Abstract
To analyze the association between non-alcoholic fatty liver disease (NAFLD) and the presence of diabetic retinopathy (DR) in patients with type 2 diabetes mellitus (T2DM).
Total 411 T2DM patients were divided into NAFLD and control groups. NAFLD was diagnosed by ultrasound. Retinopathy was diagnosed by fundus photography. All patients were screened based on medical history, physical examinations, and laboratory measurements.
The prevalence of NAFLD and DR in T2DM patients was 60.8% and 40.9%, respectively. The presence of DR was associated with diabetes duration, systolic blood pressure (SBP), glycated hemoglobin (HbA1c), and proteinuria (all P < .001) using univariate and multivariate regression analyses. The prevalence of DR was lower in patients with NAFLD than those without NAFLD (37.2% vs 46.6%, P = .065), and significantly lower in patients with moderate and severe NAFLD (30.2% vs 46.6%, P = .012; 14.3% vs 46.6%, P = .024). The presence of DR in NAFLD patients was associated with diabetes duration (P = .032) in Chi-squared analysis.
NAFLD and DR were highly prevalent in T2DM patients. Diabetes duration, SBP, HbA1c, and proteinuria were risk factors for DR in T2DM patients. The presence of DR was lower in T2DM patients with NAFLD, which was mainly due to their shorter diabetes duration.
Keywords: diabetes, diabetic retinopathy, non-alcoholic fatty liver disease
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) refers to the condition of fat accumulation in the liver unrelated to excessive alcohol consumption and any other specific causes of hepatic steatosis.[1] It has a diverse histopathological spectrum ranging from simple steatosis without significant inflammation to steatohepatitis (NASH) to various stages of fibrosis, cirrhosis, and ultimately to hepatocellular carcinoma.[2] NAFLD is strongly associated with obesity, diabetes, and insulin resistance (IR).[3] IR can facilitate the accumulation of triglycerides in the liver and is a key factor in the pathophysiology of NAFLD.[4,5] NAFLD is the most common cause for chronic liver disease in the world.[6] The pooled overall global prevalence of NAFLD diagnosed by imaging was estimated to be 25.24%.[7] The incidence of NAFLD is markedly increased with the increasing prevalence of obesity, diabetes mellitus, and the metabolic syndrome in general population. It is not only associated with increased liver-related morbidity and mortality, but also with increased mortality due to cardiovascular disease (CVD) and cancer.[8] There is now growing evidence that NAFLD is a multisystem disease, affecting extra-hepatic organs and regulatory pathways.[9] As a result, the effect of NAFLD on extra-hepatic organs has attracted more and more research interests.
NAFLD is common in individuals with type 2 diabetes (T2DM), which is present in up to 75% of patients with T2DM.[10] Current data suggest that NAFLD can increase the risk of T2DM complications, especially vascular complications.[11,12] Diabetic vascular complications can be divided into 2 categories: macrovascular and microvascular complications. Macrovascular complications include coronary artery disease and cerebrovascular disease,[13,14] while microvascular complications include retinopathy and chronic kidney disease.[15] There were more studies on the relationship between NAFLD and coronary artery disease of T2DM[16,17] and it has been demonstrated that NAFLD is an important factor for the development of coronary artery disease in patients with T2DM.[18–20] Moreover, some studies have investigated the relationship between NAFLD and chronic kidney disease of T2DM.[21,22] Diabetic retinopathy (DR) is the most common chronic complication of diabetes and one of the main causes of acquired blindness in the world.[23] The pathogenesis of DR has not yet been fully understood.[24] To date, there is very little information on the association between NAFLD and DR. The present study was to explore whether NAFLD (as diagnosed by ultrasonography) is associated with an increased risk of DR in a clinical cohort of Chinese patients with T2DM.
2. Materials and methods
2.1. Study participants
The incidence ratio of DR in NAFLD group and control group is about 1:0.64. Sample size was calculated by PASS software (PASS 11, NCSS, LLC, Kaysville, Utah) (assuming alpha = 0.1, 1-beta = 0.8, input PASS software, calculate NAFLD Group N = 250, control group N = 160). A total of 800 inpatients with T2DM from Department of Endocrinology, Beijing Tongren Hospital, Capital Medical University were initially recruited to the present cohort study between December 2014 and December 2016. The Epidemiology Ethics Committee of Beijing Tongren Hospital approved this study protocol.
2.2. Clinical measurements and laboratory procedures
Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Waist circumference was measured at the level of the umbilicus. Blood pressure was assessed in triplicate with a standard mercury manometer. Information on name, sex, age, diabetic duration, daily alcohol consumption, and smoking status of participants was obtained by systematically inquisition. Individuals completed self-administered questionnaires, related to their medical and social history and medication usage. Fasting blood glucose (FBG), hemoglobin A1C (HbA1C), blood lipid, high sensitivity C reactive protein (HCRP), proteinuria, liver enzymes were determined by standard laboratory procedures (AU5800, Beckman Coulter, Inc. 250 S. Kraemer Boulevard Brea, CA).
Eight hundred inpatients were screened for NAFLD by abdominal ultrasound. The classification of NAFLD was carried out based on the severity of fatty liver by abdominal ultrasound according to the given criteria.[25] Grade 1: no fatty liver; Grade 2 (mild): there was slight diffuse increase in the echogenicity of liver parenchyma or increased hepatorenal contrast with normal diaphragm and intrahepatic vessel borders; Grade 3 (moderate): there was moderate diffuse increase in the echoegenicity of liver parenchyma and increased hepatorenal contrast with slight impairment of diaphragm and intrahepatic vessel borders; Grade 4 (severe): in addition to the criteria for moderate steatosis, there was no visualization of posterior portion of the right lobe of liver, intrahepatic vessel borders, and diaphragm. Moreover, all patients underwent a fundus photography that was used to diagnose diabetic retinopathy according to the guidelines for clinical treatment of DR.[26]
2.3. Statistical analysis data
The SPSS statistical package 21.0 (IBM, Amund City, New York) was used for database establishment and statistical analysis. Data are presented as means ± standard deviation, median (P25, P75). The normality of variables was checked by K-S test. Differences were assessed by the unpaired t test for normally distributed variables. Differences were assessed by Wilcoxon Mann–Whitney U test for non-normally distributed variables. Categorical variables were checked by chi-square test and Fisher exact test. Multivariate logistic regression analysis was used to analyze the factors associated with DR in T2DM patients, OR, and 95% CI were calculated. A P-value <.05 was considered statistically significant.
3. Results
3.1. Study participants
Total 514 of 800 patients with T2DM were screened, who had complete clinical information. Those patients included individuals who did not consume alcohol or consumed alcohol <20 g/d. Eighty-three patients with positive serology for hepatitis B or C or with a history of chronic liver disease were excluded from the study. Patients with NAFLD did not receive any medical treatment to prevent liver injury before they were recruited to this study. Twenty patients with diabetic ketoacidosis, hyperthyroidism, hypothyroidism were excluded. Total 411 enrolled patients were divided into 2 groups (NAFLD group and control group) based on the presence of NAFLD or not, diagnosed by abdominal ultrasonography to identify fatty liver disease (Fig. 1).
Figure 1.
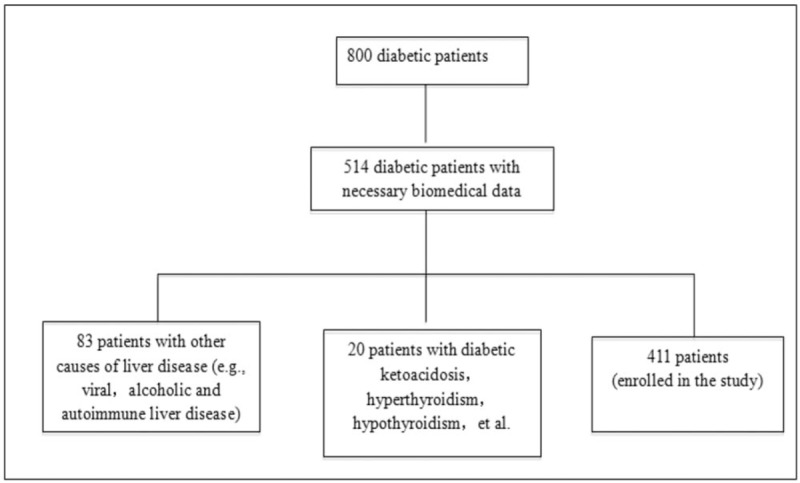
Flow chart of selection of study participants.
3.2. The clinical features and biochemical characteristics of participants
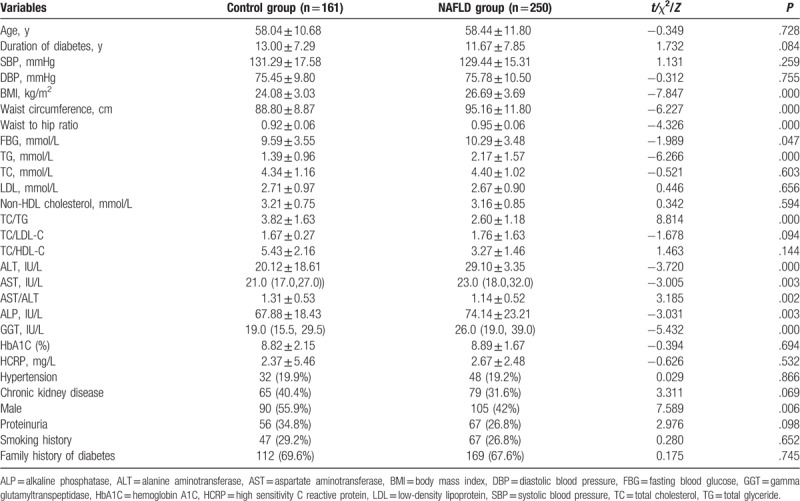
In the whole study population, the prevalence of NAFLD among the participants was about 60.8% (250/411). The FBG, BMI, waist circumference, waist to hip ratio, triglyceride (TG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and γ-glutamyl transferase (GGT) in patients with NAFLD were significantly higher than those in the control group (P < .05). The proportion of male patients in the NAFLD group was 42%, which was markedly lower than those in the control group (P < .05). There were no significant differences in age, duration of diabetes, proteinuria, systolic blood pressure (SBP), diastolic blood pressure (DBP), cholesterol, low density lipoprotein cholesterol (LDL), HbA1C, HCRP, smoking history, and family history of diabetes between 2 groups (P > .05) (Table 1).
Table 1.
Clinical and laboratory characteristics of study subjects.
3.3. Prevalence of DR in participants
As shown in Table 2, the prevalence of DR in the participants was about 40.9%. The prevalence of DR was 46.6% and 37.2% in control and NAFLD groups, respectively. Compared with the control group, the prevalence of DR in the NAFLD group is lower, but the difference was not statistically significant (P > .05).
Table 2.
Prevalence of DR in T2DM patients with different levels of NAFLD.
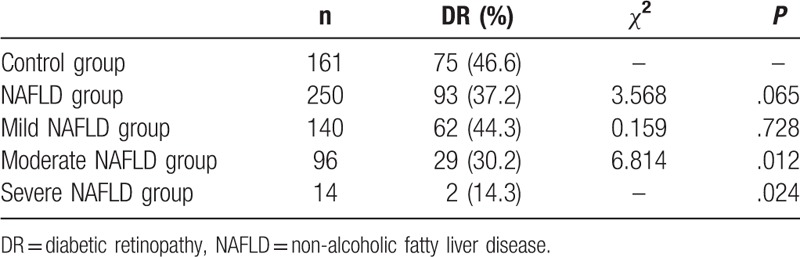
According to the given criteria,[1] the patients in the NAFLD group were further divided into mild NAFLD, moderate NAFLD, and severe NAFLD groups. Compared with the control group, the prevalence of DR in the moderate NAFLD group and the severe NAFLD group is significantly lower (P < .05), the prevalence of DR in the mild NAFLD group is lower but no statistical significance (P > .05).
3.4. Multivariable logistic regression analyses of the risk factors for DR in participants
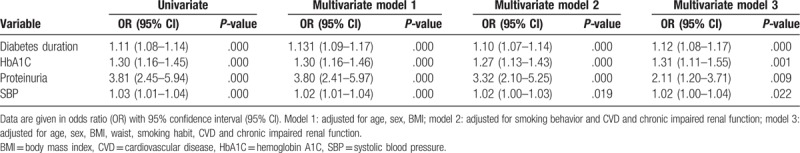
In univariate logistic regression analysis (Table 3), HbA1c, diabetes duration, proteinuria, and SBP were significantly correlated (P < .05) with increased rate of retinopathy. The correlation remained significant after adjustment for age, sex, and BMI (Table 3; model 1). Moreover, after adjustment for comorbidities including CVD and chronic impaired renal function, the correlation was still significant (P < .05) (Table 3; model 2, 3). However, there were no statistical significance in the relationship between NAFLD and age, smoking behavior, family history of diabetes, BMI, blood lipid level, lipoproteins, and DR, which were not shown in the Table 3.
Table 3.
Univariate and multivariate logistic regression analyses of DR in participants.
3.5. The differences of the DR risk factors in patients with different degree of fatty liver
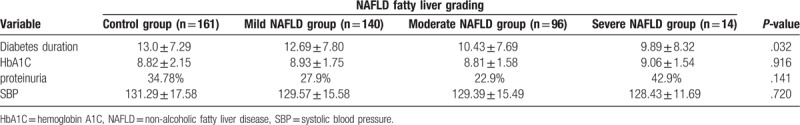
To examine the risk factors of DR in patients with different levels of fatty liver, we further graded NAFLD according to ultrasound findings. As shown in Table 4, individual diabetes duration was significantly shorter in the NAFLD group (P < .05). We also found that more severe fatty liver is, the shorter duration of diabetes is. However, the other risk factors of DR such as HbA1C, proteinuria, and SBP remained not statistically significant in patients with different levels of fatty liver.
Table 4.
The differences of the DR risk factors in T2DM patients with different degrees of fatty liver.
4. Discussion
Diabetes is a common metabolic disease with a rising global prevalence. It is estimated to affect 415 million people worldwide, which accounts for almost 10% of the global adult population.[26] Moreover, recent data predict that diabetes may further rise to almost 600 million worldwide by 2035.[27] T2DM represents about 90% of diabetes.[28] T2DM is mostly accompanied by NAFLD. The present study found that the prevalence of NAFLD in T2DM patients was 60.8%, which is similar to the 59.67% of pooled prevalence of NAFLD in T2DM patients in a meta-analysis study on 24 studies involving 35,599 T2DM patients.[29] It was well-known that the relationship between T2DM and DR is complicated because the incidence of DR in patients with T2DM varied in different studies. One study from Spain showed that the DR prevalence in 14,266 T2DM patients was 14.9%.[30] Another study from United Kingdom found that the prevalence of DR was 20.2% in 1062 patients with newly diagnosed T2DM. However, our result suggested that the prevalence of DR in T2DM patients was 40.9%. This result is consistent with the 40.5% of a study from China.[31]
As the global prevalence of diabetes increases, the number of patients with DR[32] has been estimated to reach 191.0 million by 2030[33] and diabetes will become the leading cause of vision loss and blindness in working-age adults.[26] The identification of risk factors associated with DR development is essential for developing preventive strategies. NAFLD usually coexists with T2DM and is a confirmed risk factor for the development of CVDs in patients with T2DM.[11] As a result, it is speculated that NAFLD may be a risk factor for DR in patients with T2DM. The association between NAFLD and DR in type 2 diabetes has not been studied thoroughly. Three previous studies investigated the association between NAFLD and DR in type 2 diabetes, but they presented different results. One study by Targher et al[34] showed that NAFLD is associated with increased prevalence of retinopathy and is independently associated with an increased prevalence of proliferative/laser-treated retinopathy in Italian patients with type 2 diabetes. However, another study from Korean by Kim et al[35] showed that NAFLD is inversely associated with the prevalence of DR in Korean patients with type 2 diabetes and one study by Lv et al[36] reported that NAFLD was also negatively correlated with the prevalence of DR in Chinese patients with type 2 diabetes. Our results showed that the prevalence of DR in patients with NAFLD is slightly lower than that of DR in patients without NAFLD (P > .05) but the prevalence of DR in patients with moderate and severe NAFLD was significantly lower than that of DR in patients without NAFLD (P < .05).
Risk factors for developing any DR have been described in many studies. Sasongko et al[37] found that the duration of diabetes, fasting glucose level, and hypertension are independently associated with the presence of DR in Indonesians with type 2 diabetes. Ting et al[33] found that hyperglycaemia, hypertension, hyperlipidemia, and obesity are the modifiable risk factors, while the duration of diabetes, puberty, and pregnancy are the non-modifiable risk factors for DR development and progression. In a prospective cohort study, Yun et al[38] demonstrated that glycemic control, diabetes duration, age, and albuminuria are important risk factors for DR development, but there is no significant relationship between DR and traditional serum lipid levels, the presence of hypertension, BMI. In a retrospective cross-sectional study, Yan and Ma[39] found that fasting serum glucose concentration, HbA1c level, diabetes duration, and insulin treatment are potential risk factors for DR in northern Chinese patients with T2DM. In a prospective longitudinal follow-up study, Abougalambou et al[40] found that there is a significant association between DR and diabetes duration, the presence of neuropathy, total cholesterol, and createnine clearance, but there is no significant difference between DR and age, sex, fasting plasma glucose, HbA1c, systolic BP, or diastolic BP. In the present study, we revealed that HbA1c, diabetes duration, proteinuria, SBP are risk factors for DR development, but age, smoking, family history of diabetes, BMI, and blood lipid are not associated with DR development.
We found that the incidence of DR is lower in T2DM patients with NAFLD, especially with moderate or severe NAFLD compared with that in T2DM patients without NAFLD. This result is not consistent to that NAFLD is an independent risk factor for CVD development in T2DM patients. We further analyzed those risk factors of DR in patients with different levels of NAFLD and observed that only diabetes duration is significantly associated with different levels of NAFLD. It has been demonstrated that the duration of diabetes is one of independent and the most consistent risk factors for DR.[41] The occurrence of DR increases with the duration of diabetes and an 8% increase in patients with DR in each additional year was observed with progression of diabetes.[40] As a result, shorter diabetes duration in patients with NAFLD may be the most important factor for lower incidence rate of DR.
4.1. Limitations
This study has several limitations. First, this is a cross-sectional study and cannot determine causal relationship between NAFLD and DR in patients with T2DM. Second, participants in our study were diabetic outpatients from single hospital, those results might not be generalizable to all T2DM patients. Third, the diagnosis of NAFLD and its degree was based on ultrasound imaging. The patients did not receive liver biopsy, which is the gold standard for the diagnosis and determination the degree of NAFLD. Despite these limitations, many results in present study are consistent with those reported previously by other authors. Thus, our results are valid and reliable.
4.2. Future directions
Prospective multi-community biopsy—proved NAFLD studies are required to elucidate the association between NAFLD and DR development in patients with T2DM. Especially the relationship between biopsy—confirmed NASH (the severe form of NAFLD) and DR in patients with T2DM is an important research direction in the future.
5. Conclusion
Our study demonstrated a higher incidence of DR with 40.9% in hospitalized Chinese patients with T2DM. Longer duration of diabetes, proteinuria, increased HbA1c, and SBP are risk factors for the development of DR. The presence of DR was lower in T2DM patients with NAFLD, especially patients with moderate and severe NAFLD compared with T2DM patients without NAFLD. Shorter diabetes duration may be the main cause of low rate of DR in T2DM patients with NAFLD.
Author contributions
Yutao Zhan contributed to the conception of the study; Meng Zhang and Li Li performed the data analyses and wrote the manuscript; Bei Li, Wenjun Lin and Jing Chen contributed significantly to analysis and manuscript preparation; Chuan Zhang helped perform the analysis with constructive discussions.
Conceptualization: Chuan Zhang.
Data curation: Meng Zhang.
Investigation: Jing Chen.
Methodology: Li Li.
Resources: Yutao Zhan.
Software: Bei Li, Wenjun Lin.
Footnotes
Abbreviations: ALP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, CI = confidence interval, DBP = diastolic blood pressure, DR = diabetic retinopathy, FBG = fasting blood glucose, GGT = gamma glutamyltranspeptidase, HbA1C = hemoglobin A1C, HCRP = high sensitivity C reactive protein, LDL = low-density lipoprotein, NAFLD = non-alcoholic fatty liver disease, OR = odds ratio, SBP = systolic blood pressure, T2DM = type 2 diabetes mellitus, TC = total cholesterol, TG = total glyceride.
MZ and LL have contributed equally to this work as the first authors.
CZ and YZ have contributed equally to this work as the corresponding authors.
This research was supported by the National Natural Science Foundation of China (NO: 81570515).
The authors have no conflicts of interest to declare.
References
- [1].van den Berg EH, Amini M, Schreuder TCMA, et al. Prevalence and determinants of non-alcoholic fatty liver disease in lifelines: a large Dutch population cohort. PLoS One 2017;12:e0171502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lonardo A, Nascimbeni F, Maurantonio M, et al. Nonalcoholic fatty liver disease: evolving paradigms. World J Gastroenterol 2017;23:6571–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang X, Ji X, Wang Q, et al. New insight into inter-organ crosstalk contributing to the pathogenesis of non-alcoholic fatty liver disease (NAFLD). Protein Cell 2018;9:164–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Abdelmoemen G, Khodeir SA, Zaki AN, et al. Overexpression of hepassocin in diabetic patients with nonalcoholic fatty liver disease may facilitate increased hepatic lipid accumulation. Endocr Metab Immune Disord Drug Targets 2019;19:185–8. [DOI] [PubMed] [Google Scholar]
- [5].Zelber-Sagi S, Ivancovsky-Wajcman D, FlissIsakov N, et al. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J Hepatol 2018;68:1239–46. [DOI] [PubMed] [Google Scholar]
- [6].Zhan Y, Xie P, Li D, et al. Deficiency of CKIP-1 aggravates high-fat diet-induced fatty liver in mice. Exp Cell Res 2017;355:40–6. [DOI] [PubMed] [Google Scholar]
- [7].Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- [8].Francque SM, van der Graaff D, Kwanten WJ. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J Hepatol 2016;65:425–43. [DOI] [PubMed] [Google Scholar]
- [9].Song JU, Jang Y, Lim SY, et al. Decreased lung function is associated with risk of developing non-alcoholic fatty liver disease: a longitudinal cohort study. PLoS One 2019;14:e0208736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Adams LA, Anstee QM, Tilg H, et al. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017;66:1138–53. [DOI] [PubMed] [Google Scholar]
- [11].Hazlehurst JM, Woods C, Marjot T, et al. Non-alcoholic fatty liver disease and diabetes. Metabolism 2016;65:1096–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Perumpail BJ, Khan MA, Yoo ER, et al. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol 2017;23:8263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Alkagiet S, Papagiannis A, Tziomalos K. Associations between nonalcoholic fatty liver disease and ischemic stroke. World J Hepatol 2018;10:474–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Abdeldyem SM, Goda T, Khodeir SA, et al. Nonalcoholic fatty liver disease in patients with acute ischemic stroke is associated with more severe stroke and worse outcome. J Clin Lipidol 2017;11:915–9. [DOI] [PubMed] [Google Scholar]
- [15].Mima A. Incretin-based therapy for prevention of diabetic vascular complications. J Diabetes Res 2016;2016:1379274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Idilman IS, Akata D, Hazirolan T, et al. Nonalcoholic fatty liver disease is associated with significant coronary artery disease in type 2 diabetic patients: a computed tomography angiography study. J Diabetes 2015;7:279–86. [DOI] [PubMed] [Google Scholar]
- [17].Yan LH, Mu B, Guan Y, et al. Assessment of the relationship between non-alcoholic fatty liver disease and diabetic complications. J Diabetes Investig 2016;7:889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mikolasevic I, Filipec-Kanizaj T, Mijic M, et al. Nonalcoholic fatty liver disease - a multisystem disease? World J Gastroenterol 2016;22:9488–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chang Y, Ryu S, Sung KC, et al. Alcoholic and non-alcoholic fatty liver disease and associations with coronary artery calcification: evidence from the Kangbuk Samsung Health Study. Gut 2018;pii:gutjnl-2018-317666. [DOI] [PubMed] [Google Scholar]
- [20].Duseja A, Singh SP, Saraswat VA, et al. Non-alcoholic fatty liver disease and metabolic syndrome-position Paper of the Indian National Association for the Study of the Liver, Endocrine Society of India, Indian College of Cardiology and Indian Society of Gastroenterology. J Clin Exp Hepatol 2015;5:51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhan YT, Zhang C, Li L, et al. Non-alcoholic fatty liver disease is not related to the incidence of diabetic nephropathy in Type 2 diabetes. Int J Mol Sci 2012;13:14698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Targher G, Byrne CD. Non-alcoholic fatty liver disease: an emerging driving force in chronic kidney disease. Nat Rev Nephrol 2017;13:297–310. [DOI] [PubMed] [Google Scholar]
- [23].Campos EJ, Campos A, Martins J, et al. Opening eyes to nanomedicine: where we are, challenges and expectations on nanotherapy for diabetic retinopathy. Nanomedicine 2017;13:2101–13. [DOI] [PubMed] [Google Scholar]
- [24].Zhang X, Zhao L, Hambly B, et al. Diabetic retinopathy: reversibility of epigenetic modifications and new therapeutic targets. Cell Biosci 2017;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fan JG, Jia JD, Li YM, et al. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver disease in China (Revised Edition 2010). Chin J Front Med Sci (Electronic Version) 2012;4:4–10. [Google Scholar]
- [26].Ruta LM, Magliano DJ, Lemesurier R, et al. Prevalence of diabetic retinopathy in Type 2 diabetes in developing and developed countries. Diabet Med 2013;30:387–98. [DOI] [PubMed] [Google Scholar]
- [27].Simó R, Ciudin A, Simó-Servat O, et al. Cognitive impairment and dementia: a new emerging complication of type 2 diabetes-The diabetologist's perspective. Acta Diabetol 2017;54:417–24. [DOI] [PubMed] [Google Scholar]
- [28].Tao Z, Shi A, Zhao J. Epidemiological perspectives of diabetes. Cell Biochem Biophys 2015;73:181–5. [DOI] [PubMed] [Google Scholar]
- [29].Dai W, Ye L, Liu A, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: a meta-analysis. Medicine (Baltimore) 2017;96:e8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].López M, Cos FX, Álvarez-Guisasola F, et al. Prevalence of diabetic retinopathy and its relationship with glomerular filtration rate and other risk factors in patients with type 2 diabetes mellitus in Spain. DM2 HOPE study. J Clin Transl Endocrinol 2017;9:61–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yang XF, Deng Y, Gu H, et al. C-reactive protein and diabetic retinopathy in Chinese patients with type 2 diabetes mellitus. Int J Ophthalmol 2016;9:111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Caglar C, Demir E, Kucukler FK, et al. A bibliometric analysis of academic publication on diabetic retinopathy disease trends during 1980-2014: a global and medical view. Int J Ophthalmol 2016;9:1663–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol 2016;44:260–77. [DOI] [PubMed] [Google Scholar]
- [34].Targher G, Bertolini L, Rodella S, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia 2008;51:444–50. [DOI] [PubMed] [Google Scholar]
- [35].Kim BY, Jung CH, Mok JO, et al. Prevalences of diabetic retinopathy and nephropathy are lower in Korean type 2 diabetic patients with non-alcoholic fatty liver disease. J Diabetes Investig 2014;5:170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lv WS, Sun RX, Gao YY, et al. Nonalcoholic fatty liver disease and microvascular complications in type 2 diabetes. World J Gastroenterol 2013;19:3134–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sasongko MB, Widyaputri F, Agni AN, et al. Prevalence of diabetic retinopathy and blindness in Indonesian adults with Type 2 diabetes. Am J Ophthalmol 2017;181:79–87. [DOI] [PubMed] [Google Scholar]
- [38].Yun JS, Lim TS, Cha SA, et al. Clinical course and risk factors of diabetic retinopathy in patients with Type 2 diabetes mellitus in Korea. Diabetes Metab J 2016;40:482–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yan ZP, Ma JX. Risk factors for diabetic retinopathy in northern Chinese patients with type 2 diabetes mellitus. Int J Ophthalmol 2016;9:1194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Abougalambou SS, Abougalambou AS. Risk factors associated with diabetic retinopathy among type 2 diabetes patients at teaching hospital in Malaysia. Diabetes Metab Syndr 2015;9:98–103. [DOI] [PubMed] [Google Scholar]
- [41].Penman A, Hancock H, Papavasileiou E, et al. Risk factors for proliferative diabetic retinopathy in African Americans with type 2 diabetes. Ophthalmic Epidemiol 2016;23:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]


