Abstract
Context:
Pancreatic cancer (PDAC) is one of the most challenging cancers to treat with modest recent improvements in survival from new systemic therapies. There is growing interest in individualized therapy underpinned by somatic and germline genomic alterations.
Objective:
A systematic review of data on therapies targeting somatic and germline alterations, and their downstream pathways in PDAC.
Method:
A systematic literature search was conducted using PRISMA guidelines to include relevant results published after January 1, 2008.
Results:
A total of 71 relevant studies were included. We identified 36 studies targeting the KRAS-pathway, the most common being with MEK-inhibitor therapy. Twenty-two studies were identified that evaluated platinum-based chemotherapy and PARP inhibitors in patients with deleterious mutations in DNA damage repair genes and have shown encouraging results. Immunotherapy has demonstrated activity in patients with mismatch repair deficiency/microsatellite instability.
Conclusion:
Evidence from translational and clinical research presents an exciting platform for genomic targeted therapy in PDAC. Validity for targeting BRCA with platinum and PARP inhibitors and microsatellite instability with immune therapy has been established, nonetheless, evidence for targeting the common driver oncogenes is lacking and much work is needed. Of importance is identifying the subgroup of KRAS -wild type PDAC (approximately 5%) where there is enrichment for targetable opportunities.
Keywords: Pancreatic ductal adenocarcinoma (PDAC), genomic alteration (GA), DNA damage repair, somatic mutation, germline mutation, mismatch repair (MMR), microsatellite instability
Background
Pancreatic ductal adenocarcinoma (PDAC) has gained increasing attention over the last decade as its incidence continues to rise in contrast to other solid organ malignancies, and this trend is unlikely to change with the increasing life expectancy. Despite being an uncommon solid tumor (estimated 3.2 percent of all new cancer cases in 2018) PDAC is a large contributor to the toll of cancer deaths (estimated 7.3 percent for 2018)[1, 2]. It has surpassed breast cancer as the third leading cause of cancer deaths, and with the current trend PDAC is predicted to overtake colorectal cancer to become the second leading cause of cancer-related mortality by the end of this decade[3].
The prognosis associated with PDAC has been enigmatic for years. The medical community is challenged with difficulties in early diagnosis due to delayed clinical presentation, along with lack of early diagnostic method or a consistent premalignant lesion, and the tendency to early metastasis. Collectively these are substantive constraints to better outcome. PDAC microenvironment features are characterized by marked heterogeneity with low epithelial tumor component, a dominant stroma and a lack of effector immune cells, also in part contributing to poor prognosis.
There have been subtle but definite improvements in survival, measurable in weeks to a few months with currently available multi-agent cytotoxic regimens for advanced PDAC[4–7]. However, unlike many other cancers its natural history has largely remained unchanged[3, 8]. While this trend is disappointing, it has triggered tremendous focus into the putative causes, most notably the molecular and genetic drivers of carcinogenesis. There are several genomic alterations (GA) with a primary or secondary role in tumorigenesis, including familial cancer syndromes underpinned by known single germline mutations and most individuals have mutations in key oncogenes/tumor suppressor genes. Nonetheless, translation to actionability and therapeutics from potentiality to reality for most individuals diagnosed with this disease remains to be realized.
There is growing data on the role of platinum-based chemotherapy and poly ADP ribose polymerase inhibitors (PARPi) in patients with germline mutations in the genes associated with DNA damage repair (DDR) mechanisms or homologous recombination repair (HRR)[9–12]. Somatic mutation testing on the other hand has consistently detected mutations in one or more of the tumor suppressor genes or proto-oncogenes namely, Kirsten RAS (KRAS), tumor protein P53 (P53), Mothers against decapentaplegic homolog 4 (SMAD4), cyclin-dependent kinase inhibitor 2A (CDKN2A)[13–16]. Recently, there has been tremendous research towards identification of potential targets to alter pathogenic GA, both somatic and germline or their downstream pathways. Nonetheless, there are only a few such agents approved in PDAC[17, 18].
Aims and Objectives
While there is substantive data in both translational and clinical settings addressing the potential application of genomic targeted therapies for individuals with PDAC, there is a dearth of clear evidence for specific applications in patients with GA’s to guide clinical use. Therefore, we choose to systematically review the available literature on genetically targeted therapies, specifically those targeting somatic and germline drivers of PDAC and their downstream pathways.
Methods
Preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement was followed for reporting this review[19].
Search Methods
An extensive literature search was conducted on September 5, 2018 in Medline (PubMed), Embase.com, and Cochrane Library (Wiley) by a medical librarian (JG) at Memorial Sloan Kettering (MSK), New York. Controlled vocabulary (MeSH, Emtree) and keywords were used. The searches had no language or publication type restriction. Additional keyword searches were completed using ClinicalTrials.gov and the World Health Organization’s International Clinical Trials Registry Platform. Results were limited to items published on or after January 1, 2008.
Three main categories were included in the search, combined using the Boolean operator AND: 1) pancreatic adenocarcinoma; 2) genetic alterations, including general genetic terms (e.g., genotype, genetic variation) and specific genes (e.g., KRAS, P53); and 3) therapeutics, including chemotherapy, immunotherapy, radiotherapy, and drugs and treatment more generally. All search results were saved to a citation management tool (EndNote), and the Bramer Method was used to remove duplicates[20].
For a complete list of MeSH headers and keywords, refer to the PubMed search strategy accompanying this paper.
Selection of manuscripts
The below outlined criteria were used to include and exclude studies for the systematic review. Two authors (RRS and EOR) independently screened all the abstracts for eligibility and then reviewed the full text of relevant manuscripts. The conflicts were resolved by common consensus.
Inclusion criteria:
Studies with
PDAC
Prospective or retrospective design with GA directed therapy or current therapies with GA implications,
Age 18 years and above,
Human subjects.
Clinical outcomes.
Exclusion criteria:
Studies with
Animal subjects,
In-vitro (preclinical) design or outcome,
Review articles, and
Transcriptomic, proteomic or other molecular alterations.
Results
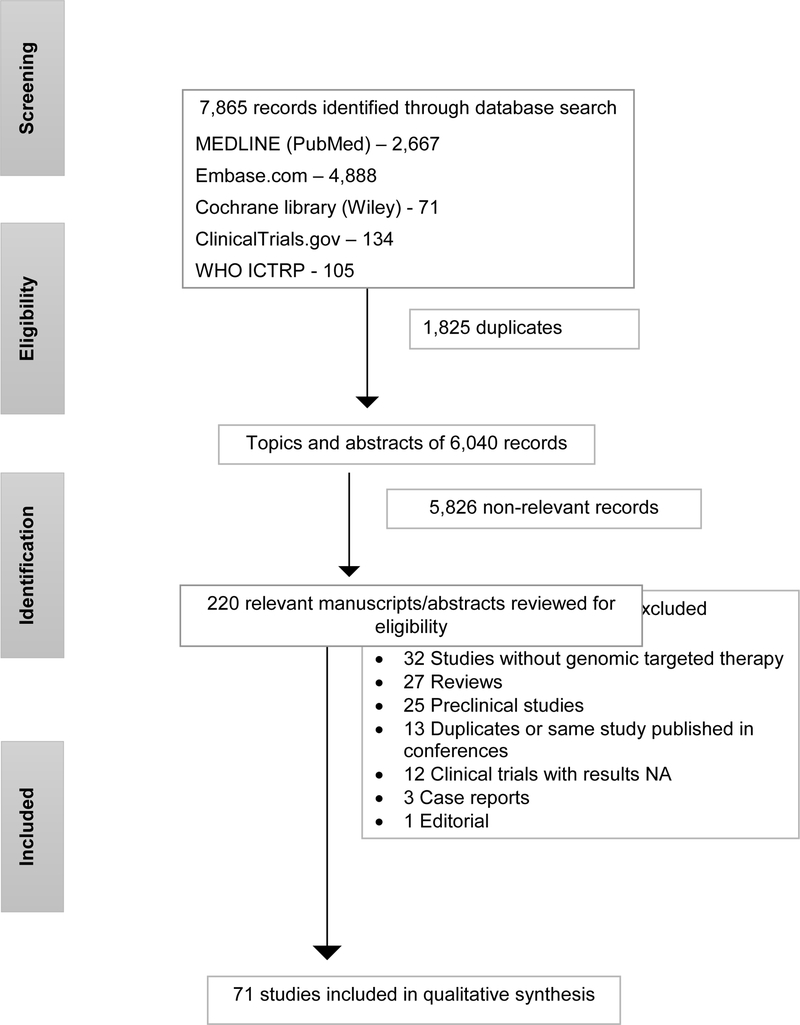
Six thousand and forty abstracts were available for review. After initial screening of the abstracts 220 were found relevant. Full-text review of these studies was performed, and 149 studies were excluded for reasons outlined in Figure 1. Thus, 71 studies were selected for final review.
Figure 1.
Flow diagram of literature search and selection of relevant studies
Somatic mutations
KRAS-targeted therapies:
Thirty-six studies were included that directly or indirectly target the KRAS pathway.
Mitogen-activated protein kinase (MEK) inhibitors
Phase 2 studies:
Three phase 2 trials evaluated MEK inhibitors in combination with gemcitabine in locally advanced and/or metastatic PDAC[21–23]. Single-agent MEK inhibitor, selumetinib was compared to capecitabine in metastatic PDAC and failed to show any survival benefit[24]. Subsequently, two phase 2 studies assessed the role of dual inhibition of KRAS downstream pathways, selumetinib in combination with erlotinib[25] and selumetinib with MK 2206 (AKT inhibitor)[26]. These clinical trials have been summarized in table 1.
Table 1.
Trials on MEK 1/2 Inhibitors
| Author, Year | Drug tested | Study design | Population | N | Outcome |
|---|---|---|---|---|---|
| Phase 1 trials | |||||
| Adjei, et al 2011 [26] | Refametinib + sorafenib | Dose escalation | HCC/non-HCC | 62 (4 PDAC) | MTD; DCR†=65.8 % |
| Infante, et al 2012[25] | Trametinib | Dose escalation | Advanced cancer | 206 (26 PDAC) | MTD, RP2D; DCR=50% |
| Bedard, et al 2015[27] | Trametinib + buparlisib | Dose escalation | RAS-/BRAF-mutant advanced cancer | 113 (24 PDAC) | MTD, RP2D; PFS=2 mo; mOS=5 mo |
| Weekes, et al 2017[28] | Cobimetinib + GDC-0994 | Dose escalation | Advanced cancer with limited options | 23 (7 PDAC) | MTD; BOR (SD+PR) = 55% |
| Phase 2 trials | |||||
| Infante, et al 2014[17] | Trametinib + Gem | Placebo controlled multicenter RCT | Untreated metastatic PDAC | 160 | mOS=8.4 Vs 6.7 mo; p= 0.453 |
| Bodoky, et al 2011[20] | Selumetinib Vs Capecitabine | Multicenter RCT | Metastatic PDAC | 70 | mOS=5.4 Vs 5.0 mo; p= 0.92 |
| Van Cutsem, et al 2018[18] | Pimasertib + Gem | Double blind multicenter RCT | Metastatic PDAC | 88 | PFS 3.7 Vs 2.8 mo; p= 0.68 |
| Van Laethem, et al 2016[19] | Refametinib + Gem | Single arm non-randomized trial | Advanced PDAC | 60 | ORR 23% |
| Ko, et al 2016[21] | Selumetinib + Erlotinib | Single arm non-randomized study | Inoperable PDAC | 46 | 24-week OS¶=58% |
| Chung, et al 2017[22] | Selumetinib + MK-2206 | Open-label RCT | Metastatic PDAC (Failed Gem) | 137 | OS: 3.9 Vs 6.7 mo; HR 1.37, p=0.15 |
Secondary outcome;
24-week OS rate of at least 43.5% was a pre-specified indicator of “Positive response”.
HCC hepatocellular carcinoma; RCT randomized controlled trial; MTD maximum tolerated dose; DCR disease control rate; RP2D recommended phase 2 dose; PFS progression free survival; mo month; mOS median overall survival; Vs versus; BOR Best overall response; SD stable disease; PR partial response; Gem gemcitabine; ORR objective response rate; HR hazard ratio.
Molecularly targeted therapy based on tumor molecular profiling versus conventional therapy for advanced cancer (SHIVA), a multicenter, open label, phase 2 randomized controlled trial (RCT) for refractory cancers assigned 195 patients with various solid tumors, including 5 with PDAC. There was no significant difference in progression free survival (PFS) or objective responses between the two arms in the overall study[27]. A retrospective analysis of 52 advanced PDAC patients for whom next generation sequencing (NGS) data was available identified 6 patients who received trametinib after experiencing three or more lines of therapy and found a PFS of 1.9 months and median overall survival (mOS) of 5.1 months. However, the main objective of the study, to find a difference in mOS based on presence or absence of KRAS or P53 was not different[28].
Phase 1 studies:
We identified four phase 1 clinical trials that evaluated the safety and efficacy of MEK inhibitors alone[29] or in combination with other modulators of KRAS pathway (phosphoinositide 3-kinase or PI3K inhibitor, Extracellular Receptor Kinase or ERK inhibitor, and multi-target kinase inhibitor, sorafenib)[30–32]. The combination of a MEK inhibitor with PI3K inhibitor or ERK inhibitor was not well tolerated with high cumulative toxicity.
Studies targeting other KRAS pathways have been summarized in Table 2.
Table 2.
Studies Targeting other KRAS Pathways
| Author, Year | Drug tested | Control | Study design | Population | N | Outcome |
|---|---|---|---|---|---|---|
| Studies targeting EGFR | ||||||
| Wang, et al 2015[44] | Erlotinib + Gem | Gem | Single center, RCT | Metastatic CT naïve PDAC | 88 | DCR=64 Vs 25%; p<0.001;mOS7.2 Vs 4.4mo; p<0.001§ |
| Philip, et al 2010[43] | Cetuximab + Gem | Gem | Phase 3 RCT | LA/metastatic PDAC | 745 | mOS 6.3 Vs 5.9 mo; p=0.23§ |
| Kim, et al 2011[41] | Panitumumab + erlotinib + Gem | Erlotinib + Gem | Phase 2 RCT | Metastatic CT naive PDAC | 92 | mOS 8.4 Vs 4.4 mo; p=0.077 |
| Chiramel, et al 2017[39] | Multiple EGFR inhibitors + CT | Standard CT | Metanalysis of RCT’s (28 studies) | LA/metastatic PDAC | 3718 | mOS (p=0.18); PFS (p=0.15) |
| Assenat, et al 2015[38] | Trastuzumab + erlotinib + Gem | NA | Phase 2, open label nonrandomized | Metastatic PDAC | 62 | DCR 74.6% |
| Fontzilas, et al 2009[40] | Gefitinib + Gem | Gem | Single arm, phase 2, nonrandomized | Advanced PDAC | 53 | mOS 4.1 mo; PFS 7.3 mo; 1-year survival 27% |
| Studies on immunologically targeting KRAS | ||||||
| Weden, et al 2010[32] | KRAS peptide vaccine | NA | Phase 2 nonrandomized | Resected PDAC | 23 | 5-year survival 29 Vs 22%; 10-year survival 20 Vs 0%‖ |
| Dueland, et al 2017[29] | TG01/GM-CSF | NA | Nonrandomized | Resected RAS-mutant PDAC | 19 | mOS 33.1 mo; DFS 13.9 mo. |
| Erickson, et al 2017[30] | TG01/GM-CSF | NA | Observational | Treatment naive unresectable PDAC | 25 | mOS5.1 (DLT)Vs 3.6 mo (no DLT); DLT 14/25 |
| Richards, et al 2012[31] | GI-4000 | Gem | Randomized, placebo-controlled | Resected RAS-mutant PDAC | 176 | RFS 41 Vs 36 wk; mOS 75 Vs 63 wk |
| Farnesyl transferase inhibition | ||||||
| Laheru, et al 2012[34] | Salirasib (FTS) + Gem | NA | Phase 1, dose escalation | CT naive LA/metastatic PDAC | 19 | mOS 6.2 mo; PFS 3.9 mo; No objective response |
| Van Custom, et al 2004[35] | Tipifarnib + Gem | Gem + placebo | Phase 3 | CT naïve advanced PDAC | 688 | mOS 193 (Tipifarnib) Vs 182 (placebo); p=0.75 |
| RNA interference (RNAi) | ||||||
| Golan, et al 2015[36] | RNAi | NA | Phase 1/2a | LA PDAC | 15 | mOS 15.4 mo; 18-mo survival 38.5% |
| Polo-like kinase 1 (PLK1) and phosphoinositide 3-kinase (PI3K) inhibitor | ||||||
| O’Neil, et al 2015[37] | Rigosertib + Gem | Gem | Multicenter, phase 2/3 RCT | Metastatic CT naïve PDAC | 160 | mOS6.1 Vs6.4 mo; Cl 0.85–1.81 |
| ERBB inhibitor | ||||||
| Heining, et al 2018[50] | Afatinib, pertuzumab, trastuzumab | NA | Retrospective analysis | KRASANT PDAC | 17 (4/17 KRAS-WT) | Objective partial response (3/3) with POD |
| ALK inhibitors | ||||||
| Singhi, et al 2017[51] | Crizotinib, ceritinib | NA | Nonrandomize d (case-series) | PDAC | 5 | SD=3/4 |
| Oncolytic virus | ||||||
| Noonan, et al 2016[47] | Pelareorep + paclitaxel/carbo platin | Paclitaxel + carboplatin | Phase 2 RCT | Metastatic PDAC | 73 | mOS 6.1 Vs 6.3 mo; p=0.6 |
| Mahalingam, et al 2018[46] | Pelareorep + Gem | NA | Single-arm, Phase 2, non-randomized | CT naive advanced PDAC | 34 | mOS 10.2 mo; SD=23/34 |
| ERK inhibitor | ||||||
| Weekes, et al 2017[49] | Cobimetinib | NA | Phase 1b, dose escalation | Advanced solid tumors | 23 | 55% (6/11) SD‡ |
KRAS status did not affect response;
compared to nonvaccinated cohort;
high cumulative toxicity.
Gem gemcitabine; RCT randomized controlled trial; CT chemotherapy; DCR disease control rate; Vs versus; mo month; mOS median overall survival; LA locally advanced; EGFR epidermal growth factor receptor; PFS progression free survival; NA not applicable; TG01 mixture of synthetic RAS peptides; GM-CSF granulocyte monocyte-colony stimulating factor; DFS disease-free survival; DLT delayed-type hypersensitivity; GI-4000 tarmogen (targeted molecular immunogen); RFS recurrence free survival; wk week; FTS S-trans, trans-farnesylthiosalicylic acid; WT wild-type; POD progression of disease; SD stable disease.
Immune Targeting of KRAS
Four studies were identified that evaluated KRAS vaccines in PDAC patients. This included three observational studies that looked at RAS peptide vaccines and one studied GI-4000, a tarmogen (targeted molecular immunogen) designed to target cells with mutant KRAS[33–36].
In addition, an open label, phase 2 trial assessed the safety and efficacy of adding personalized peptide vaccine to 41 patients with advanced PDAC whose disease had progressed following at least one line of chemotherapy. Median OS was 7.9 months and 26.8% (11/41) were alive at one year. Patients who received concurrent chemotherapy (N=33) fared better than who did not (N=8) with improved mOS (9.6 versus 3.1 months; p=0.0013)[37].
Farnesyl Transferase Inhibition
Tipifarnib, a farnesyl transferase inhibitor and S-trans, trans-farnesyl thiosalicylic acid (FTS, salirasib) that inhibits Ras-dependent growth of cells were tested in a large phase 3 trial and a phase 1 dose escalation trial in combination with gemcitabine in advanced PDAC. There was no measurable survival benefit or objective response[38, 39].
Ribonucleic Acid Interference (RNAi)
In a first in-human phase 1/2a study, Golan et al. studied the safety and efficacy of RNAi approach utilizing siG12D-LODERTM (Silenseed Ltd.) in patients with locally advanced PDAC. siG12D-LODERTM was inserted into the tumor to slowly release anti-KRASG12D siRNA. Fifteen patients were enrolled, who received standard chemotherapy in conjunction with anti-KRASG12D siRNA. Median OS was 15.4 months after a single dose of the investigational drug/device[40]. An ongoing randomized phase 2 study is evaluating gemcitabine and nab-paclitaxel with or without siG12D-LODERTM. (NCT01676259)
Polo-like kinase 1 (PLK1) and PI3K Pathway
Rigosertib, a multi-kinase inhibitor of PLK1 and PI3K, was studied in a multicenter, randomized phase 2 trial in treatment naïve metastatic PDAC in combination with gemcitabine. Majority of the tumors for which adequate sample for mutational analysis was available had mutation in KRAS while one had mutation in PI3KCA. The results are summarized in table 2[41].
Epidermal growth factor receptor (EGFR) Targeting
Seven studies, four RCT’s, two nonrandomized trials and a meta-analysis of RCT’s were found that evaluated the role of EGFR inhibition in combination with gemcitabine or other cytotoxic agents in advanced PDAC[42–48]. Although considered to a marker of tyrosine kinase inhibitor (TKI) resistance, KRAS status did not affect the outcomes[44]. The development of grade 2 or more rash was associated with improved outcome, as previously observed with TKI therapy[49].
Oncolytic Viruses
Pelareorep, a formulation of human Reovirus serotype 3 strain, which has cytotoxic effects on cancer cells with RAS oncogene mutation, was tested in combination with chemotherapy in two phase 2 RCT’s. The results are summarized in Table 2[50, 51].
ERK Inhibition
GDC0994, an oral ERK 1/2 inhibitor, was evaluated in two phase 1 trials involving 45 and 23 locally advanced or metastatic solid tumors. There was also demonstration of MAPK pathway inhibition (19 to 51%) in the paired pre- and post-treatment biopsies. GDC0994 showed some promise in advanced PDAC and BRAF-mutated colorectal cancer, although the efficacy data is limited for PDAC due to a small sample size[52, 53]. Combination of GDC0994 and cobimetinib demonstrated cumulative toxicity that were not manageable, discouraging future undertaking of trials on above combination therapy[53].
ErbB family: Epidermal Growth Factor Receptors (EGFR)
NRG1 rearrangement was identified in three of 4 KRAS wild-type PDAC in a molecular analysis of 17 PDAC patients. All had metastatic disease and received one or more prior lines of therapy. Two of the three received ERBB targeted therapy, afatinib (pan-ERBB inhibitor) and pertuzumab (monoclonal antibody that prevents interaction between ERBB receptors) while the third received trastuzumab in combination with erlotinib, nab-paclitaxel and 5-fluorouracil. All three patients showed an objective partial response (PR) at 7,8 and 12 weeks, respectively[54].
ALK Rearrangements
ALK gene translocations have been identified in 0.14 to 0.16% of PDAC[55, 56]. Although rare, they constitute a larger proportion (1.3%) of younger PDAC patients (less than 50 years). Singhi, et al performed comprehensive genomic profiling on 3,170 samples of PDAC patients and discovered ALK gene translocation in five (0.16%). Of significant and important note, all these patients were younger (<50 years) and lacked a KRAS gene alteration. Four of the 5 patients were treated with an ALK-inhibitor and three demonstrated stable disease (SD), or radiographic or biomarker response[55, 56].
NTRK fusion
Entrectinib, a TRK- and ROS-inhibitor was evaluated in three patients with PDAC (all part of a phase 2, non-randomized trial, NCT02568267), including two with TPR-NTRK fusion. All three patients showed clinical improvement with confirmed partial response in both the patients with TPR-NTRK fusion[57]. Larotrectinib, a highly selective TRK-inhibitor was evaluated in 55 selected TRK-fusion positive cancer patients (including one with PDAC). Overall response rate was 75% (13% or 7 patients complete and 62% or 34 patients partial) as determined by an independent review committee. The only patient with PDAC had 30% reduction in tumor size[58].
SMAD4
Seven studies were identified that investigated direct and indirect implications of SMAD4 expression on treatment of PDAC, however most of these address prediction of disease progression and recurrence patterns in relation to SMAD4 status. A phase 2 trial evaluated the efficacy and safety of cetuximab first in combination with gemcitabine and oxaliplatin and later with capecitabine and radiation therapy in treatment-naïve locally advanced PDAC. Median OS was 19.1 months and 1-year OS rate was 66% (Primary end-point >45% 1-year OS). SMAD4 expression was associated with local disease spread compared to metastatic spread in those with SMAD4 loss (p=0.016)[59].
A retrospective analysis of 471 resected PDAC’s demonstrated benefit from adjuvant gemcitabine-based chemotherapy in patients withSMAD4 loss (HR=0.59; 95% CI 0.42–0.82; p=0.002) compared to those with intact SMAD4[60]. In another study, intact SMAD4 correlated with improved recurrence-free survival in patients receiving erlotinib in combination with adjuvant chemo- or chemoradiotherapy compared with SMAD4 loss (17.5 versus 11.5 months; p=0.003)[61].
In a retrospective cohort of 641 advanced PDAC patients, SMAD4/DPC4 expression was associated with higher risk of locoregional recurrence and benefit from intensive local disease control in addition to systemic chemotherapy compared to those with SMAD4/DPC4 loss (HR=0.25; p=0.002)[62]. However, the above studies did not find survival difference based on SMAD4 status.
TP53
Three studies were identified that evaluated role of P53 mutation in treatment outcome. A subgroup analysis of CONKO-001 (multicenter, phase 3 randomized trial to evaluate gemcitabine in patients with PDAC following complete tumor resection)[63] cohort that received gemcitabine and overexpressed p53 in the tumor cells was compared to those with wild-type p53 expression, and found to have shorter median disease-free survival and mOS (8.5/18.2 months compared to 12.8/28.8 months; p=0.03)[64]. The above relation between P53 overexpression and diminished response to gemcitabine was not replicated in a study of 137 patients with advanced PDAC.[53] MDM2, a negative regulator of p53 expression was associated with diminished response to gemcitabine-based regimen (mOS=3.7 versus 5.8 months; p=0.048)[65].
In another analysis of patients from the CONKO-001 trial, NGS was performed on 187 patient-samples of which 97 were analyzable and 57 had a TP53 mutation which was found to be a positive predictor of benefit from adjuvant gemcitabine with improved disease-free survival (HR=0.22) compared to observation[66].
CDK pathway
There is limited clinical data on manipulation of CDK pathway in PDAC. As described below, palbociclib (CDK4/6 inhibitor) was studied in 2 patients with CDK4/6 amplification in the COMPASS trial (a prospective study to establish the feasibility of whole genome sequencing (WGS) to identify predictive genomic and transcriptomic features to guide personalized therapy), however there was no benefit in outcome[67].
Studies Evaluating Other Alterations
In a large, multicenter, non-randomized sample of 640 patients as part of the Know Your Tumor (KYT) initiative, 591 patients with PDAC histology were identified. The most common actionable GA (15%; N=92) were found in DDR genes, ATM (N=28), breast related cancer antigen (BRCA)2 (N=18), partner and localizer of BRCA2 (PALB2), Fanconi anemia complementation group (FANCA/C/G), RAD50, and checkpoint kinase 1/2 (CHEK1/2). Other actionable alterations were in ERBB2 (N=17) and isocitrate dehydrogenase (IDH1) (N= 3), PI3K/mechanistic target of rapamycin (mTOR)/AKT pathway was present in 19% of patients. Eighteen of 81 patients with wild-type KRAS had alterations in other elements of the MAPK pathway including 14 BRAF mutations. One hundred and twenty-six patients started treatment based on the KYT report, including off-label molecular targeted therapy (N=20) and clinical trial (N=26) enrollment. Patients who received matched therapy for actionable GA achieved an improved PFS (HR=0.47; p=0.03) and mOS (1.5 years versus 0.9 years) compared to those who did not[68].
Early results of the COMPASS trial indicate feasibility of prospective genomic sequencing in PDAC. Sixty-two (98%) of 63 included patients had successful WGS with a median reporting time of 35 days. Eighteen (33%) of the advanced PDAC patients had actionable GA’s. All patients received standard chemotherapy as first line therapy and 50 progressed. Five of the 50 (10%) received second line therapy based on the trial results, one with a KRAS mutation achieved disease stability with dual blockade of RAS pathway while four others, two with CDK4/6 amplification treated with palbociclib, one with high neoantigen load treated with programmed death ligand 1 (PD-L1) inhibitor and one with polyploid genome treated with PLK4 inhibitor did not experience any benefit[67].
Egeli, et al. attempted to find an association of KRAS and EGFR mutational status and the micro-RNA (miRNA) related to these GA’s with potential effect on resistance to radiotherapy. Of the six miRNAs evaluated, miR-216b and miR-217 were downregulated in tumor tissues compared to normal tissues. Fifteen patients without KRAS and EGFR mutation or induced expression did not benefit from gemcitabine or radiotherapy, including 12 patients with downregulated miR-216b expression who had reduced median survival[69].
Lowery, et al. assessed feasibility of comprehensive genetic analysis within a clinically relevant timeframe and its clinical applicability in 338 tumor samples (N=336 patients) using Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT)[70]. Median turn-around time after tissue acquisition was 20 days. Actionability scale of 1–4 was used to classify GA, where 1–2a implied standard therapeutic and 2b-4 investigational therapies including clinical trials. Using this classification 222 (68% patients) had alterations that could be or potentially be targeted (2b-4). Two of the three patients who received molecular targeted therapy had ERBB2 mutations and received trastuzumab (no response in one and details unknown in second) and one with KRAS mutation received combination of PI3K and MEK inhibitor (no response)[71].
An ongoing phase 1 trial is evaluating the role of dinaciclib in combination with an AKT inhibitor MK2206 in inoperable PDAC. (NCT01783171) Molecular Analysis for Therapy Choice (MATCH), a phase 2 trial is evaluating the benefit of genetically targeted therapy in solid tumors (including PDAC) and lymphomas that have progressed on at least one standard treatment. (NCT02465060) The study aims to evaluate different genomic targets and designed to have 30 sub-protocols to include various targets, EGFR, HER2, RAS, BRCA, mTOR, AKT, ALK, ROS, PIK3CA, MLH/MSH, BRAF, PTEN, CDK, and more.
Germline Mutation and Targeted Therapies
DNA Damage Repair (DDR) Genes
Twenty-two studies were identified that looked at the association between various DDR gene (BRCA2/1, ATM, PALB2, CHEK 1/2, ATR) mutations and therapy, particularly platinum agents and PARPi. While most of the studies are observational or retrospective, six prospective clinical trials with BRCA or PALB2 mutations were identified including two phase 1 and four phase 2, as depicted in Table 3.
Table 3.
Studies Targeting BRCA/PALB2 Mutations
| Author, Year | Drug tested | Study design | Population | N | Outcomes (Primary/secondary) |
|---|---|---|---|---|---|
| O’Reilly, et al. 2018[11] | Veliparib + Gem/Cis | Phase 1, dose escalation | Untreated BRCA/PALB2 mutated PDAC or strong FH | 17 | DLT, RP2D; mOS 23.3 Vs 11 mo (BRCA+ Vs BRCA-) |
| Kauffman, et al. 2015[99] | Olaparib | Nonrandomized phase 2 | ≥2 prior therapies, BRCA mutated | 23 PC¶ | 5/23 (21%) CR+PR; mOS 9.8 mo; PFS 4.6 mo; 8/23 SD |
| Yarchoan, et al. 2017[12] | Olaparib + Cis/mitomycin/Irinotecan | Phase 1, dose escalation | Unresectable PDAC | 18 | MTD; Grade ≥3 AE=89% |
| Domcheck, et al. 2014[9] | Rucaparib | Open label, phase 2 RCT | BRCA mutated LA/metastatic PDAC (≥1 prior treatment) | 19† | ORR3/19(16%); DCR6/19(31.6%) |
| Lowery, et al. 2018[10] | Veliparib | Single-arm phase 2 nonrandomized | BRCA/PALB2 mutated previously treated PDAC | 16 | RECIST 1.1; PFS1.7 mo.; mOS 3.1 mo.% |
| Aung, et al 2016[100] | Platinum-based CT | Prospective database | BRCA mutated PDAC | 57 | mOS 15.3 (platinum) Vs 8.3 mo (other CT) |
| Golan, et al 2017[72] | Platinum-based CT | Retrospective analysis | BRCA mutated stage 3/4 PDAC | 71 | mOS 22 (platinum) Vs 9 mo. (other CT), p=0.039 |
17 BRCA2, 5 BRCA1, 1 both BRCA2/BRCA1;
stable disease on platinum agent;
enrollment pre-terminated after an insufficient response rate as prespecified in the trial protocol.
FH family history; DLT dose limited toxicity; RP2D recommended phase 2 dose; mOS median overall survival; mo month; CR complete response; PR partial response; SD stable disease; MTD maximum tolerated dose; AE adverse event; RCT randomized controlled trial; ORR overall response rate; DCR disease control rate; RECIST Response Evaluation Criteria In Solid Tumors; CT chemotherapy; PFS progression free survival; gBRCA germline BRCA; NA not available
A multicenter, randomized, phase 2 trial is evaluating the role of adding PARPi, veliparib to the combination of cisplatin and gemcitabine in BRCA1/2- or PALB2-mutated PDAC. As part of the above trial, a nonrandomized single arm evaluated the role of single-agent veliparib in 16 previously treated, stage III/IV PDAC patients (who had received median of 2 lines of therapy) with BRCA1 (N=5) or BRCA2 (N=11) mutation. One patient had unconfirmed PR, four (25%) had SD while the rest 11 (69%) had progression. Notably, 14 patients (88%) were previously exposed to platinum agents and likely explained the poor response to the PARPi[10].
Two studies identified eight and four patients with ATM-mutated PDAC and suggested significantly improved survival with oxaliplatin-based therapy[2, 72]. A study from Japan assessed the outcome of BRCAness (BRCA2 N= 6, ATM N=4, ATR N=2, BRCA1 N=2, PALB2 N=1) in 17 PDAC patients who received oxaliplatin-based therapy. BRCAness was defined as defects in individual genes involved in HRR. Median time to treatment failure was 294 days and 52 days in BRCAness group and non-BRCAness group, respectively (p=0.027). The result is limited by small sample size[72]. The same group subsequently showed improved survival with oxaliplatin-based therapy in HRR gene mutated patients (median PFS 20.8 months versus 1.7 months; p=0.49) compared to those without HRR gene mutations. The HRR related gene mutations comprised of BRCA2 (N=10), ATM (N=8), BRCA1 (N=2), CHEK2 (N=2), ATR (N=1), and PALB2 (N=1)[2].
Several groups have retrospectively analyzed available genomic data in PDAC patients with BRCA1/2 and other DDR gene (ATM, PALB2, CHEK, ATR) mutations and demonstrated encouraging results with platinum-based therapy. Despite the retrospective design and small sample size in majority of the studies, collectively they represent data from more than 200 patients and demonstrate the role of platinum agents in the treatment of PDAC with mutation in DNA damage repair (DDR) genes[2, 72–80].
Somatic Mutations in DDR Genes
While most of the studies on DDR genes have assessed vulnerability of the tumor based on germline mutational status, a few have looked at somatic alterations as well. Shroff, et al in a single-arm, open label, phase 2 trial examined the effect of somatic or germline mutation in BRCA gene on response to rucaparib in advanced PDAC who had previously received one or two lines of therapy. Disease control rate (PR or SD at 12 weeks) was 32% (6/19) including 50% (3/6) in those who received only one prior therapy[81].
Sehdev, et al[75] assessed the effect of somatic or germline DDR gene mutations in BRCA1 (N=7), BRCA2 (N=5), PALB2 (N=3), MSH2 (N=1) and FANCF (N=1) on response to FOLFIRINOX therapy in metastatic PDAC. There is no data available to interpret results on somatic gene mutations separately. OS was improved in those with mutations in DDR genes (N=12) compared to those without mutations (N=24) (14 versus 5 months), which did not reach statistical significance (HR 0.58; p=0.08). However, multivariate logistic and Cox regression analysis determined significantly improved mOS in those with DDR gene mutations (OR=1.47; p=0.04 and HR=0.37; p=0.04).
Lowery et al. analyzed 336 PDAC patients who underwent somatic profiling at MSK for matched systemic therapy. Although a very small number of patients received matched therapy, a sizable number (N=50) had a somatic mutation in one or more DDR genes[71] Another prospective analysis of genetic data identified 15 BRCA-mutated patients. Median OS was 27.6 months in all the patients. All the 3 patients who received a PARPi and 5 of the 6 who received platinum-based chemotherapy as first line therapy for metastatic disease had at least PR by RECIST (Response Evaluation Criteria in Solid Tumors)[82].
In a prospective observational study of deep WGS in 100 PDAC’s Waddell, et al found deleterious BRCA signature gene mutations in 11 patients (BRCA1 N=2, BRCA2 N=7, and PALB2 N=2), 5 having a somatic BRCA mutation (BRCA1 N=2, BRCA2 N=3). Eight patients received platinum-based therapy including 5 with a deleterious BRCA mutation. Three of the 5 had a somatic BRCA2 mutation, two experienced exceptional response and two PR while one with somatic BRCA1 mutation had no response[73]. Loss of heterozygosity (LOH) or loss of the second allele may explain the differential response to platinum agents and PARPi in this population of PDAC with DDR gene mutations[83].
Deficient Mismatch Repair (MMR-d) and Microsatellite Instability (MSI)
Three studies with immunotherapy directed at MMR-d PDAC were found. In an analysis of 833 PDAC patients with available NGS at MSK, 7 with MMR-d were identified and all had Lynch syndrome with an underlying germline mutation. Five of the 7 patients received immunotherapy with a programmed death (PD)-1 inhibitor (N=3) or a PD-L1 inhibitor (N=2) and either had SD (N=1) or durable response (N=3)[84]. In a phase 2 study to evaluate the clinical activity of pembrolizumab in MMR-d tumors, Dung et al. demonstrated the benefit of single-agent PD-1 inhibitor in 2 MSI-high PDAC patients[85]. A retrospective review of gastrointestinal cancer patients (N=9) who received pembrolizumab (PD-1 inhibitor) for MMR-d included 2 patients with PDAC. Response data was available for one of the two patients and showed 56.7% response from baseline per RECIST criteria with time to progression >5 months[86].
Discussion
Lessons learned from failure of anti-cancer drugs across multiple malignancies at different stages of development suggest that a biomarker-driven strategy in drug selection can improve outcomes. A systematic review to evaluate reasons for experimental drug failure showed that 57% (21/37) of successful drug-programs adopted a biomarker-driven rationale compared to 16% (7/43) of failed drug-programs[87]. Similar results have been shown in phase 2 and phase 3 trials. The extrapolation of biomarker-based drug selection remains unproven in PDAC, nevertheless it is a potential strategy[88, 89].
Our qualitative analysis of the literature identifies several genomic targets that have been explored in the treatment of PDAC. While DDR gene mutations and MMR-d have demonstrated greatest potential for actionability several other targets, notably common somatic mutations await further attestation in large prospective studies to provide unambiguous answers to personalizing therapy in PDAC. The most interesting data regarding GA driven therapy relate to platinum-based therapy and PARPi in patients with germline mutations in DDR- or HHR-genes, and immune checkpoint inhibitors in patients with MMR-d genes.
Pathogenic germline mutations in BRCA1/2 and related genes are found in 4.6 to 8% of PDAC in different series[90–92]. While germline alterations in DNA double-stranded break-repair and HRR predispose to tumorigenesis, they also provide vulnerable targets for agents like platinum which induce double-strand breaks and PARPi which block single-strand break repair subsequently leading to double-strand breaks. This concept has been tested in retrospective analyses and prospective trials, further replication of the results is awaited from ongoing clinical trials[13]. Single-agent olaparib is being evaluated in a phase 3 RCT (POLO) for maintenance therapy in germline BRCA mutated metastatic PDAC whose disease has not progressed on first line platinum-based therapy. (NCT02184195) Meanwhile, a phase 2 clinical trial is evaluating veliparib combined with platinum-based therapy in patients with BRCA1/2 or PALB2 mutation. (NCT01585805)
There is a paucity of data on somatic alterations in BRCA1/2 and related genes in PDAC. This has been investigated more recently with identification of somatic mutations at varying rates in pancreatic cancer specimen across different studies, ranging from as low as about 4% in an earlier study by Chantrill, et al[93] to over 35% in a sample of 109 micro-dissected pancreatic cancer cases which identified multiple Fanconi anemia genes, ATM, CHEK2, BCLAF1, BRCA1, BRCA2[94]. The benefit of treatment with platinum agents in these tumors has been comparable to those with germline DDR gene mutations in small retrospective and prospective series[73, 75].
It has been appreciated that tumors with MMR-d have enhanced expression of mutation-associated neoantigens and strong expression of immune check-point ligands. Correspondingly, they have 10–100 times higher number of somatic mutations compared to those with proficient MMR genes[85]. This concept has been successfully manipulated in tumors like melanoma, renal cell cancers and lung cancers[85, 95, 96]. Although MMR-d is detected in a very small number (approximately 1%) of PDAC the benefit from PD-1 and PD-L1 has been shown to prolong survival in this population (OS=30–214 months)[84–86].
Mutations affecting the KRAS gene although the most common somatic gene alteration has yet to be effectively targeted. Recent discovery of KRAS-G12C inhibitors have potential, however G12D and G12V account for about 80% of PDAC KRAS mutations and G12C mutations are rare.[97, 98] Attempts to target the downstream KRAS pathways through MEK inhibitors have largely been disappointing. This is attributed to the adaptive reactivation of MAPK signaling and multiple pathway redundancy[99]. SHP2 or PTPN11 are mediators of the adaptive MAPK response to MEK inhibitor treatment, and consequent discovery of SHP2 inhibitors reopens interest in this target[100]. Dual targeting of this pathway with a MEK inhibitor and EGFR inhibitor or dual inhibition of the EGFR pathway has shown some positive results[44, 45]. Furthermore, solely targeting other KRAS pathway molecules like PI3K or mTOR have been unsuccessful. A translational study investigated the bypass mechanisms in KRAS-mutant colorectal cancer and revealed enhancement of other pathways like EGFR, ERBB2 and ERBB3 accounting for resistance to the targeted therapy. This observation suggests potential role of combining multiple pathways to overcome the resistance barrier[101]. On the other hand, tumors with a wild-type KRAS gene are found to have enrichment of kinases, like Neuregulin 1 (NRG1) rearrangement, anaplastic lymphoma kinase (ALK) rearrangement, ROS and NTRK fusions. These are attractive targets in this subset of patients with wild-type KRAS gene.
Cell cycle checkpoints and the corresponding CDK’s are vulnerable sites for oncogenesis. Preclinical xenograft and in-vitro studies have shown growth inhibition by targeting multi-CDK inhibitors. Two ongoing phase 1 trials (NCT02501902, NCT02897375) are evaluating palbociclib in combination with chemotherapy (nab-paclitaxel or cisplatin/carboplatin). Ribociclib, another CDK inhibitor, is being evaluated in phase 1/2 trials (NCT02985125, NCT02703571) in previously treated PDAC in combination with other targets, everolimus and trametinib, respectively.
Although there have been glimpses of success with some of the paths employed to target the KRAS pathway, there is little correlation between the somatic gene (KRAS, SMAD4, P53, CDKN2A) mutational status and efficacy of these tested therapies. These outcomes speak to the complex inter-relationship between drivers of oncogenesis, pathway redundancy, factors known and unknown, and activation of bypass tracks.
Severe clinical trials are on way to provide more consolidated evidence to guide individualized approach to treating PDAC. These studies have been summarized in Table 4.
Table 4.
Ongoing Clinical Trials
| Clinical trial | Drug tested | Control | Study design | Population | N¶ | Primary outcome |
|---|---|---|---|---|---|---|
| Agents targeting DNA damage repair (DDR) - PARP inhibitors | ||||||
| NCT02184195 | Olaparib (maintenance) | Placebo | Phase 3, double blind RCT | gBRCA mutant metastatic PDAC | 154 | PFS |
| NCT01585805 | Gem/cis + Veliparib | Gem/Cis | Phase 2 RCT | BRCA/PALB2 mutant PDAC | 107 | Response rate by RECIST |
| NCT02890355 | mFOLFIRI + Veliparib | mFOLFIRI | Phase 2 RCT | Metastatic PDAC | 143 | mOS |
| NCT01489865 | Veliparib + mFOLFOX | NA | Phase 1/2 | Metastatic PDAC | 79 | DLT |
| NCT03553004 | Niraparib | NA | Phase 2, single arm | Previously treated metastatic PDAC | 18 | ORR (PR+SD) |
| NCT03337087 | Rucaparib + Nal-IRI + FU + leucovorin | NA | Phase 1/1 b, open label | Metastatic GI cancers§ | 110 | DLT, ORR |
| CDK inhibitors | ||||||
| NCT02501902 | Pablociclib + nab-P | NA | Phase 1, dose escalation trial | Metastatic PDAC | 77 | DLT |
| NCT02897375 | Palbociclib + Cis or Carboplatin | NA | Phase 1, dose escalation | Advanced solid tumors | 90 | RP2D, DLT |
| NCT02703571 | Ribociclib + Trametinib | NA | Phase 1/2, nonrandomized | Advanced PDAC/CRC | 150 | ORR, DLT |
| NCT02985125 | Ribociclib + everolimus | NA | Phase 1/2, nonrandomized | Metastatic CT resistant PDAC | 44 | PFS at 8 weeks |
| MEK inhibitor | ||||||
| NCT03637491 | talazoparib + Binimetinib + avelumab | Binimetinib + avelumab | Phase 1b/2 open label, RCT | LA/metastatic RAS mutant solid tumors‖ | 127 | DLT, ORR (CR+PR) |
| P53 targeting | ||||||
| NCT02340117 | SGT53 + Gem/nab-P | NA | Phase 2, single arm | Metastatic PDAC | 28 | PFS at 5.5 month |
| Other targets/lmmunotherapy | ||||||
| NCT01676259 | Gem/nab-P ± siG12D-L0DER | Gem/nab-P | Phase 2 RCT | LA PC | 80 | PFS |
| NCT02243371 | GVAX + CY + nivolumab Vs GVAX + CY | GVAX + CY + CRS-207 | Phase 2 RCT | Previously treated metastatic PDAC | 96 | mOS |
| NCT01896869 | Ipilimumab + GVAX | NA | Phase 2 RCT | Metastatic PDAC | 83 | mOS |
| NCT02383433 | Regorafenib + Gem | NA | Phase 2, single group | Previously treated metastatic PDAC | 2 | PFS |
| NCT01652976 | Dasatinib + FOLFOX | NA | Phase 2, single group | Metastatic PDAC | 58 | PFS |
| NCT02699749 | TAK-931† | NA | Phase 1, dose escalation | Advanced solid tumors | 100 | DLT |
Estimated sample size;
Pancreatic, colorectal, gastroesophageal and biliary tract cancers;
(PDAC, NSCLC, other RAS mutant solid tumors;
CDC7 (cell division cycle 7-related protein kinase).
RCT randomized controlled trial; gBRCA germline BRCA; PFS progression free survival; RECIST Response Evaluation Criteria in Solid Tumors; mFOLFIRI modified regimen containing Folic acid, Fluorouracil, Irinotecan; mOS median overall survival; mFOLFOX modified regimen containing Folic acid, Fluorouracil, Oxaliplatin; CRS-207 live attenuated Listeria monocytogenes strain; FOLFIRINOX Folic acid, Fluorouracil, Irinotecan, Oxaliplatin; NA not applicable; DLT dose limited toxicity; ORR objective response rate; CR complete response; PR partial response; NaI-IRI liposomal irinotecan; FU fluorouracil; GI gastrointestinal; nab-P nanoparticle albumin&-bound paclitaxel; SGT53 Study of Combined Targeted p53 Gene Therapy; RP2D recommended phase 2 dose; LA locally advanced.
Ongoing endeavors to personalize therapy in PDAC has elucidated several potentially actionable somatic GA (e.g. KRAS-wild type, ALK rearrangement, MMR-d, DDR)[54, 56, 71] most of which individually account for a small fraction of PDAC population, although for individual patients there are significant implications. Detection of these sporadic GA is only feasible through widespread application of somatic and germline genetic testing in PDAC patients. Until recently, genetic testing was limited to patients with family history of hereditary breast or ovarian cancer-related cancers, or Ashkenazi Jewish ancestry. Over the last few years germline and somatic testing has been integrated at many large dedicated cancer centers and the current National Comprehensive Cancer Network (NCCN) guideline has boosted those efforts. The updated NCCN guidelines recommend consideration of routine testing for somatic and germline mutations in all individuals with a diagnosis of PDAC[102].
Conclusion
Evidence from translational and clinical research presents an exciting platform for genomic targeted therapy in PDAC. Current literature supports the use of platinum-based therapy in patients with germline mutations in BRCA-mutated PDAC and to consider PARPi therapy. Evidence is mounting that all patients with advanced PDAC should undergo both germline and somatic profiling and that there is a significant minority of patients who will benefit from a targeted therapeutic strategy. For many GA identified in PDAC beyond BRCA and MSI, it remains to be seen what the impact from targeted therapy will be. Other approaches that will yield therapeutic refinements include pathologic and transcriptomic profiling where increasing data suggests that there are several subtypes of PDAC, a classical and basal type. The latter demonstrating increased treatment resistance compared to the former. Ongoing work will help utilize this information in real-time to optimize treatment decision making.[14, 67]
Supplementary Material
Highlights.
Genomic alterations in PDAC, both germline and somatic, represent potential targeting opportunities to individualize and tailor therapy.
Attempts to target key somatic driver mutations in PDAC (KRAS, p53, SMAD4, CDKN2A) have yielded no impact on outcome.
DNA-damage repair gene mutations confer vulnerability to platinum agents and PARP-inhibitors and early promise has been identified in PDAC.
Microsatellite unstable PDAC, approximately 1% of all PDAC’s, can benefit from checkpoint point inhibitor therapy.
Identification of the KRAS wild-type subset of PDAC (about 5%) is important in view of the enrichment for actionable targets, including, ALK, ROS, NTRK, NRG-1 fusions and others.
Universal genetic profiling is recommended for patients with advanced PDAC.
Acknowledgements
Funding Support
Cancer Center Support Grant P30 −17 CA008748
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest:
A.M. Varghese: Research funding to MSK: Lilly, Taiho Pharmaceuticals, Bristol Myers Squibb, Silenseed.
K.H. Yu: Research funding to MSK: Halozyme, BMS. Consulting/Advisory: Halozyme, Ipsen.
E.M. O’Reilly: Research funding to MSK: Genentech, Roche, BMS, Halozyme, Celgene, MabVax Therapeutics, ActaBiologica, OncoMed, Momenta Pharmaceuticals, Parker Institute, AstraZenica, Silenseed, Incyte, Pfizer, Polaris, Lustgarten Foundation, NCI-CTEP. Consulting/Advisory: Cytomx, BioLineRx, Targovax, Halozyme, Celgene, Bayer, Loxo, Polaris, Sobi.
All other authors have no conflicts to declare.
Bibliography
- [1].Institute NC. Cancer Stat Facts: Pancreatic cancer. April 2018. ed: Surveillance, Epidemiology and End Results Program; 2018. [Google Scholar]
- [2].Kondo T, Kanai M, Kou T, Sakuma T, Mochizuki H, Kamada M, et al. Association between homologous recombination repair gene mutations and response to oxaliplatin in pancreatic cancer. Oncotarget. 2018;9:19817–25. 10.18632/oncotarget.24865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- [4].Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N Engl J Med. 2011;364:1817–25. 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- [5].Paulson AS, Tran Cao HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144:1316–26. 10.1053/j.gastro.2013.01.078. [DOI] [PubMed] [Google Scholar]
- [6].Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang-Gillam A, Li C-P, Bodoky G, Dean A, Shan Y-S, Jameson G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. The Lancet. 2016;387:545–57. 10.1016/S0140-6736(15)00986-1. [DOI] [PubMed] [Google Scholar]
- [8].Noone AM HN, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA SEER Cancer Statistics Review, 1975–2015, National Cancer Institute; Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018. 2018. [Google Scholar]
- [9].Domchek SM, McWilliams R, Hendifar A, Shroff RT, Leichman L, Epelbaum R, et al. A phase 2, open-label study of the PARP inhibitor rucaparib in patients with pancreatic cancer and a known deleterious BRCA mutation. Cancer research Conference: AACR special conference on pancreatic cancer: innovations in research and treatment 2014 new orleans, LA united states Conference start: 20140518 conference end: 20140521 Conference publication: (varpagings). 2015;75 10.1158/1538-7445.panca2014-b102. [DOI] [Google Scholar]
- [10].Lowery MA, Kelsen DP, Capanu M, Smith SC, Lee JW, Stadler ZK, et al. Phase II trial of veliparib in patients with previously treated BRCA-mutated pancreas ductal adenocarcinoma. Eur J Cancer. 2018;89:19–26. 10.1016/j.ejca.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].O’Reilly EM, Lee JW, Lowery MA, Capanu M, Stadler ZK, Moore MJ, et al. Phase 1 trial evaluating cisplatin, gemcitabine, and veliparib in 2 patient cohorts: Germline BRCA mutation carriers and wild-type BRCA pancreatic ductal adenocarcinoma. Cancer. 2018;124:1374–82. 10.1002/cncr.31218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yarchoan M, Myzak MC, Johnson BA 3rd, De Jesus-Acosta A, Le DT, Jaffee EM, et al. Olaparib in combination with irinotecan, cisplatin, and mitomycin C in patients with advanced pancreatic cancer. Oncotarget. 2017;8:44073–81. 10.18632/oncotarget.17237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chiaravalli M, Reni M, O’Reilly EM. Pancreatic ductal adenocarcinoma: State-of-the-art 2017 and new therapeutic strategies. Cancer Treat Rev. 2017;60:32–43. 10.1016/j.ctrv.2017.08.007. [DOI] [PubMed] [Google Scholar]
- [14].O’Kane G, Fischer S, Denroche R, Ho Jang G, Zhang A, Dodd A, et al. Integrative molecular profiling and response to chemotherapy on the COMPASS trial2019. 10.1200/JCO.2019.37.4_suppl.188. [DOI]
- [15].Puleo F, Nicolle R, Blum Y, Cros J, Elarouci N, Franchimont D, et al. Clinical application and potential usefulness of targeted next-generation sequencing on resected pancreatic ductal adenocarcinoma. 2018;78. [Google Scholar]
- [16].Qian ZR, Rubinson DA, Nowak JA, Morales-Oyarvide V, Dunne RF, Kozak MM, et al. Association of Alterations in Main Driver Genes With Outcomes of Patients With Resected Pancreatic Ductal Adenocarcinoma. 2018;4:e173420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib Plus Gemcitabine Compared With Gemcitabine Alone in Patients With Advanced Pancreatic Cancer: A Phase III Trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. 10.1200/jco.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- [18].Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD. et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med,372 (26) (2015, pp. 2509–2520. 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. Journal of the Medical Library Association : JMLA. 2016;104:240–3. 10.3163/1536-5050.104.3.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Infante JR, Somer BG, Park JO, Li CP, Scheulen ME, Kasubhai SM, et al. A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur J Cancer. 2014;50:2072–81. 10.1016/j.ejca.2014.04.024. [DOI] [PubMed] [Google Scholar]
- [22].Van Cutsem E, Hidalgo M, Canon JL, Macarulla T, Bazin I, Poddubskaya E, et al. Phase I/II trial of pimasertib plus gemcitabine in patients with metastatic pancreatic cancer. Int J Cancer. 2018. 10.1002/ijc.31603. [DOI] [PubMed] [Google Scholar]
- [23].Van Laethem JL, Riess H, Jassem J, Haas M, Martens UM, Weekes C, et al. Phase I/II Study of Refametinib (BAY 86–9766) in Combination with Gemcitabine in Advanced Pancreatic cancer. Target Oncol. 2017;12:97–109. 10.1007/s11523-016-0469-y. [DOI] [PubMed] [Google Scholar]
- [24].Bodoky G, Timcheva C, Spigel DR, La Stella PJ, Ciuleanu TE, Pover G, et al. A phase II open-label randomized study to assess the efficacy and safety of selumetinib (AZD6244 [ARRY-142886]) versus capecitabine in patients with advanced or metastatic pancreatic cancer who have failed first-line gemcitabine therapy. Invest New Drugs. 2012;30:1216–23. 10.1007/s10637-011-9687-4. [DOI] [PubMed] [Google Scholar]
- [25].Ko AH, Bekaii-Saab T, Van Ziffle J, Mirzoeva OM, Joseph NM, Talasaz AA, et al. A multicenter, open-label phase II clinical trial of combined MEK plus EGFR inhibition for chemotherapy-refractory advanced pancreatic adenocarcinoma. Clin Cancer Res. 2016;22:61–8. 10.1158/1078-0432.CCR-15-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chung V, McDonough S, Philip PA, Cardin D, Wang-Gillam A, Hui L, et al. Effect of Selumetinib and MK-2206 vs Oxaliplatin and Fluorouracil in Patients With Metastatic Pancreatic Cancer After Prior Therapy: SWOG S1115 Study Randomized Clinical Trial. JAMA Oncol. 2017;3:516–22. 10.1001/jamaoncol.2016.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Le Tourneau C, Delord JP, Gonçalves A, Gavoille C, Dubot C, Isambert N, et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): A multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. lancet Oncol. 2015;16:1324–34. 10.1016/S1470-2045(15)00188-6. [DOI] [PubMed] [Google Scholar]
- [28].Kundranda MN, Donald Peter B, Kramer K, Ai J, Tan BA, Kilmant E, et al. Next-generation sequencing (NGS) to identify potential therapeutic targets in advanced pancreatic cancer. J Clin Oncol. 2015;33 10.1200/jco.2015.33.3_suppl.357. [DOI] [Google Scholar]
- [29].Infante JR, Fecher LA, Falchook GS, Nallapareddy S, Gordon MS, Becerra C, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:773–81. 10.1016/s1470-2045(12)70270-x. [DOI] [PubMed] [Google Scholar]
- [30].Adjei AA, Richards DA, El-Khoueiry A, Becerra CHR, Stephenson JJ, Leffingwell DP, et al. Safety, pharmacokinetic, and pharmacodynamic results of BAY 86–9766, an oral MEK inhibitor, in combination with sorafenib, an oral multikinase inhibitor, in advanced cancer patients. Mol Cancer Ther. 2011;10 10.1158/1535-7163.TARG-11-A88. [DOI] [Google Scholar]
- [31].Bedard PL, Tabernero J, Janku F, Aw Z, Paz-Ares L, Vansteenkiste J, et al. A Phase Ib dose-escalation study of the oral pan-PI3K inhibitor buparlisib (BKM120) in combination with the oral MEK1/2 inhibitor trametinib (GSK1120212) in patients with selected advanced solid tumors. Clin Cancer Res. 2015;21:730–8. 10.1158/1078-0432.CCR-14-1814. [DOI] [PubMed] [Google Scholar]
- [32].Weekes CD, Lockhard A, Lorusso P, Murray E, Park E, Tagen M, et al. A Phase Ib study to evaluate the MEK inhibitor cobimetinib in combination with the ERK1/2 inhibitor GDC-0994 in patients with advanced solid tumors. AACR Annual Meeting. 2017;77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dueland S, Valle JW, Bell K, Faluyi O, Staiger H, Gjertsen TJ, et al. TG01/GM-CSF and adjuvant gemcitabine in patients with resected RAS-mutant adenocarcinoma of the pancreas. Ann Oncol. 2017;28:v227–v8. [Google Scholar]
- [34].Eriksen JA, Gladhaug IP, Rosseland A, Risberg Handeland K, Buanes T. An observational clinical study with RAS peptide vaccine TG01 evaluating immune response, safety and overall survival in patients with non-resectable pancreatic cancer. Ann Oncol. 2017;28:v409–v10. [Google Scholar]
- [35].Richards D, Muscarella P, Bekaii-Saab T, Wilfong L, Rosemurgy A, Ross S, et al. A phase 2 adjuvant trial of GI-4000 plus gemcitabine vs. Gemcitabine alone in ras+ patients with resected pancreas cancer: R1 subgroup analysis. Ann Oncol. 2012;23:iv5 22774231 [Google Scholar]
- [36].Weden S, Klemp M, Gladhaug IP, Moller M, Eriksen JA, Gaudernack G, et al. Long-term follow-up of patients with resected pancreatic cancer following vaccination against mutant K-ras. Int J Cancer.128:1120–8. 10.1002/ijc.25449. [DOI] [PubMed] [Google Scholar]
- [37].Yutani S, Komatsu N, Yoshitomi M, Matsueda S, Yonemoto K, Mine T, et al. A phase II study of a personalized peptide vaccination for chemotherapy-resistant advanced pancreatic cancer patients. Oncol Rep. 2013;30:1094–100. 10.3892/or.2013.2556. [DOI] [PubMed] [Google Scholar]
- [38].Laheru D, Shah P, Rajeshkumar NV, McAllister F, Taylor G, Goldsweig H, et al. Integrated preclinical and clinical development of S-trans, trans-Farnesylthiosalicylic Acid (FTS, Salirasib) in pancreatic cancer. Invest New Drugs. 2012;30:2391–9. 10.1007/s10637-012-9818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22:1430–8. 10.1200/JCO.2004.10.112. [DOI] [PubMed] [Google Scholar]
- [40].Golan T, Khvalevsky EZ, Hubert A, Gabai RM, Hen N, Segal A, et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget. 2015;6:24560–70. 10.18632/oncotarget.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].O’Neil BH, Scott AJ, Ma WW, Cohen SJ, Leichman L, Aisner DL, et al. A phase II/III randomized study to compare the efficacy and safety of rigosertib plus gemcitabine versus gemcitabine alone in patients with previously untreated metastatic pancreatic cancer. Ann Oncol. 2015;26:1923–9. 10.1093/annonc/mdv264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Assenat E, Mineur L, Mollevi C, Lombard-Bohas C, Mazard T, Samalin E, et al. Phase II study evaluating the association of gemcitabine, trastuzumab, and erlotinib as first-line treatment in patients with metastatic pancreatic adenocarcinoma (GATE 1). J Clin Oncol. 2015;33 10.1200/jco.2015.33.3_suppl.379. [DOI] [PubMed] [Google Scholar]
- [43].Chiramel J, Backen AC, Pihlak R, Lamarca A, Frizziero M, Tariq NU, et al. Targeting the Epidermal Growth Factor Receptor in Addition to Chemotherapy in Patients with Advanced Pancreatic Cancer: A Systematic Review and Meta-Analysis. Int J Mol Sci. 2017;18 10.3390/ijms18050909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fountzilas G, Bobos M, Kalogera-Fountzila A, Xiros N, Murray S, Linardou H, et al. Gemcitabine combined with gefitinib in patients with inoperable or metastatic pancreatic cancer: a phase II Study of the Hellenic Cooperative Oncology Group with biomarker evaluation. Cancer Invest.26:784–93. 10.1080/07357900801918611. [DOI] [PubMed] [Google Scholar]
- [45].Kim GP, Foster NR, Salim M, Flynn PJ, Moore DF, Zon R, et al. Randomized phase II trial of panitumumab, erlotinib, and gemcitabine (PGE) versus erlotinib-gemcitabine (GE) in patients with untreated, metastatic pancreatic adenocarcinoma. J Clin Oncol. 2011;29 10.1200/jco.2011.29.15_suppl.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kullmann F, Hartmann A, Stohr R, Messmann H, Dollinger MM, Trojan J, et al. KRAS mutation in metastatic pancreatic ductal adenocarcinoma: results of a multicenter phase II study evaluating efficacy of cetuximab plus gemcitabine/oxaliplatin (GEMOXCET) in first-line therapy. Oncology. 2011;81:3–8. 10.1159/000330194. [DOI] [PubMed] [Google Scholar]
- [47].Philip PA, Benedetti J, Corless CL, Wong R, O’Reilly EM, Flynn PJ, et al. Phase III Study Comparing Gemcitabine Plus Cetuximab Versus Gemcitabine in Patients With Advanced Pancreatic Adenocarcinoma: Southwest Oncology Group–Directed Intergroup Trial S0205. J Clin Oncol. 2010;28:3605–10. 10.1200/jco.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang JP, Wu CY, Yeh YC, Shyr YM, Wu YY, Kuo CY, et al. Erlotinib is effective in pancreatic cancer with epidermal growth factor receptor mutations: a randomized, open-label, prospective trial. Oncotarget. 2015;6:18162–73. 10.18632/oncotarget.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Perez-Soler R, Delord JP, Halpern A, Kelly K, Krueger J, Sureda BM, et al. HER1/EGFR inhibitor-associated rash: future directions for management and investigation outcomes from the HER1/EGFR inhibitor rash management forum. Oncologist. 2005;10:345–56. 10.1634/theoncologist.10-5-345. [DOI] [PubMed] [Google Scholar]
- [50].Mahalingam D, Goel S, Aparo S, Patel Arora S, Noronha N, Tran H, et al. A Phase II Study of Pelareorep (REOLYSIN((R))) in Combination with Gemcitabine for Patients with Advanced Pancreatic Adenocarcinoma. Cancers (Basel). 2018;10 10.3390/cancers10060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Noonan AM, Farren MR, Geyer SM, Huang Y, Tahiri S, Ahn D, et al. Randomized Phase 2 Trial of the Oncolytic Virus Pelareorep (Reolysin) in Upfront Treatment of Metastatic Pancreatic Adenocarcinoma. Mol Ther. 2016;24:1150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Varga A, Soria JC, Hollebecque A, LoRusso P, Vaishampayan U, Okrah K, et al. A first-in-human phase I study to evaluate the ERK1/2 inhibitor GDC-0994 in patients with advanced solid tumors. Eur J Cancer. 2016;69:S11 10.1016/S0959-8049(16)32624-7. [DOI] [PubMed] [Google Scholar]
- [53].Weekes CD LA, LoRusso P, Murray ER, Park E, Tagen M, Mueller L, Dokainish H, Shapiro GI, Burris HA. A Phase Ib study to evaluate the MEK inhibitor cobimetinib in combination with the ERK1/2 inhibitor GDC-0994 in patients with advanced solid tumors. AACR Annual Meeting. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Heining C, Horak P, Uhrig S, Codo PL, Klink B, Hutter B, et al. NRG1 Fusions in KRAS Wild-Type Pancreatic Cancer. Cancer Discov. 2018. 10.1158/2159-8290.cd-18-0036. [DOI] [PubMed] [Google Scholar]
- [55].Singhi A, Ali SM, Greenbowe J, Ross JS, Nguyen K, Nikiforova M, et al. A clinicopathologic study of ALK rearrangements in pancreatic ductal adenocarcinoma. Lab Invest. 2016;96:448A–9A. [DOI] [PubMed] [Google Scholar]
- [56].Singhi AD, Ali SM, Lacy J, Hendifar A, Nguyen K, Koo J, et al. Identification of Targetable ALK Rearrangements in Pancreatic Ductal Adenocarcinoma. J Natl Compr Canc Netw. 2017;15:555–62. 10.6004/jnccn.2017.0058. [DOI] [PubMed] [Google Scholar]
- [57].Pishvaian MJ, Rolfo CD, Liu SV, Multani PS, Maneval EC, Garrido-Laguna I. Clinical benefit of entrectinib for patients with metastatic pancreatic cancer who harbor NTRK and ROS1 fusions. 2018;36:521-. 10.1200/JCO.2018.36.4_suppl.521. [DOI] [Google Scholar]
- [58].Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med. 2018;378:731–9. 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Crane CH, Varadhachary GR, Yordy JS, Staerkel GA, Javle MM, Safran H, et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol.29:3037–43. 10.1200/JCO.2010.33.8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bachet JB, Marechal R, Demetter P, Bonnetain F, Couvelard A, Svrcek M, et al. Contribution of CXCR4 and SMAD4 in predicting disease progression pattern and benefit from adjuvant chemotherapy in resected pancreatic adenocarcinoma. Ann Oncol. 2012;23:2327–35. 10.1093/annonc/mdr617. [DOI] [PubMed] [Google Scholar]
- [61].Herman JM, Fan KY, Wild AT, Wood LD, Blackford AL, Donehower RC, et al. Correlation of Smad4 status with outcomes in patients receiving erlotinib combined with adjuvant chemoradiation and chemotherapy after resection for pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2013;87:458–9. 10.1016/j.ijrobp.2013.06.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Shin SH, Kim HJ, Hwang DW, Lee JH, Song KB, Jun E, et al. The DPC4/SMAD4 genetic status determines recurrence patterns and treatment outcomes in resected pancreatic ductal adenocarcinoma: A prospective cohort study 2017;8:17945–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: The conko-001 randomized trial. JAMA. 2013;310:1473–81. 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- [64].Striefler JK, Sinn M, Pelzer U, Juhling A, Wislocka L, Bahra M, et al. P53 overexpression and Ki67-index are associated with outcome in ductal pancreatic adenocarcinoma with adjuvant gemcitabine treatment. Pathol Res Pract. 2016;212:726–34. 10.1016/j.prp.2016.06.001. [DOI] [PubMed] [Google Scholar]
- [65].Yang SH, Lee JC, Guo JC, Kuo SH, Tien YW, Kuo TC, et al. Association of MDM2 expression with shorter progression-free survival and overall survival in patients with advanced pancreatic cancer treated with gemcitabine-based chemotherapy. PLoS ONE. 2017;12 10.1371/journal.pone.0180628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sinn M, Budczies J, Damm F, Lohneis P, Schmuck R, Treue D, et al. TP53 mutation predicts sensitivity to adjuvant gemcitabine in pancreatic cancer: results from the CONKO-001 study. Ann Oncol. 2017;28:v251 10.1093/annonc/mdx369. [DOI] [Google Scholar]
- [67].Aung KL, Fischer SE, Denroche RE, Jang GH, Dodd A, Creighton S, et al. Genomics-Driven Precision Medicine for Advanced Pancreatic Cancer: Early Results from the COMPASS Trial. Clin Cancer Res. 2018;24:1344–54. 10.1158/1078-0432.CCR-17-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Pishvaian MJ, Bender RJ, Halverson D, Rahib L, Hendifar AE, Mikhail S, et al. Molecular Profiling of Patients with Pancreatic Cancer: Initial Results from the Know Your Tumor Initiative. Clin Cancer Res. 2018;24:5018–27. 10.1158/1078-0432.CCR-18-0531. [DOI] [PubMed] [Google Scholar]
- [69].Egeli U, Tezcan G, Cecener G, Tunca B, Demirdogen Sevinc E, Kaya E, et al. miR-216b Targets FGFR1 and Confers Sensitivity to Radiotherapy in Pancreatic Ductal Adenocarcinoma Patients Without EGFR or KRAS Mutation. Pancreas. 2016;45:1294–302. 10.1097/mpa.0000000000000640. [DOI] [PubMed] [Google Scholar]
- [70].Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. 2015;17:251–64. 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lowery MA, Jordan EJ, Basturk O, Ptashkin RN, Zehir A, Berger MF, et al. Real-Time Genomic Profiling of Pancreatic Ductal Adenocarcinoma: Potential Actionability and Correlation with Clinical Phenotype. Clin Cancer Res. 2017;23:6094–100. 10.1158/1078-0432.CCR-17-0899. [DOI] [PubMed] [Google Scholar]
- [72].Kondo T, Kanai M, Kou T, Sakuma T, Mochizuki H, Kamada M, et al. Impact of BRCAness on the efficacy of oxaliplatin-based chemotherapy in patients with unresectable pancreatic cancer. J Clin Oncol. 2017;35 10.1200/JCO.2017.35.4_suppl.250. [DOI] [Google Scholar]
- [73].Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Vyas O, Leung K, Ledbetter L, Kaley K, Rodriguez T, Garcon MC, et al. Clinical outcomes in pancreatic adenocarcinoma associated with BRCA-2 mutation. Anti-Cancer Drugs. 2015;26:224–6. 10.1097/CAD.0000000000000178. [DOI] [PubMed] [Google Scholar]
- [75].Sehdev A, Gbolahan O, Hancock BA, Stanley M, Shahda S, Wan J, et al. Germline and Somatic DNA Damage Repair Gene Mutations and Overall Survival in Metastatic Pancreatic Adenocarcinoma Patients Treated with FOLFIRINOX. Clin Cancer Res. 2018;24:6204–11. 10.1158/1078-0432.CCR-18-1472. [DOI] [PubMed] [Google Scholar]
- [76].O’Kane GM, Borgida A, Dodd A, Aung KL, Holter S, Knox J, et al. Prognosis of familial pancreatic cancer (FPC): A matched case analysis. Ann Oncol. 2017;28:v249 10.1093/annonc/mdx369. [DOI] [Google Scholar]
- [77].Lowery MA, Stadler ZK, Ludwig E, Salo-Mullen E, D’Adamo DR, Allen PJ, et al. Clinical outcomes in pancreatic adenocarcinoma (PAC) associated with a known BRCA mutation. J Clin Oncol. 2011;29 10.1200/jco.2011.29.4_suppl.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Golan T, Sella T, O’Reilly EM, Katz MH, Epelbaum R, Kelsen DP, et al. Overall survival and clinical characteristics of BRCA mutation carriers with stage I/II pancreatic cancer. Br J Cancer. 2017;116:697–702. 10.1038/bjc.2017.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Golan T, Kanji ZS, Epelbaum R, Devaud N, Dagan E, Holter S, et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer. 2014;111:1132–8. 10.1038/bjc.2014.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Blair AB, Groot VP, Gemenetzis G, Wei J, Cameron JL, Weiss MJ, et al. BRCA1/BRCA2 Germline Mutation Carriers and Sporadic Pancreatic Ductal Adenocarcinoma. J Am Coll Surg. 2018;226:630–7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Shroff RT, Hendifar A, McWilliams RR, Geva R, Epelbaum R, Rolfe L, et al. Rucaparib Monotherapy in Patients With Pancreatic Cancer and a Known Deleterious BRCA Mutation. JCO Precision Oncology. 2018:1–15. 10.1200/po.17.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lowery MA, Kelsen DP, Stadler ZK, Yu KH, Janjigian YY, Ludwig E, et al. An emerging entity: pancreatic adenocarcinoma associated with a known BRCA mutation: clinical descriptors, treatment implications, and future directions. Oncologist. 2011;16:1397–402. 10.1634/theoncologist.2011-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lucas AL, Shakya R, Lipsyc MD, Mitchel EB, Kumar S, Hwang C, et al. High prevalence of BRCA1 and BRCA2 germline mutations with loss of heterozygosity in a series of resected pancreatic adenocarcinoma and other neoplastic lesions. Clin Cancer Res. 2013;19:3396–403. 10.1158/1078-0432.CCR-12-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Hu ZI, Varghese AM, Shia J, Zervoudakis A, Lowery MA, Yu KH, et al. Clinical characterization of pancreatic ductal adenocarcinomas (PDAC) with mismatch repair (MMR) gene mutations. J Clin Oncol. 2017;35 10.1158/1078-0432.CCR-17-3099. [DOI] [Google Scholar]
- [85].Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–20. 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Cavalieri CC, Swanson E, Whisenant JR, Weis JR, Gilcrease GW, Stenehjem DD, et al. Pembroliuzmab in gastrointestinal (GI) malignancies with defective DNA mismatch repair (dMMR): A single institution experience. J Clin Oncol. 2017;35:792-. 10.1200/JCO.2017.35.4_suppl.792. [DOI] [Google Scholar]
- [87].Jardim DL, Groves ES, Breitfeld PP, Kurzrock R. Factors associated with failure of oncology drugs in late-stage clinical development: A systematic review. Cancer Treatment Reviews. 2017;52:12–21. [DOI] [PubMed] [Google Scholar]
- [88].Seruga B, Ocana A, Amir E, Tannock IF. Failures in Phase III: Causes and Consequences. Clin Cancer Res. 2015;21:4552–60. 10.1158/1078-0432.Ccr-15-0124. [DOI] [PubMed] [Google Scholar]
- [89].Jardim DL, Schwaederle M, Wei C, Lee JJ, Hong DS, Eggermont AM, et al. Impact of a Biomarker-Based Strategy on Oncology Drug Development: A Meta-analysis of Clinical Trials Leading to FDA Approval. Journal of the National Cancer Institute. 2015;107:djv253 10.1093/jnci/djv253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2017;32:185–203.e13. 10.1016/j.ccell.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Holter S, Borgida A, Dodd A, Grant R, Semotiuk K, Hedley D, et al. Germline BRCA Mutations in a Large Clinic-Based Cohort of Patients With Pancreatic Adenocarcinoma. J Clin Oncol. 2015;33:3124–9. 10.1200/jco.2014.59.7401. [DOI] [PubMed] [Google Scholar]
- [92].Salo-Mullen EE, O’Reilly EM, Kelsen DP, Ashraf AM, Lowery MA, Yu KH, et al. Identification of germline genetic mutations in patients with pancreatic cancer. Cancer. 2015;121:4382–8. 10.1002/cncr.29664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Chantrill LA, Nagrial AM, Watson C, Johns AL, Martyn-Smith M, Simpson S, et al. Precision Medicine for Advanced Pancreas Cancer: The Individualized Molecular Pancreatic Cancer Therapy (IMPaCT) Trial. Clin Cancer Res. 2015;21:2029–37. 10.1158/1078-0432.Ccr-15-0426. [DOI] [PubMed] [Google Scholar]
- [94].Witkiewicz AK, McMillan EA, Balaji U, Baek G, Lin WC, Mansour J, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun. 2015;6:6744 10.1038/ncomms7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].M Taube J, Klein A, Brahmer J, Xu H, Pan X, H Kim J, et al. Association of PD-1, PD-1 Ligands, and Other Features of the Tumor Immune Microenvironment with Response to Anti-PD-1 Therapy2014. 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548–51. 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Patricelli MP, Janes MR, Li LS, Hansen R, Peters U, Kessler LV, et al. Selective Inhibition of Oncogenic KRAS Output with Small Molecules Targeting the Inactive State. Cancer Discov. 2016;6:316–29. 10.1158/2159-8290.Cd-15-1105. [DOI] [PubMed] [Google Scholar]
- [99].Mai TT, Lito P. A treatment strategy for KRAS-driven tumors. Nature Medicine. 2018;24:902–4. 10.1038/s41591-018-0111-x. [DOI] [PubMed] [Google Scholar]
- [100].Chen YN, LaMarche MJ, Chan HM, Fekkes P, Garcia-Fortanet J, Acker MG, et al. Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature. 2016;535:148–52. 10.1038/nature18621. [DOI] [PubMed] [Google Scholar]
- [101].Belmont PJ, Jiang P, McKee TD, Xie T, Isaacson J, Baryla NE, et al. Resistance to dual blockade of the kinases PI3K and mTOR in <em>KRAS</em>-mutant colorectal cancer models results in combined sensitivity to inhibition of the receptor tyrosine kinase EGFR. Science Signaling. 2014;7:ra107 10.1126/scisignal.2005516. [DOI] [PubMed] [Google Scholar]
- [102].NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast and Ovarian. 2018;Version 1.2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.