Abstract
Glucose and glycerol are important circulating metabolites. Due to poor ionization and/or ion suppression, the liquid chromatography-mass spectrometry (LC-MS) detection of glucose and glycerol presents challenges. Here, we propose an efficient LC-MS method of quantitative glucose and glycerol detection via enzymatic derivatization to glucose-6-phosphate and snglycerol-3-phosphate, respectively. This derivatization protocol can be used to measure the concentrations of glucose production in a plethora of sample types for metabolic analysis and is compatible with the general metabolomics workflow. This novel approach allows us to quantitatively study glucose and glycerol metabolism using stable isotope tracers in vivo.
Circulating glucose in living animals is tightly controlled to achieve euglycemia. High levels of blood glucose over a prolonged period is characterized as diabetes mellitus. Elevated glucose levels may be the result of both impaired glucose uptake in muscle/adipose tissues and increased glucose production in the liver. Obesity, which is known to be a risk factor for Type 2 diabetes, is excess body fat accumulation. Excess amounts of triglycerides are major sources of free fatty acids and glycerol through lipolysis. Free fatty acids impair insulin signaling [1] and lowers glucose uptake in the periphery. Moreover, evidence suggests glycerol plays a role in gluconeogenesis in diabetic patients [2,3]. Therefore, it is important to measure glycerol and glucose simultaneously to study their metabolism.
Various methods exist to measure glucose and glycerol respectively. One popular option is the commercially available colorimetric/fluorometric kits. These kits are easy to use, relatively sensitive and do not require specialized equipment. However, there are limitations to using these kits. Firstly, the kits are designed for very specific use. A kit measuring free glycerol in serum may not be suitable for measuring free glycerol levels in tissue samples. Secondly, the kits use coupled enzymatic assays. Whether the coupled enzymatic reactions are interfered with or inhibited by endogenous metabolites other than glycerol has not been fully characterized. Third, glycerol and glucose measurements require different kits. This increases the cost and requires more biological samples. More importantly, the kits can only measure the total level of glucose or glycerol. To measure the endogenous production rate of glucose or glycerol, stable isotope labeled tracers are often used. Stable isotope tracers cannot be used with colorimetric and fluorometric kits, making them an unsuitable option for studying dynamic metabolism. Thus, NMR [4] and mass spectrometry are the only options.
Sensitive detection and quantitation of glycerol is most typically associated with analysis using gas chromatography-mass spectrometry (GC-MS). A good number of established protocols commonly rely on silylation agents [5–8] or heptafluorobutyric anhydride [9]. Similar approaches have been applied to glucose measurements for better sensitivity and accuracy [10]. GC-MS has been a reliable and widely used method. However, the derivatization is laborious and potentially deleterious to compounds of interest. Moreover, GC-MS is not the most suitable platform for metabolomics analysis due to relatively low sensitivity and compound-specific derivatization procedures.
LC-MS is currently the most popular analysis platform measuring metabolite levels. Some reported methods involve derivatization with benzyol chloride which reacts with hydroxy groups [11,12]. These methods work well for the detection of glucose and glycerol, but other common metabolites such as lactate and malate may also react with the derivatization reagent, decreasing the compatibility of these methods with the general metabolomics workflow. Another approach is to detect glucose and glycerol as their alkali metal adducts such as [M+Na]+ [13] or [M+Cs]+ [14]. These methods do not require chemical derivaitization of the sample, however, to efficiently form these adducts mobile phase modifiers such as cesium acetate are typically added. The impact of such modifiers in typical metabolomics analysis is yet to be evaluated. The [M+Na]+ adducts can be detected without additional salt in the mobile phase, but in some systems, ours included, this adduct is not detected [15,16].
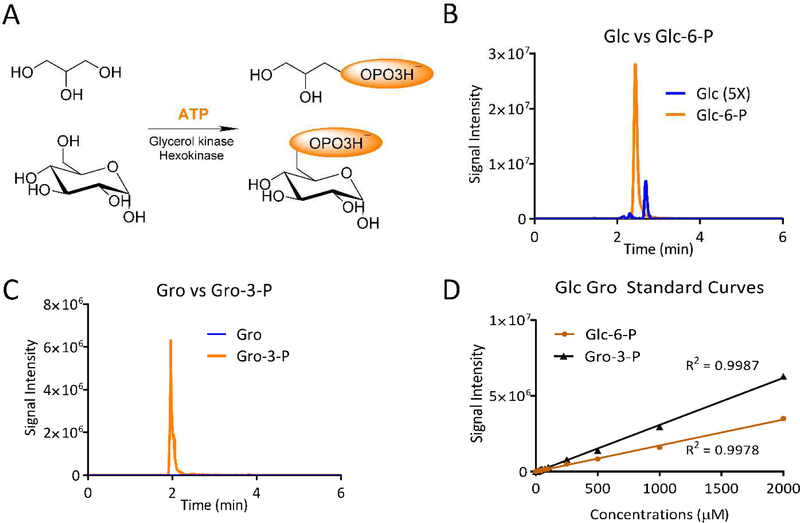
Our goal is to find a method that is easy, reliable and compatible to the general metabolomics workflow. To this end, we have developed a simple derivatization method that uses a combination of hexokinase, glycerol kinase and ATP to phosphorylate glucose and glycerol at the same time. The resulting glucose-6-phosphate (Glc-6-P) and sn-glycerol-3-phosphate (Gro-3-P) can be sensitively detected under negative ionization mode. Other metabolites in the sample are not affected by the highly specific enzymatic derivatization, making it a metabolomics-compatible method. This HILIC compatible derivatization requires a fairly short chromatography method of 6 minutes per run, allowing for good throughput. Also, using this method, we can explore the importance of glycerol in glucose production.
To make glucose and glycerol more ionizable, they were enzymatically derivatized to Glc-6-P and Gro-3-P using ATP as the phosphate donor. The enzymatic reactions involved hexokinase and glycerol kinase, both of which were active under pH 8.0, making the derivatization a simple one-pot reaction (Figure 1A). The derivatization reactions were performed in a buffer containing 25mM Tris-HCl (pH 8.0), 50mM NaCl, 10mM MgCl2, and 5mM ATP (Sigma-Aldrich A2383). Hexokinase (Sigma-Aldrich H4502) and glycerokinase (Sigma-Aldrich G6278) were diluted to 40U/mL in the same buffer with 10% sucrose. This enzyme mix can be aliquoted and stored under −80°C for up to 3 months. To each reaction, 2.5μl of this enzyme mix was added, along with 5μl of the sample to be tested. The mixture was allowed to sit at room temperature for 10 minutes, followed by the addition of 950μl of quenching solvent, which is acetonitrile/methanol/water 40/40/20 (V/V) with 0.1M formic acid (Fisher Scientific A117). The reaction mixture was then neutralized with 88μl of 15% NH4HCO3 (Sigma-Aldrich A6141, M/V), spun at 16,000g for 10min under 4°C and transferred to LC-MS vials for analysis.
Figure 1.
Enzymatic Derivatization of Glucose and Glycerol. (A) Derivatization Schematic. Glucose and glycerol are converted to glucose-6-phosphate (Glc-6-P) and sn-glycerol-3-phosphate (Gro-3-P) using hexokinase and glycerokinase and ATP. (B) Extracted ion chromatograms comparing the detection of the underivatized glucose (Glc) (m/z 179.0556) to derivatized of Glc-6-P (m/z 259.0224). The Glc intensity is enhanced 5X. (C) Extracted ion chromatograms comparing the detection of the underivatized Glycerol (Glo) (m/z 105.0190) to derivatized of Gro-3-P (m/z 171.0063). (D) Standard curves of Glc and Glo at 0μM, 5μM, 10μM, 20μM, 30μM, 40μM, 50μM, 75μM, 100μM, 250μM, 500μM, 1000μM, and 2000μM concentrations. R2=0.9978 and 0.9987 for Glc-6-P and Gro-3-P respectively.
Our LC-MS system is Thermo Q Exactive PLUS with a HESI source coupled with UltiMate 3000 UHPLC. The chromatography uses a Waters XBridge BEH Amide column (2.1mm × 150mm, 2.5μm particle size, 130Å pore size) coupled with a Waters XBridge BEH Amide XP VanGuard cartridge (2.1mm × 5mm, 2.5μm particle size, 130Å pore size) guard column. The column oven temperature was set to 25°C. The chromatography method is a 6min isocratic method using 27% solvent A (water/acetonitrile, 95/5 V/V with 20mM NH3Ac and 20mM NH3OH at pH=9.4) and 73% solvent B (water/acetonitrile, 20/80 V/V with 20mM NH3Ac and 20mM NH3OH at pH=9.4). The flow rate was 0.3mL/min. The autosampler temperature was set to 4°C and the injection volume was 5μL. The source of the mass spectrometer was set to a spray voltage of −2.7kV and sheath, auxiliary, and sweep gas flow rates of 40, 10, and 2 (arbitrary unit) respectively. The capillary temperature was set to 300°C and aux gas heater was 360°C. The S-lens RF level was 45. For a better metabolome coverage, the m/z scan range was set to 72 to 1000m/z at a resolution of 70,000 under negative polarity with AGC target of 3e6 and a maximum IT of 500ms. The derivatized Glc-6-P is eluted at 2.4min (m/z 259.0224) and Gro-3-P is eluted at 1.9min (m/z 171.0063).
Method validation was performed on linearity, LOD, LOQ, intra-day and inter-day precision for both Glc-6-P and Gro-3-P and the overall method accuracy (Table 1). These data were obtained in accordance with the pharmacokinetic analytical methods validation procedures established by Shah et al [17]. The determined LOD is 25pmol and LOQ is 50pmol for both glucose and glycerol input. The sensitivity is similar to the one reported by Rogatsky E. et al. [14] and lower than the one by Bornø A. et al. [11]. Input concentrations of 20, 80, and 4000μM were used to assess the method precision. This range represents physiological concentrations of glycerol and glucose that will be encountered in various sample types as established in published literature, as well as a low concentration that is slightly above the LOQ. Measurement of glucose, at lower concentrations, poses greater variability with respect to quantification. This is something we believe to be negligible because the physiological concentrations of glucose we observe in samples is higher and in a range where better reproducibility is observed. Also, the inter-day measurements are higher than the inter-day measurements, suggesting a batch specific standard curve is necessary to obtain the most reliable quantification.
Table 1:
Method validation results.
| Input Concentration (μM) |
Glc-6-P Intra-day
Precision (CV%) (n=10+10+10) |
Glc-6-P Inter-day
Precision (CV%) (n=30+30+30) |
Gro-3-P Intra-day
Precision (CV%) (n=10+10+10) |
Gro-3-P Inter-day
Precision (CV%) (n=30+30+30) |
Overall Accuracy (%) (n=20+20+20) |
|---|---|---|---|---|---|
| 4000 | 9.9 | 17.7 | 5.7 | 18.9 | 109 |
Comparing the signal intensities of underivatized glucose and derivatized Glc-6-P, the detection sensitivity improved 30-fold (Figure 1B). Glycerol before derivatization could not be detected even at 5mM concentration, neither did we detect Na+ adducts of glucose or glycerol under positive mode (data not shown). After the derivatization reaction, glycerol was readily detected at 50μM concentration (Figure 1C). Standard curves for derivatized glucose and glycerol showed good linearity, R2=0.9978 and R2=0.9987 for glucose and glycerol respectively, (Figure 1D). Using this method, we measured the glucose production rates of primary hepatocytes using glycerol as the gluconeogenic substrate. After a 12-hour incubation, the glycerol concentration in the culture media dropped from 2.0mM to 0.295mM, indicating significant glycerol consumption (Figure 2A). When pyruvate and lactate were used as additional gluconeogenic substrates, the glycerol concentration at the end of the 12-hour incubation was elevated in comparison to using glycerol alone as a substrate. This suggests that the addition of pyruvate and lactate decreased the overall glycerol consumption rate. However, addition of pyruvate and lactate increased the glucose production rate. In fact, when 13C3-glycerol was used as the gluconeogenic substrate, the labeling pattern of glucose showed mainly M+3 and M+6 after the correction of isotope natural abundance and tracer isotope impurity [18]. This labeling pattern is consistent with the biochemistry that glycerol makes Gro-3-P and dihydroxyacetone phosphate for glucose production and always keeps its 3-carbon backbone intact, whereas pyruvate and lactate go to TCA cycle through pyruvate carboxylase and then scramble their carbon backbones. The measured labeling pattern of glucose suggests glycerol is the major gluconeogenesis substrate even in the presence of pyruvate and lactate (Figures 2B,C).
Figure 2:
Using Enzymatic Glucose/Glycerol Derivitization to Study Circulating Metabolism in Hepatocytes and Animal Tissue. (A) In hepatocyte media, the total glycerol concentration after 12 hours. Starting concentration of glycerol was 2mM. Glycerol was added alone (Gro), or supplemented with pyruvate (5mM) and lactate (50mM) (GroPL). (B) Glucose production rate. (C) The distribution of the labeled fractions of glucose in the hepatocyte culture media. (D-E) Comparison of free glucose / total glucose (free glucose + hexose phosphate) and free glycerol / total glycerol (free glycerol + glycerol phosphate). (F-G) Labeling pattern of glycerol, glucose, and lactate after 13C-Glycerol infusion. (I) Schematic of glycerol contribution to gluconeogenesis. Glycerol contributes to gluconeogenesis directly, or by first going through the TCA cycle, which results in loss of glycerol carbon atoms. All bars are showing mean ± SEM, n=3.
Our glucose/glycerol measurement method also works well in serum and tissue samples. To this end, we evaluated a commonly used commercial biochemical kit (Sigma-Aldrich F6428) for “free glycerol” measurement. This kit uses a coupled enzymatic assay of glycerol kinase and glycerol phosphate oxidase. This method can measure free glycerol from serum or plasma samples. In tissue samples, however, the endogenous Gro-3-P will also be oxidized by glycerol phosphate oxidase and be measured incorrectly as free glycerol. To test the ratio of free glucose/glycerol and Glc-6-P/Gro-3-P, we compared the liver extract after kinase derivatization and after a mock derivatization. Glc-6-P levels in the mock derivatized samples were low and increased significantly after the derivatization (Figure 2D). This suggests that in liver tissue glucose is mainly present in the form of free glucose, not Glc-6-P. Gro-3-P levels, on the other hand, only increased ~2-fold after derivatization (Figure 2E). This demonstrates liver tissue has roughly equal molarity of free glycerol and Gro-3-P. Therefore, the commercial “free glycerol” kit that also measures Gro-3-P may overestimate the glycerol concentration if used in tissue sample glycerol measurements.
A clear advantage of using LC-MS to measure glucose and glycerol production in biological samples is the ability to detect and quantify isotopologues, which enables quantitative metabolic flux analysis using stable isotope tracers. Using this enzymatic derivatization method, we measured the contribution of glycerol as a gluconeogenic substrate in vivo. The mouse was infused with U-13C3-glycerol at 20nmols per gram body weight per minute. The infusion lasted 5 hours for the glycerol and glucose to reach metabolic steady state, followed by blood sampling from tail vein. At steady state at this infusion rate, circulating glycerol is 6.4% M+3 labeled (Figure 2F). The large portion of unlabeled glycerol suggests a high endogenous production rate of glycerol. The circulating glucose labeling pattern showed that the most abundant labeled fraction is M+3 (Figure 2G). This 13C3-glucose was made by combining a labeled 3-carbon unit and an unlabeled one at the aldolase step of gluconeogenesis. This is the pathway that incorporates the intact glycerol carbon backbone into newly synthesized glucose. Meanwhile, 1.9% of glucose showed 13C1 labeling, which suggested some further metabolism of glycerol is involved. Indeed, we observed the 13C3 labeled lactate in the serum (Figure 2H). The glycerol derived lactate has to go into TCA cycle where it loses 13C atoms through oxidative decarboxylation to become gluconeogenic intermediates. This is the major pathway that 13C1-glucose is made. Therefore, glycerol in vivo can contribute to glucose in two ways, either directly go through gluconeogenesis as a 3-carbon unit or indirectly through lactate and TCA cycles (Figure 2I).
Here, we describe a novel derivatization method for the accurate and sensitive detection of glucose and glycerol in samples. This method is fast and easy to perform and requires only 5μl of cell culture media or mouse serum sample. Moreover, our method is compatible to the general metabolomics workflow. Our enzymatic derivatization does not affect other metabolites, and we routinely use HILIC chromatography for our polar metabolomics studies. Therefore, when analyzing the derivatized samples, other metabolites such as amino acids and organic acids can still be reliably measured. Enabled by this new method, we discovered that glycerol makes significant amount of glucose in isolated primary hepatocytes and in mice.
Sensitive LC-MS quantitation of glucose and glycerol via enzymatic derivatization to glucose-6-phosphate and glycerol-3-phosphate.
Improvement on current quantitation methods and compatible metabolomic workflow and applicable to cells, serum, and tissue samples.
Our results suggest glycerol plays a key role in gluconeogenesis
Acknowledgement.
This research is supported by NIH grants P30 CA072720 and R01 DK063349.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Sears B, Perry M, The role of fatty acids in insulin resistance, Lipids Health Dis 14 (2015) 121. doi: 10.1186/s12944-015-0123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Puhakainen I, Koivisto VA, Yki-Järvinen H, Lipolysis and gluconeogenesis from glycerol are increased in patients with noninsulin-dependent diabetes mellitus, J. Clin. Endocrinol. Metab 75 (1992) 789–794. doi: 10.1210/jcem.75.3.1517368. [DOI] [PubMed] [Google Scholar]
- [3].Nurjhan N, Consoli A, Gerich J, Increased lipolysis and its consequences on gluconeogenesis in non-insulin-dependent diabetes mellitus, J. Clin. Invest 89 (1992) 169–175. doi: 10.1172/JCI115558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jin ES, Sherry AD, Malloy CR, Metabolism of glycerol, glucose, and lactate in the citric acid cycle prior to incorporation into hepatic acylglycerols., J. Biol. Chem 288 (2013) 14488–96. doi: 10.1074/jbc.M113.461947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Flakoll PJ, Zheng M, Vaughan S, Borel MJ, Determination of stable isotopic enrichment and concentration of glycerol in plasma via gas chromatography-mass spectrometry for the estimation of lipolysis in vivo., J. Chromatogr. B. Biomed. Sci. Appl 744 (2000) 47–54. http://www.ncbi.nlm.nih.gov/pubmed/10985565. [DOI] [PubMed] [Google Scholar]
- [6].Magni F, Arnoldi L, Monti L, Piatti P, Pozza G, Galli Kienle M, Determination of plasma glycerol isotopic enrichment by gas chromatography-mass spectrometry: an alternative glycerol derivative., Anal. Biochem 211 (1993) 327–8. http://www.ncbi.nlm.nih.gov/pubmed/8317711. [DOI] [PubMed] [Google Scholar]
- [7].Yang L, Kombu RS, Kasumov T, Zhu S-H, V Cendrowski A, David F, Anderson VE, Kelleher JK, Brunengraber H, Metabolomic and mass isotopomer analysis of liver gluconeogenesis and citric acid cycle. I. Interrelation between gluconeogenesis and cataplerosis; formation of methoxamates from aminooxyacetate and ketoacids., J. Biol. Chem 283 (2008) 21978–87. doi: 10.1074/jbc.M803454200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Thevis M, Guddat S, Flenker U, Schänzer W, Quantitative analysis of urinary glycerol levels for doping control purposes using gas chromatography-mass spectrometry., Eur. J. Mass Spectrom. (Chichester, Eng) 14 (2008) 117–25. doi: 10.1255/ejms.919. [DOI] [PubMed] [Google Scholar]
- [9].Gilker CD, Pesola GR, Matthews DE, A mass spectrometric method for measuring glycerol levels and enrichments in plasma using 13C and 2H stable isotopic tracers., Anal. Biochem 205 (1992) 172–8. http://www.ncbi.nlm.nih.gov/pubmed/1443555. [DOI] [PubMed] [Google Scholar]
- [10].Guo ZK, Lee WN, Katz J, Bergner AE, Quantitation of positional isomers of deuterium-labeled glucose by gas chromatography/mass spectrometry., Anal. Biochem 204 (1992) 273–82. http://www.ncbi.nlm.nih.gov/pubmed/1443525. [DOI] [PubMed] [Google Scholar]
- [11].Bornø A, Foged L, van Hall G, Glucose and glycerol concentrations and their tracer enrichment measurements using liquid chromatography tandem mass spectrometry., J. Mass Spectrom 49 (2014) 980–8. doi: 10.1002/jms.3407. [DOI] [PubMed] [Google Scholar]
- [12].Dong Y, Ma Y, Yan K, Shen L, Wang X, Xu Y, He G, Wu Y, Lu J, Yang Z, Feng F, Quantitative analysis of glycerol levels in human urine by liquid chromatography-tandem mass spectrometry., J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci 957 (2014) 30–5. doi: 10.1016/j.jchromb.2014.02.054. [DOI] [PubMed] [Google Scholar]
- [13].McIntosh TS, Davis HM, Matthews DE, A liquid chromatography-mass spectrometry method to measure stable isotopic tracer enrichments of glycerol and glucose in human serum., Anal. Biochem 300 (2002) 163–9. doi: 10.1006/abio.2001.5455. [DOI] [PubMed] [Google Scholar]
- [14].Rogatsky E, Jayatillake H, Goswami G, Tomuta V, Stein D, Sensitive LC MS quantitative analysis of carbohydrates by Cs+attachment, J. Am. Soc. Mass Spectrom 16 (2005) 1805–1811. doi: 10.1016/j.jasms.2005.07.017. [DOI] [PubMed] [Google Scholar]
- [15].Verardo G, Duse I, Callea A, Analysis of underivatized oligosaccharides by liquid chromatography/electrospray ionization tandem mass spectrometry with post-column addition of formic acid., Rapid Commun. Mass Spectrom 23 (2009) 1607–18. doi: 10.1002/rcm.4047. [DOI] [PubMed] [Google Scholar]
- [16].Khamis MM, Adamko DJ, Purves RW, El-Aneed A, Quantitative determination of potential urine biomarkers of respiratory illnesses using new targeted metabolomic approach., Anal. Chim. Acta 1047 (2019) 81–92. doi: 10.1016/j.aca.2018.09.035. [DOI] [PubMed] [Google Scholar]
- [17].Shah VP, Midha KK, Dighe S, McGilveray IJ, Skelly JP, Yacobi A, Layloff T, Viswanathan CT, Cook CE, McDowall RD, Analytical methods validation: bioavailability, bioequivalence and pharmacokinetic studies. Conference report., Eur. J. Drug Metab. Pharmacokinet 16 (1991) 249–55. doi: 10.1007/BF03189968. [DOI] [PubMed] [Google Scholar]
- [18].Su X, Lu W, Rabinowitz JD, Metabolite Spectral Accuracy on Orbitraps., Anal. Chem 89 (2017) 5940–5948. doi: 10.1021/acs.analchem.7b00396. [DOI] [PMC free article] [PubMed] [Google Scholar]