To the Editor:
Bronchiolitis is the leading cause of hospitalization in US infants.1 Rhinovirus (RV) is the second most common cause of severe bronchiolitis (ie, bronchiolitis requiring hospitalization) following respiratory syncytial virus (RSV).1 RVs are RNA viruses consisting of more than 160 genotypes that are classified into 3 species (RV-A, RV-B, and RV-C).2 RV-A and RV-C are more frequently found than RV-B in children with acute respiratory infections (ARIs) and wheezing illnesses.3, 4 Emerging evidence suggests a complex interplay between viral infection, airway microbes, and host immune response in the pathobiology of ARI. Studies have shown that RV infection in children is associated with increased detection of pathogenic bacteria in the airways.5, 6 Furthermore, detection of RV together with specific airway pathogens (eg, Moraxella catarrhalis) is associated with increased ARI and asthma symptoms.6 Recently, RV-A and RV-C were reported to differentially associate with detection of pathogenic bacteria in school-age children.7 However, no study has investigated the relationships between rhinovirus species and airway microbiota in infants, let alone infants with bronchiolitis. To address the knowledge gap, we examined the association between rhinovirus species and the nasopharyngeal airway microbiota determined by 16S rRNA gene sequencing in 774 infants with severe bronchiolitis.
This was a post hoc analysis of data from the 35th Multicenter Airway Research Collaboration (MARC-35) cohort study—a multicenter prospective cohort study of infants hospitalized for bronchiolitis. The details of the study design, setting, virus and microbiota measurements, and analysis are described in this article's Online Repository at www.jacionline.org. Briefly, 1016 infants (age <1 year) hospitalized for bronchiolitis were enrolled in 17 sites across 14 US states (see Table E1 in this article's Online Repository at www.jacionline.org). Bronchiolitis was defined according to the American Academy of Pediatrics guidelines. The institutional review boards at participating sites approved the study. Informed consent was obtained from the infants' parent or legal guardian. Nasopharyngeal samples were collected within 24 hours of hospitalization and stored at −80°C locally. These samples were processed and tested for 17 respiratory pathogens by real-time PCR and for microbiota using 16S rRNA gene sequencing at Baylor College of Medicine (Houston, Tex). Singleplex real-time PCR was used to detect RV, and positive specimens were further genotyped by using molecular typing assay at the University of Wisconsin (Madison, Wis). By using partitioning around medoids unsupervised clustering with the use of weighted UniFrac distance, 4 distinct nasopharyngeal microbiota profiles were derived as previously described.8 In the current analysis, we grouped infants into 4 mutually exclusive virus categories: solo RSV (reference), RV-A, RV-B, and RV-C. We tested the association between these virus categories and nasopharyngeal microbiota profiles by constructing multinomial logistic regression model adjusting for 8 covariates. Data were analyzed using R version 3.4.4.
Of 1016 enrolled infants, 774 were in 1 of the 4 prespecified virus categories (580 RSV-only, 91 RV-A, 12 RV-B, and 91 RV-C) and had high-quality microbiota data; they comprised the analytic sample. Overall, the median age was 2.9 months (interquartile range, 1.6-5.3), 60% were male, and 16% infants received intensive care therapy. Compared with infants with RSV-only, those with RV-A or RV-C were older and more likely to have previous breathing problems (P < .001; see Table E2 in this article's Online Repository at www.jacionline.org).
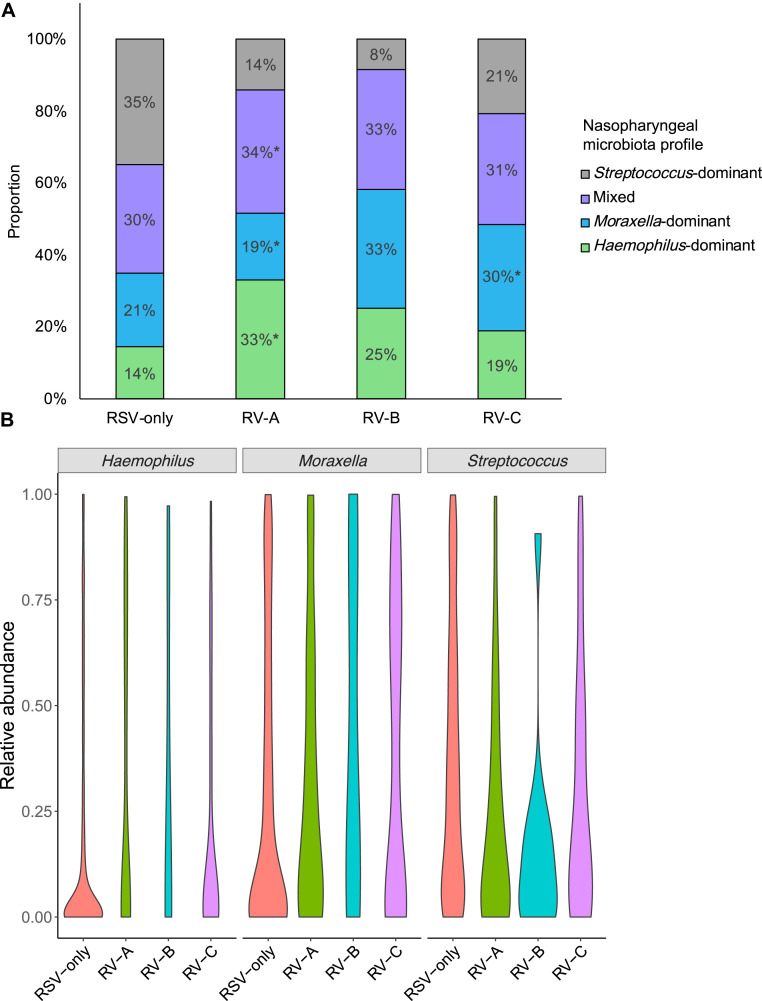
Across the virus categories, there was a significant difference in the likelihood of nasopharyngeal microbiota profiles (P < .001; see Table E3 in this article's Online Repository at www.jacionline.org). For example, while infants with RSV had the highest likelihood of Streptococcus-dominant profile (the reference virus and microbiota profile), those with RV-A had the highest likelihood of Haemophilus-dominant profile (Fig 1 , A), corresponding to an adjusted relative rate ratio of 5.67 (95% CI, 2.76-11.67; P < .001; Table I ). In contrast, infants with RV-C were more likely to have Moraxella-dominant profile than Streptococcus-dominant profile (adjusted relative rate ratio, 2.69; 95% CI, 1.39-5.20; P = .003). Similarly, at the genus-level (Fig 1, B; see Table E3 in this article's Online Repository at www.jacionline.org), compared with infants with RSV-only, those with any RV species had lower relative abundance of Streptococcus (P = .002) and those with RV-A had a higher abundance of Haemophilus (P = .002).
Fig 1.
Between-virus difference in nasopharyngeal microbiota in infants hospitalized for bronchiolitis. A, Between the 4 virus categories, the proportion of nasopharyngeal microbiota profiles differed. For example, compared with infants with RSV-only bronchiolitis, those with RV-A infection were more likely to have a Haemophilus-dominant, mixed, or Moraxella-dominant profile than a Streptococcus-dominant profile. Infants with RV-C infection were more likely to have a Moraxella-dominant profile. P values were derived from adjusted multinomial logistic regression model. Corresponding relative rate ratios are presented in Table I. *P < .05. B, Between the 4 virus categories, the distribution of relative abundance of 3 most common genera in the nasopharyngeal microbiota differed. Data are presented using violin plots, which are boxplots with a rotated kernel density plot on each side. P values adjusted for multiple comparisons are presented in Table E3.
Table I.
Unadjusted and adjusted associations of respiratory viruses (exposure) with nasopharyngeal microbiota profiles (outcome) in infants hospitalized for bronchiolitis
| Model and virus category | Microbiota profile |
||||||
|---|---|---|---|---|---|---|---|
|
Haemophilus-dominant (n = 133) |
P value |
Moraxella-dominant (n = 167) |
P value | Mixed profile (n = 239) |
P value |
Streptococcus-dominant (n = 235) |
|
| RRR (95% CI) | RRR (95% CI) | RRR (95% CI) | RRR (95% CI) | ||||
| Unadjusted model | |||||||
| RSV-only (n = 580) | Reference | Reference | Reference | Reference | |||
| RV-A (n = 91) | 5.62 (2.79-11.30) | <.001 | 2.22 (1.04-4.73) | .04 | 2.74 (1.39-5.39) | .004 | Reference |
| RV-B (n = 12) | 7.30 (0.75-71.21) | .09 | 6.79 (0.75-61.46) | .09 | 4.59 (0.51-41.50) | .17 | Reference |
| RV-C (n = 91) | 2.18 (1.08-4.40) | .03 | 2.41 (1.29-4.53) | .006 | 1.69 (0.91-3.13) | .09 | Reference |
| Adjusted model∗ | |||||||
| RSV-only (n = 580) | Reference | Reference | Reference | Reference | |||
| RV-A (n = 91) | 5.67 (2.76-11.67) | <.001 | 2.26 (1.05-4.89) | .04 | 2.74 (1.38-5.44) | .004 | Reference |
| RV-B (n = 12) | 7.50 (0.74-76.08) | .09 | 5.72 (0.62-52.71) | .12 | 4.73 (0.52-43.04) | .17 | Reference |
| RV-C (n = 91) | 1.81 (0.86-3.81) | .12 | 2.69 (1.39-5.20) | .003 | 1.57 (0.83-2.96) | .17 | Reference |
RRR, Relative rate ratio.
Multinomial logistic regression model adjusting for 8 patient-level covariates (age, sex, race/ethnicity, gestational age, siblings in the household, breast-feeding, history of breathing problems, and lifetime history of systemic antibiotic use). RSV-only infection was used as the reference of exposure (virus category), and Streptococcus-dominant microbiota profile was used as the reference for the outcome (nasopharyngeal microbiota profile).
Earlier studies reported that RV-C infection is associated with higher risks of subsequent ARI in young children4 and that enrichment of Moraxella abundance in the upper airways is related to higher frequency of ARIs.9 Furthermore, a recent analysis from RhinoGen study (310 children [aged 4-12 years] with or without asthma, using quantitative PCR for 3 bacteria) reported that RV-A and RV-C are differentially associated with increased quantity of H influenzae, M catarrhalis, and S pneumoniae.7 Our observations—for example, the association between RV-C and higher likelihood of Moraxella-dominant microbiota— corroborate these earlier findings, and extend them by applying 16S rRNA gene sequencing to the airway samples of a large multicenter prospective cohort of infants with severe bronchiolitis.
The underlying mechanisms of the virus-microbiota relationships are beyond the scope of our data. The observed associations may be causal—that is, specific respiratory virus species (eg, RV-C) alters the airway microbiota.6 Alternatively, unique microbiota profiles in conjunction with airway immune response might have contributed to susceptibility to specific virus infection. These potential mechanisms are not mutually exclusive. Despite this complexity, the identification of the association between specific virus species and airway microbiota in infants with bronchiolitis is important given their relation to subsequent respiratory health in children.
Our study has potential limitations. First, the study design precluded us from examining the relation between the temporal pattern of airway microbiota and respiratory health in children. To address this question, the cohort is currently being followed longitudinally for 6+ years with serial examinations of microbiota. Second, the current study did not have healthy controls. However, the study aim was to determine the association of virus species with microbiota among infants with bronchiolitis. Finally, although the study cohort comprised a racially/ethnically diverse US sample of infants, we must generalize the inferences cautiously beyond infants with severe bronchiolitis. Regardless, our data are highly relevant for 130,000 US children hospitalized with bronchiolitis each year.1
In summary, on the basis of this multicenter prospective cohort study of infants with severe bronchiolitis, we observed that compared with infants with RSV-only infection, infants with RV-A or RV-C infection had distinct nasopharyngeal microbiota profiles—for example, those with RV-C infection had a higher likelihood of Moraxella-dominant microbiota profile, whereas those with RV-A infection had a higher likelihood of Haemophilus-dominant profile. Although causal inferences remain premature, our data should advance research into delineating the complex interrelations between respiratory viruses, airway microbiome, and respiratory outcomes in children.
Acknowledgments
We thank the MARC-35 study hospitals and research personnel for their ongoing dedication to bronchiolitis and asthma research (Table E1), and Janice A. Espinola, MPH, Ashley F. Sullivan, MS, MPH, and Courtney N. Tierney, MPH (Massachusetts General Hospital, Boston, Mass), for their many contributions to the MARC-35 study. We also thank Joseph F. Petrosino, PhD, at Alkek Center for Metagenomics and Microbiome Research, Department of Molecular Virology and Microbiology, Baylor College of Medicine (Houston, Tex) for 16S rRNA gene sequencing analysis and Alkis Togias, MD, at the National Institutes of Health (Bethesda, Md) for helpful comments about the study results.
Footnotes
The current analysis was supported by the National Institutes of Health (Bethesda, Md; grant nos. UG3 OD-023253, U01 AI-087881, R01 AI-134940, and R01 AI-137091). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. L.T. was supported by the Finnish Medical Foundation and Päivikki and Sakari Sohlberg Foundation.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Methods
Study design, setting, and participants
This was a post hoc analysis of data from the 35th Multicenter Airway Research Collaboration (MARC-35)E1—a multicenter prospective cohort study of infants hospitalized with bronchiolitis (ie, severe bronchiolitis). MARC-35 is coordinated by the Emergency Medicine Network (EMNet), a collaboration of 245 participating hospitals.E2 Site investigators enrolled infants (age <1 year) hospitalized with bronchiolitis at 17 sites across 14 US states (Table E1) using a standardized protocol during 3 consecutive bronchiolitis seasons (from November 1 through April 30) during the period 2011 to 2014. Bronchiolitis was defined by the American Academy of Pediatrics guidelines as an acute respiratory illness with some combination of rhinitis, cough, tachypnea, wheezing, crackles, and retractionsE3 and was diagnosed by an attending physician. We excluded infants who were transferred to a participating hospital more than 24 hours after the original hospitalization, those for whom consent was provided more than 24 hours after hospitalization, or those with known heart-lung disease, immunodeficiency, immunosuppression, or gestational age of less than 32 weeks. All patients were treated at the discretion of the treating physicians. The institutional review board at each of the participating hospitals approved the study. Written informed consent was obtained from the parent or guardian.
Data collection
Clinical data (patients' demographic characteristics and family, environmental, and medical history, and details of the acute illness) were collected via structured interview and chart reviews.E1 All data were reviewed at the EMNet Coordinating Center (Boston, Mass), and site investigators were queried about missing data and discrepancies identified by manual data checks. In addition to the clinical data, blood and nasopharyngeal airway samples were collected within 24 hours of hospitalization and stored at −80°C using standardized protocols.E1 Nasopharyngeal samples were analyzed for 17 respiratory pathogens using real-time PCR assaysE4 as well as for microbiota using 16S rRNA gene sequencing at Baylor College of Medicine (Houston, Tex).
Identification of RV species
Singleplex real-time PCR was used to detect RV at Baylor College of Medicine. Of RV-positive specimens, their species and genotypes were identified by using molecular typing assay that targets a variable fragment in 5′ untranslated region of the viral genome flanked by highly conserved motifs at the University of Wisconsin (Madison, Wis).E5
16S rRNA gene sequencing of nasopharyngeal airway microbiota
16S rRNA gene sequencing methods were adapted from the methods developed for the National Institutes of Health Human Microbiome Project.E6, E7 All samples were processed with a low-biomass extraction protocol to avoid sample loss and degradation and to maximize yield. Bacterial genomic DNA was extracted using MO BIO PowerSoil DNA Isolation Kit (Mo Bio Laboratories; Carlsbad, Calif). The 16S rDNA V4 region was amplified by PCR and sequenced in the MiSeq platform (Illumina; San Diego, Calif) using the 2 × 250 bp paired-end protocol yielding pair-end reads that overlap almost completely. The primers used for amplification contain adapters for MiSeq sequencing and single-end barcodes allowing pooling and direct sequencing of PCR products.E8, E9
Sequencing read pairs were demultiplexed on the basis of unique molecular barcodes, and reads were merged using USEARCH v7.0.1090,E10 allowing 0 mismatches and a minimum overlap of 50 bases. Rarefaction curves of bacterial operational taxonomic units (OTUs) were constructed using sequence data for each sample to ensure coverage of the bacterial diversity present. Samples with suboptimal amounts of sequencing reads were resequenced to ensure that most bacterial taxa were encompassed in our analyses. 16S rRNA gene sequences were clustered into OTUs at a similarity cutoff value of 97% using the UPARSE algorithm.E11 OTUs were determined by mapping the centroids to the SILVA databaseE12 containing only the 16S V4 region to determine taxonomies. A custom script constructed a rarefied OTU table (rarefaction was performed at only 1 sequence depth) from the output files generated in the previous 2 steps for downstream analyses of alpha-diversity (eg, Shannon index) and beta-diversity (eg, weighted UniFrac).E13, E14
Quality control
The processes involving microbial DNA extraction, 16S rRNA gene amplification, and amplicon sequencing included a set of controls that enabled us to evaluate the potential introduction of contamination or off-target amplification. Nontemplate controls (extraction chemistries) were included in the microbial DNA extraction process and the resulting material was subsequently used for PCR amplification. In addition, at the step of amplification, another set of nontemplate controls (PCR-mix) was included to evaluate the potential introduction of contamination at this step. Similarly, a positive control composed of known and previously characterized microbial DNA was included at this step to evaluate the efficiency of the amplification process. Before samples (unknowns) were pooled together, sequencing controls were evaluated and the rejection criteria were the presence of amplicons in any of the nontemplate controls or the absence of amplicons in the positive control. In the present study, no amplicons were observed in the nontemplate controls and a negligible amount of raw reads was recovered after sequencing.
Outcome measure
16S rRNA gene sequencing of the nasopharyngeal airway samples from the enrolled infants (n = 1016) obtained 17,399,260 high-quality merged sequences, of which 16,685,637 (95.9%) were mapped to the database. Of 1016 infant samples, 1005 (98.9%) had sufficient sequence depth (rarefaction cutoff, 2128 reads per sample).
The primary outcome was nasopharyngeal microbiota profile. As previously described,E1 by using the partitioning around medoids methodE15 with weighted UniFrac distance, an unsupervised clustering method, we derived 4 distinct microbiota profiles: (1) Haemophilus-dominant profile, (2) Moraxella-dominant profile, (3) mixed profile, and (4) Streptococcus-dominant profile. The number of clusters was determined using the average silhouette score.E16
Statistical analyses
To examine the association of respiratory viruses with nasopharyngeal microbiota profiles, we first grouped infants into 4 mutually exclusive virus categories: RSV-only (reference), RV-A, RV-B, and RV-C. Infections with multiple RV species were excluded. We compared the patient characteristics and clinical presentation between the virus categories, by using chi-square test and Wilcoxon-Mann-Whitney test, as appropriate. We also compared the relative abundances of 10 most abundant genera between the virus categories by using 1-way ANOVA, adjusting for multiple comparisons with the use of the Benjamini-Hochberg false- discovery rate method.E17 We then tested the association of these virus categories with nasopharyngeal microbiota profiles by constructing a multinomial logistic regression model adjusting for 8 potential confounders (ie, age, sex, race/ethnicity, gestational age, siblings in the household, breast-feeding, history of breathing problems, and lifetime history of systemic antibiotic use). These potential confounders were chosen on the basis of clinical plausibility and a priori knowledge.E18, E19, E20, E4 We reported all P values as 2-tailed, with P < .05 considered statistically significant. The data were analyzed with the use of R version 3.4.4 (R Foundation, Vienna, Austria) with the phyloseq package for the microbiota analysis.
Table E1.
Principal investigators at the 17 participating sites in MARC-35
| Amy D. Thompson, MD | Alfred I. duPont Hospital for Children, Wilmington, Del |
| Federico R. Laham, MD, MS | Arnold Palmer Hospital for Children, Orlando, Fla |
| Jonathan M. Mansbach, MD, MPH | Boston Children's Hospital, Boston, Mass |
| Vincent J. Wang, MD, MHA | Children's Hospital of Los Angeles, Los Angeles, Calif |
| Michelle B. Dunn, MD | Children's Hospital of Philadelphia, Philadelphia, Pa |
| Juan C. Celedon, MD, DrPH | Children's Hospital of Pittsburgh, Pittsburgh, Pa |
| Michael Gomez, MD, MS-HCA, and Nancy Inhofe, MD | The Children's Hospital at St Francis, Tulsa, Okla |
| Brian M. Pate, MD, and Henry T. Puls, MD | The Children's Mercy Hospital & Clinics, Kansas City, Mo |
| Stephen J. Teach, MD, MPH | Children's National Medical Center, Washington, DC |
| Richard T. Strait, MD | Cincinnati Children's Hospital and Medical Center, Cincinnati, Ohio |
| Ilana Waynik, MD | Connecticut Children's Medical Center, Hartford, Conn |
| Sujit Iyer, MD | Dell Children's Medical Center of Central Texas, Austin, Tex |
| Michelle D. Stevenson, MD, MS | Kosair Children's Hospital, Louisville, Ky |
| Wayne G. Schreffler, MD, PhD, and Ari R. Cohen, MD | Massachusetts General Hospital, Boston, Mass |
| Anne K Beasley, MD | Phoenix Children's Hospital, Phoenix, Ariz |
| Thida Ong, MD | Seattle Children's Hospital, Seattle, Wash |
| Charles G. Macias, MD, MPH | Texas Children's Hospital, Houston, Tex |
Table E2.
Characteristics of 774 infants hospitalized for bronchiolitis by RV category
| Characteristic | RV |
P value | |||
|---|---|---|---|---|---|
| RSV-only (n = 580) | RV-A (n = 91) | RV-B (n = 12) | RV-C (n = 91) | ||
| Age (mo), median (IQR) | 2.7 (1.5-4.8) | 3.1 (1.9-5.7) | 3.0 (2.1-4.7) | 4.4 (2.3-7.4) | <.001 |
| Female sex | 249 (42.9) | 30 (33.0) | 6 (50.0) | 27 (29.7) | .04 |
| Race/ethnicity | .38 | ||||
| Non-Hispanic white | 267 (46.0) | 33 (36.3) | 5 (41.7) | 36 (39.6) | |
| Non-Hispanic black | 126 (21.7) | 20 (22.0) | 4 (33.3) | 26 (28.6) | |
| Hispanic | 162 (27.9) | 36 (39.6) | 3 (25.0) | 25 (27.5) | |
| Other | 25 (4.3) | 2 (2.2) | 0 (0) | 4 (4.4) | |
| Parental history of asthma | 190 (32.8) | 35 (38.5) | 4 (33.3) | 35 (38.5) | .54 |
| Maternal smoking during pregnancy | 84 (14.5) | 14 (15.4) | 1 (8.3) | 13 (14.3) | .48 |
| C-section delivery | 214 (36.9) | 25 (27.5) | 3 (25.0) | 34 (37.4) | .07 |
| Prematurity (32-37 wk) | 104 (17.9) | 18 (19.8) | 1 (8.3) | 21 (23.1) | .51 |
| Low birth weight (<2.3 kg) | 33 (5.7) | 8 (8.8) | 0 (0) | 8 (8.8) | .58 |
| Sibling in the household | 457 (78.8) | 80 (87.9) | 12 (100) | 67 (73.6) | .03 |
| Mostly breast-fed during the first 3 mo of life | 248 (42.8) | 37 (40.7) | 7 (58.3) | 33 (36.3) | .57 |
| History of a breathing problem | 76 (13.1) | 21 (23.1) | 2 (16.7) | 31 (34.1) | <.001 |
| Lifetime history of systemic antibiotic use | 153 (26.4) | 27 (29.7) | 3 (25.0) | 32 (35.2) | .36 |
| Lifetime history of corticosteroid use | 62 (10.7) | 11 (12.1) | 3 (25.0) | 21 (23.1) | .005 |
| Detected pathogens | |||||
| RSV | 580 (100) | 52 (57.1) | 10 (83.3) | 47 (51.6) | <.001 |
| RV | 0 (0) | 91 (100) | 12 (100.0) | 91 (100) | <.001 |
| Other pathogen∗ | 0 (0) | 22 (24.2) | 2 (16.7) | 24 (26.4) | <.001 |
| Clinical outcomes | |||||
| Intensive care therapy† | 93 (16.0) | 13 (14.3) | 3 (25.0) | 13 (14.3) | .78 |
| Hospital length of stay (d), median (IQR) | 2.0 (1.0-3.0) | 2.0 (1.0-3.0) | 1.5 (1.0-3.3) | 2.0 (1.0-2.5) | .09 |
IQR, Interquartile range.
Data are n (%) of infants unless otherwise indicated.
Adenovirus, bocavirus, Bordetella pertussis, enterovirus, human coronavirus NL63, OC43, 229E, or HKU1, human metapneumovirus, influenza A or B virus, Mycoplasma pneumoniae, parainfluenza virus 3.
Defined as admission to intensive care unit or use of mechanical ventilation (continuous positive airway pressure or intubation).
Table E3.
Nasopharyngeal microbiota of infants hospitalized for bronchiolitis by respiratory virus category
| Characteristic | RV |
P value | |||
|---|---|---|---|---|---|
| RSV-only (n = 580) | RV-A (n = 91) | RV-B (n = 12) | RV-C (n = 91) | ||
| Richness | |||||
| No. of genera, median (IQR) | 17 (10-25) | 13 (7-23) | 14 (7-24) | 15 (8-22) | .10 |
| Alpha-diversity | |||||
| Shannon index, median (IQR) | 0.96 (0.58-1.49) | 0.94 (0.41-1.39) | 0.67 (0.14-1.34) | 0.91 (0.57-1.28) | .20 |
| Microbiota profiles | <.001 | ||||
| Haemophilus-dominant profile | 83 (14.3) | 30 (33.0) | 3 (25.0) | 17 (18.7) | |
| Moraxella-dominant profile | 119 (20.5) | 17 (18.7) | 4 (33.3) | 27 (29.7) | |
| Mixed profile | 176 (30.3) | 31 (34.1) | 4 (33.3) | 28 (30.8) | |
| Streptococcus-dominant profile | 202 (34.8) | 13 (14.3) | 1 (8.3) | 19 (20.9) | |
| Relative abundance of 10 most abundant genera, mean ± SD | |||||
| Streptococcus | 0.35 ± 0.31 | 0.23 ± 0.26 | 0.17 ± 0.25 | 0.29 ± 0.30 | .002∗ |
| Moraxella | 0.27 ± 0.33 | 0.31 ± 0.33 | 0.42 ± 0.42 | 0.36 ± 0.36 | .10∗ |
| Haemophilus | 0.16 ± 0.28 | 0.30 ± 0.36 | 0.29 ± 0.39 | 0.19 ± 0.29 | .002∗ |
| Prevotella | 0.03 ± 0.07 | 0.02 ± 0.06 | 0.01 ± 0.01 | 0.02 ± 0.06 | .77∗ |
| Neisseria | 0.02 ± 0.07 | 0.02 ± 0.07 | 0.02 ± 0.04 | 0.02 ± 0.10 | .99∗ |
| Staphylococcus | 0.03 ± 0.10 | 0.01 ± 0.07 | 0.01 ± 0.02 | 0.02 ± 0.08 | .72∗ |
| Corynebacterium | 0.02 ± 0.08 | 0.00 ± 0.01 | 0.00 ± 0.01 | 0.00 ± 0.02 | .10∗ |
| Alloprevotella | 0.01 ± 0.05 | 0.02 ± 0.06 | 0.00 ± 0.01 | 0.01 ± 0.06 | .75∗ |
| Veillonella | 0.01 ± 0.03 | 0.01 ± 0.02 | 0.00 ± 0.00 | 0.01 ± 0.02 | .17∗ |
| Gemella | 0.01 ± 0.04 | 0.01 ± 0.02 | 0.00 ± 0.00 | 0.01 ± 0.02 | .61∗ |
IQR, Interquartile range.
Benjamini-Hochberg false-discovery rate–adjusted P value accounting for multiple comparisons.
Table E4.
Characteristics of 774 infants hospitalized for bronchiolitis by nasopharyngeal microbiota profiles
| Characteristic | Microbiota profile |
P value | |||
|---|---|---|---|---|---|
| Haemophilus-dominant (n = 133) | Moraxella-dominant (n = 167) | Mixed profile (n = 239) | Streptococcus-dominant (n = 235) | ||
| Age (mo), median (IQR) | 3.9 (2.1-7.6) | 2.9 (1.5-5.4) | 2.9 (1.7-4.8) | 2.5 (1.3-4.3) | <.001 |
| Female sex | 53 (39.8) | 71 (42.5) | 95 (39.7) | 93 (39.6) | .93 |
| Race/ethnicity | |||||
| Non-Hispanic white | 49 (36.8) | 70 (41.9) | 100 (41.8) | 122 (51.9) | .20 |
| Non-Hispanic black | 29 (21.8) | 42 (25.1) | 60 (25.1) | 45 (19.1) | |
| Hispanic | 49 (36.8) | 48 (28.7) | 68 (28.5) | 61 (26.0) | |
| Other | 6 (4.5) | 7 (4.2) | 11 (4.6) | 7 (3.0) | |
| Parental history of asthma | 44 (33.1) | 49 (29.3) | 80 (33.5) | 91 (38.7) | .18 |
| Maternal smoking during pregnancy | 17 (12.8) | 21 (12.6) | 38 (15.9) | 36 (15.3) | .43 |
| C-section delivery | 47 (35.3) | 60 (35.9) | 82 (34.3) | 87 (37.0) | .60 |
| Prematurity (32-37 wk) | 26 (19.5) | 27 (16.2) | 48 (20.1) | 43 (18.3) | .78 |
| Low birth weight (<2.3 kg) | 9 (6.8) | 12 (7.2) | 13 (5.4) | 15 (6.4) | .94 |
| Sibling in the household | 98 (73.7) | 142 (85.0) | 188 (78.7) | 188 (80.0) | .11 |
| Mostly breast-fed during the first 3 mo of life | 59 (44.4) | 79 (47.3) | 88 (36.8) | 99 (42.1) | .43 |
| History of a breathing problem | 27 (20.3) | 22 (13.2) | 44 (18.4) | 37 (15.7) | .34 |
| Lifetime history of systemic antibiotic use | 52 (39.1) | 29 (17.4) | 67 (28.0) | 67 (28.5) | .001 |
| Lifetime history of corticosteroid use | 20 (15.0) | 19 (11.4) | 30 (12.6) | 28 (11.9) | .79 |
| Virus category∗ | |||||
| RSV-only | 83 (62.4) | 119 (71.3) | 176 (73.6) | 202 (86.0) | <.001 |
| RV-A | 30 (22.6) | 17 (10.2) | 31 (13.0) | 13 (5.5) | |
| RV-B | 3 (2.3) | 4 (2.4) | 4 (1.7) | 1 (0.4) | |
| RV-C | 17 (12.8) | 27 (16.2) | 28 (11.7) | 19 (8.1) | |
IQR, Interquartile range.
Data are n (%) of infants unless otherwise indicated.
Of these, 48 had coinfection with a non-RSV/non-RV, which did not have statistically significant association with the microbiota profiles (P = .06).
Table E5.
Full results of the multivariable analysis on associations of RVs (exposure) with nasopharyngeal microbiota profiles (outcome) in infants hospitalized for bronchiolitis∗
| Variable | Microbiota profile |
||||||
|---|---|---|---|---|---|---|---|
|
Haemophilus-dominant (n = 133) |
Moraxella-dominant (n = 167) |
Mixed profile (n = 239) |
Streptococcus-dominant (n = 235) |
||||
| RRR (95% CI) | P value | RRR (95% CI) | P value | RRR (95% CI) | P value | RRR (95% CI) | |
| Virus category | |||||||
| RSV-only (n = 580) | Reference | Reference | Reference | Reference | |||
| RV-A (n = 91) | 5.67 (2.76-11.67) | <.001 | 2.26 (1.05-4.89) | .04 | 2.74 (1.38-5.44) | .004 | Reference |
| RV-B (n = 12) | 7.50 (0.74-76.08) | .09 | 5.72 (0.62-52.71) | .12 | 4.73 (0.52-43.04) | .17 | Reference |
| RV-C (n = 91) | 1.81 (0.86-3.81) | .12 | 2.69 (1.39-5.20) | .003 | 1.57 (0.83-2.96) | .17 | Reference |
| Age ≥ 6 mo | 2.82 (1.58-5.02) | <.001 | 2.09 (1.18-3.72) | .01 | 1.34 (0.78-2.29) | .29 | Reference |
| Female (vs male) sex | 1.08 (0.68-1.71) | .74 | 1.11 (0.73-1.69) | .63 | 1.01 (0.69-1.48) | Reference | |
| Race/ethnicity | .95 | ||||||
| Non-Hispanic white | Reference | Reference | Reference | Reference | |||
| Non-Hispanic black | 1.75 (0.96-3.22) | .07 | 1.58 (0.92-2.69) | .10 | 1.56 (0.96-2.53) | .07 | Reference |
| Hispanic | 1.94 (1.14-3.30) | .01 | 1.41 (0.86-2.33) | .17 | 1.28 (0.82-2.00) | .28 | Reference |
| Other | 2.19 (0.67-7.11) | .19 | 1.74 (0.57-5.31) | .33 | 1.98 (0.73-5.34) | .18 | Reference |
| Prematurity (32-37 wk) | 1.04 (0.59-1.83) | .90 | 0.83 (0.48-1.43) | .49 | 1.06 (0.67-1.70) | .80 | Reference |
| Sibling in the household | 0.65 (0.38-1.11) | .11 | 1.49 (0.86-2.58) | .16 | 0.89 (0.56-1.40) | .61 | Reference |
| Breast-feeding during the first 3 mo of life | 1.13 (0.70-1.84) | .62 | 1.26 (0.81-1.97) | .31 | 0.80 (0.53-1.20) | .28 | Reference |
| History of breathing problems before the index hospitalization | 0.80 (0.43-1.49) | .49 | 0.65 (0.35-1.22) | .18 | 1.05 (0.62-1.76) | .86 | Reference |
| Lifetime history of systemic antibiotic use | 1.39 (0.86-2.27) | .18 | 0.49 (0.29-0.82) | .007 | 0.94 (0.62-1.44) | .79 | Reference |
RRR, Relative rate ratio.
Multinomial logistic regression model adjusting for 8 patient-level covariates. RSV-only infection was used as the reference of exposure (virus category), and Streptococcus-dominant microbiota profile was used as the reference for the outcome (nasopharyngeal microbiota profile).
References
- 1.Hasegawa K., Mansbach J.M., Camargo C.A., Jr. Infectious pathogens and bronchiolitis outcomes. Expert Rev Anti Infect Ther. 2014;12:817–828. doi: 10.1586/14787210.2014.906901. [DOI] [PubMed] [Google Scholar]
- 2.Bochkov Y.A., Gern J.E. Rhinoviruses and their receptors: implications for allergic disease. Curr Allergy Asthma Rep. 2016;16:30. doi: 10.1007/s11882-016-0608-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwane M.K., Prill M.M., Lu X., Miller E.K., Edwards K.M., Hall C.B. Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. J Infect Dis. 2011;204:1702–1710. doi: 10.1093/infdis/jir634. [DOI] [PubMed] [Google Scholar]
- 4.Cox D.W., Bizzintino J., Ferrari G., Khoo S.K., Zhang G., Whelan S. Human rhinovirus species C infection in young children with acute wheeze is associated with increased acute respiratory hospital admissions. Am J Respir Crit Care Med. 2013;188:1358–1364. doi: 10.1164/rccm.201303-0498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karppinen S., Terasjarvi J., Auranen K., Schuez-Havupalo L., Siira L., He Q. Acquisition and transmission of Streptococcus pneumoniae are facilitated during rhinovirus infection in families with children. Am J Respir Crit Care Med. 2017;196:1172–1180. doi: 10.1164/rccm.201702-0357OC. [DOI] [PubMed] [Google Scholar]
- 6.Kloepfer K.M., Lee W.M., Pappas T.E., Kang T.J., Vrtis R.F., Evans M.D. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol. 2014;133:1301–1307. doi: 10.1016/j.jaci.2014.02.030. 7.e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bashir H., Grindle K., Vrtis R., Vang F., Kang T., Salazar L. Association of rhinovirus species with common cold and asthma symptoms and bacterial pathogens. J Allergy Clin Immunol. 2018;141:822–824.e9. doi: 10.1016/j.jaci.2017.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasegawa K., Mansbach J.M., Ajami N.J., Espinola J.A., Henke D.M., Petrosino J.F. Association of nasopharyngeal microbiota profiles with bronchiolitis severity in infants hospitalised for bronchiolitis. Eur Respir J. 2016;48:1329–1339. doi: 10.1183/13993003.00152-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosch A.A., de Steenhuijsen Piters W.A., van Houten M.A., Chu M., Biesbroek G., Kool J. Maturation of the infant respiratory microbiota, environmental drivers and health consequences: a prospective cohort study. Am J Respir Crit Care Med. 2017;196:1582–1590. doi: 10.1164/rccm.201703-0554OC. [DOI] [PubMed] [Google Scholar]
References
- Hasegawa K., Mansbach J.M., Ajami N.J., Espinola J.A., Henke D.M., Petrosino J.F. Association of nasopharyngeal microbiota profiles with bronchiolitis severity in infants hospitalised for bronchiolitis. Eur Respir J. 2016;48:1329–1339. doi: 10.1183/13993003.00152-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emergency Medicine Network. http://www.emnet-usa.org/ Available at: Accessed October 1, 2018.
- Ralston S.L., Lieberthal A.S., Meissner H.C., Alverson B.K., Baley J.E., Gadomski A.M. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474–e1502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- Mansbach J.M., Piedra P.A., Stevenson M.D., Sullivan A.F., Forgey T.F., Clark S. Prospective multicenter study of children with bronchiolitis requiring mechanical ventilation. Pediatrics. 2012;130:e492–e500. doi: 10.1542/peds.2012-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochkov Y.A., Grindle K., Vang F., Evans M.D., Gern J.E. Improved molecular typing assay for rhinovirus species A, B, and C. J Clin Microbiol. 2014;52:2461–2471. doi: 10.1128/JCM.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium A framework for human microbiome research. Nature. 2012;486:215–221. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Lauber C.L., Walters W.A., Berg-Lyons D., Huntley J., Fierer N. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C., Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C., Lladser M.E., Knights D., Stombaugh J., Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J. 2011;5:169–172. doi: 10.1038/ismej.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G.D., Chen J., Hoffmann C., Bittinger K., Chen Y.Y., Keilbaugh S.A. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseeuw P. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J Comput Appl Math. 1987;20:53–65. [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc (Methodological) 1995;57:289–300. [Google Scholar]
- Hasegawa K., Linnemann R.W., Mansbach J.M., Ajami N.J., Espinola J.A., Fiechtner L.G. Household siblings and nasal and fecal microbiota in infants. Pediatr Int. 2017;59:473–481. doi: 10.1111/ped.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch A.A., de Steenhuijsen Piters W.A., van Houten M.A., Chu M., Biesbroek G., Kool J. Maturation of the infant respiratory microbiota, environmental drivers and health consequences: a prospective cohort study. Am J Respir Crit Care Med. 2017;196:1582–1590. doi: 10.1164/rccm.201703-0554OC. [DOI] [PubMed] [Google Scholar]
- Teo S.M., Mok D., Pham K., Kusel M., Serralha M., Troy N. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704–715. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]