Abstract
Patients with inflammatory bowel disease have an increased risk of vitamin D deficiency and this may impact upon the disease activity. This study explored the association between serum vitamin D levels and inflammatory bowel disease in a Chinese population.
Sixty-five patients with ulcerative colitis (UC) and 50 with Crohn's disease (CD) were investigated between January 2015 and December 2016 at the Kunshan Second People's Hospital, China. A control group of 120 healthy volunteers was also selected. Serum vitamin D levels were detected and compared between groups and among patients with different disease activity.
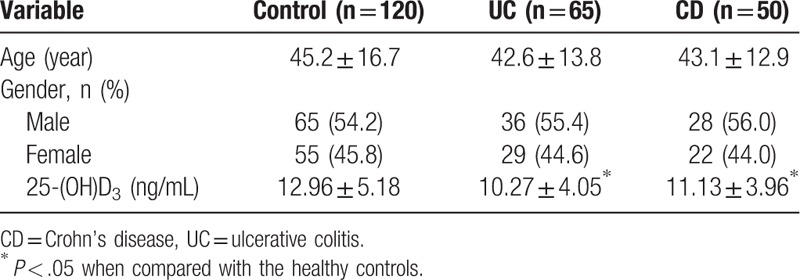
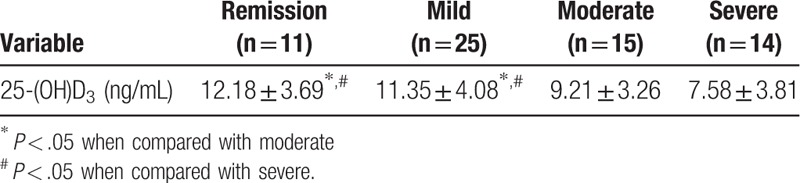
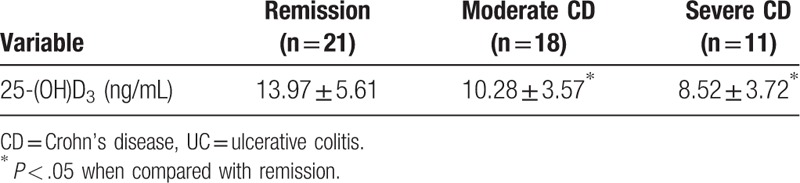
The serum vitamin D levels in the UC (10.27 ± 4.05 ng/mL) and CD (11.13 ± 3.96 ng/mL) groups were lower than in the control group (12.96 ± 5.18 ng/mL) (P < .05). In the UC group, during the moderate (9.21 ± 3.26 ng/mL) and severe (7.58 ± 3.81 ng/mL) periods, serum vitamin D levels were significantly lower compared with during remission (12.18 ± 3.69 ng/mL) and the mild period (11.35 ± 4.08 ng/mL) (P < .05). In the CD group, serum vitamin D levels were significantly lower during the moderate (10.28 ± 3.57 ng/mL) and severe (8.52 ± 3.72 ng/mL) periods compared with remission (13.97 ± 5.61 ng/mL) (P < .05).
Patients with UC and CD are both prone to vitamin D deficiency. Serum vitamin D was significantly lower with aggravating disease status. Therefore, vitamin D may be involved in the development of inflammatory bowel disease in a Chinese population.
Keywords: Crohn's disease, inflammatory bowel diseases, ulcerative colitis, vitamin D deficiency
1. Introduction
Inflammatory bowel disease (IBD) is characterized by chronic inflammation of the gastrointestinal tract but it is difficult to define because it is a complex set of debilitating disorders where physiology, microbiology, immunology, and genetics are all disrupted.[1] As a chronic disease, IBD mainly includes ulcerative colitis (UC) and Crohn's disease (CD). While the pathogenesis of IBD is unclear, it is generally agreed that environment, genetics, and infectious factors are responsible for the development of IBD,[2] and both UC and CD have an important and overlapping genetic component.[3] IBD occurrence is currently increasing worldwide although the reasons for this remain unclear.[4]
In the human body, vitamin D participates in the metabolism of calcium, phosphorus and other substances, and it is believed to be highly associated with cancer as well as other chronic diseases.[5] There appears to be an important role for vitamin D deficiency in the development of a wide range of inflammatory diseases.[6] Because IBD increases the risk of developing osteoporosis, guidelines recommend vitamin D supplementation for patients with IBD to prevent bone disease, but despite this vitamin D deficiency often occurs in IBD.[7]
The reason for deficiency of vitamin D in IBD patients is probably related to poor absorption due to the disease, but it has also been suggested that the deficiency may lead to the development of IBD and impact upon disease severity.[8] This has been supported by studies in different populations that show the association between vitamin D level and the development of IBD and its activity.[8–10] The level of vitamin D is probably not related to disease type as patients with UC and CD both have similarly low levels.[11,12] Local activation of vitamin D probably coordinates both innate and adaptive immunity in the intestinal epithelium, to promote barrier integrity and divert from the inflammatory response.[13] Nevertheless, because vitamin D deficiency is also strongly related to race and genetic background,[14] it is important to understand how vitamin D levels are associated with IBD, whether there are differences between UC and CD, and with disease activity in different populations.
In this study, patients with UC and CD receiving treatment at our hospital were selected together with 120 healthy controls, and the subjects had their serum vitamin D levels investigated. The aim was to investigate the association between vitamin D and IBD in a Chinese population.
2. Materials and methods
2.1. Patients
This study included patients with IBD who visited the Department of Gastroenterology of Kunshan Second People's Hospital (China) between January 2015 and December 2016. The clinical symptoms, imaging, and endoscopic results of all patients with IBD were consistent with the diagnosis guidelines of the World Gastroenterology Organization.[15] The inclusion criteria were: patients ≥18 years old; and patients diagnosed with UC or CD. The exclusion criteria were:
-
(1)
patients with heart, liver, renal, or other organic diseases; or
-
(2)
patients with severe malnutrition or impaired absorption.
Individuals seeking routine health checkups in our hospital were selected as healthy controls. All subjects signed an informed consent form.
2.2. Measurement of serum vitamin D
The peripheral blood of the subjects in the three groups was sampled. Serum levels of vitamin D were measured using the 25-hydroxy vitamin D enzyme-linked immunosorbent assay (ELISA) kit (IDS, UK) with a Model 680 microplate reader (Bio-Rad, USA). The levels of vitamin D were assessed according to the criteria proposed by the Institute of Medicine (IOM), with 25-(OH)D3 ≥20 ng/mL as sufficient, 10 ng/mL ≤ 25-(OH)D3 <20 ng/mL as insufficient, and 25-(OH)D3 <10 ng/mL as deficient.
2.3. Evaluation of disease activity
For patients with UC, evaluation of disease activity was done by the Mayo scoring system,[15] with the disease divided into four stages, namely remission, mild, moderate, and severe. For patients with CD, evaluation of disease activity was done by the Harvey-Bradshaw Index (HBI),[16] and the disease was divided into four stages, namely remission, mild, moderate, and severe.
2.4. Statistical analysis
Statistical analysis was performed using SPSS 17.0 (IBM, Armonk, NY, USA). Continuous variables are presented as mean ± standard deviation (SD); intergroup comparison was done using one-way analysis of variance (ANOVA). Categorical variables are presented as frequency and percentage; intergroup comparison was done using the chi-square test. Statistical significance was defined at P < .05.
3. Results
3.1. Characteristics of the subjects
The study included 65 patients with UC, 50 patients with CD, and 120 healthy controls. Among the 65 patients with UC, 36 were males and 29 were females; they were between 19–62 years and the mean age was 42.6 ± 13.8 years. Among the patients with 50 CD, 28 were males and 22 were females; they were 20–65 years and the mean age was 43.1 ± 12.9 years. Among the healthy controls, 65 were males and 55 were females; they were 19–70 years and the mean age was 45.2 ± 16.7 years. No significant difference was identified among the three groups in terms of gender, age, and other baseline data (P > .05).
The levels of serum vitamin D in patients with UC and CD were lower than in the controls, and the difference was of statistical significance (P < .05) (Table 1).
Table 1.
Baseline characteristics.
3.2. Association between serum vitamin D and disease activity in patients with UC
The levels of serum vitamin D were lower with aggravated disease activity for patients with UC. Patients with moderate and severe UC had significantly lower levels of serum vitamin D when compared with those with remission of mild UC, and the differences were significant for both comparisons (P < .05) (Table 2).
Table 2.
The association between serum vitamin D and disease activity in UC patients.
3.3. Association between serum vitamin D and disease activity in patients with CD
In this study, no patient was diagnosed with mild CD. In moderate and severe patients, the levels of serum vitamin D were significantly lower (P < .05) in comparison to those with disease remission (Table 3).
Table 3.
The association between serum vitamin D and disease activity in CD patients.
4. Discussion
The aim of this study was to investigate the levels of serum vitamin D in patients with UC and CD in comparison with healthy controls. We also investigated whether the levels of vitamin D decreased as the disease aggravated. The results showed that patients with UC and CD had significantly lower vitamin D levels than healthy controls and that the levels were the lowest in patients whose disease was currently aggravated. These results support international studies that suggest that low levels of vitamin D may play a role in IBD progression. This information provides important details about the role of vitamin D deficiency in a Chinese patient population.
Historically, IBD is a rare disease in China, but it is becoming more of a problem as its incidence is increasing over the years.[17] The commonly believed mechanism suggests that environmental factors impact on genetic susceptible individuals, causing intestinal microflora to activate both immune and non-immune pathways of the intestine, and finally causing bowel damage. Based on this, infection and immune activation are key factors for the development of IBD.[18,19]
Vitamin D is a derivative of liposoluble steroids that has biological activity. It participates in the metabolism of calcium and phosphorus, and functions to maintain levels of these two substances in the blood, thereby guaranteeing normal growth and development of the teeth, bones, and other organs.[6,20–22] Related studies showed that many human cells express vitamin D receptors and, upon bacterial infection, monocytes, macrophages, and dendritic cells recognize bacteria ligands and trigger the upregulation of vitamin D receptors as well as vitamin D hydroxylase, which then converts vitamin D into its active form, activates nuclear transcription factors, and promotes the expression of bacteriostatic peptides. Thus, the vitamin D receptor is an important biomolecule due to its role against infections and in immunoregulation.[23–27]
This study included 65 patients with UC, 50 patients with CD, and 120 healthy controls. The serum levels of vitamin D were measured and the results suggested that patients with UC and CD had significantly lower serum vitamin D in comparison to healthy controls (P < .05 for both comparisons), indicating that patients with IBD have lower levels of vitamin D than healthy individuals. Then, according to the Mayo scoring system, patients with UC were further divided into four subgroups and we found that serum vitamin D levels in patients with moderate and severe UC were significantly lower than in those with remission or mild UC (P < .05 for all comparisons), which indicated that aggravated UC was accompanied by lower serum vitamin D levels. Meanwhile, patients with CD were further divided into three subgroups according to HBI, and we found that patients with moderate and severe CD had significantly lower vitamin D in comparison to those with remission (P < .05), indicating that CD aggravation was also associated with low serum vitamin D levels. These results are supported by other studies in different patient populations that also found lower vitamin D levels in patients with IBD, and that lower levels were associated with aggravation of the disease.[8–10]
Though the evidence continues to support the significance of vitamin D in the pathogenesis of IBD,[28] the complex metabolism of vitamin D in the human body makes it difficult to clarify the mechanisms by which vitamin D affects immune response. Since this study only included a small sample size, the association between vitamin D and IBD in Chinese patients needs verification by future clinical studies. Still, we suggest that routine vitamin D monitoring should be performed for IBD patients, since most clinicians do not measure serum vitamin D and lack the awareness of vitamin D supplementation. At present, using vitamin D to prevent IBD is still in the exploratory stage.[29,30]
In clinics, there is no common agreement on the dose of vitamin D supplementation for patients with IBD, and no evidence suggests that patients with IBD without vitamin D deficiency should also take vitamin D supplementation. At the molecular level, the mechanism of vitamin D against IBD is not fully understood. Therefore, studying the mechanism mentioned above would lay foundation for the development of effective treatments.
This study has some limitations. The sample size was quite small, and it was based in a single center, so the results cannot be presumed to reflect the entire Chinese population. There was no follow-up to examine the changes in levels of vitamin D over time. For example, it would be interesting to investigate the changes in disease status (e.g., in patients that went into remission or aggravating disease) in relation to the vitamin D levels, or whether the vitamin D levels change with the change in disease status.
5. Conclusions
Patients with UC and CD had significantly lower serum vitamin D levels in comparison to healthy controls. Patients with severe disease had even lower serum vitamin D than those with mild disease. Since patients with IBD have vitamin D deficiency, vitamin D supplementation should be investigated as a potential treatment for patients with IBD.
Author contributions
Conceptualization: Jianmin Zhao.
Data curation: Jianmin Zhao, Yunfeng Wang.
Formal analysis: Yunfeng Wang, Weichang Chen.
Methodology: Jianmin Zhao, Yunfeng Wang, Qing Gu, Zhiquan Du, Weichang Chen.
Project administration: Qing Gu, Weichang Chen.
Resources: Qing Gu.
Software: Yunfeng Wang, Qing Gu, Zhiquan Du.
Supervision: Qing Gu, Weichang Chen.
Validation: Zhiquan Du, Weichang Chen.
Visualization: Zhiquan Du.
Writing – original draft: Jianmin Zhao.
Writing – review & editing: Jianmin Zhao, Yunfeng Wang, Qing Gu, Zhiquan Du, Weichang Chen.
Footnotes
Abbreviations: ANOVA = analysis of variance, CD = Crohn's disease, ELISA = enzyme-linked immunosorbent assay, HBI = Harvey-Bradshaw Index, IBD = inflammatory bowel disease, IOM = Institute of Medicine, SD = standard deviation, UC = ulcerative colitis.
This study was supported by the Science and Technology Project of Social Development in Kunshan (No. KS1622).
The authors have no conflicts of interest to declare.
References
- [1].Mulder DJ, Noble AJ, Justinich CJ, et al. A tale of two diseases: the history of inflammatory bowel disease. J Crohns Colitis 2014;8:341–8. [DOI] [PubMed] [Google Scholar]
- [2].Ananthakrishnan AN. Environmental triggers for inflammatory bowel disease. Curr Gastroenterol Rep 2013;15:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].de Lange KM, Barrett JC. Understanding inflammatory bowel disease via immunogenetics. J Autoimmun 2015;64:91–100. [DOI] [PubMed] [Google Scholar]
- [4].Burisch J, Munkholm P. Inflammatory bowel disease epidemiology. Curr Opin Gastroenterol 2013;29:357–62. [DOI] [PubMed] [Google Scholar]
- [5].Peterlik M, Cross HS. Vitamin D and calcium insufficiency-related chronic diseases: molecular and cellular pathophysiology. Eur J Clin Nutr 2009;63:1377–86. [DOI] [PubMed] [Google Scholar]
- [6].Querfeld U. Vitamin D and inflammation. Pediatr Nephrol 2013;28:605–10. [DOI] [PubMed] [Google Scholar]
- [7].Raftery T, O’Morain CA, O'Sullivan M. Vitamin D: new roles and therapeutic potential in inflammatory bowel disease. Curr Drug Metab 2012;13:1294–302. [DOI] [PubMed] [Google Scholar]
- [8].Alrefai D, Jones J, El-Matary W, et al. The association of vitamin D status with disease activity in a cohort of Crohn's disease patients in Canada. Nutrients 2017;9: E1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dumitrescu G, Mihai C, Dranga M, et al. Serum 25-hydroxyvitamin D concentration and inflammatory bowel disease characteristics in Romania. World J Gastroenterol 2014;20:2392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mouli VP, Ananthakrishnan AN. Review article: vitamin D and inflammatory bowel diseases. Aliment Pharmacol Ther 2014;39:125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Castro FD, Magalhaes J, Carvalho PB, et al. Lower levels of vitamin D correlate with clinical disease activity and quality of life in inflammatory bowel disease. Arq Gastroenterol 2015;52:260–5. [DOI] [PubMed] [Google Scholar]
- [12].Kabbani TA, Koutroubakis IE, Schoen RE, et al. Association of vitamin D level with clinical status in inflammatory bowel disease: a 5-year longitudinal study. Am J Gastroenterol 2016;111:712–9. [DOI] [PubMed] [Google Scholar]
- [13].Palmer MT, Weaver CT. Linking vitamin D deficiency to inflammatory bowel disease. Inflamm Bowel Dis 2013;19:2245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bernstein CN, Fried M, Krabshuis JH, et al. World Gastroenterology Organization Practice Guidelines for the diagnosis and management of IBD in 2010. Inflamm Bowel Dis 2010;16:112–24. [DOI] [PubMed] [Google Scholar]
- [15].Paine ER. Colonoscopic evaluation in ulcerative colitis. Gastroenterol Rep (Oxf) 2014;2:161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bennebroek Evertsz F, Hoeks CC, Nieuwkerk PT, et al. Development of the patient Harvey Bradshaw index and a comparison with a clinician-based Harvey Bradshaw index assessment of Crohn's disease activity. J Clin Gastroenterol 2013;47:850–6. [DOI] [PubMed] [Google Scholar]
- [17].Zhao J, Ng SC, Lei Y, et al. First prospective, population-based inflammatory bowel disease incidence study in mainland of China: the emergence of “western” disease. Inflamm Bowel Dis 2013;19:1839–45. [DOI] [PubMed] [Google Scholar]
- [18].Holick MF. Vitamin D deficiency. N Engl J Med 2007;315:266–81. [DOI] [PubMed] [Google Scholar]
- [19].Cantorna MT, Munsick C, Bemiss C, et al. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr 2000;130:2648–52. [DOI] [PubMed] [Google Scholar]
- [20].Garg M, Lubel JS, Sparrow MP, et al. Review article: vitamin D and inflammatory bowel disease-established concepts and future directions. Aliment Pharmacol Ther 2012;36:324–44. [DOI] [PubMed] [Google Scholar]
- [21].Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol 2007;5:1424–9. [DOI] [PubMed] [Google Scholar]
- [22].Yu S, Bruce D, Froicu M, et al. Failure of T cell homing, reduced CD4/CD8alphaalpha intraepithelial lymphocytes, and inflammation in the gut of vitamin D receptor KO mice. Proc Natl Acad Sci U S A 2008;105:20834–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Froicu M, Weaver V, Wynn TA, et al. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol 2003;17:2386–92. [DOI] [PubMed] [Google Scholar]
- [24].Cantorna MT. Why do T cells express the vitamin D receptor? Ann N Y Acad Sci 2011;1217:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ojaimi S, Skinner NA, Strauss BJ, et al. D deficiency impacts on expression of toll-like receptor-2 and cytokine profile: a pilot study. J Transl Med 2013;11:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Maruotti N, Cantatore FP. Vitamin D and the immune system. J Rheumatol 2010;37:491–5. [DOI] [PubMed] [Google Scholar]
- [27].Bamias G, Kaltsa G, Ladas SD. Cytokines in the pathogenesis of ulcerative colitis. Discov Med 2011;11:459–67. [PubMed] [Google Scholar]
- [28].Del Pinto R, Pietropaoli D, Chandar AK, et al. Association between inflammatory bowel disease and vitamin D deficiency: a systematic review and meta-analysis. Inflamm Bowel Dis 2015;21:2708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Reich KM, Fedorak RN, Madsen K, et al. Vitamin D improves inflammatory bowel disease outcomes: basic science and clinical review. World J Gastroenterol 2014;20:4934–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hlavaty T, Krajcovicova A, Payer J. Vitamin D therapy in inflammatory bowel diseases: who, in what form, and how much? J Crohns Colitis 2015;9:198–209. [DOI] [PubMed] [Google Scholar]


