Abstract
Many neurodegenerations, including those of the visual system, have complex etiologies that include roles for both neurons and glia. In the retina there is evidence that retinal astrocytes play an important role in neurodegeneration. There are several approaches for isolating and growing primary retinal astrocytes, however, they often lead to different results. In this study, we examined the influence of culture conditions on phenotypic maturation of primary, purified retinal glia. We compared retinal astrocytes and Müller glia purified by immunomagnetic separation, as differentiation between these astrocyte subtypes is critical and immuno-based methods are the standard practice of purification. We found that while time in culture impacts the health and phenotype of both astrocytes and Müller glia, the phenotypic maturation of retinal astrocytes was most impacted by serum factors. These factors appeared to actively regulate intermediate filament phenotypes in a manner consistent with the induction of astrocyte-mesenchymal transition (AMT). This propensity for retinal astrocytes to shift along an AMT continuum should be considered when interpreting resulting data. Our goal is that this study will help standardize the field so that studies are replicable, comparable, and as accurate as possible for subsequent interpretation of findings.
Keywords: astrocytes, Müller glia, retina, astrocyte-mesenchymal transition, primary culture, intermediate filaments
1. Introduction:
Retinal astrocytes are glial cells derived from neuroepithelial cells that migrate into the retina during development (Tao and Zhang, 2014; Zuchero et al., 2015). These cells take on different morphologies and functional roles depending on their environment (Jäkel et al., 2017). They are important in retinal homeostasis, including establishment of the blood-retina barrier as well as ionic and metabolic homeostasis (Jäkel et al., 2017; Bellot-Saez et al., 2018; Song et al., 2018). There is also evidence that these cells may be major players in complex neurodegenerations, including glaucoma. Glaucoma is a leading cause of blindness world-wide and has a complex etiology. Migration and activation of astrocytes has been reported at early stages of disease progression in animal models (Son et al., 2010; Ramírez et al., 2010; Tezel et al., 2012; Lye-Barthel et al., 2013; Qu and Jakobs, 2013; Formichella et al., 2014). The altered function of these cells in disease versus healthy states is an area of active investigation.
However, it is difficult to ascertain the role of these cells in vivo due to the complexity of the tissue. Therefore, many scientists have developed methods to study these cells in vitro. Since the goal is to understand the way these cells respond in vivo, there is great interest in keeping the cells as close to their in vivo phenotype as possible. Therefore, purified, primary astroctyes are more attractive than glial cell lines. Although antigen-based methods of purification, either immunomagnetic separation or immunopanning, are the standard procedure for cell isolation, there is great diversity in protocols for the culture conditions after purification. This has lead to divergent results from individual laboratories.
The establishment of primary retinal astrocyte cultures is further complicated by the presence of Müller glia, a retina-specific radial glial cell. Radial glial cells are a subtype of astrocytes present mostly during development of the CNS, but that persist in adulthood in the retina and cerebellum (Zuchero et al., 2015; Bringmann et al., 2006; Chrobak et al., 2016). Each Müller glia cell spans the entire depth of the retina from outer to inner limiting membranes and orients parallel to neural circuits (Jeon et al., 1998). In contrast, astrocytes reside only in the nerve fiber layer, where they have a perpendicular orientation to retinal ganglion cell axons (Jeon et al., 1998). In vivo comparisons of Müller glia and astrocytes illustrate many similarities in protein expression, i.e. excitatory amino acid transporters and intermediate filaments, as well as response to injury, i.e. modulation of glial fibrillary acidic protein (GFAP) and hypertrophy (Ramírez et al., 2010; Bringmann et al., 2006, 2009; Tezel et al., 2009; Zamanian et al., 2012). However, the spatiotemporal characteristics of reactivity and the functional roles of these two glial subtypes are quite distinct in both health and disease (Bringmann et al., 2006, 2009; Zamanian et al., 2012; Fernández-Sánchez et al., 2015; Nicchia et al., 2016). Retinal astrocytes are vimentin-negative and GFAP-positive (Eisenfeld et al., 1984; Molnar et al., 1984). In contrast, Müller cells are GFAP-negative, vimentin-positive and express visual cycle proteins (Bringmann et al., 2006; Luna et al., 2010). In response to injury or disease, Müller cells induce nestin and GFAP expression and also increase vimentin expression (Eisenfeld et al., 1984; Molnar et al., 1984; Bringmann et al., 2006, 2009; Luna et al., 2010). In contrast, astrocytes either up-regulate or down-regulate GFAP, depending on the stressor, and do not induce nestin expression (Tezel et al., 2009; Ramírez et al., 2010; Son et al., 2010; Luna et al., 2010; Qu and Jakobs, 2013; Formichella et al., 2014). Thus, production and maintenance of primary astrocyte cultures from retina must differentiate between Müller glia and astrocytes. This is challenging given that Müller glia are the most abundant glial cell type in the retina (Jeon et al., 1998).
We have previously published methods for isolating Müller glia and retinal astrocytes as separate cell populations, using immunomagnetic separation (Sappington et al., 2006a, 2006b; Lee et al., 2015). This antigen-based method of cell separation has been utilized routinely by us and others for studies examining everything from cell signaling and behavior to high-throughput proteomic and genomic screens (Scheef et al., 2005; Ho et al., 2014; Hosoki et al., 2015; Reyes-Aguirre et al., 2018). The goal of this study was to characterize astrocyte and Müller cell phenotypes induced by previously published culture conditions, particularly those utilized for high-throughput screening. The hope is that this will lead to a standardized protocol for culturing and performing experiments with primary retinal astrocytes and Müller glia that exhibit mature phenotypes. A common protocol and confirmation of phenotype will improve the quality of data in the field and help move the field forward faster. Primary cultures are an important part of research, but careful characterization of the cells is critical to avoid incorrect data interpretation. In our study, we found that these cells never take on a mature astrocyte morphology, if always grown in serum without culture substrates. Further, we found that the cells must be maintained in culture for at least one week to re-induce expression of astrocyte-specific markers. Finally, we did not see any added benefit of culturing in the presence of growth factors.
2. Methods and Materials:
2.1. Animals:
Pregnant Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA). Pups were sacrificed at postnatal day 1 to 3 and retinas isolated for cell collection. Animals were housed in an ALAAC approved facility at Vanderbilt University and all procedures performed according to the Vanderbilt University IACUC approved protocol.
2.2. Müller and Astrocyte Isolation:
Müller cells and astrocytes were sequentially isolated from dissociated cells, using immunomagnetic separation, as previously described (Sappington et al., 2006a, 2006b; Lee et al., 2015). Sixteen eyes from postnatal day 1 to 3 Sprague-Dawley rats were enucleated and the retinas dissected and placed on ice in a 50 ml centrifuge tube containing Dulbecco’s Modified Eagle plus 5% glucose (DMEM/Glu) (Gibco, Carlsbad, CA). After centrifugation for 6 min (70 × g at 4°C), retinas were dissociated by pipetting with 4 ml Earle’s Balanced Salt Solution (Gibco, Carlsbad, CA) containing 1 mg/ml papain (Worthington, Lakewood, NJ) and 0.01% DNase I (Worthington) and incubated for 15 min at 37°C. The cell suspension was centrifuged for 8 min (250 × g at 4°C) and the supernatant discarded. A solution of mouse IgG1 anti-CD44 (Thermo Fisher Scientific, Waltham MA, #MA5–16909) was prepared at a dilution of 8 μl antibody/500 μl DMEM/Glu and added to the pellet. The suspension was mixed and incubated on ice for 10 minutes while shaking. The cell suspension was centrifuged for 8 minutes (250 × g at 4°C) and the pellet re-suspended with 80 μl DMEM/Glu. Anti-mouse IgG1 MicroBeads (Miltenyi Biotech, Auburn, CA; #130–047–102; 20 μl) were added and incubated on ice while shaking. After 15 min, 20 ml of DMEM/Glu was added and the suspension centrifuged for 8 min (250 × g at 4°C). The supernatant was discarded, replaced with 1 ml fresh DMEM/Glu, and the cell suspension loaded into a pre-equilibrated MS column (Miltenyi Biotec; #130–042–201) that was placed in a MACS MultiStand. The column flow-through, containing astrocytes, was placed on ice. The columns were washed three times with 500 μl DMEM/Glu and the washes discarded. The columns were removed from the magnetic stand and Müller cells eluted with 1 ml of growth media consisting of DMEM/F12 Medium (Gibco #10565–018) with 10% Fetal Bovine serum (FBS; Gibco # 26140079, 0.1% gentamycin (Corning, Corning, NY; # 30005CR) and 1% G5 supplement (Thermo Fisher Scientific; #17503012). Eight μl of mouse IgM anti-astrocyte (Leinco Technologies, St. Louis, MO, #A116) was added to the cell suspension from the column flow-through and placed on ice for 10 minutes while shaking. This IgM antibody recognizes a 200-kDa surface protein isolated from human brain astrocytes. Our previous characterization of this antibody in both postnatal and adult rat retina confirmed recognition of a 200kD protein in whole retina lysates (Sappington et al., 2006). Furthermore, immunolabeling with this antibody co-localizes with that from anti-GFAP antibodies in astrocytes of the nerve fiber layer (Sappington et al., 2006). The cell suspension was centrifuged and re-suspended in DMEM/Glu. Astrocytes were isolated with anti-mouse IgM MicroBeads (Miltenyi Biotech; #130–047–302; 20 μl) as described above for the Müller cells.
2.3. Cell Culture:
Müller cells or astrocytes were placed in 6-well cell culture plates coated with Poly-L-Lysine (Sigma, St. Louis, MO; # P4707) or Nunc Lab-Tek II CC2 chamber slides (Thermo Fisher Scientific) and maintained in growth media. Cells were grown in a standard incubator containing 5% CO2. For serum-free (SF) experiments, astrocytes were washed twice and incubated in SF medium for five or seven days. For the growth factor experiments, astrocytes were washed and incubated in SF medium for two days and then treated in the absence or presence of 20 ng/ml mouse leukemia inhibitory factor (LIF; R&D Systems, Inc., Minneapolis, MN) or 20 ng/ml rat ciliary neurotrophic factor (CNTF; Thermo Fisher Scientific) for five days. For recovery experiments, SF medium from cells incubated for five days was replaced with growth media. Primary astrocytes were used at passage four or earlier.
2.4. Luciferase Promoter Assay:
The activities of vimentin and GFAP promoters in transfected astrocytes were evaluated with pGL4.10 plasmids expressing Luc2 luciferase (Promega, Madison, WI) relative to a control plasmid with CMV driving expression of Renilla luciferase (Promega pGL4.75). A 1.1 kb region of the vimentin promoter was PCR-amplified from pEMS1228 (a gift from Elizabeth Simpson; Addgene plasmid # 29114) and the GFAP promoter amplified from pZac2.1 gfaABC1D-tdTomato (a gift from Baljit Khakh, Addgene plasmid # 44332). Sanger sequencing (Genewiz, South Plainfield, NJ) was used to confirm plasmid sequences. The empty pGL4.10 plasmid without a promoter was used as the negative control.
Primary astrocytes were placed in 96-well plates and grown overnight in growth media. Wells were washed with SF medium and plasmids transfected with Fugene HD (Promega, Madison, WI) at a ratio of 4 μl Fugene/1 μg of DNA in SF medium. The Luc2 and Renilla plasmids were mixed at a 1:1 ratio and 100 ng of total DNA was introduced per well. After 6 h, the SF medium was replaced with growth media and the cells grown for 2 days. Luciferase activity was quantified with the Dual-Glo luciferase assay system (Promega) read by a GloMax microplate luminometer (Promega). The data are presented as the ratio of Luc2 activity to Renilla activity from three independent experiments.
2.5. Immunocytochemistry:
Cells were fixed with HistoChoice Tissue Fixative solution (VWR, Radnor, PA) for 10 minutes at room temperature (RT), washed with TBS, and incubated overnight in blocking buffer consisting of TBS with 2% Ig-free BSA (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA; #001–000–161). Primary antibodies were diluted in 1%BSA/TBS and incubated for 2 h at RT. Cells were labeled with rabbit anti-vimentin (Cell Signaling Technology CST, Danvers, MA; 1:100), mouse anti-GFAP (CST, 1:300), rabbit anti-GFAP (Dako/Agilent, Santa Clara, CA; 1:1000), mouse anti-Nestin (CST, 1:300), or rabbit anti-PAX2 (BioLegend, San Diego, CA; 1:200). The neurofilament antibodies from CST included mouse anti-neurofilament-M (#2838,1:200), mouse anti-neurofilament-L (#2835,1:100), rabbit anti-neurofilament-L (#2837, 1:100), and mouse anti-neurofilament-H (#2836, 1:400). Cells were washed with TBS and incubated with secondary Alexa Fluor488 or Alexa Fluor594 antibodies (Thermo Fisher Scientific; 1:1000 in 1%BSA/TBS) for 1 hour at RT. After washing with PBS, DAPI (Thermo Fisher Scientific; 1:1000 in PBS) was added to the wells and incubated at RT for 15 min. Wells were washed with PBS and then water before mounting the slides. Images were captured using an Olympus fluorescent microscope.
2.6. Activation of the JAK/STAT pathway:
Primary astrocytes grown in chamber slides were washed and incubated with SF medium for 2 days. Medium was removed and replaced with SF medium in the absence or presence of 20 ng/ml mouse LIF (R&D Systems, Inc., Minneapolis, MN) for 15 min. Cells were fixed with cold methanol and processed for immunoreactive phospho-STAT (rabbit anti-pSTAT3; #9145, CST), according to the manufacturer’s recommendations. Cells were co-immunolabeled with mouse anti-α-tubulin (#3873, CST).
3. Results:
3.1. The astrocyte isolation protocol results in highly pure glial cultures but not an astrocyte phenotype
To confirm that the immunomagnetic isolation protocol produces highly purified cultures of retinal glial subtypes, we compared expression and localization of standard cell type-specific markers for neurons and glia in primary astrocyte and Müller glia cultures produced by this method (Sappington et al., 2006a, 2006b; Lee et al., 2015). Twenty-five astrocyte isolations were examined for immunoreactive GFAP and nine were also tested for the three neurofilaments. Eight of these nine astrocyte cultures were negative for the neuronal marker, neurofilament-light (NF-L). In that one preparation only 1.5% of the culture was positive with the rabbit NF-L antibody (Figure 1A), which was found to be more sensitive than the mouse NF-L and NF-M antibodies. In contrast, NF-H immunoreactivity was not detected in this preparation (data not shown). All cells were negative for GFAP (Figure 1A), but positive for vimentin (Figure 1A). Similarly, primary Müller glia were negative for NF-L (Figure 1B) and positive for vimentin (Figure 1B). Unlike astrocytes, Müller glia cultures were positive for GFAP (Figure 1B). These data validate the ability to isolate two distinct glial cell populations from retina using immunomagnetic separation. The lack of GFAP expression in the astrocyte cultures suggests that these cells may be phenotypically immature compared to their respective Müller cell cultures.
Figure 1. Primary astrocytes and Müller cells differentially express intermediate filaments.
A,B. Representative epifluorescence micrographs of primary retinal astrocytes (A) and Müller cells (B) labeled with DAPI (blue) and immunolabeled with antibodies against neurofilament (NF-L; left), the intermediate filaments GFAP (middle), and vimentin (right). Only a single astrocyte culture displayed a few NF-L-positive cells. No other astrocyte and Müller cell cultures contained NF-L-positive cells (A, B). Both astrocyte (A) and Müller cell (B) cultures were vimentin-positive, but only Müller cell (B) cultures were GFAP-positive. Scale bar = 100μm.
3.2. Primary astrocyte and Müller glia cultures exhibit similar developmental states
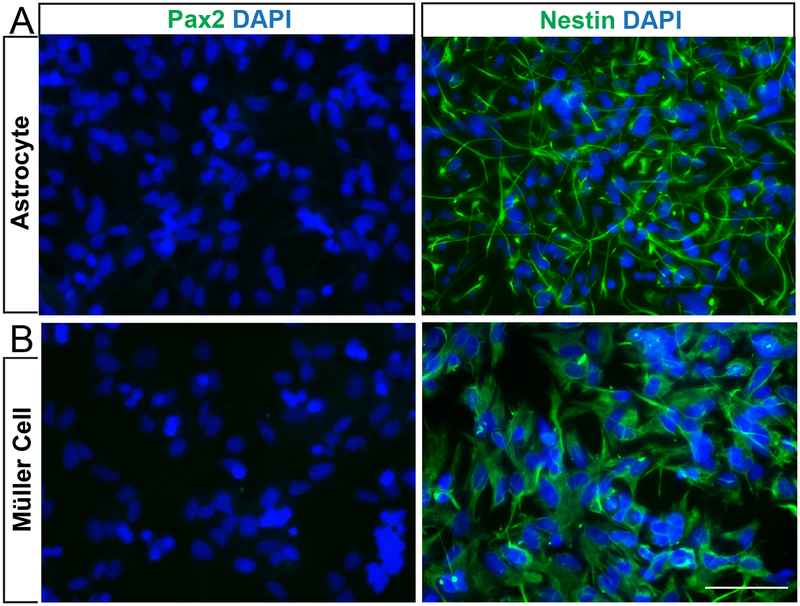
To determine whether the primary astrocyte and Müller glia cultures reverted to an immature phenotype we immunolabeled with markers of immature astrocytes, Pax2 and nestin (Mi and Barres, 1999; Tao and Zhang, 2014). Astrocyte precursor cells (APCs) in vitro are positive for Pax2 and nestin (Mi and Barres, 1999; Tao and Zhang, 2014). Further differentiation to an immature, perinatal astrocyte results in loss of Pax2 expression, but continued expression of nestin and GFAP (Mi and Barres, 1999; Tao and Zhang, 2014). Both glial subtypes were negative for Pax2 (Figure 2), but positive for nestin (Figure 2). This suggests that neither astrocytes nor Müller glia revert to full APC phenotype during cell isolation and purification.
Figure 2. Primary astrocytes and Müller cells exhibit an immature phenotype.
A,B. Representative epifluorescence micrographs of primary astrocytes (A) and primary Müller cells (B) labeled with DAPI (blue) and immunolabeled with antibodies against Pax2 (left) and nestin (right). Both primary astrocytes (A) and Müller cells (B) were nestin-positive and Pax2-negative. Scale bar = 100μm.
3.3. Isolated astrocytes do not acquire a mature phenotype with time in culture
Next, we evaluated whether maturation time in culture could influence GFAP expression in astrocytes. We cultured primary astrocytes for 5, 12, 20, or 30 days in vitro (DIV) in serum-containing media and then performed immunolabeling for nestin, vimentin and GFAP with DAPI counterstain. Astrocytes were positive for nestin immunolabeling after 5 and 12 DIV, but negative by 20 and 30 DIV (Figure 3A). We detected vimentin immunolabeling with a typical intermediate filament distribution at all time-points (Figure 3B). At 30DIV, health of the cultures drastically decreased and sparse GFAP immunoreactivity was detected (Figure 3B). However, the pattern of localization was inconsistent with typical intermediate filament labeling (Figure 3B). Instead, GFAP immunolabeling appeared concentrated in the perinuclear area (Figure 3B). To assure that the lack of labeling was not due to an insensitive antibody, we repeated the experiment using a commonly used polyclonal antibody against GFAP and obtained the same results (data not shown). These data suggest that time in culture matures astrocytes to a phenotype more consistent with perinatal astrocytes, as indicated by vimentin expression. However, the GFAP expression consistent with a fully mature phenotype is not achieved prior to 30DIV, when health of the culture is significantly compromised.
Figure 3. Time in culture does not alter intermediate filament phenotypes of primary astrocytes.
A,B. Representative epifluorescence micrographs of primary astrocytes grown for 5, 12, 20 or 30 days in vitro (DIV) in serum and immunolabeled with antibodies against nestin (green) and counterstained with DAPI (blue; A) or co-immunolabeled with antibodies against vimentin (red) and GFAP (green; B). Nestin immunolabeling decreased over time (A), while vimentin immunolabeling remained consistent across all DIV (B). GFAP was minimally detected at 20DIV and 30DIV, but appeared perinuclear rather than filamentous (B). Scale bar = 100μm.
3.4. Isolated Müller cells require time in culture to become non-reactive
To determine whether time in culture influences phenotype maturation of primary Müller cells, we performed the same time-course and immunolabeling as above in the primary astrocytes. Like primary astrocytes, Müller glia cultures were nestin-positive after both 5 and 12 DIV, but largely nestin-negative after 20 and 30 DIV (Figure 4A). In contrast, Müller glia were also vimentin-positive at 5, 12 and 20 DIV (Figure 4B). However, the intensity of this immunolabeling decreased markedly by 30 DIV, when the health of these cultures was significantly impacted (Figure 4B). Immunolabeling for GFAP was present in these cells at 5 DIV and increased transiently at 12 DIV (Figure 4C). However, GFAP labeling disappeared by 20 DIV and Müller glia remained GFAP-negative through 30 DIV (Figure 4C). Decreases in immunolabeling noted at 30 DIV for all antigens coincided with a precipitous decline in cell density (Figure 4A–C). These data suggest that, like astrocytes, Müller glia mature with time in culture. The time-course of GFAP and vimentin expression suggests that this phenotype maturation is accompanied by acquisition of a more non-reactive phenotype. However, this is offset by appreciable decline in cell viability after 20DIV. Thus, the optimal time in culture for Muller glia appears to be 12DIV to 20DIV.
Figure 4. Time in culture reduces Müller cell reactivity and promotes mature phenotype.
A-C. Representative epifluorescence micrographs of primary Müller cells grown for 5, 12, 20 or 30 days in vitro (DIV) in serum-containing media immunolabeled with antibodies against nestin (A), vimentin (B) and GFAP (C) and counterstained with DAPI (blue). Nestin (A) and GFAP (C) immunolabeling decreased substantially at 20 and 30 DIV. Vimentin immunolabeling also decreased, but not until 30 DIV (B). Scale bar = 100μm.
3.5. Serum factors inhibit GFAP expression and promote vimentin expression in primary astrocyte cultures
Unlike Müller glia, DIV is insufficient to produce a mature phenotype in retinal astrocytes that aligns with that in vivo. To determine whether culture conditions could influence primary astrocyte phenotypes, we maintained our primary cultures in serum-containing media for 5 DIV to ensure attachment and health of the isolated cells. Cells were then maintained in either serum-containing or SF media for another 5 DIV. As illustrated above, we did not detect GFAP immunolabeling in the primary astrocytes maintained in serum-containing media (Figure 5A). However, we did detect robust vimentin immunolabeling (Figure 5A). When astrocytes were maintained in SF media for 5 DIV, we observed a reversal of this phenotype, where GFAP immunolabeling was present in the expected intracellular pattern and vimentin immunolabeling was substantially reduced (Figure 5B). Interestingly, astrocytes maintained for only 2 DIV in SF media did not exhibit this reversal in phenotype (data not shown).
Figure 5. Intermediate filament phenotype of primary astrocytes is impacted by serum factors.
A-C. Representative epifluorescence micrographs of primary astrocytes maintained in serum-containing media (A), serum-free (SF) media for 5 days (B) or SF media for 5 days followed by serum-containing media for 5 days (C). Cultures were immunolabeled with antibodies against GFAP (left) and vimentin (right) and stained with DAPI (blue). Astrocytes maintained in serum-containing media were vimentin-positive and GFAP-negative (A), while astrocytes transitioned to SF media were GFAP-positive and vimentin-negative (B). Transition from SF media back to serum-containing media changed the intracellular pattern of GFAP immunolabeling from filamentous to large clumps localized in the soma surrounding nuclei (C). Vimentin immunolabeling also returned in the characteristic filamentous pattern (C). Scale bar = 100μm.
We next examined whether this phenotype reversal induced by SF media was persistent. We again ensured attachment and health of the just isolated cells by growing them for 5 DIV in serum-containing media. We then maintained the astrocytes in SF media for 5 DIV (as above) and then transitioned them back to serum-containing media for another 5 DIV. These astrocytes maintained GFAP expression (Figure 5C). However, the intracellular pattern changed from filamentous to large clumps localized in the soma surrounding nuclei (Figure 5C). Interestingly, these astrocytes also induced vimentin expression with a filamentous pattern of localization (Figure 5C). Together, these data suggest that maintaining astrocytes in SF media is sufficient to induce mature astrocytic phenotypes (i.e. GFAP expression). However, this maturation requires persistent SF conditions, as re-introduction of serum restores vimentin expression and decreases GFAP expression. This indicates that factors in serum actively influence the maturity phenotype of retinal astrocytes.
3.6. Serum factors actively modulate GFAP and vimentin promoter activation
To determine whether serum inhibits GFAP promotor activation and induces vimentin promotor activation, we transfected primary astrocytes with constructs driving luciferase from the GFAP promoter (pGFAP), the Vimentin promoter (pVim), or no promoter (control). We normalized to Renilla driven from the CMV promoter (Figure 6A). The luciferase promoter activity assay was performed 2 DIV with only 24 hours of SF conditions. Based on our GFAP immunolabeling data, 5 days in SF is required to induce GFAP expression (Figure 5). Consistent with our immunolabeling studies, high-levels of luciferase were detected from pVim in the primary astrocytes maintained in serum-containing media (Figure 6B). However, very little luciferase activity was detected with pGFAP (Figure 6B). These data suggest that factors present in serum inhibit activation of the GFAP promoter and promote activation of the vimentin promotor in purified primary retinal astrocytes.
Figure 6. Serum factors actively modulate GFAP and vimentin promoter activation in primary astrocytes.
A. Luciferase-renilla reporter constructs used to assess GFAP and vimentin promoter activity in primary astrocytes grown in serum. B. Quantification of promoter activity for GFAP and vimentin in the presence of serum reveals inactivation of the GFAP promoter that is comparable to luciferase only control. In contrast, vimentin promoter activity is 8-fold above GFAP promotor and control levels (p<0.0001; **). Luciferase is normalized to Renilla driven by the CMV promoter. C. Representative epifluorescence micrographs of primary astrocytes immunolabeled with alpha-tubulin (α-tubulin; green) and pSTAT3 (red) after culturing in SF media alone (left) or with 20ng/ml LIF (right). pSTAT3 immunolabeling is undetectable in control conditions, but is present in the nucleus following treatment with 20ng/ml LIF. Note: results for CNTF looked identical to those with LIF. Scale bar = 100μm.
To determine whether reduced activation of pGFAP reflects active inhibition by serum factors, we treated primary astrocyte cultures with CNTF and LIF. These trophic factors contribute to phenotype maturation of astrocytes and can drive expression of GFAP in astrocytes in vivo via activation and translocation of phospho-STAT3 (pSTAT3) into the nucleus (Raff et al., 1983; Mi and Barres, 1999; Nakashima et al., 1999; Purrello et al., 2002). We maintained primary astrocytes for 2 DIV in SF media with or without CNTF or LIF and examined nuclear translocation of pSTAT3 by immunocytochemistry. The results from either growth factor were identical so we show the results from LIF only (Figure 6C). To visualize the full morphology of these astrocytes, we coimmunolabeled for alpha-tubulin (α-tubulin; Figure 6C). As indicated by α -tubulin labeling, astrocytes in these culture conditions exhibit the characteristic radial astrocyte morphology, independent of treatment with LIF (Figure 6C). Cells grown in the absence of LIF were negative for pSTAT3 (Figure 6C). In contrast, treatment with LIF resulted in a large increase in nuclear pSTAT3 immunolabeling (Figure 6C). Thus, supplementation with GFAP-promoting factors is insufficient to hasten the vimentin to GFAP conversion induced by transition to a SF environment. Together, these data suggest that serum factors actively inhibit activation of the GFAP promoter and that time in absence of serum factors is critical for releasing this inhibitory effect.
4. Discussion
Astrocytes play important roles in retinal health and disease and thus, there is much interest in understanding them on a mechanistic level. As a result, many groups have isolated these cells from the neural retina to better study them. The method of isolation, duration of culture and the components of the culture media often vary between groups. In this study, we compared different culture conditions to identify the optimal method for establishing and maintaining a mature phenotype in primary astrocyte cultures purified from postnatal rat retina. We compared purified retinal astrocytes to purified Müller glia, a retina-specific radial glial cell, as differentiation between these astrocyte subtypes is critical in retinal glia cultures. We found that while time in culture impacts the phenotype of both astrocytes and Müller glia, the maturation of retinal astrocytes was most impacted by serum factors. These factors appeared to actively regulate intermediate filament phenotypes, which are indicative of both reactivity state and maturity.
In the presence of serum, both astrocytes and Müller glia express nestin and vimentin (Figures 1 and 2). These intermediate filaments are associated with APCs, immature astrocytes and reactive Müller glia (Mi and Barres, 1999; Luna et al., 2010; Tao and Zhang, 2014; Hol and Pekny, 2015). Reactive astrocytes in retina do not typically express nestin (Luna et al., 2010). The lack of Pax2 expression suggests that these purified cultures do not contain APCs, which stop expressing Pax2 by birth in rodents (Figure 2) (Mi and Barres, 1999; Tao and Zhang, 2014). While time in culture decreased expression of nestin by astrocytes, vimentin expression remained relatively stable on a per cell basis (Figure 3). Thus, astrocytes appeared to retain a reactive-like phenotype (vimentin), despite some evidence of maturation (nestin expression). In contrast, Müller glia exhibited continued expression of all three markers that peaked at 12 DIV, then decreased dramatically, along with health of cultures, by 30 DIV (Figure 4). Vimentin expression remained for longer than nestin and GFAP, lasting out to 20 DIV (Figure 4). This more stable expression of vimentin is consistent with previous observations of Müller glia in vivo (Luna et al., 2010) as well as in Müller glia cultures from multiple species (Hauck et al., 2003; Merl 2012). Thus, our data suggest that the optimal time for experimentation with primary Müller glia is between 12DIV and 20DIV.
Our marker expression data aligns with some aspects of previous studies utilizing primary retinal astrocytes. Other studies report various combinations of GFAP, vimentin, nestin and S100-beta expression in primary retinal and brain astrocytes maintained in serum-containing media (Mi and Barres, 1999; Scheef et al., 2005; Lukas and Wang, 2012; Zamanian et al., 2012; Zhang et al., 2016; Sun et al., 2017). While our detection of vimentin and nestin correlate with these studies, the detection of GFAP does not. The most notable difference between our approach and that of other studies is the sequential purification of Müller glia and astrocytes. In our experimental design, Müller glia are purified first, followed by astrocytes. Our data indicate that Müller glia do express GFAP, even at the earliest timepoint. Thus, it is possible that GFAP expression previously noted in other studies reflects the presence of Müller glia. This is especially the case for studies utilizing purification methods that do not rely on antigen expression, i.e. shaking method. Radial glia, like Müller glia, are a subset of astrocytes and thus, it is reasonable that they could be retained in these cultures.
Our data indicate that differences in culture conditions post-isolation also likely drive differences in astrocyte phenotypes. Previous studies utilizing brain astrocytes report that serum induces a reactive phenotype in vitro (Zamanian et al., 2012; Foo et al., 2011). A similar effect on astrocytes was noted in response to heparin-binding epidermal growth factor-like growth factor (HB-EGF), when tested as an alternative to serum (Puschmann et al., 2014). Accordingly, we transitioned our primary retinal astrocytes to SF media. After 5 days of SF conditions, these cells began expressing GFAP and ceased to express vimentin (Figure 5). The transition between vimentin and GFAP intermediate filament expression has significant implications for astrocyte reactivity states. Increased expression of nestin and vimentin are associated with brain astrogliosis in vivo (Hol and Pekny, 2015) and brain astrocyte reactivity in vitro (Bramanti et al., 2010). In the healthy CNS in vivo, mature astrocytes express GFAP, but not vimentin (Yang et al., 2018). Given that vimentin is primarily expressed by mesenchymal cells in mature systems, the induction of vimentin is considered evidence of astrocyte-mesenchymal transition (AMT) (Yang et al., 2018). It is hypothesized that AMT is an early event in astrogliosis (Yang et al., 2018). Combined with previous accounts identifying broad reactive phenotypes for brain astrocytes maintained in serum, our data support that primary retinal astrocytes maintained in serum also assume an early reactive phenotype with the hallmarks of AMT.
Interestingly, reversal experiments indicated that retinal astrocytes will revert to the early reactive phenotype when transitioned back to serum-containing media from SF conditions (Figure 5). This suggests that: 1) primary retinal astrocytes exist along a continuum of AMT and 2) serum factors or the absence of these factors can “push” these primary cells towards one end of the spectrum or the other. Further studies are necessary to determine whether other aspects of AMT accompany intermediate filament indicators, i.e. proliferation rate, cytokine production, gap junction formation (Yang et al., 2018).
Our data in SF conditions suggested that serum factors may directly influence the intermediate filament phenotype of retinal astrocytes (Figure 5). Thus, we examined whether serum-containing media altered activation of the GFAP and vimentin promotors (Figure 6). We found that activation of the GFAP promotor was negligible in retinal astrocytes maintained in serum-containing media, while the vimentin promotor was highly induced (Figure 6). This was consistent with our immunocytochemical findings (Figure 3) and suggests that serum factors directly influence transcription of intermediate filaments. Interestingly, exogenous treatment with two maturation factors known to induce GFAP expression in astrocytes, LIF and CNTF (Mi and Barres, 1999; Nakashima et al., 1999) was not sufficient to counteract the influence of these serum factors (Figure 6). This was despite activation and nuclear translocation of STAT3, which lies upstream of GFAP induction by these factors (Figure 6) (Mi and Barres, 1999; Nakashima et al., 1999)]. Further studies are needed to determine what factor(s) is influencing intermediate filament expression as well as the mechanisms that appear to actively inhibit GFAP expression and likely, promote AMT.
5. Conclusions
Overall, our study indicates that, while primary astrocytes can be useful to study retinal biology and disease, caution is necessary to ensure that the phenotype of these cells is considered in the interpretation of data. Key elements to be considered are purity, particularly as it relates to Müller glia, and reactivity state. Our data indicate that sequential isolation of Müller glia and astrocytes is effective for producing highly purified cultures of retinal astrocytes. In terms of reactivity state, time in culture is critical for both astrocytes and Müller glia, where a minimum of 12 DIV is necessary to reduce baseline reactivity. For astrocytes, reactivity state is further and, perhaps even more impacted, by culture conditions. In this case, SF culture media is required for a minimum of 5 days to push cells towards a more mature phenotype and continued SF conditions are necessary to maintain this phenotype. Even so, the propensity for retinal astrocytes to shift along an AMT continuum should be considered when interpreting resulting data. Our hope is that this study will help standardize the field so that studies are replicable, comparable, and as accurate as possible for subsequent interpretation of findings.
Some current purification methods may result in co-purification of both astrocytes and Müller cells.
Müller cells are initially reactive in culture after purification, but with time in culture they return to a quiescent state.
Upon isolation and culturing, astrocytes convert to phenotype consistent with induction of astrocyte-mesenchymal transition (AMT).
Factors in fetal bovine serum actively promote AMT in retinal astrocytes.
Primary astrocytes can convert back to an in vivo healthy phenotype by culturing in serum-free media.
Funding
This work was supported by the Department of Defense [W81XWH-15–1-0096 (TR), W81XWH-17–2-0055 (TR)], National Institutes of Health [NEI R01 EY022349 (TR), NIA R01 NS094595 (TR), NEI RO1EY020496 (RMS), NEI P30EY008126 (VVRC)], Research Prevent Blindness Unrestricted Funds (VEI), Potocsnak Family – CSC Research Fund (TR, RMS), Ayers Research Fund in Regenerative Visual Neuroscience (TR, RMS), Ret. Maj. General Stephen L. Jones, MD Fund (TR), Mark Pigott Fund (TR),
Abbreviations:
- AMT
astrocyte-mesenchymal transition
- CNTF
ciliary neurotrophic factor
- DMEM
Dulbecco’s Modified Eagle Media
- FBS
fetal bovine serum
- GFAP
glial fibrillary acidic protein
- Glu
glucose
- LIF
leukemia inhibitory factor
- RT
room temperature
- PBS
phosphate-buffered saline
- SF
serum-free
- TBS
tris-buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bellot-Saez A, Kékesi O, Morley JW, Buskila Y Astrocytic modulation of neuronal excitability through K+ spatial buffering. Neurosci. Biobehav. Rev, 2017, 77, 87–97. [DOI] [PubMed] [Google Scholar]
- Bramanti V, Tomassoni D, Avitabile M, Amenta F, Avola R Biomarkers of glial cell proliferation and differentiation in culture. Front Biosci. 2010,2,558–570. [DOI] [PubMed] [Google Scholar]
- Bringmann A Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006, 25, 397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Iandiev I, Pannicke T, Wurm A, Hollborn M, Wiedemann P, Osborne NN, Reichenbach A. Cellular signaling and factors involved in Muller cell gliosis: neuroprotective and detrimental effects. Prog. Retin. Eye Res. 2009, 28, 423–451. [DOI] [PubMed] [Google Scholar]
- Chrobak AA, Soltys Z Bergmann glia, long-term depression, and autism spectrum disorder. Mol Neurobiol. 2016, doi: 10.1007/s12035-016-9719-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenfeld AJ, Bunt-Milam AH, Sarthy PV. Müller cell expression of glial fibrillary acidic protein after genetic and experimental photoreceptor degeneration in the rat retina. Invest Ophthalmol Vis Sci. 1984, 25, 1321–8. [PubMed] [Google Scholar]
- Fernández-Sánchez L, Lax P, Campello L, Pinilla I, Cuenca N Astrocytes and Muller cell alterations during retinal degeneration in a transgenic model of retinitis pigmentosa. Front. Cell Neurosci. 2015, 9, 484. doi: 10.3389/fncel.2015.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo LC, Allen NJ, Bushong EA, Ventura PB, Chung W-S, Zhou L, Cahoy JD, Daneman R, Zong H, Ellisman MH, and Barres BA Development of a method for the purification and culture of rodent astrocytes. Neuron. 2011, 71, 799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formichella CR, Abella SK, Sims SM, Cathcart HM, Sappington RM Astrocyte reactivity: A biomarker for ganglion cell health in retinal neurodegeneration. J Clin Cell Immunol. 2014, 5(1), 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck SM, Suppmann S, Ueffing M. Proteomic profiling of primary retinal Muller glia cells reveals a shift in expression patterns upon adaptation to in vitro conditions. Glia 2003, 44, 251–263. [DOI] [PubMed] [Google Scholar]
- Ho KW, Lambert WS, Calkins DJ. Activation of the TRPV1 cation channel contributes to stress-induced astrocyte migration. Glia. 2014, 62,1435–1451, doi: 10.1002/glia.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hol EM, Pekny M Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol. 2015, 32, 121–130. [DOI] [PubMed] [Google Scholar]
- Hosoki A, Oku H, Horie T, Kida T, Sugiyama T, Nakamura K, Ikeda T. Changes in Expression of Nestin, CD44, Vascular Endothelial Growth Factor, and Glutamine Synthetase by Mature Müller Cells After Dedifferentiation. J Ocul Pharmacol Ther. 2015, 31, 476–81, doi: 10.1089/jop.2014.0117. [DOI] [PubMed] [Google Scholar]
- Jäkel S, Dimou L Glial Cells and Their Function in the Adult Brain: A Journey through the History of Their Ablation. Front. Neurosci 2017, 11, 24. doi: 10.3389/fncel.2017.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon C-J, Strettoi E, Masland RH The major cell populations of the mouse retina. J. Neurosci 1998, 18, 8936–8946, doi: 10.1523/jneurosci.18-21-08936.1998(1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Duncan DS, Echevarria FD, McLaughlin WM, Hatcher JB, Sappington RM Pressure-induced Alterations in PEDF and PEDF-R Expression: Implications for Neuroprotective Signaling in Glaucoma. J. Clin. Exp. Ophthalmol 2015, 5(6), 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas TJ, Wang AL Isolation and Culture of Astrocytes from the Retina and Optic Nerve. Richard Milner (ed.), Astrocytes: Methods and Protocols, Methods in Molecular Biology. 2012. 814, Chapter 8 DOI 10.1007/978-1-61779-452-0_8. [DOI] [PubMed] [Google Scholar]
- Luna G, Lewis GP, Banna CD, Skalli O, Fisher SK. Expression profiles of nestin and synemin in reactive astrocytes and Müller cells following retinal injury: a comparison with glial fibrillar acidic protein and vimentin. Mol Vis. 2010, 27, 2511–23. [PMC free article] [PubMed] [Google Scholar]
- Lye-Barthel M, Sun D, Jakobs TC Morphology of astrocytes in a glaucomatous optic nerve. Invest Ophthalmol Vis Sci. 2013, 54(2), 909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merl J, Ueffing M, Hauck SM, von Toerne C. Direct comparison of MS-based label-free and SILAC quantitative proteome profiling strategies in primary retinal Muller cells. Proteomics. 2012, 12, 10. [DOI] [PubMed] [Google Scholar]
- Mi H, Barres BA Purification and characterization of astrocyte precursor cells in the developing rat optic nerve. J. Neurosci 1999, 19(3), 1049–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar ML, Stefansson K, Marton LS, Tripathi RC, Molnar GK. Distribution of S-100 protein and glial fibrillary acidic protein in normal and gliotic human retina. Exp Eye Res. 1984, 38, 27–34. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Kawabata M, Miyazono K, Taga T Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science. 1999, 284, 479–482. [DOI] [PubMed] [Google Scholar]
- Nicchia GP, Pisani F, Simone L, Cibelli A, Mola MG, Dal Monte M, Frigeri A, Bagnoli P, Svelto M Glio-vascular modifications caused by Aquaporin-4 deletion in the mouse retina. Exper. Eye Res. 2016, 146, 259–268. [DOI] [PubMed] [Google Scholar]
- Purrello VS, Cormaci G, Denaro L, Reale S, Costa A, Lalicata C, Sabbatini M, Marchetti B, Avola R Effect of growth factors on nuclear and mitochondrial ADP-ribosylation processes during astroglial cell development and aging in culture. Mech. Ageing. Develop 2002, 123, 511–520. [DOI] [PubMed] [Google Scholar]
- Puschmann TB, Zandén C, Lebkuechner I, Philippot C, de Pablo Y, Liu J, Pekny MJ HB-EGF affects astrocyte morphology, proliferation, differentiation and the expression of intermediate filament proteins. J. Neurochem (2014) 128, 878–889. doi: 10.1111/jnc.12519. [DOI] [PubMed] [Google Scholar]
- Qu J, Jakobs TC The Time Course of Gene Expression during Reactive Gliosis in the Optic Nerve. PLoS One. 2013, 8(6), e67094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC, Abney ER, Cohen J, Lindsay R, Noble M Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J. Neurosci 1983, 3, 1289–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez AI, Salazar JJ, de Hoz R, Rojas B, Gallego BI, Salinas-Navarro M, Alarcón-Martínez L, Ortín-Martínez A, Avilés-Trigueros M, Vidal-Sanz M, Triviño A, Ramírez JM Quantification of the effect of different levels of IOP in the astroglia of the rat retina ipsilateral and contralateral to experimental glaucoma. Invest Ophthalmol Vis Sci. 2010, 51(11), 5690–6. [DOI] [PubMed] [Google Scholar]
- Reyes-Aguirre LI, Quintero H, Estrada-Leyva B, Lamas M. In Vitro Assays for Mouse Müller Cell Phenotyping Through microRNA Profiling in the Damaged Retina. Methods Mol Biol. 2018,1753, 305–315, doi: 10.1007/978-1-4939-7720-8_21. [DOI] [PubMed] [Google Scholar]
- Sappington RM, Chan M, Calkins DJ Interleukin-6 protects retinal ganglion cells from pressure-induced death. Invest. Ophthalmol. Vis. Sci 2006a, 47, 2932–2942. [DOI] [PubMed] [Google Scholar]
- Sappington RM, Calkins DJ Pressure-induced regulation of IL-6 in retinal glial cells: Involvement of the ubiquitin/proteasome pathway and NFkB. Invest. Ophthalmol. Vis. Sci 2006b, 47, 3860–3869. [DOI] [PubMed] [Google Scholar]
- Scheef E, Wang S, Sorenson CM, Sheibani N Isolation and characterization of murine retinal astrocytes. Mol. Vis 2005, 11, 613–24. [PubMed] [Google Scholar]
- Son JL, Soto I, Oglesby E, Lopez-Roca T, Pease ME, Quigley HA, Marsh-Armstrong N Glaucomatous optic nerve injury involves early astrocyte reactivity and late oligodendrocyte loss. Glia. 2010, 58(7), 780–9. [DOI] [PubMed] [Google Scholar]
- Song I, Dityatev A Crosstalk between glia, extracellular matrix and neurons. Brain Res Bull. 2018, 136, 101–108. doi: 10.1016/j.brainresbull.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Sun X, Hu X, Wang D, Yuan Y, Qin S, Tan Z, Gu Y, Huang X, He C, Su Z Establishment and characterization of primary astrocyte culture from adult mouse brain. Brain Res Bull. 2017, 132, 10–19. [DOI] [PubMed] [Google Scholar]
- Tao C, Zhang X Development of astrocytes in the vertebrate eye. Dev Dyn. 2014, 243(12), 1501–1510. doi: 10.1002/dvdy.24190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G The role of glia, mitochondria, and the immune system in glaucoma. Invest Ophthalmol Vis Sci. 2009, 50, 1001–1012. [DOI] [PubMed] [Google Scholar]
- Tezel G, Yang X, Luo C, Cai J, Powell DW An astrocyte-specific proteomic approach to inflammatory responses in experimental rat glaucoma. Invest Ophthalmol Vis Sci. 2012, 28, 53(7), 4220–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ren J Sun, Y, Xue Y, Zhang Z, Gong A, Wang B, Zhong Z, Cui Z, Xi Z, Yang G-Y, Sun Q, Bian L A connexin43/YAP axis regulates astroglial-mesenchymal transition in hemoglobin induced astrocyte activation. Cell Death Differen. 2018, 25, 1870–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, and Barres BA Genomic analysis of reactive astrogliosis. J. Neurosci 2012,32,6391–6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sloan SA, Clarke LE, Grant GA, Hayden Gephart MG, Barres BA Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron. 2016, 89, 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchero JB, Barres BA Glia in mammalian development and disease. Development. 2015, 142, 3805–3809. doi: 10.1242/dev.129304 [DOI] [PMC free article] [PubMed] [Google Scholar]