Abstract
CD4 T cell activation is critical to the initiation of adaptive immunity. CD4 T cells are also the main targets of HIV infection and their activation status contributes to the maintenance and outcome of infection. While the role of activation in the differentiation and proliferation of CD4 T cells is well studied its impact on the processing and MHC-I presentation of epitopes and immune recognition by CD8 T cells are not investigated. Here we show that the expression and hydrolytic activities of cellular peptidases are increased upon TCR-dependent and MHC-peptide activation of primary CD4 T cells from healthy or HIV-infected persons. Changes in peptidase activities altered the degradation patterns of HIV antigens analyzed by mass spectrometry, modifying the amount of MHC-I epitopes produced, the antigenicity of the degradation products and the coverage of antigens by degradation peptides presentable by MHC-I. The computational analysis of 2237 degradation peptides generated during the degradation of various HIV antigenic fragments in CD4 T cells identified cleavage sites that were predictably enhanced, reduced or unchanged upon cellular activation. Epitope processing and presentation by CD4 T cells may be modulated by the activation state of cells in a sequence-dependent manner. Accordingly, cellular activation modified endogenous antigen processing and presentation and killing of HIV-infected CD4 T cells by CD8 T cells in a way that mirrored differences in in vitro epitope processing. The clearance of HIV-infected cells may rely on different immune responses according to activation state during HIV infection.
Introduction
T cell activation stimulated by TCR ligation is an early and fundamental step in the initiation of immune responses (1). The interaction between MHC-peptide displayed by T cells and cognate TCR induces cellular activation of naïve resting T cells, proliferation and differentiation into various subsets. CD4 T cells are also the targets of HIV infection and the activation state affects their susceptibility to infection (2–4), their capacity to become viral reservoirs or to re-express HIV (5, 6). While transduction signals and transcriptional regulations involved in T cell activation have been dissected, the role of activation state in shaping epitope processing and MHC-I presentation by CD4 T cells has not been investigated despite its important role for immune recognition.
The degradation of proteins into peptides defines the immunopeptidome available for display by MHC-I or MHC-II and eventually triggers immune recognition. Protein degradation involves proteasomes, post-proteasomal aminopeptidases, endopeptidases or carboxypeptidases (7) in the cytosol, and aminopeptidases in the endoplasmic reticulum (ER) for MHC-I presentation (8) while different endolysosomal aminopeptidases and cathepsins degrade proteins in the MHC-II and MHC-I cross-presentation pathways (9, 10). The expression and activities of the antigen processing machinery are modulated by various cytokines such as interferon gamma (11), TLR ligands (12–14), oxidative stress (15), viral infections (16), drugs (17–19) and varies according to cell types (13, 14, 20). Modulations of peptidase hydrolytic activities by drugs or among cell types affect degradation patterns of antigens and epitope production, leading to reduction or enhancement of epitope production and changes in T cell recognition.
In the HIV-infected population cellular activation is highly variable, usually higher during acute infection where viral load is the highest, reduced by antiviral treatments (21) and modulated by co-infections (22), microbial translocation (23) and HIV shedding in the genital tract or residual replication during ART treatment (24). Activation of CD4 T cells during early infection is predictive of CD4 count evolution (25) and CD4 recovery during antiretroviral therapy (ART) (26). Infection of resting CD4 T cells is mostly abortive while productive infection mostly occurs in activated CD4 T cells (27, 28), which may partly be due to the variable expression and activity of multiple host restriction factors (29). HIV infection of CD4 T cells can modulate the activation state of neighboring cells and increase cell-to-cell spread of the virus (30). While cellular activation triggers HIV re-expression from latently infected cells proposed strategies to flush out HIV reservoirs aim at reactivating HIV expression without inducing cellular activation (6, 31). Thus, HIV antigens could be found in different metabolic environments in CD4 T cells but the impact of CD4 T cell activation on epitope processing and presentation and immune recognition has not been assessed despite its importance for the clearance of infected cells.
In this study, we compared the expression and hydrolytic activities of various cellular peptidases involved in antigen processing. We showed that TCR-dependent, mitogen- or MHC-peptide-induced cellular activation increased most peptidase activities, leading to modification in antigen degradation patterns in a sequence-dependent manner, variations in MHC-I epitope production and cytotoxic T cell responses. Cellular activation altered endogenous processing and presentation by HIV-infected CD4 T cells and the subsequent epitope-specific CD8 T cell-mediated killing in a pattern mirroring the changes observed during in vitro degradation. These data show that antigen presentation in HIV infection is variable according to activation state and may affect CD8 T cell immune recognition and clearance of infected cells.
Material and methods
Ethics statement
Cells were isolated from buffy coats of anonymous healthy blood donors, obtained from the Massachusetts General Hospital blood center (Boston, MA) after approval by the Partners Human Research Committee under protocol 2005P001218 or from HLA-typed blood donors after written informed consent and approval under protocol 2010P002121 for HIV negative donors, and protocols 2006P000849 and 2003P001894 for HIV positive donors (Boston, MA). HIV-infected donors included 22 HIV controllers (average viral load 120 copies/ml, average CD4 count 736, average age 47), 14 Chronic untreated (7390 copies/ml, CD4: 533, average age 43.5), 18 Chronic treated (<50 copies/ml, CD4: 520, average age 49); ranges for each parameter are indicated in supplemental figure 2.
Primary cells and cell lines
CD4 T cells were isolated by negative selection from fresh PBMCs using a CD4+ T cell enrichment magnetic isolation kit (StemCell Technologies). Primary CD4 T cells were cultured in R10+ medium (RPMI (Sigma) supplemented with Penicillin/Streptomycin, Glutamine and 1% (v/v) HEPES (Sigma) and in the presence of 50 Units/mL of Interleukin-2 (IL-2) (NIH AIDS Research and Reference Reagents Program). In vitro stimulation was achieved by addition of anti-CD3/CD28 coupled to magnetic beads (Life Technologies) at a ratio of 1:1 bead to T cell, with 25 ng/mL of PHA (Remel) or the addition of the cognate peptide at the desired concentration. EBV-immortalized B cell lines were obtained from the Ragon Institute Repository and kept in culture in R10+ medium. Cytotoxic CD8 T cell clones and CD4 T cell clones were isolated by limited dilution, maintained in R10+ medium supplemented with IL-2 and stimulated for proliferation with irradiated PBMCs and 12F6 or OKT3 antibodies (eBioscience), respectively.
Flow Cytometry
Cell viability was measured using LIVE/DEAD Fixable Blue Dead Cell Stain Kit (Invitrogen). Unstimulated and CD3/CD28- or PHA-stimulated CD4 T cells were stained with CD4-PerCpCy5.5 and HLA-ABC-APC (BD Biosciences) to assess purity (94–98%) and CD38-FITC, CD69-PE, CD25-PerCpCy5.5 and HLA-DR-APC (BD Biosciences) to measure surface activation marker levels. Cellular activation levels were calculated by first gating for CD25 versus CD69 on the unstimulated sample on live, CD4-positive cells and applying the same gates to the CD3/CD28-stimulated sample. A similar gating strategy was applied to the CD38 versus HLA-DR analysis. For co-culture experiment of CD4 T cell clones with B cell lines, cells were stained with CD4-BV605, CD19-APC-Cy7, CD3-Pacific Blue (BD Biosciences), and HLA-ABC-AlexaFluor 700 (eBioscience) in addition to the cellular activation markers. Cellular activation levels were calculated by gating for CD25 versus CD69 on the CD3-positive, CD19-negative, CD4-positive cells. For degranulation experiments of HLA-B57-expressing CD4 T cell clone with CD8 T cell clones, cells were stained with CD4-BV605, CD8-APC-Cy7, CD3-Pacific Blue and CD107a-PeCy7 (BD Biosciences). HIV-1 infection was assessed by GFP expression and HIV-1 p24 intracellular protein expression using KC57-RD1 HIV p24 antibody (Beckman-Coulter). CD107a-positive cells were gated from the live, CD3-positive, CD4-negative, CD8-positive cells and non-HIV-infected cells were used to calculate background degranulation. Samples were run on a FACS Calibur or a BD 4 Laser LSRII flow cytometer and analyzed using FlowJo software (FlowJo, LLC).
Expression of the antigen processing machinery in CD4 T cells
Cytosolic extracts were prepared from matched unstimulated and CD3/CD28- or PHA-stimulated CD4 T cells by 0.125% digitonin permeabilization in ice-cold lysis buffer as reported previously (14, 17, 18, 32). Protein concentration was assessed by Bio-Rad protein assay, according to manufacturer’s protocol.
Extracts analyzed by denaturing Western blot were probed with the following antibodies: β-actin (Abcam), Leucine aminopeptidase (Abcam), proteasome subunits S1 and S4 (Abcam); proteasome subunits α2, S6a, β1, β1i, β2, β4, β5, β5i, PA28a, and PA28b (Enzo Life Sciences); thimet oligopeptidase (TOP) and Endoplasmic reticulum aminopeptidase 1 (ERAP1) (Santa Cruz Biotechnology); Endoplasmic reticulum aminopeptidase 2 (ERAP2) (R&D Systems); β2i and tripeptidyl peptidase II (TPP2) (ProteinTech). Protein bands were visualized by dual infrared fluorophore Odyssey Infrared Imaging System, and quantified by densitometry (Li-Cor Biosciences). Beta-actin was used as a loading control to compare protein expression levels. We used equivalent amounts of cytosolic extracts, as determined by actin normalization, to degrade HIV peptides.
Measurement of peptidase activities in live CD4 T cells
The proteolytic activities of proteasomal caspase-like (50 μM Z-LLE-AMC; EMD Millipore), tryptic (50 μM Boc-LRR-AMC; Bachem), chymotryptic (50 μM Suc-LLVY-AMC; Bachem), aminopeptidases (50 μM H-Leu-AMC; Bachem), cathepsin S and omnicathepsins (50 μM Z-VVR-AMC and Z-FR-AMC, respectively; Enzo Life Sciences) were measured in 25×103 live CD4 T cells with peptidase-specific fluorogenic substrates as in (13, 14, 17, 18). The specificity of each catalytic reaction was checked by pre-incubating cells for 30 min with inhibitors of proteasome (10 µM MG132; Enzo Life Sciences), aminopeptidases (120 µM Bestatin; Sigma-Aldrich), cathepsin S (10 μM ZFL-COCHOO, Calbiochem), or omnicathepsins (5 μM E64, Enzo Life Sciences), before adding each substrate. The rate of fluorescence emission, which is proportional to the proteolytic activity, was measured every 600 s at 37 °C in a Victor-3 Plate Reader (Perkin Elmer), as in (13, 14, 18).
In vitro peptide degradation and mass spectrometry analysis
Peptides (Biosynthesis, Texas) were purchased and their purity was assessed by mass spectrometry. All peptides were >90% pure. 1 nmol of highly purified peptides was degraded in 15 μg of CD4 T cell extract at 37 °C in pH7.4 degradation buffer as in (14, 17). Aliquots were taken at various time points and the reaction was stopped by addition of 5% (v/v) of Formic acid (Thermo Scientific). The degradation products were purified by trichloroacetic acid (TCA) (Sigma) precipitation (final concentration 5% (v/v)) and identified by in-house mass spectrometry as in (13, 17, 18, 32). Briefly, equal amounts of the purified degradation products were injected into a NanoLC Ultra-HPLC (Eksigent) for salt removal and separation, then online nanosprayed into an LTQ Orbitrap Discovery mass spectrometer (Thermo) for identification. Peptides were separated in a Nano cHiPLC column (75 um x 15 cm ChromXP c18-CL 5 um 300 Å; Eksigent) over a gradient of 2–60% buffer B (buffer A: 0.1% (v/v) formic acid in MS-grade water (Fisher Scientific)); buffer B: 0.1% (v/v) formic acid in MS-grade acetonitrile (Fisher Scientific) in 95 min with a conserved flow rate of 400 nl/min. Mass spectra were recorded in the 370–2000 Daltons range. In the tandem mass spectrometry mode, the eight most intense peaks were selected with a window of 1 Dalton and fragmented using a collision voltage of 35 V and helium as collision gas. Peaks in the mass spectra were searched against the source peptide databases with Proteome Discoverer (version 1.3; Thermo) and quantitatively analyzed. For a given peptide, the integrated area under the peak is proportional to the relative abundance of the peptide in the sample as previously shown (13, 32). Each sample was run on the mass spectrometer at least twice.
Antigenicity of the degradation peptides
The degradation products purified after TCA precipitation as in (33, 34) were diluted in serum-free RPMI, and the pH of the solution was readjusted to 7.4. HLA-matched EBV-transformed B cells loaded with 51Cr (Perkin Elmer) were pulsed with the purified degradation products or with various amounts of optimal peptides and used as targets in a 4 h chromium release assay with HLA-matched, epitope-specific effector CTL clones at an E:T ratio of 4:1 as in (14). The lysis percentage was calculated by comparison of target B cells pulsed with degradation products with B cells pulsed with medium only or B cells lysed with 5% triton detergent (Perkin Elmer) as in (14, 35).
Endogenous processing and presentation assay to CD8 T cells
HLA-B57+ primary CD4 T cells were either stimulated with anti-CD3/CD28 or kept in culture with no stimulation. The magnetic anti-CD3/CD28 beads were removed at 48 h post-stimulation and, for each experiment, 2 million of non-stimulated and CD3/CD28-stimulated CD4 T cells were infected with 20 μg HIV Gag p24 equivalent of NL4–3-ΔEnv-GFP virus pseudotyped with Vesicular Stomatitis Virus glycoprotein (VSV-g) in the presence of 5 μg/mL polybrene (Sigma) by spinfection for 1 h at 2000 xg as in (36). At 3, 24 and 48 h post-infection, CD4 T cells were plated with epitope-specific CD8 T cells at a ratio of 1:5 (CD4:CD8) and an aliquot of CD4 T cells was also harvested to measure HIV-1 infection through GFP and HIV-1 p24 intracellular expression, cellular activation, and HLA class I surface expression by flow cytometry. After 30 min of co-culture, CD107a-PeCy7 (BD Biosciences) was added to each well to measure CTL degranulation. After 6 h cells were harvested for CD8 flow cytometry staining.
Statistical analysis
Experimental data were analyzed using Microsoft Excel and GraphPad Prism version 5 (GraphPad Software). Spearman’s rank correlation was used to examine bivariate associations and Wilcoxon signed rank test was used to compare measurements between paired non-stimulated and stimulated samples. All p values are two-sided and p values lower than 5% were considered statistically significant.
Results
TCR-dependent and MHC-peptide-mediated activation of primary CD4 T cells increased cellular peptidase activities.
We investigated the effects of cellular activation on the antigen processing machinery of primary CD4 T cells using CD3/CD28-stimulation as a proxy for TCR engagement and mode of activation. The level of cellular activation of primary CD4 T cells from healthy donors was assessed by flow cytometry through surface expression of activation markers CD25, CD69, CD38 and HLA-DR. Consistently, the CD3/CD28-stimulated cells expressed more CD25+ and CD69+ at their surface starting 6 h post-stimulation and reaching maximum expression at 24h and sustained for at least 24 more hours (Figure 1A). The increased expression of activation markers at 48h post-stimulation was consistently significant (data not shown), and in accordance with other studies (37–40).
Figure 1:
CD3/CD28-stimulation increases peptidase activities in primary CD4 T cells. A. The surface expression of CD25 (circle) and CD69 (triangle) was monitored by flow cytometry in non-stimulated (open) and CD3/CD28-stimulated (plain) CD4 T cells at 8, 24 and 48h. Results are shown for n=3-4 healthy donors. B. The peptidase activities (proteasomal chymotryptic (circle), tryptic- (triangle) and caspase-like (inversed triangle), aminopeptidase (square), lysosomal cathepsin S (dark circle) and Omnicathepsin (diamond) were measured with peptidase-specific fluorogenic substrates in the same samples at 8, 24 and 48h post-stimulation. The fold change in activities was calculated by dividing the peptidase activity for the stimulated samples by their paired non-stimulated counterparts. n=4 healthy donors. C. Peptidase activities in paired non-stimulated (open circles) and CD3/CD28-stimulated (filled circles) primary CD4 T cells measured at 48h post-stimulation. Results are shown for n=15-17 healthy donors. Wilcoxon matched-pairs signed rank t test were performed (* p<0.05, ** p<0.01, *** p<0.001). D. Peptidase activities were plotted against the percentage of CD25+ CD4+ T cells in each experiment. n=10-12 healthy donors. Correlation was calculated by Spearman test (r>0.5 and p<0.02). E. The surface expression of CD25 was monitored by flow cytometry in CD4 T cells stimulated with increasing concentrations of a cognate peptide (0, 0.001, 0.01, 0.1, 1 and 10 μg/mL) presented by the autologous B cell line at 48h post-stimulation. Results are shown for n=3 CD4 T cell clones. F. The surface expression of CD25 was monitored by flow cytometry in CD4 T cells stimulated by autologous B cell lines incubated with no peptide (open circle), the cognate peptide at 1 μg/mL (filled square), an irrelevant peptide at 1 μg/mL (open square) or CD3/CD28 (filled circle) at 8, 24 and 48h in the left panel. Results are shown for n=3 CD4 T cell clones. The fold change in the percentage of CD25-positive CD4 T cells (Percentage of stimulated sample divided by percentage of non-stimulated sample) is represented on the right panel for the irrelevant peptide (white), the matched peptide (grey) and CD3/CD28-stimulated CD4 T cell clones. G. Peptidase activities were plotted against the percentage of CD25+ CD4+ T cells for a representative CD4 T cell clone across the different peptide concentrations tested. Correlation was calculated by Spearman test (r>0.5 and p<0.035).
Next, we assessed in live CD4 T cells the hydrolytic activities of several enzymes involved in antigen processing using a fluorometric assay developed in our laboratory (13, 14, 17, 18). Using peptidase-specific fluorogenic peptidic substrates we measured the three proteasome hydrolytic activities (chymotryptic, tryptic-like, caspase-like), aminopeptidases, omnicathepsin (cysteine cathepsins B, C, F, K, L, L2, O, S and X) and cysteine cathepsin S in resting or stimulated live cells at 4, 24 and 48 h post-stimulation. CD3/CD28-stimulation did not significantly change the peptidase activities at 6 h and 24 h post-stimulation while expression of CD25 was already higher than in resting CD4 T cells (Figure 1B). At 48 h post-stimulation when the maximum expression of CD25 was reached, all the cellular peptidase activities in 15–17 healthy donors were significantly increased (p<0.001 for the proteasomal, aminopeptidase and cathepsin S activities and p<0.02 for omnicathepsin activity) (Figure 1C). Interestingly, the fold changes in hydrolytic peptidase activities (ratio of CD3/CD28-stimulated cells over matching non-stimulated cells) were higher for the proteasomal caspase-like and the omnicathepsin activities, increased by up to 247-fold and 66.5-fold respectively. These activities are typically low at baseline in primary CD4 T cells. In comparison, the aminopeptidase, proteasomal chymotryptic-like, tryptic-like and cathepsin S hydrolytic activities increased by 4.6- to 6.7-fold. The temporal disconnect between the immediate signal transduction following CD3 and CD28 engagement and expression of surface activation markers and the increased peptidase activities at 48 h post-stimulation suggests that the observed changes may be linked to an increased in protein expression and not a direct modulation of enzymatic activity.
The increases in aminopeptidase, proteasomal chymotrytic and caspase-like hydrolytic activities were directly proportional to the percentage of activated CD25+ CD4+ T cells in the 10–12 samples (r>0.5 and p<0.02) (Figure 1D). Cellular peptidase activities except cathepsins positively correlated with the percentage of CD69+, CD25+CD69+, CD38+, HLA-DR+ and CD38+HLA-DR+ CD4 T cells, implying that the activated CD4 T cell population drives the overall increase in peptidase activity.
We also assessed the effect of activation mediated by the lectin phytohemagglutinin (PHA) (41) on cellular peptidase activities (supplemental figure 1). The maximal cellular activation was achieved 96 h post-stimulation (supplemental figure 1A). Antigen processing activities were increased although with lesser fold changes (2- to 3- fold for CD3/CD28-stimulated over non-stimulated) (supplemental figure 1B) and the increase was proportional to the number of activated cells (supplemental figure 1C). Since immune activation is highly variable in HIV-infected persons we aimed to assess how activatable CD4 T cells from various HIV-infected donors are and determine if activation still modifies antigen processing activities in CD4 T cells from infected donors. We tested the aminopeptidases and proteasome peptidase activities in live primary CD4 T cells isolated from HIV-infected patients (supplemental figure 2). All the groups responded to PHA-stimulation by an increase in peptidase activities. The aminopeptidase activities were significantly increased in paired primary CD4 T cells from controllers (n=8) and treated patients (n=12) (p<0.02). The proteasomal chymotrypic, caspase-like and tryptic-like activities were also significantly increased upon PHA-stimulation for controllers (n=8–18), ART-treated patients (n=8–11) and healthy donors (n=8–9) (p<0.02). None of the peptidase activities were significantly affected by PHA-stimulation in the HIV-infected untreated group (n=4–9) (supplemental figure 2A). The different groups were similarly responsive to PHA-stimulation as assessed by fold change between non-stimulated and stimulated peptidase activities (data not shown). In the HIV controllers and treated patient groups but not for the HIV+ untreated donors, the increase in peptidase activities significantly correlated with increasing percentages of CD25+ CD4 T cells as observed for healthy donors (supplemental figure 2B).
We then assessed if a more physiologically relevant stimulation, MHC-peptide binding, affected antigen processing activities. We used a co-culture assay where autologous B cells were loaded with HIV-1 peptides before being mixed with matched HIV-specific CD4 T cell clones and measuring CD4 T cell activation and peptidase activities. The 3 selected CD4 T clones were specific for HIV-1 Gag peptides: Gag-p24 WIILGLNKIVRMYSPTSI (aa 133–150) restricted by DRB1*0404, Gag-p24 YVDRFYKTLRAEQASQEV (aa 164–181) restricted by DRB*1101 and Gag-p15 TAPPEESFRFGEETTTPSQK aa 93 –112 restricted by DRB1*0401. Increasing concentrations (0.001–10 μg/mL) of cognate peptides upregulated expression of surface CD25 on CD4 T cells (Figure 1E). After a 48 h incubation with 0.1 μg/mL of cognate peptide, 80–98% of the CD4 T cells expressed CD25 and the maximum expression levels were reached with 1–10 μg/mL (97.7–99.9%). We compared the level of CD4 T cellular activation at 8, 24 and 48 h post-stimulation with B cells pulsed with no peptide, 1 μg /mL of irrelevant or cognate (matched) peptide (Figure 1F left panel). After 8 h of co-culture, the CD4 T cell clones stimulated with cognate peptides or aCD3/CD28 showed an upregulation of CD25 surface expression (35–45% and 40–60% respectively) and plateaued at >80% between 24–48 hours. At 48 h post-stimulation, aCD3/CD28-stimulation gave similar levels of activation as the matched peptide. The fold change (stimulated/non-stimulated) in percentage of CD25-positive CD4 T cells was higher for CD3/CD28-stimulation (2.1–3.2-fold) than for the matched peptide (1.6–1.9-fold) and no change for irrelevant peptide (1-fold) (Figure 1 Fright panel). Finally, we measured the peptidase activities of the CD4 T cell clones following peptide-dependent stimulation (Figure 1 G). The aminopeptidase, tryptic-like and omnicathepsin hydrolytic activities positively correlated with increasing concentration of cognate peptides and thus with increased cellular activation (r>0.89, p<0.034). Altogether these data show that TCR-dependent, mitogen- and cognate MHC-peptide-induced cellular activation modify cellular peptidase activities in CD4 T cells.
Increased peptidase expression upon CD3/CD28-stimulation contributes to the increased peptidase activities
Since the increase in peptidase activities lagged >24 h after CD4 T cell activation it may involve an increase in protein expression. We assessed the expression of various cellular peptidases by dual infrared fluorophore Western Blot in extracts from paired non-stimulated and CD3/CD28-stimulated primary CD4 T cells at 48 h post-stimulation (14). We measured the expression of 14 proteasomal subunits including 6 catalytic, 2 structural core subunits and 5 lid subunits, 3 aminopeptidases and 2 post-proteasomal peptidases. The expression of proteins was calculated as a ratio over actin. The levels of proteasome catalytic β1, β2, β5, the non-catalytic α2 and β4, the constitutive 19S lid S1, S4 and S6α and the immunoproteasome 11S lid PA28α and PA28β subunits were significantly increased at 48 h post- CD3/CD28 stimulation (p<0.01) (Figure 2). The expression of the immunoproteasome catalytic subunits β1i, β2i, and β5i was not significantly impacted by CD3/CD28-stimulation. The expression levels of the post-proteasomal peptidases thymet oligopeptidase (TOP) and tripeptidyl peptidase II (TPP2) were significantly increased upon CD3/CD28-stimulation 48 h post-stimulation (p<0.01) while that of ER-resident aminopeptidases ERAP1, ERAP2 and leucine aminopeptidase LAP was not significantly modified (Figure 2). The increased expression of proteasomes subunits and several post-proteasomal peptidases is in agreement with previously documented increased RNA transcription of proteasomes upon CD3/CD28-stimulation (42) and may contribute to the increased peptidase activities.
Figure 2:

The expression of proteasomal subunits and post-proteasomal peptidases is modified upon CD3/CD28-stimulation. The expression of 13 proteasomal subunits (catalytic subunit of the 20S core beta1, beta2, beta5; non-catalytic subunits of the 20S core alpha2, and beta4; constitutive proteasome 19S subunits S1, S4 and S6alpha; catalytic subunits of the immunoproteasome beta1i, beta2i and beta5i; and immunoproteasome 11S lid subunits PA28alpha and PA28beta), 3 aminopeptidases (ERAP1, ERAP2, LAP) and 2 post-proteasomal proteases (TOP, TPPII) were assessed in paired non-stimulated (open circles), CD3/CD28-stimulated (filled circles) CD4 T cell samples by Western blot. The ratios of protein of interest over actin (loading control) are presented for each sample pair. N=7-9 healthy donors. Wilcoxon matched-pairs signed rank t tests were performed (* p<0.05, ** p<0.01).
The increase in cellular peptidase activities in CD3/CD28-stimulated CD4 T cells alters antigen processing.
We assessed the consequences of higher hydrolytic activities upon CD3/CD28-stimulation on the processing of HIV peptides in CD4 T cells. We compared the degradation of synthetic HIV peptides in matched extracts from resting or CD3/CD28-stimulated CD4+ T cells using an in vitro degradation assay previously developed in our laboratory (14, 17, 33, 43). Cellular extracts used for peptide degradation contained all the cellular peptidases involved in antigen processing in each condition. The assay was shown to recapitulate endogenous epitope processing while providing additional information on degradation patterns and epitope production (17, 33, 35). The degradation peptides generated at different time points were purified, identified and quantified by mass spectrometry. Each identified peptide is associated with a peak intensity that varies with the amount of peptide (14, 17, 43).
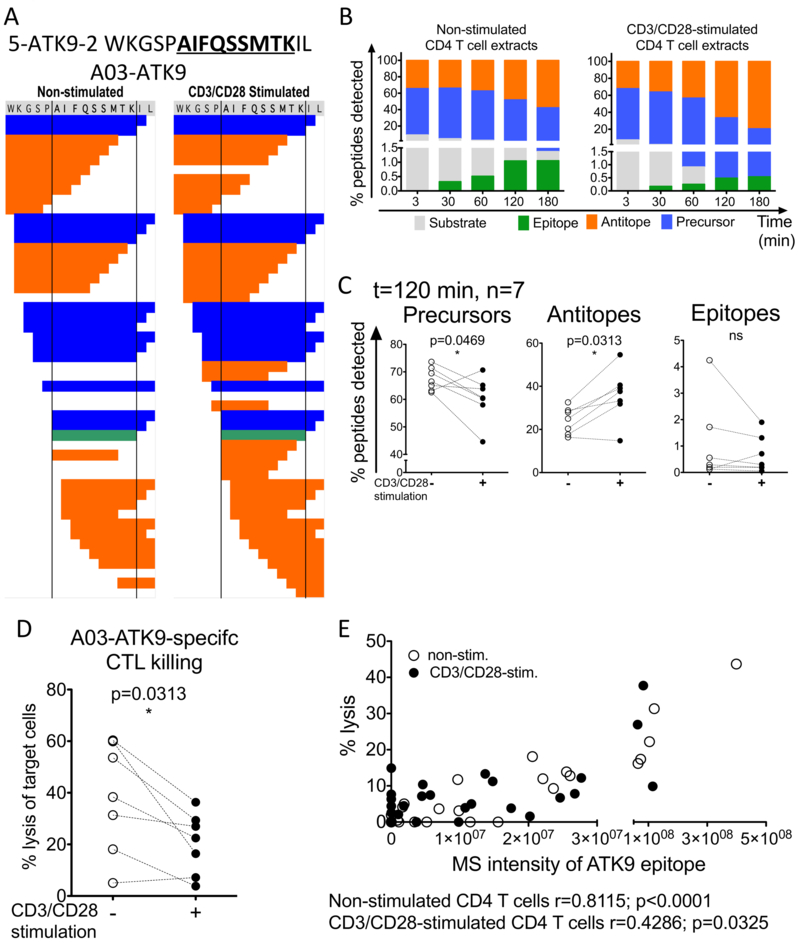
HIV-1 reverse transcriptase (RT) extended 16-mer peptide 5-ATK9–2 WKGSPAIFQSSMTKIL (aa 153–168) that contains the HLA-A*03:01/11:01-restricted optimal epitope AIFQSSMTK (A03/11-ATK9, (44)) was degraded in equal amount of resting and CD3/CD28-stimulated CD4+ T cells paired cellular extracts (figure 3). The number of fragments obtained after a 2 h degradation was in the same range for both resting and CD3/CD28-stimulated extracts (n=40 and 48 respectively) and included peptides unique to each condition (figure 3A). Peptides included the substrate (the original 16-mer), optimal epitope A03/11-ATK9, precursors (fragments with N- and/or C-extensions that can be further processed into the optimal epitope) and antitopes (peptides cleaved within the epitopic sequence) (figure 3A-B). The 16-mer substrate was degraded faster in CD3/CD28-stimulated extracts than in non-stimulated extracts (Figure 3B). Less ATK9 epitope (1.05, 1.06% vs. 0.49, 0.54%) and more antitopes (48.1, 57.7% vs. 66.43, 79.38%) were produced in the CD3/CD28-stimulated extracts at 120 and 180min. Across degradations in 7 different donors we consistently obtained fewer precursors and optimal ATK9 and more antitopes produced by the CD3/CD28-stimulated primary CD4+ T cell extracts (Figure 3C), in agreement with the faster kinetics of degradation of the original peptide.
Figure 3:
CD3/CD28-stimulation decreased HLA-A03/11-ATK9 epitope production. A. Representative degradation patterns of 5-ATK9-2 (HIV-1 RT, aa 153-168) during 120min degradation in paired non-stimulated (left) and CD3/CD28-stimulated cytosolic extracts. The degradation products were identified by mass spectrometry and classified as original substrate (grey), optimal epitope (green), precursors or peptides encompassing the optimal epitope (blue) and antitopes, peptides in which the optimal epitope has been cleaved (orange). B. Quantification of the degradation products at 3, 30, 60, 120 and 180min in paired non-stimulated and CD3/CD28-stimulated cytosolic extracts. C. Percentage of precursors (left), antitopes (middle) and epitopes (right) produced in paired non-stimulated (open circles), CD3/CD28-stimulated (filled circles) CD4 T cell extracts for each donor (n=7) at t= 120 min. Wilcoxon matched-pairs signed rank t tests were performed (* p<0.05). D. The degradation products purified from degradation in paired non-stimulated (open circles), CD3/CD28-stimulated (filled circles) CD4 T cell extracts were loaded onto HLA-A03 EBV-transformed B cell targets. Lysis of the target cells was measured by 51Cr release assay upon killing by cognate HLA-A03 ATK9-specific CTLs. Results are shown for n=7 donors. Wilcoxon matched-pairs signed rank t tests were performed (* p<0.05). E. Percentage of cell target lysis was plotted against the optimal epitope ATK9 MS peak intensity in each experiment. Results shown for n=7 donors. Correlation was calculated by Spearman test (r>0.4 and p<0.05).
Among the fragments generated, both the optimal epitope A03/11-ATK9 and extended precursors may contribute to the pool of antigenic peptides recognized by cognate CD8 T cells (32, 45, 46). We compared the antigenicity of the degradation products generated in resting and activated CD4 T cells with a CTL killing assay, providing a MS-independent measure of peptide antigenicity (33, 43). The purified degradation products were loaded onto 51Cr-labelled HLA-A03/11-matched B cells used as APC in a killing assay with ATK9-specific CTL. The degradation products produced in CD3/CD28-stimulated CD4+ T cell extracts at 120 min were consistently less antigenic than those produced in resting extracts in 7 donors (0.21–1.4-fold change between CD3/CD28-activated vs non-stimulated extracts, p=0.0313) (Figure 3D). The antigenicity of the degradation products positively correlated with the production of optimal A03/11-ATK9 epitope measured by mass spectrometry (Figure 3E), showing that, in activated CD4 T cells antigen processing led to the destruction of ATK9 and ATK9-containing antigenic peptides and resulted in less CTL-mediated killing.
To assess if the changes in degradation patterns affected peptides beyond this HIV epitope, we analyzed the size of degradation products identified, concentrating on 3 size classes: peptides shorter than 7aa (too short to bind MHC), 8 to 12aa (potential MHC class I binders) and 13 to 15aa long (potential MHC class I precursors) as done in (13, 18). While the numbers of peptides identified increased over time, the proportion of the different size classes did not change significantly between the resting and CD3/CD28-stimulated extracts (Figure 4A). At 120 min, the CD3/CD28-stimulated fragments significantly produced less 8–12aa long peptides (p=0.0469, n=7) and more fragments <7aa (p=0.0313, n=7) (Figure 4B), in line with the faster degradation of the precursor into shorter fragments in activated extracts.
Figure 4:
The degradation patterns of 5-ATK9-2 vary according to activation state of cell extracts. A. Quantification of the degradation products at 3, 30, 60, 120 and 180min in paired non-stimulated (left) and CD3/CD28-stimulated (right) cytosolic extracts according to peptide lengths (shorter fragments <7aa in gray, MHC class I potential binders 8-12aa in green and potential MHC-I precursors 13-15aa in orange). The numbers of peptides identified at each time point is indicated above each bar. B. Percentage of short fragments (left) and MHC class I binders (right) produced in paired non-stimulated (open circles), CD3/CD28-stimulated (filled circles) CD4 T cell extracts for each donor (n=7). Wilcoxon matched-pairs signed rank t tests were performed (* p<0.05). C. Cleavage patterns of 5-ATK9-2 after degradation in non-stimulated (open bars) and CD3/CD28-stimulated (filled bars) at 180min showing the relative amount of fragments starting (N-terminus, top) or ending (C-terminus, bottom) at each residue. Stars (blue for decrease, red for increase) indicate statistically significant differences in cleavage sites between degradation in non-stimulated and CD3/CD28-stimulated extracts. D. Percentage of fragments starting (top) or ending (bottom) at the amino acids highlighted in panel C. Results are shown for n=10 donors. Wilcoxon matched-pairs signed rank t tests were performed (* p<0.05, ** p<0.01, *** p<0.001).
To further analyze the degradation patterns of 5-ATK9–2 in resting and activated CD4 T cell extracts we quantified the relative amount of all degradation fragments starting (N-terminus) or ending (C-terminus) at each aa as in (13, 18), illustrating decreased or increased cleavage sites across the sequence (Figure 4C). At 180 min, significantly fewer peptides started at W1, K2, G3 and S4 in the CD3/CD28-stimulated extracts (Figures 4C top and 4D top) whereas more peptides starting at A6, I7, F8, Q9, S10 and M12 were detected in the degradation with CD3/CD28-stimulated extracts in 17 donors (p<0.05). Enhanced cleavages within ATK9 (A6-S10) explained the decrease in antigenic A03/11-ATK9 peptides in the CD3/CD28-stimulated extracts. For the C-terminus, there were significantly less peptides ending in F8, Q9, M12 and K14 and more peptides ending with I15 (p<0.05) in the CD3/CD28-stimulated extracts (Figures 4C and D bottom panels). The higher expression and peptidases activities driven by CD3/CD28-stimulation resulted in changes in HIV peptide processing with faster degradation of the substrate into short peptides <7aa, and fewer potential class I binders (8–12aa peptides) and antigenic A03/11-ATK9-containing peptides.
To further assess the effect of cellular activation on HIV antigen processing we performed in vitro degradation experiments on 17 additional long HIV peptides located in Gag, Nef, Vif, Vpr, which contain multiple well-characterized CD4+ and CD8+ T cell epitopes (44, 47). We selected for illustration the HIV-1 Gag-derived peptides p24–10-35m (MVHQAISPRTLNAWVKVVEEKAFSPEVIPMFAALS, Gag p24 aa 10–45), which contains 11 MHC-I-restricted epitopes (Figure 5A) (47). The degradation of p24–10-35m in resting or CD3/CD28-activated extracts yielded similar number of peptides (n=185 vs. 187). Similarly, to the results obtained for the RT peptide, we observed significant differences in the proportion of cleavage sites generated in paired extracts from resting and CD3/CD28-stimulated CD4 T cells. For example, in the degradation of p24–10-35mer, more peptides starting with I6, P8, L11, N12, A13, V15, E20, K21 were produced in CD3/CD28-stimulated extracts of 17 donors and less peptides starting with R9, E19, E26, M30 (Figure 5B) (p<0.05). Several C-terminal cleavage sites were also variably generated (data not shown). Out of 11 known HIV epitopes contained in p24–10-35m all were produced as N-extended precursor peptides, 9 were produced as optimal epitopes, and peptide production was affected by cellular activation. For instance, we observed in extracts from 11 donors a significantly higher production of B57-KI8 (KAFSPEVI) peptide in the CD3/CD28-stimulated extracts (Figure 5C), more B44-EV9-containing, less B57-ISW9-containing, less A26-EL9-containing and less Cw01-VL8-containing N-extended precursors (p<0.05) (Figure 5D).
Figure 5:
Resting and CD3/CD28-stimulated CD4 T cell extracts process HIV1 Gag p24-10-35m differently. A. Sequence of HIV-1 Gag p24-10-35m and map of the MHC class I optimal epitopes. B. Cleavage patterns of p24-10-35m after degradation in non-stimulated (open bars) and CD3/CD28-stimulated (filled bars) at 120min showing the relative amount of fragments starting (N-terminus cleavage site) at each amino acid residue. Stars indicate the statistically significant difference between degradation in non-stimulated and CD3/CD28-stimulated extracts. N=11 donors. C. Percentage of HLA-B57-KI8 optimal epitope produced by degradation of p24-10-35m peptide in paired non-stimulated (open circles), CD3/CD28-stimulated (filled circles) CD4 T cell extracts (n=11 donors). Wilcoxon matched-pairs signed rank t tests were performed (* p<0.05, ** p<0.01, *** p<0.001). D. Percentage of N-extended precursor fragments of HLA-B44-EV9, HLA-B57-ISW9, HLA-A26-EL9 and Cw01-VL8 (left to right) detected after degradation of p24-10-35m peptide in paired non-stimulated (open circles), CD3/CD28-stimulated (filled circles) CD4 T cell extracts (t= 120 min, n=11 donors). Wilcoxon matched-pairs signed rank t test were performed (* p<0.05, ** p<0.01). E. Heat map representing the numbers (top) or relative amount (bottom) of all 8-11aa fragments encompassing each residue. One representative experiment out of 11 is shown. (F) Heat map representing the relative amount of all peptides <7aa (top), and 12-18aa (bottom) peptides containing a specific amino acid residue. One representative experiment out of 11 is shown.
We assessed whether activation and subsequent changes in antigen processing activities altered the production of peptides of various lengths and their distribution across the sequence. We mapped the amount of potential MHC-I-binding peptides (8–11mers) and precursors to MHC-I peptides (12–18mers) and short peptides (<7aa) produced in CD3/CD28-stimulated and resting CD4 T cells extracts in representative donors (Figure 5E-F). The distribution of 8–11aa identified fragments across the sequence was similar in number during degradation in resting and activated extracts (Figure 5E, top line), but differed in relative amount (Figure 5E, bottom line). The degradation of p24–10-35m in CD3/CD28-stimulated extracts generated more 8–11mers in its N-terminal region compared to resting conditions whereas the C-terminal part of the sequence was less variable. The distribution of degradation peptides <7aa varied across the sequence but was similar between resting and activated extracts (Figure 5F, top line). Degradation peptides of 12–18aa were located mostly in the second half of the p24–10-35m sequence and produced in higher abundance during degradation in activated extracts (Figure 5F, bottom line).
We compared the degradation of 17 additional fragments of 19–35aa from Gag, Vif, Vpr, Nef proteins and monitored the production of 73 known HIV epitopes and matching N-extended versions. Two epitopes and 15 N-extended epitopes were produced in similar amounts in resting and activated extracts, 21 epitopes were made in variable amounts according to activation status, and 8 were made only in non-activated or activated extracts, showing that differences in epitope production upon activation affect most epitopes (data not shown). Overall the higher expression and hydrolytic peptidase activities observed in CD3/CD28-stimulated CD4 T cell extracts translated to changes in the size and the nature of the peptides produced, altered cleavage patterns of long peptides, and differential production of known epitopes and potential class I and class II binders.
Changes in degradation patterns upon cellular activation are sequence-specific
We hypothesized that changes in degradation patterns upon cellular activation are driven by identifiable and reproducible modulations of specific cleavage sites. We performed cytosolic degradation of 24 long peptides located in Gag, Vif, Vpr or Nef in matching resting and activated cytosolic extracts of primary CD4 T cells (Table 1). They yielded 2,237 degradation peptides framed by 784 cleavage sites, 260 of which were distinct sequences of aa pairs. In order to identify cleavage sites altered across antigens we selected 17–20 long peptides as training set (table 1; bottom). We used the remaining long peptides as a validation dataset and determined if all possible cleavage sites common with the training set were experimentally altered the way identified by the training set. For each long peptide degradation of the training set, we quantified the relative amount of each degradation peptide generated in resting and activated cell extracts. Each degradation peptide is associated with 2 cleavage sites (N- and C-terminus for internal peptides) and one for peptides including the N- or C- terminus of the long fragment). The cleavage site score of each motif was calculated as the relative amount of all peptides containing the specific motif in the training set during the degradation in non-stimulated or activated extracts. We assessed the changes in cleavage site hydrolysis upon cellular activation by comparing the scores obtained for the paired unstimulated and CD3/CD28-stimulated samples (figure 6A). For each training set, we calculated the change in cleavage as the ratio between cleavage score obtained with the CD3/CD28-stimulated sample by the one obtained for the paired non-stimulated extracts. Upon cellular activation, the hydrolysis of certain motifs was increased (ratio > 1.1, red area above the diagonal), others decreased (ratio < 0.9, blue area below the diagonal) or unchanged (within 10% variation upon activation as 0.9< ratio < 1.1, white area around the diagonal). For instance, in dataset 1 all peptides except p17 peptides (18 peptides) were assigned to the training set. In this training set 230 aa pairs corresponding to distinct cleavage sites were assigned scores leading to the identification of 110 cleavage sites increased upon activation, 52 decreased and 68 unchanged (i.e. within 10% variation upon activation). The validation dataset 1 containing the 6 remaining long p17 peptides included 92 cleavages sites, 72 (78.3%) of which were common to the training set. Out of the common cleavage sites we correctly identified 69.44% of changes in cleavage site intensity upon activation, (32 being increased, 15 decreased, 3 unchanged; figure 6B). Similarly, dataset 4 is represented in Figure 6 panels C and D. With 110 cleavage sites common between training and validation set (67.27%), we correctly identified 62.16% of the changes in cleavage site intensity upon activation (23 increased, 13 decreased and 6 unchanged; figure 6D), showing the reproducibility of the changes in cleavage sites across different HIV proteins. To further validate the reproducibility of the changes we swapped long peptides across training and validation data sets creating 7 datasets, some keeping one protein as validation set (datasets 1–4 with p15, p17, p24, Nef peptides in validation sets), others with random distribution of long peptides between the training and validation sets (datasets 5–7), creating 74–134 cleavage sites to analyze (Table 2). We correctly predicted 60–75% activation-induced modulations in cleavage site intensity (increase, decrease, no change) (Table 2). The amino acid pairs that appeared more than 4 times in the 24 long peptides used for degradation were selected for further analysis. The 21 cleavage sites consistently affected in the same manner upon CD3/CD28-stimulation in most datasets (6 or 7 out of 7 datasets) are reported in Table 3. Many changes in cleavage sites induced by cellular activation are reproducible across multiple sequences, suggesting that these changes are solely or mostly driven by the 2 aa constituting the cleavage site. Changes in hydrolysis of cleavage sites that were not reproducible across datasets may correspond to motifs where neighboring residues and/or location of the cleavage sites within the sequence may play a more decisive role in the efficiency of hydrolysis or possibly involving different peptidases across different fragments.
Table 1: Peptides used in degradation experiments for figure 6.
24 long HIV-1 peptides (19-35mers) were used in degradation experiments. The location of the peptides in the sequence are in reference to the HIV-1 subtype B HXB clone2 laboratory-adapted strain used in the Los Alamos database.
| Peptide code |
HIV-1 Protein of origin |
Location in HXB2 HIV-1 |
Sequence |
|---|---|---|---|
| 1 | Gag p17 | 8-39 | LSGGELDRWEKIRLRPGGKKKYKLKHIVWASR |
| 2 | Gag p17 | 29-49 | YKLKHIVWASRELERFAVNPG |
| 3 | Gag p17 | 43-62 | RFAVNPGLLETSEGCRQILG |
| 4 | Gag p17 | 67-86 | SLQTGSEELRSLYNTVATLY |
| 5 | Gag p17 | 92-114 | IEIKDTKEALDKIEEEQNKSKKK |
| 6 | Gag p17/p24 | 120-132/ 1-12 | ADTGNNSQVSQNYPIVQNIQGQMVH |
| 7 | Gag p24 | 10-44 | MVHQAISPRTLNAWVKVVEEKAFSPEVIPMFAALS |
| 8 | Gag p24 | 43-70 | LSEGATPQDLNTMLNTVGGHQAAMQMLK |
| 9 | Gag p24 | 73-96 | INEEAAEWDRVHPVHAGPIAPGQM |
| 10 | Gag p24 | 91-117 | IAPGQMREPRGSDIAGTTSTLQEQIGW |
| 11 | Gag p24 | 161-184 | FRDYVDRFYKTLRAEQASQEVKNW |
| 12 | Gag p24 | 119-150 | TNNPPIPVGEIYKRWIILGLNKIVRMYSPTSI |
| 13 | Gag p24 | 194-217 | ANPDCKTILKALGPAATLEEMMTA |
| 14 | Gag p15 | 1-24 | AEAMSQVTNPATIMIQKGNFRNQR |
| 15 | Gag p15 | 64-87 | TERQANFLGKIWPSHKGRPGNFLQ |
| 16 | Gag p15 | 86-110 | LQSRPEPTAPPEESFRFGEETTTPS |
| 17 | Gag p15 | 114-137 | EPIDKELYPLASLRSLFGSDPSSQ |
| 18 | Vpr | 25-43 | ELKNEAVRHFPRIWLHGLG |
| 19 | Vif | 71-90 | GLHTGERDWHLGQGVSIEWR |
| 20 | Nef | 1-30 | MGGKWSKSSVIGWPTVRERMRRAEPAADRV |
| 21 | Nef | 59-87 | AQEEEEVGFPVTPQVPLRPMTYKAAVDL |
| 22 | Nef | 103-137 | SQRRQDILDLWIYHTQGYFPDWQNYTPGPGVRYPL |
| 23 | Nef | 160-184 | ENTSLLHPVSLHGMDDPEREVLEWR |
| 24 | Nef | 187-206 | FDSRLAFHHVARELHPEYFKNC |
Figure 6:
Cellular activation reproducibly modulates specific cleavage sites. A. The degradation of 18 long HIV peptides in matching resting and CD3/CD28-stimulated cytosolic extracts yielded 1664 fragments and 238 distinct cleavages sites. Each fragment was quantified and relative changes in cleavage sites framing each fragment were scored in resting and activated cells, showing motifs that were increased (red, n=110), decreased (grey, n=52)) or unchanged (0-10% modulation, n=76) upon cellular activation. B. In 6 additional long HIV peptides yielding 92 cleavage sites of which 72 were also found in the training set. 50 motifs (or 69.44% of total) were accurately predicted to increase (red, n=32), decrease (blue, n=15), and remain unchanged (n=3) upon cellular activation. Inset zooms in on motifs scoring between 0-0.2. C. The degradation of 19 long HIV peptides in matching resting and CD3/CD28-stimulated cytosolic extracts yielded 1766 fragments and 226 distinct cleavages sites. Each fragment was quantified and relative changes in cleavage sites framing each fragment were scored in resting and activated cells, showing motifs that were increased (red, n=109), decreased (grey, n=56)) or unchanged (n=54) upon cellular activation. D. In 5 additional long HIV peptides yielding 110 cleavage sites of which 74 were also found in the training set. 55 motifs (or 69.6% of total) were accurately predicted to increase (red, n=40), decrease (blue, n=11), and remain unchanged (n=4) upon cellular activation. Inset zooms in on motifs scoring between 0-0.2.
Table 2: Prediction of cleavage sites altered upon cellular activation.
Degradation products yielded from 24 long HIV peptides (described in Table 1) were analyzed for changes in cleavage site intensity upon cellular activation. Degradation peptides from training sets of 18-20 long peptides were used to identify changes in cleavage sites and validation sets of 5-7 remaining peptides were used to test the predictability of changes in cleavage site intensity (increase/decrease/unchanged).
| Peptides in training set (total number) |
Peptides in validation set (number of peptides) |
Cleavage sites in validation sets |
Common cleavage sites between training and validation sets |
Accurately predicted changes in cleavage sites intensity |
|
|---|---|---|---|---|---|
| Dataset 1 | 7-24 (18 Pp) |
1-6 (p17) (6 Pp) |
92 | 78.26% | 69.44% |
| Dataset 2 | 1-13, 17-24 (20 Pp) |
14-17 (p15) (5 Pp) |
74 | 68.92% | 70.59% |
| Dataset 3 | 1-6, 14-24 (17 Pp) |
7-13 (p24) (7 Pp) |
134 | 64.93% | 70.11% |
| Dataset 4 | 1-19 (19 Pp) |
20-24 (Nef) (5 Pp) |
110 | 67.27% | 62.16% |
| Dataset 5 | 1, 3-8, 11-15, 17-18, 20-21,23-24 (18 Pp) |
2, 9-10, 16, 19, 22 (6 Pp) |
112 | 75.89% | 60.00% |
| Dataset 6 | 2-6, 8-11, 13-19, 21, 23-24 (19 Pp) |
1, 7, 12, 20, 22 (5 Pp) |
125 | 67.20% | 75.00% |
| Dataset 7 | 1, 6-17, 20-24 (18 Pp) |
2-5, 18-19 (6 Pp) |
84 | 84.52% | 69.01% |
Table 3: Summary of cleavage sites altered upon cellular activation.
Amino acid pairs that appeared at least 4 times in the full dataset (24 peptides) and that were consistently affected in the same manner upon CD3/CD28-stimulation were selected (6 or 7 out of 7 datasets in agreement). The ranges of the cleavage score ratio (cleavage site score from CD3/CD28-stimulated divided by score of the matching non-stimulated sample) are also reported for the datasets.
| Amino acid pair |
Number of instances in 24 peptides |
Number of cleavage sites observed |
Trend observed |
Datasets in agreement (out of 7) |
Cleavage score ratio range |
|---|---|---|---|---|---|
| EE | 11 | 7 | Increased | 7 | 1.5-2.25 |
| ER | 5 | 5 | Increased | 7 | 1.7-2.65 |
| LK | 5 | 5 | Increased | 6 | 1.28-3.24 |
| TL | 5 | 5 | Increased | 6 | 1.30-1.75 |
| YK | 5 | 5 | Increased | 6 | 1.21-1.62 |
| LH | 5 | 4 | Increased | 7 | 1.24-1.49 |
| LN | 4 | 3 | Increased | 6 | 1.74-1.89 |
| QN | 4 | 3 | Increased | 6 | 3.56-4.19 |
| EV | 4 | 4 | Increased | 6 | 2.22-2.43 |
| GQ | 4 | 4 | Increased | 7 | 2.08-4.9 |
| PI | 4 | 4 | Increased | 6 | 1.26-2.1 |
| QA | 4 | 4 | Increased | 7 | 1.28-1.48 |
| TS | 4 | 4 | Decreased | 6 | 0.73-0.76 |
| EL | 6 | 6 | Decreased | 6 | 0.66-0.78 |
| SQ | 6 | 5 | Decreased | 6 | 0.4-0.62 |
| SL | 6 | 3 | Decreased | 7 | 0.44-0.71 |
| AS | 4 | 4 | Decreased | 7 | 0.3-0.76 |
| PV | 4 | 4 | Unchanged | 7 | 0.99-1.12 |
| AA | 6 | 6 | Unchanged | 7 | 0.98-1.03 |
| PE | 5 | 4 | Unchanged | 6 | 0.86-0.94 |
| AE | 4 | 4 | Unchanged | 6 | 0.9-0.92 |
The changes in peptidase activities induced by cellular activation modify endogenous antigen processing and epitope presentation by HIV-infected CD4 T cells to CD8 T cells.
To assess if cellular activation of CD4 T cells affects endogenous processing and presentation of epitopes to CD8 T cells we compared the processing of two HIV-1 Gag p24 epitopes (HLA-B57 KF11 KAFSPEVIPMF and HLA-B57-TW10 TSTLQEQIGW) from extended precursors in extracts of unstimulated and CD3/CD28-stimulated HLA-B57+ CD4 T cells to their endogenous processing and presentation by unstimulated and activated HIV-infected CD4 T cells from the same donor to epitope-specific CD8 T cells (Figure 7).
Figure 7:

Cellular activation changes endogenous antigen processing and epitope presentation by HIV-infected CD4 T cells to CD8+ T cells.
A. Relative amount of N-extended (up to 3 aa) epitopes produced during the degradation of HIV-1 Gag-p24 5-TW10-3 (left) and 5-KF11-3 (right) in extracts of paired unstimulated (open symbols) and CD3/CD28-stimulated (filled symbols) HLA-B57+ CD4 T cells at 2, 5 and 8 h. The production of the optimal epitope is marked by a filled or open star for non-stimulated and CD3/CD28-stimulated conditions respectively. Results are shown for one representative experiment. B. Percentage of degranulating CD107a+ CD8 T cells, HLA-B57 TW10 (left) and HLA-B57-KF11 (right) after incubation with unstimulated (open symbols) or CD3/CD28-stimulated (filled symbols) HLA-B57+ CD4 T cells infected with HIV-1 NL4-3-ΔEnv-GFP pseudotyped with VSVg. CD107a degranulation of CTL was measured at 3, 24 and 48 h post-infection after 6 h of incubation at a 5:1 CTL:CD4 ratio. The results of n=5 for TW10 and n=4 for KF11 Infection and degranulation experiments are shown. C. Percentage of CD107a+ TW10- (left) and KF11-specific (right) CD8 T cells after incubation with peptide-pulsed unstimulated (open symbols) and CD3/CD28-stimulated (filled symbols) HLA-B57+ CD4 T cells. CD4 T cells were pulsed with increasing concentrations (0, 0.0002, 0.002, 0.02, 0.2, 2 μg/mL) of TW10 (left) or KF11 (right). Percentage of CD107a+ CD8 T cells were measured after 6 h of incubation at a 5:1 CTL:CD4 ratio. Results are shown for one representative experiment. D. The antigenic peptide equivalent, TW10 (left) and KF11 (right), displayed at the surface of HIV-infected unstimulated (open symbols) and CD3/CD28-stimulated (filled symbols) HLA-B57+ CD4 T cells, TW10 (left) and KF11 (right), was calculated for each time point using the peptide titration curves. The results of n=5 (TW10) and n=4 (KF11) infection and degranulation experiments are shown.
The degradation of Gag p24 peptides 5-KF11–3 KVVEEKAFSPEVIPMFSAL (aa 25–43) and 5-TW10–3 DIAGTTSTLQEQIGWMTN (aa 103–120) over 8 hours showed faster degradation rates of the substrates in CD3/CD28-stimulated extracts than in their unstimulated counterparts (not shown). The production of up to 3 aa N-extended precursors of TW10 (which have been detected at the surface of HLA-B57 HIV-infected cells (48)) peaked at 2 hours during the degradation in activated extracts and was 1.6-fold higher than in extracts from resting CD4 T cells (Figure 7A left panel). Optimal TW10 was detected at 2, 5 and 8 hours only in activated extracts (black stars). In extracts from resting CD4 T cells 5-TW10–3 degradation was slower, yielding only N-extended precursors and peaked later at 5 hours (Figure 7A left panel). The degradation of 5-KF11–3 yielded 2.8-fold more KF11 peptides in extracts from activated cells than resting CD4 T cells (figure 7A right) but remained lower than that of TW10 precursors (0.15% vs. 0.5% respectively of total amount of degradation peptides). The optimal KF11 peptide (star) was detected at 2 hours, higher in activated cells, and not detected at later timepoints due to cleavage sites within KF11. Differences in degradation patterns and kinetics between 5-KF11–3 and 5-TW10–3 were in accordance with the low degradability of the TW10 area and higher degradability of the KF11 area in p24 (18, 34, 49), and the unusually long intracellular half-life of TW10 peptide that we previously identified in PBMC (49), dendritic cells and macrophages (13, 14).
Unstimulated and CD3/CD28-activated CD4 T cells from the same donor were infected in single round infections with HIV-1ΔEnv pseudotyped with VSVg and expressing GFP and degranulation of CD107a by TW10- and KF11-specific CTL were measured at 3, 24 and 48 h post-infection (Figure 7B). This allowed us to compare the endogenous processing and presentation from two epitopes within the protein. Single round infection rates were low at 3 hours post-infection and plateaued at 4–5% at 24–48 hours. MHC-I levels were 2.3-fold higher in activated cells than in resting cells and remained constant at 3, 24 and 48 h post-infection. TW10-specific CTL degranulation peaked at 24 hours (35% CD107a+ CTL) and was higher in the presence of HIV-infected activated CD4 T cells (2.1-fold at 24 hours and 1.5-fold at 48 hours), mirroring the higher production of N-extended TW10 and/or optimal TW10 (Figure 7B left panel). KF11-specific CTL activation was low at 3 hours, peaked at 24 hours and was 6.1-fold higher in the presence of HIV-infected activated CD4 T cells than unstimulated cells before decreasing at 48 hours in accordance with the degradation of the epitopes at later time points (Figure 7B right panel).
The endogenous presentation of 2 epitopes from the same protein to CTL summarizes differences in antigen processing, MHC-I levels and peptide affinity for HLA-B57 and the TCR of CTL. To parse out the contribution of these parameters, we quantified the relative presentation of antigenic peptides by resting and activated HIV-infected CD4 T cells. We measured equivalent TW10 or KF11 peptide concentrations presented by infected CD4 T cells by comparing CD107a degranulation of CTL after incubation with HIV-infected resting or activated CD4 T cells (Figure 7B), to that of uninfected resting or activated CD4 T cells pulsed with increasing amount of TW10 or KF11 peptide (Figure 7C). The peptide titration on both uninfected resting or activated CD4 T cells showed that CD107a degranulation increased with peptide concentration and was equivalent in the presence of resting or activated CD4 T cells despite the 2-fold higher expression of MHC-I on activated cells (figure 7C). This is likely due to the high binding affinity of both TW10 and KF11 peptides to HLA-B57 (49–52) and thus ruled out a sole contribution of MHC-I levels in differences observed in CTL degranulation between HIV-infected resting and activated CD4 T cells. Similar CTL degranulation percentages were observed for TW10 and KF11 clones specifically at low peptide concentration more relevant to endogenous peptide presentation and were similar to titrations previously done by chromium release assay (49). Therefore, differences in CTL responses against TW10 and KF11 at a given time point are mostly driven by differences in antigen processing than functional avidity of the two CTL clones.
The TW10 peptide equivalent presented by HIV-infected activated CD4 T cells was higher than that observed in resting HIV-infected CD4 T cells, peaked at 24 hours and was maintained at 48 hours (Figure 7D left panel). In resting cells equivalent TW10 peptide presentation was lower and increased steadily in accordance with the slower processing kinetics in these cells (shown in Figure 7B). The KF11 peptide equivalent presented by HIV-infected activated CD4 T cells peaked at 24 hours and was 4-fold higher than that of matching unstimulated CD4 T cells (Figure 7D right panel). At 48 hours despite similar % of infected cells and similar levels of MHC-I as 24 hours earlier, the KF11 equivalent presented HIV-infected activated cells decreased in accordance with the faster degradation of KF11-containing peptides in activated extracts. The equivalent TW10 peptide presentation was 10-fold higher than that of KF11, in agreement with the higher intracellular stability and superior processing of TW10-containing peptides identified during in vitro peptide degradation. These data show that differences in antigen processing activities between HIV-infected resting and CD3/CD28-activated CD4 T cells that modulated epitope processing in vitro also alter endogenous processing and presentation of HIV epitopes by HIV-infected CD4 T cells and the kinetics and efficiency of CTL recognition.
Discussion
This study shows that cellular activation of CD4 T cells affects the expression and activity of the proteolytic machinery, the in vitro degradation patterns of antigens and production of epitopes in cytosolic extracts, and the CD8 T cell responses after endogenous antigen processing and presentation by HIV-infected primary CD4 T cells.
T cell activation through TCR-dependent or -independent pathways leads to profound changes in the transcription profiles of CD4 T cells identifying genes actively repressed, activated or unmodified during either resting or activated stages of CD4 T cells (42, 53). The transcription of several proteasome subunits was enhanced in line with the increased protein expression we observed (42, 53). The retention of unspliced nuclear RNA and their subsequent release under activation contribute to the changes in mRNA expression induced by cellular activation. Interestingly the retention and release of RNA encoding proteasome subunits were the highest among all gene groups (54). Certain non-ATPase proteasome subunits of the 19S cap act as transactivators of genes such as CIIT (55, 56) and could further influence changes in the RNA expression of the antigen processing and presentation machinery. Our study supports a link between increased proteasome expression and increased hydrolytic activity. However, for aminopeptidases we observed an increased activity but no increase in ERAP1 or ERAP2 expression, suggesting that the increased hydrolytic activity is due to an enhanced expression of other cytosolic peptidases or changes in proteins regulating peptidase activities.
Activation of CD4 T cells through TCR or mitogen stimulation or through cognate peptide-MHC stimulation modified the degradation of antigens into epitopes. T cell activation altered peptidase activities in multiple compartments (ER, cytosol and lysosomes), which will affect degradations of antigens or pathogens trafficking in these compartments. Since the changes in cleavage sites are sequence-specific and seen across several HIV proteins they are likely to alter the processing of both self- and pathogen-derived antigens. Changes in the transcriptome and subsequently in the proteome, variations in proteasome and protease activities are likely to alter the self-derived MHC-peptidome, as suggested by the presentation of a peptide from IL-1 whose expression is induced by activation of Jurkat T cells (57). Pathogen-derived peptides presented by MHC represent a minority of MHC-bound peptides (32, 45, 46) and changes in the sequence, length or amount of MHC-bound peptides may affect immune recognition. We showed here that cellular activation affects the pool of antigenic peptides identified in in vitro degradation as well as the endogenous antigen processing and presentation by HIV-infected CD4 T cells and the subsequent killing by epitope-specific CD8 T cells. The kinetics and production of peptides depend on cellular hydrolytic activities in the compartment where the antigen is located as well as sequence within and outside epitopes (33, 35, 49, 58). Changes in cleavage site intensity were sequence-specific and reproducible across different antigens, suggesting that many changes are solely driven by the sequence and will eventually allow us to build bioinformatics tools to predict changes in degradation patterns of HIV variants. Aside from cleavage sites defining the degradation fragments, the variable intracellular stability of peptides -also defined by motifs- contributes to the amount of epitopes available for presentation as shown for TW10 and KF11 epitopes in this study and in (49). Altogether motifs outside and within epitopes define the kinetics and amount of peptides available for MHC loading and CTL recognition. Changing the hydrolytic environment upon activation will act as rheostat and may favor the faster production of epitopes containing motifs associated with stability while increasing the degradation of less stable peptides. Conversely lower peptidase activities would slow the processing of epitopes with stability motifs but may reduce the degradation of intracellularly less stable peptides. Accordingly, we observed that cellular activation enhanced the cytosolic production of some epitopes while decreasing others, including some previously identified as intracellularly unstable peptides (49). Such changes in the pool of in vitro processed peptides altered their antigenic potential measured by T cell killing. Beyond the known epitopes cellular activation changed the distribution of degradation peptides along the antigen, suggesting that beyond the alterations in the amount of known epitopes produced inside cells activation might lead to the presentation of additional peptides or possibly lack of presentation of others. Others and we showed that healthy, cancerous and HIV-infected cells present not only optimal epitopes but also extended peptides (32, 45, 46, 59–62). The changes in degradation patterns observed in extracts from resting vs. activated cells could potentially alter the ratio of optimal vs. extended peptides displayed by cells and the efficiency of TCR binding. Accordingly, we showed that endogenous antigen processing and presentation of two epitopes from the same protein by HIV-infected CD4 T cells was modulated by cellular activation and modulated the kinetics and efficiency of CTL recognition. While changes in the surface peptidome of resting and activated cells remain to be defined, we expect that changes in peptides or amount of peptides displayed by MHC, and dynamic changes in surface MHC-peptide upon activation could more globally affect CD8 T cell recognition of target cells.
In HIV-infected persons cellular activation is highly variable within and across patients, higher during acute infection (25), reduced by ART (21), increased during viral blips in treated chronic infection (63), and modulated by co-infections and microbial translocation (22, 23). Susceptibility to activation mediated by mitogens and TLR-inducing activation is variable across healthy individuals due to polymorphisms in IL2 and other activation pathways (53). While productive HIV infection occurs mostly in activated cells (27, 28) CD4 T cells harboring latent provirus are mostly differentiated resting memory CD4 T cells (64). Cure strategies to flush HIV reservoirs rely on latency-reserving agents triggering re-expression of HIV provirus without inducing cellular activation, followed by immune clearance (6, 31). The differences in cellular activation levels between productive infection and LRA-mediated reactivation of reservoirs may lead to distinctive processing of HIV antigens and presentation of different peptides after provirus reactivation. These variations may contribute to the limited capacity of pre-existing HIV-specific immune responses to clear reactivated reservoirs (65, 66) and the need to broaden immune responses and elicit additional immune responses of distinct peptide specificity for the clearance of HIV reservoirs.
Supplementary Material
Acknowledgments
The authors thank current and past lab members, N. Joshi for help running the recent mass spectrometry experiments, Drs. F. Pereyra and D. Kavanagh for stimulating discussions and input on the manuscript and Drs. A. Piechocka-Trocha and D. Kaufmann for the generous gift of CD8 T cell clones and CD4 T cell clones.
The project was funded by grants AI122947 and AI131912 from NIAID to SLG.
Abbreviations
- ART
antiretroviral treatments
- ER
endoplasmic reticulum
- ERAP
ER-resident aminopeptidases
References
- 1.Smith-Garvin JE, Koretzky GA, and Jordan MS. 2009. T cell activation. Annual review of immunology 27: 591–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chun TW, Chadwick K, Margolick J, and Siliciano RF. 1997. Differential susceptibility of naive and memory CD4+ T cells to the cytopathic effects of infection with human immunodeficiency virus type 1 strain LAI. J Virol 71: 4436–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldauf HM, Pan X, Erikson E, Schmidt S, Daddacha W, Burggraf M, Schenkova K, Ambiel I, Wabnitz G, Gramberg T, Panitz S, Flory E, Landau NR, Sertel S, Rutsch F, Lasitschka F, Kim B, Konig R, Fackler OT, and Keppler OT. 2012. SAMHD1 restricts HIV-1 infection in resting CD4(+) T cells. Nat Med 18: 1682–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruffin N, Brezar V, Ayinde D, Lefebvre C, Schulze Zur Wiesch J, van Lunzen J, Bockhorn M, Schwartz O, Hocini H, Lelievre JD, Banchereau J, Levy Y, and Seddiki N. 2015. Low SAMHD1 expression following T-cell activation and proliferation renders CD4+ T cells susceptible to HIV-1. AIDS 29: 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosmane NN, Kwon KJ, Bruner KM, Capoferri AA, Beg S, Rosenbloom DI, Keele BF, Ho YC, Siliciano JD, and Siliciano RF. 2017. Proliferation of latently infected CD4+ T cells carrying replication-competent HIV-1: Potential role in latent reservoir dynamics. J Exp Med 214: 959–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margolis DM, Garcia JV, Hazuda DJ, and Haynes BF. 2016. Latency reversal and viral clearance to cure HIV-1. Science 353: aaf6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen XZ, Billet S, Lin C, Okwan-Duodu D, Chen X, Lukacher AE, and Bernstein KE. 2011. The carboxypeptidase ACE shapes the MHC class I peptide repertoire. Nat Immunol 12: 1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rock KL, York IA, and Goldberg AL. 2004. Post-proteasomal antigen processing for major histocompatibility complex class I presentation. Nat Immunol 5: 670–677. [DOI] [PubMed] [Google Scholar]
- 9.Blum JS, Wearsch PA, and Cresswell P. 2013. Pathways of antigen processing. Annual review of immunology 31: 443–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rucevic M, Boucau J, Dinter J, Kourjian G, and Le Gall S. 2014. Mechanisms of HIV Protein Degradation into Epitopes: Implications for Vaccine Design. Viruses 6: 3271–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beninga J, Rock KL, and Goldberg A. 1998. Interferon-gamma can stimulate post-proteasomal trimming of the N terminus of an antigenic peptide by inducing leucine aminopeptidase. J Biol Chem 273: 18734–18742. [DOI] [PubMed] [Google Scholar]
- 12.Crespo MI, Zacca ER, Nunez NG, Ranocchia RP, Maccioni M, Maletto BA, Pistoresi-Palencia MC, and Moron G. 2013. TLR7 triggering with polyuridylic acid promotes cross-presentation in CD8alpha+ conventional dendritic cells by enhancing antigen preservation and MHC class I antigen permanence on the dendritic cell surface. J Immunol 190: 948–960. [DOI] [PubMed] [Google Scholar]
- 13.Dinter J, Duong E, Lai NY, Berberich MJ, Kourjian G, Bracho-Sanchez E, Chu D, Su H, Zhang SC, and Le Gall S. 2015. Variable Processing and Cross-presentation of HIV by Dendritic Cells and Macrophages Shapes CTL Immunodominance and Immune Escape. PLoS Pathog 11: e1004725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinter J, Gourdain P, Lai NY, Duong E, Bracho-Sanchez E, Rucevic M, Liebesny PH, Xu Y, Shimada M, Ghebremichael M, Kavanagh DG, and Le Gall S. 2014. Different antigen processing activities in dendritic cells, macrophages and monocytes lead to uneven production of HIV epitopes and affect CTL recognition. Journal of Immunology 193: 4322–4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trujillo JA, Croft NP, Dudek NL, Channappanavar R, Theodossis A, Webb AI, Dunstone MA, Illing PT, Butler NS, Fett C, Tscharke DC, Rossjohn J, Perlman S, and Purcell AW. 2014. The cellular redox environment alters antigen presentation. J Biol Chem 289: 27979–27991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loureiro J, and Ploegh HL. 2006. Antigen presentation and the ubiquitin-proteasome system in host-pathogen interactions. Adv Immunol 92: 225–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kourjian G, Xu Y, Mondesire-Crump I, Shimada M, Gourdain P, and Le Gall S. 2014. Sequence-Specific Alterations of Epitope Production by HIV Protease Inhibitors. J Immunol 192: 3496–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kourjian G, Rucevic M, Berberich MJ, Dinter J, Wambua D, Boucau J, and Le Gall S. 2016. HIV Protease Inhibitor-Induced Cathepsin Modulation Alters Antigen Processing and Cross-Presentation. J Immunol 196: 3595–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andre P, Groettrup M, Klenerman P, de Giuli R, Booth BL Jr., Cerundolo V, Bonneville M, Jotereau F, Zinkernagel RM, and Lotteau V. 1998. An inhibitor of HIV-1 protease modulates proteasome activity, antigen presentation, and T cell responses. Proc Natl Acad Sci U S A 95: 13120–13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butz EA, and Bevan MJ. 1998. Differential presentation of the same MHC class I epitopes by fibroblasts and dendritic cells. J Immunol 160: 2139–2144. [PubMed] [Google Scholar]
- 21.Krebs SJ, and Ananworanich J. 2016. Immune activation during acute HIV infection and the impact of early antiretroviral therapy. Curr Opin HIV AIDS 11: 163–172. [DOI] [PubMed] [Google Scholar]
- 22.Shmagel KV, Saidakova EV, Shmagel NG, Korolevskaya LB, Chereshnev VA, Robinson J, Grivel JC, Douek DC, Margolis L, Anthony DD, and Lederman MM. 2016. Systemic inflammation and liver damage in HIV/hepatitis C virus coinfection. HIV Med 17: 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mudd JC, and Brenchley JM. 2016. Gut Mucosal Barrier Dysfunction, Microbial Dysbiosis, and Their Role in HIV-1 Disease Progression. J Infect Dis 214 Suppl 2: S58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishizaka A, Sato H, Nakamura H, Koga M, Kikuchi T, Hosoya N, Koibuchi T, Nomoto A, Kawana-Tachikawa A, and Mizutani T. 2016. Short Intracellular HIV-1 Transcripts as Biomarkers of Residual Immune Activation in Patients on Antiretroviral Therapy. J Virol 90: 5665–5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narvaez AB, Hunt P, Martin JN, Kahn JO, Levy J, McGrath MS, and Hecht FM. 2004. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 104: 942–947. [DOI] [PubMed] [Google Scholar]
- 26.Nakanjako D, Ssewanyana I, Mayanja-Kizza H, Kiragga A, Colebunders R, Manabe YC, Nabatanzi R, Kamya MR, and Cao H. 2011. High T-cell immune activation and immune exhaustion among individuals with suboptimal CD4 recovery after 4 years of antiretroviral therapy in an African cohort. BMC infectious diseases 11: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doitsh G, and Greene WC. 2016. Dissecting How CD4 T Cells Are Lost During HIV Infection. Cell host & microbe 19: 280–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munoz-Arias I, Doitsh G, Yang Z, Sowinski S, Ruelas D, and Greene WC. 2015. Blood-Derived CD4 T Cells Naturally Resist Pyroptosis during Abortive HIV-1 Infection. Cell host & microbe 18: 463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu WW, Han MJ, Chen D, Chen L, Guo Y, Willden A, Liu DQ, and Zhang HT. 2013. Genome-wide search for the genes accountable for the induced resistance to HIV-1 infection in activated CD4+ T cells: apparent transcriptional signatures, co-expression networks and possible cellular processes. BMC Med Genomics 6: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Len AC, Starling S, Shivkumar M, and Jolly C. 2017. HIV-1 Activates T Cell Signaling Independently of Antigen to Drive Viral Spread. Cell reports 18: 1062–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasmussen TA, and Lewin SR. 2016. Shocking HIV out of hiding: where are we with clinical trials of latency reversing agents? Curr Opin HIV AIDS 11: 394–401. [DOI] [PubMed] [Google Scholar]
- 32.Rucevic M, Kourjian G, Boucau J, Blatnik R, Garcia Bertran W, Berberich MJ, Walker BD, Riemer AB, and Le Gall S. 2016. MHC-bound HIV Peptides Identified from Various Cell Types Reveal Common Nested Peptides and Novel T Cell Responses. J Virol 90: 8605–8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Gall S, Stamegna P, and Walker BD. 2007. Portable flanking sequences modulate CTL epitope processing. J Clin Invest 117: 3563–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaithilingam A, Lai NY, Duong E, Boucau J, Xu Y, Shimada M, Gandhi M, and Le Gall S. 2013. A simple methodology to assess endolysosomal protease activity involved in antigen processing in human primary cells. BMC cell biology 14: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang SC, Martin E, Shimada M, Godfrey SB, Fricke J, Locastro S, Lai NY, Liebesny P, Carlson JM, Brumme CJ, Ogbechie OA, Chen H, Walker BD, Brumme ZL, Kavanagh DG, and Le Gall S. 2012. Aminopeptidase Substrate Preference Affects HIV Epitope Presentation and Predicts Immune Escape Patterns in HIV-Infected Individuals. J Immunol 188: 5924–5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohammadi P, Desfarges S, Bartha I, Joos B, Zangger N, Munoz M, Gunthard HF, Beerenwinkel N, Telenti A, and Ciuffi A. 2013. 24 hours in the life of HIV-1 in a T cell line. PLoS Pathog 9: e1003161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Motamedi M, Xu L, and Elahi S. 2016. Correlation of transferrin receptor (CD71) with Ki67 expression on stimulated human and mouse T cells: The kinetics of expression of T cell activation markers. J Immunol Methods 437: 43–52. [DOI] [PubMed] [Google Scholar]
- 38.Reddy M, Eirikis E, Davis C, Davis HM, and Prabhakar U. 2004. Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: an in vitro model to monitor cellular immune function. J Immunol Methods 293: 127–142. [DOI] [PubMed] [Google Scholar]
- 39.Biselli R, Matricardi PM, D’Amelio R, and Fattorossi A. 1992. Multiparametric flow cytometric analysis of the kinetics of surface molecule expression after polyclonal activation of human peripheral blood T lymphocytes. Scand J Immunol 35: 439–447. [DOI] [PubMed] [Google Scholar]
- 40.Graessel A, Hauck SM, von Toerne C, Kloppmann E, Goldberg T, Koppensteiner H, Schindler M, Knapp B, Krause L, Dietz K, Schmidt-Weber CB, and Suttner K. 2015. A Combined Omics Approach to Generate the Surface Atlas of Human Naive CD4+ T Cells during Early T-Cell Receptor Activation. Molecular & cellular proteomics : MCP 14: 2085–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiefenthaler G, and Hunig T. 1989. The role of CD2/LFA-3 interaction in antigen- and mitogen-induced activation of human T cells. Int Immunol 1: 169–175. [DOI] [PubMed] [Google Scholar]
- 42.Hess K, Yang Y, Golech S, Sharov A, Becker KG, and Weng NP. 2004. Kinetic assessment of general gene expression changes during human naive CD4+ T cell activation. Int Immunol 16: 1711–1721. [DOI] [PubMed] [Google Scholar]
- 43.Lazaro E, Godfrey SB, Stamegna P, Ogbechie T, Kerrigan C, Zhang M, Walker BD, and Le Gall S. 2009. Differential HIV epitope processing in monocytes and CD4 T cells affects cytotoxic T lymphocyte recognition. J Infect Dis 200: 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Llano A, Williams A, Olvera A, Silva-Arrieta S, and Brander C. 2013. Best-Characterized HIV-1 CTL Epitopes: The 2013 Update In HIV Molecular Immunology 2013. Yusim K, Korber B, Brander C, Barouch DH, de Boer R, Haynes BF, Koup RA, Moore JP, Walker BD, and Watkins DI, eds. Los Alamos National Laboratory, Theoretical Biology and Biophysics, Los Alamos, New Mexico: 3–19. [Google Scholar]
- 45.Yaciuk JC, Skaley M, Bardet W, Schafer F, Mojsilovic D, Cate S, Stewart CJ, McMurtrey C, Jackson KW, Buchli R, Olvera A, Cedeno S, Plana M, Mothe B, Brander C, West JT, and Hildebrand WH. 2014. Direct interrogation of viral peptides presented by the class I HLA of HIV-infected T cells. J Virol 88: 12992–13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ternette N, Yang H, Partridge T, Llano A, Cedeno S, Fischer R, Charles PD, Dudek NL, Mothe B, Crespo M, Fischer WM, Korber BT, Nielsen M, Borrow P, Purcell AW, Brander C, Dorrell L, Kessler BM, and Hanke T. 2016. Defining the HLA class I-associated viral antigen repertoire from HIV-1-infected human cells. Eur J Immunol 46: 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yusim K, Korber B, Brander C, Barouch D, de Boer R, Haynes BF, Koup R, Moore JP, Walker BD, and Watkins DI. 2016. HIV Molecular Immunology 2015. Los Alamos National Laboratory, Theoretical Biology and Biophysics, Los Alamos, New Mexico. [Google Scholar]
- 48.Pymm P, Illing PT, Ramarathinam SH, O’Connor GM, Hughes VA, Hitchen C, Price DA, Ho BK, McVicar DW, Brooks AG, Purcell AW, Rossjohn J, and Vivian JP. 2017. MHC-I peptides get out of the groove and enable a novel mechanism of HIV-1 escape. Nat Struct Mol Biol 24: 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lazaro E, Kadie C, Stamegna P, Zhang SC, Gourdain P, Lai NY, Zhang M, Martinez SM, Heckerman D, and Le Gall S. 2011. Variable HIV peptide stability in human cytosol is critical to epitope presentation and immune escape. J. Clin. Invest. 121: 2480–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berger CT, Frahm N, Price DA, Mothe B, Ghebremichael M, Hartman KL, Henry LM, Brenchley JM, Ruff LE, Venturi V, Pereyra F, Sidney J, Sette A, Douek DC, Walker BD, Kaufmann DE, and Brander C. 2011. High-functional-avidity cytotoxic T lymphocyte responses to HLA-B-restricted Gag-derived epitopes associated with relative HIV control. J Virol 85: 9334–9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedrich D, Jalbert E, Dinges WL, Sidney J, Sette A, Huang Y, McElrath MJ, and Horton H. 2011. Vaccine-induced HIV-specific CD8+ T cells utilize preferential HLA alleles and target-specific regions of HIV-1. J Acquir Immune Defic Syndr 58: 248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kloverpris HN, Payne RP, Sacha JB, Rasaiyaah JT, Chen F, Takiguchi M, Yang OO, Towers GJ, Goulder P, and Prado JG. 2013. Early antigen presentation of protective HIV-1 KF11Gag and KK10Gag epitopes from incoming viral particles facilitates rapid recognition of infected cells by specific CD8+ T cells. J Virol 87: 2628–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ye CJ, Feng T, Kwon HK, Raj T, Wilson MT, Asinovski N, McCabe C, Lee MH, Frohlich I, Paik HI, Zaitlen N, Hacohen N, Stranger B, De Jager P, Mathis D, Regev A, and Benoist C. 2014. Intersection of population variation and autoimmunity genetics in human T cell activation. Science 345: 1254665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ni T, Yang W, Han M, Zhang Y, Shen T, Nie H, Zhou Z, Dai Y, Yang Y, Liu P, Cui K, Zeng Z, Tian Y, Zhou B, Wei G, Zhao K, Peng W, and Zhu J. 2016. Global intron retention mediated gene regulation during CD4+ T cell activation. Nucleic Acids Res 44: 6817–6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Truax AD, Koues OI, Mentel MK, and Greer SF. 2010. The 19S ATPase S6a (S6’/TBP1) regulates the transcription initiation of class II transactivator. J Mol Biol 395: 254–269. [DOI] [PubMed] [Google Scholar]
- 56.Bhat KP, and Greer SF. 2011. Proteolytic and non-proteolytic roles of ubiquitin and the ubiquitin proteasome system in transcriptional regulation. Biochim Biophys Acta 1809: 150–155. [DOI] [PubMed] [Google Scholar]
- 57.Frumento G, Corradi A, Ferrara GB, and Rubartelli A. 1997. Activation-related differences in HLA class I-bound peptides: presentation of an IL-1 receptor antagonist-derived peptide by activated, but not resting, CD4+ T lymphocytes. J Immunol 159: 5993–5999. [PubMed] [Google Scholar]
- 58.Tenzer S, Peters B, Bulik S, Schoor O, Lemmel C, Schatz MM, Kloetzel PM, Rammensee HG, Schild H, and Holzhutter HG. 2005. Modeling the MHC class I pathway by combining predictions of proteasomal cleavage, TAP transport and MHC class I binding. Cell Mol Life Sci 62: 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ben Dror L, Barnea E, Beer I, Mann M, and Admon A. 2010. The HLA-B*2705 peptidome. Arthritis Rheum 62: 420–429. [DOI] [PubMed] [Google Scholar]
- 60.Trolle T, McMurtrey CP, Sidney J, Bardet W, Osborn SC, Kaever T, Sette A, Hildebrand WH, Nielsen M, and Peters B. 2016. The Length Distribution of Class I-Restricted T Cell Epitopes Is Determined by Both Peptide Supply and MHC Allele-Specific Binding Preference. J Immunol 196: 1480–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bassani-Sternberg M, and Gfeller D. 2016. Unsupervised HLA Peptidome Deconvolution Improves Ligand Prediction Accuracy and Predicts Cooperative Effects in Peptide-HLA Interactions. J Immunol. [DOI] [PubMed] [Google Scholar]
- 62.Samino Y, Lopez D, Guil S, Saveanu L, van Endert PM, and Del Val M. 2006. A long N-terminal-extended nested set of abundant and antigenic major histocompatibility complex class I natural ligands from HIV envelope protein. J Biol Chem 281: 6358–6365. [DOI] [PubMed] [Google Scholar]
- 63.Zoufaly A, Kiepe JG, Hertling S, Hufner A, Degen O, Feldt T, Schmiedel S, Kurowski M, and van Lunzen J. 2014. Immune activation despite suppressive highly active antiretroviral therapy is associated with higher risk of viral blips in HIV-1-infected individuals. HIV Med 15: 449–457. [DOI] [PubMed] [Google Scholar]
- 64.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK, and Sekaly RP. 2009. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 15: 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, Zhang H, Margolick JB, Blankson JN, and Siliciano RF. 2012. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 36: 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deng K, Pertea M, Rongvaux A, Wang L, Durand CM, Ghiaur G, Lai J, McHugh HL, Hao H, Zhang H, Margolick JB, Gurer C, Murphy AJ, Valenzuela DM, Yancopoulos GD, Deeks SG, Strowig T, Kumar P, Siliciano JD, Salzberg SL, Flavell RA, Shan L, and Siliciano RF. 2015. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature 517: 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.