Abstract
Background
Multi-center randomized controlled trials (RCTs) for asthma management that incorporate usual care regimens could benefit from standardized application of evidence-based guidelines.
Objective
To evaluate performance of a computerized decision support tool, Asthma Control Evaluation and Treatment (ACET) Program, to standardize usual care regimens for asthma management in RCTs.
Methods
Children and adolescents with persistent, uncontrolled asthma, living in urban census tracts were recruited into 3 multi-center RCTs (each with a usual care arm) between 2004 and 2014. A computerized decision support tool scored asthma control and assigned an appropriate treatment step based on published guidelines. Control level determinants (symptoms, rescue medication use, pulmonary function measure, adherence estimates) were collected at visits and entered into the ACET Program. Changes in control level and treatment steps were examined during the trials.
Results
At screening, over half the participants were rated as not or poorly controlled. The proportion of participants who gained good control between screening and randomization increased significantly in all three trials. Between 51% and 70% were well-controlled by randomization. The proportion of well-controlled participants remained constant or improved slightly from randomization until the last post-treatment visit. Night symptoms were the most common control level determinant; there were few (<1%) instances of complete overlap of factors. FEV1 was the driver of control level assignment in 30% of determinations.
Conclusion
The ACET decision support tool facilitated standardized asthma assessment and treatment in multicenter RCTs and was associated with attaining and maintaining good asthma control in most participants.
Keywords: asthma guidelines, asthma control, decision support, inner-city asthma
Graphical Abstract

Capsule summary
The acceptance and performance of a guideline-based, computerized decision support tool to assess and score asthma control and then provide appropriate treatment step for inner-city children and adolescents with poorly controlled asthma was excellent.
Introduction
Asthma is a common, complex and costly chronic condition in the U.S. resulting in nearly 2 million acute-care visits and $56 billion in overall costs each year1–3. Uncontrolled asthma disproportionately affects children from minority groups (predominately African American), low-income families, and single-parent households with financial hardship and familial strain 4–6. Successful asthma management is often complex, typically requiring repeated administration of multiple medications, and using complicated devices. National and international evidence-based guidelines have been developed to assist healthcare providers and patients gain control of asthma; however, they are often not implemented7–12.
Implementation of evidence-based, clinical practice guidelines for managing chronic diseases is challenging in both primary care and specialist care settings13,14. Although guidelines for the diagnosis and management of asthma, (National Heart, Lung and Blood Institute (NHLBI) and produced by the National Asthma Education and Prevention Program (NAEPP) Expert Panel Report (EPR)7 were published in 1991 and updated in 2007, dissemination strategies, educational promotions, seminars and numerous supplemental materials have not resulted in effective guideline implementation 9,10,13. It is also unclear how consistent application of guidelines using standardized data collection and decision making would improve long-term asthma control.
The NIAID-funded Inner-City Asthma Consortium (ICAC), initiated in 2002, seeks to (1) identify and evaluate immune-based therapy for treatment of asthma in inner-city children, (2) identify and determine forms of immune-based therapies most likely to promote disease prevention and control, and (3) determine both the overall and potentially unique mechanisms of immune-based therapies associated with asthma pathogenesis in inner-city children15. We designed RCTs that used an innovative paradigm; asthma control was assessed and treatment adjusted at regular intervals throughout the trial duration in both the intervention and usual care control arms. A computerized decision support tool to operationalize the NAEPP asthma guidelines to both gain and maintain asthma control throughout ICAC trials was designed. The treatment algorithm included elements from the EPR2 and EPR3 guidelines: day and night symptom frequency, short-acting beta agonist (SABA) rescue use, FEV1, and systemic corticosteroid treatment of acute exacerbations.
It was our objective to have the computerized decision support tool permit effective and standardized longitudinal treatment step determination required to gain and maintain asthma control among high-risk inner-city children and adolescents. This report focuses on implementation and performance of the decision support tool to collect components of asthma control and then to adjust appropriate treatment among participants enrolled in three published ICAC multicenter trials using independent patient cohorts. Each trial implemented a usual care regimen, standardized across the ICAC centers, to address the trial aims: 1) value of a biomarker to guide treatment as compared with usual care alone; 2) benefit of an immune-based therapy when added to usual care over 12 months; and 3) benefit of immune-based versus step-up therapy for prevention of severe fall exacerbations when added to usual care.
Methods
Participants with persistent, uncontrolled asthma, ages 6 to 20 years, living in urban census tracts with at least 20% poverty were recruited at ten geographically dispersed, ICAC clinical centers between September 2004 and May 2014; each center had 2-4 investigators certified to conduct protocol visits. Participants all had a physician diagnosis or a history of asthma symptoms for ≥ 1 year. All trials used a computerized decision support tool constructed to assign an asthma control level, and an appropriate treatment step. The trials were the Asthma Control Evaluation (ACE), the Inner City Anti-IgE Treatment for Asthma (ICATA), and the Preventative Omalizumab or Step-up Therapy for Severe Fall Exacerbations (PROSE) trials (Table I). Protocol designs and outcomes are published elsewhere16–18.
Table I –
General Characteristics of the three ICAC clinical trials
| ACE (n=546) | ICATA (n=419) | PROSE (n=478) | |
|---|---|---|---|
| Study Run - in Duration (mean, days) | 20 | 30 | 145 |
| Treatment Duration | 46 weeks | 60 weeks DB, 24 weeks OL | 16 weeks, from school start |
| Ages | 12-20 years | 6-20 years | 6-17 years |
| Primary Outcome | Mean of max. Sx days per 2 weeks recall at each visit (16) | Mean of max. Sx days per 2-week recall at each visit (17) | Asthma exacerbations within 90 days of school start (18) |
| Study Medications | Guidelines-based care alone vs. Guidelines-based care plus Feno measurements; all study meds provided | Omalizumab vs. placebo (study provided), when added to guidelines-based care (meds prescribed via insurance) | Omalizumab (study provided) vs. ICS boost vs placebo, when added to guidelines – based care (meds prescribed via insurance) |
| Estimation of Adherence | Diskus ® built-in dose counter + structured questionnaire | Structured questionnaire | Diskus® built-in dose counter for ICS boost, structured questionnaire for usual care inhaler, injection records for omalizumab |
| Control Level Determination | Sx, SABA rescue, FEV, adherence (4 levels; Level 1 = well controlled, Levels 2 = not well controlled, Level 3&4 = poorly controlled)) | Sx, SABA rescue, FEV, adherence (4 levels; Level 1 = well controlled, Levels 2 = not well controlled, Level 3& 4 = poorly controlled) | Sx, SABA rescue, FEV1, adherence, courses of systemic corticosteroids since last visit (4 levels; Level 1 = well-controlled, Levels 2 = not well controlled, Level 3&4 = poorly controlled) |
| Treatment Steps | 0-6 | 0-6 | 0-5 |
Consistent with NAEPP guidelines,7 asthma control determinants were collected at each visit and entered into the Asthma Control Evaluation &Treatment (ACET) Program. The ACET program, including asthma control levels, linkage to treatment steps, and criteria for prescribing oral corticosteroids for asthma exacerbations, was developed by a small group of ICAC clinical investigators who were not involved in care delivery at the trial sites. Training scenarios and case studies using ACET were then provided to the ICAC site investigators and staff, via manuals of operation and protocol orientations. ACET calculated overall asthma control level based on the collected determinants and generated treatment step options according to standardized algorithms defined a priori in the protocols; algorithms were similar for the baseline, randomization, and follow-up visits. Asthma control level was based on three determinants: days with symptoms or albuterol rescue use; nights of sleep disruption or albuterol use for awakening; and FEV1. The ACET Program utilized in the PROSE trial also included oral corticosteroid treatment for acute exacerbations in the 6 months prior to the screening visit and in the intervals between subsequent visits. Symptoms were based on two-week recall at the visit and were scored on a scale of 1 to 4, with a score of 1 representing good control. The highest determinant defined the overall asthma control level (Table IIa). ACET was used to adjust treatment level starting with the screening visit in all trials. Study physicians were given the opportunity to over-ride the treatment algorithm at each visit. Reasons for changes in ACET Program recommendations included physician corrected symptoms and adherence data16–18, or clinical judgment resulting from findings on physical examination. Control levels were then matched with medication treatment steps (Table IIA, IIB) derived from the EPR2 guidelines and ranged from step 0 (as needed albuterol only) to step 6 (high dose ICS plus LABA plus a second controller medication). There were slight differences in the treatment steps among the trials, most notably in the PROSE trial that did not use low dose ICS+LABA as a treatment step18 (Table I, IIb). Although all investigational medications were provided for ICAC trial participants, regularly prescribed guidelines-based medications for usual care were obtained by participants in the ICATA and PROSE trials from retail pharmacies via insurance coverage; all usual care guidelines-based asthma medications were provided to ACE trial participants.
Table II -.
Asthma Control Level (A) and Treatment Step (B) Overview by Clinical Trial. Cells that differ between studies are shaded. Day and night symptoms are determined from participant recall, based on the 2-week interval directly preceding the study visit.
| A. | ||||
|---|---|---|---|---|
| Control Level | Day Symptoms* | Night Symptoms** | FEV1 (% predicted) | Systemic corticosteroids *** |
| 1 | 0-3 days | 0-1 night | ACE: ≥ 80 ICATA/PROSE: ≥ 85 | PROSE: 0 |
| 2 | 4-9 days | 2 nights | ACE: ≥ 80 ICATA/PROSE: 80-84 | PROSE: 1 |
| 3 | 10-13 days | 3-4 nights | 70-79 | - |
| 4 | 14 days | 5-14 nights | < 70 | - |
| B. | |||
|---|---|---|---|
| Step | ACE | ICATA | PROSE |
| 0 | albuterol prn | albuterol prn | albuterol/levalbuterol prn |
| 1 | fluticasone DPI 100mcg qd | budesonide DPI 180mcg qd | Flovent DPI 50mcg bid |
| 2 | fluticasone DPI 100mcg bid | budesonide DPI 180mcg bid | Flovent DPI 100mcg bid |
| 3 | Advair® DPI 100/50mcg bid | budesonide DPI 360mcg bid | Flovent DPI 250mcg bid |
| 4 | Advair® DPI 250/50mcg bid | Advair® DPI 250mcg/50mcg bid | Advair® DPI 250/50mcg bid |
| 5 | Advair® DPI 500/50mcg bid | Advair® DPI 250mcg/50mcg bid + montelukast qd | Advair® DPI 500/50mcg bid |
| 6 | Advair® DPI 500/50mcg bid plus either low dose theophylline or montelukast qd | Advair® DPI 500mcg/50mcg bid + montelukast qd | Not applicable (see reference 18) |
Maximum number of days with asthma symptoms and/or days with rescue albuterol use/two weeks.
Maximum of number of nights of sleep disruption due to asthma and/or nights use of albuterol for awakening/two weeks.
Courses of systemic corticosteroids in last 6 months for screening visit and since the last study visit after screening. DPI – dry powder inhaler
All three trials had an intervention group and control (guidelines-based usual care) group. Data from each trial include all participants (intervention and guidelines-based usual care) and all visits for the evaluation of and change in asthma control from screening to randomization. For the ACE and ICATA trials, data are also presented on the change in control from the first post-randomization visit to the last visit for participants in the guidelines-based usual care group only. This control level data was used to adjust treatment step after randomization throughout the trials. No post-randomization data are presented for PROSE, due to the study cohort design.
Adherence was estimated either by monitoring medication use from built-in dose counters on controller medications, and/or administration of a structured questionnaire by trained ICAC coordinators. Adherence was greater than 80% in all three trials and did not vary significantly throughout the trials.16–18. Mean ACE adherence was 86.6% (SD 27.7)16; ICATA controller adherence was 84.6% in the omalizumab arm and 88.6% in the placebo injection arm17. Median PROSE guidelines-directed usual care adherence was 92.1% (IQR 82.2-97.9%); with median inhaler counter information for ICS-boost use 82.4% (IQR 51.6-115.4%)18.
Definition of Asthma Control Level:
Well controlled asthma was defined based on determinants in the impairment domain only for ACE and ICATA and in addition, for PROSE, systemic corticosteroids use (risk domain Table IIa). Asthma was determined to be well-controlled, i.e. control level 1, Table IIa, when a participant had minimal day symptoms (0-3 days in the previous 2 weeks), 0-1 night symptoms in the previous 14 nights, and an FEV1 ≥ 80-85% predicted.
Statistical Analyses
Descriptive statistics are presented as frequencies and percentages for categorical variables and mean (standard deviation) or median (first – third quartile) according to the distribution of the continuous variable. Normality was determined by assessing the skewness of variables visually. Demographics, baseline characteristics and clinical factors influencing the ACET Program were compared between studies using the Kruskal-Wallis test for continuous and Chi-Square test for categorical variables. To visualize the relationships (intersections, unions and disjoints) between each ACET impairment domain we generated area-proportional Venn diagrams with circles. All statistical analyses were conducted within the R software environment version 3.4.1 and figures constructed using lattice19 and eulerr add-on library20.
Results
Data were available on 1,443 participants distributed among the 3 trials16–18. Participants were predominantly Black or Hispanic and highly sensitized to common aeroallergens. There were a few, small, but statistically significant differences among the groups, some of which (age, asthma duration) were related to differences in study design and key inclusion criteria. Over half the participants resided in households with annual income less than $15,000. In addition, asthma morbidity was high, with mean ACT score < 20 and approximately 4 days of symptoms per two weeks reported across all trials. ACE trial participants were slightly more symptomatic compared to those in ICATA and PROSE. Pulmonary function showed mild obstruction evidenced by slightly reduced FEV1/FVC in all trials; although FEV1 was in the normal range, and there were no differences in spirometry measures among the trials (Table E1).
At the screening visit, less than 30% of participants were rated as control level 1 (well controlled)(Table E 2). Using the treatment algorithms, the proportion of participants who then gained asthma control (control level 1) between the screening and randomization visits increased significantly in all trials; participants who gained control came from all 3 higher control levels in similar proportions (Figure 1). Between 51% (ICATA and PROSE) and 70% (ACE) were classified as control level 1 at the randomization visit (Figure 1; Table E2 A). Moreover, the proportion of well-controlled participants remained constant or improved slightly from randomization until the last post-treatment visit for ACE and ICATA. Fewer patients remained poorly controlled over the duration of the treatment period, with the largest decline occurring between screening and randomization visits (Interactive Figure 1: https://rhoinc.github.io/asthma-control-graphic/; Table E2 A and B). In all trials, the mean treatment step recommended by the ACET Program increased from screening to randomization visit and the corresponding control level improved (Table E2 A and B). For ACE and ICATA, application of the ACET Program was associated with stable or slight further improvement in control level with a corresponding decrease in treatment step by trial end. In PROSE, the protocol prohibited a decrease in treatment step during the 16-week treatment intervention phase and therefore data are not shown for the end of trial results; in addition, the PROSE primary outcome was prevention of fall asthma exacerbations and not symptom control (Table I).
Figure 1 -.
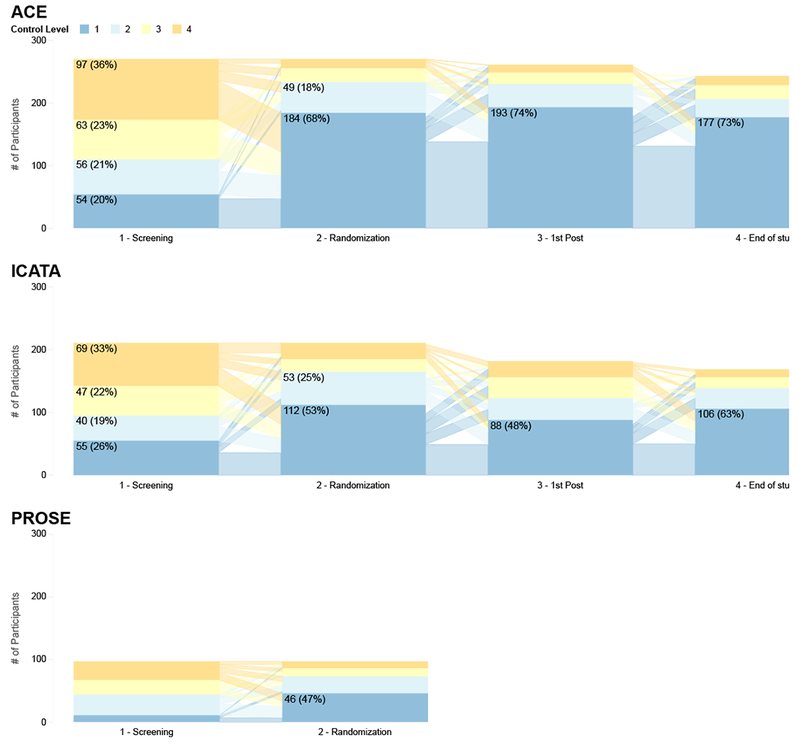
Asthma Control Level by Trial Visit. Proportion and number of study participants who were determined to be at Control Level 1-4 at screening visit, randomization, first and last post-randomization visits. In general, participants that finished at CL 1 came from CL 1 to 4 at Screening. CL1 in similar proportions: Control level 1 (well controlled); CL2: Control Level 2; CL3: Control Level 3; CL4: Control Level 4 (poorly controlled). An interactive version of the figure can be found at https://rhoinc.github.io/asthma-control-graphic/.
Although ACET Program treatment adjustments made at the screening visit were sufficient to gain control for most participants, over one third required further increase in treatment step at the randomization visit. The initial treatment step remained unaltered at randomization for a variable proportion of participants, ranging from 52% (ACE) to 22% (ICATA). Fewer than 20% of participants required two or more treatment step increases at randomization. The mean duration of the run-in period varied among the trials due to design differences. For ACE and ICATA, the mean run-in period was 20 and 30 days, respectively. PROSE had a substantially longer run in (mean 145 days) to allow for cohort assembly and multiple visits to adjust treatment and stabilize care prior to the “start of school” study drug administration.
The contribution of the various determinants (day symptoms, night symptoms, and pulmonary function) of overall asthma control level was examined. Since all factors needed to be concordant to achieve level 1, we examined the determinants driving control attribution at levels 2, 3, and 4. As shown in Figure 2, an individual determinant was more likely the driver of control levels. Night symptoms were the most common determinant, and there were few (<1%) instances of complete overlap. Moreover, FEV1 was the driver of control level in approximately 30% of determinations. The contributions of FEV1 and night symptoms were more evident among those groups with poorer control (Figure E1,2). Variability among the trials with respect to the primary determinant of control level was due to differences in protocol design. More specifically, the apparent small proportion of instances in ACE in which spirometry alone determined control (Figure E1), was due to two treatment steps (Steps 1 and 2) using the same FEV1 cut point (80%). Irrespective of protocol design differences, data show that the ACET Program performed consistently to help gain asthma control with standardized medication adjustments across sites in all three ICAC trials, and reduction of participants remaining in control level 4 (Figure 1, Table E2 A, p-value for each trial was p<0.001 for control gain between screening and randomization) .
Figure 2 -.
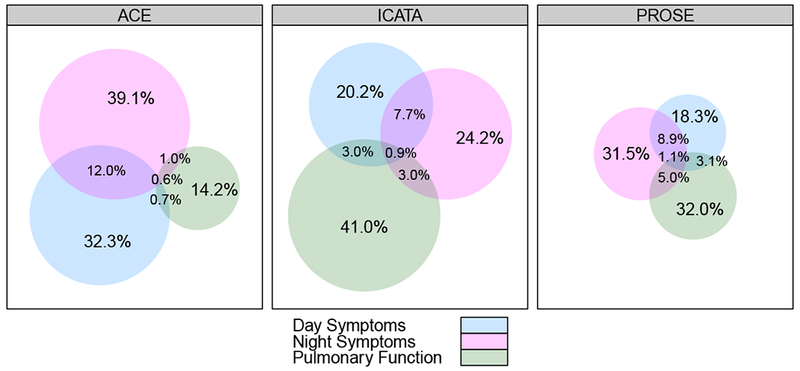
Venn Diagrams showing asthma control determinants contributing to Asthma Control Level. Proportionate contributions of day symptoms, night symptoms and FEV1 for Control Levels 2-4. Size of circle indicates proportion of contribution; data are from all included study visits.
Among over 3,000 visits in each trial with ample opportunity for ACET Program application, study physician over-rides occurred in relatively few instances (Table IV). The study physician infrequently revised the symptom report (8.1%); but when change occurred, about one-third of the time it resulted in control level alteration (Table IV). Likewise, study physicians agreed with the ACET Program step recommendations in 90% or more of the visits. Poor medication adherence affected treatment algorithm choices in 5-10% of visits (Table IV). In PROSE, reported treatment with systemic corticosteroids was used as a factor in the treatment algorithm in 15% of applications.
Table IV-.
Performance of the ICAC ACET Program, all visits and all treatment arms.
| Overall (n=10025) | ACE (n=3173) | ICATA (n=3399) | PROSE (n=3453) | p-value | |
|---|---|---|---|---|---|
| M.D. vs coordinator disagree regarding initial symptom data | 8.1% | 4.6% | 8.2% | 11.0% | <0.001 |
| M.D. correction of symptom data resulting in change in Control Level | 2.7% | 1.6% | 2.6% | 3.7% | <0.001 |
| M.D. correction of adherence data collected by coordinator | 1.6% | 0.4% | 3.2% | 1.2% | <0.001 |
| M.D. disagrees with algorithm-recommended treatment options | 7.7% | 4.5% | 8.1% | 10.2% | <0.001 |
| Used treatment algorithm for adherence <50% | 7.3% | 7% | 10% | 5% | <0.001 |
| Corticosteroids use as a factor in treatment algorithm* | - | - | 15% |
Only applicable using updated guidelines in PROSE. N represents total number of ACET Program applications within each trial.
Discussion
We provide evidence that when current asthma treatment guidelines are operationalized for usual care arms using a computerized decision support tool during multi-center RCT visits, good asthma control is gained and maintained in most inner-city children, adolescents, and young adults. Parent and patient-provided answers to questions about day and night symptoms, along with FEV1, yielded necessary information to determine asthma control level. Despite studying a high-risk population, between 50-70% of participants gained asthma control within 3-5 weeks after enrollment with careful adherence to ACET Program step recommendation. Moreover, asthma control was maintained throughout the trial duration by over 50% of the participants. A higher proportion of ACE participants gained control between screening and randomization compared to ICATA and PROSE. This finding may in part be due to supplying all guidelines-based controllers to ACE participants and only prescribing these medications in the others. Nonetheless, the ACET Program performed well in all trials, in spite of differences in study entry criteria and design, suggesting its effectiveness to standardize usual asthma care regimens in large RCTs and applicability for comparable study designs.
The allergists and pulmonologists who reviewed ACET results agreed with algorithm performance to a high degree. Disagreements about symptom reports resulted in a change in control level determination in less than 10 % of ACET Program applications; override of the step decision occurred between 5-10% in over 10,000 control and treatment assessments. Strict adherence to protocols in clinical trials is essential to obtaining accurate and meaningful data in comparing treatments, especially when new approaches are added to standard of care. The ACET electronic decision support algorithm provided a mechanism to accurately monitor treatment decisions, minimize variability in decision making across multiple centers, study the alignment of decision support with clinical judgment, and determine when adjustments were made due to poor participant adherence versus symptom or exacerbation occurrences.
ICAC developed the ACET Program to operationalize the NAEPP guidelines before the publication of official step recommendations. We designed an easy to apply framework that used patient responses to standardized questions and FEV1 to determine asthma control level and medication step. Our workflow included entry of patient/parent-reported responses into the program by research assistants and brief validation by the investigator. An asthma treatment plan with specific controller medications and doses was immediately provided; physician verification and either acceptance or revision of treatment step was possible. ACET Program performance was stable over the course of three clinical trials with discrete patient cohorts ranging from ages 6-20 years, suggesting a robust, easy to use algorithm. Importantly, once the ACET Program provided an initial treatment step, substantial percentage of trial participants, largely high-risk, inner-city asthmatics, gained control. Further treatment adjustments using ACET throughout the trial continued to maintain asthma control for 6-12 months in the majority of participants.
ACET relied on day and night asthma symptom self-reports and/or SABA rescue use over the 2 weeks prior to the visit, as well as the addition of FEV1 and systemic corticosteroid courses. An important feature of ACET is that control levels were based on the highest number of days and nights with symptoms, the lowest FEV 1 measure, and estimated medication adherence, rather than a composite score (such as ACT, ACQ). Of note, the contribution of each asthma control determinant was not equivalent and varied somewhat according to study design differences. For instance, spirometry alone influenced the control level and subsequent treatment step in a minority of instances in the ACE trial, where only 2 treatment steps could be defined by spirometry. In addition, ACE participants had to demonstrate good control (level 1) for 2 consecutive visits to step down treatment, which greatly reduced the number of participants who decreased treatment step. PROSE had a much longer run-in (mean of 145 days vs 20 or 30 in ACE and ICATA) and this accommodated either 2 or 3 treatment step adjustments prior to randomization. Nevertheless, the ACET Program performed in a consistent, robust fashion even though minor differences in study design influenced treatment patterns among the three ICAC trials.
There are some limitations to our study. First, all ICAC trial participants were managed using ACET and therefore, there was no non-ACET control group. As observed in other RCTs, asthma control may improve as a result of trial participation alone (regression to the mean) without the application of computerized decision support30. An RCT designed to compare the ACET Program to standard care without decision support would be necessary to validate the effectiveness of ACET. However, advantages of ACET use include standardized data collection of symptoms, lung function measures, exacerbations, and alignment of treatment with these parameters. Population, patient, and clinician level data can be obtained allowing for longitudinal measure of protocol adherence and treatment response.
Next, all analyses were conducted in the context of RCTs, with care provided by asthma specialists at regularly scheduled visits. It is unclear if the same degree of ACET use would occur in primary care or non-research settings 31,32 The high fidelity of ACET performance may have been due in part to clinical trial infrastructure. Patients who elect to join clinical trials may have different psychosocial and environmental characteristics than the non-participant population. Although we do not have data on non-participants, the three ICAC RCTs had a variety of entry criteria and these differences should help minimize some of the bias and enhance generalizability.
Although EPR3 guidelines have been widely disseminated, implementation by health care providers and treatment plan use by patients has lagged behind. Previous studies indicate that even when patients meet criteria for daily controller medications, up to half report not being prescribed or using a controller11,21 .In addition, many patients report frequent SABA use suggesting less than optimal asthma control22,23. Equipping health care providers with tools that facilitate determination of asthma control and then linking control level to an evidence-based treatment step care plan could improve compliance with guidelines and appropriate use of controller medications13,14. From an asthma population management perspective, ACET use would allow more specific data quantitation and identification of patients who were not well controlled and facilitate evaluation of those parameters and potential reasons contributing to those causes; e.g., frequent exacerbations that are refractory to conventional therapy, persistent symptoms due to inadequate controller adherence, and overall poor control with many features that would lead to assessment of the environment and social determinants of health.
Our ACET Program could be adapted to clinical practice with a few modifications. In ambulatory settings, patient-reported data may be completed in the waiting room or at visit check-in by clinic/office personnel, and verified by a prescriber. Nevertheless, system integration of the treatment algorithm into the EHR would be necessary to optimize its use. Other investigators have developed such tools but have largely focused on implementation, rather than actual impact on clinical outcomes27,28. Decision support tools are often unwieldy for prescribers to use owing to the need to shift from paper-based to electronic-based data collection, complex interfaces, and lack of direct linkage from control assessment to suggested treatment 9,14,25,29 An asthma decision support tool similar to the ACET Program was integrated into the EHR (EPIC™) at a large children’s hospital through a link to an external internet based program31. Symptom data and SABA use are directly added to the program by the patient/parent, with spirometry data directly imported from the EHR. The decision support is run by the health care provider or ancillary personnel. In addition, ACET data can allow clinicians to focus improvement efforts on which component of poor control (symptoms, exacerbations, lung function) is most prominent.
Implementation of the guidelines-based ACET Program to standardize asthma control level determination and appropriate treatment step, supported gaining and maintaining asthma control in high-risk inner-city asthmatic children and adolescents participating in RCTs. ACET Program use in future ICAC protocols, as well as other RCTs, could facilitate high-fidelity to treatment algorithms in both clinical efficacy and comparative effectiveness protocols. ACET use in clinical settings also could facilitate more extensive implementation of guidelines-based care among children with health disparities and traditionally more difficult to control asthma.
Supplementary Material
Table III –
Treatment Step Overview by Clinical Trial for Participants with Acceptable Adherence
| Control Level | ACE | ICATA | PROSE |
|---|---|---|---|
| 1 |
First visit at control level 1 Continue same controller regimen Consecutive visits at control level 1 Decrease controller regimen by 1 step. |
Decrease controller regimen by 1 step. | |
| 2 | Increase controller regimen by 1 step | ||
| 3 | Increase controller regimen by 2 steps |
No systemic corticosteroids since the last visit Increase controller regimen by 1 step** ≥1 course(s) of systemic corticosteroids since the last visit Increase controller regimen by 2 steps** |
|
| 4 | Increase controller regimen by 3 steps** | Increase controller regimen by 2 steps** | |
Full details in study protocols posted included as supplemental material.
Treatment step includes prednisone at the physician’s discretion in combination with a treatment increase
Clinical Implications.
A computerized decision support tool for pediatric asthma management that standardizes assessment and treatment steps can improve control among high-risk patients enrolled in multicenter RCTS; clinical adaptation is also feasible.
Acknowledgment Section
Funding:
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract numbers NO1-AI-25496, NO1-AI-25482, HHSN272200900052C, HHSN272201000052I, 1UM1AI114271-01, UM1AI109565, and UM2AI117870. Additional support was provided by the National Center for Research Resources, and National Center for Advancing Translational Sciences, National Institutes of Health, under grants NCRR/NIH RR00052, M01RR00533, UL1RR025741, UL1TR000451, UL1RR024982, M01RR00071, 1UL1RR024156, 5M01RR020359-04, UL1RR031988, 1ULRR025771, 1UL1RR025780, 1UL1RR031988, UL1TR000150, UL1TR000077-04, UL1TR001105, 1UL1RR025780, UL1TR000154, NCATS/NIH UL1TR001082, UL1TR000040, UL1TR000075, and NIH NIAID 5R01AI098077. The authors gratefully acknowledge receiving donated product for these studies from Lincoln Diagnostics (skin testing materials), GlaxoSmithKline (GSK) (study drug), Novartis Pharmaceuticals (study drug and EpiPens), SC Johnson (household pest control), Mylan, Inc. (EpiPens), and BF Ascher & Co Inc. (Ayr nasal rinse).
Financial Disclosure Statement:
W. Busse reports personal fees from Boston Scientific, ICON, Novartis, Glaxo SmithKline, Genentech, Roche, Boehringer-Ingelheim, Sanofi Genzyme, AstraZeneca, Teva, 3M, PrEPBiopharm, Circassia, Regeneron, Peptinnovate, and Elsevier outside the submitted work. C.M. Kercsmar reports personal fees from Glaxo SmithKline outside the submitted work. C.A. Sorkness reports institutional grants from Novartis during the conduct of study and institutional grants from NIH-NHLBI outside the submitted work. R.S. Gruchalla reports personal fees from Consulting Massachusetts Medical Society, as well as employment at the Center for Biologics Evaluation and Research without monetary compensation, outside the submitted work. M. Kattan reports personal fees from Novartis Pharma outside the submitted work. A.H. Liu reports personal fees from Merck Sharp & Dohme and uncompensated involvement with Glaxo SmithKline as a Data Monitoring Committee member for an asthma study outside the submitted work. G.T. O’Connor reports personal fees from AstraZeneca and institutional grants from Janssen Pharmaceuticals and NIH outside the submitted work. J.A. Pongracic reports provision of study drug from Glaxo SmithKline, Teva, Merck, Boehringer-Ingelheim, and Genentech/Novartis for research studies outside the submitted work. S.J. Szefler reports personal fees from Merck and Glaxo SmithKline; money paid to institution from Boehringer-Ingelheim, Genentech, Aerocrine, Novartis, AstraZeneca, Daiichi Sankyo, Roche, and Teva; and a grant from Glaxo SmithKline outside the submitted work. S.J. Teach reports personal fees from Novartis for consulting and royalties from Up to Date, as well as grants from NIH/NHLBI, PCORI, the Fight for Children Foundation, and EJF Philanthropies outside the submitted work. R.A. Wood reports employment at Johns Hopkins University, royalties from Up to Date, and grants from NIH, DBV, Aimmune, Astellas, and HAL-Allergy outside the submitted work. E.M. Zorrati reports personal fees from Wayne State University and an institutional grant from the NIH outside the submitted work. P.J. Gergen, A. Calatroni, G.R. Bloomberg, and J.J. Wildfire have nothing to disclose outside the submitted work. All authors, with the exception of P.J. Gergen, report grants from NIH/NIAID during the conduct of study.
Abbreviations:
- ACE
Asthma Control Evaluation
- ACET
Asthma Control Evaluation & Treatment
- ACT
Asthma Control Test
- CL
Control Level
- EPR
Expert Panel Report
- FEV1
Forced expiratory volume in 1 second
- FVC
Forced Vital Capacity
- ICAC
Inner-City Asthma Consortium
- ICATA
Inner-City Anti-IgE Therapy for Asthma
- ICS
Inhaled corticosteroid
- LABA
Long-acting beta-agonist
- NAEPP
National Asthma Education and Prevention Program
- NIAID
National Institute of Allergy and Infectious Diseases
- PROSE
Preventative Omalizumab or Step-up Therapy for Severe Fall Exacerbations
- SABA
Short-acting beta-agonist
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Carolyn M. Kercsmar, Cincinnati Children’s Hospital.
Christine A. Sorkness, University of Wisconsin School of Medicine and Public Health.
Agustin Calatroni, Rho Federal Systems Division, Inc..
Peter J. Gergen, Division of Allergy, Immunology, and Transplantation, National Institutes of Health.
Gordon R. Bloomberg, Washington University School of Medicine.
Rebecca S. Gruchalla, University of Texas Southwestern Medical Center.
Meyer Kattan, Columbia University College of Physicians and Surgeons.
Andrew H. Liu, Children’s Hospital Colorado.
George T. O’Connor, Boston University School of Medicine.
Jacqueline A. Pongracic, Children’s Memorial Hospital.
Stanley J. Szefler, National Jewish Health and University of Colorado Denver School of Medicine.
Stephen J. Teach, Children’s National Medical Center.
Jeremy J. Wildfire, Rho Federal Systems Division, Inc..
Robert A. Wood, Johns Hopkins University School of Medicine.
Edward M. Zoratti, Henry Ford Health System.
William W. Busse, University of Wisconsin School of Medicine and Public Health.
References
- 1.Asthma Facts and Figures: Asthma and Allergy Foundation of America; [cited 2011]. Available from: http://aafa.org/display.cfm?id=8&sub=42
- 2.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005-2009. Natl Health Stat Report. 2011;(32):1–14. [PubMed] [Google Scholar]
- 3.Sullivan PW, Slejko JF, Ghushchyan VH, Sucher B, Globe DR, Lin SL, et al. The relationship between asthma, asthma control and economic outcomes in the United States. J Asthma. 2014;51:769–778. doi: 10.3109/02770903.2014.906607. [DOI] [PubMed] [Google Scholar]
- 4.Rand CS, Apter AJ. Mind the widening gap: have improvements in asthma care increased asthma disparities? The Journal of allergy and clinical immunology. 2008;122(2):319–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck AF, Huang B, Simmons JM, Moncrief T, Sauers HS, Chen C, et al. Role of financial and social hardships in asthma racial disparities. pediatrics. 2014. March;133(3):431–9. doi: 10.1542/peds.2013-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halterman JS, Aligne CA, Auinger P, McBride JT, Szilagyi PG. Inadequate therapy for asthma among children in the United States. Pediatrics. 2000;105(1 Pt 3):272–276. [PubMed] [Google Scholar]
- 7.National Institutes of Health. Heart, Lung and Blood Institute. National Asthma Education and Prevention Program Expert Panel Report 3. Guidelines for the diagnosis and management of asthma. Full report 2007. Available from https://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln.pdf.
- 8.Global Strategy for Asthma Management and Prevention, Updated 2015. http://www.ginasthma.org
- 9.Wisnivesky JP, Lorenzo J, Lyn-Cook R, Newman T, Aponte A, Kiefer E, et al. Barriers to adherence to asthma management guidelines among inner-city primary care providers. Annals of Allergy, Asthma and Immunology. 2008;101(3), 264–270. doi: 10.1016/S1081-1206(10)60491-7. [DOI] [PubMed] [Google Scholar]
- 10.Janson S, Weiss K. A national survey of asthma knowledge and practices among specialists and primary care physicians. Journal of Asthma. 2005;41(3), 343–348. [DOI] [PubMed] [Google Scholar]
- 11.Navaratnam P, Jayawant SS, Pedersen CA, Balkrishnan R. Physician adherence to the national asthma prescribing guidelines: Evidence from national outpatient survey data in the United States. Annals of Allergy, Asthma and Immunology. 2008;100(3), 216–221. [DOI] [PubMed] [Google Scholar]
- 12.Butz AM, Tsoukleris M, Donithan M, et al. Patterns of inhaled anti-inflammatory medication use in young underserved children with asthma. Pediatrics. 2006;118:2504–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cloutier MM. Asthma management programs for primary care providers: increasing adherence to asthma guidelines. Curr Opin Allergy Clin Immunol 2016. April;16(2):142–7. [DOI] [PubMed] [Google Scholar]
- 14.Okelo SO, Butz AM, Sharma R, Diette GB, Pitts SI, King TM, Linn ST, Reuben M,Chelladurai Y, Robinson KA. Interventions to modify health care provider adherence to asthma guidelines: a systematic review. Pediatrics. 2013. September;132(3):517–34. doi: 10.1542/peds.2013-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Busse WW, Mitchell H. Addressing issues of asthma in inner-city children. J Allergy Clin Immunol 2007;119:43–9. [DOI] [PubMed] [Google Scholar]
- 16.Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, O’Connor GT, Morgan WJ, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med 2011. March 17;364(11):1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ Jr, Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol 2015. December;136(6):1476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarkar Deepayan. Lattice: multivariate data visualization with R. Springer Science & Business Media, 2008. [Google Scholar]
- 20.Larsson J Version 1.0.0 [Software] eulerr: Area-Proportional Euler Diagrams R package. 2016. Available from: https://cran.r-project.org/package=eulerr [Google Scholar]
- 21.Kit BK, Simon AE, Ogden CL, Akinbami LJ. Trends in preventive asthma medication use among children and adolescents, 1988-2008. Pediatrics. 2012;129(1):62–9. doi: 10.1542/peds.2011-1513. [DOI] [PubMed] [Google Scholar]
- 22.Slejko JF, Ghushchyan VH, Sucher B, Globe DR, Lin SL, Globe G, et al. Asthma control in the United States, 2008-2010: indicators of poor asthma control. J Allergy Clin Immunol 2014;133(6):1579–87. doi: 10.1016/j.jaci.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 23.Fuhlbrigge A, Reed ML, Stempel DA, Ortega HO, Fanning K, Stanford RH. The status of asthma control in the U.S. adult population. Allergy Asthma Proc 2009;30:529–33. [DOI] [PubMed] [Google Scholar]
- 24.Tamblyn R, Ernst P, Winslade N, Huang A, Grad R, Platt RW, et al. Evaluating the impact of an integrated computer-based decision support with person-centered analytics for the management of asthma in primary care: a randomized controlled trial. J Am Med Inform Assoc 2015;22(4):773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhn L, Reeves K, Taylor Y, Tapp H, McWilliams A, Gunter A, et al. Planning for action: The impact of an asthma action plan decision support tool integrated into an electronic health record (EHR) at a large healthcare system. J Am Board Fam Med 2015; 28:382–393. [DOI] [PubMed] [Google Scholar]
- 26.Bell LM, Grundmeier R, Localio R, Zorc J, Fiks AG, Zhang X, et al. Electronic health record-based decision support to improve asthma care: a cluster-randomized trial. Pediatrics. 2010;125(4):770–7. [DOI] [PubMed] [Google Scholar]
- 27.Hoeksema LJ, Bazzy-Asaad A, Lomotan EA, Edmonds DE, Ramírez-Garnica G, Shiffman RN, et al. Accuracy of a computerized clinical decision-support system for asthma assessment and management. J Am Med Inform Assoc 2011;18(3):243–50. doi: 10.1136/amiajnl-2010-000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lomotan EA, Hoeksema LJ, Edmonds DE, Ramírez-Garnica G, Shiffman RN, Horwitz LI. Evaluating the use of a computerized clinical decision support system for asthma by pediatric pulmonologists. Int J Med Inform 2012;81(3):157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dexheimer JW, Borycki EM, Chiu KW, Johnson KB, Aronsky D. A systematic review of the implementation and impact of asthma protocols. BMC Med Inform Decis Mak 2014. 9;14:82. doi: 10.1186/1472-6947-14-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans R, Gergen PJ, Mitchell H, Kattan M, Kercsmar CM, Crain E, et al. A randomized clinical trial to reduce asthma morbidity among inner-city children: Results of the National Cooperative Inner City Asthma Study. J Pediatr 1999;135:332–338. [DOI] [PubMed] [Google Scholar]
- 31.Dexheimer JW, Gu L, Guo Y, Johnson LH, Kercsmar CM. Design and implementation of the asthma treat smart system in a pediatric institution. Knowledge Management & E-Learning. 2015;7(3), 353–366. [Google Scholar]
- 32.Backer V, Nepper-Christensen S, Nolte H. Quality of care in patients with asthma and rhinitis treated by respiratory specialists and primary care physicians: a 3-year randomized and prospective follow-up study. Ann Allergy Asthma Immunol 2006;97(4):490–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


