Abstract
Background:
Signal Transducer and Activator of Transcription 3 (STAT3) gain-of-function germline mutations are associated with diverse clinical manifestations, including autoimmune cytopenia, lymphadenopathy, immunodeficiency, endocrinopathy, and enteropathy. We describe a new feature: raised intracranial pressure with papilledema.
Materials and Methods:
Report of two cases.
Results:
The first patient had a de novo heterozygous c.2144C>T (p.Pro715Leu) mutation in the STAT3 gene. At age 1 she had papilledema with marked sheathing of the proximal vessels on the optic discs. Follow-up 8 years later showed chronic papilledema, cystoid macular edema, and vision loss. The second patient had a de novo heterozygous c.2147C>T (p.Thr716Met) mutation. At age 12 he developed papilledema, which recurred despite treatment. In both patients, repeated sampling of the cerebrospinal fluid demonstrated a lymphocytic pleocytosis.
Conclusions:
Papilledema can occur as a manifestation of STAT3 gain-of-function mutation, sometimes accompanied by prominent vascular sheathing and cystoid macular edema. The mechanism may be chronic meningeal infiltration by white blood cells, impairing cerebrospinal fluid absorption.
Keywords: Evans Syndrome, cystoid macular edema, transcription factor, signal transducer and activator of transcription
Introduction
Signal Transducer and Activator of Transcription (STAT) proteins constitute a seven member family involved in the regulation of DNA transcription (1). Through activation by Janus kinases (Jak), STAT molecules serve as classic transcription factors by engaging DNA regulatory elements (2). The discovery of the JAK-STAT pathway has enhanced the understanding of a multitude of human diseases, by yielding new insights into the mechanisms that control cellular proliferation, differentiation, and apoptosis (3,4).
STAT3 (OMIM *102582) has the highest mutation frequency among the STAT transcription factors (2). Germline STAT3 loss-of-function mutations are associated with hyper-IgE syndrome (OMIM #147060), formerly known as Job’s syndrome, manifested by eczema, chronic skin abscesses, and recurrent bouts of pneumonia (5). Germline STAT3 gene gain-of-function mutations cause early onset autoimmune and lymphoproliferative disease (OMIM #615952). The mechanism is not fully understood, but involves aberrant cytokine signaling (6). Findings include autoimmune hemolytic anemia, thrombocytopenia, neutropenia, hypogammaglobulinemia, hepatosplenomegaly, lymphadenopathy, immunodeficiency, inflammatory bowel disease, interstitial lung disease, endocrinopathy, arthritis, eczema, alopecia, hepatitis, and short stature (7,8).
Although STAT3 mutations are associated with pervasive systemic manifestations, ocular signs have been recognized in only a single case, reported twice (9,10). A 17-year-old female with STAT3 gain-of-function mutation c.1974G>C (p.Lys658Asn) was diagnosed with bilateral posterior uveitis and cystic macular edema leading to severe visual impairment. Here we broaden the phenotype associated with STAT3 gain-of-function by describing two patients with different mutations who each developed papilledema.
Methods -- Case Studies
Patient 1:
A 20-month-old girl was referred to our pediatric neuro-ophthalmology service for evaluation of an esotropia which had developed at age 4 months. She was born at 34 weeks gestation and hospitalized for 10 days to treat jaundice, bradycardia, and failure to thrive. There was spasticity of the lower extremities, suggesting the possibility of cerebral palsy. At age 4 months a helmet was prescribed for right occipital plagiocephaly. MR imaging at age 9 months showed a serpentine course of the optic nerves in both orbits with dilation of the nerve sheaths. The ventricles were normal in size. The pituitary gland was small, but anterior and posterior lobes were present. Signal characteristics of the gray and white matter were unremarkable.
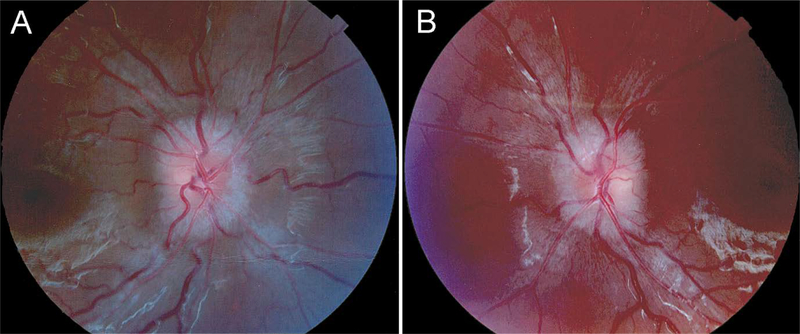
On examination, the child could not yet walk. She fixated and tracked small targets with each eye. Pupils were normal. The extraocular eye movements appeared full, but cooperation was limited. There were 25–30 prism-diopters of alternating esotropia, measured by the relative position of the corneal light reflexes. Cycloplegic refraction was plano OU. Fundus examination showed chronic optic disc swelling with gliotic sheathing of the proximal arterioles and venules (Figure 1). The findings were more severe in the left eye.
Figure 1.
Patient 1, fundi at age 20 months. A. Right eye shows a blurred, elevated optic disc with trace vascular sheathing. B. Left eye shows more swelling, with pronounced sheathing of arterioles and venules. The macula and periphery were normal in both eyes.
Opening pressure on lumbar puncture under general anesthesia was initially 30 cm of water, but declined after several minutes to 19 cm of water. There were 14 white cells/ml (21% neutrophils, 78% lymphocytes), with a normal protein and glucose. The diagnosis of papilledema was made and a ventriculoperitoneal shunt was recommended. However, the ambiguous opening pressure reading and presence of white cells fostered uncertainty. The parents declined shunting and the child was subsequently lost to follow up.
At age 9 the child was referred back to our clinic. She had been chronically ill during the intervening 7 years. At age 2 she had developed autoimmune hemolytic anemia and thrombocytopenia. She also had hepatosplenomegaly and hypogammaglobulinemia, leading to the diagnosis of Evans Syndrome. Treatment was initiated with corticosteroids and rituximab. She remained on chronic immunosuppression with oral prednisolone and periodic infusions of IVIG. Multiple infections occurred, including Clostridium difficile colitis, Escherichia coli urosepsis with respiratory failure, and Pseudomonas aeruginosa pneumonia. She had chronic diarrhea associated with norovirus infection, adenovirus infection, and bowel inflammation requiring periodic total parenteral nutrition.
At age 6 she was examined by an ophthalmologist who reported persistent optic disc swelling. A lumbar puncture was repeated, yielding an opening pressure of 31 cm of water. There were 7 white cells/ml with a normal protein and glucose. Treatment was commenced with a low dose of acetazolamide. At age 7 whole exome sequencing revealed a germline STAT3 de novo heterozygous gain-of-function mutation c.2144C>T (p.Pro715Leu). Treatment was initiated with tocilizumab, which was later replaced with tofacitinib.
At the time of her second visit to our clinic she was being treated with acetazolamide, prednisone, trimethoprim/sulfamethoxazole, ceftazidime, caspofungin, ursodiol, fluconazole, mirtazapine, IVIG, and tofacitinib. The height was 111 cm and the weight was 30.3 kg, yielding a BMI of 24.6 kg/m2. She had a Cushingoid appearance. The best corrected visual acuity was 20/60 in the right eye and 20/200 in the left eye. There was no afferent pupillary defect. The extraocular eye movements were full. Ocular alignment seemed orthotropic but stereopsis could not be demonstrated. Slit lamp examination showed no iritis. Humphrey threshold testing revealed enlarged blindspots, with visual field defects extending to fixation (Figure 2). There was no vitritis. Both fundi showed chronic papilledema, marked gliosis, vascular sheathing, hemorrhage, and cystoid macular edema (Figure 3), confirmed by optical coherence tomography. MR imaging supported the diagnosis of papilledema, with flattening of the posterior globes, protrusion of the optic discs into the vitreous, and dilation of the optic nerve sheaths (Figure 4A). The pituitary was hypoplastic (Figure 4B). The aqueduct of Sylvius was patent with normal ventricles. An MR venogram showed no thrombosis or focal narrowing. A CT scan showed a short segment of synostosis involving the right lambdoid suture. Again, a ventriculoperitoneal shunt was recommended and declined.
Figure 2.
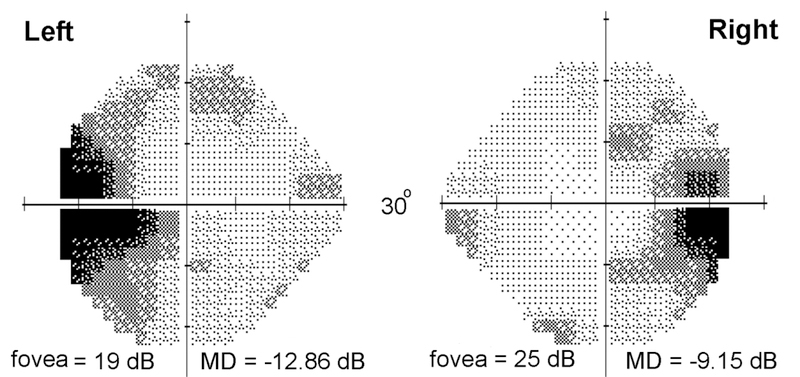
Patient 1, Humphrey 24–2 threshold visual fields. There is enlargement of the blind spots and depression of foveal sensitivity, more marked in the left eye than the right eye.
Figure 3.
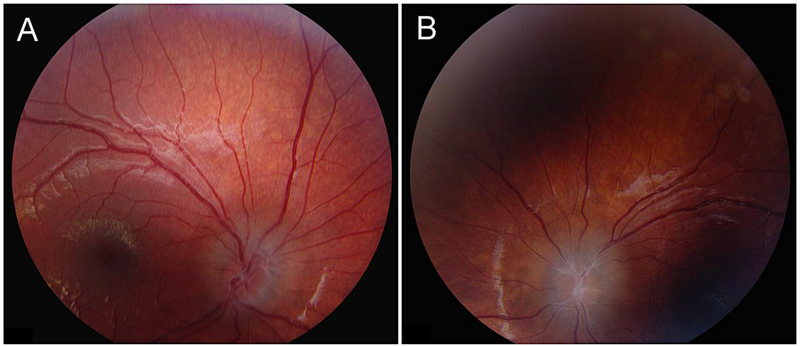
Patient 1, fundi at age 9 years. A. Right fundus shows chronic papilledema, with an extensive peripapillary ring of fluid. There is a fresh splinter hemorrhage inferotemporally and a cluster of fine preretinal hemorrhages just above the optic disc. All the arterioles and venules show extensive sheathing proximally. B. Left fundus shows similar findings, but more severe. There is macular wrinkling and cystoid edema.
Figure 4.
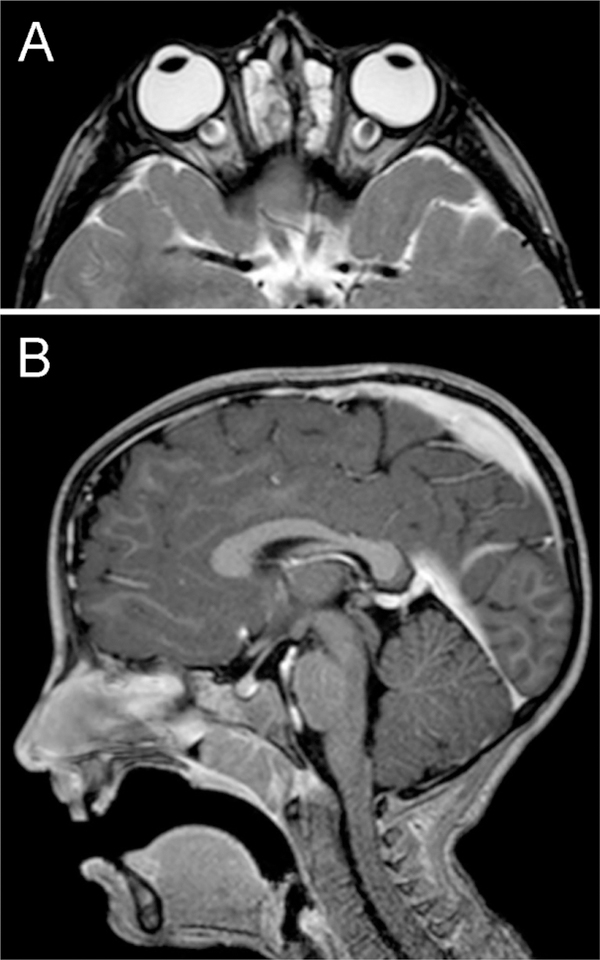
Patient 1, MR imaging. A. Axial T2-weighted image showing flattening of globes, swelling of optic discs into the vitreous, and dilation of the nerve sheaths. There is chronic ethmoid sinusitis. B. Sagittal T1-weighted image with gadolinium enhancement showing a small pituitary gland but anterior and posterior lobes with normal signal characteristics. The ventricles, craniocervical junction, and meninges were normal.
Patient 2:
A 12-year-old boy was hospitalized for malaise, headaches, blurred vision, hepatosplenomegaly and lymphadenopathy. He was born at term by Caesarian section, weighing 4.51 kg. Meconium aspiration required a short stay in the neonatal intensive care unit. At age 3 he underwent tonsillectomy and adenoidectomy. He was generally healthy during his childhood, although he received treatment periodically for allergic rhinitis, asthma, and eczema.
Evaluation revealed anemia, neutropenia, and idiopathic thrombocytopenia. Fundus examination showed marked bilateral optic disc swelling. A lumbar puncture was performed under sedation, yielding an opening pressure of 45 cm of water. There were 14 white cells/ml (97% lymphocytes, 3% monocytes), with a normal protein and glucose. He was thought to have an autoimmune lymphoproliferative syndrome and was treated with acetazolamide, prednisone, and mycophenolate mofetil. Over the subsequent year the papilledema resolved completely and nerve fiber layer thickness measurements returned to normal. Treatment with acetazolamide and prednisone was halted but he continued to receive mycophenolate mofetil and monthly infusions of IVIG. Other medications included cetirizine, diphenhydramine, montelukast, fluticasone nasal spray, loratadine, and ondansetron.
Seven months later the patient returned to the eye clinic reporting transient visual obscurations. The height was 161 cm and the weight was 62.1 kg, for a BMI of 23.8 kg/m2. The visual acuity was 20/20 in each eye without correction. There was no afferent pupillary defect. The extraocular eye movements were full. Ocular alignment was orthotropic. Slit lamp examination was normal. Humphrey 30–2 threshold tests revealed slightly enlarged blindspots. There was symmetrical optic disc swelling (Figure 5), without vitritis, vascular sheathing, or macular edema. Brain MR imaging was normal. A lumbar puncture under sedation yielded an opening pressure of 36 cm of water with 10 white cells/ml (3% neutrophils, 91% lymphocytes, 6% monocytes), with a normal protein and glucose. Treatment was restarted with acetazolamide 500 mg bid. The papilledema gradually resolved and acetazolamide treatment was discontinued after one year. Over a follow up period of two years the papilledema has not recurred. His condition remains stable on a regimen of IVIG infusion every 6 weeks and mycophenolate mofetil 500 mg bid.
Figure 5.
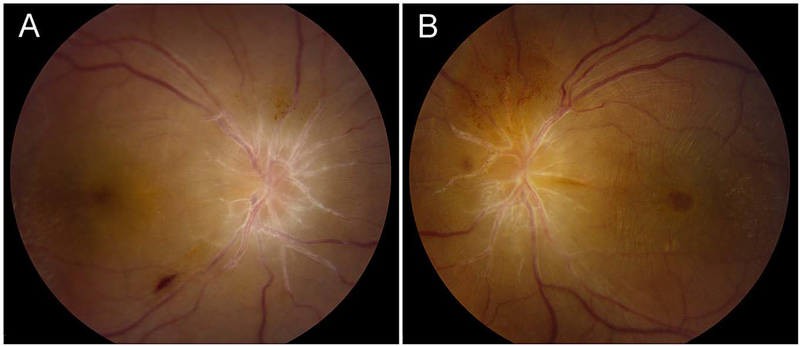
Patient 2, fundus images. A. Right eye and B. Left eye show papilledema. The reddish hue of the left fundus image is a digital file artifact.
At age 15 whole exome sequencing revealed a germline STAT3 heterozygous de novo gain-of-function mutation c.2147C>T (p.Thr716Met).
Discussion
These two cases demonstrate that papilledema can be among the clinical findings encountered in patients harboring mutations causing STAT3 gain of function. In both patients a raised lumbar opening pressure was measured by spinal puncture in the lateral decubitus position. In Patient 1 the initial lumbar puncture yielded an ambiguous result because the pressure reading declined to normal during the procedure. However, the diagnosis of papilledema was supported strongly by MR imaging, which showed bulging of the optic nerve sheaths. It was also confirmed by a second lumbar puncture years later.
In 1951 Evans and colleagues described a cohort of 29 patients with a spectrum of acquired hemolytic anemia and primary thrombocytopenic purpura (11). Patients with Evans Syndrome also manifest neutropenia, hepatosplenomegaly, immunodeficiency, recurrent infections, interstitial lung disease, and eczema (12,13). Autoimmune lymphoproliferative syndrome has been included as a feature of Evans Syndrome (14). Before genetic testing revealed STAT3 mutations, our first patient received a diagnosis of Evans Syndrome and our second patient received a diagnosis of autoimmune lymphoproliferative syndrome.
In a recent study of 181 patients with Evans Syndrome, two were reported to have raised intracranial pressure (15). Both patients had lymphocytosis of the cerebrospinal fluid and vasculitic brain lesions visible on MR imaging. In these patients, STAT3 testing was not performed and fundus appearance was not described. It is noteworthy, however, that raised intracranial pressure was present with cerebrospinal pleocytosis, as in both our patients.
Evans Syndrome is a descriptive entity, encompassing a variety of presentations and genetic causes. Genetic testing of 18 children with Evans Syndrome yielded two with STAT3 gain-of-function mutations (16). One patient had keratoconjunctivitis, but neither had papilledema. Patients with autoimmune lymphoproliferative syndrome were excluded from this cohort. Broader inclusion criteria for Evans Syndrome might have increased the percentage of patients harboring STAT3 mutations.
A total of 25 cases of STAT3 gain-of-function mutation has been reported, including our 2 cases (6,9,10,16,17). As mentioned earlier, one patient reported twice had severe vision loss from bilateral uveitis and cystoid macular edema (9,10). Therefore, we estimate that vision threatening eye disease occurs in about 12% (3/25) of germline STAT3 gain-of-function patients, with papilledema in 8% (2/25). Care of these patients is complex, and often focused on other medical issues, so the prevalence of eye findings may be under recognized.
Data are too fragmentary to conclude that any particular germline STAT3 gain-of-function mutation is more likely to result in papilledema. One other patient with a c.2144C>T (p.Pro715Leu) mutation has been reported, without papilledema (16). Five other cases with a c.2147C>T (p.Thr716Met) mutation have been reported, all without papilledema (6,9,16). It is striking that the two patients we encountered had very different ocular findings. Patient 1 had marked gliotic sheathing of the retinal vessels, as they emerged from the optic discs, giving rise to a highly unusual, distinctive fundus appearance. She eventually developed cystoid macular edema, an uncommon feature in papilledema. Her clinical course was unrelenting, eventually resulting in serious vision loss. In contrast, the papilledema in Patient 2 had a typical appearance. Although it recurred once it eventually resolved, for unexplained reasons. As more cases of papilledema from STAT3 gain-of-function are discovered, perhaps certain mutations will become associated with characteristic fundus findings.
Peripapillary glial proliferation and vascular sheathing occur in post-papilledema atrophy (18), but they were present in Patient 1 on initial examination, when she had intact visual function and no optic disc pallor. After central nervous system damage, STAT3 is activated in microglia and endothelial cells, and it promotes the migration of reactive astrocytes to injury sites (19–22). STAT3 signaling mediates astrocyte reaction in optic nerve injury (23,24). We speculate that in the c.2144C>T (p.Pro715Leu) gain-of-function mutation, STAT3 causes exuberant astrogliosis when the optic discs are subjected to raised intracranial pressure
The mechanism of papilledema in our two patients is obscure. Neither had hydrocephalus, dural venous sinus thrombosis, or brain lesions. Patient 1 had a short segment of occipital craniosynostosis, but it was insignificant. Both were overweight, but neither fit the typical profile of pseudotumor cerebri. In patient 1, papilledema was present at a very young age, suggesting that it was a primary finding produced by STAT 3 mutation, not a secondary effect of a subsequent infection, a medication side effect, or anemia. The only feature common to both our patients was the presence in the cerebrospinal fluid of a lymphocytic pleocytosis, a finding also reported in patients with Evans syndrome and raised intracranial pressure. It is possible that papilledema was caused by chronic meningeal lymphocytic infiltration impeding the absorption of cerebrospinal fluid.
These two cases illustrate that papilledema and raised intracranial pressure are potential manifestations of mutations causing STAT3 gain of function. Ophthalmological screening of patients with STAT3 mutations may be useful because papilledema can be asymptomatic and hence remain occult.
Acknowledgments
This work was supported by grants EY 029703 (J.C.H.), EY02162 (Beckman Vision Center) from the National Eye Institute and by an unrestricted grant from Research to Prevent Blindness. We thank Anthony T. Moore for critical review of the manuscript.
References
- 1.Stark GR, Darnell JE Jr. The JAK-STAT pathway at twenty. Immunity. 2012;36(4):503–14. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18(4):374–84. doi: 10.1038/ni.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Consonni F, Dotta L, Todaro F, Vairo D, Badolato R. Signal transducer and activator of transcription gain-of-function primary immunodeficiency/immunodysregulation disorders. Curr Opin Pediatr. 2017;29(6):711–17. doi: 10.1097/MOP.0000000000000551. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects. Drugs. 2017;77(5):521–46. doi: 10.1007/s40265-017-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogel TP, Milner JD, Cooper MA. The Ying and Yang of STAT3 in Human Disease. J Clin Immunol. 2015;35(7):615–23. doi: 10.1007/s10875-015-0187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milner JD, Vogel TP, Forbes L, Ma CA, Stray-Pedersen A, Niemela JE, Lyons JJ, Engelhardt KR, Zhang Y, Topcagic N, et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood. 2015;125(4):591–9. doi: 10.1182/blood-2014-09-602763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forbes LR, Milner J, Haddad E. Signal transducer and activator of transcription 3: a year in review. Curr Opin Hematol. 2016;23(1):23–7. doi: 10.1097/MOH.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillmer EJ, Zhang H, Li HS, Watowich SS. STAT3 signaling in immunity. Cytokine Growth Factor Rev. 2016;31:1–15. doi: 10.1016/j.cytogfr.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flanagan SE, Haapaniemi E, Russell MA, Caswell R, Allen HL, De Franco E, McDonald TJ, Rajala H, Ramelius A, Barton J, et al. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nat Genet. 2014;46(8):812–14. doi: 10.1038/ng.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haapaniemi EM, Kaustio M, Rajala HL, van Adrichem AJ, Kainulainen L, Glumoff V, Doffinger R, Kuusanmaki H, Heiskanen-Kosma T, Trotta L, et al. Autoimmunity, hypogammaglobulinemia, lymphoproliferation, and mycobacterial disease in patients with activating mutations in STAT3. Blood. 2015;125(4):639–48. doi: 10.1182/blood-2014-04-570101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans RS, Takahashi K, Duane RT, Payne R, Liu C. Primary thrombocytopenic purpura and acquired hemolytic anemia; evidence for a common etiology. AMA Arch Intern Med. 1951;87(1):48–65. [DOI] [PubMed] [Google Scholar]
- 12.Mantadakis E, Farmaki E. Natural History, Pathogenesis, and Treatment of Evans Syndrome in Children. J Pediatr Hematol Oncol. 2017;39(6):413–19. doi: 10.1097/MPH.0000000000000897. [DOI] [PubMed] [Google Scholar]
- 13.Michel M, Chanet V, Dechartres A, Morin AS, Piette JC, Cirasino L, Emilia G, Zaja F, Ruggeri M, Andres E, et al. The spectrum of Evans syndrome in adults: new insight into the disease based on the analysis of 68 cases. Blood. 2009;114(15):3167–72. doi: 10.1182/blood-2009-04-215368. [DOI] [PubMed] [Google Scholar]
- 14.Teachey DT, Manno CS, Axsom KM, Andrews T, Choi JK, Greenbaum BH, McMann JM, Sullivan KE, Travis SF, Grupp SA. Unmasking Evans syndrome: T-cell phenotype and apoptotic response reveal autoimmune lymphoproliferative syndrome (ALPS). Blood. 2005;105(6):2443–8. doi: 10.1182/blood-2004-09-3542. [DOI] [PubMed] [Google Scholar]
- 15.Pincez T, Neven B, Le Pointe HD, Varlet P, Fernandes H, Gareton A, Leverger G, Leblanc T, Chambost H, Michel G, et al. Neurological Involvement in Childhood Evans Syndrome. J Clin Immunol. 2019; doi: 10.1007/s10875-019-0594-3. [DOI] [PubMed] [Google Scholar]
- 16.Besnard C, Levy E, Aladjidi N, Stolzenberg MC, Magerus-Chatinet A, Alibeu O, Nitschke P, Blanche S, Hermine O, Jeziorski E, et al. Pediatric-onset Evans syndrome: Heterogeneous presentation and high frequency of monogenic disorders including LRBA and CTLA4 mutations. Clin Immunol. 2018;188:52–57. doi: 10.1016/j.clim.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Nabhani S, Schipp C, Miskin H, Levin C, Postovsky S, Dujovny T, Koren A, Harlev D, Bis AM, Auer F, et al. STAT3 gain-of-function mutations associated with autoimmune lymphoproliferative syndrome like disease deregulate lymphocyte apoptosis and can be targeted by BH3 mimetic compounds. Clin Immunol. 2017;181:32–42. doi: 10.1016/j.clim.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Brodsky MC. Pediatric Neuro-Ophthalmology. 3rd ed. NY: Springer; 2016, page 219. [Google Scholar]
- 19.Kang W, Hebert JM. Signaling pathways in reactive astrocytes, a genetic perspective. Mol Neurobiol. 2011;43(3):147–54. doi: 10.1007/s12035-011-8163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Planas AM, Soriano MA, Berruezo M, Justicia C, Estrada A, Pitarch S, Ferrer I. Induction of Stat3, a signal transducer and transcription factor, in reactive microglia following transient focal cerebral ischaemia. Eur J Neurosci. 1996;8(12):2612–8. [DOI] [PubMed] [Google Scholar]
- 21.Satriotomo I, Bowen KK, Vemuganti R. JAK2 and STAT3 activation contributes to neuronal damage following transient focal cerebral ischemia. J Neurochem. 2006;98(5):1353–68. doi: 10.1111/j.1471-4159.2006.04051.x. [DOI] [PubMed] [Google Scholar]
- 22.Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28(28):7231–43. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun D, Moore S, Jakobs TC. Optic nerve astrocyte reactivity protects function in experimental glaucoma and other nerve injuries. J Exp Med. 2017;214(5):1411–30. doi: 10.1084/jem.20160412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S, Li W, Wang W, Zhang SS, Huang P, Zhang C. Expression and activation of STAT3 in the astrocytes of optic nerve in a rat model of transient intraocular hypertension. PLoS One. 2013;8(1):e55683. doi: 10.1371/journal.pone.0055683. [DOI] [PMC free article] [PubMed] [Google Scholar]