Abstract
Background:
Increasing HPV vaccination rates may decrease the disproportionately high HPV-associated disease incidence and mortality in African Americans (AA) and lower socioeconomic individuals. Data from a community-based participatory research (CBPR) study addressing immunization disparities among 19-35 month old children was analyzed to identify ancillary benefits in HPV immunization rates for adolescent siblings.
Methods:
Sub-study analysis inclusion criteria: AA (N=118), 13-17 years old, younger sibling enrolled in parent study, and enrolled ≥ 9 months. Parent/caregiver interventions included: a web-based immunization toolkit with information on age-appropriate vaccines; a multimedia community outreach campaign; and reminder mailings. HPV up-to-date (UTD) status was defined as Wisconsin Immunization Registry (WIR) documentation of at least 3 HPV vaccines. McNemar’s test compared pre/post intervention HPV status. Two dependent proportions testing compared the proportion of adolescents that became UTD in the study cohort, City of Milwaukee, and State of Wisconsin.
Results:
Parents/caregivers perceived that 92% of adolescents were HPV-UTD, while only 24% had a WIR-verified HPV-UTD status. Baseline UTD status of the younger siblings 19-35 month old 4:3:1:3:3:1:4 antigen series was 63%, which increased to 86% at study completion. Adolescent’s HPV-UTD immunization status increased from 30 (25%) at enrollment to 54 (46%) at study completion [p=0.004]. A statistically significant larger proportion of adolescents became HPV-UTD in the study cohort (20%) compared to the City of Milwaukee [14%, p=0.042] and the State of Wisconsin [14%, p=0.046].
Conclusions:
A culturally-tailored CBPR approach targeting parents/caregivers of younger AA children can have significant ancillary benefit to increase HPV immunization rates in adolescent siblings.
Keywords: Human Papilloma Virus (HPV), Health Disparities, Immunizations, Community-Based Participatory Research (CBPR), Adolescents
INTRODUCTION
Human papillomavirus (HPV) is the most common sexually transmitted infection in the United States [1], affecting 42.5% of people 18-59 years old [2]. While many infections are asymptomatic and resolve spontaneously, persistent HPV infection can cause cervical and vaginal cancer in women, penile cancer in men, and anal and oropharyngeal cancer in both men and women [3]. Three different HPV vaccines have been developed that offer protection from HPV subtypes 16 and 18, which cause the majority of HPV-associated cancers [4]. If the HPV vaccine is administered before initial exposure to HPV, it is very effective at preventing HPV-associated cancers [5]. Despite the HPV vaccine being added to the recommended immunization schedule in 2006 for adolescent females [6] and 2011 for adolescent males [7], national HPV vaccine adoption rates remain low, with only 34.7% of 13-17 year olds having 3 or more doses in 2015 [8]. In Milwaukee County, immunization rates are even lower, with only 26.4% of 13-18 year olds having 3 or more doses in 2015 [9].
Tremendous inequalities in HPV-associated disease exist [10]. In the U.S., cervical cancer incidence and mortality are 25% and 95% higher, respectively, among African American (AA) women [11]. In Wisconsin, AA women have an 80% higher incidence of cervical cancer, compared with white women [12]. Cervical cancer incidence is also disproportionately higher in low-income populations. Counties with poverty levels above 20% have nearly double the cervical cancer incidence compared with counties with poverty levels less than 10% [11].
One proposed mechanism for decreasing HPV-associated disease disparities is ensuring higher vaccination coverage rates [10]. Therefore, exploring methods of increasing HPV vaccination rates is a key public health priority. Researchers need to understand factors that contribute to under-vaccination rates and interventions that will eliminate immunization disparities [13]. Prior vaccine intervention studies have largely focused on written information handouts targeting more educated populations. There is a need for more studies with culturally-tailored interventions that reach a diverse population to address vaccine disparities, with vaccine uptake as the primary outcome for the intervention [14].
While prior studies have used a community-based participatory research (CBPR) approach to tackle health problems such as cervical cancer prevention [15], few studies have evaluated the effectiveness of a CBPR approach to enhance immunization rates [16]. To our knowledge, no studies have evaluated the utilization of CBPR to increase HPV immunization rates. Minkler suggested that a significant factor contributing to an increase in immunization coverage for children is community ownership as fostered by the CBPR approach [17]. Effective community and academic collaboration necessitates an in-depth understanding of circumstances which can facilitate the adoption of best practices [18]. Therefore, it was hypothesized that a CBPR approach could be effective in AA and low-income populations for increasing immunization rates in the City of Milwaukee. From 2012-2016, approximately 39% of the population in Milwaukee was AA, and the median household income was approximately $36,801, with 28.4% of individuals living in poverty [19].
CHIMC Background
Community Health Improvement for Milwaukee’s Children (CHIMC) is a multi-phase CBPR study with a goal of eliminating childhood immunization disparities in Milwaukee, Wisconsin [20, 21, 22, 23]. This 11-year study began in 2005 with Phase I as a three-year community assessment, infrastructure building, and pilot phase targeting two zip codes with the lowest immunization rates in the City of Milwaukee [21]. Phase II, CHIMC-Save Lives Immunize (CHIMC-SLI!), was an intervention phase to decrease immunization disparities among children and youth less than 14 years old from 2008 to 2013 that expanded to four target zip codes [20]. Phase III, CHIMC Take Control Immunize (CHIMC-TCI!), was the dissemination phase of the study to address immunization disparities among children <4 years old and adolescents 10-18 years old from 2013 to 2016 that expanded to 10 target zip codes. This manuscript reports a sub-study analysis for HPV immunization rates among adolescents 13-17 years old living in the same households as younger siblings 19-35 months old enrolled in the CHIMC-TCI! parent-study. The objective of this sub-study was to determine if CHIMC interventions aimed at eliminating immunization disparities for younger siblings 19-35 months old had any added ancillary benefit on HPV immunization rates among older siblings 13-17 years old. The parent study refers to eliminating immunization disparities among 19-35 month old children and the sub-study refers to a subset of enrolled adolescents 13-17 years old that had a sibling 19-35 months old enrolled in the parent study.
METHODS
CBPR and Knowledge-to-Action Approach
Guided by the CBPR principles, CHIMC-TCI! applied the Knowledge-to-Action (KTA) framework to design culturally-tailored communication tools for community-wide dissemination. CBPR is a research approach that encourages equitable partnerships between the community and academic partners to engage community members in driving the solutions to health problems [24]. The KTA framework is a dynamic and iterative process that highlights the importance of relating evidence/knowledge gained to local contexts [25, 26]. The goal of CHIMC-TCI! was to disseminate promising practices identified during the first two phases of the study into local community-based organizations (CBOs) and public service providers in order to build greater community capacity and impact a larger population of children and families.
From 2013-2016, the CHIMC-TCI! research team continued to utilize the infrastructure developed in Phase I to ensure collaboration, co-learning, and cultural-relevancy in all phases of the study. This included a steering team, executive committee, and community forward team (CFT). Steering team provided oversight for all elements of implementation and included community leaders/agency representatives, health educators, public health officials, researchers, physicians, nurses, parents, and other community residents. Executive committee members were responsible for overall administrative duties of the study and included the principal investigator, CBO members such as executive directors, co-investigators, research coordinator, and community coordinator. The CFT provided input into all aspects of the study in order to ensure effective community ownership. During CHIMC-TCI!, two CFT workgroups were created: Communication Strategies & Tactics and Dissemination & Evaluation. Work groups met monthly and were designed to engage CFT members to gain their continued input and perspectives.
Design
A pre and post quasi-experimental design was used to compare HPV immunization rates before and after CHIMC-TCI! interventions. Primary outcome measured for this sub-study was HPV-up-to-date (UTD) vaccine status, defined as receiving 3 or more doses of the HPV vaccine. Vaccination status was obtained through the Wisconsin Immunization Registry (WIR), an online database used and authorized by Wisconsin public health officials to track immunizations. Results were compared with baseline data from the Wisconsin Department of Health Services for a similar cohort of children and adolescents in the City of Milwaukee and State of Wisconsin. Participants were enrolled in CHIMC-TCI! from May 2014 – November 2015. Immunization data was recorded at enrollment and then quarterly until January 2016.
Enrollment and Baseline Data Collection
CHIMC-TCI! participants were recruited using a convenience sample obtained through outreach at 10 community satellite sites including five Women, Infants, and Children (WIC) Supplemental Nutrition Program Sites and five United Neighborhood Centers of Milwaukee (UNCOM) Agencies. Parents/caregivers who resided in targeted zip codes and had children ≤4 years in their households were eligible for enrollment, regardless if their child had an UTD or BEHIND immunization status. Households with children meeting enrollment criteria and an adolescent between 10-18 years old were eligible for tracking of their immunization status inclusive of the HPV vaccine. After parental consent, adolescents 10-18 years old assented prior to staff accessing their immunization records. Eligible populations encompassed the following 10 central City of Milwaukee zip codes: 53203, 53205, 53206, 53208, 53209, 53210, 53212, 53216, 53218, and 53233. These zip codes encompassed large populations of AA and low-income families where lower immunization rates have been documented. Families were excluded from enrollment if they did not have at least one child ≤4 years old, or if they did not reside in one of the 10 targeted zip codes.
Adolescents were eligible for inclusion in this HPV sub-study analysis if they met the following criteria: AA, 13-17 years old, had a younger sibling 19-35 months old enrolled in the parent study, and enrolled in CHIMC-TCI! a minimum of nine months. The 13-17 year old group was selected due to the availability of comparable data for that age group in the city of Milwaukee and state of Wisconsin. The nine month enrollment period was selected as it provided a reasonable amount of time for adolescents to complete the 3 dose HPV vaccination series. Upon enrollment, parents/caregivers completed a baseline demographics and parent/caregiver assessment survey to assess immunization attitudes/beliefs, perceived immunization status of each child/adolescent, self-efficacy, and social support factors. Parents/caregivers were then exposed to several interventions to expand parents’/caregivers’ knowledge about the benefits and risks of childhood and adolescent vaccinations. All research activities conducted for this study were approved through the Institutional Review Board (IRB) at Children’s Hospital of Wisconsin.
Interventions
Communication tools that were designed as part of CHIMC-TCI! dissemination plan included: CHIMC-TCI! Parent Toolkit, a multimedia campaign, an interactive eLearning Café, and reminder mailings.
CHIMC-TCI! Parent Toolkit:
CHIMC-TCI! Parent Toolkit was offered on a website and contained a list of locations where immunization information can be accessed and agencies offering Vaccines For Children (VFC) [27]. The website was launched on July 1st, 2014. The Parent Toolkit had six components as shown in Table 1. Toolkit components were developed based on information gathered during the previous phases of CHIMC and were adapted with involvement of staff, CFT members, and community agency representatives. CHIMC steering team members reviewed and voted to finalize each component. Upon enrollment, parents/caregivers were trained on how to access the website and guided through an orientation of information provided within the Toolkit. Parents/caregivers were also given opportunities to enhance their knowledge on immunizations by completing an interactive eLearning Café which was accessible on the website. The eLearning Café consisted of four modules: (1) Introduction; (2) Immunizations for Ages Birth to Four years; (3) Catch-Up Immunizations; and (4) Immunizations for Ages 10-18 years old.
Table 1.
CHIMC-TCI! Parent Toolkit Components (www.chimcmke.org)
| Advisory Committee Immunization Program (ACIP) Recommended Immunization Schedules |
| Look Up Your Child’s Immunization Record |
| Free and Low-Cost Clinics for Vaccines For Children (VFC) Eligible Participants |
| Health Care Appointment Checklist |
| Immunization Websites |
| Frequently Asked Questions |
Multimedia Campaign:
As part of a multimedia campaign, walking billboards were developed with items including: t-shirts, water bottles, pencils, tote bags, and other insignia to promote the CHIMC-TCI! study and to facilitate name recognition [23]. Focus groups were conducted to guide the CHIMC-TCI! research team on the development of social media strategies with consultation from Marquette University’s Diederich College of Communication faculty. Focus group participants were identified through outreach and enrollment activities led by CFT and study staff at local CHIMC-TCI! sites. Consent occurred at the time of focus groups. Academic and community organization partners co-facilitated each focus group in English and participants received childcare and a $25 gift card. A total of 4 focus groups, with 8 to 10 participants each, were held from January to June 2015. Focus groups recommended the development of a Facebook Page with information on the CHIMC-TCI! study and immunizations to facilitate access to the greater Milwaukee community. At enrollment, all parents/caregivers were invited to “like” the CHIMC-TCI! Facebook Page which was initiated June 25, 2015. Followers could receive updates on the study, a link to access the online parental toolkit, as well as read featured news stories about CHIMC-TCI!.
Reminder Mailings:
To promote compliance with childhood/adolescent immunization schedules, study staff identified all children/adolescents with a BEHIND immunization status quarterly based on WIR data. Postcards were then mailed to families to remind parents/caregivers of their child’s/adolescent’s immunization status. Reminder mailings were sent quarterly resulting in a total of five mailings. CFT members designed all mailings to ensure the delivery of the most simplified, yet culturally-relevant messages.
Immunization Collaborations:
To disseminate knowledge to the greater metropolitan area of Milwaukee, the CHIMC-TCI! research team and CFT members became active members and/or leaders of two collaborative organizations: Immunize Milwaukee Coalition, and Milwaukee Succeeds Immunization Network. Immunize Milwaukee Coalition is an independent, not-for-profit, community coalition focused on innovative education, communication, and collaboration with the goal of improving and sustaining vaccination rates in metro-Milwaukee [28]. Milwaukee Succeeds Immunization Network is a collaborative effort of community leaders focused on ensuring vaccination rates for children 9-35 months old in Milwaukee in an effort to reduce the incidence of immunization-preventable diseases and foster school readiness [29]. The CHIMC-TCI! research team and CFT members partnered with both organizations to share lessons learned from their analysis. Additionally, coalition members promoted the adoption of CHIMC-TCI! Parent Toolkit throughout greater Milwaukee.
Data Collection and Analysis
For sub-study analysis, HPV immunization statuses of adolescents were recorded as UTD or BEHIND upon enrollment and quarterly until the end of the study in January 2016. All immunization records were obtained and verified through WIR. HPV-UTD statuses were analyzed pre/post intervention and compared with a similar cohort in the City of Milwaukee and State of Wisconsin. Comparison groups consisted of AA adolescents with the same dates of birth as the sub-study group to control for secular trends in immunization rates. Data was provided by the Wisconsin Department of Health Services.
SPSS version 24 (IBM Software, Chicago, IL, USA) was used to analyze the data. McNemar’s test was used to compare parents perceived versus WIR-verified UTD status, and pre/post intervention HPV status. Parents’/caregivers’ baseline demographics, attitudes/beliefs about vaccines, perceived racism, social support factors, and self-efficacy were analyzed using Fisher’s exact test. Two dependent proportions testing was used to compare the proportion of adolescents that became UTD in sub-study cohort, compared with the City of Milwaukee, and the State of Wisconsin. Sub-study adolescents were assumed to be included within the Milwaukee and State of Wisconsin data, therefore that data was removed before comparison analysis. Unadjusted p-values of <0.05 were reported as statistically significant.
RESULTS
Baseline Assessment
A convenience sample was obtained that yielded n = 1,857 children, 404 adolescents and n = 1,335 parents/caregivers for the CHIMC parent-study. After using the inclusion criteria of 13-17 years old, enrolled in CHIMC-TCI! a minimum of nine months, and AA, a final sample of n = 118 adolescents was obtained. A diagram of inclusion criteria can be seen in Figure 1. CHIMC-TCI! parents/caregivers (n=118) were all AA (100%); female (92%); low-income, earning <$30,000 a year (83%); had an education level of high school graduate/GED or less (54%); and were unemployed (56%). Demographics of parents/caregivers can be seen in Table 2.
Figure 1:
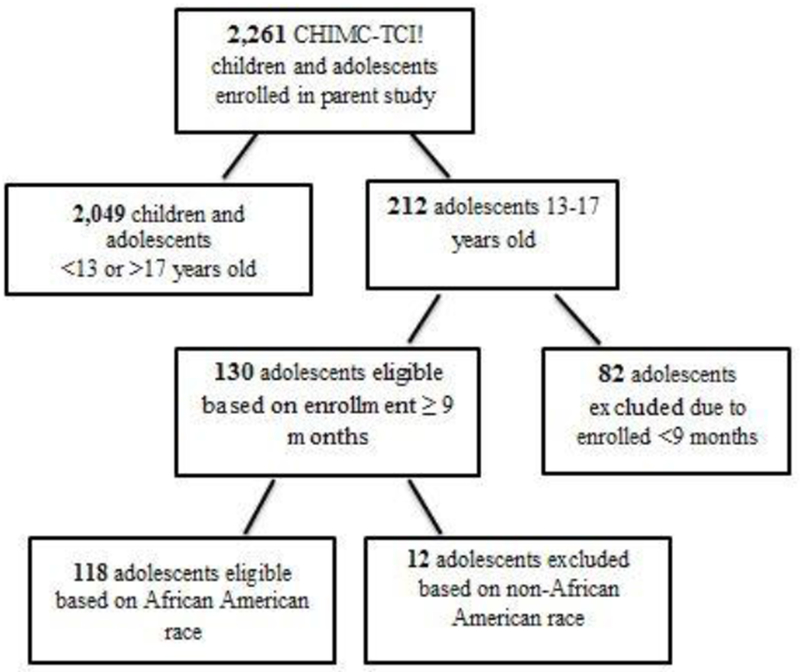
Flow diagram of sub-study analysis inclusion and exclusion criteria.
Table 2.
Demographic Characteristics of 118 Parents/Caregivers.
| N Eval | N (%) | |
|---|---|---|
| Gender | 118 | |
| Female | 109 (92) | |
| Male | 9 (8) | |
| Race | 118 | |
| African American | 118 (100) | |
| Household Income | 115 | |
| <$30,000 | 95 (83) | |
| $30,0000 - $49,999 | 14 (12) | |
| $50,000 - $74,999 | 6 (5) | |
| Education Level | 118 | |
| Some high school or Less | 20 (17) | |
| High school diploma/GED | 44 (37) | |
| Some college but no diploma | 30 (25) | |
| College graduate or higher | 24 (21) | |
| Employment Status | 117 | |
| Unemployed | 65 (56) | |
| Employed | 52 (44) |
Comparison groups obtained from Wisconsin Department of Health Services consisted only of AA adolescents 13-17 years old. There was an overall similar percent of female and male adolescents in each group. Proportion of female adolescents for each group was: 57% among CHIMC-TCI!; 50% among the City of Milwaukee; and 49% for the State of Wisconsin.
At the time of enrollment, parents/caregivers were asked whether their adolescent was UTD on immunizations. Parents/caregivers perceived that 92% of adolescents were HPV-UTD, while only 24% of adolescents had a WIR-verified HPV-UTD status, [p≤0.001].
Baseline UTD status was significantly associated with favorable parental immunization attitudes/beliefs. Those that were UTD pre/post intervention were more confident with safety of childhood immunizations (97%), compared with those that were not UTD pre/post-intervention (79%) [p=0.032]. Those that were UTD pre/post intervention agreed more that unvaccinated children may get a disease such as measles (93%), compared with those that were not UTD pre/post intervention (57%) [p=0.001].
HPV Sub-study:
In the sub-study cohort, a statistically significant increase in HPV-UTD status was observed from 30 (25%) at enrollment to 54 (46%) at parent-study completion [p=0.004]. A larger percentage of adolescents in the sub-study cohort (46%) had an HPV-UTD immunization status at study completion compared with adolescents in the City of Milwaukee (29%), and in the State of Wisconsin (29%). Results are shown in Table 3. In addition, a larger statistically significant proportion of adolescents became UTD in the sub-study cohort (20%) compared with the City of Milwaukee [14%, p=0.042] and State of Wisconsin [14%, p=0.046]. Results are shown in Figure 2. E-learning Café analysis was not completed as only a small subset of parents/caregivers completed the interactive educational session (n = 22).
Table 3.
Demographic characteristics and intervention outcome by group for African Americans 13-17 years old.
| CHIMC-TCI! N = 118 | City of Milwaukee N = 25,918 | P-valuea | State of Wisconsin N = 41,630 | P-valueb | |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | |||
| Gender | |||||
| Female | 67 (57) | 12,862 (50) | 20,567 (49) | ||
| Male | 51 (43) | 13,056 (50) | 21,063 (51) | ||
| Vaccination Status | |||||
| UTD Pre-CHIMC-TCI! | 30 (25) | 3,877 (15) | 0.003 | 6,244 (15) | 0.003 |
| UTD Post-CHIMC-TCI! | 54 (46) | 7,493 (29) | ≤0.001 | 12,065 (29) | ≤0.001 |
| Brought UTD | 24 (20) | 3,616 (14) | 0.042 | 5,821 (14) | 0.046 |
Comparison between CHIMC-TCI! and City of Milwaukee
Comparison between CHIMC-TCI! and State of Wisconsin
Figure 2.
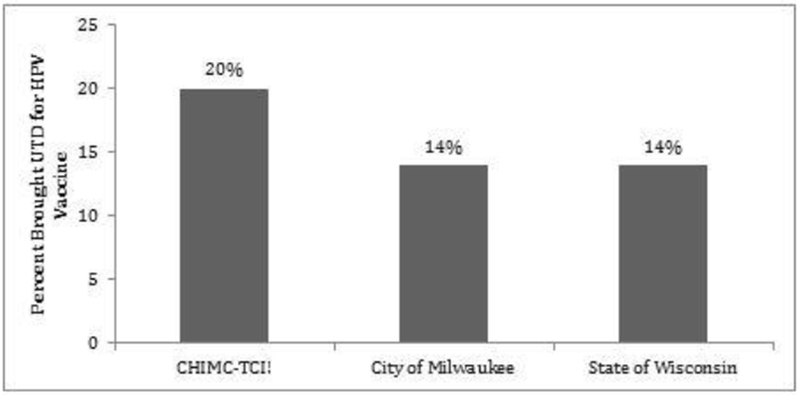
Percent of 13-17 year old African American adolescents who were brought UTD for the HPV vaccine.
HPV immunization statuses in the sub-study adolescent cohort were compared to their matched 19-35 month old siblings for 4:3:1:3:3:1:4 antigen series immunization statuses. Baseline WIR-verified UTD status of the younger sibling cohort (n=64) for the 4:3:1:3:3:1:4 antigen series was 63%, which increased to 86% at parent-study completion.
DISCUSSION
This sub-study demonstrates benefits of a CBPR approach using a KTA framework as an alternative to traditional population-based biomedical research designs. A randomized control trial was not performed as community partners advocated against this design due to expressed desires to have the study benefit as many children/adolescents as possible. Other studies have shown immunization rates will increase if you make the health-related message relevant to the community most impacted [16]. These studies demonstrated that creating culturally-tailored health messages can have ancillary benefits. HPV immunization rates in low-income, AA adolescents significantly increased and should be considered to reduce vaccination disparities in similar communities. Furthermore, this parent-study demonstrates that by designing culturally-tailored interventions, the CBPR approach can be used effectively to address community-relevant health problems.
An interesting finding was that parents/caregivers perceived that 92% of adolescents were HPV-UTD, while only 24% had a WIR-verified HPV-UTD status. Therefore, a significant ancillary benefit was realized from educating parents/caregivers on how to access immunization records, as well as sending reminder mailings that notified parents/caregivers of their children’s/adolescent’s immunization status. Future studies should assess the impact of solely informing at-risk communities of immunization status, as there was a significant discrepancy in perceived and verified immunization status.
One strength of this sub-study was the use of comparison groups. In a recent review of community-based interventions to increase HPV immunization rates, Niccolai and Hansen (2015) emphasized that rigorous intervention designs include a comparison group [30]. In our sub-study, the cohort group was compared to the City of Milwaukee and the State of Wisconsin. These comparison groups allow for a better assessment of the parent-study impact by comparing the rate of HPV vaccine uptake across groups over time. However, comparison groups were not a true control group as other community-wide immunization initiative may have impacted immunization rates outside of our sub-study cohort and therefore the true intervention effect may have been larger than our data shows.
In addition, another strength was use of verified immunization statuses. Many prior studies have only evaluated a population’s intent to vaccinate with few studies actually verifying immunization status pre and post-intervention [14]. Given the large discrepancies between parent/caregiver perceived immunization status and actual documented immunization status discussed earlier, a great strength of this parent-study is the ability to obtain verified immunization statuses from the WIR rather than relying on self-reported vaccination status.
A limitation of this study is the inability to determine which intervention was most effective. Participants were exposed to multiple interventions, and therefore, results could be attributed to any of these interventions. Future studies should separate interventions to determine which is most effective. Another limitation is the convenience sampling on a small group of adolescents. Future studies need to ensure that a similar intervention is effective on larger populations using a randomized sample. Furthermore, this study did not take into consideration health care providers’ practices when it comes to HPV and adolescents. The HPV vaccine is not consistently recommended to parents by health care providers [31, 32], and therefore, future studies should evaluate providers’ practices as a physician’s recommendation is cited as a major decision factor [33]. Finally, comparison data made available by the Wisconsin Department of Health Services has two limitations. First, race is not always noted within the WIR, therefore immunization rates may be underestimated. Second, the WIR cannot identify if an individual has moved from the registry, therefore numbers may not be full representations of who lives in each specific area.
It should be recognized that the primary focus of CHIMC-TCI! was on eliminating immunization disparities among 19-35 month old children. Therefore, enrollment numbers were much higher for younger children than adolescents. Future research should focus on enrolling a larger number of adolescents, and evaluate the impact on all age appropriate adolescent vaccines including Meningococcal, TDaP and HPV. Interventions specifically targeting HPV and adolescents might result in an even larger benefit than demonstrated in this sub-study.
The parent-study enrollment ended in January 2016 and in October 2016 the HPV immunization recommendations changed. Adolescents receiving their first HPV dose before 15 years old are now considered HPV-UTD after only 2 doses [34]. In addition, the second HPV dose now needs to be 6-12 months after the first dose, compared with 1-2 months with the 3-dose regimen [34, 5]. This sub-study did not evaluate which participants received two doses before age 15, and instead focused only on HPV-UTD status defined as 3 or more doses which was the recommendation at that time. Future studies should focus on recommendation change impact and encourage adolescents to become vaccinated before age 15 as completion rates may increase given there is one less dose to obtain.
CONCLUSION
To address large HPV-associated disease disparities, public health officials need to continue to design and implement interventions focused on increasing HPV immunization rates in populations where disparities exist. Future studies need to examine the best culturally-tailored interventions for increasing HPV immunization rates. The CBPR approach fosters collaboration and partnership between academic institutions and communities which are imperative to ensure interventions are community-desired and culturally-relevant.
ACKNOWLEDGEMENTS:
We wish to acknowledge the CHIMC team for their contributions to the work described in this manuscript. Primary partners in this research study were Medical College of Wisconsin; Children’s Hospital of Wisconsin Immunization Committee; City of Milwaukee Health Department; State of Wisconsin Department of Health Services - Immunization Program; United Neighborhood Centers of Milwaukee – COA Youth & Family Center, Next Door, Neighborhood House of Milwaukee, Silver Spring Neighborhood Center & Norcott Neighborhood House; Milwaukee County WIC Program; & Diederich College of Communication: Marquette University. Research reported in this publication was sponsored by the National Institute On Minority Health And Health Disparities of the National Institutes of Health under Award Number R24MD001812. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICTS OF INTEREST: Authors of this manuscript verify that they have no conflicts of interest.
Contributor Information
Tyler Lennon, Department of Pediatrics, Johns Hopkins University School of Medicine, 1800 Orleans Street, Baltimore, MD 21287.
Constance Gundacker, Department of Pediatrics, Medical College of Wisconsin, 8701 West Watertown Plank Road, Milwaukee, WI 53226, cgundacker@mcw.edu Phone: (414) 955-7656.
Melodee Nugent, Department of Pediatrics, Medical College of Wisconsin, 8701 West Watertown Plank Road, Milwaukee, WI 53226, mlnugent@mcw.edu Phone: (414) 955-7632.
Pippa Simpson, Department of Pediatrics, Medical College of Wisconsin, 8701 West Watertown Plank Road, Milwaukee, WI 53226, psimpson@mcw.edu.
Norma K. Magallanes, Global Health News Wire, Use Our Intel, P.O. Box 1972, Vienna, VA 22183, norma.magallanes@globalhealthnewswire.com.
Christal West, Community Forward Team Member, 2444 North 21st Street, Milwaukee, WI. 53206, christalwest1@gmail.com.
Earnestine Willis, Department of Pediatrics, Director, Center for the Advancement of Underserved Children, Medical College of Wisconsin, 8701 West Watertown Plank Road, Milwaukee, WI 53226, ewillis@mcw.edu Phone: (414) 955-4131 Fax: (414) 955-6385.
References:
- 1.Satterwhite CL, Torrone E, Meites E, et al. (2013). Sexually transmitted infections among US women and men: Prevalence and incidence estimates, 2008. Sexually Transmitted Diseases. 40(3), 187–193. [DOI] [PubMed] [Google Scholar]
- 2.McQuillan G, Kruszon-Moran D, Markowitz LE, et al. (2017). Prevalence of HPV in adults aged 18–69: United States, 2011–2014. NCHS data brief, no 280. Hyattsville, MD: National Center for Health Statistics. [Google Scholar]
- 3.Viens LJ, Henley SJ, Watson M, et al. (2016). Human papillomavirus-associated cancers - United States, 2008–2012. Morbidity and Mortality Weekly Report. 65(26), 661–666. [DOI] [PubMed] [Google Scholar]
- 4.Petrosky E, Bocchini J, Hariri S, et al. (2015). Use of 9-Valent Human Papillomavirus (HPV) Vaccine: Updated HPV Vaccination Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 64(11), 300–304. [PMC free article] [PubMed] [Google Scholar]
- 5.Markowitz LE, Dunne EF, Saraiya M, et al. (2014). Human Papillomavirus Vaccination: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report. 63(RR05), 1–30. [PubMed] [Google Scholar]
- 6.Markowitz LE, Dunne EF, Saraiya M, et al. (2007). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 56(RR-2), 1–24. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. (2011). Recommendations on the use of quadrivalent human papillomavirus vaccine in males--Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report. 60(50), 1705–1708. [PubMed] [Google Scholar]
- 8.Reagan-Steiner S, Yankey D, Jeyarajah J, et al. (2016). National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years — United States, 2015. Morbidity and Mortality Weekly Report. 65, 850–858. [DOI] [PubMed] [Google Scholar]
- 9.Wisconsin Immunization Program. (2017). Vaccination coverage among Wisconsin adolescents aged 13 through 18 years, by vaccine, county of residence and year. https://www.dhs.wisconsin.gov/publications/p02004.pdf; Accessed 07.26.17.
- 10.Brisson M, Drolet M, Malagón T. (2013). Inequalities in human papillomavirus (HPV)–associated cancers: implications for the success of HPV vaccination. Journal of the National Cancer Institute. 105(3), 158–161. [DOI] [PubMed] [Google Scholar]
- 11.Jeudin P, Liveriht E, del Carmen MG, Rebecca B. (2013). Race, ethnicity and income as factors for HPV vaccine acceptance and use. Human Vaccines & Immunotherapeutics.. 9(7), 1413–1420. [DOI] [PubMed] [Google Scholar]
- 12.Wisconsin Department of Health Services. (2014). Wisconsin Cancer Data Bulletin. https://www.dhs.wisconsin.gov/publications/p0/p00379.pdf; Accessed 07.26.17.
- 13.Gelman A, Miller E, Schwarz EB, et al. (2013). Racial disparities in human papillomavirus vaccination: Does access matter? Journal of Adolescent Health. 53(6), 756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu LY, Bonhomme LA, Cooper SC, et al. (2014). Educational interventions to increase HPV vaccination acceptance: a systematic review. Vaccine. 32(17), 1901–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin S, Glover SH, Williams AW, Brandt HM. (2009). Participatory evaluation of community-based HPV and cervical cancer prevention and control efforts. Journal of the South Carolina Medical Association. 105(7), 309–317. [PMC free article] [PubMed] [Google Scholar]
- 16.Findley SE, Irigoyen M, Sanchez M, et al. (2006). Community-based strategies to reduce childhood immunization disparities. Health Promotion Practice. 7(3), 191S–200S. [DOI] [PubMed] [Google Scholar]
- 17.Minkler M, Blackwell AG, Thompson M, Tamir H. (2003). Community-Based Participatory Research: implications for public health funding. American Journal of Public Health. 93(8), 1210–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viswanath K, Breen N, Meissner H, et al. (2006). Cancer knowledge and disparities in the information age. Journal of Health Communication. 11(S1), 1–17. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Census Bureau. 2016 Population Estimates. (2016). https://www.census.gov/quickfacts/fact/table/milwaukeecitywisconsin/RHI125216; Accessed 03.23.18.
- 20.Willis E, Sabnis S, Hamilton C, et al. (2016). Improving Immunization Rates Through Community-Based Participatory Research: Community Health Improvement for Milwaukee’s Children Program. Prog Community Health Partnersh. 10(1), 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willis E, Ngui E, Johnson C, et al. (2008). Navigating the Complexity of Relationships in Community-based Participatory Research (CBPR). In: Stanton B et al. , ed. The Uncharted Path from Clinic-Based to Community-Based Research. 1st ed New York, NY: Nova Science Publishers; 161–181. [Google Scholar]
- 22.Gray-Murray J, Leary M, Watts M, et al. (2012). Field Methods for Discovering practical wisdom: The Microdynamics of Going Beyond Technical Rationality in Real-World Practice. Int Q Community Health Educ. 33(1), 39–53. [DOI] [PubMed] [Google Scholar]
- 23.Nguie E, Hamilton C, Nugent M, et al. (2015). Evaluation of a social marketing campaign to increase awareness of immunizations for urban low-income children. WMJ. 114(1), 10–15. [PMC free article] [PubMed] [Google Scholar]
- 24.O’Fallon LR, Dearry A. (2002). Community-Based Participatory Research as a tool to advance environmental health sciences. Environmental Health Perspective. 110(S2), 155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straus S, Tetroe J, Graham ID. (2009). Knowledge Translation in Health Care: Moving Forward from Evidence to Practice. 1st ed Hoboken, NJ: BMJ. [Google Scholar]
- 26.Graham ID, Logan J, Harrison MD, et al. (2006). Lost in Knowledge Translation: Time for a Map? Journal of Continuing Education in the Health Professions. 26(1), 13–24. [DOI] [PubMed] [Google Scholar]
- 27.Community Health Improvement for Milwaukee’s Children. (2014). CHIMC Moving towards a healthy community. http://www.chimcmke.org/; Accessed 7.12.17.
- 28.Immunization Action Coalition. Immunization Coalitions Network. (2018). https://www.immunizationcoalitions.org/; Accessed 3.5.18
- 29.Milwaukee Succeeds. Milwaukee Succeeds, cradle to career. (2016). http://milwaukeesucceeds.org/; Accessed 3.5.18
- 30.Niccolai LM, Hansen CE. (2015). Practice- and Community-Based Interventions to Increase Human Papillomavirus Vaccine Coverage: A Systematic Review. JAMA Pediatr. 169(7), 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finney Rutten LJ, St. Sauver JL, Beebe TJ, et al. (2017). Association of both consistency and strength of self-reported clinician recommendation for HPV vaccination and HPV vaccine uptake among 11- to 12-year-old children. Vaccine. 35(45), 6122–6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vadaparampil ST, Malo TL, Sutton, et al. (2016). Missing the Target for Routine Human Papillomavirus Vaccination: Consistent and Strong Physician Recommendations Are Lacking for 11- to 12-year-Old Males. Cancer Epidemiol Biomarkers Prev. 25(10), 1435–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown B, Gabra MI, Pellman H. (2017). Reasons for acceptance or refusal of Human Papillomavirus Vaccine in a California pediatric practice. Papillomavirus Res. 3, 42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meites E, Kempe A, Markowitz LE. (2016). Use of a 2-Dose Schedule for Human Papillomavirus Vaccination - Updated Recommendations of the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report. 65, 1405–1408. [DOI] [PubMed] [Google Scholar]


