Abstract
Nur77 (Nr4a1) belongs to a small family of orphan nuclear receptors that are rapidly induced by B cell receptor (BCR) stimulation, yet little is known about its function in B cells. We have previously characterized a reporter of Nr4a1 transcription, Nur77-eGFP, in which GFP expression faithfully detects antigen encounter by B cells in vitro and in vivo. Here we report that Nur77 expression correlates with the degree of self-reactivity, counter-selection, and anergy among individual B cell clones from two distinct BCR Tg mouse models, but is dispensable for all of these tolerance mechanisms. However, we identify a role for Nur77 in restraining survival of self-reactive B cells in the periphery under conditions of competition for a limiting supply of the survival factor BAFF. We find that Nur77-deficiency results in the progressive accumulation of self-reactive B cells in the mature repertoire with age and is sufficient to break B cell tolerance in VH3H9 heavy chain Tg mice. We thus propose that Nur77 is upregulated in self-reactive B cells in response to chronic antigen stimulation and selectively restricts their survival, gradually pruning self-reactivity from the mature repertoire to impose a novel layer of peripheral B cell tolerance.
Keywords: Nur77, Nr4a1, BAFF, B cell tolerance, autoreactivity, autoantibodies, anergy
INTRODUCTION
Self-reactive B cell receptors (BCRs) are a frequent byproduct of random VDJ recombination. Moreover, despite layered tolerance mechanisms including receptor editing and central deletion, self-reactive B cells persist in the periphery of healthy mice and humans (1, 2). How are such cells normally kept in check and restrained from mounting a productive immune response to spurious stimulation by endogenous antigens? It has long been known that self-reactive B cells are rendered functionally unresponsive (“anergic”) by a combination of antigen receptor downregulation and by transcriptional and post-translational mechanisms that serve to suppress BCR signal transduction (3). Finally, self-reactive B cells have a heightened dependence upon the B cell survival factor BAFF, and have a reduced lifespan in the periphery under competitive conditions, yet the molecular mechanisms accounting for this are incompletely understood (4, 5).
Nr4a1–3 encode a small family of orphan nuclear hormone receptors (Nur77, Nurr1, and Nor1 respectively) with significant homology in their DNA- and ligand-binding domains (6). Nr4a1–3 were initially cloned as primary response genes, and are rapidly induced in response to antigen receptor stimulation in lymphocytes (7–9). The Nr4a1–3 gene products are thought to function as constitutively active transcription factors with no known endogenous ligand (6). Indeed, crystal structures of their Drosophila ortholog and of murine Nurr1 reveal that the ligand binding pocket is occluded by conserved hydrophobic side chains (10, 11). Furthermore, the ligand binding pocket of Nurr1 adopts a constitutively active conformation similar to that of other ligand-bound nuclear receptors (11). In addition to important roles as transcriptional regulators of Treg and myeloid cell fate (12–14), the Nr4a family mediate TCR-induced apoptosis (15, 16), and are essential for negative selection of self-reactive thymocytes (17, 18). It has been argued that Nr4a family members mediate apoptosis (at least in part) independently of their DNA-binding capacity by translocating to the cytosol, binding to Bcl2 and inducing a conformational change that exposes the BH3-only domain of Bcl2 (19, 20). Yet, although Nur77 and its family members are also upregulated by BCR stimulation, relatively little is known about their role in B cells (7, 21).
We previously characterized a BAC Tg reporter mouse line in which eGFP is under the control of the regulatory region of Nr4a1 (Nur77-eGFP) (22). We showed that Nur77-eGFP expression in reporter lymphocytes scales with the intensity and duration of BCR and TCR stimulation in vitro, and is highly upregulated in antigen-specific lymphocytes after immunization or infection in vivo (23–25). In addition to dynamic induction of Nur77-eGFP with strong BCR stimuli, we showed that its expression scales in proportion to self-reactivity among naturally occurring self-reactive B cells (22). Importantly, we established that such steady-state Nur77-eGFP expression scales with the strength of BCR signal transduction, requires endogenous antigen recognition, and is independent of microbiota (22, 26). More recently, we showed that naturally self-reactive B-1a cells also upregulate Nur77-eGFP in response to chronic self-antigen stimulation, and found that Nur77 plays a critical negative regulatory role in B-1a cells by restricting the generation of natural IgM plasma cells under steady state conditions (27). However, the function of Nur77 in B-2 cells is unknown.
Here we take advantage of the Nur77-eGFP reporter to show that Nur77 is upregulated in self-reactive B cells from two distinct murine models of B cell anergy: the hen egg lysozyme (HEL) model, in which monoclonal Ig-HEL B cells develop in the context of soluble cognate antigen (sHEL), and the VH3H9 heavy chain (HC) model in which DNA-reactive B cells can be tracked in the context of a polyclonal repertoire on the basis of endogenous light chain expression (28, 29). We show that Nur77-eGFP expression correlates with the self-reactivity, editing, deletion, and anergy of individual B cell clones. We go on to show that Nur77 itself is dispensable for editing and deletion in the VH3H9 model system, and that it is largely dispensable for IgM downregulation, anergy, and follicular exclusion in both model systems. However, we find that Nur77 restricts the survival of self-reactive B cells in the periphery by promoting antigen-induced cell death in a cell intrinsic manner. This can be overcome by the soluble B cell survival factor BAFF. Despite generation of a highly self-reactive B cell repertoire, layered tolerance mechanisms ensure that VH3H9 HC Tg mice do not develop autoantibodies. We find that Nur77 contributes to removal of the most highly self-reactive B cells from the repertoire of these mice as they age, and loss of Nur77 is sufficient to break tolerance in this model. We thus show that Nur77 is upregulated in self-reactive B-2 cells in response to chronic antigen stimulation, and is critical to maintain tolerance by restricting the survival of these cells, particularly in the setting of competition with less self-reactive cells for a limited supply of BAFF.
MATERIALS AND METHODS
Mice.
Nur77-eGFP mice, IgHEL Tg (MD4), and sHEL Tg (ML5) mice were previously described (22, 29). Site-directed VH3H9 HC Tg mice have been previously described and were generously shared by Anthony DeFranco (28). Nr4a1fl/fl mice were generously shared by Pierre Chambon and Catherine Hedrick (13). Mb1 Cre, Nr4a1−/−, C57BL/6, and CD45.1+ BoyJ mice were from The Jackson Laboratory (30, 31). All strains were fully backcrossed to C57BL/6 genetic background. All mice were housed in a specific pathogen-free facility at UCSF according to University and National Institutes of Health guidelines.
Antibodies and Reagents.
Streptavidin (SA) and Abs to B220, CD3, CD19, CD21, CD23, CD93 (AA4.1), CD45.1, CD45.2, IgM, IgM[a], IgM[b], IgD, IgD[a], IgD[b], Igκ, Igλ1 and Igλ1,2,3 conjugated to biotin or fluorophores (Biolegend, eBiosciences, BD, Miltenyi Biotech or Tonbo); Antibodies for intra-cellular staining, pErk Ab (clone 194g2) and pS6 Ab (clone 2F9), were from Cell Signaling Technologies, Nur77 Ab conjugated to PE (clone 12.14) manufactured by Invitrogen, purchased from eBioscience. Donkey anti-rabbit secondary Ab conjugated to APC was from Jackson Immunoresearch. Stimulatory antibodies: Goat anti-mouse IgM F(ab’)2 was from Jackson Immunoresearch. Anti-IgD was from MD Biosciences.; ELISA reagents: Costar Assay Plate, 96 Well Clear, Flat bottom half area high binding, polystyrene(Corning), SA-HRP, anti-mouse IgM, IgH+L, IgG unlabeled or conjugated to biotin or HRP (Southern Biotech), TMB (Sigma). C10 refers to complete culture media prepared with RPMI-1640 + L-glutamine (Corning-Gibco), Penicillin Streptomycin L-glutamine (Life Technologies), HEPES buffer [10mM] (Life Technologies), B-Mercaptoethanol [55mM] (Gibco), Sodium Pyruvate [1mM] (Life Technologies), Non-essential Amino acids (Life Technologies), 10% heat inactivated FBS (Omega Scientific). Recombinant BAFF 20ng/ml (R&D).
Flow Cytometry and data analysis.
Cells were stained with indicated antibodies and analyzed on a Fortessa (Becton Dickson). Data analysis was performed using FlowJo (v9.7.6) software (Treestar Incorporated, Ashland, OR). Statistical analysis and graphs were generated using Prism v6 (GraphPad Software, Inc). Student’s unpaired T test was used to calculate p values and mean ± SEM is displayed in all graphs. *p<0.05, **p<0.005, ***p<0.0005, ****p<0.00005. Figures were prepared using Illustrator CS6 v16.0.0.
Intracellular staining to detect pErk, pS6, or endogenous Nur77.
Following stimulation, cells were fixed in 2% paraformaldehyde for 10 minutes, permeabilized on ice with 100% methanol for 30 minutes, and stained with anti-pErk or anti-pS6 or anti-Nur77 followed by lineage markers and secondary antibodies if needed (40 minutes incubation for primary and secondary staining). Antibody concentrations were previously described (26).
Intracellular Calcium Flux.
Cells were loaded with 5 μg/mL Indo-1 AM (Life Technologies) and stained with lineage markers for 15 minutes. Cells were rested at 37 C for 2 minutes, and Indo-1 fluorescence was measured by FACS immediately prior to and after stimulation to determine intracellular calcium.
Live/dead staining.
LIVE/DEAD Fixable Near-IR Dead Cell Stain kit (Invitrogen). Reagent was reconstituted as per manufacturer’s instructions, diluted 1:1000 in PBS, and cells were stained at a concentration of 2×106 cells /100μL on ice for 10 minutes.
Vital dye loading.
Cells were loaded with CellTrace Violet (CTV; Invitrogen) per the manufacturer’s instructions except 5 ×106 cells/ml rather than 1 × 106 cells/ml.
In Vitro B Cell Culture and Stimulation.
Splenocytes or lymphocytes were harvested into single cell suspension, subjected to red cell lysis using ACK buffer, loaded with CTV as described above, and plated at a concentration of 5 × 105 cells/200 μL in round bottom 96 well plates in complete RPMI media with stimuli for 1–3 days. In vitro cultured cells were stained using fixable near IR live/dead stain (Invitrogen) per manufacturer’s instructions.
ELISA.
Serum antibody titers for total IgG, and anti-dsDNA IgG were measured by ELISA. For total IgG, 96-well plates (Costar) were coated with 1 μg/mL anti-IgH+L (Jackson). Sera were diluted serially, and total IgG was detected with anti-IgG-HRP (Southern Biotech). dsDNA plates were generated by serially coating plates with 100 μg/mL poly-L-lysine (Sigma Aldrich) and 0.2 U/mL poly dA-dT (Sigma Aldrich) in 0.1 M Tris-HCL pH 7.6. Sera were diluted serially on dsDNA plates, and autoantibodies were detected as above. All ELISA plates were developed with TMB (Sigma) and stopped with 2N sulfuric acid. Absorbance was measured at 450 nm.
Bone marrow chimeras.
Host mice were irradiated with 530 rads x 2, 4 hours apart, and injected IV with either 106 donor IgHEL BM to generate naïve or anergic B cell populations, or with 106 donor A mixed with 106 donor B BM cells to generate competitive chimeras. Chimeras were sacrificed 10 weeks after reconstitution, and cells were assayed as described above.
Cell sorting.
Single cell suspensions were generated from spleens and stained as described above. B cells were sorted using a FACs Aria II (Becton Dickinson). For IgHEL/sHEL chimeras, mature B cells were gated as live/single/B220+/CD21low/CD23+. For VH3H9 Tg mice, B cells were gated as live/single/B220+/CD21low/CD23+ and then either κ or λ expressing B cells were sorted.
Adoptive transfer.
Splenocytes from either IgHEL/sHEL chimeras or IgHEL Tg+ mice (MD4 line) were harvested into single cell suspensions and subjected to red blood cell lysis with ACK buffer. Cells were mixed at a 1:1 ratio with CD45.1 splenocytes, loaded with CTV as described above, and between 2.5×106 and 5×106 cells in 200 μL total volumes were injected into either ML5 Tg+ or Tg- C57BL/6 host mice via the tail vein.
BrdU labeling and staining.
7 week-old mice were treated with 0.8μg/ml BrdU in drinking water for 6 days. Cells were stained to detect both surface markers and BrdU incorporation as per manufacturer’s protocol (BrdU Flow Kit, BD).
Immunohistology.
Spleens were first fixed in 4% PFA for 3 hours at room temperature, then sequentially immersed in 18% and 30% sucrose, and finally embedded in OCT (Fisher) and flash-frozen using dry ice/EtOH bath. Five-to-seven-micron sections were cut using a Leica CM1900 cryostat and stored at −80C prior to staining. Sections were blocked with 5% rat serum for 1 hour at RT, and then stained for 1 hour at RT with FITC B220 (clone RA3–6B2), APC anti-CD3 (clone 17A2), and either biotin-conjugated anti-λ1 light chain (clone R11–153), or PE anti-IgDa (clone REA484) to identify adoptively transferred IgHEL Tg B cells. Images were captured with a Zeiss Axio Imager M2 widefield fluorescence microscope. Images were processed using Zen Pro (Zeiss) and Adobe Photoshop (Adobe).
Light chain cloning and sequencing.
B cells were sorted using a FACs ARIAII as described above. RNA was isolated via Trizol (Life technologies) / chloroform phase separation. cDNA was generated and PCR amplified through the use of Onestep RT PCR kit (Qiagen), with degenerate PCR primers to amplify lambda light chain sequences (see Supplemental Table 1 for primers)(32). The cDNA was then run on a 2% agarose gel, and extracted with the Qiaquick Gel Extraction kit (Qiagen). cDNA was then inserted into the PCR-2.1 Topo vector through the use of the Topo-TA kit (Invitrogen), and transformed into Top10 competent E. coli (Invitrogen) via heat shock. Colonies were expanded at 37°C overnight, and were then picked and PCR amplified using M13 primers from the Topo-TA kit. The amplified DNA was then treated with recombinant Shrimp Alkaline Phosphatase and Exonuclease 1 (New England Biolabs), and sent to ELIM Biopharm for sequencing. The returned sequences were then uploaded to IMGT_V-QUEST (IGMT.org) for VJ alignment and light chain identification.
qPCR.
Sorted B cell populations as noted were harvested into Trizol (Invitrogen), and stored at −80°C. RNA was extracted via phenol phase separation. cDNA was prepared with Superscript III kit (Invitrogen). qPCR reactions were run on a QuantStudio 12K Flex thermal cycler (ABI) using either Taqman (Life Technologies) assays or SYBR Green detection (see Supplemental Table 1 for list of primers).
RESULTS
Endogenous antigen recognition is necessary and sufficient for Nur77-eGFP expression in B cells
MD4/ML5 mice are engineered to co-express two transgenes (Tgs); MD4 mice harbor a BCR Tg with high affinity for its cognate antigen hen egg lysozyme (IgHEL), while ML5 mice constitutively express soluble HEL protein (sHEL) under the control of the metallothionine promoter (29). Although follicular B cells develop in mice harboring both Tgs, surface IgM on these cells is massively downregulated, they are unresponsive to acute antigen stimulation (“anergic”), and they do not differentiate into antibody-secreting cells at steady state or in response to immunization (29, 33–35). We previously introduced the Nur77-eGFP reporter into this model system and showed that reporter B cells lose Nur77-eGFP expression when they express IgHEL in the absence of cognate antigen (22). In contrast, forced constitutive expression of soluble cognate antigen upregulates Nur77-eGFP, establishing that endogenous antigen is both necessary and sufficient for Nur77-eGFP expression in naïve, follicular B-2 cells (Fig 1A, B). Recently published microarray data from Goodnow and colleagues confirm that endogenous Nr4a1 is highly upregulated in anergic B cells from IgHEL/sHEL double Tg mice (Supplemental Fig 1A) (36). Importantly, consistent with the extremely high affinity of IgHEL B cells for their cognate antigen HEL (10−10 M)(29), Nur77-eGFP expression in IgHEL / sHEL double Tg reporter B cells was much higher than in wild type (WT) reporter B cells (Fig 1A, B, Supplemental Fig 1B). This demonstrates that, as expected, the auto-reactivity of IgHEL / sHEL double Tg reporter B cells far exceeds that of mature naïve follicular B cells that normally populate the periphery. This is consistent with prior evidence that upregulation of Nur77-eGFP correlates with the degree of self-reactivity in the natural diverse B cell repertoire (22), and highlights that highly self-reactive B cells resembling IgHEL / sHEL are normally excluded from the peripheral repertoire of healthy mice.
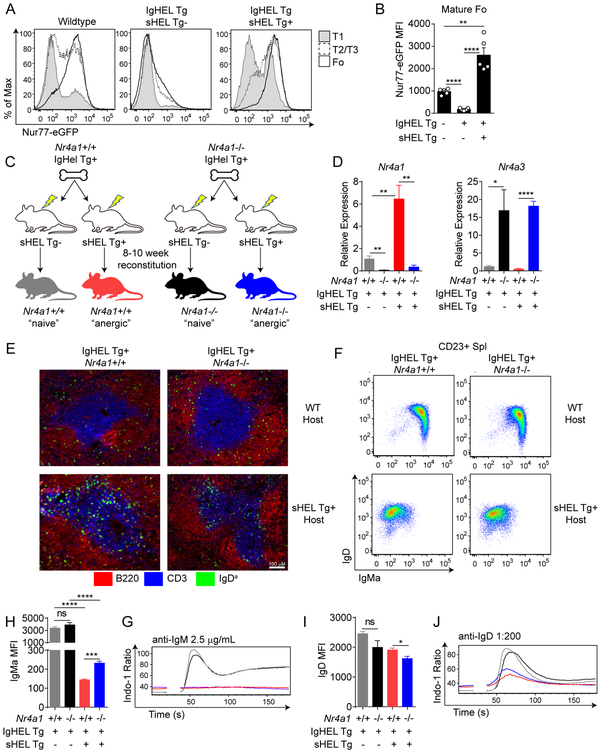
Figure 1. Nur77 is upregulated, but dispensable for anergy, in IgHEL B cells chronically exposed to soluble cognate antigen.
A. B220+ splenic B cells from WT, IgHEL Tg, and IgHEL/sHEL Tg Nur77-eGFP mice were stained with CD23 and CD93 to identify T1(CD23− CD93+), T2/3 (CD23+ CD93+) and Follicular (CD23+ CD93−) subsets. Histograms represent Nur77-eGFP expression in these cells and are representative of at least 5 independent experiments. B. Quantification of Nur77-eGFP MFI as shown in Fig 1A. C. Schematic representation of IgHEL/sHEL Tg chimera generation. Lethally irradiated wildtype or sHEL Tg hosts were reconstituted with 5×106 bone marrow cells from either Nr4a1+/+ or Nr4a1−/− IgHEL Tg hosts. After at least 10 weeks of reconstitution, mice were sacrificed for further analysis. D. Mature CD23+ splenic B cells were sorted from IgHEL chimeras. Relative Nr4a1 and Nr4a3 mRNA expression was determined via qPCR. N=4 for each group. E. Representative spleen sections depicting localization of adoptive transferred Nr4a1+/+ or Nr4a1−/− IgHEL Tg B cells (IgDa+) into WT or sHEL Tg hosts after 12 hours. Data are representative of two independent mice of each of four conditions. F. Representative flow plots showing IgM and IgD expression on splenic CD23+ mature B cells from chimeras. G, I. Quantification of IgMa and IgD MFI as shown in Fig 1F. H, J. Splenic B cells from IgHEL/sHEL Tg chimeras were loaded with Indo-1 dye. Ratiometric assessment of intracellular calcium was assessed by flow cytometry after stimulation with anti-IgM (2.5 μg/mL, H) or anti-IgD (1:200 dilution, J). Plots are representative of 3 (D) or 4 (G, I) separate mice per group.
Nur77 is dispensable for anergy of IgHEL B cells chronically exposed to soluble cognate antigen
In order to define the function of Nur77 in anergic IgHEL B cells, we transplanted bone marrow from Nr4a1−/− or Nr4a1+/+ IgHEL Tg (MD4) mice into irradiated WT and sHEL Tg (ML5) hosts in order to generate chimeras harboring either “naïve” or “anergic” IgHEL B cells respectively (Fig 1C). After at least 10 weeks of reconstitution, we sorted CD23+ splenic B cells from these mice and observed, as expected, upregulation of Nr4a1 transcript in “anergic” IgHEL Tg B cells from ML5 hosts relative to “naïve” Tg B cells from hosts lacking cognate antigen expression (Fig 1D). Importantly, although we observed a compensatory increase in Nr4a3 transcript in the absence of Nr4a1, we did not detect antigen-dependent upregulation of Nr4a3 (Fig 1D). By contrast, Nr4a2 transcript was undetectable by qPCR suggesting minimal expression in this model (DNS).
Although “anergic” IgHEL / sHEL B cells upregulate the maturation marker CD23 and populate the periphery, they retain expression of CD93 (AA4.1) and assume a “T3-like” phenotype that has been described in other model systems and in naturally occurring anergic B cells (3, 37). However, Nr4a1−/− “anergic” IgHEL / sHEL B cells begin to downregulate CD93 and exhibit a more “mature” phenotype than Nr4a1+/+ anergic B cells (Supplemental Fig 1C, D). “Anergic” IgHEL/sHEL B cells are excluded from the marginal zone compartment which is populated sparsely by IgMb escapees expressing endogenous HC, and this is not influenced by Nur77 expression (Supplemental Fig 1D, data not shown).
Cyster et al. originally reported that either anergic or naïve IgHEL B cells adoptively transferred into sHEL Tg hosts harboring competitor B cells are excluded from B cell follicles and localize to T cell zone (or the T/B boundary depending on experimental design) (38, 39). We next sought to assess whether Nur77 modulated the follicular exclusion of self-reactive B cells. We adoptively transferred either IgHEL Tg Nr4a1+/+ or IgHEL Tg Nr4a1−/− splenocytes into WT and sHEL Tg hosts and assessed their localization after 12 hours. As previously described, IgHEL Tg B cells transferred into WT hosts localize to the B cell follicles and are excluded from the T cell zone, while the reverse is true when they are transferred into sHEL Tg hosts (Fig 1E). Nur77 expression was dispensable for this phenomenon (Fig 1E).
IgM but not IgD BCR expression is highly downregulated on the surface of “anergic” IgHEL / sHEL B cells irrespective of Nur77 expression (Fig 1F, Supplemental Fig 1D). However, such IgM downregulation is subtly but significantly impaired in the absence of Nur77 while the opposite is true for IgD (Fig 1G, I). Consistent with profound downregulation of surface IgM, calcium entry triggered by ligation of the IgM BCR is markedly dampened in “anergic” IgHEL / sHEL B cells (33). Impaired calcium entry is unaltered by Nur77 expression despite subtle changes in surface IgM (Fig 1G, H). Importantly, calcium entry is also dampened downstream of IgD BCR stimulation in “anergic” IgHEL / sHEL B cells irrespective of Nur77 expression, even though surface IgD receptor levels are minimally altered relative to “naïve” IgHEL B cells (Fig 1I, J). This demonstrates that Nur77 expression is dispensable for anergic rewiring downstream of the BCR. Elevated basal calcium levels are a well-described feature of self-reactive B cells and are thought to arise from chronic occupancy of the BCR by endogenous antigen (3). “Anergic” IgHEL / sHEL B cells exhibit elevated basal calcium relative to “naïve” IgHEL Tg B cells, and this is also independent of Nur77 (Fig 1H, J).
In addition to phenotypic and functional changes induced by chronic cognate antigen stimulation, an entire transcriptional program is upregulated in anergic IgHEL B cells that notably includes the primary response genes Egr 1–3 and the pro-apoptotic factor Bcl2l11 (Bim) (36). Nur77 is dispensable for the induction of these transcripts and does not significantly modulate their expression (Supplemental Fig 1E–H), suggesting that this transcriptional program is not globally disrupted in the absence of Nur77.
Although Nur77 subtly modulates the phenotype of “anergic” IgHEL B cells to render them slightly more “naïve-like”, it is dispensable for the three most well-studied and prominent features of anergy in this model: follicular exclusion, IgM downregulation and dampened signal transduction downstream of the BCR.
Nur77 limits survival of “anergic” IgHEL B cells chronically exposed to cognate HEL Ag
It has been previously demonstrated that “anergic” IgHEL / sHEL B cells have a very high turnover rate in vivo and are rapidly lost in competition with WT B cells (38–40). Increased dependence upon a limiting supply of the B cell survival factor BAFF accounts for their competitive elimination (4, 5). However, the molecular mechanism that links chronic antigen stimulation to apoptosis in anergic B cells is incompletely understood. Nur77 plays an important role in negative selection of self-reactive thymocytes (17). We therefore hypothesized that Nur77 might contribute to the impaired survival of “anergic” IgHEL B cells in competitive settings. To test this, we assessed in vitro survival of “anergic” IgHEL B cells from sHEL hosts relative to congenically-marked CD45.1 WT B cells, co-cultured either in the presence or absence of exogenous BAFF. “Anergic” IgHEL B cells survive poorly relative to WT B cells, and this is largely rescued by addition of recombinant BAFF (Fig 2A, B). Strikingly, survival of “anergic” IgHEL B cells is significantly improved in the absence of Nur77 expression, but this advantage is no longer evident with addition of excess BAFF (Fig 2A, B). Importantly, Nur77 does not modulate in vitro survival of “naïve” IgHEL Tg B cells harvested from non-Tg host chimeras (Fig 2C, D). These data demonstrate that Nur77 contributes to the reduced survival of self-reactive B cells in vitro.
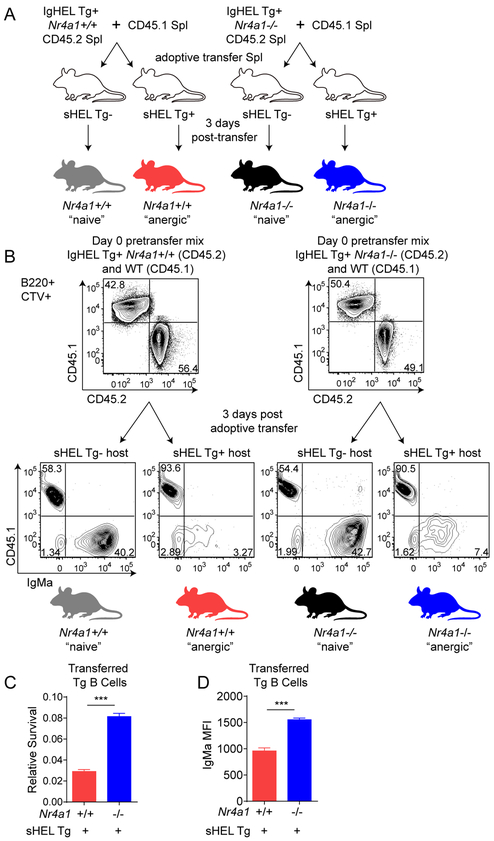
Figure 2. Nur77 limits survival of “anergic” IgHEL Tg B cells chronically exposed to cognate HEL in vitro and in vivo Ag.
A-D. Lymph nodes were harvested from IgHEL/sHEL Tg chimeras generated as described in Fig 1C. Tg CD45.2+ B cells were mixed 1:1 with CD45.1+ WT B cells and plated at a total concentration of 5×106 cells per well. These cells were incubated for up to 2 days with or without 20 ng/mL BAFF. The CD45.2/CD45.1 ratio was calculated at each time point and normalized to the CD45.2/CD45.1 cell input ratio. N=4 for each group. E. Schematic for experimental setup used for Fig. 2F-I. IgHEL/sHEL Tg chimeras were generated as in Fig 1C. Splenocytes from IgHEL/sHEL Tg chimeras and CD45.1 WT mice were loaded with the vital dye Cell Trace Violet (CTV) and combined 1:1. 5×106 total splenocytes were adoptively co-transferred into either WT or sHEL Tg mice via I.V. injection. 2 days after adoptive transfer, mice were sacrificed, and spleens were harvested for surface marker analysis via flow cytometry. Donor cells were identified as CTV+, and IgHEL Tg and CD45.1 WT B cells were separated based on CD45.1 and CD45.2 expression. F. Representative plots shown. G. Nur77-eGFP MFI of CTV+, CD45.2+ and Nur77-eGFP+ B cells isolated from sHEL Tg+ and sHEL Tg- host mice, N=3 per group. H. IgMa MFI of adoptively transferred B cells, N=3 per group. I. Ratio of transferred IgHEL Tg cells compared to competitively transferred CD45.1 WT cells normalized to the input ratio. N=3 per group.
We next sought to define the B cell-intrinsic role of Nur77 in the competitive elimination of anergic B cells in vivo. To do so, we mixed congenically marked CD45.1+ WT splenocytes in a fixed ratio with “anergic” IgHEL B cells that were either sufficient or deficient for Nur77 (generated as described in Fig 1C), loaded cells with the fluorescent vital dye CellTrace Violet (CTV) to facilitate tracking, and adoptively transferred aliquots into either WT or sHEL Tg (ML5) hosts (see Fig 2E, F for schematic of experimental design). Two days after adoptive transfer into WT hosts lacking cognate antigen expression, “anergic” IgHEL B cells lost Nur77-eGFP expression and upregulated surface IgM, suggesting that chronic self-antigen exposure was required to maintain Nur77 expression in self-reactive B cells and that certain features of anergy in this model are reversible (Fig 2G, H). As previously reported, rapid loss after adoptive transfer of “anergic” IgHEL B cells (identified as CTV+, CD45.2+, IgDa+) relative to co-transferred CD45.1+ WT B cells depended on persistent exposure to self-antigen stimulation (Fig 2F, I) (39). Strikingly, Nur77-deficiency partially and selectively ameliorated competitive elimination of anergic B cells exposed to chronic self-antigen (Fig 2F, I).
Cyster and colleagues further showed that such competitive elimination of IgHEL B cells in the presence of chronic self-antigen was separable from anergy per se (38). To isolate the contribution of Nur77 to this process, we performed the competitive adoptive transfer assay described in Fig 2E with “naïve” rather than “anergic” IgHEL B cells (see schematic Fig 3A). “Naïve” IgHEL B cells transferred into sHEL hosts, but not WT hosts, exhibit massive downregulation of surface IgMa, assuming an “anergic”-like phenotype (Fig 3B). In the absence of self-antigen, adoptively transferred “naïve” IgHEL B cells survived comparably to WT competitors and this was not altered by Nur77 (Fig 3B). However, after transfer into sHEL hosts, “naïve” IgHEL B cells were rapidly lost, and this loss was partially rescued by deletion of Nur77 (Fig 3B, C). Concomitantly, surface IgMa expression remained higher on residual IgHEL B cells transferred into sHEL hosts in the absence of Nur77 (Fig 3D). Taken together, our data suggests that Nur77 contributes to antigen-induced cell death of autoreactive B cells in vitro and in vivo.
Figure 3. Nur77 limits survival of “naïve” B cells chronically exposed to their cognate antigen in vivo.
A. Schematic of experimental set up. Splenocytes from IgHEL Tg and WT CD45.1 mice were loaded with the vital dye Cell Trace Violet (CTV) and combined 1:1. 5×106 total splenocytes were adoptively co-transferred into either WT or sHEL Tg hosts via I.V. injection. 3 days after adoptive transfer, mice were sacrificed, and spleens were harvested for surface marker analysis via flow cytometry. Donor cells were identified as CTV+, and IgHEL Tg and WT CD45.1 B cells were separated based on CD45.1 and CD45.2 staining. B. Representative plots shown. C. Ratio of transferred IgHEL Tg cells compared to competitively transferred CD45.1 WT cells normalized to the input ratio. N=3 per group. D. IgMa MFI of IgHEL Tg B cells recovered from adoptively transferred hosts. N=3 per group.
DNA-reactive reporter B cells upregulate Nur77-eGFP early during their development
The IgHEL / sHEL model of B cell autoreactivity is extremely powerful because expression of model self-antigen can be manipulated genetically. However, because this model system tracks a monoclonal repertoire of B cells and incorporates a very high affinity, extremely abundant, and non-physiological neo-self-antigen, it fails to capture several important tolerance mechanisms. We therefore took advantage of the VH3H9 model system in which DNA-specific B cells develop within a polyclonal repertoire, and can be tracked by surface staining for specific light chains. VH3H9 is a heavy chain (HC) originally cloned from the MRL/lpr mouse model of lupus that biases the entire BCR repertoire towards DNA-reactivity, most strongly when paired with endogenous λ1 light chain (41–43). This heavy chain has been introduced into the endogenous BCR locus to generate site-directed (sd) VH3H9 Tg mice in which the majority of B cells early in development express the VH3H9 HC paired with endogenous LCs, and subsequently can undergo isotype switching and somatic hypermutation (28). Although it has been shown the secondary rearrangement of the HC in this sd model can occur, anti-idiotype analysis confirms that the vast majority of splenic B cells express the VH3H9 HC on the C57BL/6 background (28, 42, 44). Small numbers of B cells expressing dsDNA-reactive Vλ1 BCRs persist in the periphery in sd-VH3H9 Tg mice despite counter-selection (42, 45, 46). Residual DNA-reactive B cells in this model are deeply anergic and sd-VH3H9 mice do not secrete significant titers of dsDNA Ab (42, 44, 47–49). However, on susceptible backgrounds where tolerance is broken, autoantibodies can develop (42, 44, 50).
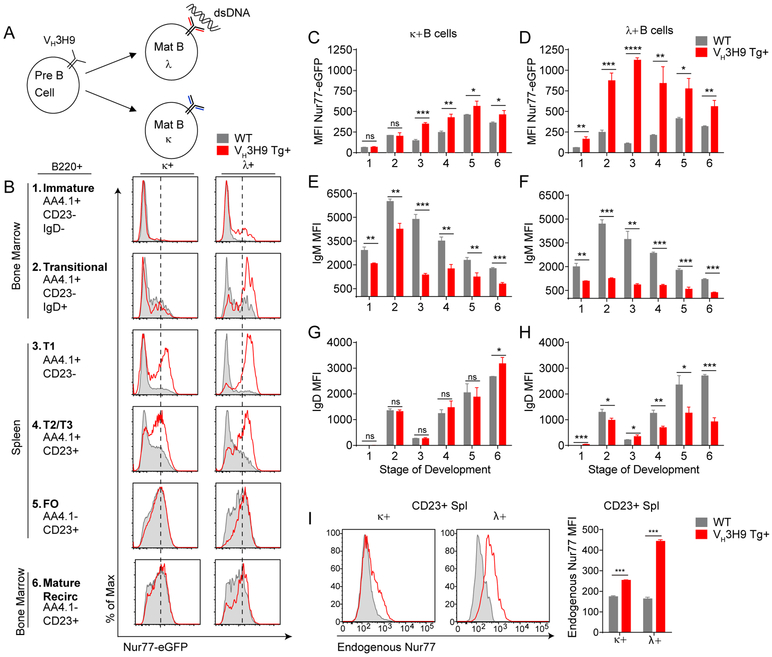
We generated Nur77-eGFP reporter mice harboring the site-directed (sd) VH3H9 Tg (Fig 4A, B). Vλ B cells (the majority of which express Vλ1 and are highly self-reactive) are the products of vigorous receptor editing during bone marrow development in sd-VH3H9 Tg mice, and exhibit marked downregulation of the IgM and IgD BCRs (41, 42, 44–46). Despite this BCR downregulation, we found that Vλ B cells from these mice turned on Nur77-eGFP much earlier in development, and expressed higher levels in maturity relative to either Vκ B cells from sd-VH3H9 Tg mice or WT B cells from non-Tg reporter mice (Fig 4B–H, Supplemental Fig 2A). These data are consistent with chronic, intense endogenous antigen stimulation of dsDNA-reactive Vλ1 B cells throughout their development, and moreover demonstrate that Nur77-eGFP expression correlates with self-reactivity in a polyclonal repertoire.
Figure 4. DNA-reactive reporter B cells upregulate Nur77-eGFP early during their development in VH3H9 Tg mice.
A. Schematic of relative autoreactivity amongst different light chain pairings in VH3H9 Tg B cells. B. Histograms represent Nur77-eGFP expression across B cell development in Vκ or Vλ B cells in either WT Nur77-eGFP or VH3H9 Tg Nur77-eGFP mice. Histograms are representative of at least 3 separate experiments. C-D. Quantification of Nur77-eGFP from histograms from Fig 4B. N=3 for each group. E-H. Quantification of IgM or IgD MFI across B cell development from Vκ or Vλ expressing B cells in either WT or VH3H9 Tg Nur77-eGFP mice. N=3 for each group. I. (Left) Histograms represent endogenous Nur77 protein expression in Vκ or Vλ expressing B cells from either WT or VH3H9 Tg mice. Histograms are representative of 3 different mice. (Right) Quantification of endogenous Nur77 MFI from histograms.
Nur77-eGFP reporter expression parallels endogenous Nr4a1 transcript upregulation in IgHEL / sHEL self-reactive B cells (Fig 1D). We wanted to confirm that reporter expression across the polyclonal repertoire of sd-VH3H9 Tg mice is similarly faithful. We assessed endogenous Nur77 expression by flow cytometric detection of intra-cellular protein, and confirmed high endogenous Nur77 protein expression in dsDNA-reactive VH3H9-Vλ B cells relative to both VH3H9 -Vκ B cells and WT B cells (Fig 4I). Nr4a1 transcript was similarly higher in Vλ B cells relative to Vκ B cells in VH3H9 Tg mice, as was Nr4a3, while Nr4a2 transcript was undetectable (Supplemental Fig 2B). However, the dynamic range of Nur77-eGFP reporter expression was much broader than either endogenous protein or transcript.
Nur77-eGFP expression correlates with counter-selection of self-reactive B cells
Although Vλ B cells express much higher levels of Nur77-eGFP than Vκ B cells in sd-VH3H9 Tg mice, we nevertheless observed a broad range of Nur77-eGFP expression even among Vλ B cells. Since the B cell repertoire in VH3H9 Tg mice is polyclonal owing to use of endogenous light chains, we wanted to determine whether Nur77-eGFP expression corresponded to the degree of self-reactivity across this repertoire. To do so, we tracked specific sub-populations of B cells according to heavy and light chain expression and correlated Nur77-eGFP expression with engagement of various tolerance mechanisms.
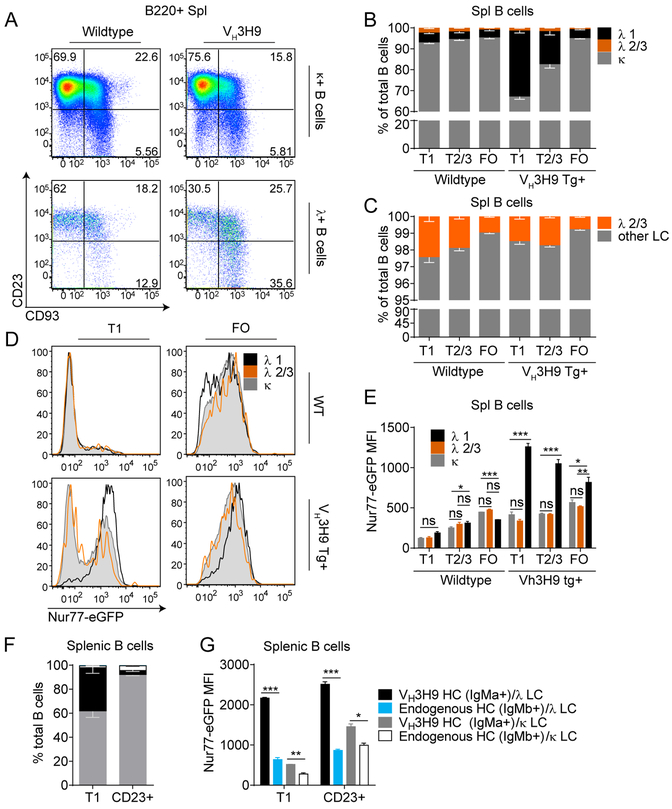
DNA-reactive Vλ1 B cells are markedly expanded in the immature B cell compartment and the splenic T1 compartment of VH3H9 Tg mice due to robust receptor editing (Fig 5A, B)(42, 45, 46). However, because of their self-reactivity, Vλ1 B cells in VH3H9 Tg mice exhibit extremely high turnover in vivo such that few “mature” CD23+ Vλ1 B cells are found under steady-state conditions, while less self-reactive Vκ B cells are progressively enriched as B cell maturation proceeds (Fig 5A, B) (45). Most remaining CD23+ Vλ1 B cells retain high surface expression of the immature/transitional B cell marker CD93 (Fig 5A, Supplemental Fig 2C)(42). Thus, Vλ1 B cells are efficiently counter-selected and progressively pruned from the developing B cell repertoire of sd-VH3H9 Tg mice (Fig 5A, B).
Figure 5. Nur77-eGFP expression correlates with counter-selection of self-reactive B cells in VH3H9 Tg mice.
A. Representative flow plots showing the relative sizes of developmental compartments containing Vκ or Vλ expressing splenic B cells in either WT or VH3H9 Tg Nur77-eGFP mice. Plots are representative of several experiments with at least N=3 mice per experiment. B. Splenic B cells from either WT or VH3H9 Tg mice were concurrently stained with an anti-pan Vλ and an anti-Vλ1 antibody to identify B cells expressing different Vλ light chains. B cells were also stained with surface markers to identify different developmental stages, and light chain usage across B cell development was charted. N=3 mice per group. % total Vλ1 p values: WT T1 vs Tg T1: ***; Tg T1 vs Tg T2/3: **; Tg T2/3 vs Tg FO: **. C. % total Vλ2/3 p values: Tg T1 vs Tg T2/3: ns; Tg 2/3 vs Tg FO: ***. D. Histograms represent Nur77-eGFP expression of B cells expressing various light chains in either WT or VH3H9 Tg Nur77-eGFP mice. E. Quantification of Nur77-eGFP MFI as shown in Fig 5D. N=3 mice per group. F. Splenic B cells were stained IgMb to identify B cells expressing the endogenous HC VH3H9 Tg mice. N=3 mice per group. % total VH3H9 Tg/ Vλ T1 vs CD23+ p value: ****; % total endogenous HC/ Vκ T1 vs CD23+ p value: **. G. Quantification of the Nur77-eGFP MFI of B cell subsets identified in F. N=3 mice per group.
Although the majority of Vλ+ B cells in sd-VH3H9 Tg mice express Vλ1, a small fraction express Vλ2 or 3 (see schematic of murine λ light chain recombination, Supplemental Fig 2D, E). By contrast to DNA-reactive Vλ1 B cells, these non-Vλ1 B cells (i.e. Vλ2+ and/or Vλ3+) are not vigorously counter-selected relative to the rest of the B cell repertoire and express lower levels of CD93 (Fig 5C, Supplemental Fig 2C). These observations suggest that Vλ1 B cells are much more self-reactive than both Vλ2/3 and Vκ B cells in sd-VH3H9 Tg mice. Consistent with these data, Vλ1 B cells from reporter sd-VH3H9 Tg mice indeed express much higher levels of Nur77-eGFP than Vλ2/3 and Vκ B cells during development (Fig 5D, E).
Interestingly, we observed a bimodal distribution of GFP expression among immature non-Vλ1 B cells (i.e. Vλ2+ and/or Vλ3+) in sd-VH3H9 Tg mice (Fig 5D). One explanation for this heterogeneity is that distinct Vλ LC paired with VH3H9 HC confer differing degrees of self-reactivity, and there is extensive literature to support this; mice harboring VH3H9 HC Tg paired with a Vλ2 LC Tg on a RAG−/− genetic background develop a monoclonal repertoire of B cells with dsDNA reactivity, an accelerated in vivo turnover rate, and impaired BCR signal transduction suggestive of anergy, implying that this LC confers significant autoreactivity (48, 49). By contrast, certain endogenous light chains (termed “editors”) have been previously reported to “veto” or limit DNA-reactivity when paired with the VH3H9 heavy chain, and included among these is Vλ3 (previously known as VλX)(51, 52). To determine whether immature “editor” Vλ3+ B cells indeed express less GFP as we might predict based on reduced DNA-reactivity, we sorted immature splenic T1 B cells from reporter sd-VH3H9 Tg mice on the basis of GFP expression, and subsequently cloned and sequenced λ light chains express by GFP-high and GFP-low cells. Because the combinatorial complexity of the murine lambda light chain repertoire is extremely restricted (see schematic of murine λ light chain recombination, Supplemental Fig 2D), we reasoned that Sanger sequencing would generate a sufficient number of sequences to resolve this question. Indeed, we found that the “editor” Vλ3 light chains were exclusively found in the GFP-low fraction, and confirmed that our data were not affected by primer bias (Supplemental Fig 3F, G).
Although most B cells in sd-VH3H9 Tg mice express the Tg heavy chain (identified in our study as IgHa and in prior work with an anti-idiotype Ab to exclude significant secondary V chain rearrangement), there are a small number of “escapee” B cells that instead express an endogenous heavy chain (IgHb) (Fig 5F, Supplemental Fig 2H) (44). We took advantage of this clonal diversity to explore how non-autoreactive IgHb B cells expressing endogenous HC compete with IgHa self-reactive B cells in a polyclonal repertoire, and to correlate this with Nur77-eGFP expression. Interestingly, although IgHb cells account for less than 3.5% of the immature T1 compartment, they are enriched in the more mature CD23+ B cell compartment (Fig 5F, Supplemental Fig 2H). Importantly, we confirm that non-autoreactive IgHb cells exhibit the lowest levels of Nur77-eGFP in the VH3H9 B cell repertoire (Fig 5G). We conclude that the Nur77-eGFP reporter can faithfully identify a range of self-reactivity in a polyclonal repertoire and that this corresponds to the extent of counter-selection of specific clones during B cell development.
Nur77-eGFP expression correlates with B cell anergy
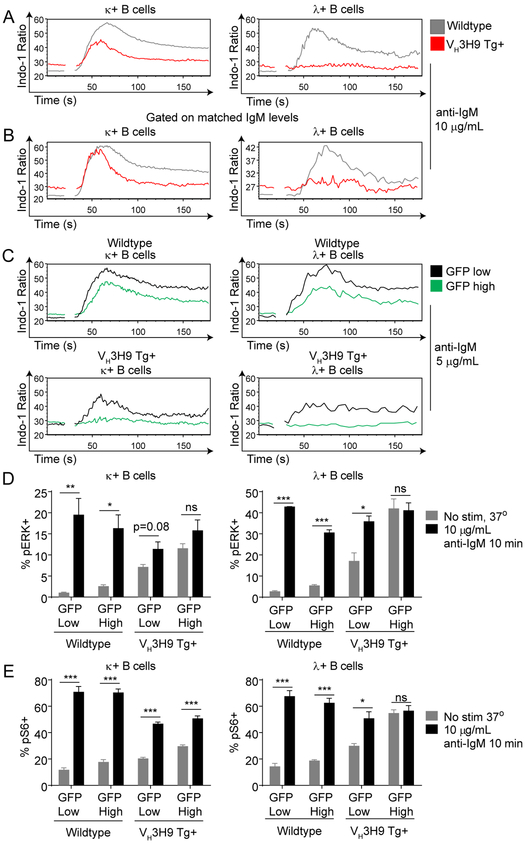
Despite triggering sequential tolerance mechanisms, including receptor editing and counter-selection, self-reactive B cells populate the periphery of sd-VH3H9 Tg mice (41, 42, 45, 46). Yet these cells remain quiescent, at least in part because of suppressed BCR signal transduction or “anergy” (47–49). Vκ B cells and Vλ B cells from sd-VH3H9 Tg mice have elevated levels of basal calcium relative to WT B cells, suggestive of chronic BCR occupancy by endogenous antigen (Fig 6A). Vκ B cells from these mice exhibit impaired intra-cellular calcium entry in response to BCR ligation, while VH3H9 Tg Vλ B cells are almost entirely refractory to BCR stimulation (Fig 6A)(47). Moreover, this is not merely attributable to downregulation of the BCR but rather reflects downstream re-wiring, as these defects are not corrected by gating on matched levels of surface IgM (Fig 6B, Supplemental Fig 3A). We wanted to determine whether there is heterogeneity in the degree of functional anergy among Vκ B cells and Vλ B cells from sd-VH3H9 Tg mice and whether this correlates with self-reactivity as marked by Nur77-eGFP. As previously observed in Nur77-eGFP reporter B cells with an unrestricted repertoire, we found that GFP-high B cells from sd-VH3H9 Tg mice exhibit impaired calcium entry in response to BCR ligation relative to GFP-low cells (Fig 6C) (22). Remarkably, by gating on GFP-low Vλ B cells, we can identify a small population that retains signaling capacity (Fig 6C). We presume that these cells may correspond to non-Vλ1 B cells based on Nur77-eGFP expression, and in particular may include the “editor” Vλ3 (51).
Figure 6. Nur77-eGFP expression correlates with B cell anergy in VH3H9 Tg mice.
Splenic B cells from WT and VH3H9 Tg Nur77-eGFP mice were loaded with Indo-1 dye. Ratiometric assessment of intracellular calcium was determined by flow cytometry. Samples were run for 30 seconds to assess basal calcium flux, and then anti-IgM was added to stimulate the cells. A. Spleens were harvested from WT or VH3H9 transgenic Nur77-eGFP mice, and intracellular calcium entry was assessed after stimulation with 10 μg/mL anti-IgM. Data is representative of at least 3 independent experiments. B. Intracellular calcium entry was assessed as in Fig 6A. A narrow IgM gate was drawn to assess intracellular calcium entry on cells with matched IgM levels. Data is representative of at least 3 independent experiments. C. Splenic B cells from WT or VH3H9 transgenic Nur77-eGFP mice were harvested, and intracellular calcium entry was assessed after stimulation with 5 μg/mL anti-IgM. The top and bottom 10% Nur77-eGFP expressing cells were gated as GFP-high and GFP low respectively. Data is representative of at least 3 independent experiments. D. Spleens from Nur77-eGFP VH3H9 Tg+ and Tg- mice were stimulated at 37° with 10 μg/mL IgM for 10 minutes. Phosphorylated ERK expression was determined via intracellular flow cytometry staining. N=5 for each group. E. Spleens from Nur77-eGFP VH3H9 Tg+ and Tg- mice were stimulated at 37° with 10 μg/mL anti-IgM for 45 minutes. Phosphorylated S6 expression was determined via intracellular flow cytometry staining. N=5 for each group
To extend these observations to other BCR-dependent signaling pathways, we assessed both basal and BCR-inducible phosphorylation of Erk and S6. We similarly observe elevated basal Erk and S6 phosphorylation in B cells from sd-VH3H9 Tg mice, which is particularly marked in Vλ B cells (Fig 6D, E). As seen with calcium entry, GFP-high Vλ B cells exhibit virtually no BCR-inducible signaling in response to BCR ligation, while GFP-low Vλ B cells retain some signaling capacity. Taken together, these data argue that Nur77-eGFP expression correlates with biochemical “anergy”, and suggests that the reporter indeed detects the degree of self-reactivity across a polyclonal BCR repertoire.
Nur77 is dispensable for receptor editing and central deletion of DNA-reactive B cells
We have shown that Nur77-eGFP reporter expression in self-reactive B cells correlates with the degree of chronic antigen stimulation and consequently with a series of tolerance mechanisms, including receptor editing, counter-selection, and anergy. We next hypothesized that Nur77 itself might play a role in restraining spurious immune responses to self-antigens by regulating specific tolerance mechanisms. To test this, we generated sd-VH3H9 Tg mice deficient for Nur77. We systematically probed the layered B cell tolerance mechanisms that are triggered by chronic stimulation of DNA-reactive B cells by endogenous antigen in these mice.
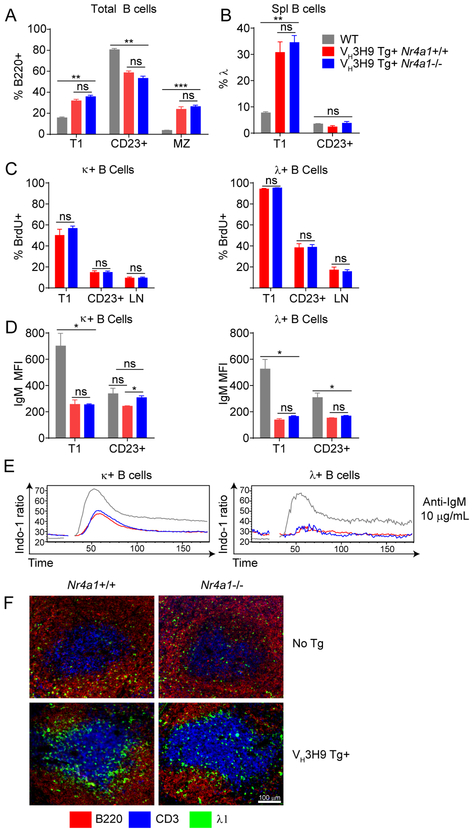
B cell development in mice with an unrestricted BCR repertoire is grossly unaffected by deletion of Nr4a1, with or without competition from Nr4a1-sufficient B cells (Supplemental Figs 3B, C). This is unaltered by introducing the non-self-reactive IgHEL BCR Tg in order to restrict and fix the BCR repertoire (Supplemental Fig 3D). Forcing expression of the self-reactive VH3H9 heavy chain skews B cell compartment size relative to WT mice, most notably by expanding the MZ compartment, but this distribution is not altered by expression of Nur77 (Fig 7A) (42, 45).
Figure 7. Nur77 is dispensable for editing, deletion, IgM downregulation and anergy in VH3H9 Tg mice.
A-B. Spleens were harvested from Nr4a1+/+ and Nr4a1−/− VH3H9 Tg mice as well as WT mice. Splenic developmental subsets and Vλ usage was determined through flow cytometry. N=3 for each group C. Non-transgenic and VH3H9 Tg mice were fed BrdU-laced water for 6 days. Spleens and lymph nodes were harvested for flow cytometry analysis. N=3 mice for each group. D. Spleens were harvested from WT, Nr4a1+/+ VH3H9 Tg and Nr4a1−/− VH3H9 Tg mice and stained for flow cytometry analysis. IgM and IgD MFI on Vκ and Vλ expressing B cells was quantified. N=3 for each group. E. Splenic B cells from WT, Nr4a1+/+ and Nr4a1−/− mice were loaded with Indo-1 dye. Ratiometric assessment of intracellular calcium was determined by flow cytometry following stimulation with 10 μg/mL anti-IgM. Plots are representative of 3 independent experiments. F. Representative spleen sections depicting localization of Vλ1 B cells in Nr4a1+/+ or Nr4a1−/− mice with or without the VH3H9 Tg. Data are representative of two independent mice of each of four conditions.
Since lambda light chains represent a terminal editing option and are used relatively infrequently in WT C57BL/6 mice, their high frequency among immature VH3H9 Tg B cells reflects robust receptor editing triggered by the highly autoreactive heavy chain (Fig 5B) (45, 46). Since Nur77-eGFP expression is upregulated early during bone marrow development in DNA-reactive VH3H9 Tg B cells that have edited to express Vλ (Figs 4B–D), we first asked whether Nur77 played a role in this process. We detected no significant differences in light chain editing between Nr4a1+/+ and Nr4a1−/− sd-VH3H9 Tg mice as reflected by Vλ light chain usage among immature T1 splenic B cells emerging from the bone marrow, suggesting that Nur77 expression is dispensable for this process (Figs 7B).
Since Nur77 has been shown to play an important role in the negative selection of self-reactive thymocytes, we hypothesized that it might play an analogous role in B cells (17, 53). In the sd-VH3H9 system, highly autoreactive Vλ1-expressing B cells are largely excluded from the more mature CD23+ B cell compartment and this was not rescued by deletion of Nr4a1 (Fig 7B) (42, 45). Indeed, Vλ1 B cells in VH3H9 Tg mice have a very short half-life in vivo as measured by BrdU incorporation (45). We further asked whether Nur77 regulates the steady-state turnover of immature and mature Vλ1 B cells in VH3H9 Tg mice. To do so in a sensitive manner, we treated VH3H9 Tg mice sufficient or deficient for Nur77 with BrdU water for 6 days. This strategy efficiently labels developing B cells undergoing a proliferative burst at the pre-B stage of development in the bone marrow. Since developing and naive B cells do not divide after the pre-B stage, this labeling strategy is commonly used to assess in vivo turnover of developing and mature resting B cell populations (54). BrdU incorporation is much higher in Vλ B cells than in Vκ B cells in VH3H9 Tg mice, indicating higher turnover and short half-life of highly autoreactive Vλ B cells (Fig 7C). In both B cell populations, BrdU incorporation is higher among immature (T1) CD23- B cells than more mature CD23+ B cells, consistent with a relatively short transit time through the T1 stage of development (Fig 7C). However, we see no difference in turnover of Vλ or Vκ B cells in the absence of Nur77, suggesting that counter-selection is intact and Nur77 is not required to mediate antigen-induced deletion of immature B cells during B cell development (Fig 7C).
Nur77 is dispensable for IgM downregulation, anergy, and follicular exclusion of DNA-reactive B cells
Since Nur77-eGFP reporter expression marks functional heterogeneity across the mature B cell repertoire in both WT and sd-VH3H9 Tg mice (Fig 6), we next sought to determine whether Nur77 regulates B cell anergy. As in the IgHEL / sHEL model system, IgM downregulation was largely unaffected by Nur77-deficiency (Fig 7D). Moreover, elevated basal calcium and dampened inducible calcium signaling in either Vλ or Vκ B cells from sd-VH3H9 Tg mice relative to non-Tg WT B cells were also unaffected by Nur77 expression (Fig 7E). Similar to the IgHEL / sHEL model, highly self-reactive VH3H9 Tg Vλ1 B cells localize to the T/B interface (45). We compared localization of Vλ1 B cells in VH3H9 Tg and WT mice either expressing or lacking Nur77. We observed enrichment of VH3H9 Tg Vλ1 B cells at the T/B boundary, and showed that this was independent of Nur77 (Fig 7F).
Nur77 limits survival of DNA-reactive mature B cells in vitro
Although Nur77 expression is dispensable for “central” deletion of immature DNA-reactive B cells in VH3H9 Tg mice (Fig 7B, C), the molecular pathways governing central deletion of B cells and peripheral turnover of self-reactive B cells may differ. Indeed, BAFF expression regulates the latter, but not the former, while Bim is implicated in both processes, albeit to a different extent (4, 5, 50, 55–57). Moreover, we found that Nur77 upregulation in peripheral CD23+ IgHEL Tg B cells exposed to chronic cognate antigen stimulation serves to limit their survival in vitro and in vivo (Fig 2). Therefore, we sought to determine whether Nur77 might play an analogous role in regulating the survival of mature VH3H9 Tg B cells. Since the vast majority of mature (CD23+) B cells in VH3H9 Tg mice express Vκ LC, our initial analysis focused on this modestly self-reactive B cell population. Similar to in vitro survival assays that we performed with “anergic” IgHEL/sHEL B cells, we assessed in vitro survival of VH3H9 Tg B cells relative to co-cultured congenically-marked WT B cells, either with or without addition of exogenous BAFF. As seen with self-reactive IgHEL / sHEL B cells (Figs 2A–D), VH3H9 Tg B cells survive poorly relative to WT and this is rescued with supplemental BAFF (Figs 8A, B). However, Nur77 is dispensable for this cell loss (Fig 8A). We reasoned that this might be due to a more modest degree of self-reactivity, and consequently lower Nur77 expression in VH3H9 Tg Vκ B cells (Fig 4B–D). Indeed, upon exogenous BCR stimulation of cultured B cells to further upregulate Nur77 expression, we saw a dramatic and significant survival advantage for Nr4a1−/− VH3H9 Tg B (Fig 8C). Importantly, the selective advantage enjoyed by VH3H9 Vκ B cells lacking Nur77 was almost entirely eliminated by BAFF supplementation, arguing that this competitive advantage was largely due to enhanced survival rather than enhanced proliferation (Fig 8D). By contrast, elimination of VH3H9 Tg Vλ B cells was extremely rapid with or without exogenous BCR stimulation, and was not rescued by loss of Nur77 (Supplemental Figs 3E–H).
Figure 8. Nur77 limits survival of DNA-reactive mature B cells in vitro and in vivo, and maintains tolerance in VH3H9 Tg mice.
A-D. Lymph nodes were harvested from Nr4a1+/+ and Nr4a1−/− VH3H9 Tg mice, and mixed 1:1 with lymph nodes from WT CD45.1 mice. 5×106 total cells were incubated for up to 3 days with either media alone or 10 μg/mL anti-IgM stim, in the absence or presence of 20 ng/mL BAFF. The ratio of VH3H9 Tg B cells to WT CD45.1 B cells was calculated and normalized to the input ratio at Day 0. N=3 for each group E-F. Mixed bone marrow chimeras were generated by reconstituting lethally irradiated IgHa WT mice with either Nr4a1−/− or Nr4a1+/+ CD45.2 bone marrow mixed 1:1 with WT CD45.1 bone marrow. 13 weeks after reconstitution, lymph nodes were harvested from the chimeras, and incubated with either media alone or 5 μg/mL anti-IgM for up to 75 hours. Donor cells were separated from host cells via expression of IgHa, and the ratio of CD45.2 to CD45.1 cells was calculated and normalized to the ratio of transferred bone marrow cells. N=5 for each group G. Spleens were harvested from either Nr4a1+/+ or Nr4a1−/− VH3H9 Tg mice, and total B cell numbers were determined. N ≥ 13 for each group. H. Vλ usage was determined in Nr4a1+/+ and Nr4a1−/− VH3H9 Tg mice via flow cytometry. N ≥ 3 for each group. I-J. Serum was collected from 14-week Nr4a1+/+ and Nr4a1−/− VH3H9 Tg mice via tail bleed, and both total IgG titer and relative anti-dsDNA IgG titer was determined via ELISA. N ≥ 10 for each group.
Nur77 mediates antigen-induced cell death in a B cell-intrinsic manner
Since Nr4a1−/− mice and radiation chimeras reconstituted with Nr4a1−/− bone marrow lack Nur77 expression in multiple hematopoietic lineages, we next wanted to determine whether Nur77 regulates antigen-induced cell death in a B cell-intrinsic manner. To do so, we took two independent approaches. We first generated two distinct sets of chimeras; irradiated hosts were reconstituted with Nr4a1+/+ CD45.1+ bone marrow mixed 1:1 either with CD45.2+ Nr4a1−/− or CD45.2+ Nr4a1+/+ bone marrow. We assessed relative in vitro survival of B cells harvested from these mixed chimeras. Neither Nur77-sufficient nor Nur77-deficient CD45.2+ B cells exhibited any competitive advantage relative to CD45.1+ B cells in the absence of BCR stimulation (Fig 8E). However, Nr4a1−/− CD45.2+ B cells (but not Nr4a1+/+ CD45.2+ controls) markedly outcompeted CD45.1+ B cells in cultures stimulated through the BCR (Fig 8F). Since Nr4a1−/− CD45.2+ B cells develop in a common milieu in vivo with competitor Nr4a1+/+ CD45.1+ B cells harvested from the same host, yet exhibit a survival advantage in co-culture, we can conclude that Nur77 mediates antigen-induced B cell death in a cell-intrinsic manner, rather than indirectly by regulating a cell-extrinsic B cell survival factor. Secondly, we performed analogous assays with an independent genetic model in which Nur77 is conditionally deletion exclusively in B cells (Mb1 Cre Nr4a1fl/fl) to further support this conclusion (Supplemental Figs 3I, J).
Nur77 limits survival of DNA-reactive mature B cells in vivo
We next wanted to determine whether the survival advantage conferred by Nur77-deficiency in vitro resulted in any change in the homeostasis of self-reactive VH3H9 Tg B cells in vivo. Strikingly, although we had seen no change in the relative size of B cell compartments in Nr4a1−/− and Nr4a1+/+ VH3H9 Tg mice (Fig 7A), we observed a significant increase in the total number of VH3H9 Tg B cells in the absence of Nur77 (Fig 8G). Importantly, this increase required a profoundly self-reactive B cell repertoire, as we observed no effect of Nur77 expression on B cell number in mice with an unrestricted B cell repertoire (Fig 8G). These data argue that Nur77 preferentially restrains the survival of self-reactive B cells in vivo.
One predicted consequence of this model would be a shift in the BCR repertoire of Nur77-deficient mice over time to include more self-reactive clones. To assess this, we examined the prevalence of mature Vλ B cells in sd-VH3H9 Tg mice at different ages. As shown earlier (Fig 7B), loss of Nur77 did not rescue the robust counter-selection of these DNA-reactive cells in young VH3H9 Tg mice (Fig 8H). However, we observed that these highly self-reactive B cells were progressively lost in VH3H9 Tg mice over time – consistent with their relatively short half-life in the periphery (Fig 7C) – and that this loss was partially ameliorated by deletion of Nr4a1 (Fig 8H). These data argue collectively that Nur77 regulates the survival of self-reactive B cells and consequently helps to shape the mature B cell repertoire.
Nur77 maintains B cell tolerance in VH3H9 Tg mice
DNA-reactive B cells are actively pruned from the mature B cell repertoire in sd-VH3H9 Tg mice, and those that persist are restrained by BCR downregulation and suppression of BCR signal transduction (Figs 5–7) (42, 45). As a result, IgG dsDNA autoantibodies do not develop at appreciable levels in these mice on the C57BL/6 genetic background (42, 44). Since we have shown that Nur77 functions to limit the survival and persistence of self-reactive B cells in the peripheral repertoire, we wanted to determine whether this had a functional consequence for immune tolerance. Total IgG Ab titers were comparable in in VH3H9 Tg mice expressing or lacking Nur77 (Fig 8I). However, we detected early accumulation of dsDNA IgG autoantibodies in the absence of Nur77 (Fig 8J). This suggests that Nur77 plays a critical role in maintaining B cell tolerance in the setting of a self-reactive BCR repertoire.
DISCUSSION
Here we sought to define the expression and function of the orphan nuclear hormone receptor Nur77 in self-reactive B cells. Nur77 is induced by antigen receptor stimulation both in vitro and in vivo (22, 25). As a result, endogenous Nr4a1 transcript, endogenous Nur77 protein, and Nur77-eGFP reporter expression are all upregulated in self-reactive B cells exposed to chronic stimulation by endogenous antigens. Owing to its long half-life relative to endogenous Nur77, the Nur77-eGFP reporter is particularly sensitive to endogenous antigen stimulation, and consequently spans a broad dynamic range across the B cell repertoire. Nur77-eGFP expression is particularly high in B cells with reactivity to high affinity (or avidity) endogenous antigens, such as HEL-specific B cells exposed to soluble HEL and VH3H9 HC / Vλ1 LC DNA-reactive B cells. By contrast, more modestly self-reactive VH3H9 HC / Vκ LC B cells express lower reporter levels while non-self-reactive “naïve” IgHEL B cells express very little GFP, suggesting that Nur77-eGFP expression scales with the degree of self-reactivity. Indeed, among polyclonal populations of B cells, Nur77-eGFP expression correlates with engagement of tolerance mechanisms such as editing, counter-selection, IgM downregulation and anergy. These data suggest that the reporter may serve as a useful tool in the study of B cell tolerance, and that endogenous Nur77 expression might be exploited to identify and perhaps target self-reactive B cells in patients.
Since Nur77 is upregulated by chronic antigen stimulation, we sought to explore its function in self-reactive B cells. To this end, we systematically probed central and peripheral B cell-intrinsic tolerance mechanisms in the presence or absence of Nur77 in two distinct murine models of B cell self-reactivity. We showed that Nur77 was dispensable for receptor editing, central deletion, IgM downregulation, follicular exclusion, and anergy. However, in surprising contrast to central deletion of immature B cells, we identified a selective role for Nur77 in regulating the survival of self-reactive mature B cells in vitro and in vivo in both model systems. Because the Nr4a family play roles in multiple immune cell populations, we took advantage of mixed cultures, adoptive transfer models, competitive bone marrow chimeras, and conditional deletion of Nr4a1 in B cells in order to establish that Nur77 acts in a cell-intrinsic manner to restrict the survival of mature self-reactive B cells (Figs 2, 3, 8A–F). However, we cannot exclude an independent role for Nur77 in other cell lineages in the maintenance of tolerance in VH3H9 Tg mice in vivo.
It remains to be formally established whether other Nr4a family members play redundant roles in self-reactive B cells. Nr4a family members are co-expressed and exhibit redundant biological functions in both myeloid and Treg cells (12, 13, 58). Indeed, thymic negative selection and development of regulatory T cells is largely intact in Nr4a1−/− mice (13, 30). By contrast, expression of a dominant negative construct of Nur77 impairs negative selection of thymocytes (17). Similarly, T cell-specific deletion of multiple Nr4a family members leads to complete loss of the regulatory T cell lineage, and germline deletion of both Nr4a1 and 3 results in acute myeloid leukemia in a gene dose-dependent manner (12, 13, 58). We fail to detect Nr4a2 transcript in self-reactive B cells, but observe compensatory upregulation of Nr4a3 in Nr4a1-deficient B cells (Fig 1D). Although published RNAseq datasets suggest that Nr4a1 is considerably more abundant than Nr4a3 in B cells (21), and we find that Nr4a3 is not markedly enriched in anergic relative to naïve IgHEL B cells (Fig 1C), we cannot exclude the possibility that expression of Nor1 (Nr4a3) partially compensates for the loss of Nur77, thereby obscuring additional functions of the Nr4a family in B cell tolerance.
Nr4a gene products were first shown to play a role in mediating antigen-induced cell death in T cell hybridomas in vitro via misexpression of either full length or truncated dominant negative constructs (15, 16). Although a role for both Nur77 and Nor1 in mediating negative selection of thymocytes in vivo was subsequently revealed using analogous approaches (17, 18), Nr4a family members have not yet been shown to regulate antigen-induced cell death of mature primary T cells. Indeed, although Nur77 was shown to restrain TCR-induced proliferation of primary CD4 and CD8 T cells, a role in antigen-induced cell death was not identified (59–61). Although such a role remains possible, and may yet be unmasked by more sensitive assays and/or deletion of redundant family members, its physiologic significance remains uncertain. By contrast, we fail to identify a role for Nur77 in central deletion of immature B cells, but show that it limits survival of self-reactive mature B cells by mediating antigen-induced cell death under conditions of limiting BAFF.
Although Nur77 and family members have well-defined DNA-binding motifs and function as transcription factors, differential gene expression analysis has failed to identify compelling transcriptional targets that account for their role in thymic negative selection (53, 62). Rather, it has been shown by several labs that Nur77 can drive apoptosis by translocating from the nucleus to mitochondria, directly associating with Bcl-2 family members and inducing a conformation change that exposes their BH3 domains, thereby converting these pro-survival factors into “killers” (19, 63, 64). Moreover, Nur77 can do so independent of its DNA binding activity, and the interaction domains of Nur77 and Bcl-2 have been mapped in vitro (19, 20). It has been suggested that this is the mechanism by which Nur77 mediates thymic negative selection in vivo (64), and it is possible that an analogous mechanism may be relevant in self-reactive B cells.
Work in humans and mice has identified at least two distinct developmental stages at which self-reactive B cells can be eliminated from the repertoire in response to auto-antigen engagement (1–3). It has been argued that one specialized function of central tolerance is to eliminate BCRs that recognize certain damage-associated molecular patterns (or DAMPs), such as nucleic acids, precisely because such antigens can coopt endosomal TLR signaling to break tolerance in the periphery. Indeed, DNA-reactive human B cells are efficiently pruned from the repertoire at an early checkpoint (1). Moreover, genetic evidence in mice has established a role for TLR-dependent signaling, together with antigen-dependent BCR signaling, in central tolerance of B cells that recognize nucleic acids (47, 65). It is possible that BCR and TLR signaling may also synergize to drive the extremely high level of Nur77-eGFP reporter expression that we observe in immature VH3H9 HC / Vλ1 LC DNA-reactive B cells. Nevertheless, TLR stimulation is not required for Nur77 induction nor for Nur77 function in the IgHEL model, as the neo-self-antigen HEL does not contain TLR ligands.
Not only the purpose, but also the molecular mechanisms that mediate central and peripheral deletion of self-reactive B cells diverge. These two tolerance checkpoints are sensitive to differences in self-antigen avidity. IgHEL B cells exposed to membrane-associated cognate antigen HEL, and 3–83 Tg B cells exposed to cell-surface H-2Kk are deleted early during bone marrow development, and their survival can be rescued by overexpression of either Bcl2 or Bcl-XL (66–71). By contrast, IgHEL B cells exposed to soluble rather than membrane-bound self-antigen bypass this early checkpoint, and persist in the periphery in a state of functional unresponsiveness (29, 33–35, 69). However, they exhibit a very high turnover rate in vivo, and have an extremely short half-life (approximately 12 hours) when placed in competition with non-self-reactive B cells (39, 40). Such self-reactive B cells are highly dependent upon BAFF for their survival; under conditions of limiting BAFF, as in a replete polyclonal B cell repertoire, highly self-reactive B cells are rapidly eliminated, while an excess supply of BAFF rescues their survival (4, 5, 50, 55). By contrast, BAFF supply is not thought to modulate central deletion of immature self-reactive B cells, because its receptor is not upregulated until later in development. Chronic antigen stimulation of self-reactive B cells upregulates expression of the BH3-only family member Bim, and this may account, in part, for their increased BAFF-dependence (4). Deletion of Bim rescues survival of self-reactive IgHEL B cells exposed to soluble HEL, but has a much more modest impact on central deletion triggered by membrane-associated self-antigen (56, 57). How does Nur77 intersect with these mechanisms?
Since Nur77 is thought to promote apoptosis at least in part by binding to Bcl2 or Bcl-B, but not to Bcl-XL (19, 20, 63), the relative abundance of individual Bcl2 family members may control the sensitivity of immature and mature B cell subsets to Nur77. Bcl-XL is preferentially expressed in, and required for survival of, immature B cells, while mature B cells upregulate Bcl2A1 genes and are shown to be dependent upon Bcl2 and Mcl1 for their survival (72). It is possible that downregulation of Bcl-XL and upregulation of other Bcl2 family members renders mature B cells uniquely sensitive to Nur77-mediated cell death.
We show that Nur77 couples chronic antigen stimulation to reduced survival of mature (but not immature) self-reactive B cells under conditions of limiting BAFF supply, and suggest that this may contribute to their increased dependence upon BAFF supply. Irrespective of Nur77 expression, an excess supply of BAFF is sufficient to rescue survival of such self-reactive B cells. This may not be a unique feature of BAFF; other co-stimulatory signals capable of supporting B cell survival, such as IL-4 or CD40L, may similarly rescue survival of self-reactive B cells. We propose that Nur77 may function broadly to restrict the survival of self-reactive B cells that receive ‘signal 1’ via chronic self-antigen stimulation in the absence of ‘signal 2’.
B cells in the periphery have a reduced half-life that seems to scale in proportion to self-reactivity and inversely with BAFF supply. Why are mildly self-reactive B cells retained in the periphery rather than efficiently deleted? It has been proposed that they may serve of as a reservoir of protective specificities that support host defense by filling “holes” in the repertoire that might otherwise be exploited by pathogens. Indeed, this is thought to be the case for HIV; it has been demonstrated that certain bNAbs to HIV and their germline precursors are highly self-reactive and trigger tolerance, while, conversely, impaired tolerance can facilitate generation of bNabs (73, 74). Moreover, it has been shown that self-reactive B cells can be “redeemed” and may contribute to protective immunity after mutating away from self-reactivity (75–77). However, preserving self-reactive B cells in the mature repertoire must be balanced against the risk of generating autoantibodies. Since Nur77 expression scales with antigen stimulation, we propose that it couples self-reactivity to B cell half-life and thus gradually shapes the mature B cell repertoire in order to balance the demands of host defense against the risk of autoimmunity.
Not only does Nur77 represent a useful marker of B cell autoreactivity in vivo, but it may represent a novel therapeutic target in B cell-mediated autoimmune disease. Recent work from Sanz and colleagues demonstrated that unmutated germline BCRs from the naïve B cell compartment are recruited directly into the circulating plasmablast pool during lupus disease flares, and are thought to contribute to dsDNA autoantibodies detected in the serum of patients at times of high disease activity (78). Selective depletion of self-reactive mature B cells may effectively restrain this process, especially as it is thought that such autoantibodies are secreted by short-lived plasma cells (79). Indeed, this forms part of the rationale for use of BAFF-depletion as a therapeutic strategy to treat lupus. By analogy, agonist ligands that enhance Nur77 function or selectively modulate its Bcl2-binding interaction could subtly prune self-reactive B cells from the peripheral repertoire while largely preserving normal humoral immunity, and may therefore have a future role in the management of B cell-mediated autoimmune diseases.
Supplementary Material
KEY POINTS.
Nur77 expression correlates with the degree of autoreactivity of individual B cells
Nur77 is dispensible for receptor editing, central deletion, and anergy
Nur77 restrains the survival of mature self-reactive B cells
Acknowledgements
We thank Al Roque for help with mouse husbandry. We thank Art Weiss and Tony DeFranco for feedback and suggestions.
Funding:
NIAID 5T32AI007334–28 (CT), HHMI Medical Research Fellows program (JH), NSF Graduate Research Fellowship 1650113 (MN), NIAMS R01AR069520 (JZ), NIAMS K08AR059723 (JZ), Rheumatology Research Foundation (JZ)
References:
- 1.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, and Nussenzweig MC. 2003. Predominant autoantibody production by early human B cell precursors. Science 301: 1374–1377. [DOI] [PubMed] [Google Scholar]
- 2.Nemazee D 2017. Mechanisms of central tolerance for B cells. Nat Rev Immunol 17: 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cambier JC, Gauld SB, Merrell KT, and Vilen BJ. 2007. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat Rev Immunol 7: 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu H-B, and Cyster JG. 2004. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity 20: 441–453. [DOI] [PubMed] [Google Scholar]
- 5.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, and Brink R. 2004. Excess BAFF Rescues Self-Reactive B Cells from Peripheral Deletion and Allows Them to Enter Forbidden Follicular and Marginal Zone Niches. Immunity 20: 785–798. [DOI] [PubMed] [Google Scholar]
- 6.Winoto A, and Littman DR. 2002. Nuclear hormone receptors in T lymphocytes. Cell 109 Suppl: S57–66. [DOI] [PubMed] [Google Scholar]
- 7.Mittelstadt PR, and DeFranco a. L.. 1993. Induction of early response genes by cross-linking membrane Ig on B lymphocytes. Journal of immunology (Baltimore, Md. : 1950) 150: 4822–4832. [PubMed] [Google Scholar]
- 8.Hazel TG, Nathans D, and Lau LF. 1988. A gene inducible by serum growth factors encodes a member of the steroid and thyroid hormone receptor superfamily. Proceedings of the National Academy of Sciences 85: 8444–8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milbrandt J 1988. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron 1: 183–188. [DOI] [PubMed] [Google Scholar]
- 10.Baker KD, Shewchuk LM, Kozlova T, Makishima M, Hassell A, Wisely B, Caravella JA, Lambert MH, Reinking JL, Krause H, Thummel CS, Willson TM, and Mangelsdorf DJ. 2003. The Drosophila orphan nuclear receptor DHR38 mediates an atypical ecdysteroid signaling pathway. Cell 113: 731–742. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Benoit G, Liu J, Prasad S, Aarnisalo P, Liu X, Xu H, Walker NPC, and Perlmann T. 2003. Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature 423: 555–560. [DOI] [PubMed] [Google Scholar]
- 12.Mullican SE, Zhang S, Konopleva M, Ruvolo V, Andreeff M, Milbrandt J, and Conneely OM. 2007. Abrogation of nuclear receptors Nr4a3 andNr4a1 leads to development of acute myeloid leukemia. Nat Med 13: 730–735. [DOI] [PubMed] [Google Scholar]
- 13.Sekiya T, Kashiwagi I, Yoshida R, Fukaya T, Morita R, Kimura A, Ichinose H, Metzger D, Chambon P, and Yoshimura A. 2013. Nr4a receptors are essential for thymic regulatory T cell development and immune homeostasis. Nature Immunology 14: 230–237. [DOI] [PubMed] [Google Scholar]
- 14.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, and Hedrick CC. 2011. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C− monocytes. Nature Immunology 12: 778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu ZG, Smith SW, McLaughlin KA, Schwartz LM, and Osborne BA. 1994. Apoptotic signals delivered through the T-cell receptor of a T-cell hybrid require the immediate-early gene nur77. Nature 367: 281–284. [DOI] [PubMed] [Google Scholar]
- 16.Woronicz JD, Calnan B, Ngo V, and Winoto a.. 1994. Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature 367: 277–281. [DOI] [PubMed] [Google Scholar]
- 17.Calnan BJ, Szychowski S, Ka-Ming Chan F, Cado D, and Winoto A. 1995. A role for the orphan steroid receptor Nur77 in apoptosis accompanying antigen-induced negative selection. Immunity 3: 273–282. [DOI] [PubMed] [Google Scholar]
- 18.Cheng LE, Chan FK, Cado D, and Winoto A. 1997. Functional redundancy of the Nur77 and Nor-1 orphan steroid receptors in T-cell apoptosis. The EMBO Journal 16: 1865–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin B, Kolluri SK, Lin F, Liu W, Han Y-H, Cao X, Dawson MI, Reed JC, and Zhang X.-k.. 2004. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell 116: 527–540. [DOI] [PubMed] [Google Scholar]
- 20.Banta KL, Wang X, Das P, and Winoto A. 2018. B cell lymphoma 2 (Bcl-2) residues essential for Bcl-2’s apoptosis-inducing interaction with Nur77/Nor-1 orphan steroid receptors. J. Biol. Chem 293: 4724–4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fowler T, Garruss AS, Ghosh A, De S, Becker KG, Wood WH, Weirauch MT, Smale ST, Aronow B, Sen R, and Roy AL. 2015. Divergence of transcriptional landscape occurs early in B cell activation. Epigenetics Chromatin 8: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zikherman J, Parameswaran R, and Weiss A. 2012. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature 489: 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Au-Yeung BB, Smith GA, Mueller JL, Heyn CS, Jaszczak RG, Weiss A, and Zikherman J. 2017. IL-2 Modulates the TCR Signaling Threshold for CD8 but Not CD4 T Cell Proliferation on a Single-Cell Level. J. Immunol 198: 2445–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Au-Yeung BB, Zikherman J, Mueller JL, Ashouri JF, Matloubian M, Cheng DA, Chen Y, Shokat KM, and Weiss A. 2014. A sharp T-cell antigen receptor signaling threshold for T-cell proliferation. Proceedings of the National Academy of Sciences 111: E3679–E3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mueller J, Matloubian M, and Zikherman J. 2015. Cutting edge: An in vivo reporter reveals active B cell receptor signaling in the germinal center. J. Immunol 194: 2993–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noviski M, Mueller JL, Satterthwaite A, Garrett-Sinha LA, Brombacher F, and Zikherman J. 2018. IgM and IgD B cell receptors differentially respond to endogenous antigens and control B cell fate. eLife 7: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huizar J, Tan C, Noviski M, Mueller JL, and Zikherman J. 2017. Nur77 Is Upregulated in B-1a Cells by Chronic Self-Antigen Stimulation and Limits Generation of Natural IgM Plasma Cells. Immunohorizons 1: 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen C, Nagy Z, Prak EL, and Weigert M. 1995. Immunoglobulin heavy chain gene replacement: a mechanism of receptor editing. Immunity 3: 747–755. [DOI] [PubMed] [Google Scholar]
- 29.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink R. a., Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, and Raphael K. 1988. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature 334: 676–682. [DOI] [PubMed] [Google Scholar]
- 30.Lee SL, Wesselschmidt RL, Linette GP, Kanagawa O, Russell JH, and Milbrandt J. 1995. Unimpaired thymic and peripheral T cell death in mice lacking the nuclear receptor NGFI-B (Nur77). Science (New York, N.Y.) 269: 532–535. [DOI] [PubMed] [Google Scholar]
- 31.Hobeika E, Thiemann S, Storch B, Jumaa H, Nielsen PJ, Pelanda R, and Reth M. 2006. Testing gene function early in the B cell lineage in mb1-cre mice. Proc Natl Acad Sci U S A 103: 13789–13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiller T, Busse CE, and Wardemann H. 2009. Cloning and expression of murine Ig genes from single B cells. Journal of Immunological Methods 350: 183–193. [DOI] [PubMed] [Google Scholar]
- 33.Cooke MP, Heath AW, Shokat KM, Zeng Y, Finkelman FD, Linsley PS, Howard M, and Goodnow CC. 1994. Immunoglobulin signal transduction guides the specificity of B cell-T cell interactions and is blocked in tolerant self-reactive B cells. The Journal of experimental medicine 179: 425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodnow CC, Crosbie J, Jorgensen H, Brink RA, and Basten A. 1989. Induction of self-tolerance in mature peripheral B lymphocytes. Nature 342: 385–391. [DOI] [PubMed] [Google Scholar]
- 35.Adams E, Basten A, and Goodnow CC. 1990. Intrinsic B-cell hyporesponsiveness accounts for self-tolerance in lysozyme/anti-lysozyme double-transgenic mice. Proceedings of the National Academy of Sciences of the United States of America 87: 5687–5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabouri Z, Perotti S, Spierings E, Humburg P, Yabas M, Bergmann H, Horikawa K, Roots C, Lambe S, Young C, Andrews TD, Field M, Enders A, Reed JH, and Goodnow CC. 2016. IgD attenuates the IgM-induced anergy response in transitional and mature B cells. Nat Commun 7: 13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merrell KT, Benschop RJ, Gauld SB, Aviszus K, Decote-Ricardo D, Wysocki LJ, and Cambier JC. 2006. Identification of Anergic B Cells within a Wild-Type Repertoire. Immunity 25: 953–962. [DOI] [PubMed] [Google Scholar]
- 38.Cyster JG, and Goodnow CC. 1995. Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral B cell fate. Immunity 3: 691–701. [DOI] [PubMed] [Google Scholar]
- 39.Cyster JG, Hartley SB, and Goodnow CC. 1994. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature 371: 389–395. [DOI] [PubMed] [Google Scholar]
- 40.Fulcher DA, and Basten A. 1994. Reduced life span of anergic self-reactive B cells in a double-transgenic model. J Exp Med 179: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erikson J, Radic MZ, Camper SA, Hardy RR, Carmack C, and Weigert M. 1991. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature 349: 331–334. [DOI] [PubMed] [Google Scholar]
- 42.Fields ML, Hondowicz BD, Wharton GN, Adair BS, Metzgar MH, Alexander ST, Caton AJ, and Erikson J. 2005. The regulation and activation of lupus-associated B cells. Immunological reviews 204: 165–183. [DOI] [PubMed] [Google Scholar]
- 43.Radic MZ, Mascelli MA, Erikson J, Shan H, and Weigert M. 1991. Ig H and L chain contributions to autoimmune specificities. J Immunol 146: 176–182. [PubMed] [Google Scholar]
- 44.Sekiguchi DR, Jainandunsing SM, Fields ML, Maldonado MA, Madaio MP, Erikson J, Weigert M, and Eisenberg RA. 2002. Chronic graft-versus-host in Ig knockin transgenic mice abrogates B cell tolerance in anti-double-stranded DNA B cells. J Immunol 168: 4142–4153. [DOI] [PubMed] [Google Scholar]
- 45.Mandik-Nayak L, Bui A, Noorchashm H, Eaton A, and Erikson J. 1997. Regulation of anti-double-stranded DNA B cells in nonautoimmune mice: localization to the T-B interface of the splenic follicle. The Journal of experimental medicine 186: 1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gay D, Saunders T, Camper S, and Weigert M. 1993. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med 177: 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nickerson KM, Christensen SR, Cullen JL, Meng W, Luning Prak ET, and Shlomchik MJ. 2013. TLR9 Promotes Tolerance by Restricting Survival of Anergic Anti-DNA B Cells, Yet Is Also Required for Their Activation. The Journal of Immunology 190: 1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noorchashm H, Bui A, Li HL, Eaton A, Mandik-Nayak L, Sokol C, Potts KM, Pure E, and Erikson J. 1999. Characterization of anergic anti-DNA B cells: B cell anergy is a T cell-independent and potentially reversible process. Int Immunol 11: 765–776. [DOI] [PubMed] [Google Scholar]
- 49.Roark JH, Bui A, Nguyen KA, Mandik L, and Erikson J. 1997. Persistence of functionally compromised anti-double-stranded DNA B cells in the periphery of non-autoimmune mice. Int Immunol 9: 1615–1626. [DOI] [PubMed] [Google Scholar]
- 50.Ota M, Duong BH, Torkamani A, Doyle CM, Gavin AL, Ota T, and Nemazee D. 2010. Regulation of the B Cell Receptor Repertoire and Self-Reactivity by BAFF. The Journal of Immunology 185: 4128–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y, Louzoun Y, and Weigert M. 2004. Editing anti-DNA B cells by Vlambdax. J Exp Med 199: 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen C, Radic MZ, Erikson J, Camper SA, Litwin S, Hardy RR, and Weigert M. 1994. Deletion and editing of B cells that express antibodies to DNA. The Journal of Immunology 152: 1970–1982. [PubMed] [Google Scholar]
- 53.Fassett MS, Jiang W, D’Alise a. M., Mathis D, and Benoist C. 2012. Nuclear receptor Nr4a1 modulates both regulatory T-cell (Treg) differentiation and clonal deletion. Proceedings of the National Academy of Sciences 109: 3891–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allman DM, Ferguson SE, Lentz VM, and Cancro MP. 1993. Peripheral B cell maturation. II. Heat-stable antigen(hi) splenic B cells are an immature developmental intermediate in the production of long-lived marrow-derived B cells. The Journal of Immunology 151: 4431–4444. [PubMed] [Google Scholar]
- 55.Hondowicz BD, Alexander ST, Quinn WJ, Pagán AJ, Metzgar MH, Cancro MP, and Erikson J. 2007. The role of BLyS/BLyS receptors in anti-chromatin B cell regulation. International Immunology 19: 465–475. [DOI] [PubMed] [Google Scholar]
- 56.Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, and Strasser A. 2003. Loss of the Pro-Apoptotic BH3-only Bcl-2 Family Member Bim Inhibits BCR Stimulation–induced Apoptosis and Deletion of Autoreactive B Cells. J Exp Med 198: 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oliver PM, Vass T, Kappler J, and Marrack P. 2006. Loss of the proapoptotic protein, Bim, breaks B cell anergy. J Exp Med 203: 731–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sekiya T, Kondo T, Shichita T, Morita R, Ichinose H, and Yoshimura A. 2015. Suppression of Th2 and Tfh immune reactions by Nr4a receptors in mature T reg cells. J Exp Med 212: 1623–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nowyhed HN, Huynh TR, Thomas GD, Blatchley A, and Hedrick CC. 2015. Cutting Edge: The Orphan Nuclear Receptor Nr4a1 Regulates CD8 +T Cell Expansion and Effector Function through Direct Repression of Irf4. The Journal of Immunology 195: 3515–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liebmann M, Hucke S, Koch K, Eschborn M, Ghelman J, Chasan AI, Glander S, Schädlich M, Kuhlencord M, Daber NM, Eveslage M, Beyer M, Dietrich M, Albrecht P, Stoll M, Busch KB, Wiendl H, Roth J, Kuhlmann T, and Klotz L. 2018. Nur77 serves as a molecular brake of the metabolic switch during T cell activation to restrict autoimmunity. Proc. Natl. Acad. Sci. U.S.A 4: 201721049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Myers DR, Lau T, Markegard E, Lim HW, Kasler H, Zhu M, Barczak A, Huizar JP, Zikherman J, Erle DJ, Zhang W, Verdin E, and Roose JP. 2017. Tonic LAT-HDAC7 Signals Sustain Nur77 and Irf4 Expression to Tune Naive CD4 T Cells. Cell Rep 19: 1558–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajpal A, Cho YA, Yelent B, Koza-Taylor PH, Li D, Chen E, Whang M, Kang C, Turi TG, and Winoto A. 2003. Transcriptional activation of known and novel apoptotic pathways by Nur77 orphan steroid receptor. The EMBO Journal 22: 6526–6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luciano F, Krajewska M, Ortiz-Rubio P, Krajewski S, Zhai D, Faustin B, Bruey J-M, Bailly-Maitre B, Lichtenstein A, Kolluri SK, Satterthwait AC, Zhang X.-k., and Reed JC. 2007. Nur77 converts phenotype of Bcl-B, an antiapoptotic protein expressed in plasma cells and myeloma. Blood 109: 3849–3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thompson J, and Winoto A. 2008. During negative selection, Nur77 family proteins translocate to mitochondria where they associate with Bcl-2 and expose its proapoptotic BH3 domain. J Exp Med 205: 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kuraoka M, Snowden PB, Nojima T, Verkoczy L, Haynes BF, Kitamura D, and Kelsoe G. 2017. BCR and Endosomal TLR Signals Synergize to Increase AID Expression and Establish Central B Cell Tolerance. Cell Rep 18: 1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fang W, Weintraub BC, Dunlap B, Garside P, Pape KA, Jenkins MK, Goodnow CC, Mueller DL, and Behrens TW. 1998. Self-reactive B lymphocytes overexpressing Bcl-xL escape negative selection and are tolerized by clonal anergy and receptor editing. Immunity 9: 35–45. [DOI] [PubMed] [Google Scholar]
- 67.Hartley SB, Cooke MP, Fulcher DA, Harris AW, Cory S, Basten A, and Goodnow CC. 1993. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell 72: 325–335. [DOI] [PubMed] [Google Scholar]
- 68.Lang J, Arnold B, Hammerling G, Harris AW, Korsmeyer S, Russell D, Strasser A, and Nemazee D. 1997. Enforced Bcl-2 expression inhibits antigen-mediated clonal elimination of peripheral B cells in an antigen dose-dependent manner and promotes receptor editing in autoreactive, immature B cells. J Exp Med 186: 1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, and Goodnow CC. 1991. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature 353: 765–769. [DOI] [PubMed] [Google Scholar]
- 70.Nemazee D, and Buerki K. 1989. Clonal deletion of autoreactive B lymphocytes in bone marrow chimeras. Proceedings of the National Academy of Sciences 86: 8039–8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nemazee DA, and Bürki K. 1989. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature 337: 562–566. [DOI] [PubMed] [Google Scholar]
- 72.Vikström IB, Slomp A, Carrington EM, Moesbergen LM, Chang C, Kelly GL, Glaser SP, Jansen JHM, Leusen JHW, Strasser A, Huang DCS, Lew AM, Peperzak V, and Tarlinton DM. 2016. MCL-1 is required throughout B-cell development and its loss sensitizes specific B-cell subsets to inhibition of BCL-2 or BCL-XL. Cell Death Dis 7: e2345–e2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schroeder KMS, Agazio A, Strauch PJ, Jones ST, Thompson SB, Harper MS, Pelanda R, Santiago ML, and Torres RM. 2017. Breaching peripheral tolerance promotes the production of HIV-1–neutralizing antibodies. J Exp Med 214: 2283–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Verkoczy L, Alt FW, and Tian M. 2017. Human Ig knockin mice to study the development and regulation of HIV-1 broadly neutralizing antibodies. Immunological Reviews 275: 89–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burnett DL, Langley DB, Schofield P, Hermes JR, Chan TD, Jackson J, Bourne K, Reed JH, Patterson K, Porebski BT, Brink R, Christ D, and Goodnow CC. 2018. Germinal center antibody mutation trajectories are determined by rapid self/foreign discrimination. Science 360: 223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reed JH, Jackson J, Christ D, and Goodnow CC. 2016. Clonal redemption of autoantibodies by somatic hypermutation away from self-reactivity during human immunization. J Exp Med 213: 1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sabouri Z, Schofield P, Horikawa K, Spierings E, Kipling D, Randall KL, Langley D, Roome B, Vazquez-Lombardi R, Rouet R, Hermes J, Chan TD, BRINK R, Dunn-Walters DK, Christ D, and Goodnow CC. 2014. Redemption of autoantibodies on anergic B cells by variable-region glycosylation and mutation away from self-reactivity. Proceedings of the National Academy of Sciences 111: E2567–E2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tipton CM, Fucile CF, Darce J, Chida A, Ichikawa T, Gregoretti I, Schieferl S, Hom J, Jenks S, Feldman RJ, Mehr R, Wei C, Lee FE, Cheung WC, Rosenberg AF, and Sanz I. 2015. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nature Immunology 16: 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hale M, Rawlings DJ, and Jackson SW. 2018. The long and the short of it: insights into the cellular source of autoantibodies as revealed by B cell depletion therapy. Current Opinion in Immunology 55: 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.