapy in most other academic hospitals in South Korea. This paradigm of “early chemotherapy strategy” might not be applicable for patients in different medical systems. Besides, early chemotherapy initiation strategy was mainly driven by specific surgeons at a single institution that an interdisciplinary effort by medical and surgical oncologists to plan an early initiation of chemotherapy was not maintained. This significantly erodes the generalizability. These limitations should be evaluated in a prospective study design.
In conclusion, the strategy of initiating chemotherapy for colon cancer before discharge after surgery is feasible. Implementing an early chemotherapy initiation strategy might even prevent unnecessary delays that can in turn decrease OS. However, our study failed to demonstrate that an early initiation strategy could translate to better clinical outcomes. One of the main reasons might be the relatively lower rate of chemotherapy delays in our group compared to other studies; thus, the positive impact of this strategy might be neutralized. It should be further validated whether this strategy has any benefits if used in regions wherein delays of chemotherapy are more common. Our study may facilitate further prospective studies of in-hospital early chemotherapy initiation coordinated between surgeons and medical oncologists and could be used as an initial experience of early chemotherapy strategy before launching a randomized controlled trial.
Acknowledgment
We would like to thank Editage (https://www.editage.co.kr) for their English language editing service.
Author contributions
Conceptualization: Jeonghyun Kang.
Data curation: Jeonghyun Kang, Su-Weon Chong, Eun Jung Park, Seung Hyuk Baik, Kang Young Lee.
Formal analysis: Jeonghyun Kang, Su-Weon Chong.
Methodology: Jeonghyun Kang.
Supervision: Eun Jung Park, Seung Hyuk Baik, Kang Young Lee.
Validation: Jeonghyun Kang.
Writing – original draft: Jeonghyun Kang, Su-Weon Chong.
Writing – review and editing: Jeonghyun Kang.
Jeonghyun Kang orcid: 0000-0001-7311-6053.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, BMI = body mass index, CI = confidence interval, LOH = length of hospital stay, OR = odds ratio, OS = overall survival.
JK and S-WC contributed equally to this work as co-first authors.
The authors have no conflicts of interest to disclose.
References
- [1].Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343–51. [DOI] [PubMed] [Google Scholar]
- [2].Malietzis G, Mughal A, Currie AC, et al. Factors implicated for delay of adjuvant chemotherapy in colorectal cancer: a meta-analysis of observational studies. Ann Surg Oncol 2015;22:3793–802. [DOI] [PubMed] [Google Scholar]
- [3].Des Guetz G, Nicolas P, Perret GY, et al. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer 2010;46:1049–55. [DOI] [PubMed] [Google Scholar]
- [4].Biagi JJ, Raphael MJ, Mackillop WJ, et al. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA 2011;305:2335–42. [DOI] [PubMed] [Google Scholar]
- [5].Kang KM, Hong KS, Noh GT, et al. Optimal time of initiating adjuvant chemotherapy after curative surgery in colorectal cancer patients. Ann Coloproctol 2013;29:150–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jung YB, Kang J, Park EJ, et al. Time to initiation of adjuvant chemotherapy in colon cancer: comparison of open, laparoscopic, and robotic surgery. J Laparoendosc Adv Surg Tech A 2016;26:799–805. [DOI] [PubMed] [Google Scholar]
- [7].Poylin V, Curran T, Lee E, et al. Laparoscopic colectomy decreases the time to administration of chemotherapy compared with open colectomy. Ann Surg Oncol 2014;21:3587–91. [DOI] [PubMed] [Google Scholar]
- [8].Chan A, Woods R, Kennecke H, et al. Factors associated with delayed time to adjuvant chemotherapy in stage iii colon cancer. Curr Oncol 2014;21:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bayraktar UD, Chen E, Bayraktar S, et al. Does delay of adjuvant chemotherapy impact survival in patients with resected stage II and III colon adenocarcinoma? Cancer 2011;117:2364–70. [DOI] [PubMed] [Google Scholar]
- [10].Wasserman DW, Boulos M, Hopman WM, et al. Reasons for delay in time to initiation of adjuvant chemotherapy for colon cancer. J Oncol Pract 2015;11:e28–35. [DOI] [PubMed] [Google Scholar]
- [11].Lima IS, Yasui Y, Scarfe A, et al. Association between receipt and timing of adjuvant chemotherapy and survival for patients with stage III colon cancer in Alberta, Canada. Cancer 2011;117:3833–40. [DOI] [PubMed] [Google Scholar]
- [12].Czaykowski PM, Gill S, Kennecke HF, et al. Adjuvant chemotherapy for stage III colon cancer: does timing matter? Dis Colon Rectum 2011;54:1082–9. [DOI] [PubMed] [Google Scholar]
- [13].Ahmed S, Ahmad I, Zhu T, et al. Early discontinuation but not the timing of adjuvant therapy affects survival of patients with high-risk colorectal cancer: a population-based study. Dis Colon Rectum 2010;53:1432–8. [DOI] [PubMed] [Google Scholar]


