Capsule Summary
Gamma tocopherol may be beneficial as a primary or complementary therapeutic agent in conditions with neutrophilic airway inflammation. Its effects are not modified by BMI or GSTM1 genotype, allowing for its use in a broader population.
Keywords: gamma tocopherol, endotoxin, LPS, asthma, BMI, obesity, GSTM1, airway inflammation
To the Editor:
In an era of personalized and precision medicine, efficacy data of therapeutic agents targeting airway inflammation are increasingly taking into account effect modifiers to optimize treatment strategies. Neutrophilic airway inflammation is often less responsive to corticosteroid treatment(1), and there is a great unmet need for non-steroidal therapies to target this specific type of inflammation. Obesity is a well-known risk factor for specific asthma phenotypes and negatively affects asthma severity and control, partially as a result of increased airway neutrophilic inflammation(2). Likewise, gene deletion polymorphisms of glutathione-S-transferase Mu1 (GSTM1), a vital antioxidant enzyme, increase the neutrophilic inflammatory response to a variety of air pollutants(3), including ozone and inhaled lipopolysaccharide (LPS), a major component of ambient air particulate matter. Asthmatic children with the GSTM1 null polymorphism experienced reduced ozone-induced decrements in FEF25-75% when supplemented with antioxidant vitamin C and vitamin E (alpha-tocopherol)(4).
Our group has identified gamma tocopherol (γT), the dietary isoform of Vitamin E with unique anti-inflammatory and anti-oxidant properties(5), as a safe and inexpensive therapy that can mitigate neutrophilic airway inflammation. We previously reported that γT supplementation attenuated airway neutrophil recruitment following inhaled LPS challenge in both healthy volunteers and mild asthmatics(6, 7). In this report, we sought to determine if BMI and/or GSTM1 genotype modify the effect of γT supplementation on mitigating LPS-induced airway neutrophilic inflammation.
Eighteen healthy volunteers (HV) and 23 mild asthmatic volunteers (AV), not on daily inhaled controller therapies, were enrolled in two randomized, double-blind, placebo-controlled crossover trials in which each subject received 1200 mg daily of a γT-enriched supplement or safflower oil tablet (placebo) for 7 (HV) or 14 (AV) consecutive days. This was followed by an inhaled LPS challenge using 20,000 endotoxin units of Clinical Center Reference Endotoxin provided by the National Institutes of Health Clinical Center(6, 7). Sputum samples were obtained following γT (or placebo) supplementation but prior to LPS challenge (post-treatment) and again 6 hours after inhaled LPS challenge (post-challenge). The change in LPS-induced sputum neutrophilia (PMNs) following γT treatment (and placebo) in each population was calculated as follows: post-challenge % sputum PMNs – post-treatment % sputum PMNs. The nonparametric Wilcoxon Rank Sum Test, with crossover design taken into account, was used to compare the effect of each treatment on LPS-induced airway neutrophil recruitment in the total population consisting of HV and AV. Linear mixed effects models were then used to assess the presence of effect modification by categorical BMI (normal weight <25, or overweight >25) and GSTM1 genotype (null or sufficient) while adjusting for effects of treatment sequence, treatment, and period. Criterion for significance was taken to be p<.05 with corresponding 95% confidence intervals (CIs) provided.
Demographic data for both HV and AV included in this post hoc analysis are presented in Table 1. During the placebo period of each study, there was no effect modification by categorical BMI [p=0.67 (−14.25, 9.12)] or GSTM1 genotype [p=0.33 (−18.25, 5.95)] on sputum PMN recruitment following inhaled LPS challenge using simple linear regression (data not shown). This indicates that the airway inflammatory response to LPS (without any treatment) was not affected by either BMI or GSTM1 genotype.
Table 1.
Demographic Characteristics
| Healthy Volunteers (N=13) |
Mild Intermittent Asthmatics (N=18) |
|
|---|---|---|
| Age (years), median (range) | 23 (20-48) | 25.5 (20-47) |
| Sex (Female/Male) | 8/5 | 14/4 |
| Race | 10 Caucasian 2 African-American 1 Asian |
11 Caucasian 4 African-American 2 Asian 1 Native-American |
| BMI (kg/m2), median (range) | 22.5 (20.4-34.3) | 26.3 (20.2-41.7) |
| Categorical BMI (<25/≥25) | 9/4 | 8/10 |
| GSTM1 Genotype (Null/Sufficient) | 5/5; 3 unknown | 8/9; 1 unknown |
| Atopic, N (%) | 1 (8%) | 13 (72%) |
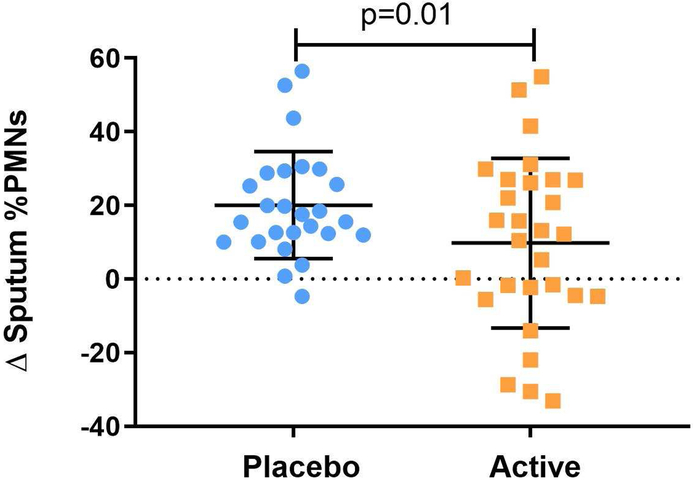
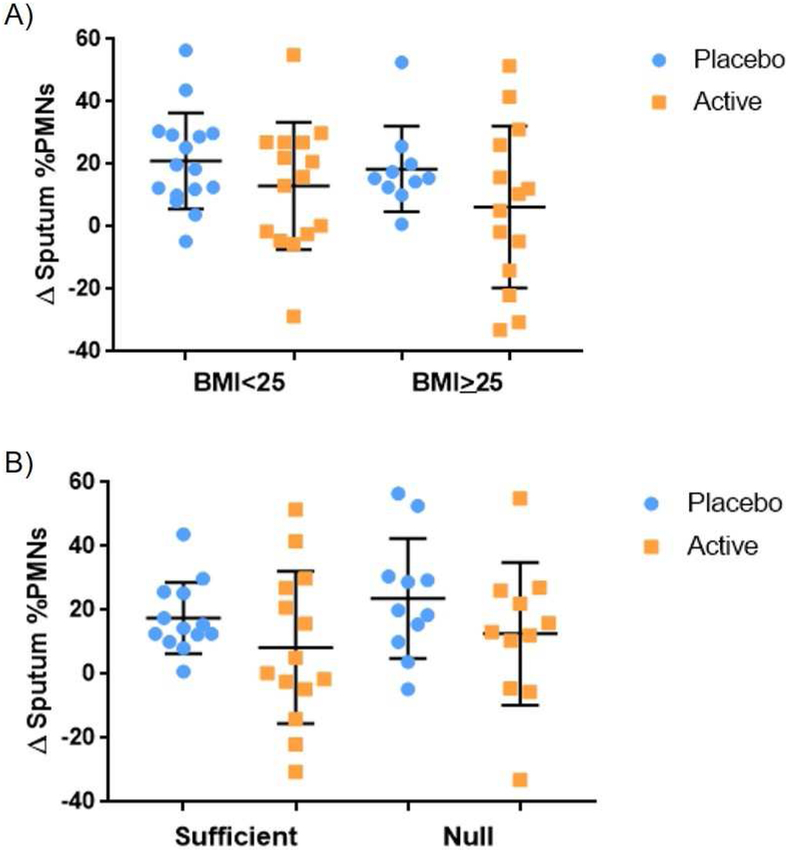
Active treatment with γT attenuated sputum PMN recruitment following inhaled LPS challenge in both HV and AV (Figure 1)(6, 7). Adjusted linear mixed effects models for the crossover study design detected no effect modification by categorical BMI (<25 or ≥25) [p=0.60 (−14.24, 8.13); Figure 2a] or GSTM1 genotype [p=0.40 (−16.02, 6.20); Figure 2b]. In addition, adjusted linear mixed effects models including categorical BMI, GSTM1 genotype, and presence of asthma as independent variables yielded similar results [p=0.44 (−17.43, 7.46), 0.51 (−15.49, 7.57), 0.69 (−14.40, 9.49), respectively]. These analyses indicate that the effect of γT on LPS-induced airway neutrophilic inflammation in HV and AV is not modified by BMI or GSTM1 genotype.
Figure 1:
Effect of γT treatment on LPS-induced sputum neutrophilia in both healthy volunteers and mild allergic asthmatics using nonparametric Wilcoxon Rank Sum Test.
Figure 2:
Effect of γT treatment on LPS-induced sputum neutrophilia stratified by categorical BMI (<25: normal vs ≥25: overweight) (a) and GSTM1 genotype (null vs sufficient) (b).
To our knowledge, this is the first report that examines whether two known risk factors for asthma and airway inflammation, increased BMI and GSTM1 null genotype, modify the effect of γT supplementation on reduction of LPS-induced neutrophilic airway inflammation. Contrary to our original hypotheses, these factors did not influence either the response to inhaled LPS or the protective effect of γT. Though Alexis et al. previously reported a linear correlation between airway PMN response to inhaled LPS and BMI(8), stratification by specific BMI categories, which is clinically more applicable, was not performed, thus not allowing for direct comparison of results. Additionally, Dillon and colleagues(3) noted comparable increases in sputum %PMNs in GSTM1 null and sufficient healthy volunteers similar to our data. As previous studies have shown that GSTM1 null asthmatic children experience less ozone-induced reductions in lung function with increased antioxidant consumption(4), it is possible that the mitigation of neutrophilic airway inflammation by γT is primarily mediated through its anti-inflammatory rather than its antioxidant properties.
We acknowledge a few limitations to our analyses. Overall, our sample sizes for both HV and AV are small. While combining the two populations for our analyses resulted in normal distribution of data, larger sample sizes in each individual population may uncover effects not seen in our analyses and allow for stratification of additional baseline characteristics, such as atopy. Both populations were predominantly female and Caucasian, limiting the generalizability of our results. Finally, we chose to stratify our results by a BMI of <25 or ≥25 rather than a cutoff of ≥30 that identifies obesity as few subjects in our studies met the definition of obesity. Future work should focus on the efficacy of therapeutics in obese individuals with airway inflammation.
As precision and personalized medicine becomes more relevant with improved diagnostic and therapeutic tactics, identifying the characteristics of populations most likely to respond to various treatments is increasingly important. Given that 70% of adults in the United States are overweight or obese(9), identifying therapies that can target neutrophilic inflammation in this population is especially salient. Our analyses suggest that γT may be beneficial as a primary or complementary treatment in predominantly-neutrophilic airway diseases, and that this benefit exists regardless of BMI or GSTM1 genotype, thus, allowing for its use in a broader population.
Acknowledgments
Sources of support: This work was supported by 5T32AI007062-39, 5T32GM086330, NHLBI R01 135235; NIEHS grants R01ES023349, K23-ES021745, P30ES010126; R01 ES025124 and EPA cooperative agreement CR 83578501.
Footnotes
Conflicts of Interest: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002;57(10):875–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Telenga ED, Tideman SW, Kerstjens HA, Hacken NH, Timens W, Postma DS, et al. Obesity in asthma: more neutrophilic inflammation as a possible explanation for a reduced treatment response. Allergy. 2012;67(8):1060–8. [DOI] [PubMed] [Google Scholar]
- 3.Dillon MA, Harris B, Hernandez ML, Zou B, Reed W, Bromberg PA, et al. Enhancement of systemic and sputum granulocyte response to inhaled endotoxin in people with the GSTM1 null genotype. Occupational and environmental medicine. 2011;68(10):783–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreno-Macias H, Romieu I. Effects of antioxidant supplements and nutrients on patients with asthma and allergies. J Allergy Clin Immunol. 2014;133(5):1237–44; quiz 45. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci U S A. 2000;97(21):11494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez ML, Wagner JG, Kala A, Mills K, Wells HB, Alexis NE, et al. Vitamin E, gamma-tocopherol, reduces airway neutrophil recruitment after inhaled endotoxin challenge in rats and in healthy volunteers. Free Radic Biol Med. 2013;60:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burbank AJ, Duran CG, Pan Y, Burns P, Jones S, Jiang Q, et al. Gamma tocopherol-enriched supplement reduces sputum eosinophilia and endotoxin-induced sputum neutrophilia in volunteers with asthma. The Journal of allergy and clinical immunology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexis NE, Peden DB. Inflammatory response of the airway to inhaled endotoxin correlates with body mass index in atopic patients with asthma but not in normal volunteers. The Journal of allergy and clinical immunology. 2006;117(5):1185–6. [DOI] [PubMed] [Google Scholar]
- 9.National Center for Health S. Health, United States. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville (MD): National Center for Health Statistics (US); 2017. [PubMed] [Google Scholar]