Abstract
Post-traumatic stress disorder (PTSD) induced by life-threatening medical events has been associated with adverse physical and mental health outcomes, but it is unclear whether early interventions to prevent the onset of PTSD after these events are efficacious. We conducted a systematic review to address this need. We searched six biomedical electronic databases from database inception to October 2018. Eligible studies used randomized designs, evaluated interventions initiated within 3 months of potentially traumatic medical events, included adult participants, and did not have high risk of bias. The 21 included studies (N = 4,486) assessed a heterogeneous set of interventions after critical illness (9), cancer diagnosis (8), heart disease (2), and cardiopulmonary surgery (2). Fourteen psychological, 2 pharmacological, and 5 other-type interventions were assessed. Four of the psychological interventions emphasizing cognitive behavioral therapy or meaning-making, 1 other-type palliative care intervention, and 1 pharmacological-only intervention (hydrocortisone administration) were efficacious at reducing PTSD symptoms relative to control. One early, in-hospital counseling intervention was less efficacious at lowering PTSD symptoms than an active control. Clinical and methodological heterogeneity prevented quantitative pooling of data. While several promising interventions were identified, strong evidence of efficacy for any specific early PTSD intervention after medical events is currently lacking.
Keywords: posttraumatic stress disorder (PTSD), medical events, prevention, intervention, systematic review
Posttraumatic stress disorder (PTSD) occurs in a substantial proportion of patients who have experienced extremely frightening medical events. PTSD is characterized by profound psychological distress, intrusive symptoms of re-experiencing of the trauma, including thoughts, flashbacks, or nightmares, avoidance of trauma reminders, negative alterations in cognition and mood, and hyperarousal or elevated trauma-related reactivity (American Psychiatric Association, 2013). The literature on medical event-induced PTSD has accelerated over the last 30 years (Edmondson et al., 2012; Shalev, Schreiber, Galai, & Melmed, 1993; Tedstone & Tarrier, 2003; Vilchinsky, Ginzburg, Fait, & Foa, 2017), but relatively less is known about interventions for PTSD due to potentially traumatic internal events such as cancer diagnosis, cardiac events, and acute life-threatening illness compared to the extensive research on PTSD due to external traumas such as combat, terrorism, sexual assault, and natural disasters.
Some researchers have speculated that medical event-induced PTSD was initially overlooked because the Diagnostic and Statistical Manual of Mental Disorders, third edition (DSM-III)(American Psychiatric Association, 1987) required external examples of precipitating events (e.g., assault, torture, combat, floods, car accidents) (Kutz, Garb, & David, 1988). The subsequent edition, DSM-IV, expanded the scope of qualifying traumas to include internal, medical events (e.g., diagnosis of a life-threatening illness was an explicitly provided example) (American Psychiatry Association, 1994). The current DSM-5 limits the types of medical events internal to one’s body that can trigger PTSD to be only those that are sudden and catastrophic (American Psychiatric Association, 2013).
A variety of medical events (e.g., myocardial infarction, cardiac arrest, critical illness requiring admission to the intensive care unit) meet this stringent criterion due to their common manifestation as acute and life-threatening events. Indeed, approximately 25% of patients with a suspected acute coronary syndrome (ACS) reported feeling like they were going to die at the time of their visit to the emergency department for evaluation of the cardiac symptoms (White, Edmondson, Umland, Sanchez, & Chang, 2017). Even a cancer diagnosis, which has been questioned as a possible cause of PTSD (e.g., Smith, Redd, Peyser, & Vogl, 1999), can be viewed as a traumatic event that potentially satisfies Criterion A if receipt of the cancer diagnosis is experienced as “exposure to… threatened death” (i.e., Criterion A1 for PTSD diagnosis; American Psychiatric Association, 2013, p. 271). We endorse the important point made by Bonanno and Mancini (2008) that highly distressing life events should be considered as “potentially traumatic events” as long as they are experienced as traumatic by some people. Furthermore, preliminary evidence suggests that the pattern of psychological symptoms following acute medical events are best described in terms of PTSD. For instance, Vilchinsky et al. (2017) conclude that cardiovascular disease-induced PTSD is a valid diagnostic entity based on data regarding its prevalence, risk factors, and consequences. For the purposes of this review, we sought to understand the efficacy of early interventions for reducing validated measures of PTSD symptoms after potentially traumatic medical events, including ones that do not uniformly meet the narrow Criterion-A classification of the DSM-5 for a medical-event trauma across all patients who experience it.
Whether or not PTSD is the appropriate diagnostic label, symptoms of extreme psychological distress in the form of PTSD-like symptoms after medical events are important phenomena to investigate (Sumner & Edmondson, 2018). Throughout the manuscript we use the term PTSD rather than a qualified term (e.g., “a PTSD-like disorder”) or an alternative DSM-5 diagnosis (e.g., adjustment disorder) because, other than definitively and uniformly meeting Criterion A, a substantial number of patients meet diagnostic criteria for PTSD after medical events as assessed by standard, validated metrics of PTSD symptoms (Abbey, Thompson, Hickish, & Heathcote, 2015; Edmondson et al., 2012; Presciutti et al., 2018; Tedstone & Tarrier, 2003; Vilchinsky et al., 2017; von Känel, Baumert, Kolb, Cho, & Ladwig, 2011). Furthermore, all studies in this systematic review asked patients about distressing symptoms in response to a distinct potentially traumatic medical event, thereby supporting the appropriateness of investigating medical event-induced PTSD specifically.
The prevalence of PTSD due to medical trauma is highly variable across medical events, with estimates ranging from at least 5% to as high as 59% for a variety of medical events (Abbey et al., 2015; Edmondson et al., 2012; Kangas, Henry, & Bryant, 2005; Presciutti et al., 2018; Tedstone & Tarrier, 2003; Vilchinsky et al., 2017; von Känel et al., 2011). Given the high frequency of acute medical events, PTSD due to medical events overall is estimated to affect approximately one million US adults each year (Sommer, Mota, Edmondson, & El-Gabalawy, 2018).
Consequences of PTSD extend well beyond mental distress. First, PTSD triggered by acute medical events is associated with decreased medication adherence (Kronish, Edmondson, Li, & Cohen, 2012; Shemesh et al., 2004; Wasson et al., 2018), an especially maladaptive health behavior for secondary prevention. Second, medical event-induced PTSD is associated cross-sectionally with a 50% higher number of comorbid physical health conditions than PTSD induced by other kinds of trauma (Sommer et al., 2018). Third, medical event-induced PTSD is associated with increased risk of recurrent medical events and mortality, a finding that has been observed in patients with PTSD induced by ACS events (Edmondson et al., 2012).
Given the pervasiveness of medical event-induced PTSD and its serious, costly consequences, a clinically important question is whether PTSD can be prevented rather than treated or simply managed (Forneris et al., 2013; Howlett & Stein, 2016; Kearns, Ressler, Zatzick, & Rothbaum, 2012; Sijbrandij, Kleiboer, Bisson, Barbui, & Cuijpers, 2015). Successful PTSD treatment approaches have been well characterized (Bisson et al., 2007; Hoffman et al., 2018; Watts et al., 2013), but less is known about preventing PTSD. This deficit in knowledge is especially true for medical event-induced PTSD. Multiple researchers have asserted that halting PTSD’s development requires a very timely intervention (Kornor et al., 2008; Kutz et al., 1988). One potentially fortunate aspect of medical-event induced PTSD from the perspective of prevention research is that its presentation in medical settings constitutes an opportunity for facilitating early intervention.
Prior systematic and non-systematic reviews have evaluated the efficacy of interventions intended to prevent PTSD due to a wide array of causes (Forneris et al., 2013; Howlett & Stein, 2016; Kearns et al., 2012; Sijbrandij et al., 2015). Although these reviews did not explicitly exclude PTSD induced by traumatic medical events, the vast majority of reviewed studies investigated the prevention of PTSD due to non-medical types of trauma. A separate systematic review of medical event-induced PTSD is needed for four reasons. First, the nature of PTSD due to internal versus external traumas may differ substantially in ways that are relevant to the success or failure of preventive interventions. Prominent symptom features of medical event-induced PTSD are fear of recurrence (Fait et al., 2018; Simard, Savard, & Ivers, 2010) and fear of an internal threat (Edmondson, 2014; Meli et al., 2017), and the same interventions may work differently for traumatic events that provoke these symptoms. Second, acceptability of treatments may differ for PTSD due to medical events; this information has not yet been systematically assessed for early intervention of PTSD, whether due to medical or non-medical events. For example, psychological therapies are generally preferred relative to other intervention types by people who would undergo an intervention for PTSD (Tarrier, Liversidge, & Gregg, 2006), but it is unknown whether patients who experience terrifying medical events would perceive initiating a psychological therapy for posttraumatic stress symptoms to be agreeable when the primary concern may be the physical illness. Third, with medical event-induced PTSD, the medical setting can influence the onset of PTSD (Chang, Sumner, Haerizadeh, Carter, & Edmondson, 2016; Edmondson, 2014; Edmondson et al., 2014; Edmondson, Shimbo, Ye, Wyer, & Davidson, 2013). Unique opportunities exist for health system-based interventions that may fundamentally alter the setting in which the traumatic medical event evolves. Fourth, patients undergoing potentially traumatic medical events have the potential to be identified for interventions at a critically early stage as they typically present in medical settings.
The goal of the present review was to evaluate the acceptability and efficacy of early interventions for preventing medical event-induced PTSD. Elevated PTSD symptoms, regardless of whether they meet PTSD diagnostic criteria, have been associated with adverse medical prognosis and negative health behaviors (Edmondson et al., 2012; Kronish et al., 2012; Shemesh et al., 2004). Accordingly, we assessed the strength of evidence for PTSD preventive interventions with respect to reducing PTSD symptoms as well as preventing a PTSD diagnosis. A secondary aim was to compare the strength of evidence for very early interventions (< 1 month since trauma) with moderately early interventions (> 1 month and < 3 months since trauma). The critical period during which PTSD symptoms are malleable is not yet known, especially in the case of medical event-induced PTSD, although most PTSD symptoms manifest within a 3-month timeframe (Forneris et al., 2013). Third, we characterized the patient acceptability and compliance rates for these early interventions in medical contexts. Fourth, we examined whether there were differences in the strength of evidence for early interventions directed at all patients exposed to acute medical events compared to early interventions targeted at patients at high risk for developing PTSD (e.g., those with high levels of distress shortly after the potentially traumatic event). Fifth, we assessed whether there was any evidence that early interventions may be psychologically harmful.
Method
Search strategy
This systematic review was conducted under the guidance of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria (Moher, Liberati, Tetzlaff, Altman, & Group, 2009). The systematic review was registered on PROSPERO, the international prospective register of systematic reviews (PROSPERO 2016:CRD42016037666). A literature search of six biomedical electronic databases (Ovid MEDLINE, EMBASE, The Cochrane Library, CINAHL, PsycINFO, and PILOTS) was conducted from database inception to November 2017. All relevant subject headings and free-text terms were used to represent PTSD AND either medical illness, OR specific medical conditions. Terms were applied to limit to randomized controlled trials (RCTs; see Appendix A in Supplementary Materials for complete search strategies). Ongoing studies were sought through clinicaltrials.gov and the WHO International Clinical Trials Registry Platform. Additional records were identified by scanning the reference lists of relevant studies and by employing the Similar Articles feature in PubMed and the Cited Reference Search in ISI Web of Science. The searches were not limited by language.
Study Selection
To be eligible for this review, studies had to fulfill the following inclusion criteria: (1) randomized controlled design; (2) interventions designed to prevent PTSD symptoms; (3) patients who had experienced recent, potentially traumatic medical events including a new diagnosis of a life-threatening illness; (4) intervention initiated within 3 months of the medical event; and (5) patients over 18 years of age. The exclusion criteria were: (1) studies that examined interventions for PTSD induced by caregiver distress; (2) studies that examined PTSD induced by a non-medical event that resulted in medical conditions (e.g., burns from a fire, combat-related injuries); and (3) studies that examined PTSD related to medical procedures but without ongoing, internal threat of recurrence after the event (e.g., pregnancy complications). Studies that included a mixed population (i.e., people who experienced a potentially traumatic medical event and those who experienced other kinds of potentially traumatic events) were also excluded unless the group with medical events could be analyzed separately. Finally, after the initial eligibility assessment was completed based on the criteria above, studies were excluded for which risk of bias was rated as either high or unclear for more than four of the seven bias criteria of the Cochrane Risk of Bias Assessment Tool for RCTs (see the Risk of bias evaluation section below for details) (Higgins & Altman, 2008). Given the relative paucity of literature evaluating early interventions for PTSD in medical populations, we wished to broadly review interventions that may be helpful for preventing medical event-induced PTSD, and thus, we did not require PTSD to be the sole primary outcome of the trial. Nevertheless, the trial needed to be designed to investigate the influence of the early intervention on PTSD symptoms, at least as a secondary outcome.
Two investigators independently reviewed all citations identified through the literature search using a predefined protocol. Articles that clearly did not meet inclusion criteria were excluded at the title-and-abstract level. The remaining articles were selected for full-text review by two independent investigators. Disagreements regarding the selection of articles were resolved through discussion, and full consensus was achieved at each stage of review.
Data extraction
Two investigators independently extracted data from the eligible studies. Key data extracted included: title of study, first author, year and country of publication, study sample size, demographic characteristics of the study sample (age, gender, race, marital status, and education level), description of the PTSD intervention, description of the control group, duration of the trial, diagnostic tool used to assess PTSD symptoms, intervention effect estimates on PTSD symptoms or diagnosis, proportion of eligible patients who agreed to participate, descriptors of compliance with the intervention, and PTSD outcome status (primary, co-primary, or secondary).
Risk of bias evaluation
Two investigators independently classified each study as low risk of bias, high risk of bias, or unclear risk of bias for each of seven criteria in the Cochrane Risk of Bias Assessment Tool for RCTs (Higgins & Green, 2011). Studies were considered to be at overall high risk of bias if they had more than four out of seven criteria with either high or unknown bias. Specifically, risk of bias was assessed in the following seven criteria within six domains of potential bias: selection bias (improper sequence generation or concealment), performance bias (lack of blinding of participants or personnel to PTSD outcomes), detection bias (blinding of the assessors of PTSD outcomes), attrition bias, selective outcome reporting, and other bias. Discrepancies were resolved by consensus.
Results
Literature Search
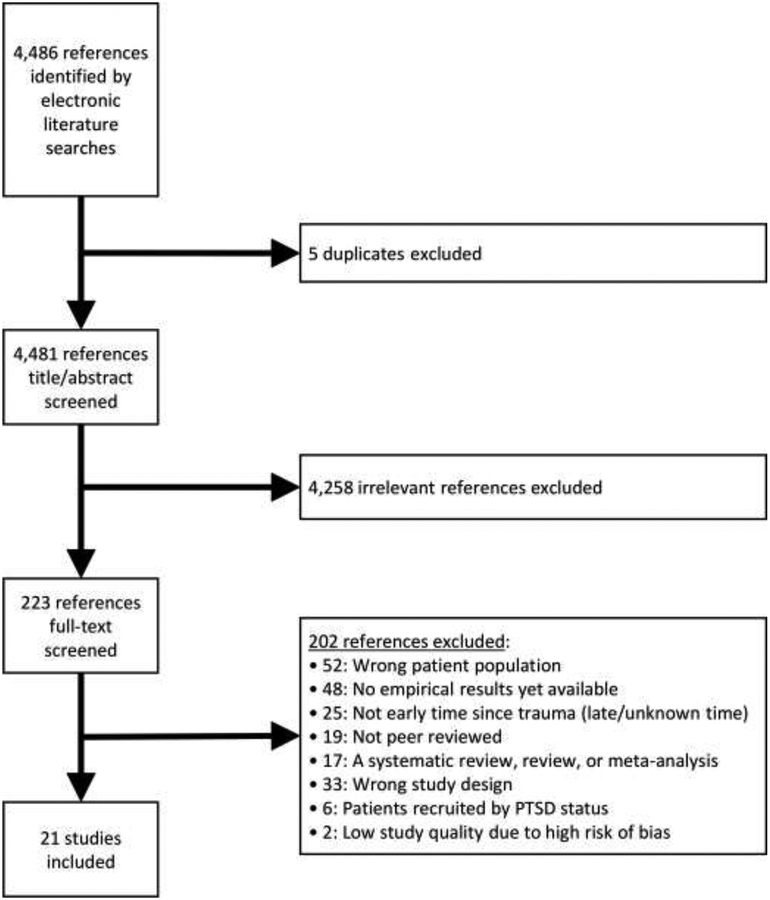
Figure 1 depicts the literature search and selection strategy, which yielded 4,486 studies, of which five duplicates were removed. After screening titles and abstracts, we selected 223 articles for full-text review and ultimately included 21 (3,423 total patients) in the systematic review (see Table 1).
Figure 1.
Flowchart of the systematic literature review
Note. PTSD = posttraumatic stress disorder.
Table 1.
Characteristics of Eligible Studies
| Study | Country | Medical Event |
Sample Size |
Intervention Timing |
Intervention Type |
No. of Intervention Sessions |
Outcome Measure |
Follow-Up Time |
Baseline Distress or High PTSD Risk Required |
PTSD Outcome Status |
Proportion of Eligible Participants Who Agreed to Participate |
Compliance with Intervention |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angell, 2003 | United States | Breast cancer diagnosis | 98 | Moderately Early (>1 month and ≤ 3 months) | Community-based workbook-joumal (administered by breast cancer patients themselves) | Variable (workbook use depended on individual participants’ engagement) | PCL-S | 3* | No (all comers) | Secondary | 83.3% | Not Reported |
| Antoni, 2006 | United States | Breast cancer diagnosis | 199 (ITT) | Moderately Early (>1 month and ≤ 3 months) | Group CBT (administered by postdoctoral fellows and predoctoral trainees) | 10 sessions | IES | 6*, 12 | No (all comers) | Primary | 57.2% | Participants completed an average of 70.8% of the intervention sessions. |
| Chan, 2005 | China | Gynecological cancer diagnosis | 155 (ITT) | Very Early (≤ 1 month) | Individualized psychotherapy (administered by clinical psychologists) | Variable (~2 times/month during active treatment and ~2 times/three-month-period during follow-up) | IES | 3, 6, 9, 12, 15, 18* | No (all comers) | Co-Primary | 96.9% | 2 patients in the intervention group completed 0% of the sessions. The proportion of completed sessions for the rest of the intervention group was not reported. |
| Cox, 2018a | United States | Critical illness and admission to ICU with cardiorespiratory failure | 136 (ITT) | Very Early (≤ 1 month) | CBT via telephone- and web-based coping skills training (administered by clinical psychologists) | 6 telephone sessions plus continuously available web content | IES-R | 3*, 6 | No (all comers) | Secondary | 22.9% | Participants completed an average of 45.0% of the intervention sessions. |
| Cox, 2018b | United States | Critical illness and admission to ICU with cardiorespiratory failure | 69 | Very Early (≤ 1 month) | Mobile-based mindfulness training (administered by a trained psychologist) / telephone-based mindfulness training (administered by a trained psychologist)b | Mobile-based: 1 introductory telephone session and 4 interactive mobile sessions; Telephone based: 4 telephone sessions |
PTSS-10 | 1*, 3 | No (all comers) | Secondary | 73.9% | All sessions were completed by 92% and 93% of participants in the mobile-based and telephone-based intervention groups, respectively. |
| Demoule, 2017 | France | Critical illness and admission to ICU | 34 | Very Early (≤ 1 month) | Earplugs and eye mask (administered by bedside nurses) | Variable (mean of ~7 nights) | IES-R | 3* | No (all comers) | Secondary | 40.8% | 9 patients in the intervention group (28.1%) completed less than 100% of the intervention (i.e., not wearing earplugs for the entire night). The rest of the patients in the intervention group (71.9%) completed 100% of the intervention. |
| El-Jawahri, 2017 | United States | Cancer-related hematopoietic stem-cell transplant treatment | 134 | Very Early (≤ 1 month) | Enhanced palliative care management (administered by two nurse practitioners and one physician) | Variable (median of 8 palliative care visits; range: 4–40) | PCL-C | 6* | No (all comers) | Co-Primary | 86.0% | All patients in the intervention group saw palliative care clinicians at least twice per week. |
| Giese-Davis, 2016 | United States | Breast cancer diagnosis | 104 | Moderately Early (>1 month and ≤ 3 months) | Peer counseling (administered by 30 trained women with previous diagnosis of breast cancer) | Variable (possible weekly meetings for up to 6 months; mean length of ~5 months) | PCL-C | 3, 6, 12* | No (all comers, but excluded significant psychiatric history) | Co-Primary | 71.7% | N/A (no minimum requirement, flexible number of encounters) |
| Irvine, 2011 | Canada | Receipt of first implantable cardioverter defibrillator | 193 (ITT) | Very Early (≤ 1 month) | Telephone CBT (administered by doctoral students in clinical psychology and exercise sciences) | 8 telephone counseling sessions | IES-R | 6, 12* | No (all comers) | Co-Primary | 66.1% | Not Reported |
| Jensen, 2016 | Denmark | Critical illness and admission to ICU | 329 (ITT) | Very Early (≤ 1 month) | Intensive care recovery program: illness narratives, person-centered communication, guided self-determination therapy, trauma-focused CBT (administered by trained study nurses) | 3 consultations with intervention-trained nurses (1 before 3-month follow up) | HTQ-IV | 3*, 12 | No (all comers) | Secondary | 79.6% | 88% of patients in intervention group received at least one consultation, and 71% received all three consultations. |
| Johansson, 2008 | Sweden | Prostate, gastrointestinal, or breast cancer diagnosis | 339 | Very Early (≤ 1 month) | Individual support / group rehabilitation / individual support and group rehabilitation (administered by trained psychologists and dieticians)b | 3 sessions (median) for individual support; 9 sessions for group rehabilitation | IES | 3, 6, 12, 24* | No (all comers) | Co-Primary | 76.7% | For patients in the individual-support intervention group, the median number of psychologist contacts was 3 (range: 1–24). For patients in the group-rehabilitation intervention (GR), 80% of patients participated in 5 or more (out of 9) group sessions. For patients in the individual-plus-group-rehabilitation group: 132 (67%) patients participated in group sessions. Individual session participation not reported. |
| Jones, 2003 | England | Critical illness and admission to ICU | 114 | Very Early (≤ 1 month) | Rehabilitation workbook with advice and exercises (administered by a research nurse) | Variable (6-week rehabilition workbook program depended on individual participants’ engagement) | IES | 2*, 6 | No (all comers) | Co-Primary | Indeterminate | The authors reported that most participants adhered to the intervention. |
| Jones, 2010 | Denmark, Italy, Norway, Portugal, Sweden, and England | Critical illness and admission to ICU | 322 | Moderately Early (>1 month and ≤ 3 months) | ICU diary (written and administered by research nurses, doctors, and the patients’ family members) | Variable (1 session of explanation and receipt of ICU diary; subsequent use depended on individual participants’ engagement) | PDS | 3* | No (all comers, but excluded significant psychiatric history) | Primary | 84.6% | Only 1 patient did not read the ICU diary. |
| Kangas, 2013 | Australia | Head and neck cancer diagnosis | 35 (ITT) | Very Early (≤ 1 month) | CBT (administered by Masters-level clinical psychologists) | 7 sessions (6 once/week followed by a booster session one month after 6th session) | PCL-C, CAPS | 1, 6, 12* | Yes (required significant psychological distress at baseline) | Co-Primary | 87.5% | Participants completed an average of 85.0% of the intervention sessions. |
| Kok, 2016 | The Netherlands | Cardiac surgery with cardiopulmonary bypass | 118 (ITT) | Very Early (≤ 1 month) | High-dose dexamethasone (administered by the surgical team after anesthesia) | 1 intraoperative dose (1 mg/kg) | SRIP | 18–48* | No (all comers) | Primary | 22.3% | Not reported |
| Manne, 2007 | United States | Gynecological cancer diagnosis | 353 (ITT) | Moderately Early (>1 month and ≤ 3 months) | Individual therapy promoting coping and communication / supportive counseling (administered by social workers and psychologists)b | 7 sessions (6 in-person and 1 telephone booster) | IES | 3, 6, 9* | No (all comers) | Co-Primary | 41.4% | For patients in the individual-therapy intervention group, 98 (80.3%) attended some sessions. For patients in the supportive-counseling intervention group, 99 (82.5%) attended some sessions. |
| Schmidt, 2016 | Germany | Critical illness of sepsis and admission to ICU | 218 | Very Early (≤ 1 month) | Enhanced primary care management (administered by ICU nurses as case managers working with consulting physicians) | Variable (all intervention participants received at least 5 additional monitoring calls) | PTSS-10 | 6*, 12 | No (all comers) | Secondary | 80.6% | 104 patients (70.3%) in the intervention group received the planned intervention at high levels of intervention integrity. |
| Treggiari, 2009 | Switzerland | Critical illness and admission to ICU | 102 | Very Early (≤ 1 month) | Light sedation (vs. deep sedation; administered by ICU nurses) | Variable (~7 days) | IES-R or PCL (ranked and then combined into single measure) | 0.25, 1* | No (all comers) | Co-Primary | 61.2% | N/A (compliance did not depend on participants) |
| von Känel, 2018 | Switzerland | Acute coronary syndrome event | 183 (ITT) | Very Early (≤ 1 month) | Trauma-focused CBT (administered by Master-level psychologists and medical students) | 1 session and subsequent self-guided use of information booklet | CAPS, PDS | 3* | Yes (required acute distress during cardiac event) | Primary | 62.5% | Not Reported |
| Walsh, 2015 | Scotland | Critical illness and admission to ICU | 160 | Moderately Early (>1 month and ≤ 3 months) | Intensified rehabilitation care (administered by ward-based therapists and trained research assistants) | Variable (increased daily support depended on individual patients’ needs) | DTS | 3*, 6, 12 | No (all comer) | Secondary | 66.6% | Not Reported |
| Weis, 2006 | Germany | Cardiac surgery with cardiopulmonary bypass | 28 | Very Early (≤ 1 month) | Stress-dose hydrocortisone (administered by the surgical team before anesthesia and intravenously thereafter) | 4 consecutive days of drug infusion (Day 0: 100-mg dose, Day 1: 10 mg/h for 24 hours, Day 2: 5 mg/h for 24 hours, Day 3: 20 mg × 3, Day 4: 10 mg × 3) | PTSS-10 | 6* | Yes (required high risk as indexed by low preoperative left ventricular ejection fraction and long expected duration of cardiopulmonary bypass surgery) | Co-Primary | Indeterminate | Not Reported |
Note. Reported sample sizes correspond to the relevant analysis of PTSD outcomes for this systematic review. However, for intention-to-treat (ITT) analyses, all initially assigned participants are included in that total.
For each included study the asterisk mark the earliest post-intervention time point at which PTSD was assessed.
Baseline N is indicated because the pre-surgery N was not reported.
For three included studies there were multiple types of intervention studied in comparison to the control group.
Abbreviations: CAPS = Clinician Administered PTSD Scale; CBT = cognitive behavioral therapy; DTS = Davidson Trauma Scale; HTQ-IV = Harvard Trauma Questionnaire Part IV; ICU = intensive care unit; IES-R = Impact of Events Scale; IES-R = Impact of Events Scale-Revised; PCL-C = PTSD Checklist-Civilian Version; PDS = Posttraumatic Diagnostic Scale; PTSD = posttraumatic stress disorder; PTSS-10 = Posttraumatic Stress Symptom 10 Question Inventory (modified version for Weis et al., 2006); SRIP = Self-Rating Inventory for PTSD.
Characteristics of the Included Studies
The analyzed sample size varied widely among the 21 studies (range: 28–353), as did the number of months into the study at which PTSD was assessed (range: <1–48 months). The mean ages of participants ranged from 44 to 69 years. The 21 studies were conducted in 15 countries. Fifteen studies involved interventions beginning within 1 month of the medical event; six involved interventions beginning between 1 and 3 months after the event. Across intervention type, PTSD was the primary outcome in 19.0% of the studies, the co-primary outcome in 47.6%, and a secondary outcome in 33.3%.
The most common target populations were patients with cancer diagnoses (8 studies), patients who survived critical illness (e.g., sepsis) that required a stay in the intensive care unit (9), and patients who had a recent, serious cardiac event (i.e., ACS) or who required urgent cardiac surgery (cardiopulmonary bypass or first-time receipt of implantable cardioverter-defibrillator) (4). Within the cancer trauma type, study populations included patients with breast cancer, gynecological cancer, head and neck cancer, and mixed cancer types.
Three trials were restricted to participants with elevated early symptoms of distress (Kangas et al., 2013; von Känel et al., 2018; Weis et al., 2006). Specifically, one study specified that participants have elevated early symptoms of cancer-related PTSD, anxiety, or depression just days after cancer diagnosis (Kangas et al., 2013). Since the DSM-5 specifies that PTSD cannot be diagnosed until symptoms have lasted at least one month, this study’s measurement of PTSD-relevant symptoms just after cancer diagnosis did not constitute a true diagnostic assessment of PTSD (American Psychiatric Association, 2013). One study specified that eligible participants have had high distress about their myocardial infarction as measured by elevated pain and either elevated fear of dying or elevated helplessness on numeric rating scales (von Känel et al., 2018). One study required that eligible participants be at increased risk of chronic stress symptoms due to low preoperative left-ventricular ejection fraction (i.e., < 35%) or a long expected duration of cardiopulmonary bypass surgery (i.e., > 97 minutes) (Weis et al., 2006). The remaining 14 studies included an “all-comer” approach with respect to variability in psychological distress at enrollment. Two of these studies excluded people having a prior PTSD diagnosis at enrollment (Giese-Davis et al., 2016; Jones et al., 2010).
Intervention Characteristics
The types of early PTSD interventions were heterogeneous across the 21 studies: interventions that were partly or entirely psychological in their intended mechanism in 14 studies, purely pharmacological interventions in two, and any other kinds of interventions involving the health system in five. We grouped the following clusters of interventions under the larger category of psychological interventions: interventions best characterized as CBT; those with mixed elements that included components of CBT; mindfulness interventions; and interventions comprising social support, positive meaning-making, and/or coping advice. These groups of studies are described in detail below.
Among the five studies testing CBT interventions, Cox (2018) administered a telephone-based coping skills CBT intervention for patients after discharge from the ICU; Irvine et al. (2011) adapted a CBT intervention for patients after first receipt of an implantable cardioverter-defibrillator (ICD); Kangas et al. (2013) administered a CBT intervention to patients with head and neck cancer; von Känel et al. (2018) tested the effects of single-session counseling with an emphasis on cognitive restructuring in patients with ACS (this trauma-focused intervention encouraged patients to think about their trauma’s meaning with discussion topics including what “trauma” and “PTSD” mean, as well as how to cope with common traumatic reactions and symptoms). Similarly, Antoni et al. (2006) used a CBT-based intervention that emphasized cognitive restructuring in patients with breast cancer, but this intervention occurred in the context of group therapy. Among these interventions characterized primarily by CBT, control conditions varied in whether they were active or inactive (i.e., not controlling for attention). Only the control condition used by Irvine et al. (2011) was inactive (routine medical care; see Table 2).
Table 2.
Summary of Strength of Evidence by Study, Intervention Characteristics, and Target Population
| Intervention | Control | Population | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | PTSD Outcome Status | Type | Type | Cancer | ICU | Cardiac | Strength of Evidence | Standardized Effect Size | ||
| CBT | ||||||||||
| Antoni, 2006 | Primary | Group CBT | Educational program about coping with breast cancer | X | + (only significant for intrusion symptoms; no effect of group on avoidance symptoms) | 0.43 (intrusion symptoms); Not Available (avoidance symptoms) | ||||
| Cox, 2018a | Secondary | Individual CBT | Educational program about understanding critical illness | X | ° | −0.16 | ||||
| Kangas, 2013 | Co-Primary | Individual CBT | Supportive counseling and educational program about head and neck cancer | X | ° | 0.36 | ||||
| Irvine, 2011 | Co-Primary | Individual CBT | Routine medical care | X | ++ (the intervention was associated with decreased PTSD total symptoms, and avoidance symptoms, but not intrusion or hyperarousal symptoms) | 0.29 | ||||
| von Känel, 2018 | Primary | Individual CBT | Stress counseling | X | - (clinician-rated PTSD was comparable for two groups, but self-reported PTSD symptoms higher for intervention vs. control) | 0.13 (clinician-administered); −0.39 (self-reported) |
||||
| Mixed Components | ||||||||||
| Chan, 2005 | Co-Primary | Individualized psychotherapy with CBT components | Routine medical care | X | ° | Not Available | ||||
| Jensen, 2016 | Secondary | Illness narratives, person-centered communication, guided self-determination therapy, and CBT components | Routine medical care | X | ° | Not Available | ||||
| Johansson, 2008 | Co-Primary | Enhanced primary care support, nutrition guidance, and light physical training, and CBT components | Routine medical care | X | ° | −0.22 (avoidance symptoms for individual and group support); 0.00 (intrusion symptoms for individual and group support); −0.13 (avoidance symptoms for individual support); −0.15 (intrusion symptoms for individual support); −0.12 (avoidance symptoms for group support); 0.17 (intrusion symptoms for group support); |
||||
| Manne, 2007 | Co-Primary | Educational materials and CBT components (1st intervention group) | Routine medical care | X | ° | Not Available | ||||
| Mindfulness | ||||||||||
| Cox, 2018b | Secondary | Mindfulness training (mobile-based or telephone-based) | Educational program about understanding critical illness | X | ° | 0.08 (mobile mindfulness vs. educational control); 0.08 (telephone mindfulness vs. educational control) |
||||
| Social support, positive meaning-making, and/or coping advice | ||||||||||
| Angell, 2003 | Secondary | Community-based workbook-j ournal | Routine medical care with educational materials | X | ° | Not Available | ||||
| Giese-Davis, 2016 | Co-Primary | Peer counseling | Routine medical care | X | ° | 0.00 | ||||
| Jones, 2003 | Co-Primary | Rehabilitation workbook | Routine medical care | X | ++ (the intervention was associated with decreased PTSD total symptoms) | 0.43 | ||||
| Jones, 2010 | Primary | ICU diary | Routine medical care | X | + (the intervention was associated with decreased incidence of new-onset PTSD but not with reduction in PTSD total symptoms) | Not Available (PTSD total symptoms); 0.87 (new-onset PTSD diagnosis) | ||||
| Manne, 2007 | Co-Primary | Supportive counseling (2nd intervention group) | Routine medical care | X | ° | Not Available | ||||
| Altered medical management or restructured environment | ||||||||||
| Demoule, 2017 | Secondary | Earplugs and eye masks | Routine medical care | X | ° | 0.43 | ||||
| El-Jawahri, 2017 | Co-Primary | Enhanced palliative care | Routine medical care | X | ++ (the intervention was associated with decreased PTSD total symptoms) | 0.43 | ||||
| Schmidt, 2016 | Secondary | Enhanced primary care | Routine medical care | X | ° | 0.16 | ||||
| Treggiari, 2009 | Co-Primary | Light sedation level | Deep sedation level | X | (+) (the intervention was marginally associated with improved PTSD total symptoms) | 0.34 | ||||
| Walsh, 2015 | Secondary | Enhanced rehabilitation care | Routine medical care with self-help rehabilitation manual | X | ° | −0.05 | ||||
| Pharmacological | ||||||||||
| Kok, 2016 | Primary | Dexamethasone drug administration | Normal saline solution placebo | X | ° | 0.11 | ||||
| Weis, 2006 | Co-Primary | Hydrocortisone drug administration | Normal saline solution placebo | X | ++ (the intervention was associated with decreased PTSD total symptoms) | 1.08 | ||||
Note. Bold rows indicate the presence of significant effects for at least one class of PTSD symptoms. The standardized effect size is equivalent to Cohen’s d and was computed, as needed, based on group differences at the relevant follow-up time specified for each study in Table 1. Positive effect sizes indicate greater reduction of PTSD symptoms for the intervention relative to the control group. Regarding the effect size reported for Johansson et al. (2008), the intervention with combined individual and group support is compared to standard care.
indicates partial support for intervention efficacy (only one subscale).
indicates strong support for intervention efficacy (significant for PTSD total symptoms).
indicates a marginally significant effect supporting intervention efficacy (.10 < p < .05).
indicates partial support for intervention harm (opposite pattern as intended).
indicates no significant evidence of intervention efficacy or harm.
Abbreviations: CBT = cognitive behavioral therapy; PTSD = posttraumatic stress disorder.
Four other studies also relied on CBT principles, but these interventions did not consist primarily of CBT (i.e., they emphasized other important elements). Chan et al. (2005) used a psychotherapy intervention for patients with gynecological cancer that was tailored to each patient and that featured specific elements (e.g., techniques for managing stress, pain, and distress and CBT principles to manage mood symptoms). Notably, this personalized intervention emphasized not asking patients to discuss their feelings about cancer if the topic felt uncomfortable. Johansson et al. (2008) used individual-support and group-based interventions, following a new cancer diagnosis, that used non-CBT elements of enhanced primary care support, nutrition guidance, and light physical training—as well as CBT techniques (e.g., identifying and challenging negative thoughts). Manne et al. (2007) used an intervention for women diagnosed with gynecological cancer that included CBT components (e.g., cognitive restructuring, at-home behavioral tasks) and non-CBT educational materials about gynecological cancer and its effects on sexual functioning. Jensen et al. (2016) used a multifaceted intervention in patients with a critical illness and admission to the ICU. It centered on nurse-led dialogues (to help the patient construct an illness narrative) and elements of trauma-focused CBT. It included post-ICU information pamphlets and reflection sheets with guided self-determination exercises.
The remaining studies used diverse psychological and pharmacological interventions. Within this especially mixed group of PTSD-prevention approaches, six studies (including the second intervention of Manne et al., 2007) used non-CBT psychological interventions. Five of the six used interventions comprising social support, positive meaning-making, and/or coping advice. Jones et al. (2010) used ICU diaries completed by hospital staff and patients’ families to improve patients’ understanding of their experiences after critical illnesses. Giese-Davis (2016) used an intervention involving one-on-one emotional support and advice between breast-cancer patients and women who had undergone similar experiences and were at least one year from their diagnosis. Angell et al. (2003) used a workbook intervention for cancer patients that involved contributing to a shared journal written for and by women with breast cancer. Jones et al. (2003) used a multi-component rehabilitation intervention for post-ICU patients after critical illness that included advice and associated exercises for handling psychological and physical issues. Manne et al. (2007) investigated a supportive counseling intervention intended to boost patients’ self-esteem, emotional expression, sense of autonomy, and existing coping behaviors. Finally, among studies using a psychological approach to PTSD prevention, Cox et al. (2018b) administered a mindfulness intervention by telephone or mobile device to post-ICU patients.
Five studies tested interventions involving altered medical management or environmental restructuring. Specifically, Schmidt et al. (2016) used enhanced primary-care management in survivors of sepsis after ICU discharge; it entailed increased monitoring of patients’ physical symptoms (e.g., sensory defects, swallowing difficulty, trouble with fine motor tasks) and mental symptoms (e.g., fear, avoidance due to negative emotion). Case managers and consulting physicians provided extra support in clinical decision-making for patients in the intervention group by communicating with patients’ primary care physicians. Similarly, Walsh et al. (2015) used an intervention involving enhanced rehabilitation efforts for patients after ICU discharge: increased patient-specific treatments related to mobilization, exercise, diet therapy, occupational therapy, or speech/language therapy. Demoule et al. (2017) administered an ICU-based intervention for critically ill patients involving nighttime use of earplugs and eye masks, hypothesized to reduce PTSD and other emotional symptoms by improving sleep quality in the ICU. Treggiari et al. (2009) systematically varied the level of sedation (light vs. deep) in endotracheally intubated ICU patients by altering the titration goals of sedating medications, to ensure that patients in the intervention were awake and aware rather than asleep. These investigators hypothesized that light sedation would reduce PTSD symptoms by minimizing the occurrence of delusional, traumatic memories of ICU experiences.
Two studies used an intervention with a purely pharmacological mechanism of action. Because functioning of the hypothalamic-pituitary-adrenal axis is disrupted in PTSD (e.g., higher cortisol reactivity and lower cortisol suppression; De Kloet et al., 2006), these interventions were hypothesized to restore stress hormone homeostasis, potentially impeding the downstream occurrence of traumatic memory symptoms as well (Yehuda, 2009). Kok et al. (2016) administered a single, high, intraoperative dose of dexamethasone in cardiac patients immediately before cardiopulmonary bypass surgery. Weis et al. (2006) administered a stress dose of hydrocortisone in high-risk cardiac patients immediately before and for 4 days after cardiopulmonary bypass surgery.
Risk of Bias Assessment
Figure 2 summarizes each study’s risk of bias, which was low for most of the seven categories in most studies. However, given the inherent inability to blind participants and, especially, treatment providers, most studies (76.2%) had high risk of bias for blinding of participants and personnel. This imperfect blinding may have influenced outcomes because of the frequent lack of a placebo-control condition that was well matched to the intervention condition. As a result, participants in the control group may have realized that they received only usual care. The same was true for blinding of the assessors of outcomes (61.9% of studies), mainly because participants themselves were the assessors of self-reported PTSD symptoms. Finally, attrition bias was high, given the incomplete outcome data for 47.6% of studies. The remaining four sources of bias were either low or indeterminate across the 21 studies. Across all included studies, 16 out of 119 total characteristics (11.6%) had indeterminate bias. Seven studies (33.3%) had high risk of bias in three of the seven categories (Cox, Hough, Carson, et al., 2018; Cox, Hough, Jones, et al., 2018; Demoule et al., 2017; Jensen et al., 2016; Manne et al., 2007; Schmidt et al., 2016; Walsh et al., 2015).
Figure 2.
Risk of bias of eligible studies
Note. Plus signs indicated low risk of bias. Minus signs indicate high risk of bias. Question marks indicate unclear risk of bias.
Efficacy of Early Interventions to Prevent PTSD
We determined that the included studies were not appropriate for meta-analysis due to heterogeneity of study interventions, control groups, and patient populations. Below we provide the key inferential statistics, to the extent reported, that were associated with each significant or null finding. Table 2 presents a summary of efficacy findings organized by intervention type and type of medical population.
CBT interventions.
Two of the five interventions consisting primarily of CBT showed significant evidence of efficacy in reducing PTSD symptoms. Antoni et al. (2006) used latent growth curve modeling to show that intrusive thoughts at 6 months were lower for cancer patients who completed the group-based CBT intervention relative to the informational control, z = 2.38, p < .03, Cohen’s d = 0.43. However, there was no parallel difference in avoidance symptoms, which declined over time similarly for both groups. Tests of overall PTSD symptom total scores were not reported. Similarly, but with a differing pattern of implicated symptoms, Irvine (2011) showed a main effect of the CBT intervention group relative to the usual care group on 12-month total PTSD symptoms in cardiac patients with ICDs, F(1, 180) = 3.92, p < .05, ηp2 = 0.02, an effect that was driven by avoidance symptoms and not intrusive or hyperarousal symptoms. In contrast, Cox et al. (2018a) showed no effect of CBT-based coping skills training on total PTSD symptoms, p = .22, Cohen’s d = −0.16 (greater improvement in symptoms for the educational control). Kangas et al. (2013) showed no interaction of group and time on total PTSD symptoms in head-and-neck cancer patients across the 12-month study for CBT vs. supporting counseling, F = 1.08, p = .36. Likewise, von Känel et al. (2018) showed no evidence of efficacy for the trauma-focused, cognitive-restructuring counseling in cardiac patients. Three-month PTSD symptoms assessed by a clinician interview were comparable for the trauma-focused CBT intervention relative to stress counseling, Cohen’s d = 0.13, p = .40. Unexpectedly, self-reported 3-month total PTSD symptoms in this same study were significantly higher in the intervention group (M = 6.54, 95% CI [4.95, 8.14]) vs. the stress-counseling group (M = 3.74, 95% CI [2.39, 5.08]), Cohen’s d = −0.39, p = .02. Thus, findings differed for this study depending on the measure and outcome assessor: a null effect for clinician-rated PTSD and a significant effect in the opposite of the hypothesized direction for patient-rated PTSD.
Interventions using mixed approaches that included CBT components.
There was no evidence of PTSD prevention in the four studies with mixed approaches. Chan et al. (2005) showed that PTSD symptoms across the 18-month study were nonsignificantly lower for the individually tailored psychotherapy intervention relative to the control group in gynecological cancer patients. This lack of efficacy was evident for intrusion symptoms (group difference = −0.27, SE = 1.21, p = .82) and avoidance symptoms (group difference = − 0.30, SE = 1.20, p = .81). Johansson et al. (2008) showed no interaction of group and time across the 24-month study on intrusion symptoms, F = 0.3, p = .8, or avoidance symptoms, F = 1.3, p = .3, for a mixed intervention involving elements of CBT, primary care support, nutrition guidance, and physical training in cancer patients. Manne et al. (2007) showed that total PTSD symptoms decreased substantially over time for gynecological cancer patients across all three groups (coping and communication therapy, supportive counseling, and usual care), t = −5.72, p < .01. There were nonsignificant interactions of group and time on total PTSD symptoms, intrusion symptoms, and avoidance symptoms across the 9-month study (inferential statistics not reported). Jensen et al. (2016) showed that 3-month total PTSD symptoms in post-ICU patients did not differ for the multifaceted intervention relative to standard care, absolute difference = 0.24 [−2,07, 2.55], p = .84.
Interventions using social support, positive meaning-making, or coping advice.
PTSD prevention efficacy findings were mixed for this set of interventions with two showing evidence of PTSD symptom reduction. Jones et al. (2003) showed that 2-month total PTSD symptoms were significantly lower for post-ICU patients who received the advice-based self-help rehabilitation manual intervention than for control patients who received standard rehabilitation care only, F(1, 112) = 5.24, p = .03. Jones et al. (2010) showed that changes in total PTSD symptoms assessed by diagnostic interview from 1-month to 3-month after ICU admission did not differ for intervention patients who received ICU diaries relative to control patients, p = 0.74 (Mann-Whitney U test). Critically, however, the ICU diary intervention was associated with a lower incidence of new-onset PTSD diagnosis at 3 months (5%) relative to the control condition (13.1%), χ2 = 7.15, p = .02. Two studies showed null effects. Specifically, Angell et al. (2003) showed no main effect of workbook intervention relative to usual care on breast cancer patients’ 3-month total PTSD symptoms, p < .05. Giese-Davis et al. (2016) showed a null interaction of group and time on total PTSD symptoms across the 12-month study for cancer patients who engaged in one-on-one meetings with peer navigators relative to patients with usual care, B = −0.0002, 95% CI [−0.39, 0.38], p = .96.
Mindfulness interventions.
Relative to the educational control program, neither the mobile-based, Cohen’s d = 0.08, nor the telephone-based mindfulness interventions, Cohen’s d = 0.08, showed improved PTSD symptoms at 1 month (Cox et al., 2018b). The reductions in total PTSD symptoms from baseline to 1 month were comparable for mobile-based mindfulness (−1.7), telephone-based mindfulness (−1.7), and educational control (−1.0).
Interventions using altered medical management or environmental restructuring.
Just one intervention in this set showed evidence of efficacious PTSD symptom reduction. Specifically, the palliative care intervention of El-Jawahri et al. (2017) improved PTSD symptoms relative to usual care, adjusted mean group difference = 4.02 (95% CI: 0.86 – 7.18), p = .013. In contrast, Demoule et al. (2017) showed that the earplugs-and-eye-mask intervention (median = 11, interquartile range: 5–18) was associated with nonsignificantly lower 90-day total PTSD symptoms than routine care in ICU patients (median = 16, interquartile range: 9–27), p = .15. Schmidt et al. (2016) reported that baseline-to-6-month change in total PTSD symptoms did not differ between post-ICU patients in the enhanced management intervention (with monitoring of sepsis-relevant symptoms) and those in usual care (group difference = −1.8, 95% CI [−4.8, 1.2], p = .24). Treggiari et al. (2009) showed a marginally significant difference in 1-month total PTSD symptoms for ICU patients in light (intervention) relative to deep (comparison group) sedation (group difference = −10, 95% CI [−20.9, 2.0], p = .07). However, the groups did not differ in the proportion with a 1-month PTSD diagnosis computed from self-reported symptoms, p = .83. Walsh et al. (2015) showed that 3-month total PTSD symptoms in post-ICU patients were nonsignificantly higher for the enhanced rehabilitation intervention relative to usual care (group difference = 0, 95% CI [−4, 3], p = .83).
Intervention using pharmacology.
One of the two eligible studies with a purely pharmacological intervention demonstrated evidence of efficacy. Cardiac-surgery patients assigned to stress-dose hydrocortisone administration reported lower 6-month total PTSD symptoms (median = 15.5, interquartile range [14.8 – 21.8]) than those assigned to placebo (median = 25.5, interquartile range [16.8 – 33.0]), p = .03 (Weis et al., 2006). In contrast, Kok et al. (2016) did not demonstrate efficacy of a single, pre-surgical dose of dexamethasone in cardiac patients for preventing PTSD diagnosis assessed 1.5 to 4 years later, OR = 0.82 (95% CI: 0.55 – 1.20), p = 0.30. However, a subgroup analysis revealed that the drug was associated with lower prevalence of PTSD diagnosis among women, OR = 0.23 (95% CI: 0.07 – 0.72), p < .01, but not men, OR = 0.93 (95% CI: 0.56–1.49), p = .76.
Discussion
Our systematic review of early interventions to prevent medical event-induced PTSD revealed a relative paucity of research in this area. Only 21 relevant studies with low-to-moderate risk of bias addressed this important issue for different types of traumatic medical events using a variety of interventional approaches. There is currently no early intervention with strong evidence for reducing PTSD after acute medical events. The majority of the interventions tested thus far have not resulted in statistically significant benefits. Nevertheless, there were some promising findings that suggest directions for future research. Interventions with at least partial evidence of limiting medical event-induced PTSD were as follows: group CBT for cancer patients; individual CBT and stress-dose hydrocortisone for cardiac patients; enhanced palliative care for cancer patients; advice via rehabilitation workbook and receipt of an ICU diary for survivors of critical illness. The hydrocortisone treatment and the ICU diary showed large effect sizes for PTSD symptom reduction, but it should be noted that both interventions pertained to only one trial each, and the latter effect concerned new-onset PTSD diagnosis but not PTSD total symptoms. The efficacious CBT-based, palliative-care, and rehabilitation-workbook interventions showed small to moderate effect sizes. Notably, there was no compelling support for interventions of mixed type. Furthermore, acceptability and compliance with many of these interventions was relatively high, suggesting the possibility of broad uptake of early PTSD interventions, should clearly efficacious interventions eventually emerge.
Addressing the Aims of the Systematic Review
Aim 1: Assess the preventability of medical event-induced PTSD symptoms.
Given the heterogeneity of patient populations and efficacious interventions, it is challenging to identify a common set of active ingredients across interventions. That said, there was a signal that more intensive interventions may be needed. For example, within CBT interventions, the two efficacious ones involved a higher number of sessions (8–10) than the unsuccessful ones (1–7). With respect to the interventions involving exogenous glucocorticoids, the multi-day dosing intervention was superior to the single-dose intervention. This review revealed a notable absence of certain evidence-based trauma-focused interventions for PTSD, such as cognitive processing therapy, prolonged exposure, and written exposure therapy (Powers, Halpern, Ferenschak, Gillihan, & Foa, 2010; Resick et al., 2008; Sloan, Marx, Lee, & Resick, 2018). Future research is much needed to test these interventions’ efficacy when applied specifically toward preventing medical event-induced PTSD.
These findings should be considered from the perspective of whether PTSD was a primary or secondary outcome measure because some interventions were developed to influence PTSD symptoms directly and others indirectly (e.g., PTSD prevention via improved sleep quality in the ICU). Notably, all six studies that showed evidence for PTSD symptom reduction targeted PTSD as a primary or co-primary outcome. There was evidence for PTSD symptom reduction in two of the four studies for which PTSD was the sole primary outcome. In contrast, none of the seven studies for which PTSD was a secondary outcome showed evidence for PTSD symptom reduction. Although all efficacious studies had PTSD symptoms as a primary or co-primary outcome, fewer than half of studies with PTSD symptoms as a primary or co-primary outcome were efficacious.
Aim 2: Evaluate whether efficacy varies for early vs. later interventions.
We found no strong indication that the timing of early PTSD interventions mattered. There was evidence of efficacy for both the early (< 1 month; El-Jawahri et al., 2017; Irvine et al., 2011; Jones et al., 2003; Weis et al., 2006) and later (> 1 month and < 3 months; Antoni et al., 2006; Jones et al., 2010) interventions. However, all four of the studies with the strongest evidence favoring PTSD prevention intervened within the first month of the potentially traumatic medical event, and the hydrocortisone intervention, in particular, began extremely early, on the very day of the potentially traumatic cardiac surgery. Kearns et al. (2012) suggested that the most effective time window to intervene may be within hours, not weeks, of the trauma, but this possibility requires empirical support.
Aim 3: Characterize patient acceptability of early interventions in medical settings.
The average intervention acceptability rate was high; the majority of eligible participants enrolled in the RCTs (66.4%; see Table 1 for the breakdown by study). Thus, many patients who had recently experienced potentially traumatic medical events found the proposed study procedures acceptable, at least initially. Acceptability was greatest for the category of mixed interventions with CBT components (M = 73.7%, SD = 23.3%) and for interventions involving social support, positive meaning-making, and coping advice (M = 70.3%, SD = 20.1%). Acceptability was somewhat lower for interventions involving altered medical management and restructured environments (M = 67.0%, SD = 17.8%) and for interventions characterized primarily by CBT (M = 59.2%, SD = 23.3%). It was lowest for purely pharmacological interventions (22.3% for Kok et al., 2016; indeterminate for Weis et al., 2006). Future research should determine whether pharmacological interventions for PTSD prevention are less acceptable to medical patients, especially given that this type of intervention shows promise of efficacy.
Compliance with the interventions was generally high, but reporting gaps limited the ability to assess this question fully. Nevertheless, among studies that did report compliance information, most participants completed the majority of the intended intervention sessions or components (see Table 1).
Aim 4: Examine efficacy for studies of high-risk patients vs. all-comers.
One of the potential pitfalls of early interventions is that PTSD symptoms often naturally recover for many people in the acute aftermath of a trauma or may even be minimal shortly after the event (Bonanno, 2004; Rothbaum, Foa, Riggs, Murdock, & Walsh, 1992). As a result, some advocate for targeting individuals at high risk of developing PTSD, rather than administering interventions to all individuals. This review revealed preliminary evidence that efficacy may differ for interventions focusing on people with high risk for PTSD relative to interventions applied to people with acute medical events more generally. In contrast to expectations derived from the larger literature on the prevention of PTSD due to a variety of causes (Howlett & Stein, 2016), our review did not show the most evidence of CBT’s efficacy among people with high distress at baseline. Instead, the only two efficacious CBT interventions allowed all-comers with respect to early symptoms of distress, whereas the two studies of CBT interventions that enrolled only high-distress participants were actually not efficacious. However, the hydrocortisone intervention was successful with the enrollment of only participants who were believed to be at elevated risk of developing PTSD. The collective meaning of these findings should be interpreted cautiously, and future research should examine how initial distress symptoms may influence the efficacy of PTSD prevention strategies.
Aim 5: Identify any evidence that early interventions may be harmful for PTSD.
Just one study showed any evidence consistent with the risk that early interventions may be harmful with respect to worsened PTSD symptoms. This effect was evident only for self-reported and not clinician-assessed symptoms (von Känel et al., 2018). As considered above, these participants were limited to cardiac patients who experienced particularly high levels of early distress. One important consideration is that this study relied on a 1-session intervention with follow-up using a self-guided information booklet. It may be that single-session trauma-focused CBT interventions are potentially harmful, even in the context of providing follow-up, CBT-related information. However, no firm conclusions can be drawn because this result pertains to a single study, the clinician-rated PTSD symptoms did not differ by group, the comparison of interest was an active control that may have reduced PTSD symptoms more than a passive control would have done, and both groups showed low PTSD symptoms at follow-up.
Comparisons with the Broader Literature on PTSD Prevention
These preliminary findings for medical event-induced PTSD prevention should be considered in light of growing evidence from the broader literature that PTSD can be prevented via certain psychological and pharmacological interventions. A systematic review on interventions to prevent PTSD after a wide range of traumas beyond medical events focused on studies in which preventive interventions were administered within 3 months of trauma exposure, as PTSD symptoms generally onset within this timeframe (Forneris et al., 2013). Although most studies included in that review were too heterogeneous to meta-analyze, three studies were examined in a meta-analysis of trauma-focused CBT for individuals with acute stress disorder, an early manifestation of posttraumatic stress symptoms within the first month of a trauma, before PTSD can be diagnosed. Trauma-focused CBT reduced intrusion and avoidance PTSD symptoms more than a supportive counseling condition. A more recent review concluded that CBT showed evidence for preventing PTSD, and this effect was most clear among people with elevated acute stress disorder symptoms following the trauma (Howlett & Stein, 2016). In contrast, single-session psychological debriefing interventions were not efficacious. The authors of this same review concluded that collaborative care interventions to prevent PTSD showed potential but needed further research before a firm conclusion could be reached. A separate meta-analysis revealed that pharmacological interventions in general did not prevent PTSD, but hydrocortisone in particular showed a large effect size in reducing risk of PTSD development (Sijbrandij et al., 2015). In totality, our results were largely consistent with these prior results from the broader literature on PTSD prevention in that CBT and hydrocortisone emerged as promising pharmacological interventions. Additionally, our review expanded on the range of possible interventions by showing efficacy for two social-support interventions and one palliative-care intervention formulated specifically for the aftermath of serious medical events (El-Jawahri et al., 2017; Jones et al., 2010; Jones et al., 2003).
Another concern about early interventions is that PTSD symptoms may be exacerbated rather than reduced for some people. For example, a debriefing intervention soon after potentially traumatic events showed no evidence of reducing PTSD symptoms in the short term, and in fact, PTSD symptoms were actually worse for the intervention group in a subset of studies that followed people over a long timeframe (Rose, Bisson, Churchill, & Wessely, 2002). However, no debriefing interventions were tested for medical event-induced PTSD symptoms, and we saw only very limited evidence that early interventions could be psychologically harmful for PTSD symptoms.
Limitations
Although this review suggests several promising interventions for preventing PTSD induced by medical events, there were gaps in the literature base that limited our conclusions. First, most studies relied on patient-reported PTSD symptoms rather than symptoms ascertained during diagnostic interviews. Some variability in the results across studies may be attributable to this factor. As mentioned above, one of the studies yielded findings that differed in surprising ways depending on the measure and outcome assessor (null effect for clinician-rated PTSD and significant effect in opposite of hypothesized direction for patient-rated PTSD) (von Känel et al., 2018). Second, the analyzed sample size for some studies was underpowered to detect small effects (Demoule et al., 2017; Weis et al., 2006). This limitation was acceptable in light of our goal to conduct a systematic review of the literature relevant to preventing PTSD in those with potentially traumatic medical events. Third, the results of this review should be interpreted cautiously as the literature is currently characterized by high levels of heterogeneity in many areas: types of medical trauma, types of intervention strategy, complexity of intervention, number of intervention sessions, duration of follow-up period, control/placebo elements, and statistical approach. Furthermore, the positive results were often limited to particular subscales of PTSD symptoms rather than total symptoms. Finally, although it could not be assessed for this preliminary review due to lack of standardized effect sizes, there is a potential risk of publication bias that would lend further need to interpret findings cautiously.
Future Directions
There are several directions for future research in addition to the ones noted above. One potential goal of future research could be to apply an experimental medicine approach to developing and testing PTSD interventions in which interventions are first tested based on their ability to influence proximal targets that constitute potential mechanisms by which interventions reduce PTSD symptoms after acute medical events. This approach is now being recommended in behavior change research (Sumner, Beauchaine, & Nielsen, 2018).
Further work is needed to test specific mechanisms that underlie the success of PTSD interventions, as has been argued and explored by Tryon (2005) with respect to exposure therapy. For example, do seemingly disparate prevention interventions (e.g., CBT and ICU diaries) actually share some of the same efficacious mechanisms (e.g., fear habituation, improved self-efficacy)? Likewise, do seemingly similar interventions (e.g., one-on-one CBT and group-based CBT) actually have different efficacious mechanisms?
Even after empirically supported interventions have been developed, greater consistency is needed regarding the procedural details and active ingredients within each intervention type. This consistency will allow the field to draw more firm conclusions about efficacy of particular PTSD prevention interventions. A prime example is the category of interventions that consist primarily of CBT elements. Even in this relatively narrow category, there are wide procedural discrepancies (e.g., which CBT elements are emphasized, number of sessions) that hinder the ability to state definitively whether and which form of CBT is efficacious for prevention of medical event-induced PTSD. A meta-analysis will be warranted and useful once a sizable number of high-quality, well powered studies exist that share clearly specified intervention components and for which PTSD is a primary outcome measure.
Future research should examine how intervention efficacy varies for individuals. Further work is needed to determine if efficacy depends on baseline distress or perhaps fear of death. The differences in trajectories of PTSD symptoms over the months following traumatic events (e.g., resilient, recovery, delayed-reaction, and chronic-distress trajectories; Bryant, 2015) point to the difficulty of early identification of people most in need of PTSD prevention efforts because symptoms develop late for some people. Nevertheless, identifying factors that predict greater likelihood of membership in chronic or delayed onset PTSD vs. resilient or spontaneous recovery PTSD trajectories (e.g., lower education; Bonanno et al., 2012) may be useful for identifying patients most likely to benefit from early PTSD interventions.
Future research may also attempt to tease apart whether distinct early interventions are needed to prevent PTSD due to life-threatening medical events that result in ongoing elevated risk of recurrent events (e.g., ACS, stroke; Edmondson, 2014) versus PTSD due to temporary, but highly disturbing hospital experiences (e.g., delusional, threatening interpretations of intubation due to prolonged sedation in the ICU; witnessing deaths of other patients in the emergency department; Jones et al., 2007; Jones, Griffiths, Humphris, & Skirrow, 2001; McGiffin, Galatzer-Levy, & Bonanno, 2016). This distinction, however, is difficult to make in practice due in part to the frequent co-occurrence of potentially traumatic medical conditions and distressing aspects of the medical environment.
Future research might test sequential combinations of evidence-based, early pharmacological and psychological interventions. For example, it may be beneficial to examine whether two of the promising interventions in this review—i.e., hydrocortisone treatment within a week of medical trauma and CBT intervention within 1 month of the trauma—may be effectively administered for the same patients to achieve prevention outcomes. Of course, special safety considerations would need to be taken using these approaches in this sensitive medical population.
Finally, future research is needed to test extremely early interventions in the hospital setting within hours or days—rather than weeks or months—of distressing medical events. Initial findings suggest that such early interventions may hold promise for preventing PTSD in response to traumas other than medical events (Kearns et al., 2012). Applying this type of research to medical populations can test whether an initial minimization of traumatic memory consolidation (Kearns et al., 2012), for example, can be achieved and leveraged to prevent the subsequent development of medical event-induced PTSD.
Concluding Remarks
In light of extensive literature showing that PTSD is common after acute medical events and contributes to adverse outcomes, the time has come for additional resources to be devoted to PTSD prevention research. This review revealed preliminary and limited evidence that elevated PTSD symptoms due to life-threatening medical events might be preventable. There is at least partial support for the potential efficacy of interventions that may eventually prevent elevated PTSD symptoms related to cancer diagnosis, critical care for life-threatening illness, and stressful cardiac procedures. However, across studies, the substantial heterogeneity of interventions and contexts obscured the ability to draw conclusions about the promise of specific interventions. Fortunately, PTSD caused by internal, medical events has one crucially beneficial feature that is generally not shared by PTSD caused by external events. Medical professionals who can aid in the administration of prompt, standardized interventions are often present soon after or sometimes even before the onset of potentially traumatic medical events. Examples include physicians revealing life-threatening cancer diagnoses, conducting stressful ICU procedures, and performing cardiac surgeries. This line of research has the potential to transform medical care by incorporating evidence-based, in-hospital protections against the development of dangerous and debilitating PTSD symptoms.
Supplementary Material
Highlights.
Interventions to prevent PTSD symptoms due to terrifying medical events were reviewed
Interventions were multifaceted and characterized by heterogeneous components
Acceptability of early intervention was high among ICU, cancer, and cardiac patients
Some CBT, meaning-making, palliative, and drug interventions reduced PTSD symptoms
Research is needed to identify optimal PTSD prevention strategies after medical events
Funding:
This work was supported by the National Heart, Lung, and Blood Institute (R01 HL1223368, R01 HL117832, and R01 HL132347, and K01 HL130650).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: None.
References
- Abbey G, Thompson SB, Hickish T, & Heathcote D (2015). A meta-analysis of prevalence rates and moderating factors for cancer-related post-traumatic stress disorder. Psycho-Oncology, 24(4), 371–381. doi: 10.1002/pon.3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (1987). Diagnostic and statistical manual of mental disorders; revised (DSM-III-R). Washington DG. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®): American Psychiatric Pub. [Google Scholar]
- American Psychiatry Association. (1994). Diagnostic and statistical manual of mental disorders (DSM-IV). American Psychiatry Association, Washington, DC. [Google Scholar]
- Angell KL, Kreshka MA, Coy R, Donnelly P, Turner-Cobb JM, Graddy K, … Koopman C (2003). Psychosocial intervention for rural women with breast cancer. Journal of General Internal Medicine, 18(7), 499–507. doi: 10.1046/j.1525-1497.2003.20316.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Wimberly SR, Lechner SC, Kazi A, Sifre T, Urcuyo KR, … Guellati S (2006). Reduction of cancer-specific thought intrusions and anxiety symptoms with a stress management intervention among women undergoing treatment for breast cancer. American Journal of Psychiatry, 163(10), 1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson JI, Ehlers A, Matthews R, Pilling S, Richards D, & Turner S (2007). Psychological treatments for chronic post-traumatic stress disorder: Systematic review and meta-analysis. The British Journal of Psychiatry, 190(2), 97–104. doi: 10.1192/bjp.bp.106.021402 [DOI] [PubMed] [Google Scholar]
- Bonanno GA (2004). Loss, trauma, and human resilience: Have we underestimated the human capacity to thrive after extremely aversive events? American Psychologist, 59(1), 20–28. doi: 10.1037/0003-066X.59.1.20 [DOI] [PubMed] [Google Scholar]
- Bonanno GA, & Mancini AD (2008). The human capacity to thrive in the face of potential trauma. Pediatrics, 121(2), 369–375. [DOI] [PubMed] [Google Scholar]
- Bonanno GA, Mancini AD, Horton JL, Powell TM, LeardMann CA, Boyko EJ, … Smith TC (2012). Trajectories of trauma symptoms and resilience in deployed US military service members: Prospective cohort study. The British Journal of Psychiatry, 200(4), 317–323. doi: 10.1192/bjp.bp.111.096552 [DOI] [PubMed] [Google Scholar]
- Bryant RA (2015). Chapter 7: Early intervention after trauma In Schnyder U & Cloitre M (Eds.), Evidence Based Treatments for Trauma-Related Psychological Disorders (pp. 125–142). Cham, Switzerland: Springer. [Google Scholar]
- Chan Y, Lee PW, Fong DY, Fung AS, Wu LY, Choi AY, … Wong L (2005). Effect of individual psychological intervention in Chinese women with gynecologic malignancy: A randomized controlled trial. Journal of Clinical Oncology, 23(22), 4913–4924. doi: 10.1200/JCO.2005.02.069 [DOI] [PubMed] [Google Scholar]
- Chang BP, Sumner JA, Haerizadeh M, Carter E, & Edmondson D (2016). Perceived clinician–patient communication in the emergency department and subsequent post-traumatic stress symptoms in patients evaluated for acute coronary syndrome. Emergency Medicine Journal, 626–631. doi: 10.1136/emermed-2015-205473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CE, Hough CL, Carson SS, White DB, Kahn JM, Olsen MK, … Porter LS (2018). Effects of a telephone-and web-based coping skills training program compared with an education program for survivors of critical illness and their family members. A randomized clinical trial. American journal of respiratory and critical care medicine, 197(1), 66–78. doi: 10.1164/rccm.201704-0720OC [DOI] [PubMed] [Google Scholar]
- Cox CE, Hough CL, Jones DM, Ungar A, Reagan W, Key MD, … Greeson JM (2018). Effects of mindfulness training programmes delivered by a self-directed mobile app and by telephone compared with an education programme for survivors of critical illness: A pilot randomised clinical trial. Thorax, 0, 1–10. Advance online publication. doi: 10.1136/thoraxjnl-2017-211264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet C, Vermetten E, Geuze E, Kavelaars A, Heijnen C, & Westenberg H (2006). Assessment of HPA-axis function in posttraumatic stress disorder: Pharmacological and non-pharmacological challenge tests, a review. Journal of Psychiatric Research, 40(6), 550–567. doi: 10.1016/j.jpsychires.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Demoule A, Carreira S, Lavault S, Pallanca O, Morawiec E, Mayaux J, … Similowski T (2017). Impact of earplugs and eye mask on sleep in critically ill patients: A prospective randomized study. Critical Care, 21(1), 284–292. doi: 10.1186/s13054-017-1865-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D (2014). An enduring somatic threat model of posttraumatic stress disorder due to acute life-threatening medical events. Social and personality psychology compass, 8(3), 118–134. doi: 10.1111/spc3.12089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, Kronish IM, Wasson LT, Giglio JF, Davidson KW, & Whang W (2014). A test of the diathesis-stress model in the emergency department: Who develops PTSD after an acute coronary syndrome? Journal of Psychiatric Research, 53, 8–13. doi: 10.1016/j.jpsychires.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, Richardson S, Falzon L, Davidson K, Mills M, & Neria Y (2012). Posttraumatic stress disorder prevalence and risk of recurrence in acute coronary syndrome patients: A meta-analytic review. PLoS ONE [Electronic Resource], 7(6), e38915. doi: 10.1371/journal.pone.0038915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, Shimbo D, Ye S, Wyer P, & Davidson KW (2013). The association of emergency department crowding during treatment for acute coronary syndrome with subsequent posttraumatic stress disorder symptoms. JAMA internal medicine, 173(6), 472–475. doi: 10.1001/jamainternmed.2013.2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Jawahri A, Traeger L, Greer JA, VanDusen H, Fishman SR, LeBlanc TW, … Rhodes A (2017). Effect of inpatient palliative care during hematopoietic stem-cell transplant on psychological distress 6 months after transplant: Results of a randomized clinical trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 35(32), 3714–3721. doi: 10.1200/JCO.2017.73.2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fait K, Vilchinsky N, Dekel R, Levi N, Hod H, & Matetzky S (2018). Cardiac-disease-induced PTSD and fear of illness progression: Capturing the unique nature of disease-related PTSD. General Hospital Psychiatry, 53, 131–138. doi: 10.1016/j.genhosppsych.2018.02.011 [DOI] [PubMed] [Google Scholar]
- Forneris CA, Gartlehner G, Brownley KA, Gaynes BN, Sonis J, Coker-Schwimmer E, … Woodell CL (2013). Interventions to prevent post-traumatic stress disorder: A systematic review. American Journal of Preventive Medicine, 44(6), 635–650. doi: 10.1016/j.amepre.2013.02.013 [DOI] [PubMed] [Google Scholar]
- Giese-Davis J, Bliss-Isberg C, Wittenberg L, White J, Star P, Zhong L, … Spiegel D (2016). Peer-counseling for women newly diagnosed with breast cancer: A randomized community/research collaboration trial. Cancer, 122(15), 2408–2417. doi: 10.1002/cncr.30036 [DOI] [PubMed] [Google Scholar]
- Higgins J, & Altman D (2008). Assessing risk of bias in included studies In Higgins JP & Green S (Eds.), Cochrane handbook for systematic reviews of interventions: Cochrane book series (pp. 187–241). Chichester, West Sussex, England: John Wiley & Sons Ltd. [Google Scholar]
- Higgins J, & Green S (2011). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. http://handbook.cochrane.org.
- Hoffman V, Middleton J, Feltner C, Gaynes B, Weber R, Bann C, … Green J (2018). Psychological and Pharmacological Treatments for Adults With Posttraumatic Stress Disorder: A Systematic Review Update Comparative Effectiveness Review No. 207. (Prepared by the RTI International-University of North Carolinaat Chapel Hill Evidence-based Practice Center under Contract No. 290–2015-00011-I for AHRQ and PCORI.) AHRQ Publication No. 18-EHC011-EF. PCORI Publication No. 2018-SR-01. Rockville, MD: Agency for Healthcare Research and Quality; May 2018. Posted final reports are located on the Effective Health Care Program search page. doi: 10.23970/AHRQEPCCER207 [DOI] [PubMed] [Google Scholar]
- Howlett JR, & Stein MB (2016). Prevention of trauma and stressor-related disorders: A review. Neuropsychopharmacology, 41(1), 357–369. doi: 10.1038/npp.2015.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine J, Firestone J, Ong L, Cribbie R, Dorian P, Harris L, … Cameron D (2011). A randomized controlled trial of cognitive behavior therapy tailored to psychological adaptation to an implantable cardioverter defibrillator. Psychosomatic medicine, 73(3), 226–233. doi: 10.1097/PSY.0b013e31820afc63 [DOI] [PubMed] [Google Scholar]
- Jensen JF, Egerod I, Bestle MH, Christensen DF, Elklit A, Hansen RL, … Overgaard D (2016). A recovery program to improve quality of life, sense of coherence and psychological health in ICU survivors: a multicenter randomized controlled trial, the RAPIT study. Intensive care medicine, 42(11), 1733–1743. doi: 10.1007/s00134-016-4522-1 [DOI] [PubMed] [Google Scholar]
- Johansson B, Brandberg Y, Hellbom M, Persson C, Petersson L, Berglund G, & Glimelius B (2008). Health-related quality of life and distress in cancer patients: Results from a large randomised study. British Journal of Cancer, 99(12), 1975–1983. doi: 10.1038/sj.bjc.6604789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, Bäckman C, Capuzzo M, Egerod I, Flaatten H, Granja C, … Griffiths RD (2010). Intensive care diaries reduce new onset post traumatic stress disorder following critical illness: A randomised, controlled trial. Critical Care, 14, R168. doi: 10.1186/cc9260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, Bäckman C, Capuzzo M, Flaatten H, Rylander C, & Griffiths R (2007). Precipitants of post-traumatic stress disorder following intensive care: A hypothesis generating study of diversity in care. Intensive care medicine, 33(6), 978–985. doi: 10.1007/s00134-007-0600-8 [DOI] [PubMed] [Google Scholar]
- Jones C, Griffiths RD, Humphris G, & Skirrow PM (2001). Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Critical Care Medicine, 29(3), 573–580. [DOI] [PubMed] [Google Scholar]
- Jones C, Skirrow P, Griffiths RD, Humphris GH, Ingleby S, Eddleston J, … Gager M (2003). Rehabilitation after critical illness: A randomized, controlled trial. Critical Care Medicine, 31(10), 2456–2461. doi: 10.1097/01.CCM.0000089938.56725.33 [DOI] [PubMed] [Google Scholar]
- Kangas M, Henry JL, & Bryant RA (2005). The course of psychological disorders in the 1st year after cancer diagnosis. Journal of consulting and clinical psychology, 73(4), 763. doi: 10.1037/0022-006X.73.4.763 [DOI] [PubMed] [Google Scholar]
- Kangas M, Milross C, Taylor A, & Bryant RA (2013). A pilot randomized controlled trial of a brief early intervention for reducing posttraumatic stress disorder, anxiety and depressive symptoms in newly diagnosed head and neck cancer patients. Psycho-Oncology, 22(7), 1665–1673. doi: 10.1002/pon.3208 [DOI] [PubMed] [Google Scholar]
- Kearns MC, Ressler KJ, Zatzick D, & Rothbaum BO (2012). Early interventions for PTSD: A review. Depression and Anxiety, 29(10), 833–842. doi: 10.1002/da.21997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok L, Hillegers MH, Veldhuijzen DS, Cornelisse S, Nierich AP, van der Maaten JM, … Dieleman JM (2016). The effect of dexamethasone on symptoms of posttraumatic stress disorder and depression after cardiac surgery and intensive care admission: Longitudinal follow-up of a randomized controlled trial. Critical Care Medicine, 44(3), 512–520. doi: 10.1097/CCM.0000000000001419 [DOI] [PubMed] [Google Scholar]
- Kornor H, Winje D, Ekeberg O, Weisaeth L, Kirkehei I, Johansen K, & Steiro A (2008). Early trauma-focused cognitive-behavioural therapy to prevent chronic post-traumatic stress disorder and related symptoms: A systematic review and meta-analysis. BMC Psychiatry, 8, 81. doi: 10.1186/1471-244X-8-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronish IM, Edmondson D, Li Y, & Cohen BE (2012). Post-traumatic stress disorder and medication adherence: Results from the Mind Your Heart study. Journal of Psychiatric Research, 46(12), 1595–1599. doi: 10.1016/j.jpsychires.2012.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutz I, Garb R, & David D (1988). Post-traumatic stress disorder following myocardial infarction. General Hospital Psychiatry, 10(3), 169–176. [DOI] [PubMed] [Google Scholar]
- Manne SL, Rubin S, Edelson M, Rosenblum N, Bergman C, Hernandez E, … Winkel G (2007). Coping and communication-enhancing intervention versus supportive counseling for women diagnosed with gynecological cancers. Journal of consulting and clinical psychology, 75(4), 615–628. doi: 10.1037/0022-006X.75.4.615 [DOI] [PubMed] [Google Scholar]
- McGiffin JN, Galatzer-Levy IR, & Bonanno GA (2016). Is the intensive care unit traumatic? What we know and don’t know about the intensive care unit and posttraumatic stress responses. Rehabilitation psychology, 61(2), 120–131. doi: 10.1037/rep0000073 [DOI] [PubMed] [Google Scholar]
- Meli LA, Alcantara C, Sumner JA, Swan B, Chang BP, & Edmondson D (2017). Enduring somatic threat perceptions and PTSD symptoms in survivors of cardiac events. Journal of Health Psychology, 1–10. doi: 10.1177/1359105317705982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, & Group P (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, & Foa EB (2010). A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clinical Psychology Review, 30(6), 635–641. doi: 10.1016/j.cpr.2010.04.007 [DOI] [PubMed] [Google Scholar]
- Presciutti A, Verma J, Pavol M, Anbarasan D, Falo C, Brodie D, … Claassen J (2018). Posttraumatic stress and depressive symptoms characterize cardiac arrest survivors’ perceived recovery at hospital discharge. General Hospital Psychiatry, 53, 108–113. doi: 10.1016/j.genhosppsych.2018.02.006 [DOI] [PubMed] [Google Scholar]
- Resick PA, Galovski TE, Uhlmansiek MOB, Scher CD, Clum GA, & Young-Xu Y (2008). A randomized clinical trial to dismantle components of cognitive processing therapy for posttraumatic stress disorder in female victims of interpersonal violence. Journal of consulting and clinical psychology, 76(2), 243–258. doi: 10.1037/0022-006X.76.2.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SC, Bisson J, Churchill R, & Wessely S (2002). Psychological debriefing for preventing post traumatic stress disorder (PTSD). Cochrane Database of Systematic Reviews, Issue 2 Art. No.: CD000560. doi: 10.1002/14651858.CD000560. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Foa EB, Riggs DS, Murdock T, & Walsh W (1992). A prospective examination of post-traumatic stress disorder in rape victims. Journal of traumatic stress, 5(3), 455–475. [Google Scholar]
- Schmidt K, Worrack S, Von Korff M, Davydow D, Brunkhorst F, Ehlert U, … Scherag A (2016). Effect of a primary care management intervention on mental health–related quality of life among survivors of sepsis: a randomized clinical trial. Jama, 315(24), 2703–2711. doi: 10.1001/jama.2016.7207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev AY, Schreiber S, Galai T, & Melmed RN (1993). Post-traumatic stress disorder following medical events. British Journal of Clinical Psychology, 32(2), 247–253. [DOI] [PubMed] [Google Scholar]
- Shemesh E, Yehuda R, Milo O, Dinur I, Rudnick A, Vered Z, & Cotter G (2004). Posttraumatic stress, nonadherence, and adverse outcome in survivors of a myocardial infarction. Psychosomatic medicine, 66(4), 521–526. doi: 10.1097/01.psy.0000126199.05189.86 [DOI] [PubMed] [Google Scholar]
- Sijbrandij M, Kleiboer A, Bisson JI, Barbui C, & Cuijpers P (2015). Pharmacological prevention of post-traumatic stress disorder and acute stress disorder: A systematic review and meta-analysis. The Lancet Psychiatry, 2(5), 413–421. doi: 10.1016/S2215-0366(14)00121-7 [DOI] [PubMed] [Google Scholar]
- Simard S, Savard J, & Ivers H (2010). Fear of cancer recurrence: Specific profiles and nature of intrusive thoughts. Journal of Cancer Survivorship, 4(4), 361–371. doi: 10.1007/s11764-010-0136-8 [DOI] [PubMed] [Google Scholar]
- Sloan DM, Marx BP, Lee DJ, & Resick PA (2018). A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: A randomized noninferiority clinical trial. JAMA Psychiatry, 75(3), 233–239. doi: 10.1001/jamapsychiatry.2017.4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MY, Redd WH, Peyser C, & Vogl D (1999). Post-traumatic stress disorder in cancer: a review. Psycho-Oncology: Journal of the Psychological, Social and Behavioral Dimensions of Cancer, 8(6), 521–537. [DOI] [PubMed] [Google Scholar]
- Sommer JL, Mota N, Edmondson D, & El-Gabalawy R (2018). Comorbidity in illness-induced posttraumatic stress disorder versus posttraumatic stress disorder due to external events in a nationally representative study. General Hospital Psychiatry, 88–94. doi: 10.1016/j.genhosppsych.2018.02.004 [DOI] [PubMed] [Google Scholar]
- Sumner JA, Beauchaine T, & Nielsen L (2018). A mechanism-focused approach to the science of behavior change: An introduction to the special issue. Behaviour Research and Therapy, 101, 1–2. doi: 10.1016/j.brat.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, & Edmondson D (2018). Refining our understanding of PTSD in medical settings. General Hospital Psychiatry, 53, 86–87. doi: 10.1016/j.genhosppsych.2018.05.001 [DOI] [PubMed] [Google Scholar]
- Tarrier N, Liversidge T, & Gregg L (2006). The acceptability and preference for the psychological treatment of PTSD. Behaviour Research & Therapy, 44(11), 1643–1656. doi: 10.1016/j.brat.2005.11.012 [DOI] [PubMed] [Google Scholar]
- Tedstone JE, & Tarrier N (2003). Posttraumatic stress disorder following medical illness and treatment. Clinical Psychology Review, 23(3), 409–448. doi: 10.1016/S0272-7358(03)00031-X [DOI] [PubMed] [Google Scholar]
- Treggiari MM, Romand J-A, Yanez ND, Deem SA, Goldberg J, Hudson L, … Weiss NS (2009). Randomized trial of light versus deep sedation on mental health after critical illness. Critical Care Medicine, 37(9), 2527–2534. doi: 10.1097/CCM.0b013e3181a5689f [DOI] [PubMed] [Google Scholar]
- Tryon WW (2005). Possible mechanisms for why desensitization and exposure therapy work. Clinical Psychology Review, 25(1), 67–95. doi: 10.1016/j.cpr.2004.08.005 [DOI] [PubMed] [Google Scholar]
- Vilchinsky N, Ginzburg K, Fait K, & Foa EB (2017). Cardiac-disease-induced PTSD (CDIPTSD): A systematic review. Clinical Psychology Review, 55, 92–106. doi: 10.1016/j.cpr.2017.04.009 [DOI] [PubMed] [Google Scholar]
- von Känel R, Barth J, Princip M, Meister-Langraf RE, Schmid J-P, Znoj H, … Schnyder U (2018). Early Psychological Counseling for the Prevention of Posttraumatic Stress Induced by Acute Coronary Syndrome: The MI-SPRINT Randomized Controlled Trial. Psychotherapy and psychosomatics, 87(2), 75–84. doi: 10.1159/000486099 [DOI] [PubMed] [Google Scholar]
- von Känel R, Baumert J, Kolb C, Cho E-YN, & Ladwig K-H (2011). Chronic posttraumatic stress and its predictors in patients living with an implantable cardioverter defibrillator. Journal of Affective Disorders, 131, 344–352. doi:doi: 10.1016/j.jad.2010.12.002 [DOI] [PubMed] [Google Scholar]
- Walsh TS, Salisbury LG, Merriweather JL, Boyd JA, Griffith DM, Huby G, … Lewis SC (2015). Increased hospital-based physical rehabilitation and information provision after intensive care unit discharge: The RECOVER randomized clinical trial. JAMA internal medicine, 175(6), 901–910. doi: 10.1001/jamainternmed.2015.0822 [DOI] [PubMed] [Google Scholar]
- Wasson LT, Shaffer JA, Edmondson D, Bring R, Brondolo E, Falzon L, & Kronish IM (2018). Posttraumatic stress disorder and nonadherence to medications prescribed for chronic medical conditions: A meta-analysis. Journal of Psychiatric Research. doi: 10.1016/j.jpsychires.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, & Friedman MJ (2013). Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. Journal of Clinical Psychiatry, 74(6), e541–e550. doi: 10.4088/JCP.12r08225 [DOI] [PubMed] [Google Scholar]
- Weis F, Kilger E, Roozendaal B, Dominique J-F, Lamm P, Schmidt M, … Schelling G (2006). Stress doses of hydrocortisone reduce chronic stress symptoms and improve health-related quality of life in high-risk patients after cardiac surgery: A randomized study. The Journal of Thoracic and Cardiovascular Surgery, 131(2), 277–282. doi: 10.1016/j.jtcvs.2005.07.063 [DOI] [PubMed] [Google Scholar]
- White M, Edmondson D, Umland R, Sanchez G, & Chang BP (2017). Patent perceptions of stress during evaluation for Acs in the Ed. The American journal of emergency medicine, 35(2), 351–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R (2009). Status of glucocorticoid alterations in post-traumatic stress disorder. Annals of the New York Academy of Sciences, 1179(1), 56–69. doi: 10.1111/j.1749-6632.2009.04979.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.