Abstract
Aims:
Higher plasma uric acid (PUA) concentrations are associated with renal vasoconstriction, renin angiotensin aldosterone system (RAAS) activation and vascular stiffness, however the timeline for these associations in type 1 diabetes (T1D) is not clear. We aimed to compare the changes in such associations over a wide range of T1D duration.
Materials and Methods:
PUA, glomerular filtration rate (GFRINULIN), effective renal plasma flow (ERPFPAH), vascular stiffness parameters (aortic augmentation index [AIx], carotid AIx, carotid femoral pulse wave velocity [cfPWV]), plasma renin and aldosterone were measured during a euglycemic clamp in participants with T1D: 27 adolescents (age 16.8±1.9yrs), 52 young adults (25.6±5.5yrs) and 66 older adults (65.7±7.5yrs).
Results:
PUA was highest in patients with the longest T1D duration: 197±44 in adolescents vs. 264±82μmol/L in older adults (p<0.001). Higher PUA correlated with lower GFR only in older adults, even after correcting for age, HbA1c and sex (β=−2.12±0.56, p=0.0003), but not in adolescents or young adults. Higher PUA correlated with lower carotid AIx (β=−1.90, p=0.02) in adolescents. In contrast, PUA correlated with higher cfPWV (p=0.02) and higher plasma renin (p=0.01) in older adults with T1D.
Conclusions:
The relationship between higher PUA with lower GFR, increased arterial stiffness and RAAS activation was observed only in older adults with longstanding T1D. T1D duration may modify the association between PUA, renal hemodynamic function and RAAS activation, leading to renal vasoconstriction and ischemia. Further work must determine whether pharmacological PUA lowering prevents or reverses injurious hemodynamic and neurohormonal sequelae of longstanding T1D, thereby improving clinical outcomes.
Keywords: Type 1 Diabetes, Plasma Uric Acid, Renal Function, Arterial Stiffness, RAAS
INTRODUCTION
Evidence from animal and human data links plasma uric acid (PUA) with a number of key pathways involved in the pathogenesis of diabetic complications, such as metabolic abnormalities, cardiovascular disease and kidney injury, which may be a consequence of PUA-mediated inflammation and renin angiotensin aldosterone system (RAAS) activation 1. PUA - even within the normal range (200–430μmol/l for men and 140–360 mol/l for women) - is independently associated with endothelial dysfunction, arterial stiffness, impaired renal function 2,3, early glomerular filtration rate (GFR) loss 4 and elevated albumin excretion in adults with T1D and in the general adult population 1. Accordingly, the potential renal and vascular protective effects of PUA lowering are being investigated in a T1D population with microalbuminuria in the Protecting Early Renal Function Loss (PERL) Study (NCT02017171) 5.
We recently showed that PUA concentrations are lower in adolescents and young adults with T1D 6 compared to healthy controls 7, likely due to the uricosuric effect with decreased uric acid reabsorption mediated by glycosuria 7. Despite lower PUA concentrations in participants with T1D, PUA negatively correlated with eGFR in adolescents with T1D 6 and with GFRINULIN and effective renal plasma flow (ERPF) in young adults with T1D 7,8, which may be driven by PUA-mediated renal vasoconstriction 7,8. Higher PUA levels within the normal range were also correlated with higher blood pressure in young adults with uncomplicated T1D 7. Our previous data were, however, limited to adolescent and young adult T1D cohorts without any overt complications, and did not include older patients with much longer duration of disease. To our knowledge, there is no study to date that has examined the effects of diabetes duration across the lifespan on the relationships between normal PUA levels, renal and vascular function or a study that examined such associations in patients with ≥50 years of T1D duration. In this analysis, our aim was to examine the relationship between normal PUA levels, renal hemodynamic function, arterial stiffness and plasma renin and aldosterone over a wide range of T1D durations in adolescent, young adult and older adult patients with T1D.
MATERIALS AND METHODS
Subject Inclusion Criteria and Study Preparation
We conducted this post-hoc analysis to compare the associations between PUA, renal hemodynamic function, arterial stiffness and plasma RAAS markers in patients with T1D: adolescents (16.8±1.9 years, n=27), young adults (25.6±5.5 years, n=52) and older adults (65.7±7.5 years, n=66) using stored plasma samples from previously conducted studies 9–16. Correlation analysis was also performed on the combined cohort when data from all 3 T1D groups were pooled, which allowed us to study the associations over a wider range of PUA concentrations. All older adults from the Canadian Study of Longevity in Type 1 Diabetes underwent RAAS inhibitor (ACE inhibitors, angiotensin receptor blockers, direct renin inhibitors, aldosterone antagonists) washout 30 days prior to the study measurements. All studies were performed after a 7 days sodium-replete diet consisting of ≥150 mmol/day sodium to avoid circulating RAAS activation, volume contraction, between-subject heterogeneity and in an attempt to keep study conditions similar to typical North American dietary patterns. Pre-study protein intake was restricted to ≤1.5 g/kg/day protein for 7 days prior to the study visit to avoid the hyperfiltration effect of high protein diets 17. All study participants were instructed to avoid caffeine- containing products and to have the same light breakfast on the morning of each study visit. Studies were carried out in accordance with the Declaration of Helsinki, all study participants gave their informed consent and the study was approved by the University Health Network or SickKids Hospital research ethics boards.
Assessment of Renal Hemodynamic Function
Participants were studied in controlled euglycemic clamp conditions (blood glucose was maintained between 4–6 mmol/L). After the clamped glycemic concentration was achieved for approximately 3 hours, blood samples were collected for baseline circulating RAAS mediators (plasma renin concentration and aldosterone). Following collection of baseline blood samples, inulin and PAH were administered and 1.5 hours later blood samples were collected for inulin and PAH. Renal hemodynamic function (GFR and ERPF) was measured using inulin and PAH clearance according to the plasma disappearance technique 13,18. The mean of the final 2 clearance periods represented baseline GFR and ERPF, expressed per 1.73 m2. The following parameters were calculated:
Assessment of Arterial Stiffness Measures
Arterial stiffness measures were performed after the clamped euglycemic concentration was achieved for approximately 3 hours. Arterial compliance was measured non-invasively using a Sphygmocor device (SphygmoCor, AtCor Medical Systems Inc., Sydney, Australia). Right carotid artery waveforms were recorded with a high-fidelity micromanometer (SPC-301, Millar Instruments) and using the validated transfer function, corresponding central aortic pressure waveform data was generated. Mean arterial pressure (MAP) and heart rate were determined using the integral software. Augmentation index, an estimate of arterial stiffness, was calculated as the difference between the second systolic peak and inflection point, expressed as a percentage of the central pulse pressure corrected to a heart rate of 75 beats per minute. The aortic pulse wave velocity was measured by sequentially recording ECG-gated right carotid and radial artery waveforms. Our group has published and validated the use of the SphygmoCor device previously 19.
Statistical Analyses
Descriptive data are presented as mean ± standard deviation (SD), unless otherwise specified. Renin and aldosterone concentrations are presented as median with interquartile range due to the non-parametric nature of these data. To assess for between-group differences, analysis of variance with post-hoc Tukey’s test was used. Non-parametric Kruskali-Wallis test was used to compare plasma renin and aldosterone levels. Pearson correlation coefficients were used to assess the relationship between renal parameters, arterial stiffness, blood pressure, plasma markers and PUA. Linear regression analysis was used to assess the impact of covariates on continuous outcomes. Based on known factors that influence PUA, potentially relevant clinical characteristics that were included as the covariates in regression analyses were GFR, sex, HbA1c and age. Regression coefficient parameters β±standard error (SE) are reported and represent the change in PUA associated with a one-unit increase of the indicated independent variable. Statistical significance was defined as p<0.05. All statistical analyses were performed using SAS v9.1.3 and GraphPad Prism software (version 5.0).
RESULTS
Baseline Demographic Characteristics and PUA levels
Sex distribution was similar between adolescent (T1D duration 12.6±2.8 years), young adult (T1D duration 14.1±6.8 years) and older adult T1D patients (T1D duration 54.6±5.7 years). Older patients had higher BMI and better glycemic control as measured by HbA1c compared to adolescents and young adults (Table 1). PUA was lower in T1D adolescents (197±44μmol/L) compared to young (241±60μmol/L) and older T1D adults (264±82μmol/L, Figure 1).
Table 1.
Baseline Demographic Characteristics of Adolescent, Young Adult Patients and Older Adults with Type 1 Diabetes (T1D).
| Parameter | Adolescent T1D (n=27) |
Young Adult T1D (n=52) | Older Adults T1D (n=66) |
|---|---|---|---|
| Sex (% male) | 12 (44%) | 28 (54%) | 30 (45%) |
| Age (years) | 16.8±1.9#& | 25.6±5.5*& | 65.7±7.5*# |
| Diabetes duration (years) | 12.6±2.8& | 14.1±6.8& | 54.6±5.7*# |
| Body mass index (kg/m2) | 24.8±3.5& | 25.1±3.3& | 26.6±3.9*# |
| Hemoglobin A1c – mmol/mol (%) | 67.9±11.3& (8.4±1.0)& |
62.2±13.9& (7.8±1.3) & |
57.0±9.3*# (7.4±0.8)*# |
| PUA (μmol/L) | 197±44#& | 241±60* | 264±82* |
| Renal Hemodynamic Function | |||
| ERPF (mL/min/1.73m2) | 737±177#& | 657±130*& | 447±101*# |
| GFR (mL/min/1.73m2) | 139±32#& | 118±20*& | 103±17*# |
| Filtration fraction | 0.195±0.053& | 0.185±0.037& | 0.240±0.04*# |
| RBF (mL/min/1.73m2) | 1216±275#& | 1067±214*& | 689±165*# |
| RVR (mmHg/L/min) | 0.067±0.015#& | 0.079±0.022*& | 0.136±0.035*# |
| Systemic Hemodynamic Function | |||
| HR (bpm) | 71±12 | 67±11 | 66±10 |
| SBP (mmHg) | 115±11& | 112±9& | 131±16*# |
| DBP (mmHg) | 63±8& | 66±7 | 68±5* |
| Arterial Stiffness Measures | |||
| PWA – Radial AIx (%) | 10.4±12.3#& | −3.6±11.3*& | 24.8±6.7*# |
| PWA – Carotid AIx (%) | 6.8±14.3& | −0.2±14.2& | 25.8±8.3*# |
| PWV – Radial-Carotid (m/s) | 6.6±1.1& | 7.2±1.1& | 8.4±1.7*# |
| PWV –Carotid-Femoral (m/s) | 5.3±1.2& | 5.8±1.0& | 11.3±4.3*# |
| Plasma RAAS Markers | |||
| Renin | 0.16 (0.09–0.31)#& | 5.35 (3.68–9.45)*& | 11.30 (5.90–17.63)*# |
| Aldosterone | 108 (81–140) | 78 (38–120)& | 150 (102–217)# |
n, number of participants.
p<0.05 vs. Adolescent T1D;
p<0.05 vs. Young Adult T1D;
p<0.05 vs. Older Adults T1D; HC: healthy controls; T1D: type 1 diabetic patients.
Figure 1. Plasma uric acid (PUA) levels in Adolescent (n=27), Young Adult Patients (n=52) and Older Adults with Type 1 Diabetes (T1D, n=66).
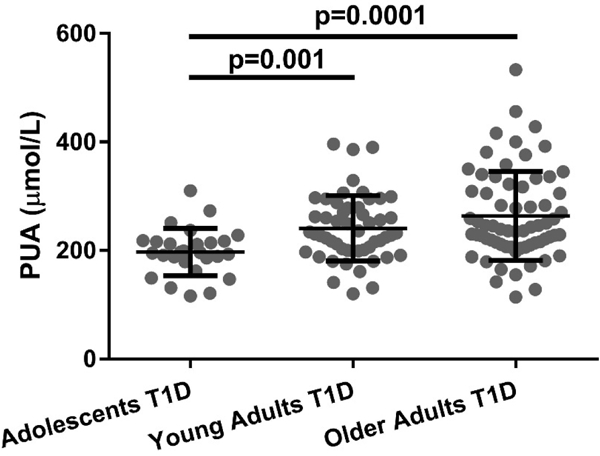
Data are presented as mean±SD.
ERPF, GFR, RBF decreased and RVR increased in a step-wise fashion when comparing adolescents to young adults to older adults with T1D. Older adults with T1D had high SBP compared to the other two groups and higher DBP compared to adolescents. Older adults with T1D had highest radial and carotid AIx, radial to carotid and cfPWV, whereas radial AIx was lowest in young adults. Plasma renin increased step-wise when comparing adolescents vs. young adults vs. older adults with T1D, and plasma aldosterone was significantly lower in young adults compared to older adults.
PUA Associations with Renal Hemodynamic Function
In the combined T1D cohort, higher PUA was associated with lower GFR after adjustment for sex, HbA1c and age (β±SE=−0.85±0.24, p=0.0006) and with higher RVR after adjustment for GFR, sex, HbA1c and age (β±SE=564.6±225.1, p=0.01, Figure 2). When the 3 different age groups were analysed separately, the association between GFR and PUA only remained significant in the older adult group with T1D (β±SE=−2.12±0.56, p=0.0003).
Figure 2. The relationship between glomerular filtration rate (GFR), effective renal plasma flow (ERPF), renal vascular resistance (RVR) and plasma uric acid (PUA) in combined cohorts with type 1 diabetes (T1D, n=145, panels A,B,C), adolescents (n=27, panels D,E,F), young adults (n=52, panels G,H,I), older adults with T1D (n=66, panels J,K,L).
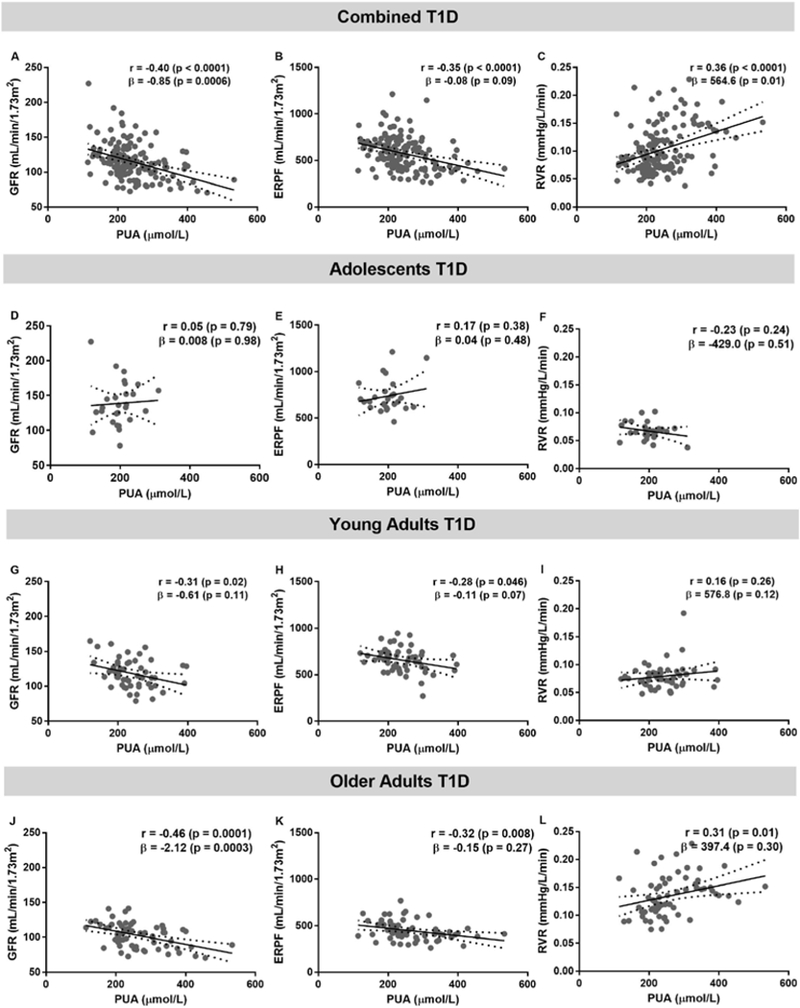
Pearson correlation analysis was used to obtain r and its associated p value. The β coefficient with the associated p value were obtained from the regression analysis. The covariates were GFR, sex, HbA1c and age. In the PUA and GFR regression analysis, the covariates were sex, HbA1c and age. Linear regression models are presented as mean (solid line) and 95% confidence interval of the mean (dashed lines).
PUA Associations with Systemic Hemodynamic Function and Arterial Stiffness
After adjustments for covariates, associations between PUA, SBP, DBP and HR were not significant (Figure 3). After adjustment for GFR, sex, HbA1c and age, higher cfPWV was associated with higher PUA (β±SE =5.31±1.71, p=0.002, Figure 4) in the combined T1D cohort, after separating data by age groups this association remained significant only in older adults with T1D (β±SE=5.11±2.05, p=0.02). In adolescents with T1D lower carotid AIx associated with higher PUA concentrations (β±SE=−1.90±0.61, p=0.02).
Figure 3. The relationship between systolic (SBP), diastolic (DBP) blood pressure, heart rate (HR) and plasma uric acid (PUA) in combined cohorts with type 1 diabetes (T1D, n=145, panels A,B,C), adolescents (n=27, panels D,E,F), young adults (n=52, panels G,H,I), older adults with T1D (n=66, panels J,K,L).
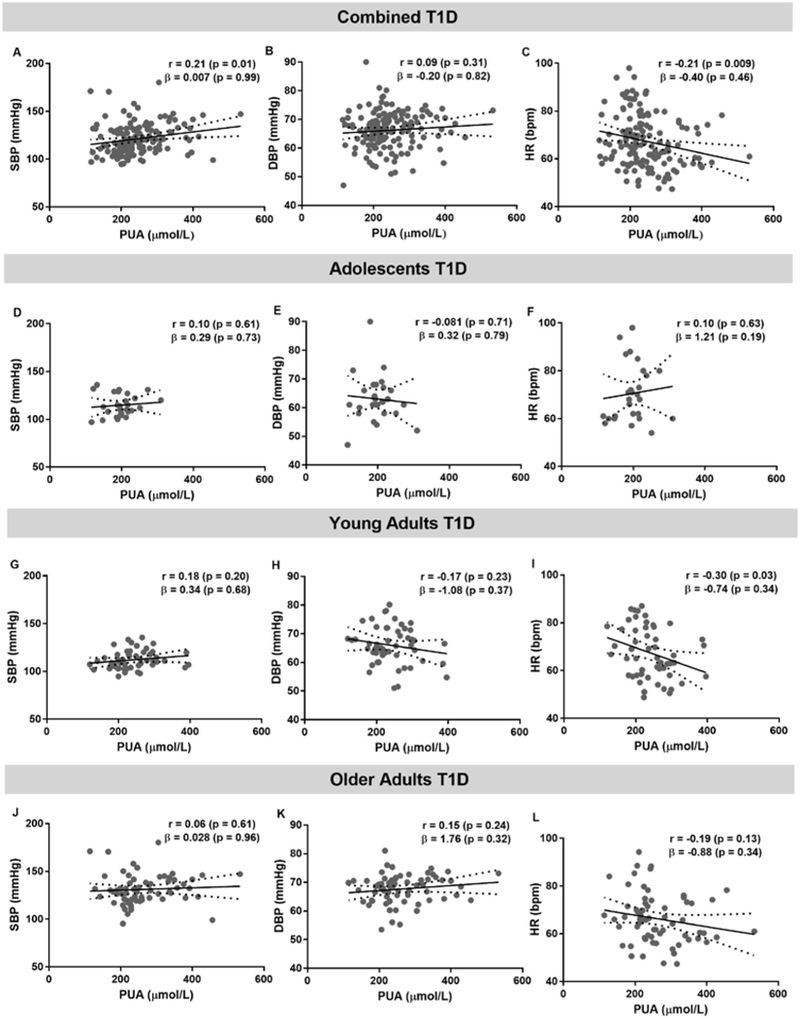
Pearson correlation analysis was used to obtain r and its associated p value. The β coefficient with the associated p value were obtained from the regression analysis. The covariates were GFR, sex, HbA1c and age. In the PUA and GFR regression analysis, the covariates were sex, HbA1c and age. Linear regression models are presented as mean (solid line) and 95% confidence interval of the mean (dashed lines).
Figure 4. The relationship between radial augmentation index (AIx), carotid AIx, carotid radial pulse wave velocity (crPWV), carotid femoral pulse wave velocity (cfPWV) and plasma uric acid (PUA) in combined cohorts with type 1 diabetes (T1D, panels A,B,C), adolescents (panels D,E,F), young adults (panels G,H,I), older adults with T1D (panels J,K,L).
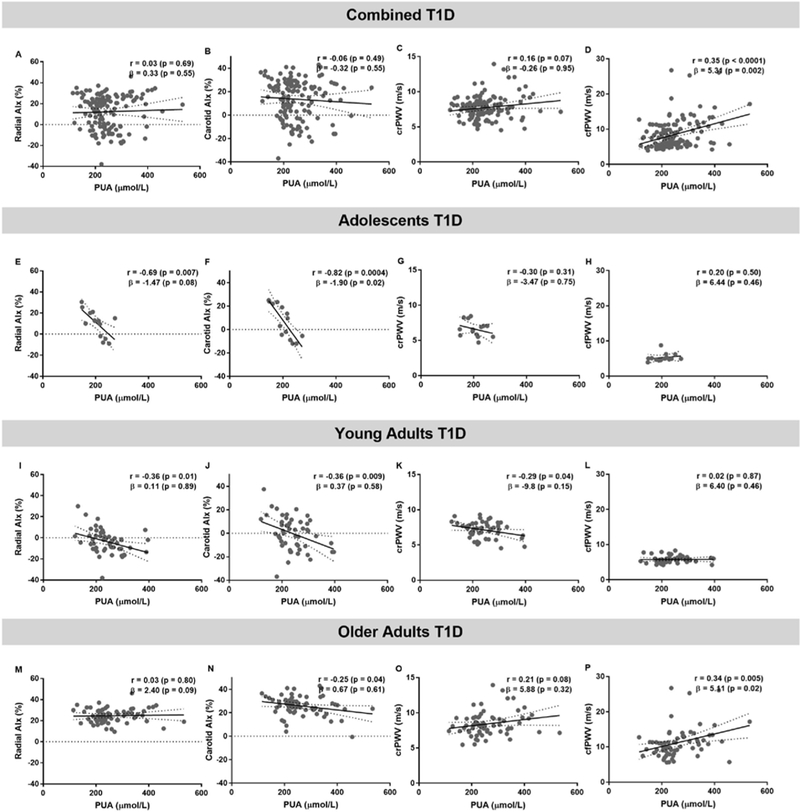
Pearson correlation analysis was used to obtain r and its associated p value. The β coefficient with the associated p value were obtained from the regression analysis. The covariates were GFR, sex, HbA1c and age. In the PUA and GFR regression analysis, the covariates were sex, HbA1c and age. Linear regression models are presented as mean (solid line) and 95% confidence interval of the mean (dashed lines).
PUA Associations with Plasma RAAS Markers
In the combined T1D cohort, higher PUA was associated with higher plasma renin and aldosterone levels (r=0.26, p=0.003 and r=0.19, p=0.04 respectively, Figure 5). The association trend between PUA and plasma aldosterone remained in the younger T1D patients (r=0.27, p=0.05) and between PUA and plasma renin in older adults with T1D (r=0.33, p=0.01).
Figure 5. The relationship between plasma renin, aldosterone and plasma uric acid (PUA) in combined cohorts with type 1 diabetes (T1D, panels A,B), adolescents (panels C,D), young adults (panels E,F), older adults with T1D (panels G,H).
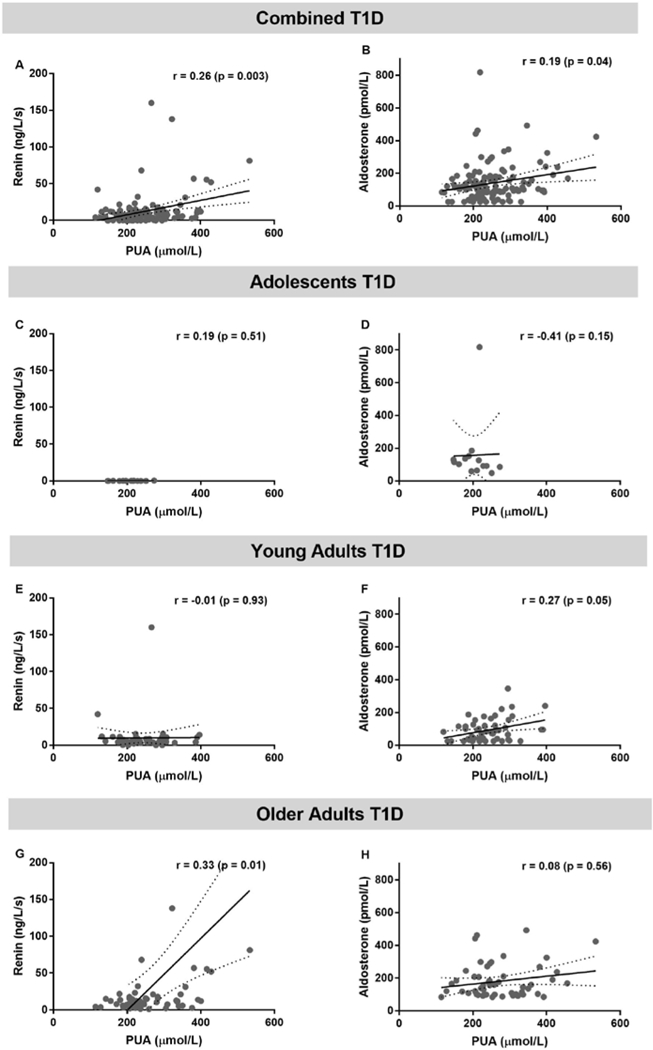
SPEARMAN correlation analysis was used to obtain r and its associated p value. Linear regression models are presented as mean (solid line) and 95% confidence interval of the mean (dashed lines).
DISCUSSION
PUA levels are consistently associated with renal and cardiovascular complications in patients with T1D, and predict incident albuminuria, rapid eGFR decline, diabetic retinopathy and coronary artery calcification 20,21. We previously showed that even within normal range PUA concentrations negatively correlate with GFR in adolescents and young adults with T1D 6–8, and with higher blood pressure in young adults with uncomplicated T1D 7. To facilitate the understanding of the role of PUA in the pathogenesis of diabetic complications across a wide range of T1D durations and to improve our strategy of implementing PUA lowering therapy, we examined the relationship between PUA and changes in renal hemodynamic function, arterial stiffness and plasma renin and aldosterone in adolescents, young adults and older adults with ≥50 years of T1D duration.
In our cohort, patients with the longest T1D duration had higher PUA concentrations. This was perhaps a result of better glycemic control of the older adult T1D patients as assessed by the HbA1c levels. Increased urinary glucose excretion during hyperglycemia is thought to be the key mechanism responsible for PUA lowering in patients with diabetes due to a stimulatory effect of urinary glucose on the GLUT 9 isoform 2 transporter on the apical membrane of the proximal tubule, which increases UA excretion in in vitro studies 22. We have previously demonstrated that impairing proximal tubule glucose reabsorption increases fractional excretion of uric acid in adults with T1D 7. Therefore, better glycemic control in our older adult T1D patients meant that lower level of hyperglycemia would reduce uricosuria leading to higher PUA concentrations compared to adolescents and young adults with T1D. Alternatively, if GFR is a determinant of PUA excretion, it is possible that lower GFR in the older adult T1D cohort leads to higher PUA levels compared to the adolescents with higher GFR and lower PUA concentrations.
A relationship between PUA and renal function decline was reported in previous longitudinal studies. Older patients with T1D and microalbuminuria show a significant correlation between baseline PUA levels and early eGFR loss over a 6-year time period 4. Moreover, each 60 μmol/L increase in PUA from baseline was shown to elevate the risk of micro- or macro-albuminuria by 80% in 652 normoalbuminuric patients over a 6 year follow up period 23. Even in normoalbuminuric patients with T1D, mildly elevated PUA is an independent predictor of early eGFR loss 24. We also showed that higher PUA concentrations within the normal range are associated with lower GFR and ERPR in young uncomplicated adult patients with T1D and with lower estimated GFR in adolescents with T1D 6. In our current analysis, using measured, gold standard GFR assessments, PUA only associated with renal hemodynamic parameters in the younger cohorts in univariable, but not multivariable analyses. In contrast, higher PUA correlated with lower GFR in older adults, even after correcting for age, HbA1c and sex. Therefore, longer duration of T1D may modify the association between PUA and renal hemodynamic function through renal vasoconstriction resulting in ischemia 8,25. T1D duration may also influence facilitative transporters that move uric acid bidirectionally depending on substrate gradients between interstitial compartment, cytosol and lumen of the nephron, which may vary along the proximal tubule segments. Alternatively, this association may occur based on decreased renal clearance leading to lower PUA, especially given that older adults with in the T1D cohort had the lowest ERPF, GFR, RBF and highest RVR compared to adolescents and young adults. In this case, the lack of association in younger T1D cohorts may be a result of a smaller sample size and a lower range of baseline GFR values.
Increased PUA concentrations were associated with intimal medial thickness, endothelial dysfunction and vascular stiffness in patients with hypertension, atherosclerosis and type 2 diabetes, and also in healthy controls 1, which all play an important role in the pathogenesis of hypertension, cardiovascular disease and chronic kidney disease 2,26. We previously reported a modest, univariable association between higher PUA and higher blood pressure within the normal range in young adults with uncomplicated T1D 7,27, but such an association was absent in adolescents with T1D 6. In our current cohort of patients, after adjusting for covariates, we did not observe any associations between PUA and blood pressure parameters. Moreover, blood pressure was controlled in our older adult cohort, where a large proportion of patients were studied after a RAAS inhibitor washout period for 30 days and some were treated with calcium channel blockers. Our findings suggest that although our patient cohorts were relatively uncomplicated in terms of blood pressure management, the relationship between PUA and blood pressure could be altered over a long duration of disease. Interestingly, in terms of factors regulating blood pressure, we observed that higher PUA correlated with lower carotid AIx (a measure of arterial stiffness) in the adolescent T1D cohort, while in older adults with T1D higher PUA correlated with higher cfPWV and higher plasma renin. This observation is consistent with a previous finding, where hypouricemia has also been associated with endothelial dysfunction in otherwise healthy patients with a URAT1 loss-of-function mutation 28. It is possible that a PUA balance may be needed for an optimal arterial stiffness. Such a U-shaped relationship was also suggested by observations in healthy Japanese subjects, where mild hyperuricemia and mild hypouricemia were significantly associated with lower GFR and ERPF 29. Similarly, our previous findings in a study examining the effects of PUA lowering with febuxostat in young uncomplicated patients with T1D suggested the need for an optimal PUA balance, whereby PUA concentrations are high enough to limit the renal vasoconstrictive response to hyperglycemia, but not excessively elevated in a range that lowers renal function and increases blood pressure 10.
Our study does have limitations. Although our patient cohorts have significantly different T1D duration, these are still relatively uncomplicated homogenous study groups with controlled blood pressure and no overt renal disease. This limits the generalizability of our findings to other populations, such as those with proteinuria and clinical nephropathy. We also acknowledge that in our older adult T1D group there maybe a survivorship bias, where study participants had to survive 50 years with T1D to be eligible for the study. Additionally, the lack of data for healthy control participants without diabetes does not allow us to conclusively determine if our observations are a result of diabetes duration or age. Moreover, future studies should examine the effects of glycemic parameters on PUA levels. To minimize the effects of small sample size, we used careful pre-study preparation and gold standard methods to quantify renal hemodynamic function. We also acknowledge that there are baseline differences between groups in glycemic control (HbA1c), blood pressure, BMI, and medication intake, however we believe these differences represent the natural progression of T1D that contributes to the outcomes presented in our analyses. Finally, our study is a retrospective cross-sectional analysis of associations between PUA, renal hemodynamics and arterial stiffness; moreover, the small correlation values may not necessarily translate to clinical significance. Therefore, our findings do not determine causality of the observed relationships and further prospective interventional studies are needed to determine mechanisms and clinical significance of our observations.
Despite its limitations, to our knowledge, our study was the first to characterize associations between PUA, renal hemodynamic function and arterial stiffness over a wide range of T1D duration. Only in older adults with longstanding T1D, the relationship between higher PUA and lower GFR, increased arterial stiffness and RAAS activation was observed. Our data suggests that duration of T1D may modify the association between PUA, GFR and activation of the RAAS, key mechanisms contributing to renal vasoconstriction and ischemia. Ongoing clinical trials and further work are required to determine if pharmacologic lowering of PUA can improve clinical outcomes by decreasing both renal injury and activation of injurious neurohormones.
ACKONOWLEDGEMENTS
Funding
The Canadian Study of Longevity in Diabetes was funded by the JDRF (Operating Grant No. 17–2013-312). Y.L. is supported by a Canadian Diabetes Association (Diabetes Canada). Fellowship. J.A.L. was supported by a CaRE (Cardio-Renal-Endocrine) post-doctoral fellowship by the Division of Nephrology, Department of Medicine, University Health Network, University of Toronto, Toronto, Ontario, Canada. A.A. is a recipient of a Diabetes Investigator Award from Diabetes Canada.
Footnotes
DISCLOSURE SUMAMRY
P.B. receives salary and research support by NIH/NIDDK (T32-DK063687, K23 DK116720–01), in addition to research support by Thrasher Foundation, Juvenile Diabetes Research Foundation (JDRF), International Society of Pediatric and Adolescent Diabetes (ISPAD), Colorado Clinical & Translational Sciences Institute (CCTSI) and Center for Women’s Health Research at University of Colorado. J.A.L. has received either consulting fees or speaking honorarium or both from Novo Nordisk, Eli Lilly & Co, Merck Sharp & Dohme, and AstraZeneca. G.B. has received speaker honoraria from Johnson & Johnson. H.A.K. has received support from Sanofi. A.A. has received research support from Boehringer Ingelheim and AstraZeneca, is listed as an inventor on a patent application (WO 2015/128453) from Boehringer Ingelheim, has received an unrestricted educational grant from Eli Lilly and has received honoraria from Novo Nordisk, Eli Lilly and Boehringer Ingelheim, Abbott and Dexcom. B.A.P. has received speaker honoraria from Medtronic, Johnson & Johnson, Roche, GlaxoSmithKline Canada, Novo Nordisk, and Sanofi; has received research grant support from Medtronic and Boehringer Ingelheim; and serves as a consultant for NeuroMetrix. D.Z.I.C. has received consulting fees or speaking honorarium or both from Janssen, Boehringer Ingelheim-Eli, Lilly, AstraZeneca, Merck, and Sanofi, and has received operating funds from Janssen, Boehringer Ingelheim-Eli, Lilly, AstraZeneca and Merck.
REFERENCES
- 1.Lytvyn Y, Perkins BA, Cherney DZ. Uric acid as a biomarker and a therapeutic target in diabetes. Can J Diabetes. 2015;39(3):239–246. [DOI] [PubMed] [Google Scholar]
- 2.Lewis G, Maxwell AP. Risk factor control is key in diabetic nephropathy. The Practitioner. 2014;258(1768):13–17, [PubMed] [Google Scholar]
- 3.Rosolowsky ET, Ficociello LH, Maselli NJ, et al. High-normal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol. 2008;3(3):706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ficociello LH, Rosolowsky ET, Niewczas MA, et al. High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabetes care. 2010;33(6):1337–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maahs DM, Caramori L, Cherney DZ, et al. Uric acid lowering to prevent kidney function loss in diabetes: the preventing early renal function loss (PERL) allopurinol study. Curr Diab Rep. 2013;13(4):550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lytvyn Y, Mahmud FH, Daneman D, et al. Association Between Plasma Uric Acid Levels and Cardiorenal Function in Adolescents With Type 1 Diabetes. Diabetes Care. 2016;39(4):611–616. [DOI] [PubMed] [Google Scholar]
- 7.Lytvyn Y, Skrtic M, Yang GK, Yip PM, Perkins BA, Cherney DZ. Glycosuria-mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. American journal of physiology Renal physiology. 2015;308(2):F77–83. [DOI] [PubMed] [Google Scholar]
- 8.Lytvyn Y, Škrtić M, Yang GK, et al. Plasma Uric Acid Effect on Glomerular Hemodynamic Profile of Patients with Uncomplicated Type 1 Diabetes Mellitus. Diabetic Medicine. 2015; [DOI] [PubMed] [Google Scholar]
- 9.Sochett EB, Cherney DZ, Curtis JR, Dekker MG, Scholey JW, Miller JA. Impact of renin angiotensin system modulation on the hyperfiltration state in type 1 diabetes. Journal of the American Society of Nephrology : JASN. 2006;17(6):1703–1709. [DOI] [PubMed] [Google Scholar]
- 10.Lytvyn Y, Har R, Locke A, et al. Renal and Vascular Effects of Uric Acid Lowering in Normouricemic Patients With Uncomplicated Type 1 Diabetes. Diabetes. 2017;66(7):1939–1949. [DOI] [PubMed] [Google Scholar]
- 11.Cherney DZ, Sochett EB, Miller JA. Gender differences in renal responses to hyperglycemia and angiotensin-converting enzyme inhibition in diabetes. Kidney international. 2005;68(4):1722–1728. [DOI] [PubMed] [Google Scholar]
- 12.Miller JA, Curtis JR, Sochett EB. Relationship between diurnal blood pressure, renal hemodynamic function, and the renin-angiotensin system in type 1 diabetes. Diabetes. 2003;52(7):1806–1811. [DOI] [PubMed] [Google Scholar]
- 13.Cherney DZ, Miller JA, Scholey JW, et al. The effect of cyclooxygenase-2 inhibition on renal hemodynamic function in humans with type 1 diabetes. Diabetes. 2008;57(3):688–695. [DOI] [PubMed] [Google Scholar]
- 14.Cherney DZ, Scholey JW, Nasrallah R, et al. Renal hemodynamic effect of cyclooxygenase 2 inhibition in young men and women with uncomplicated type 1 diabetes mellitus. American journal of physiology Renal physiology. 2008;294(6):F1336–1341. [DOI] [PubMed] [Google Scholar]
- 15.Cherney DZ, Miller JA, Scholey JW, et al. Renal hyperfiltration is a determinant of endothelial function responses to cyclooxygenase 2 inhibition in type 1 diabetes. Diabetes care. 2010;33(6):1344–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovshin JA, Boulet G, Lytvyn Y, et al. Renin-angiotensin-aldosterone system activation in long-standing type 1 diabetes. JCI Insight. 2018;3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones SL, Kontessis P, Wiseman M, et al. Protein intake and blood glucose as modulators of GFR in hyperfiltering diabetic patients. Kidney international. 1992;41(6):1620–1628. [DOI] [PubMed] [Google Scholar]
- 18.Cherney D, Reich H, Lai V, et al. Hyperfiltration and the effect of nitric oxide inhibition on renal hemodynamic and endothelial function in humans with uncomplicated type 1 diabetes mellitus. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2012;August 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cherney DZ, Lai V, Scholey JW, Miller JA, Zinman B, Reich HN. Effect of direct renin inhibition on renal hemodynamic function, arterial stiffness, and endothelial function in humans with uncomplicated type 1 diabetes: a pilot study. Diabetes care. 2010;33(2):361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjornstad P, Maahs DM, Rivard CJ, et al. Serum uric acid predicts vascular complications in adults with type 1 diabetes: the coronary artery calcification in type 1 diabetes study. Acta Diabetol. 2014;51(5):783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer TM, Nordestgaard BG, Benn M, et al. Association of plasma uric acid with ischaemic heart disease and blood pressure: mendelian randomisation analysis of two large cohorts. BMJ (Clinical research ed). 2013;347:f4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chino Y, Samukawa Y, Sakai S, et al. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharmaceutics & drug disposition. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jalal DI, Rivard CJ, Johnson RJ, et al. Serum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: findings from the Coronary Artery Calcification in Type 1 Diabetes study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2010;25(6):1865–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krolewski AS, Niewczas MA, Skupien J, et al. Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria. Diabetes care. 2014;37(1):226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazzali M, Kanellis J, Han L, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. American journal of physiology Renal physiology. 2002;282(6):F991–997. [DOI] [PubMed] [Google Scholar]
- 26.Stanton RC. Clinical challenges in diagnosis and management of diabetic kidney disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;63(2 Suppl 2):S3–21. [DOI] [PubMed] [Google Scholar]
- 27.Bjornstad P, Paul Wadwa R, Sirota JC, et al. Serum uric acid and hypertension in adults: a paradoxical relationship in type 1 diabetes. Journal of clinical hypertension (Greenwich, Conn). 2014;16(4):283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugihara S, Hisatome I, Kuwabara M, et al. Depletion of Uric Acid Due to SLC22A12 (URAT1) Loss-of-Function Mutation Causes Endothelial Dysfunction in Hypouricemia. Circulation journal : official journal of the Japanese Circulation Society. 2015;79(5):1125–1132. [DOI] [PubMed] [Google Scholar]
- 29.Uedono H, Tsuda A, Ishimura E, et al. U-shaped relationship between serum uric acid levels and intrarenal hemodynamic parameters in healthy subjects. American journal of physiology Renal physiology. 2017: [DOI] [PubMed] [Google Scholar]


