Abstract
Background:
Asthma phenotypes are currently not amenable to primary prevention or early intervention because their natural history cannot be reliably predicted. Clinicians remain reliant on poorly predictive asthma outcome tools due to a lack of better alternatives.
Objective:
To develop a quantitative, personalized tool to predict asthma development in young children.
Methods:
Data from the Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS, n=762) birth cohort were utilized to identify factors that predicted asthma development. The Pediatric Asthma Risk Score (PARS) was constructed by integrating demographic and clinical data. The sensitivity and specificity of PARS were compared to the Asthma Predictive Index (API) and replicated in the Isle of Wight (IOW) birth cohort.
Results:
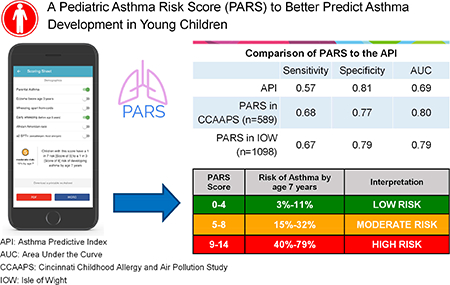
PARS reliably predicted asthma development in CCAAPS (sensitivity=0.68, specificity=0.77). While both the PARS and API predicted asthma in high-risk children, PARS had improved ability to predict asthma in children with mild to moderate asthma risk. In addition to parental asthma, eczema and wheezing apart from colds, variables that that predicted asthma in PARS included early wheezing (OR=2.88; 95%CI 1.52–5.37), sensitization to ≥2 food and/or aeroallergens (OR=2.44 95%CI 1.49–4.05) and African-American race (OR=2.04 95%CI 1.19–3.47). PARS was replicated in IOW (sensitivity=0.67, specificity=0.79), demonstrating that it is a robust, valid and generalizable asthma predictive tool.
Conclusions:
The PARS performed better than the API in the children with mild to moderate asthma. This is significant as these children are the most common, the most difficult to predict, and may be the most amenable to prevention strategies.
Keywords: asthma prediction score, persistent wheezing, sensitization, childhood asthma
Capsule Summary:
PARS relies on clinical and demographic data collected in the office setting. PARS better predicted children at moderate risk for asthma compared to the API, likely the most common and the most difficult to predict.
Graphical Abstract
INTRODUCTION
Asthma affects 25.7 million people in the US including 7.0 million children1, and its global pharmacotherapeutic costs exceed $5 billion per year2. Primary prevention of asthma has been identified as a key public health goal to decrease morbidity, mortality, and economic burden of disease. Recently, an Asthma Birth Cohort Workshop, jointly sponsored by the National Institute of Allergy and Infectious Disease (NIAID), the National Heart, Lung, and Blood Institute (NHLBI), and the European Commission Framework Program for Research and Technological Development 7 (Mechanisms of the Development of Allergy, MeDALL), was convened to review the findings from asthma/allergy birth cohorts and identify key knowledge gaps and research priorities. In their summary, they conclude that current asthma phenotypes are not amenable to primary prevention or early intervention because “their natural history cannot be reliably predicted” 3. They identified that a key research priority need is to develop better tools that reliably predict the development of asthma in young children and better align natural history with mechanisms. Several tools have tried to address this need. The most widely used and most validated is the Asthma Predictive Index (API), which was developed by Castro-Rodriguez et al in 20004. The stringent definition of the API has a high specificity (96%), but relatively low sensitivity (28%)4. As such, while it is useful for predicting which children will not develop asthma, it leaves much room for improvement in terms of identifying children who will.
Our group and others have attempted to improve the API by making the criteria more stringent, adding additional criteria, or through the development of new predictive indices. These have resulted in a marginal improvement in the ability to forecast which children will develop asthma 5–15. These additional criteria have ranged from sensitization to aero- and food allergens 7, 9 to the addition of non-invasive measures such as FeNO 11–13 and inclusion of environmental exposures 15. Further improvements will help enable identification of those who would benefit most from preventative interventions before they develop disease. Herein, we utilize the Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS) birth cohort to develop a new personalized predictive algorithm that integrates clinical and demographic factors, and compare this new tool directly to the API. We then replicated our findings in an independent birth cohort, the Isle of Wight (IOW) birth cohort.
METHODS
Primary Population (CCAAPS)
Subjects were obtained from participants in CCAAPS, a birth cohort of 762 infants born to atopic parent(s) between 2001 and 2003 in Cincinnati, Ohio and Northern Kentucky16. Infants were identified by birth records. Eligible parents had at least one allergy symptom and were skin prick test (SPT) positive to at least one aeroallergen16. Children were examined annually at ages 1, 2, 3, 4 and 7 years of age for the development of allergic disease and objectively evaluated for asthma development at age 7 years. At each annual exam, parents reported symptoms and frequency of wheeze, wheezing apart from colds, and skin and allergy symptoms were recorded. Children objectively assessed for asthma at age 7 were included in this analysis (n=589).
Asthma Determination in CCAAPS
Asthma was defined at age 7 years in CCAAPS by reported symptoms and objective measures of lung function17. Spirometry testing was performed according to ATS-ERS guidelines18. Each child participant completed at least four acceptable maneuvers after the spirometers were verified for volume accuracy. Children were defined as having asthma if the parent reported asthma symptoms (tight or clogged chest or throat in the past 12 months, difficulty breathing or wheezy after exercise, wheezing or whistling in the chest in the previous 12 months, or a previous doctor’s diagnosis of asthma) and the child demonstrated either significant airway reversibility (>12% increase in FEV1) or a positive methacholine challenge test result17.
Eczema, Allergic Rhinitis, and Wheeze Definitions in CCAAPS
During the clinical exam, the children were defined as having eczema if the parent(s) reported frequent skin scratching for ≥6 months AND ≥6 months of redness/red spots, raised bumps, or rough dry skin in the first three years of life 19. The children were defined as having allergic rhinitis (AR) if the clinician indicated a “probable” or “definitive” diagnosis of AR at the age 1, 2 or 3 years clinical exam based on SPT results and symptoms. Early wheezing was defined as any parental report of wheeze in the first 3 years of life. Early frequent wheezing was defined as ≥10 episodes of wheezing in the past 12 months (top 15th percentile) at the ages 1, 2 or 3 years clinical exams. Wheezing without a cold was defined as present if the parental reported total number of episodes of wheezing minus the number of wheezing episodes that occurred after a cold was >0 at ages 1, 2 and 3 years.
Skin Prick Testing in CCAAPS
At each exam, CCAAPS children underwent SPT to 15 aeroallergens (meadow fescue, timothy, white oak, maple mix, American elm, red cedar, short ragweed, Alternaria, Aspergillus fumigatus, Penicillium mix, Cladosporium), cat, dog, German cockroach [Blattella germanica], and dust mite mix Dermatophagoides farinae and Dermatophagoides pteronyssinus]), and two foods (cow’s milk and hen’s egg)16. A positive SPT was defined as a wheal ≥3mm larger than the saline control after 15 minutes.
Replication Cohort (IOW)
The replication population consisted of children (n=1,456) born and enrolled between January 1, 1989 and February 28, 1990 in the Isle of Wight (IOW), a UK whole population birth cohort study20, 21. Approval for the study was obtained from the Local Research Ethics Committee. Children were phenotyped for asthma at ages 1, 2, 4 and 10 years, with asthma diagnosis at age 10 (n=1,368) based on a minimum criteria of physician-diagnosed asthma plus wheeze in the previous 12 months, using a validated questionnaire22. At every follow-up, detailed questionnaires were completed with the parents for each child regarding asthma and allergy prevalence. Skin-prick testing (SPT) was performed in children at 1, 2 and 4 years of age (n=1,098) to a panel of common inhaled and food allergens (Biodiagnostics, Reinbek, Germany). This included house dust mite (Dermatophagoides pteronyssinus), grass pollen mix, cat and dog epithelia, Alternaria alternata, Cladosporium herbarum, cow’s milk, hen’s egg, soya, cod, wheat and peanut, plus histamine and physiological saline to act as positive and negative controls, respectively. Mean wheal diameter of 3 mm greater than the negative control was regarded as a positive reaction. Eczema was defined as chronic or chronically relapsing, itchy dermatitis lasting >6 weeks with characteristic morphology and distribution.
Statistical Analyses
The prevalence of each potential predictor in asthmatics and non-asthmatics was evaluated and logistic regression was performed to assess the significance of each predictor on asthma. All the potential predictors were defined using data collected during the first 3 years of life and asthma was defined at age 7 years. All potential predictors were included in the logistic regression model at the first step. Backward selection was used to develop the final PARS model, with a p value cutoff point at 0.05 and the odds ratio (OR) for each predictor calculated. A weight was assigned to each predictor by rounding the OR to the nearest whole number. These weights were then used to calculate the PARS for each subject in the CCAAPS cohort. To predict the asthma risk using PARS, a logistic regression model of asthma on PARS was conducted to calculate the predicted asthma risk.
The original API published by Castro-Rodriguez et al. has arguably been the gold standard to which predictive indices are compared, has been highly replicated, and many studies utilize it for patient selection23, 24. The API had both a loose and a stringent definition with the loose definition having a greater sensitivity.4 The loose definition was defined by being an “early wheezer” plus one major criteria or two minor criteria. The stringent definition was defined by being an “early frequent wheezer” and one major or two minor criteria4. These predictive criteria were then applied to “active asthma” defined at ages 6, 8, 11 and 13 years in children participating in the Tucson Children’s Respiratory Study.4 Since the asthma diagnosis in CCAAPS was performed at age 7 years, we compared our results to the API at age 6 years. We applied the loose and stringent API index to the CCAAPS cohort, with the exception of eosinophilia as a minor criterion since that measure was not available. In addition, we applied the modified API (mAPI), and index that uses more objective criteria than the API, to the CCAAPS populations, again with the exception of the eosinophilia criteria. We compared our results to the diagnostic utility of the mAPI at age three for asthma diagnosis at age 6 since this had the greatest sensitivity14.
Logistic regression was used to evaluate AUC for the continuous PARS measures and the sensitivity, specificity and predictive values were estimated using a threshold = 6 (the point that maximized sensitivity and specificity). Model discrimination was evaluated by the area under the receiver operator characteristics (ROC) curve. Model precision was evaluated by the Hosmer-Lemeshow goodness-of-fit statistic. Area under the curve (AUC) was calculated and compared to assess discriminatory power of API and PARS. For replication in IOW, weights summed by the predictors for each subject were used to calculate PARS. All the analyses were performed in R25.
RESULTS
Demographics and Clinical Attributes of the CCAAPS Cohort
Of the 762 active participants in the CCAAPS cohort, 589 were objectively assessed for asthma development at age 7 years, and the prevalence of asthma at age 7 years was 16% (n=95, Table I). We evaluated parental asthma, eczema, early wheezing, wheezing apart from colds, early frequent wheezing, AR, race, sex and SPT status as potential factors in our score since these contribute to asthma risk and are easily assessed during outpatient visits. The children who had asthma at age 7 years were more likely to have a parent(s) with asthma (p=0.0005), have eczema ≤age 3 years (p=0.0004), wheeze apart from colds, have early wheeze and early frequent wheezing (all p<0.0001), have a probable or definitive clinician diagnosis of AR in the first 3 years of life (p=0.0016), be African-American (p=0.0004) and be polysensitized (have two or more positive SPTs to aeroallergens or foods, p=0.0001) compared to children who did not have asthma at age 7 years (Table I).
Table I.
Demographic and Clinical Characteristics During the First 3 Years of Life in Asthmatics and Non-Asthmatics in CCAAPS.
| Non-asthma (N=494) | Asthma (N=95) | p value* | |
|---|---|---|---|
| Clinical Risk Factors | |||
| Eczema before age 3 years | 24.0% (118) | 42.6% (40) | 0.0004 |
| Wheezing apart from colds | 12.0% (59) | 45.3% (43) | <0.0001 |
| Early wheezing (before age 3 years) | 29.4% (145) | 68.4% (65) | <0.0001 |
| Early frequent wheezing | 10.3% (51) | 37.9% (36) | <0.0001 |
| AR (clinician diagnosis probable or definite) | 35.1% (172) | 52.7% (49) | 0.0016 |
| SPT+ to ≥ 1 aeroallergen | 53.5% (264) | 71.6% (68) | 0.0009 |
| SPT+ to ≥ 1 food allergen | 16.2% (80) | 26.3% (25) | 0.02 |
| SPT+ to aero/food allergens (≥2 SPT+) | 38.3% (189) | 60.0% (57) | 0.0001 |
| Personal Risk Factors | |||
| Parental asthma | 37.7% (186) | 56.8% (54) | 0.0005 |
| African-American Race | 19.4% (96) | 36.8% (35) | 0.0004 |
| Male Sex | 53.6% (265) | 61.1% (58) | 0.18 |
AR= allergic rhinitis, SPT=skin prick test
P value was obtained by logistic regression model
Application of the original API and mAPI to the CCAAPS Cohort
As a reference, the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive and negative likelihood ratios and AUC published by Castro-Rodriguez et al. for the API in the Tucson Children’s Respiratory Study at age 6 years is shown in Table IIa4. In addition, the sensitivity, specificity and positive and negative likelihood ratios are included for the mAPI14 from age three characteristics predicting asthma at age 6 (Table IIa). We applied the criteria for the loose API, stringent API and mAPI criteria to the CCAAPS cohort. The loose API criteria applied to CCAAPS yielded results identical to the published results (Table IIb), with the sensitivity and specificity at 0.57 and 0.81, respectively. The PPV was higher in the CCAAPS cohort (0.37 compared to 0.26) while the NPV was slightly higher in the original Tucson cohort (0.94 compared to 0.91). The AUC were identical at 0.69 for both the CCAAPS and Tucson cohorts (Table IIa and b). Using the stringent criteria, the CCAAPS cohort had slightly higher sensitivity, PPV and AUC, but slightly lower specificity and NPV (Table IIa and b). The mAPI applied to CCAAPS had much higher sensitivity than the original (0.47 compared to 0.17), but lower specificity (0.87 compared to 0.99). Since the loose API yielded superior results in terms of sensitivity and AUC, all comparisons between PARS and the API are performed with the loose API definition.
Table II.
Application of Published API and mAPI Criteria to the CCAAPS and IOW Birth Cohorts.a. Published API of Asthma at Age 6 years in the Tucson Children’s Respiratory Study and mAPI Evaluated in the Childhood Origins of Asthma Cohort
| a. Published API of Asthma at Age 6 years in the Tucson Children’s Respiratory Study and mAPI Evaluated in the Childhood Origins of Asthma Cohort | |||||||
| Sensitivity | Specificity | PPV | NPV | LR+ | LR- | AUC (95%CI) | |
| Loose API Index | 0.57 | 0.81 | 0.26 | 0.94 | 2.94 | 0.54 | 0.69 (0.64 – 0.74) |
| Stringent API Index | 0.28 | 0.96 | 0.48 | 0.92 | 7.39 | 0.75 | 0.62 (0.57 – 0.66) |
| mAPI | 0.17 | 0.99 | - | - | 21.0 | 0.84 | - |
| b. Application of Published API and mAPI Criteria to CCAAPS and IOW Cohorts. | |||||||
| Sensitivity | Specificity | PPV | NPV | LR+ | LR- | AUC (95%CI) | |
| mAPI (CCAAPS) | 0.47 | 0.87 | 0.41 | 0.90 | 3.54 | 0.61 | 0.67 (0.62 – 0.72) |
| Stringent API Index (CCAAPS) | 0.34 | 0.93 | 0.49 | 0.88 | 5.03 | 0.71 | 0.64 (0.59 – 0.68) |
| Loose API Index (CCAAPS) | 0.57 | 0.81 | 0.37 | 0.91 | 2.98 | 0.53 | 0.69 (0.64 – 0.74) |
| Stringent API Index (IOW) | 0.29 | 0.95 | 0.50 | 0.89 | 6.03 | 0.75 | 0.62 (0.59 – 0.65) |
| Loose API Index (IOW) | 0.47 | 0.86 | 0.37 | 0.91 | 3.47 | 0.61 | 0.67 (0.63 – 0.70) |
| c. Comparison of Loose API to PARS Model in the CCAAPS and IOW Cohorts | |||||||
| Sensitivity | Specificity | PPV | NPV | LR+ | LR- | AUC (95%CI) | |
| Loose API Index | 0.57 | 0.81 | 0.26 | 0.94 | 2.94 | 0.54 | 0.69 (0.64 – 0.74) |
| PARS in CCAAPS (at cut-point of 6*) | 0.68 | 0.77 | 0.37 | 0.93 | 3.02 | 0.41 | 0.80 (0.75 – 0.84) |
| PARS in IOW (at cut-point of 6*) | 0.67 | 0.79 | 0.36 | 0.93 | 3.25 | 0.41 | 0.79 (0.75 – 0.83) |
API=Asthma Predictive Index, AUC=area under the curve, CCAAPS=Cincinnati Childhood Allergy and Air Pollution Study, IOW=Isle of Wight Study, LR+=Positive Likelihood Ratio, LR-=Negative Likelihood Ratio, mAPI=modified API, NPV=negative predictive value, PARS=Pediatric Asthma Risk Score, PPV=positive predictive value.
cut-points were chosen to maximize the sensitivity and specificity (see Figure 2).
Development of the PARS
There were three variables in the original univariate screen that evaluated SPT results (Table I). As all three were significant, we opted to include the polysensitization variable (≥2 SPT+ to aero or food allergens) as prior studies have demonstrated that individuals with sensitivity to two or more allergens are at a higher risk of asthma26 and because the number of SPTs was highly significant in our cohort (Table III). The ORs for each factor were then calculated. A weight was assigned to each factor by rounding the OR to the nearest whole number. These weights were then summed to calculate a PARS for each subject in the CCAAPS cohort. The scores range from 0–14 with scores of 1 and 13 unattainable given the weighting of the ORs. A PARS scoring sheet that includes the decision tool, as well as the interpretive data is included in Repository E Figure I.
Table III.
Multivariate Logistic Model of Factors Predicting Asthma in the CCAAPS Cohort.
| Factor | p value | Coefficient | Odds Ratio (95% CI) | Weight |
|---|---|---|---|---|
| Parental asthma | 0.009 | 0.65 | 1.92(1.17 – 3.16) | 2 |
| Eczema before age 3 years | 0.02 | 0.61 | 1.97 (1.09– 3.06) | 2 |
| Wheezing apart from colds | 0.004 | 0.97 | 2.64 (1.39 – 5.13) | 3 |
| Early wheezing (before age 3 years) | 0.001 | 1.06 | 2.88 (1.52– 5.37) | 3 |
| SPT+ to ≥2 aero and/or food allergens | 0.0004 | 0.89 | 2.44 (1.49 – 4.05) | 2 |
| African American Race | 0.009 | 0.71 | 2.04 (1.19 – 3.47) | 2 |
*Sex and allergic rhinitis were removed by backward elimination at p > 0.05.
Observed and Predicted PARS with Asthma Risk at age 7 years in the CCAAPS Cohort
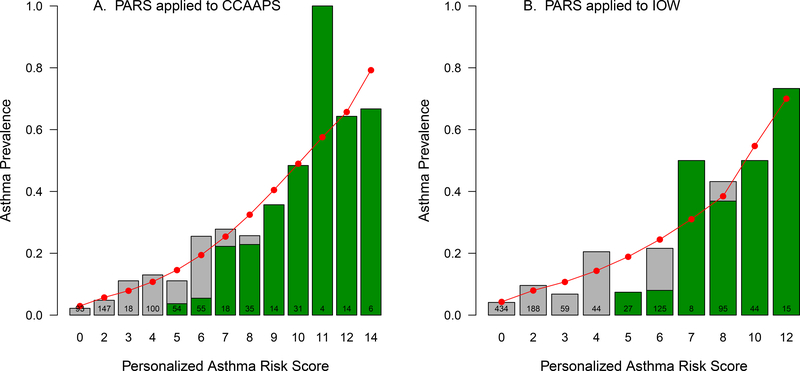
The gray bars in Figure 1A display the observed distribution of the PARS in the CCAAPS cohort and the asthma prevalence at age 7 years. The predicted values are depicted by the circles connected by the red line. The predicted risk of asthma ranged from 3% for children with a PARS = 0 to 79% for children with a PARS = 14 (Figure 1A). The predicted and observed scores show a high level of precision (Supplementary Table I), reflected by a p-value = 0.89 for the Hosmer-Lemeshow goodness of fit statistic (data not shown). The green shaded portion of each bar reflects the proportion of asthmatic children that were predicted to have asthma according to the original loose definition of the API for each level of PARS. For PARS of 7–14, there was strong concordance with the API (≥80%, Figure 1, Supplemental Table I). In contrast, for PARS of <7, there was poor concordance with the API (average of 9%, Figure 1A, Supplemental Table I). The PARS was superior to API in predicting asthma in children with lower risk scores.
Figure 1:
Predicted (closed circle) versus observed (gray bars) asthma prevalence by asthma prediction score in CCAAPS (A) and IOW (B). The green shading depicts the proportion of children that were predicted to have asthma according to the original loose definition of the API of those that were observed to have asthma.
Comparison of Published API to PARS
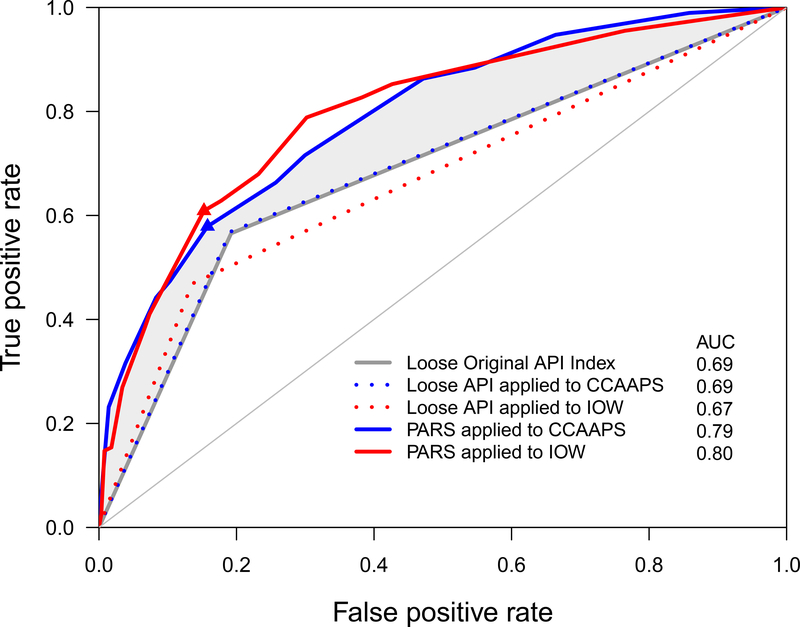
In order to compare the discriminatory power of PARS, the AUC from the published loose API was compared to the PARS. Figure 2 depicts the ROC curves. The solid gray ROC curve was reported in the original API paper using the loose index at age 6 years in the Tucson study data4. The blue dotted ROC curve was obtained by applying the loose API index to CCAAPS. The solid blue ROC curve was obtained by applying the PARS model to the CCAAPS birth cohort. The shaded gray area between the gray and blue curves shows the proportion of children that were missed by the API but detected by PARS. The AUC from the original API was 0.69 ± 0.0264 (Figure 2, solid gray line), which was identical to the AUC calculated when we applied the published loose API to the CCAAPS data (AUC=0.69 ± 0.027, blue dotted line, Figure 2). The PARS model was significantly higher (AUC=0.80 ± 0.025) than both the original loose API model (p=0.003) and the model applying the loose API index to the CCAAPS data (p=0.004), suggesting that the PARS better discriminates between asthma and non-asthma compared to the original loose API.
Figure 2:
Comparison of ROC curves between API and PARS. The dotted lines indicate API applied to the two cohorts; solid lines indicate PARS applied to the two cohorts. Blue lines indicate CCAAPS and red lines indicate IOW. Model discrimination was evaluated by the area under ROC curve. Model discrimination for the CCAAPS PARS model was excellent (AUC=0.80; p<0.001) and was significantly higher than the API loose index (p=0.002), and also was higher than the model applying the loose API to CCAAPS (p=0.003). Model discrimination for the IOW PARS model was excellent (AUC=0.80; p<0.001) and was significantly higher than the API loose index (p=0.0004), and also was higher than the model applying the loose API to IOW (p<0.0001). Blue and red triangles are the points at which sensitivity and specificity were assessed for PARS in CCAAPS (≥7) and IOW (≥6), respectively. The shaded gray area between the green and blue lines shows the proportion of children that were missed by the API but detected by PARS.
We then compared the sensitivity, specificity, PPV and NPV of the PARS model to the original loose API. We evaluated the sensitivity and specificity of the PARS model at a cut point of 6, (Figure 2, blue triangle). Sensitivity and PPV of PARS were both increased by 11% at 0.68 and 0.37 (Table IIc) compared to 0.57 and 0.26 for the loose API (Table IIc), respectively. The specificity was marginally decreased and the NPV was almost identical to the loose API (Table IIa and c).
Replication of PARS in the IOW Cohort
To determine whether the PARS model is robust across different populations and demonstrate its validity, we applied both the stringent and loose API index as well as the PARS in a second independent cohort, the IOW birth cohort study. IOW is a general population birth cohort on a different continent and the children were recruited 10 years prior to CCAAPS. The gray bars in Figure 1B display the observed distribution of the PARS in the IOW cohort and asthma prevalence at age 10 years. The predicted values are depicted by the circles connected by the red line. The predicted risk of asthma ranged from 4% for children with a PARS of 0 to 70% for children with a PARS of 12 (Figure 1B, Supplemental Table I). The predicted and observed scores show a high level of precision (Supplemental Table I), reflected by a p-value of 0.99 for the Hosmer-Lemeshow goodness of fit statistic (data not shown). Similar to what was observed for CCAAPS, PARS of ≥7 displayed strong concordance with the API in IOW children (≥85%, Figure 1B, Supplemental Table I). In contrast, for PARS of <7 there was poor concordance with the API (average of 23%, Figure 1B, Supplemental Table I).
The observed distribution of the PARS in the IOW cohort and the asthma prevalence at age 10 years performed very similarly as PARS applied to CCAAPS. The dotted red ROC curve was obtained by applying the loose API to the IOW birth cohort (AUC (0.67 ± 0.019, Figure 2), which was similar to AUCs of the original loose API and the loose API applied to the CCAAPS cohort (both 0.69, Table IIa and b). The solid red ROC curve was obtained by applying the PARS model to the IOW cohort (AUC (0.79 ± 0.020), Figure 2). The PARS model was again superior to the loose API in the IOW cohort (AUC 0.79 versus 0.67, p < 0.0001, Figure 2). The PARS model applied to the IOW cohort had a greater sensitivity (0.67) than the original loose API (0.57) and similar sensitivity to the PARS model applied to CCAAPS (0.68, Table IIc), highlighting the validity and robustness of the PARS model. The PPVs were similar in the CCAAPS and IOW PARS models but are both greater than the PPV of the loose API index model. The models were similar with respect to specificity and NPV.
DISCUSSION
For clinicians and researchers, the ability to accurately predict which children will develop asthma is a challenge. Asthma prediction matrices use a combination of major and minor criteria to give a binary yes/no as to whether or not a child will develop asthma. However, personalized prediction tools are needed that take into consideration the individual combination of risk factors in order to better estimate the spectrum of asthma risk. Therefore, we developed and validated PARS, a continuous risk score for asthma which has increased sensitivity over the API and mAPI and captures children with mild to moderate risk. PARS was developed by systematically determining risk factors for asthma in a multivariate model. Beyond API risk factors, we have improved detection by including poly-sensitization, early wheezing (before age 3 years), and African American race. Notably, the improved prediction was evident in children with mild to moderate asthma risk, whom are not predicted to have asthma by the API.
PARS is superior to the API with an 11% increase in sensitivity. This increase is due to improved prediction in children with mild to moderate asthma risk. Specifically, the API identifies children at the highest risk for developing asthma, as shown by 100% agreement between the API and PARS scores of ≥9. However, 43.2% of asthmatics in CCAAPS missed by the API had scores <9, indicating a mild to moderate risk of asthma. Children with mild to moderate risk have fewer risk factors and may be the most likely to respond favorably to prevention strategies. This is critical because the API and the mAPI are used to populate asthma prevention trials. One of these was the Prevention of Early Asthma in Kids (PEAK) trial, which sought to determine if the natural course of childhood asthma could be altered in children aged 2–3 years by treating with inhaled fluticasone propionate for two years. The anti-inflammatory therapy, although effective in preventing symptoms, did not change the natural history of asthma outcomes after cessation of therapy. The continuous nature of the PARS score would enable clinical trials to be populated with children with varying asthma risk. It is critical to correctly identify children across the spectrum of asthma risk since the efficacy of preventions and interventions may be higher in those with mild to moderate asthma risk.
Importantly, we replicated the results of the PARS model in the IOW cohort with almost identical sensitivity, specificity, PPV and NPV, highlighting the robustness of the model in a distinct population. The IOW is a population birth cohort in contrast to CCAAPS, which is a high-risk birth cohort such that each participant has at least one atopic parent. Further, IOW is on a different continent, separated in time (children were recruited 10 years prior to CCAAPS), and does not include African-Americans. Even with substantial differences between the studies, PARS was superior to the API and able to reliably predict asthma risk in both CCAAPS and IOW, highlighting the validity and broad applicability of the PARS tool.
Polysensitization (≥2 aero or food allergens) was most predictive of asthma in PARS than aeroallergen or food sensitization alone. Polysensitization reflects a greater degree of atopy so this finding is not unexpected. Indeed, both children and adults are more likely to have asthma with increasing odds ratios as the number of positive tests increase26. Further, in asthmatics, total IgE is higher in subjects that are polysensitized than those that are monosensitized27.The API did not include sensitization, and the mAPI defined sensitization to ≥1 aeroallergen as a major criteria and sensitization to food (egg, milk, peanut) as a minor criteria28. We recognize that skin prick testing may not be routinely performed in general pediatric care. However, even without the skin testing information, a pre-test risk score can be calculated and a post-test range can be estimated. Further, the PARS performs superiorly to the API (AUC 0.67) without the SPT criteria in both CCAAPS and IOW (AUC 0.78 and AUC 0.72, respectively), further supporting the robustness of the model.
We also included race in the PARS. In CCAAPS race was a risk factor for asthma, consistent with prior work finding higher rates of asthma and asthma severity29–31, and poorer control32 in African Americans. The cause of this association is likely multifactorial and may have both a genetic and environmental basis. Flores et al, utilizing genetic analysis of ancestral informative markers in a study of self-reported African Americans, found African ancestry to be associated with asthma30, supporting a role of genetics in the increased risk. However, in Greater Cincinnati where CCAAPS was recruited, there are still marked racial disparities. Specifically, African Americans more likely to live in poverty33 and have higher exposures to traffic pollution34. These disparities are often seen across the United States as well35, 36. Thus, while race is an important risk factor, it is likely a result of both underlying genetic risk as well as sociodemographic factors. However, in areas which are much more racially homogenous such as IOW, the PARS still retains excellent predictive ability.
While our study compared PARS to API, there are substantial advantages of PARS when compared to other predictive models. In 2015, a systematic review of 30 predictive models for asthma development in children was performed37 and our PARS model either out performs (had lower AUC, sensitivity or PPV) and/or is less invasive (biologic sampling, spirometry, and blood draw) than all of the published models. Therefore, the PARS model is the most accurate, non-invasive asthma predictive tool to date. Since a blood count and a differential are not routinely part of the routine allergy or asthma workup, the PARS may be more clinically useful and readily applicable in an office setting. In order to facilitate easy implementation of PARS in clinical and research settings, we have included a PARS scoring sheet that includes the decision tool, as well as the clinical interpretations. Further, a PARS web application, which provides fast and easy calculation of the PARS, is accessible at: https://pars.research.cchmc.org. Through 6 simple yes/no questions, the application calculates the risk score and provides the interpretation of the results. The responsive design permits viewing on all devices formats/screen sizes. In addition, the groundwork has been laid that allows for easy communication with 3rd party applications such as EMRs through RESTful web services.
PARS has some opportunities for improvement. First, to ensure maximal generalizability, additional studies should be performed evaluating PARS in other racial/ethnic groups specifically Hispanic or Latino populations where rates of asthma are higher than whites or African-Americans.38 Further, we welcome additional studies including African Americans given that the CCAAPS population is ~20% African-American, limiting our ability to perform race specific analyses. Second, there is no agreement in the literature regarding when prevention strategies should be instituted, and it is possible that the optimal window may be before age three. In any case, PARS will be useful to identify children for early clinical intervention and potential disease modification strategies. Third, to minimize recall bias of parental report of wheeze, clinicians are encouraged to collect information about whether the child has been wheezing at each well child visit. Lastly, environmental factors were not included in PARS at this time. While we recognize the importance of the environment in asthma risk39, how to uniformly estimate exposures to ensure accuracy and generalizability while minimizing burden and cost is still an ongoing debate.
In conclusion, we have developed a new asthma risk assessment scoring system that can quickly and easily be utilized in the clinical setting to assess asthma risk in children. The PARS performed better than the original API and mAPI, and is particularly better able to distinguish among the mild to moderate scoring patients, which are arguably the most difficult group to predict.
Supplementary Material
Supplemental Figure1: Pediatric Asthma Risk Score (PARS) Scoring Sheet.
Supplemental Table1 I. Observed and Predicted Risk of Asthma by PARS and Agreement between PARS and API
Key Messages:
We have developed the Pediatric Asthma Risk Score (PARS) that relies on factors that are routinely collected in the assessment of a child being evaluated for allergy and/or asthma.
PARS had an improved ability to predict asthma development in children with fewer risk factors who are likely amenable to respond favorably to prevention strategies, therefore, may be a more useful clinical and research tool.
Calculating PARS does not require blood tests and can be easily implemented in an office setting.
To facilitate easy implementation of PARS in clinical and research settings, we have included a PARS scoring sheet that includes the decision tool, as well as the clinical interpretations. Further, A PARS web application, which provides fast and easy calculation of the PARS, is accessible at: https://pars.research.cchmc.org.
Acknowledgments
Acknowledgments:
We would like to thank the CCAAPS and IOW participating children and their families.
Funding: This work was funded by National Institutes of Health grants 2U19AI70235 (GKKH, JBM, LJM), R01ES011170 (GKL, GKKH, DIB, JEL), R01HL082925 (SHA, RJK), T32ES010957 (ES) and R01ES019890 (PHR).
Abbreviations Used:
- API
Asthma Predictive Index
- AR
Allergic Rhinitis
- AUC
Area Under the Curve
- CCAAPS
Cincinnati Childhood Allergy and Air Pollution Study
- IOW
Isle of Wight
- LR
Likelihood Ratio
- Mapi
Modified Asthma Predictive index
- OR
Odds Ratio
- PARS
Pediatric Asthma Risk Score
- ROC
Receiver Operator Characteristics
- SPT
Skin Prick Test
- PPV
Positive Predictive Value
- NPV
Negative Predictive Value
Footnotes
Conflict of Interest Statement: The authors have declared that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief 2012:1–8. [PubMed] [Google Scholar]
- 2.Palmer LJ, Cookson WO. Genomic approaches to understanding asthma. Genome Res 2000; 10:1280–7. [DOI] [PubMed] [Google Scholar]
- 3.Savenije OE, Kerkhof M, Koppelman GH, Postma DS. Predicting who will have asthma at school age among preschool children. J Allergy Clin Immunol 2012; 130:325–31. [DOI] [PubMed] [Google Scholar]
- 4.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 2000; 162:1403–6. [DOI] [PubMed] [Google Scholar]
- 5.Amin P, Levin L, Epstein T, Ryan P, LeMasters G, Khurana Hershey G, et al. Optimum predictors of childhood asthma: persistent wheeze or the Asthma Predictive Index? J Allergy Clin Immunol Pract 2014; 2:709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caudri D, Wijga A, CM AS, Hoekstra M, Postma DS, Koppelman GH, et al. Predicting the long-term prognosis of children with symptoms suggestive of asthma at preschool age. J Allergy Clin Immunol 2009; 124:903–10 e1–7. [DOI] [PubMed] [Google Scholar]
- 7.Kurukulaaratchy RJ, Matthews S, Holgate ST, Arshad SH. Predicting persistent disease among children who wheeze during early life. European Respiratory Journal 2003; 22:767–71. [DOI] [PubMed] [Google Scholar]
- 8.Devulapalli CS, Carlsen KC, Haland G, Munthe-Kaas MC, Pettersen M, Mowinckel P, et al. Severity of obstructive airways disease by age 2 years predicts asthma at 10 years of age. Thorax 2008; 63:8–13. [DOI] [PubMed] [Google Scholar]
- 9.Guilbert TW, Morgan WJ, Zeiger RS, Bacharier LB, Boehmer SJ, Krawiec M, et al. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. J Allergy Clin Immunol 2004; 114:1282–7. [DOI] [PubMed] [Google Scholar]
- 10.Klaassen EM, van de Kant KD, Jobsis Q, van Schayck OC, Smolinska A, Dallinga JW, et al. Exhaled biomarkers and gene expression at preschool age improve asthma prediction at 6 years of age. Am J Respir Crit Care Med 2015; 191:201–7. [DOI] [PubMed] [Google Scholar]
- 11.Singer F, Luchsinger I, Inci D, Knauer N, Latzin P, Wildhaber JH, et al. Exhaled nitric oxide in symptomatic children at preschool age predicts later asthma. Allergy 2013; 68:531–8. [DOI] [PubMed] [Google Scholar]
- 12.Balinotti JE, Colom A, Kofman C, Teper A. Association between the Asthma Predictive Index and levels of exhaled nitric oxide in infants and toddlers with recurrent wheezing. Arch Argent Pediatr 2013; 111:191–5. [DOI] [PubMed] [Google Scholar]
- 13.Bloemen K, Koppen G, Govarts E, Colles A, Van Den Heuvel R, Nelen V, et al. Application of non-invasive biomarkers in a birth cohort follow-up in relation to respiratory health outcome. Biomarkers 2010; 15:583–93. [DOI] [PubMed] [Google Scholar]
- 14.Chang TS, Lemanske RF, Jr., Guilbert TW, Gern JE, Coen MH, Evans MD, et al. Evaluation of the modified asthma predictive index in high-risk preschool children. J Allergy Clin Immunol Pract 2013; 1:152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iossifova YY, Reponen T, Ryan PH, Levin L, Bernstein DI, Lockey JE, et al. Mold exposure during infancy as a predictor of potential asthma development. Annals of Allergy Asthma & Immunology 2009; 102:131–7. [DOI] [PubMed] [Google Scholar]
- 16.LeMasters GK, Wilson K, Levin L, Biagini J, Ryan P, Lockey JE, et al. High prevalence of aeroallergen sensitization among infants of atopic parents. J Pediatr 2006; 149:505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reponen T, Vesper S, Levin L, Johansson E, Ryan P, Burkle J, et al. High environmental relative moldiness index during infancy as a predictor of asthma at 7 years of age. Ann Allergy Asthma Immunol 2011; 107:120–6. [DOI] [PubMed] [Google Scholar]
- 18.National Asthma Education and Prevention Program (NAEPP) NHLBI. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. NIH Publication No. 07–4051.: National Institutes of Health, National Heart, Lung and Blood Institute, 2007:213–76. [Google Scholar]
- 19.Epstein TG, LeMasters GK, Bernstein DI, Ericksen MB, Martin LJ, Ryan PH, et al. Genetic variation in small proline rich protein 2B as a predictor for asthma among children with eczema. Ann Allergy Asthma Immunol 2012; 108:145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurukulaaratchy RJ, Fenn MH, Waterhouse LM, Matthews SM, Holgate ST, Arshad SH. Characterization of wheezing phenotypes in the first 10 years of life. Clin Exp Allergy 2003; 33:573–8. [DOI] [PubMed] [Google Scholar]
- 21.Arshad SH, Holloway JW, Karmaus W, Zhang H, Ewart S, Mansfield L, et al. Cohort Profile: The Isle Of Wight Whole Population Birth Cohort (IOWBC). Int J Epidemiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet 1998; 351:1225–32. [PubMed] [Google Scholar]
- 23.Castro-Rodriguez JA. The Asthma Predictive Index: a very useful tool for predicting asthma in young children. J Allergy Clin Immunol 2010; 126:212–6. [DOI] [PubMed] [Google Scholar]
- 24.Wi CI, Krusemark EA, Voge G, Sohn S, Liu H, Ryu E, et al. Usefulness of asthma predictive index in ascertaining asthma status of children using medical records: An explorative study. Allergy 2018; 73:1276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria; 2007. [Google Scholar]
- 26.Burbach GJ, Heinzerling LM, Edenharter G, Bachert C, Bindslev-Jensen C, Bonini S, et al. GA(2)LEN skin test study II: clinical relevance of inhalant allergen sensitizations in Europe. Allergy 2009; 64:1507–15. [DOI] [PubMed] [Google Scholar]
- 27.Kim KW, Kim EA, Kwon BC, Kim ES, Song TW, Sohn MH, et al. Comparison of allergic indices in monosensitized and polysensitized patients with childhood asthma. J Korean Med Sci 2006; 21:1012–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guilbert TW. Identifying and managing the infant and toddler at risk for asthma. J Allergy Clin Immunol 2010; 126:417–22. [DOI] [PubMed] [Google Scholar]
- 29.Ater D, Bar BE, Fireman N, Fireman E, Shai H, Tasher D, et al. Asthma-predictive-index, bronchial-challenge, sputum eosinophils in acutely wheezing preschoolers. Pediatr Pulmonol 2014; 49:952–9. [DOI] [PubMed] [Google Scholar]
- 30.Flores C, Ma SF, Pino-Yanes M, Wade MS, Perez-Mendez L, Kittles RA, et al. African ancestry is associated with asthma risk in African Americans. PLoS One 2012; 7:e26807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vergara C, Caraballo L, Mercado D, Jimenez S, Rojas W, Rafaels N, et al. African ancestry is associated with risk of asthma and high total serum IgE in a population from the Caribbean Coast of Colombia. Hum Genet 2009; 125:565–79. [DOI] [PubMed] [Google Scholar]
- 32.Thakur N, Barcelo NE, Borrell LN, Singh S, Eng C, Davis A, et al. Perceived Discrimination Associated With Asthma and Related Outcomes in Minority Youth: The GALA II and SAGE II Studies. Chest 2017; 151:804–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohio ULoGC. The State of Black Cincinnati 2015: Two Cities. 2015.
- 34.Newman NC, Ryan P, Lemasters G, Levin L, Bernstein D, Hershey GK, et al. Traffic-related air pollution exposure in the first year of life and behavioral scores at 7 years of age. Environ Health Perspect 2013; 121:731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mikati I, Benson AF, Luben TJ, Sacks JD, Richmond-Bryant J. Disparities in Distribution of Particulate Matter Emission Sources by Race and Poverty Status. Am J Public Health 2018; 108:480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.JL S, KR F, MA K. Income and Poverty in the United States: 2016 In: U.S. Census Bureau CPR, P60–259, ed. Washington, DC: US Government Printing Office, 2017. [Google Scholar]
- 37.Luo G, Nkoy FL, Stone BL, Schmick D, Johnson MD. A systematic review of predictive models for asthma development in children. BMC Med Inform Decis Mak 2015; 15:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beckett WS, Belanger K, Gent JF, Holford TR, Leaderer BP. Asthma among Puerto Rican Hispanics: a multi-ethnic comparison study of risk factors. Am J Respir Crit Care Med 1996; 154:894–9. [DOI] [PubMed] [Google Scholar]
- 39.Salam MT, Li YF, Langholz B, Gilliland FD, Children’s Health S. Early-life environmental risk factors for asthma: findings from the Children’s Health Study. Environ Health Perspect 2004; 112:760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure1: Pediatric Asthma Risk Score (PARS) Scoring Sheet.
Supplemental Table1 I. Observed and Predicted Risk of Asthma by PARS and Agreement between PARS and API