Abstract
Background:
This meta-analysis aimed to evaluate the effect of dexmedetomidine on prognosis in patients with sepsis.
Methods:
Computer-related electronic databases were searched, including PubMed, Embase, Web of Science, the Cochrane Library, and the China National Knowledge Infrastructure, from the date of database construction to January 2019. Stata 12.0 was used to perform a meta-analysis of short-term mortality [intensive care unit (ICU) mortality or 28-day mortality], ICU length of stay, and mechanical ventilation. Mortality was expressed using risk ratio (RR) and 95% confidence interval (CI). ICU length of stay and mechanical ventilation were expressed as weighted mean difference (WMD) and 95% CIs.
Results:
We finally included 8 randomized controlled trials in this meta-analysis. Compared with the control group, the dexmedetomidine group had a lower occurrence of 28-day mortality (RR, 0.49; 95% CI, 0.35 to 0.69; P = .000) and ICU mortality (RR, 0.44; 95% CI, 0.23 to 0.84; P = .013). However, there was no statistically significant difference for the length of hospital stay (WMD, −0.05; 95% CI, −0.59 to 0.48; P = .840) and mechanical ventilation time (WMD, 1.05; 95% CI, −0.27 to 2.37; P = .392) between dexmedetomidine group and control group.
Conclusions:
In patients with sepsis, dexmedetomidine can reduce the short-term mortality of patients, but could not shorten the ICU length of stay and mechanical ventilation time. More clinical randomized controlled trials are needed to verify the efficacy and safety of dexmedetomidine on the length of hospital stay and mechanical ventilation time.
Keywords: dexmedetomidine, meta-analysis, sepsis
1. Introduction
Sepsis is the systemic inflammatory response syndrome caused by infection.[1,2] Despite advances in supportive care, the mortality rate in patients with severe sepsis continues to exceed 30%.[3] Sepsis is characterized by inflammatory response, including tumor necrosis factor-α, interleukin 1β, and interleukin-6.[4,5] Studies have shown that reduced levels of serum inflammatory factors could improve patient mortality.[6]
Dexmedetomidine, a highly selective α2-adrenergic agonist, is a unique sedative agent compared with γ-aminobutyric acid receptor agonists.[7,8] Compared with other sedative drugs, dexmedetomidine has not only excellent sedative and analgesic effects, but also less inhibition effects on respiratory and circulatory function.[9] Dexmedetomidine also has effects on inhibiting inflammatory response and protecting organ function.[10] Several randomized controlled trials (RCTs) have compared dexmedetomidine to placebo.[11,12] Many of these trials contained relatively small cohorts and demonstrated inconsistent outcomes. This uncertainty leads to the determination of whether to adopt dexmedetomidine for treatment sepsis.
Thus, we undertook a meta-analysis to evaluate whether dexmedetomidine is superior to placebo with respect to 28-day mortality, intensive care unit (ICU) mortality, length of hospital stay, and mechanical ventilation time. We hypothesized that dexmedetomidine results in 28-day mortality and ICU mortality than placebo in sepsis or septic shock patients.
2. Materials and methods
The meta-analysis was based on the Cochrane Handbook for Systematic Reviews of Interventions[13] and was prepared in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist guidelines.[14] Ethical approval is unnecessary because it is a review of previously published articles and does not involve any treatment of individual patient data.
2.1. Search strategy
PubMed, Embase, Web of Science, the Cochrane Library, and the China National Knowledge Infrastructure were systematically conducted up to January 2019. All of the comparative studies were involved in sepsis or septic shock patients. The following keywords were used: “septic,” “septic shock,” “Toxic Shock,” “Toxic Shock Syndrome,” “dexmedetomidine,” “MPV-1440,” “MPV 1440,” “MPV1440,” “Precedex,” “Dexmedetomidine Hydrochloride,” and “Hydrochloride.” There are no language or geographical restrictions.
2.2. Inclusion criteria
The meta-analysis met the following criteria: the target populations were patients diagnosed with septic or septic shock; the intervention was dexmedetomidine and comparison study was administration with saline or placebo; the study design performed RCTs; and the outcomes were the 28-day mortality, ICU mortality, length of hospital stay, and mechanical ventilation time. Studies that report at least 1 result were included, and those without results were excluded. The duplicates of published literature, letters, comments and letters, and comments and abstracts were excluded.
2.3. Assessment of methodological quality
Two reviewers independently assessed the methodological qualities of the study using the Cochrane Collaboration for Systematic Reviews. The 7 items were sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of the outcome assessment, incomplete outcome data, selective reporting, and other biases. The overall methodological quality of each aspect was measured as “low risk of bias,” “high risk of bias,” and “unclear risk of bias” according to the Cochrane Handbook.[13]
2.4. Data extraction and outcome measures
Full texts of studies that met the inclusion criteria were thoroughly reviewed. Two reviewers independently extracted the eligibility study results from the predefined data fields. The differences were resolved through discussion to reach a consensus. The following information was extracted: the first author, published date, age, number of participants, Acute Physiology and Chronic Health Evaluation (APACHE) scores, outcomes, and follow-up.
2.5. Data synthesis
Statistical analyses were performed using Stata 12.0 (Stata Corp., College Station, TX). The continuous data, such as the length of stay and mechanical ventilation time, and the weighted mean difference (WMD) with 95% confidence interval (CI), were calculated. The dichotomous data, such as the 28-day mortality and ICU mortality were calculated by risk ratio (RR) and 95% CI. Heterogeneity test was assessed using the chi-squared test and I2 statistic. If the chi-squared test was above 0.05 or the I2 was below 50%, the fixed effects model was used. A random effects model was used if the chi-squared test was below 0.05 or the I2 was above 50%. Publication bias was independently assessed using funnel plots of the urinary tract infection. Subgroup analysis was performed for 28-day mortality based on risk of bias (low vs unclear/high), APACHE II scores (≤20 vs >20), and follow-up (≤3 vs >3 months). We also performed sensitivity analysis by omitting 1 study at a time to test the stability of the pooled results.
3. Results
3.1. Search results
The flow chart of the study inclusion and exclusion was shown in Figure 1. A total of 266 potentially relevant studies were identified through the search strategy, and 234 articles were read when excluding the duplicates. After reading title and abstracts of the included articles, 226 articles were excluded according to our inclusion criteria. Finally, 8 RCTs[11–20] were finally included after reading the full text.
Figure 1.
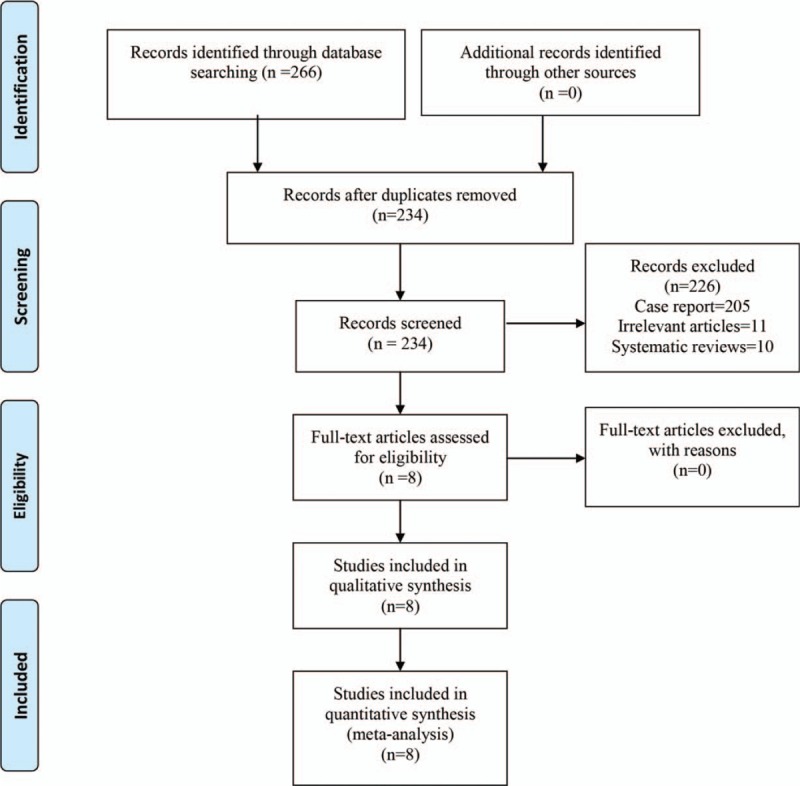
PRISMA flow chart of retrieved studies. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
3.2. Study characteristics
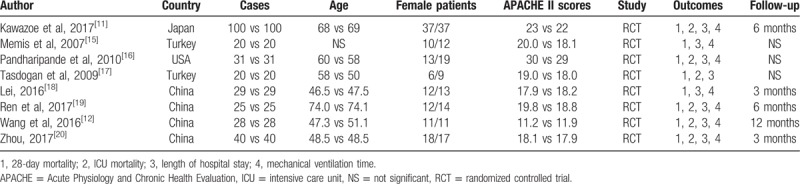
Study characteristics of the included studies can be seen in Table 1. Published years of the included studies ranged from 2007 to 2017. Sample sizes ranged from 20 to 100. Age of the included patients ranged from 46.5 to 74.1. Female patients ranged from 6 to 37. APACHE II scores ranged from 11.2 to 23.
Table 1.
General characteristic of the included studies.
3.3. Risk of bias of the included studies
Risk of bias summary and risk of bias graph can be seen in Figures 2 and 3, respectively. Random sequence generation was low in 5 studies, with unclear risk of bias in 2 studies and high risk of bias in 1 study. Allocation concealment was with low risk of bias in 4 studies and the rest were all with unclear risk of bias. Blinding of the participant was with low risk of bias in 4 studies. Blinding of the outcome assessment was with low risk of bias in 4 studies. Only 1 study report section of the data was listed as unclear risk of bias. Other biases were all with low risk.
Figure 2.
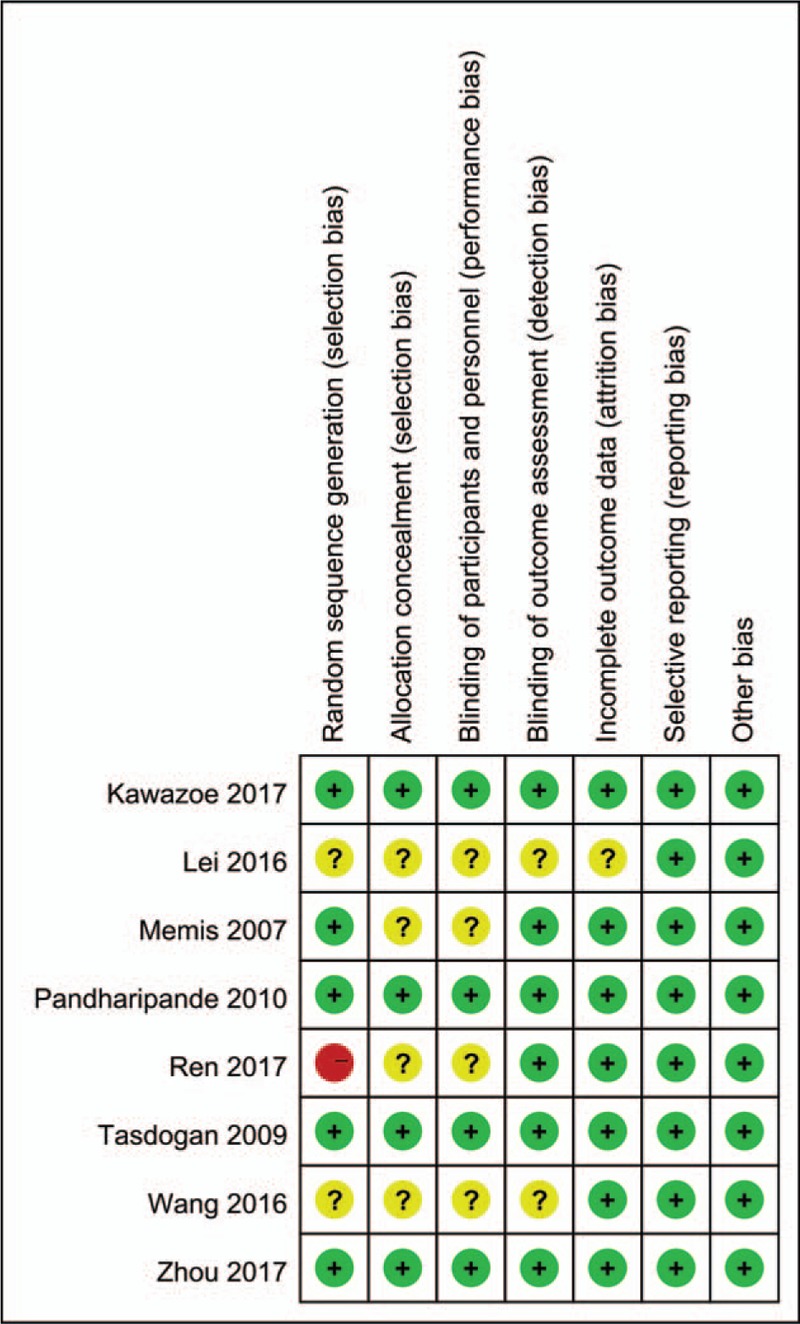
Risk of bias summary; “+” denotes low risk of bias; “−” denotes high risk of bias; “?” denotes unclear risk of bias.
Figure 3.
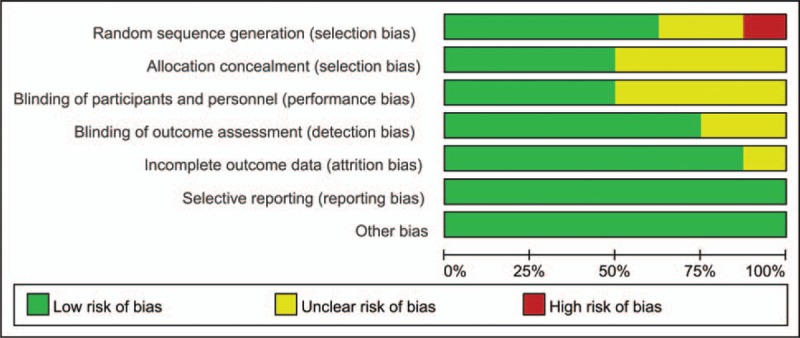
Risk of bias graph of the included studies.
3.4. Results of meta-analysis
3.4.1. 28-day mortality
Seven studies reported relevant data on the 28-day mortality. Compared with the control group, dexmedetomidine group had a lower occurrence of 28-day mortality (RR, 0.49; 95% CI, 0.35 to 0.69; P = .000; Fig. 4). The pooled data show little statistical heterogeneity, thus the fixed model was used (P = .160, I2 = 35.2%; Fig. 4).
Figure 4.
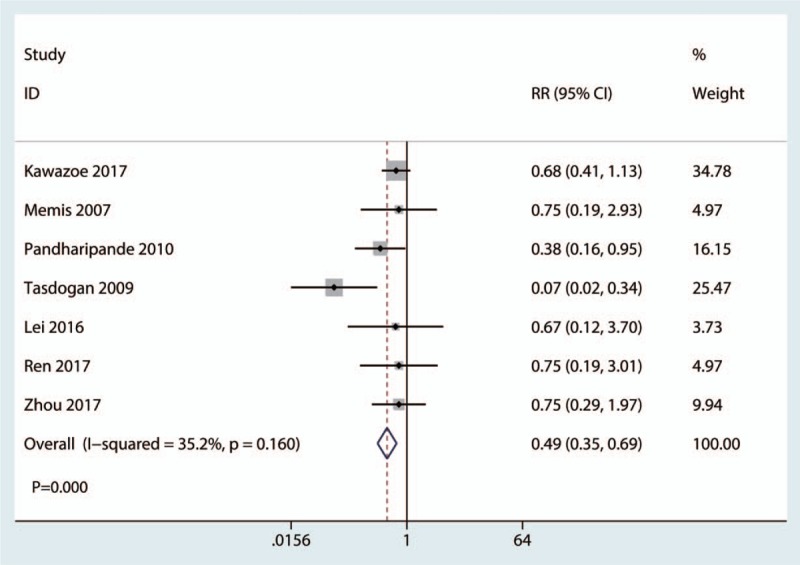
Forest plot comparing the 28-day mortality between the 2 groups.
3.4.2. ICU mortality
Five studies reported relevant data on the ICU mortality. Meta-analysis result showed that dexmedetomidine could significantly decreased ICU mortality than placebo group (RR, 0.44; 95% CI, 0.23 to 0.84; P = .013; Fig. 5). The pooled data did not show statistical heterogeneity, thus the fixed model was used (P = .808, I2 = 0.0%; Fig. 5).
Figure 5.
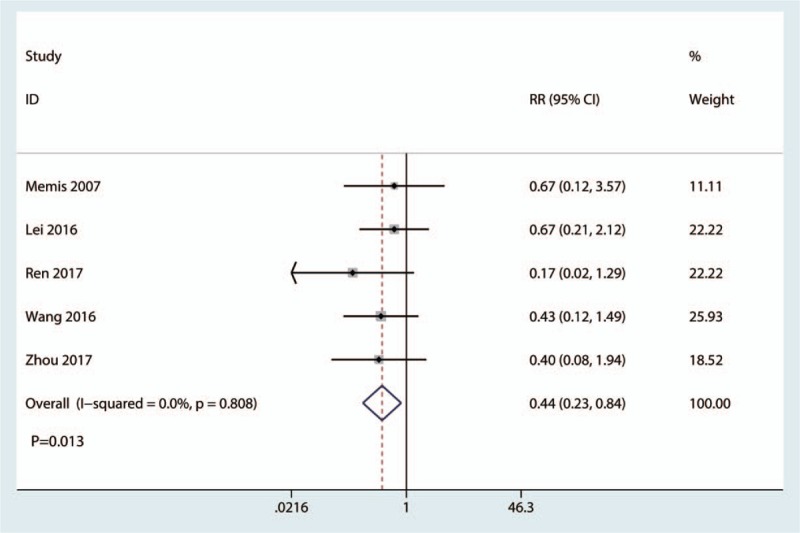
Forest plot comparing ICU mortality between the 2 groups. ICU = intensive care unit.
3.4.3. Length of hospital stay
All studies reported relevant data on the length of hospital stay. The meta-analysis showed that there was no significant difference between the 2 groups in terms of the length of hospital stay (WMD, −0.05; 95% CI, −0.59 to 0.48; P = .840; Fig. 6). The pooled data did not show statistical heterogeneity, thus the fixed model was used (P = .798, I2 = 0.0%; Fig. 6).
Figure 6.
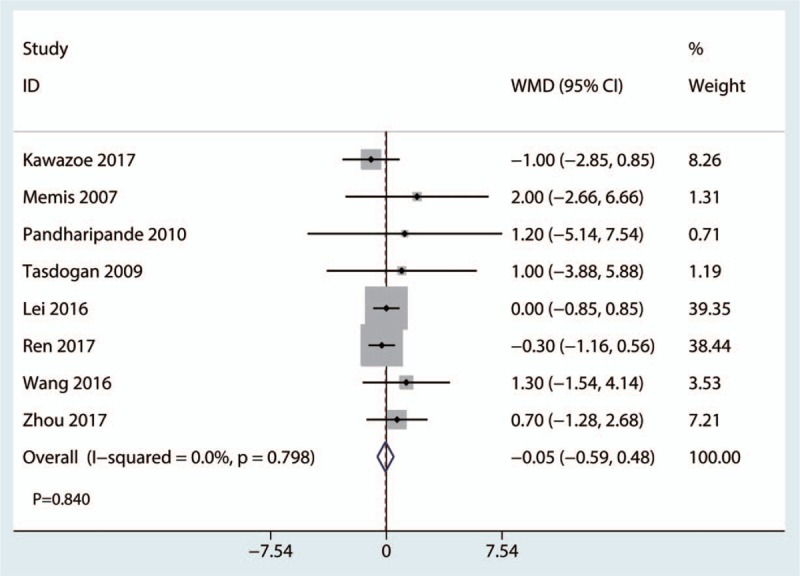
Forest plot comparing length of hospital stay between the 2 groups.
3.4.4. Mechanical ventilation time
Four studies reported relevant data on mechanical ventilation time. There was no statistically significant difference between dexmedetomidine group and control group in terms of the mechanical ventilation time (WMD, 1.05; 95% CI, −0.27 to 2.37; P = .392; Fig. 7). The pooled data did not show statistical heterogeneity, thus the fixed model was used (P = .392, I2 = 0.0%; Fig. 7).
Figure 7.
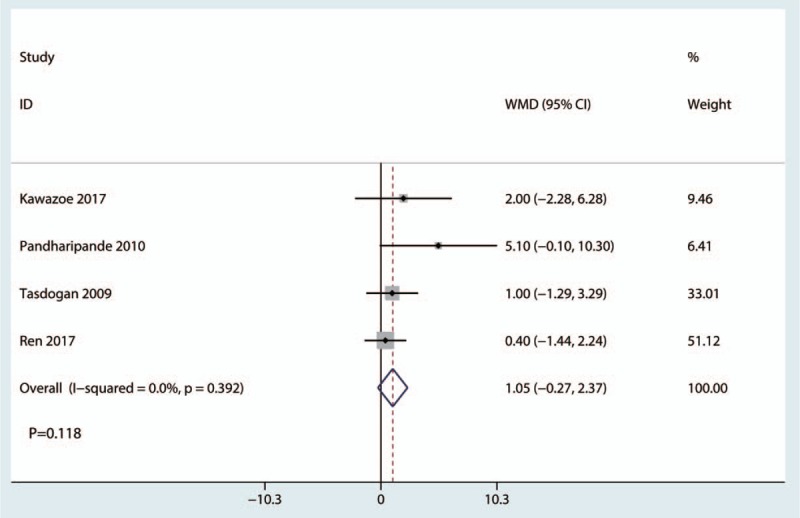
Forest plot comparing the mechanical ventilation time between the 2 groups.
3.4.5. Subgroup analysis, sensitivity analysis, and publication bias
Table 2 presents the results of subgroup analyses. The findings of decreased 28-day mortality were consistent in all subgroup analyses except for the follow-up duration subgroups. Sensitivity analysis result was shown in Figure 8. Among most of the studies, the heterogeneity results were not obviously altered after sequentially omitting each studies, indicating that our results were statistically reliable. For the meta-analysis of dexmedetomidine on 28-day mortality, there was no evidence of publication bias by inspection of the funnel plot (Fig. 9) and formal statistical tests (Egger test, P = .634; Begg test, P = .552).
Table 2.
Subgroup analysis for 28-day mortality.
Figure 8.
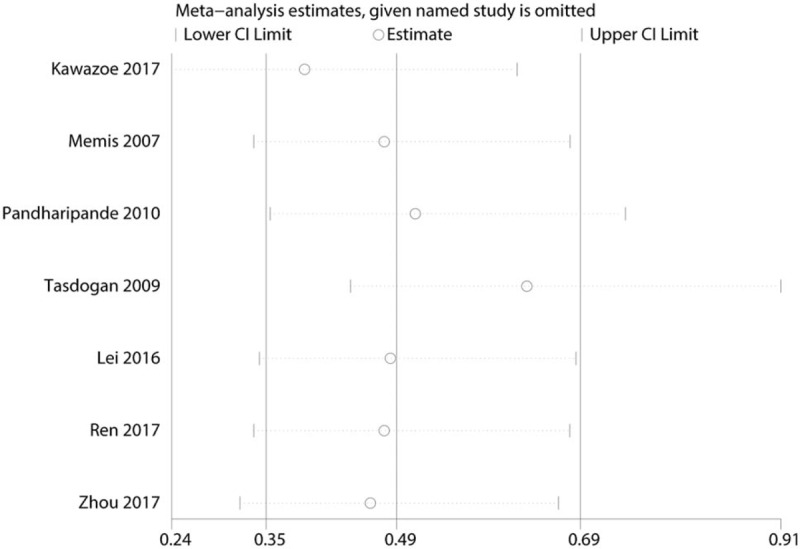
Sensitivity analysis of the 28-day mortality.
Figure 9.
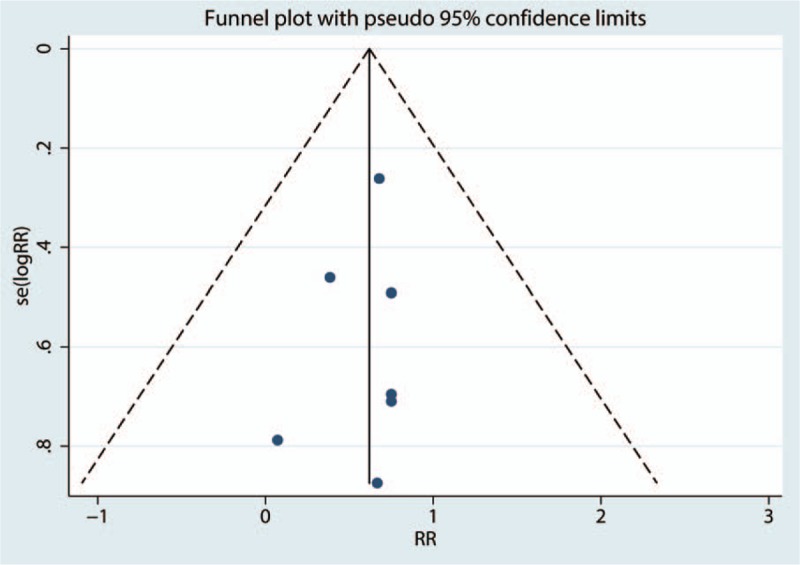
Funnel plot of the 28-day mortality.
4. Discussion
Our meta-analysis comprehensively and systematically reviewed the current available literature and found that dexmedetomidine compared with placebo significantly reduced 28-day mortality and ICU mortality for sepsis or septic shock patients; and dexmedetomidine has no benefit on the length of hospital stay and mechanical ventilation time.
This is the first meta-analysis that compares dexmedetomidine vs placebo for sepsis or septic shock patients. According to our inclusion criteria, we finally included 586 sepsis or septic shock patients. Results showed that dexmedetomidine could significantly reduce 28-day mortality. Taniguchi et al[21] revealed that dexmedetomidine dose dependently attenuated extremely high mortality rates and increased plasma cytokine concentrations after endotoxin injection in rats model. The author concluded that dexmedetomidine administration may be effective during sepsis. Riker et al[22] found that dexmedetomidine-treated patients spent less time on the ventilator, experienced less delirium, and developed less tachycardia and hypertension. Jiang et al[23] conducted a meta-analysis about dexmedetomidine for ischemic brain injury patients. Results showed that dexmedetomidine could reduce the release of inflammatory mediators and neuroendocrine hormones as well as maintain intracranial homoeostasis. The function of inflammatory mediators of dexmedetomidine could explain that dexmedetomidine has a beneficial role in reducing the mortality of sepsis or septic shock patients.
We then compared the length of hospital stay between dexmedetomidine and control groups. Results found that there was no significant difference between the 2 groups in terms of the length of hospital stay. Patanwala et al[24] found that use of dexmedetomidine was associated with increased lengths of ICU and hospital stay. However, this was a retrospective study and author also stated that future prospective trials are needed to confirm their conclusions.
Dexmedetomidine has no benefit on mechanical ventilation time when compared with the control group. Pandharipande et al[16] found that dexmedetomidine significantly increased mechanical ventilation time than the placebo group. When compared with propofol for sedation in the ICU, dexmedetomidine may increase the length of hospital stay.[24] Obviously, prolonged length of hospital stay may increase the economic costs for the patients. Ren et al[19] found that administration of dexmedetomidine could significantly decrease the length of hospital stay when compared with the control group. We further compared dexmedetomidine vs placebo for mechanical ventilation time. Results found that dexmedetomidine has no benefit on mechanical ventilation time.
This study has several advantages. First, this is the first meta-analysis that includes only RCTs with strict inclusion criteria. Second, we identified 28-day mortality as the primary outcome and further performed subgroup analysis and sensitivity analysis to further increase the robust of our meta-analysis. Third, the study found that the dexmedetomidine could significantly decrease the 28-day mortality and ICU mortality.
There were also several limitations to this study. First, the number of included studies and the sample size were relatively few in this meta-analysis. Second, there were no consistent criteria for sepsis or septic shock, and this may cause clinical heterogeneity. Third, follow-up duration was relatively short, and long-term follow-up RCTs were needed to identify the adverse effects of dexmedetomidine. Therefore, more high-quality articles are needed to confirm the above conclusions. Fourth, some studies combined dexmedetomidine with opioids or benzodiazepines for treatment of sepsis; thus, real effects of single administration with dexmedetomidine for sepsis need to be explored further.
5. Conclusions
Based on the current evidence, this meta-analysis showed that dexmedetomidine could significantly decrease the 28-day mortality and ICU mortality than placebo in sepsis or septic shock patients. More clinical RCTs are needed to verify the efficacy and safety of dexmedetomidine on the length of hospital stay and mechanical ventilation time.
Author contributions
Conceptualization: Wen-Qing Zhang.
Data curation: Wen-Qing Zhang.
Software: Peng Zheng.
Supervision: Peng Zheng, Wei Yang.
Writing – original draft: Xiaohong Zhan, Wei Yang.
Writing – review & editing: Po Xu, Xiaohong Zhan.
Footnotes
Abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation, CI = confidence interval, ICU = intensive care unit, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCTs = randomized controlled trials, RR = risk ratio, WMD = weighted mean difference.
W-QZ and PX contributed equally to this work and are listed as co-first authors.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Rochwerg B, Alhazzani W, Sindi A, et al. Fluid resuscitation in sepsis: a systematic review and network meta-analysis. Ann Intern Med 2014;161:347–55. [DOI] [PubMed] [Google Scholar]
- [2].Lu H, Wen D, Wang X, et al. Host genetic variants in sepsis risk: a field synopsis and meta-analysis. Crit Care 2019;23:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sun J, Marwah G, Westgarth M, et al. Effects of probiotics on necrotizing enterocolitis, sepsis, intraventricular hemorrhage, mortality, length of hospital stay, and weight gain in very preterm infants: a meta-analysis. Adv Nutr 2017;8:749–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bosch NA, Cohen DM, Walkey AJ. Risk factors for new-onset atrial fibrillation in patients with sepsis: a systematic review and meta-analysis. Crit Care Med 2019;47:280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhou J, Yang D, Liu K, et al. Systematic review and meta-analysis of the protective effect of resveratrol on multiple organ injury induced by sepsis in animal models. Biomed Rep 2019;10:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chi C, Buys N, Li C, et al. Effects of prebiotics on sepsis, necrotizing enterocolitis, mortality, feeding intolerance, time to full enteral feeding, length of hospital stay, and stool frequency in preterm infants: a meta-analysis. Eur J Clin Nutr 2018;[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ji F, Li Z, Nguyen H, et al. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation 2013;127:1576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kang K, Gao Y, Wang SC, et al. Dexmedetomidine protects against lipopolysaccharide-induced sepsis-associated acute kidney injury via an alpha7 nAChR-dependent pathway. Biomed Pharmacother 2018;106:210–6. [DOI] [PubMed] [Google Scholar]
- [9].Qiu R, Yao W, Ji H, et al. Dexmedetomidine restores septic renal function via promoting inflammation resolution in a rat sepsis model. Life Sci 2018;204:1–8. [DOI] [PubMed] [Google Scholar]
- [10].Ma Y, Yu XY, Wang Y. Dose-related effects of dexmedetomidine on immunomodulation and mortality to septic shock in rats. World J Emerg Med 2018;9:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kawazoe Y, Miyamoto K, Morimoto T, et al. Effect of dexmedetomidine on mortality and ventilator-free days in patients requiring mechanical ventilation with sepsis: a randomized clinical trial. JAMA 2017;317:1321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang L, Yao L, Zou H, et al. Effects of dexmedetomidine on inflammatory responses in sepsis patients. Modern J Integr Trad Chin Western Med 2016;25:544–5. [Google Scholar]
- [13].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;2:g7647. [DOI] [PubMed] [Google Scholar]
- [15].Memis D, Hekimoglu S, Vatan I, et al. Effects of midazolam and dexmedetomidine on inflammatory responses and gastric intramucosal pH to sepsis, in critically ill patients. Br J Anaesth 2007;98:550–2. [DOI] [PubMed] [Google Scholar]
- [16].Pandharipande PP, Sanders RD, Girard TD, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care 2010;14:R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tasdogan M, Memis D, Sut N, et al. Results of a pilot study on the effects of propofol and dexmedetomidine on inflammatory responses and intraabdominal pressure in severe sepsis. J Clin Anesth 2009;21:394–400. [DOI] [PubMed] [Google Scholar]
- [18].Lei XLY. Effect of dexmedetomidine in protecting cardiac muscle in patients with sepsis. J Community Med 2016;14:11–4. [Google Scholar]
- [19].Ren D, Lin M, Xiao X, et al. Effect of dexmedetomidine on expression of inflammation related genes in patients with sepsis. Contemp Med 2017;23:1–4. [Google Scholar]
- [20].Zhou P. Effect of dexmedetomidine on mortality in patients with sepsis: a randomized clinical trial. Xinxueguanbing Fangzhi Zhishi 2017;12:73–4. [Google Scholar]
- [21].Taniguchi T, Kurita A, Kobayashi K, et al. Dose- and time-related effects of dexmedetomidine on mortality and inflammatory responses to endotoxin-induced shock in rats. J Anesth 2008;22:221–8. [DOI] [PubMed] [Google Scholar]
- [22].Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 2009;301:489–99. [DOI] [PubMed] [Google Scholar]
- [23].Jiang L, Hu M, Lu Y, et al. The protective effects of dexmedetomidine on ischemic brain injury: a meta-analysis. J Clin Anesth 2017;40:25–32. [DOI] [PubMed] [Google Scholar]
- [24].Patanwala AE, Erstad BL. Comparison of dexmedetomidine versus propofol on hospital costs and length of stay. J Intensive Care Med 2016;31:466–70. [DOI] [PubMed] [Google Scholar]


