Abstract
Background and aim:
Unsuccessful defibrillation shocks adversely affect survival from out-of-hospital cardiac arrest (OHCA). Ventricular fibrillation (VF) waveform analysis is the tool-of-choice for the non-invasive prediction of shock success, but surrogate markers of perfusion like end-tidal CO2 (EtCO2) could improve the prediction. The aim of this study was to evaluate EtCO2 as predictor of shock success, both individually and in combination with VF-waveform analysis.
Materials and methods:
In total 514 shocks from 214 OHCA patients (75 first shocks) were analysed. For each shock three predictors of defibrillation success were automatically calculated from the device files: two VF-waveform features, amplitude spectrum area (AMSA) and fuzzy entropy (FuzzyEn), and the median EtCO2 (MEtCO2) in the minute before the shock. Sensitivity, specificity, receiver operating characteristic (ROC) curves and area under the curve (AUC) were calculated, for each predictor individually and for the combination of MEtCO2 and VF-waveform predictors. Separate analyses were done for first shocks and all shocks.
Results:
MEtCO2 in first shocks was significantly higher for successful than for unsuccessful shocks (31 mmHg/25 mmHg, p<0.05), but differences were not significant for all shocks (32 mmHg/29 mmHg, p>0.05). MEtCO2 predicted shock success with an AUC of 0.66 for first shocks, but was not a predictor for all shocks (AUC 0.54). AMSA and FuzzyEn presented AUCs of 0.76 and 0.77 for first shocks, and 0.75 and 0.75 for all shocks. For first shocks, adding MEtCO2 improved the AUC of AMSA and FuzzyEn to 0.79 and 0.83, respectively.
Conclusions:
MEtCO2 predicted defibrillation success only for first shocks. Adding MEtCO2 to VF-waveform analysis in first shocks improved prediction of shock success. VF-waveform features and MEtCO2 were automatically calculated from the device files, so these methods could be introduced in current defibrillators adding only new software.
Keywords: Ventricular fibrillation, shock outcome prediction, end-tidal CO2 (EtCO2), out-of-hospital cardiac arrest, amplitude spectrum area (AMSA), fuzzy entropy
1. INTRODUCTION
Cardiopulmonary resuscitation (CPR) and electrical defibrillation are the cornerstones of therapy for out-of-hospital cardiac arrest (OHCA). Defibrillation shocks are the only effective way to terminate ventricular fibrillation (VF) and restore a perfusing rhythm. However, the mechanisms of defibrillation are not fully understood [1], and many electrical shocks are unsuccessful. Unsuccessful shocks may cause myocardial damage [2], and interruptions in chest compressions to deliver the shock are associated with a rapid decrease in coronary perfusion pressure [3]. All these factors adversely affect survival, and compromise the success of later shocks in prolonged cardiac arrest [2–4].
VF waveform analysis has been extensively studied as a non-invasive tool to predict shock success, and thus optimize CPR/defibrillation therapy [5]. Many VF waveform features have been proposed for this purpose [6–9], ranging from classical amplitude, slope or spectral analyses of VF [6, 10], to quantitative measures of its non-linear nature such as Poincare plot [11] and detrended fluctuation analyses [12], fractal dimension [13] or Hurst and Scaling exponents [13, 14]. The best known VF-waveform feature is Amplitude Spectrum Area (AMSA), a measure of amplitude and frequency distribution of VF [15, 16]. Recently, VF waveform regularity and predictability measures based on entropy estimates have been shown to accurately predict shock success [17, 18], and Fuzzy Entropy (FuzzyEn) was identified as the most accurate predictor based on entropy estimates outperforming other classical predictors [17].
Advanced life support guidelines recommend continuous use of end-tidal carbon dioxide (EtCO2) to monitor quality of CPR, confirm endotracheal intubation, detect return of spontaneous circulation and guide the rescuer during CPR [19]. EtCO2, measured as the peak value of the capnogram at the end of exhalation, is a surrogate indicator of coronary perfusion during CPR [20]. Abrupt increments of EtCO2 and higher values of EtCO2 have been associated with restoration of spontaneous circulation, quality of CPR and patient outcome [21–26]. Higher EtCO2 has been linked to shock success in animal models [20], and positive correlations between AMSA and EtCO2 have been found in animal data [27] and during a prolonged case of refractory VF in OHCA [28]. Although the evidence is not conclusive, the mean EtCO2 in the minute preceding defibrillation is associated to defibrillation success [29], and the accuracy of defibrillation success prediction may be increased by combining EtCO2 and VF-waveform features [30].
The aim of this study was to evaluate the value of capnography for the prediction of defibrillation success using OHCA data, and to develop automatic prediction methods based on the capnogram. For that purpose the median EtCO2 (MEtCO2) in the minute before the shock was used. The assessment was done in two ways, using MEtCO2 alone, and combining MEtCO2 with AMSA and FuzzyEn to evaluate its aggregate value for the prediction of shock success.
2. MATERIALS AND METHODS
2.1. Data materials
The study dataset was obtained from OHCA patients treated by the DFW Center for Resuscitation Research (UTSW, Dallas, Texas) and the Tualatin Valley Fire and Rescue (Portland, Oregon) between 2010 and 2016. Data collected using the MRx monitor-defibrillator (Philips Medical Systems, Andover, MA, USA) was analysed. MRx data included the ECG, compression depth from a CPR-assist pad, and the capnogram acquired using Microstream technology (sidestream acquisition). The ECG was recorded with a 0–50 Hz bandwith, 250 Hz sampling rate and a resolution of 1.03 µV per least significant bit. The capnogram was acquired with a sampling rate of 40/125 Hz and a resolution of 0.004 mmHg per bit. Cases were included in the study if the patient presented VF cardiac arrest, and had at least one defibrillation attempt with: concurrent recordings of capnogram (to compute MEtCO2) and compression depth (to assess CPR quality) in the 1-minute interval before the shock, an artifact free ECG in the 5-sec interval before the shock to compute the VF-waveform features, and 1-minute post shock ECG to annotate whether the shock was successful.
All shocks were visually reviewed and annotated by two experienced biomedical engineers (EA, UI). Shock success was defined as the appearence of sustained QRS complexes with a rate above 40 beats/min within 60-seconds after the shock [16]. The appearence of sustained QRS complexes after the shock has been a widely accepted criterion for the definition of shock success in many previous studies [8, 29–33]. Figure 1 shows two representative examples. In the example above the patient presents a rhythm with sustained QRS complexes (rate around 60 beats/min) after the shock, the bottom example shows a case with refractory VF. In both shocks there was a minute of end-tidal CO2 data before the shock to compute MEtCO2.
Figure 1.
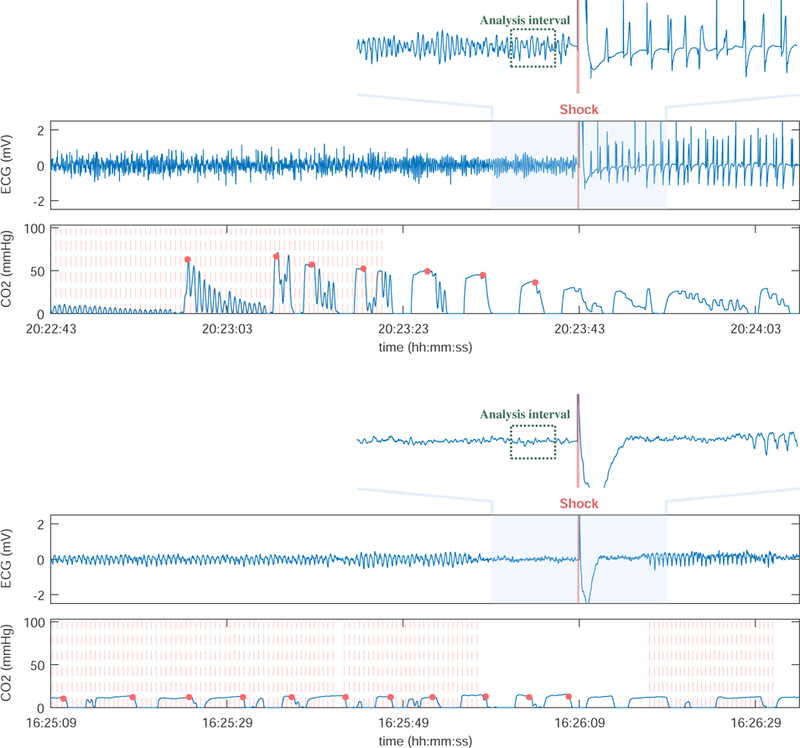
Representative examples of two shocks, a successful shock (top) and an unsuccessful shock with refractory VF (below). VF-waveform features (AMSA and FuzzyEn) were computed in the 2-sec analysis interval with a 1-sec guard before the shock. MEtCO2 and the CPR metrics were computed in the 1-min interval before the shock. Chest compressions obtained from the compression depth are marked as vertical red lines, and the EtCO2 values for each ventilation as red dots.
2.2. Predictors of defibrillation success
Three predictors of defibrillation success were computed, MEtCO2 and two established VF waveform predictors, AMSA and FuzzyEn. MEtCO2 was calculated using a 1-minute interval before the shock. First, ventilations were automatically detected in the capnogram using the algorithm proposed in [34], which is very accurate even in the presence of chest compressions. Then, the EtCO2 value for each ventilation was obtained as the maximum value of the CO2 waveform during the alveolar plateau (see Fig 1). MEtCO2 was the median value of the EtCO2 values. VF waveform features were computed using a 2-sec interval before the shock, leaving a 1-sec guard interval to avoid interferences (see Fig 1). The ECG was bandpass filtered (0.5–30 Hz) using forward-backward filtering and an order 8 elliptic filter to remove baseline oscillations and high frequency noise. The spectral amplitudes of the ECG were computed using the Fast Fourier Transform, and AMSA was obtained in the 2–48 Hz frequency range as , where Ak is the amplitude corresponding to frequency fk [16]. FuzzyEn quantifies the regularity of VF by analyzing repetitive patterns along the waveform. VF-amplitude was considered in the calculation of FuzzyEn as proposed in [17, 18], because VF-amplitude has been shown to correlate to the state of the myocardium [35].
2.3. Statistical analysis
CPR quality metrics associated with every shock were computed using the 1-minute interval before the shock. The mean chest compression rate (CR) and depth (CD), were obtained using the compression depth signal from the CPR assist pad, the mean ventilation rate (VR) was obtained from the ventilations detected in the capnogram. CPR quality metrics, MEtCO2, AMSA and FuzzyEn are reported as median (interquartile range, IQR) for successful and unsuccessful shocks because their distributions did not pass the Anderson-Darling normality test. Distributions for successful and unsuccessful shocks were compared using the Mann-Whitney U-test, and differences were considered statistically significant for p<0.05.
Univariate logistic regression analyses were done for all predictors and CPR quality metrics. The parameters that showed significant differences (p<0.05) were further included in multivariate logistic regression models. The analyses were done separately for first shocks and all shocks. Data was randomly partitioned patient-wise into training (60%) to fit the models, and test (40%) to report the results. The process was repeated 100 times to obtain the statistical distribution of the performance metrics. For patients with multiple shocks (all shocks group) generalized estimating equations with time adjusted correlations were used to account for repeated measures [36]. The performance of the models was evaluated in terms of Sensitivity (Se), the capacity to identify successful shocks, and specificity (Sp), the capacity to identify unsuccessful shocks. ROC curves were obtained and the Area Under the Curve (AUC) was used to compare the accuracy of the models [37]. The optimal point in the ROC curve was determined using the Youden index, which gives equal importance to Se and Sp [38]. All calculations were done using the Statistics and Machine Learning toolbox from MATLAB (Mathworks Inc, Natick, MA, USA).
3. RESULTS
A total of 1933 cases from the study period were analyzed and 214 met the inclusion criteria. In the study dataset the mean (SD) duration of the cases was 41 (15) min, 77% of patients were male, and the median (IQR) age was 60 (51–71) years. A total of 76 patients achieved ROSC, and 12% survived. There were 514 shocks that met the inclusion criteria, 196 were successful and 318 were not. The median number of shocks included per patient was 2 (1–3). In 75 cases first shocks were available, 33 of which were successful.
Table 1 compares the distributions of the CPR quality variables for successful and unsuccessful shocks, and the p-value of an univariate analysis for each individual variable. There were no significant differences in CR and VR, although CD was significantly larger for unsuccessful shocks (4.7 cm vs 4.9 cm). Similar values were observed for the subgroup of first shocks. The univariate analyses for the CPR quality metrics confirmed these results, with p-values for the models based on CR and VR not significant for both all shocks and first shocks groups, and significant for CD in both groups.
Table 1.
Distributions of the CPR quality parameters for all shocks and for the first shocks. The abbreviations are: VR (ventilation rate), CR (compression rate) and CD (compression depth).
| All shocks |
First shocks |
|||||
|---|---|---|---|---|---|---|
| Success | No Success | p | Success | No Success | p | |
| VR (min−1) | 10.0 (7.0 – 12.0) | 9.0 (7.0 – 12.0) | > 0.05 | 9.0 (5.7 – 10) | 8.0 (6.0 – 9.0) | > 0.05 |
| CR (min−1) | 107 (100 – 115) | 107 (100 – 115) | > 0.05 | 109 (100 – 115) | 107 (100–114) | > 0.05 |
| CD (cm) | 4.7 (4.1 – 5.2) | 4.9 (4.3 – 5.4) | < 0.05 | 4.4 (3.9 – 5.0) | 5.0 (4.4 – 5.4) | < 0.05 |
Figure 2 compares the distributions (boxplots) of the shock success predictors for successful and unsuccessful shocks, both for all shocks (top) and first shocks (bottom). VF-waveform features were significantly different between successful and unsuccessful shocks in all cases. MEtCO2 was larger for successful shocks in both cases, but the difference between successful and unsuccessful shocks was only statistically significant for the subgroup of first shocks. For this subgroup the median MEtCO2 value was 31.0 (22.5–50.3) mmHg for successful shocks and 25.0 (11.3–34.5) mmHg for unsuccessful shocks. Figure 3 shows the proportion (raw percentages) of successful and unsuccessful first shocks for three ranges of MEtCO2. MEtCO2 under 20 mmHg are associated to low quality CPR [39, 40], and values above 40 mmHg to ROSC [40]. We observed a large increase in the proportion of successful shocks from 25% for MEtCO2<20 mmHg to over 60% for MEtCO2>40 mmHg.
Figure 2.
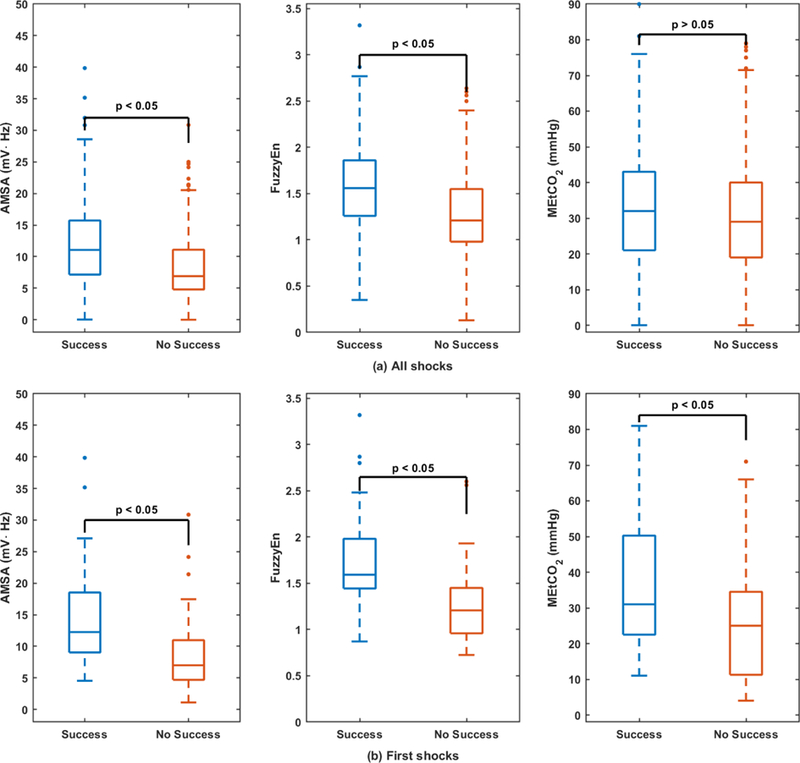
Boxplots of the defibrillation success predictors, for all shocks (top) and for the first shocks (bottom).
Figure 3.
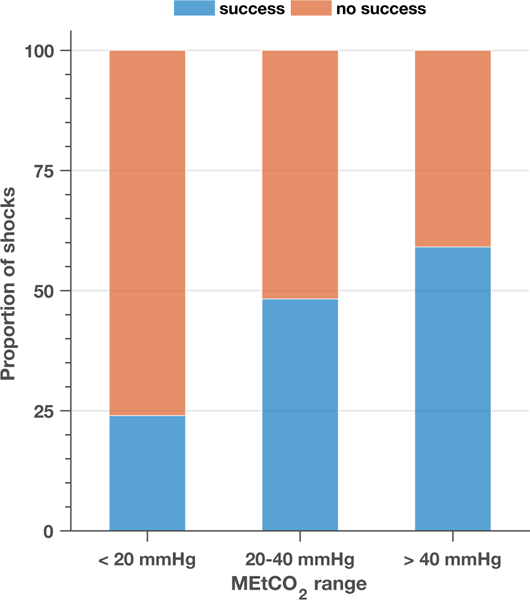
Distribution of first shocks as successful or not successful for three representative intervals of MEtCO2.
Table 2 summarizes the results of the univariate and multivariate analyses with the test set for all shocks and first shocks. VF waveform features, AMSA and FuzzyEn, were significant (p<0.05) for first shocks and all shocks. AMSA was not significant in multivariate models that combined AMSA and FuzzyEn, so AMSA and FuzzyEn were considered separately in multivariate analyses (Table 2). For all shocks CD was significant, but MEtCO2 was not and it was not considered for multivariate analyses. Adding CD to AMSA or FuzzyEn increased the AUC by less than 1-point. For first shocks both MEtCO2 and CD were significant, but MEtCO2 contributed more than CD to multivariate models. The AUC of AMSA/FuzzyEn increased by 3/6 points when MEtCO2 was added, but adding CD only increased the AUC of AMSA by 3 points, showing no improvement for FuzzyEn. For AMSA, MEtCO2 and CD contributed independently but did not outperform the model with FuzzyEn and MEtCO2 which had the largest AUC for first shocks. Figure 4 shows how VF-waveform features and MEtCO2 values are distributed, and how first successful shocks concentrate on high values of the VF-waveform predictors and MEtCO2 (north-east corner). For first shocks MEtCO2 contributes to the separability of successful and unsuccessful shocks.
Table 2.
ROC curve analysis for the four individual predictors and for the combination of VF-waveform predictors, MEtCO2 and CD when each one alone is significant. All features were significant with a p < 0.05, except MEtCO2 for all shocks (*), so MEtCO2 combinations for all shocks are not reported. The median(IQR) of the distributions are reported for the AUC, Se and Sp with the test set, after repeating training/test partitioning 100 times. The Se/Sp are given for the optimal point (Youden index)
| Data base |
||||||
|---|---|---|---|---|---|---|
| All shocks |
First shocks |
|||||
| AUC | Se (%) | Sp (%) | AUC | Se (%) | Sp (%) | |
| Univariate | ||||||
| AMSA | 0.75 (0.72 – 0.75) | 79.8 (71.4 – 85.5) | 63.2 (58.5 – 71.6) | 0.76 (0.72 – 0.82) | 82.1 (76.2 – 88.4) | 59.9 (52.9 – 64.7) |
| FuzzyEn | 0.75 (0.73 – 0.77) | 73.6 (67.1 – 78.8) | 69.4 (64.1 – 75.6) | 0.77 (0.73 – 0.82) | 83.0 (78.1 – 89.5) | 62.5 (58.8 – 70.6) |
| MEtCO2 | 0.54 (0.51 – 0.57)* | 48.1 (42.0 – 69.3) | 66.1 (43.3 – 72.1) | 0.66 (0.60 – 0.71) | 69.1 (57.9 – 86.5) | 53.0 (38.3 – 64.7) |
| CD | 0.58 (0.56 – 0.60) | 56.2 (34.6 – 75.7) | 62.7 (42.2 – 82.8) | 0.65 (0.61 – 0.72) | 76.9 (53.8 – 92.3) | 58.8 (35.3 – 85.3) |
| Multivariate | ||||||
| AMSA+CD | 0.75 (0.73 – 0.77) | 78.5 (72.1 – 84.3) | 63.7 (59.2 – 70.3) | 0.76 (0.72 – 0.81) | 69.2 (61.5 – 84.6) | 82.4 (70.6 – 88.2) |
| AMSA+MEtCO2 | - | - | - | 0.79 (0.75 – 0.85) | 85.1 (81.2 – 89.8) | 60.7 (52.9 – 67.6) |
| AMSA+MEtCO2 +CD | - | - | - | 0.82 (0.77 – 0.86) | 92.3 (84.6 – 96.2) | 70.6 (64.7 – 82.4) |
| FuzzyEn+CD | 0.76 (0.73 – 0.78) | 79.5 (75.8 – 82.2) | 65.1 (60.6 – 69.2) | 0.80 (0.74 – 0.85) | 84.6 (76.9 – 92.3) | 76.5 (70.6 – 82.4) |
| FuzzyEn+MEtCO2 | - | - | - | 0.83 (0.78 – 0.88) | 85.9 (80.5 – 91.6) | 65.4 (58.8 – 70.6) |
| FuzzyEn+MEtCO2 +CD | - | - | - | 0.83 (0.78 – 0.88) | 84.6 (76.9 – 92.3) | 82.4 (70.6 – 88.2) |
Figure 4.
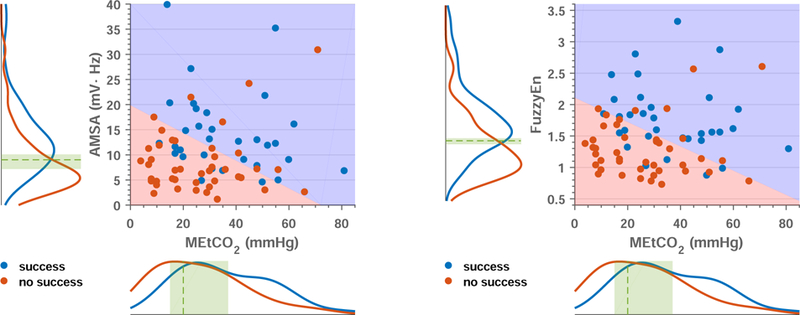
Analysis of the models for the prediction of first shock success based on VF-waveform features combined with MEtCO2. The scatter plots show how the pair of values for each shock are distributed, and in both cases successful shocks (blue dots) fall in the north-east side indicating that they correspond to high values of MEtCO2 and the VF-waveform predictor. Unsuccessful shocks (red dots) concentrate in the south-west corner, corresponding to low values of MEtCO2 and the VF-waveform predictor. The blues and red areas are separated by the median decision boundary corresponding to 100 replicas of the logistic regression classifier. The curves on the sides are a non-parametric estimation of the probability distribution of each variable (AMSA left, FuzzyEn right, and MEtCO2 bottom) for successful (blue) and unsuccessful (red) shocks. The dashed green line and area indicate the median (IQR) of the thresholds for 100 replicas, corresponding to AMSA, 9.0 (7.2–10.1) mVHz, FuzzyEn, 1.4 (1.4–1.5), and MEtCO2, 20 (15–37) mmHg, respectively.
4. DISCUSSION
This study provides new evidence on value of MEtCO2 for the prediction of defibrillation success. MEtCO2 showed predictive power only for first shock attempts, for which it also added predictive power to VF-waveform features. Furthermore, all MEtCO2 calculations and VF-feature calculations were performed automatically using the waveforms recorded by the monitor defibrillator, and could therefore be integrated into current equipment adding only new software.
Many studies have analysed the value of EtCO2 in cardiac arrest as a surrogate marker of perfusion, with emphasis on its value to monitor CPR quality [21, 22] or to provide an early indication of return of spontaneous circulation [23–25, 41]. However, little is known on its value to predict defibrillation success. In our data, low MEtCO2 values in the first shock were associated to unsuccessful shocks, and higher MEtCO2 values were an indication of an increased probability of successful shocks (Fig 3). First shocks with MEtCO2 values under 11 mmHg (n=10) were always unsuccessful, while 60% were successful for MEtCO2 above 40 mmHg (n=22). Our low MEtCO2 cutoff for unsuccessful shocks is similar to the values observed by Savastano et al [29], and confirm an MEtCO2 value around 10 mmHg may be used as an indication to delay the shock. These findings are aligned with the European [19] and American [40] guidelines which suggest 10 mmHg as an indicator of good quality CPR. However, our cutoffs for successful shocks were higher than the 31 mmHg proposed by Savastano et al. This suggests that differences in the measurements of EtCO2 might be expected when different devices and technologies are used. Further analyses are needed to define universal EtCO2 thresholds.
We found an accuracy for the prediction of defibrillation success for first shocks using MEtCO2 (AUC of 0.66) similar to the one reported by Savastano et al [29] for all shocks (AUC of 0.67). However, our analysis showed that MEtCO2 was not useful as a predictor for all shocks (AUC of 0.54). EtCO2 values may be affected by many factors in recurrent VF and prolonged cardiac arrest, such as quality of CPR, type of CPR (manual/mechanical ventilation and manual/mechanical compressions), ventilation rate, compression site on the sternum or pharmacological treatment [42, 43]. In our data, compression and ventilation rates were similar in successful and unsuccessful shocks, but compression depth was significantly larger for unsuccessful shocks. Differences in manual chest compressions may also be associated to rescuer changes, that may affect the position of the hands on the chest and the quality of CPR. For the subgroup of unsuccessful shocks, median MEtCO2 values in all shocks were significantly larger than in first shocks (see Fig 2), 29 mmHg to 25 mmHg (p<0.05). In Savastano et al [29] patients were mechanically ventilated and mechanically compressed, so there were no differences in CPR quality, which may explain why MEtCO2 was a predictor of shock success for all shocks. Our findings stress the importance of controlling for additional variables like CPR quality when using MEtCO2 for the prediction of defibrillation success. The effects may be larger for patients with prolonged therapy, i.e. shocks other than the first shock.
VF-waveform analysis is the tool-of-choice for the non-invasive prediction of defibrillation success [6, 8, 9, 16]. The AUCs obtained in this study for AMSA and FuzzyEn are in line with previous findings, which are in the 0.60–0.85 range [9, 12, 16, 30, 44, 45]. FuzzyEn following the definition introduced in [17] slightly outperformed AMSA, although the AUCs were very similar. Most importantly, when MEtCO2 had prognostic value, it also added valuable information to the VF-waveform features. For first shocks, the addition of MEtCO2 significantly increased the AUC for AMSA and FuzzyEn, with an increase of 3-points for AMSA and 6-points for FuzzyEn. These results suggest that MEtCO2 could be used as a complementary tool to VF-waveform analysis to predict defibrillation in first shocks, confirming some previous findings with very small datasets [30]. The inclusion of CD in the predictive model produced a smaller increase in AUC of AMSA and no increase for FuzzyEn.
Finally, MEtCO2 was automatically obtained using a capnogram based ventilation detector [34], and a simple definition of EtCO2 as the maximum CO2 value in the alveolar plateau. When we compared the MEtCO2 values obtained from manual and automatic EtCO2 measurements, we found a median (IQR) error of −1 (−3–0) mmHg, and a median unsigned error of 2 (1–4) mmHg. This confirms MEtCO2 can be automatically determined before the shock, and then be used to improve the prediction of defibrillation success in the subgroup of first shocks. The method could therefore be integrated into current monitor-defibrillators that use capnography modules simply adding software.
4.1. Limitations
Three were the main limitations of this study. First, the capnogram was not available in the initial shocks of some cases, in which it was introduced once the advanced airway had been placed. Second, the capnogram was acquired using Microstream (sidestream) technology. EtCO2 measurements using other devices or technologies (mainstream, nasal cannulae, ...) may be slightly different. Third, no information on ROSC after every shock was available, so the criterion to define successful shocks was based on the appearence of sustained QRS complexes in the post-shock interval. This is the most frequently used criterion in the studies on the prediction of shock success.
5. Conclusions
MEtCO2 was only a predictor of defibrillation success for first shocks, and it added prognostic value to VF-waveform features. The method introduced in this study is the first fully automatic method that combines MEtCO2 values and VF-waveform features for the prediction of defibrillation success.
Acknowledgements
This work received financial support from the Spanish Ministerio de Economía y Competitividad, project TEC2015-64678-R, jointly with the Fondo Europeo de Desarrollo Regional (FEDER), from the University of the Basque Country via Ayuda a Grupos de Investigacioón GIU17/03 and the grant PIF15/190, and also was partially supported by NIH grant HL 077887 (AHÍ).
Conflict of interest
Dr. Idris receives research grants from the US National Institutes of Health (NIH) and serves as an unpaid volunteer on the American Heart Association National Emergency Cardiovascular Care Committee and the HeartSine, Inc. Clinical Advisory.
Dr. Daya has received grant support from the US NIH and has served as an unpaid consultant for Philips Healthcare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dosdall DJ, Fast VG, Ideker RE. Mechanisms of defibrillation. Annual review of biomedical engineering 2010; 12:233–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Xie J, Weil MH, Sun S, et al. High-energy defibrillation increases the severity of postresuscitation myocardial dysfunction. Circulation 1997;96(2):683–688. [DOI] [PubMed] [Google Scholar]
- [3].Steen S, Liao Q, Pierre L, Paskevicius A, Sjo¨berg T. The critical importance of minimal delay between chest compressions and subsequent defibrillation: a haemodynamic explanation. Resuscitation 2003;58:249–258. [DOI] [PubMed] [Google Scholar]
- [4].Cheskes S, Schmicker RH, Christenson J, et al. Perishock pause: an independent predictor of survival from out-of-hospital shockable cardiac arrest. Circulation 2011;124(1):58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ristagno G, Mauri T, Cesana G, et al. Amplitude spectrum area to guide defibrillation: a validation on 1617 patients with ventricular fibrillation. Circulation 2015;131(5):478–487. [DOI] [PubMed] [Google Scholar]
- [6].Neurauter A, Eftestøl T, Kramer-Johansen J, et al. Prediction of countershock success using single features from multiple ventricular fibrillation frequency bands and feature combinations using neural networks. Resuscitation 2007;73(2):253–263. [DOI] [PubMed] [Google Scholar]
- [7].Endoh H, Hida S, Oohashi S, Hayashi Y, Kinoshita H, Honda T. Prompt prediction of successful defibrillation from 1-s ventricular fibrillation waveform in patients with out-of-hospital sudden cardiac arrest. J Anesth 2011; 25(1):34–41. [DOI] [PubMed] [Google Scholar]
- [8].Firoozabadi R, Nakagawa M, Helfenbein ED, Babaeizadeh S. Predicting defibrillation success in sudden cardiac arrest patients. J Electrocardiol 2013;46(6):473–479. [DOI] [PubMed] [Google Scholar]
- [9].He M, Chen B, Gong Y, Wang K, Li Y. Prediction of defibrillation outcome by ventricular fibrillation waveform analysis: a clinical review. J Clinic Experiment Cardiol S 2013;10(2). [Google Scholar]
- [10].Marn-Pernat A, Weil MH, Tang W, Pernat A, Bisera J. Optimizing timing of ventricular defibrillation. Crit Care Med 2001;29(12):2360–2365. [DOI] [PubMed] [Google Scholar]
- [11].Gong Y, Lu Y, Zhang L, Zhang H, Li Y. Predict Defibrillation Outcome Using Stepping Increment of Poincare Plot for Out-of-Hospital Ventricular Fibrillation Cardiac Arrest. Biomed Res Int 2015;2015:493472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lin LY, Lo MT, Ko PCI, et al. Detrended fluctuation analysis predicts successful defibrillation for out-of-hospital ventricular fibrillation cardiac arrest. Resuscitation 2010;81(3):297–301. [DOI] [PubMed] [Google Scholar]
- [13].Sherman LD, Callaway CW, Menegazzi JJ. Ventricular fibrillation exhibits dynamical properties and self-similarity. Resuscitation 2000;47(2):163–173. [DOI] [PubMed] [Google Scholar]
- [14].Callaway CW, Sherman LD, Scheatzle MD, Menegazzi JJ. Scaling structure of electrocardiographic waveform during prolonged ventricular fibrillation in swine. Pacing Clin Electrophysiol 2000;23(2):180–191. [DOI] [PubMed] [Google Scholar]
- [15].Povoas HP, Weil MH, Tang W, Bisera J, Klouche K, Barbatsis A. Predicting the success of defibrillation by electrocardiographic analysis. Resuscitation 2002;53:77–82. [DOI] [PubMed] [Google Scholar]
- [16].Ristagno G, Li Y, Fumagalli F, Finzi A, Quan W. Amplitude spectrum area to guide resuscitation-a retrospective analysis during out-of-hospital cardiopulmonary resuscitation in 609 patients with ventricular fibrillation cardiac arrest. Resuscitation 2013;84:1697–1703. [DOI] [PubMed] [Google Scholar]
- [17].Chicote B, Irusta U, Alcaraz R, et al. Application of Entropy-Based Features to Predict Defibrillation Outcome in Cardiac Arrest. Entropy 2016;18(9):313. [Google Scholar]
- [18].Chicote B, Irusta U, Aramendi E, et al. Fuzzy and Sample Entropies as Predictors of Patient Survival Using Short Ventricular Fibrillation Recordings during out of Hospital Cardiac Arrest. Entropy 2018;20(8):591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Soar J, Nolan JP, B¨ottiger BW, et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 3. Adult advanced life support. Resuscitation 2015;95:100–147. [DOI] [PubMed] [Google Scholar]
- [20].Von Planta M, Von Planta I, Weil MH, Bruno S, Bisera J, Rackow EC. End tidal carbon dioxide as an haemodynamic determinant of cardiopulmonary resuscitation in the rat. Cardiovascular research 1989; 23:364–368. [DOI] [PubMed] [Google Scholar]
- [21].Chen JJ, Lee YK, Hou SW, Huang MY, Hsu CY, Su YC. End-tidal carbon dioxide monitoring may be associated with a higher possibility of return of spontaneous circulation during out-of-hospital cardiac arrest: a population-based study. Scandinavian journal of trauma, resuscitation and emergency medicine 2015;23:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gudipati CV, Weil MH, Bisera J, Deshmukh HG, Rackow EC. Expired carbon dioxide: a noninvasive monitor of cardiopulmonary resuscitation. Circulation 1988;77:234–239. [DOI] [PubMed] [Google Scholar]
- [23].Hartmann SM, Farris RWD, Di Gennaro JL, Roberts JS. Systematic Review and Meta-Analysis of End-Tidal Carbon Dioxide Values Associated With Return of Spontaneous Circulation During Cardiopulmonary Resuscitation. Journal of intensive care medicine 2015;30:426–435. [DOI] [PubMed] [Google Scholar]
- [24].Pokorná M, Necas E, Kratochvíl J, Skripský R, Andrlík M, Franek O. A sudden increase in partial pressure end-tidal carbon dioxide (P(ET)CO(2)) at the moment of return of spontaneous circulation. The Journal of emergency medicine 2010;38:614–621. [DOI] [PubMed] [Google Scholar]
- [25].Lui CT, Poon KM, Tsui KL. Abrupt rise of end tidal carbon dioxide level was a specific but non-sensitive marker of return of spontaneous circulation in patient with out-of-hospital cardiac arrest. Resuscitation 2016; 104:53–58. [DOI] [PubMed] [Google Scholar]
- [26].Sanders AB, Kern KB, Otto CW, Milander MM, Ewy GA. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. A prognostic indicator for survival. JAMA 1989;262:1347–1351. [PubMed] [Google Scholar]
- [27].Segal N, Metzger AK, Moore JC, et al. Correlation of end tidal carbon dioxide, amplitude spectrum area, and coronary perfusion pressure in a porcine model of cardiac arrest. Physiological reports 2017;5(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Azeli Y, Herrero J, Fortuny G, et al. Variation and correlation of end–tidal CO2 and amplitude spectrum area in a refractory ventricular fibrillation. A case from the ReCaPTa study. Resuscitation 2018;122:e19–e20. [DOI] [PubMed] [Google Scholar]
- [29].Savastano S, Baldi E, Raimondi M, et al. End-tidal carbon dioxide and defibrillation success in out-of-hospital cardiac arrest. Resuscitation 2017;121:71–75. [DOI] [PubMed] [Google Scholar]
- [30].Shandilya S, Ward K, Kurz M, Najarian K. Non-linear dynamical signal characterization for prediction of defibrillation success through machine learning. BMC Med Inform Decis Mak 2012;12:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Van Alem AP, Chapman FW, Lank P, Hart AAM, Koster RW. A prospective, randomised and blinded comparison of first shock success of monophasic and biphasic waveforms in out-of-hospital cardiac arrest. Resuscitation 2003;58:17–24. [DOI] [PubMed] [Google Scholar]
- [32].Koster RW, Walker RG, van Alem AP. Definition of successful defibrillation. Critical care medicine 2006; 34:S423–S426. [DOI] [PubMed] [Google Scholar]
- [33].Ristagno G, Gullo A, Berlot G, Lucangelo U, Geheb E, Bisera J. Prediction of successful defibrillation in human victims of out-of-hospital cardiac arrest: a retrospective electrocardiographic analysis. Anaesthesia and intensive care 2008;36:46–50. [DOI] [PubMed] [Google Scholar]
- [34].Aramendi E, Elola A, Alonso E, et al. Feasibility of the capnogram to monitor ventilation rate during cardiopulmonary resuscitation. Resuscitation 2017;110:162–168. [DOI] [PubMed] [Google Scholar]
- [35].Weaver WD, Cobb LA, Dennis D, Ray R, Hallstrom AP, Copass MK. Amplitude of ventricular fibrillation waveform and outcome after cardiac arrest. Ann Intern Med 1985;102(1):53–55. [DOI] [PubMed] [Google Scholar]
- [36].Sternberg MR, Hadgu A. A GEE approach to estimating sensitivity and specificity and coverage properties of the confidence intervals. Statistics in medicine 2001;20(9–10):1529–1539. [DOI] [PubMed] [Google Scholar]
- [37].Zou KH, O’Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation 2007;115(5):654–657. [DOI] [PubMed] [Google Scholar]
- [38].Ruopp MD, Perkins NJ, Whitcomb BW, Schisterman EF. Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection. Biometrical Journal: Journal of Mathematical Methods in Biosciences 2008;50(3):419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Meaney PA, Bobrow BJ, Mancini ME, et al. Cardiopulmonary resuscitation quality: improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation 2013;128(4):417–435. [DOI] [PubMed] [Google Scholar]
- [40].Link MS, Berkow LC, Kudenchuk PJ, et al. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015;132:S444–S464. [DOI] [PubMed] [Google Scholar]
- [41].Sehra R, Underwood K, Checchia P. End tidal CO2 is a quantitative measure of cardiac arrest. Pacing and clinical electrophysiology : PACE 2003;26:515–517. [DOI] [PubMed] [Google Scholar]
- [42].Qvigstad E, Tømte Ø, Sunde K, et al. Changing hand position during manual chest compressions in cardiac arrest affects the hemodynamic response: a clinical pilot study 2011.
- [43].Heradstveit BE, Sunde K, Sunde GA, Wentzel-Larsen T, Heltne JK. Factors complicating interpretation of capnography during advanced life support in cardiac arrest–a clinical retrospective study in 575 patients. Resuscitation 2012;83:813–818. [DOI] [PubMed] [Google Scholar]
- [44].Coult J, Sherman L, Kwok H, Blackwood J, Kudenchuk PJ, Rea TD. Short ECG segments predict defibrillation outcome using quantitative waveform measures. Resuscitation 2016;109:16–20. [DOI] [PubMed] [Google Scholar]
- [45].Indik JH, Conover Z, McGovern M, et al. Association of amplitude spectral area of the ventricular fibrillation waveform with survival of out-of-hospital ventricular fibrillation cardiac arrest. Journal of the American College of Cardiology 2014;64(13):1362–1369. [DOI] [PubMed] [Google Scholar]


