Abstract
Connective tissue is one of the four major types of animal tissue and plays essential roles throughout the human body. Genetic factors, aging, and trauma all contribute to connective tissue dysfunction and motivate the need for strategies to promote healing and regeneration. The goal of this Review is to link a fundamental understanding of connective tissues and their multiscale properties to better inform the design and translation of novel biomaterials to promote their regeneration. We discuss major clinical problems in adipose tissue, cartilage, dermis, and tendon that inspire the need to replace native connective tissue with biomaterials. We then detail multiscale structure-function relationships in native soft connective tissues that may be used to guide material design. Several biomaterials strategies to improve healing of these tissues that incorporate biologics and are biologic-free are reviewed. Finally, we highlight important guidance documents and standards (ASTM, FDA, EMA) that are important to consider for translating new biomaterials into clinical practice.
Keywords: collagen, structure-function, multiscale, therapeutics, translation, regeneration, regulation
1. Introduction
Throughout the human body, fibrous matrices, non-fibrous matrices, and cells form important connections to adjacent tissues (termed “connective tissues”) that provide support and protection to organs. The specific composition and structure of connective tissues dictate their mechanical properties and govern interactions with cellular components. For example, the primary structural load bearing component in connective tissues is the protein collagen, whose Greek derivative, “kola” (glue) and “gen” (producing) exemplifies its mechanical and structural role in the ECM. Connective tissues that experience high mechanical loads, such as tendon, have dense and aligned collagen in contrast to adipose tissue. Collagen functions synergistically with other ECM proteins including proteoglycans, elastin, and fibronectin to assemble tissue structure and support its function.
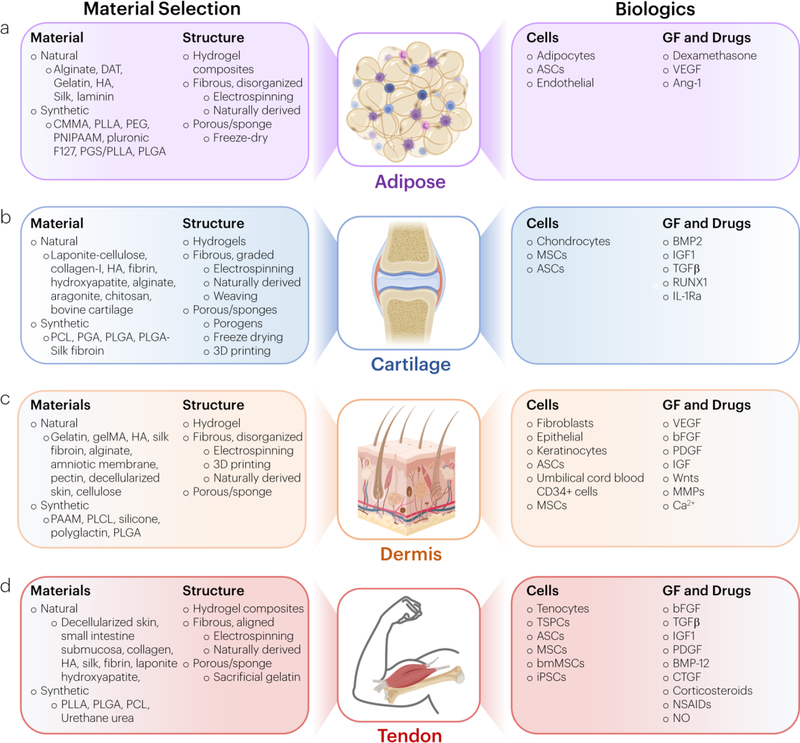
Aging, genetic diseases, and trauma may result in connective tissue pathology and the need for regenerative or replacement therapies. Biomaterials that mimic and heal connective tissues represent an exciting strategy to restore native tissue properties. For example, materials may be used to template regeneration, replace damaged tissue, or deliver biologics and other therapeutics. Each approach has scientific and translational merits and pitfalls that depend on its target tissue. The goal of this Review is to link a fundamental understanding of connective tissue diseases and their multiscale properties to inform the design and translation of novel biomaterials. The remainder of the review first discusses the multiscale structure-function relationships in native soft connective tissues, with examples of adipose, cartilage, dermis, and tendon (Figure 1). We then examine several biomaterials strategies to improve healing that both incorporate biologics and are biologic-free. Finally, we highlight many important standards (ASTM, FDA, EMA) that may be important when considering translation of biomaterials for connective tissue disorders to the clinic.
Figure 1: Anatomy and disease state affect connective tissue structure-function properties.
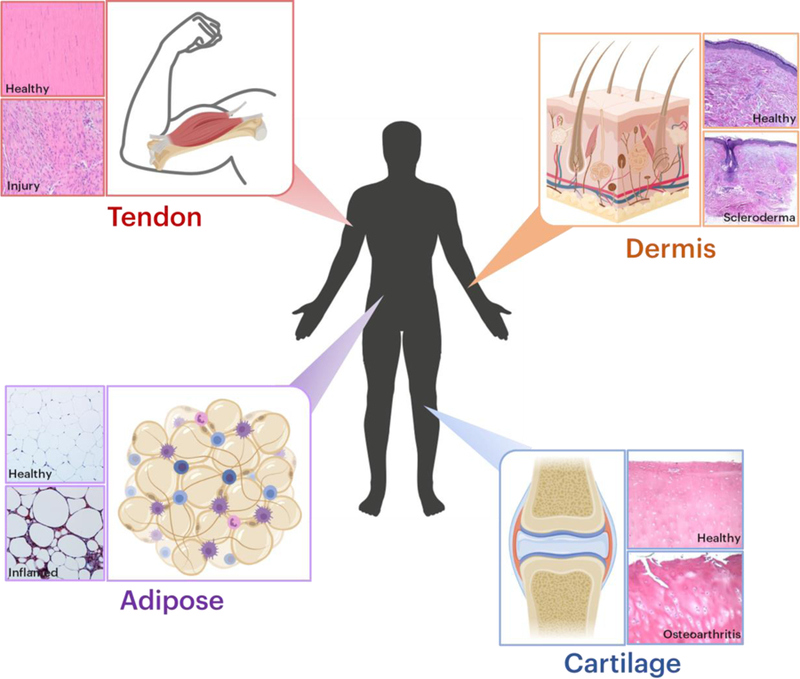
Adipose tissue, cartilage, dermis, and tendon are all examples of different types of connective tissues. In each panel group, the top panel depicts native tissue histology and the bottom panel depicts pathological conditions. Histological images were reproduced with permission.[98–100]
1.1. Connective Tissue Structure and Composition Overview
Connective tissues are broadly classified into the connective tissues proper (loose/areolar, adipose, reticular, dense) and special connective tissues (cartilage, bone, blood).[1] Loose connective tissue (low fiber to cell ratio) such as adipose tissue fills space between muscle sheaths and is soft compared to dense regular connective tissues (high fiber to cell ratio), such as tendon (connects muscle to bone). Dense irregular connective tissue carries a more disorganized pattern in fiber structure and is present in tissues such as the dermis of the skin and fibrous sheath of bone (periosteum). Reticular connective tissue contains a network of fibers, fibroblasts, and macrophages that constructs tissues such as adipose tissue, liver, bone marrow, and spleen.
The most abundant protein in mammals is collagen, and it serves as the main structural component of connective tissues. Collagen is widely used for medical applications including cardiology, cosmetic surgery, bone grafts, tissue regeneration, reconstructive surgery, wound care, dietary supplements, and is also used in basic science studies.[2] Collagen protein (Diameter (D) =1.5nm, Molecular weight (Mw) ~300kDa)[3] is formed from a triple helix stabilized by hydrogen bonds rich in the amino acid hydroxyproline that enables its twisting. Collagen molecules aggregate through entropic, electrostatic, and hydrophobic interactions to form collagen fibrils (D=100–500nm), which are staggered during assembly to create overlap regions that repeat every 67 nm (“D-banding”). Collagen exists in fibrillar forms (types I, II, III, V, XI), fibril-associated collagens with interrupted triple helices (FACITs), and non-fibrillar forms (MACITs, MULTIPLEXINs) that vary with tissue type and function.[2] For example, collagen type-I (fibril forming) is of the main component of tendon, fascia, and bone, whereas collagen type-IV constructs adipose tissue. Upon mineralization, collagen can form rigid tissues such as bone. Collagen crosslinking (at the molecule and fibril levels)[4–6] further stabilizes fibrils and impacts soft tissue mechanical properties.
Elastin is an ECM protein abundant in tissues requiring elastic recoil after mechanical loading, such as blood vessels, tendon, and skin. Its fibers are composed of aggregated tropoelastin molecules (D=10–12nm, Mw=72kDa)[7] that appear as rope-like structures at the nanoscale. Unlike collagen, elastin’s amino acid composition contains 75% hydrophobic residues that makes hydration critical for elasticity.[8, 9] Elastin-based biomaterials are engineered using decellularized tissue, purified insoluble elastin, synthetic mimics, and elastin-like polypeptides.[8]
Fibronectin (FN) is a glycoprotein (D=5–20nm, Mw=440kDa) that mediates many cell-ECM interactions controlling cell adhesion, migration, differentiation, and growth.[10, 11] The arginine-glycine-aspartic acid (RGD) peptide motif of FN is primarily involved in cell adhesion. Beyond binding to cell surfaces through cell surface integrin receptors, FN also binds to other ECM molecules including collagen, fibrin, and heparan sulfate proteoglycans.[10] FN is critical throughout development and adulthood; an inactivated FN gene results in embryonic lethality, and altered expression of FN is associated with disease states including fibrosis and cancer.
Proteoglycans (PGs) are highly glycosylated proteins that play important mechanical, structural, and regulatory roles in connective tissues. PGs are composed of a core protein covalently bonded to glycosaminoglycan (GAG) side chains that exist in many forms and vary across tissues and length scales. Aggrecan (Mw= 2–3MDa)[12] is an example of a large PG that is highly abundant in cartilage and plays important roles in tissue mechanical function. Smaller proteoglycans, termed small leucine-rich proteoglycans (SLRPs), are the largest class of PGs by gene number and function as structural components and signaling molecules.[13] The GAG chains extending from the core protein are highly polar linear unbranched polysaccharides composed of an amino sugar and uronic sugar that attract water, lubricate surfaces, and absorb mechanical energy. PGs play distinct roles in intracellular, cell surface, pericellular, and extracellular spaces.[13] At the cell and pericellular levels, heparin sulfate proteoglycans help modify growth factors and maintain morphogen gradients during development and regeneration. In tissues, chondroitin- and dermatin-sulfate proteoglycans contribute to structure and mechanical strength against compression through fluid retention and generation of osmotic pressure due to the negatively charged sulfate groups on GAG chains in these PGs.[13, 14] Their negative charges also promote the binding of cations and can impede diffusion of molecules through the ECM.
1.2. Clinical Problems Overview
Genetic, immune, traumatic, and age-induced factors all contribute to connective tissue pathology (Table 1), primarily affecting limbs, joints, skin, and the cardiovascular system. As these disease states are a challenge for modern day medicine, they motivate novel tissue engineering strategies, biomaterials, and therapeutics. Importantly, for successful implementation, it is necessary to understand not only the pathological state, but also the native tissue structure-function properties to provide benchmarks for engineering design strategies.
Table 1:
Pathology and phenotypes of connective tissue disorders.
| Pathology | Tissues Affected | Tissue Phenotype | Mechanism | Treatment |
|---|---|---|---|---|
| Marfan’s Syndrome [15–17, 19–21] | Limbs, digits, spine, heart, lungs, eyes, bone | Elongated limbs and digits, flexibility, scoliosis | Fibrillin-1 | Beta blockers, surgery, avoid exercise |
| Ehler’s Danlos Syndrome [22–25] | Skin, aorta, spine, cartilage | Loose joints, stretchy tissues, abnormal scarring | Col1a1, col1a2, col3a1, col5a1, Tnxb | Physical therapy, pain relief, surgery |
| Osteogenesis Imperfecta [26] | Bone, eye, joints, heart, teeth, lung, ears | Brittle bones, blue tinge in eye, short height, loose joints, hearing loss, aortic dissection | Col 1–3 | Bisphosphonates |
| Myxomatous Degeneration [27, 28] | Mitral valve | Displacement of thickened mitral valve leaflet into left atrium | Fibrosis and excess dermatan sulfate | Beta blockers, blood thinners, surgical replacement |
| Rheumatoid Arthritis [31–33] | Joints, skin, eyes, lungs, heart, vessels | Women (40–60yo); Inflamed, thickened synovium; | Immune system attacks synovium, mTORC1 | NSAIDs, DMARDS, biologics, JAK inhibitors |
| Lupus [34–36] | Joints, skin, kidneys, blood, brain, heart, lungs | Women (15–45yo); flares, fatigue, fever, joint pain, swelling, rash, skin lesions, dry eyes, headaches | Autoimmune; Vitamin D HLA; IRF5, PTPN22, STAT4, CDKN1A, ITGAM, BLK, NFSF4, BANK1 | NSAIDs, antimalarial drugs, corticosteroids, immunosuppresants, biologics |
| Scleroderma [34, 37–42] | Skin, vessels, muscle, internal organs | Women (mid age) Thickened skin, increased tissue stiffness, calcium deposits | HLA; collagen synthesis, T lymphocyte activation | Vitamin D |
| Osteoarthritis [65–70] | Cartilage | Reduced modulus, reduced GAG | Chronic overuse, aging, trauma, IL1β, TNF, Cox2, MMPs | Physical therapy, NSAID, corticosteroid, surgery |
| Tendinopathy [76–85] | Tendon | Reduced modulus, hypercellularity, fiber disorganization | Chronic overuse, aging, trauma, IL1β, IL4, IL-17, TNF | Physical therapy, NSAID, corticosteroid, surgery |
1.2.1. Impact of Genetic Diseases on Connective Tissues
Many genetic diseases have detrimental effects on connective tissues. Understanding disease onset and progression is necessary to design targeted therapies and establish validated models systems representative of in vivo pathology to evaluate therapeutic efficacy. We next review several genetic diseases and discuss current clinical treatments. These present clinical opportunities for material strategies to improve connective tissue dysfunction in individuals with genetic diseases.
Marfan’s Syndrome affects 1–2 in 10 thousand people each year and is due to mutations in fibrillin-1, a glycoprotein in the ECM that affects elastic fiber homeostasis.[15, 16] Patients with Marfan’s Syndrome present with elongated limbs and digits,[17] scoliosis,[17] speech disorders (due to high plate and receding chin),[18] and heart complications.[19, 20] Some patients with Marfan’s Syndrome experience reduced range of motion in the hip and the presence of a protruding femoral head, which can lead to premature osteoarthritis.[17, 21] Similar to Marfan’s Syndrome, individuals with Ehler’s Danlos Syndrome (EDS) present with loose joints and abnormal scarring. However, phenotypic presentation of EDS is much broader, with up to eleven abnormally expressed genes that cause stretchy skin, aortic dissection, joint dislocation, chronic pain, and early osteoarthritis.[22–24] These features are tested using the Ghent or Brighton criteria, and disease subsets are commonly classified as hypermobile, classical, vascular, periodontal, and myopathic.[25] Osteogenesis Imperfecta is a collagen specific rare genetic disease (mutations in col1a1 or col1a2 genes), primarily resulting in brittle bones, as well as shorter height, loose joints, hearing loss, decrease in breathing efficiency, brittle teeth, and aortic dissection.[26] Finally, myxomatous degeneration, or floppy mitral valve syndrome, is caused by a thickened spongiosa layer that occurs in concert with calcification and excess production of dermatan sulfate, which further weakens the valve leaflets and adjacent tissue. Several genes and signaling pathways have been implicated in this disease, including collagens, elastin, GAGs, SLRPs, and Wnts.[27] The prevalence of myxomatous degeneration ranges from 8–15% and increases with age and in patients with other cardiovascular risk factors.[28] Although no optimal treatment currently exists, common remedies include beta-blockers (propranolol, atenolol, calcium channel or ACE inhibitors),[29] bisphosphonates,[30] surgical intervention, and reduced exercise.
1.2.2. Impact of Autoimmune Diseases on Connective Tissues
Autoimmune diseases such as rheumatoid arthritis (RA), lupus, and scleroderma have devastating effects on connective tissues. RA is a chronic inflammatory disorder affecting 0.1–1% of individuals worldwide.[31] RA affects several tissues including the joints, skin, eyes, lungs, heart, and blood vessels.[32] In RA, the immune system attacks the synovium (lining of membranes surrounding joints) causing it to become inflamed and thickened, which can result in degenerate cartilage, bone, and tendons, and altered joint biomechanics.[33] In Lupus, patients present with joint pain and swelling, a butterfly rash on the face, or skin lesions.[34] Lupus may be attributed to both genetic (HLA gene[34]) and environmental (e.g., vitamin D[35]) factors. There are 16,000 new cases of lupus reported each year in the US, and it is ~9 times more common in women.[36] Scleroderma (systemic sclerosis) affects 75,000–100,000 people in the United States[37] and is primarily characterized by excessive collagen type I and III synthesis.[38] Patients with scleroderma present with thickened and stiff skin,[39] and poor blood flow.[39] If symptoms are specific to skin distal to the elbows and knees, this is termed CREST (calcinosis, Raynaud’s phenomenon, esophagealdysmotility, sclerodactyly, teleangiectasia) syndrome.[34, 40] Scleroderma may be caused by vascular and immune abnormalities that initiate impaired angiogenesis, endothelial damage, and upregulation of adhesion molecules that chemoattract leukocytes, fibroblasts, and myofibroblasts resulting in tissue fibrosis. Additionally, scleroderma may be initiated by genetic mutations in the HLA gene[41] and environmental factors (e.g., exposure to silica[42]). Current treatments for autoimmune diseases affecting connective tissues include NSAIDs,[43] corticosteroids,[44] immunosuppressants,[45] vitamin D,[46] and other biologics.
1.2.3. Impact of Aging and Trauma on Connective Tissues
Aging and trauma contribute to aberrant connective tissue phenotypes. White adipose tissue (WAT) ranges from 2–70% of total body weight and is redistributed with aging from subcutaneous to visceral depots,[47] which affects metabolism,[48] accumulation of macrophages,[49, 50] and telomere lengths.[50] Since growth hormone is lipolytic, growth hormone deficient and resistant mice increase in body fat with aging.[51] Aging also causes adipose progenitor cell dysfunction[52] senescent cell accumulation,[53] ectopic lipid deposition,[54, 55] and adipose tissue miRNA processing.[56] Indeed, several interventions aimed to reduce aged-induced changes in WAT include caloric restriction, growth hormone reduction, and estradiol.[57, 58] In contrast, brown adipose tissue (BAT) activity is inversely related to body mass index (BMI)[59, 60] and may be protective against childhood and adult obesity.[61] At birth, BAT accounts for 5% of total body weight and is essential for thermoregulation since newborns cannot shiver to produce heat. As thermogenesis is initiated, BAT lipid stores decrease within days after birth and are not increased again until adolescence. During adulthood, BAT decreases, which may be associated with declines in growth hormone, estrogen, and androgen.[62–64]
Aging and traumatic injury can cause osteoarthritis (OA). Derived from the Greek terms “arthron” (joint) and “itis” (inflammation), OA is the continuous and slow degradation of cartilage, bone, and synovium and is associated with tissue avascularity, joint inflammation, aberrant remodeling, and reduced joint mobility.[65] The primary individuals impacted by OA are the elderly, females over age 45, those with prior joint injuries, as well as those with poor joint alignment and biomechanics, genetic factors, and obesity.[66] In the United States, it is estimated that OA affects over 20% of the population above age 45 and 50% of those over age 65.[67] Joints with OA may display an ECM with decreased collagen and PG content, fibrillation, increased water content, formation of bony protrusions (osteophytes), synovial inflammation, meniscal damage, joint capsule hypertrophy, and subchondral bone thickening.[65] In addition to age induced OA, excessive loading trauma can induce cartilage damage and subsequent OA.[68] Damage to other ligaments or tendons such as the anterior cruciate ligament (ACL) can also promote OA and meniscal damage.[69, 70]
In skin, the production of hormones during puberty activates sebaceous and sweat glands. However, over time, these glands decrease in activity together with changes in skin wrinkling, sagging, gray hair, and baldness.[71] Additionally, aging results in decreased skin strength and elasticity, which are attributed to decreased elastin and collagen:elastin ratios and increased collagen crosslinking.[71] Traumatic injury to the dermis also alters its structure-function properties. Inferior wound healing affects millions of people worldwide,[72] with added complications due to diabetes,[72] aging,[72] and smoking.[73] Although tissue regeneration occurs during development,[74] tissue repair and scar development occur during adult wound healing.[75]
Tendon disorders are devastating injuries that result in significant pain, lost productivity, and high healthcare costs. Repeated overuse, aging, and traumatic injury are major risk factors for tendinopathy or tendon rupture. Tendinopathy in the rotator cuff affects 4–6% of the population between the ages of 25–64, with increased frequency in laborers (19%)[76] and athletes (69%).[77, 78] Several tendons, particularly those highly load bearing[79–81] are at increased risk of tendinopathy. Excessive mechanical loading[76, 82–85] may result in tendon thickening and increased vascularization,[86] and mechanical cues can drive tenocytes and tendon stem/progenitor cells (TSPCs) towards non-tenogenic lineages.[87, 88] Altered collagen content, decreased fiber organization, and non-tendon ECM deposition (calcification, ossification, lipid accumulation, deposits of proteoglycans) have been identified in histological sections from tendinopathic human tissue.[89] Furthermore, with increases in age past adulthood, tendon exhibits changes in collagen structure,[90, 91] decreased modulus,[92, 93] increased cross sectional area,[93–95] and decreased cell density.[93, 96] Tendon degeneration and ultimate rupture can occur throughout the tendon, and its muscle and bone attachments.[97]
2. Multiscale Structure-Function Relationships in Native Soft Connective Tissues
2.0. Quantifying Multiscale Structure-Function Relationships in Native Tissue
Knowledge of the native ECM is critical to accurately identify and understand disease pathology and engineer biomaterials to mimic and heal tissues. Therefore, the study of structure-function relationships in tissues has remained a key component to establish proper design benchmarks. Unfortunately, there is no one-size-fits-all approach to evaluate structure-function relationships as several testing methods are used depending on the length and mechanical scale of interest. This makes it imperative that one considers the hierarchical scale(s) desired to benchmark, ranges of potential expected values, and testing factors that may influence results. For example, many human tendons can withstand thousands of Newtons of loading, yet cell-ECM forces are ~12 orders of magnitude smaller (nN)[101] and subcellular forces between myosin and actin are less than 1 pN.[102] Additionally, many biological tissues are anisotropic, viscoelastic, and poroelastic, which has major implications for material property values derived from mechanical testing as these will depend on the direction of loading, stress magnitude, loading mode, and loading rate applied. Human tissues have various collagen compositions and a wide range of compression moduli varying from 0.1 kPa in brain tissue to over 1000 kPa in bone.[103, 104] Here, we provide an overview of methods to evaluate multiscale structure-function relationships commonly used throughout this Review.
Assessment of multiscale structure-function relationships at various length scales in naïve tissues and engineered constructs is accomplished using a variety of techniques (Figure 2). In vivo macroscale mechanical properties are approximated using combinations of imaging based methods, such as biplane radiography,[105] MRI,[106] and cinePC MRI,[107, 108] ultrasonography (B-mode, elastography, shear-wave elastography),[109, 110] gait analysis,[111] or dynamometry.[110] By combining image-based methods to quantify tissue deformations with external force assessment (e.g., ground reaction forces), inverse dynamics and finite element methods can approximate tissue and joint stresses. If tissue samples are available, direct mechanical assessment ex vivo is possible using mechanical testing equipment in tension, compression, shear, or torsion. Selection of the specific testing method depends on the in vivo loading environment for the tissue of interest. For example, tendons typically function in uniaxial tension, unlike cartilage that is compressed. Several mechanical properties may be evaluated including quasi-static mechanical properties (Young’s modulus, toe/linear modulus, transition strain, maximum stress and strain), viscoelastic properties (percent relaxation, relaxation half-life), dynamic properties (dynamic modulus and tangent of loss angle), and fatigue properties (laxity, cycle to failure).[112] Due to the nonlinear response of stress and strain with applied loading in fibrous tissues, tissue properties are commonly evaluated during the toe, transition, and linear regions. Rheology is also used to determine the shear modulus of materials.[113]
Figure 2: Connective tissue and biomaterial assessment varies across length scales.
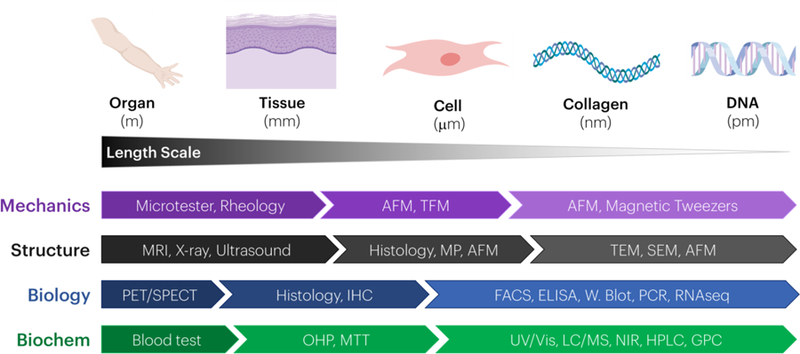
Methods to quantify material mechanics, structure, biology, and biochemistry vary when going from the whole organ (meter) to DNA (picometer) levels. The methods listed are representative examples for the different modes of analysis listed.
At the microscale, increased precision is necessary to capture mechanical properties at these smaller length scales. Tissue and cell biomechanics is commonly evaluated using atomic force microscopy (AFM) whereby samples can be evaluated mechanically in native fluid environments, with several testing and length scale evaluations possible depending on the testing mode (e.g., tapping v. contact) and cantilever tip (colloid, pyramid).[114, 115] Functionalization of the cantilever tip can also allow local mechanical characterization of specific proteins such as collagen.[114] To determine the traction force generated by cells, traction force microscopy (TFM) is commonly used.[116] In this method, cells are embedded in a matrix of known mechanical properties (e.g., polyacrylamide) and gel displacements are tracked over time to back calculate forces. Methods to determine forces exerted by cells on their substrates have several strengths and limitations.[117] Finally, magnetic tweezers may be used to evaluate mechanical properties at the picometer level, including assessment of DNA.[118]
Multiscale structural property assessment is important to both design biomaterials that may mimic native tissue structure and evaluate whether biomaterials used as therapies restore native tissue architecture. Macroscale tissue structure is commonly evaluated with MRI, x-ray, and ultrasound. Several MRI pulse sequences (T1, T2) can define anatomical features of body tissues that depend on water content (e.g., bone, cartilage, tendon, fat)[119] and infer glycosaminoglycan distributions in tissues such as cartilage (T2*, T1ρ).[120, 121] Ultrasound can monitor soft tissue structures with low acoustic impedance, such as fetuses, abdominal features, and tendons. Higher frequency ultrasound (>10MHz) imaging may provide increased resolution of smaller structures such as tendon fascicles that are superficial to the skin.[122] Tissue level structure, particularly collagen alignment, porosity and fiber size, is evaluated with intact tissues or histological sections imaged with polarized light imaging,[112, 123] multiphoton confocal imaging,[124] or AFM.[125] At the nanoscale, transmission electron microscopy, scanning electron microscopy,[126] and AFM[114] are used to define features not visible by conventional microscopes, such as the D-banding of collagen.[125]
Several testing methods evaluate aspects of tissue biology and biochemistry. Positron emission tomography (PET) and single photon emission computed tomography (SPECT) detect changes in metabolic activity in vivo. These methods commonly incorporate structural imaging to detect metabolically active tumors in patients with cancer, as well as to detect osteoarthritis. At the tissue level, histological sections stain for many tissue and cell markers using immunohistochemistry. If tissues are available, they may be assessed for total collagen content or cell viability. Cells from tissues can be isolated and evaluated for their expression of many cell surface markers. Proteins within tissues are evaluated quantitatively with ELISAs, Western blots, and mass spectrometry. Gene expression is evaluated following RNA isolation using quantitative real time polymerase chain reaction (qRT-PCR) as well as RNAseq. Further evaluation of tissue composition (e.g., crosslinking), component molecular weights, and particle size are evaluated with high performance liquid chromatography, gel permeation chromatography, and dynamic light scattering.
2.1. Adipose Tissue Structure-Function Relationships
Adipose tissue has important functions throughout the body, including energy storage, nutrient sensing, temperature regulation, immune modulation, wound healing, and tissue regeneration.[127, 128] It is present in both unilocular (white, WAT) and multilocular (brown, BAT) forms and is composed of adipocytes (fat cells), adipose derived stem cells (ASCs), and immune cells.[128, 129] Adipocytes are surrounded by a thin (100nm) basement membrane containing type IV collagen[130] and a sheath of fibrillar collagen (I, III, V, VI), laminin, and proteoglycans[131] that function to dissipate external stresses.[132] Adipose tissue is deposited throughout the body in the dermis, breasts, bone marrow, face, and adjacent to the mesentery. The mechanical modulus of adipose tissue can range substantially, and is approximately 2kPa in the breast[133] and 600kPa in the plantar fat pad.[134]
Throughout the body adipose tissue interacts with neighboring tissues and plays important roles in tissue homeostasis and function. In the dermis, hair follicles are in contact with WAT, and reciprocal interactions occur between WAT size and hair follicle cycling.[135–137] Hair follicle regeneration and dermal healing is disrupted when adipocyte differentiation is inhibited,[138] and the addition of adipocyte precursors can activate new hair growth.[138] Adipose tissue in the acetabular fossa of the hip and deep to the patellar tendon of the knee[139, 140] serves biomechanical roles by dissipating stress and reducing friction[141] and may also play a role in adipokine and paracrine signaling during osteoarthritis and joint inflammation.[142, 143] In mammary tissue, WAT and the mammary gland epithelium both remodel during pregnancy, lactation, and ovulation.[144, 145] For example, adipocyte lineage cells promote epithelial proliferation and branching, alveolar and ductal morphogenesis, and differentiation into milk protein producing cells.[146–148] During breast cancer, tumors have decreased adipocyte cellularity and lipid content,[149, 150] and can provide lipids and cytokines to regulate tumor cells and activate metabolic pathways to stimulate tumor cell invasion.[151, 152] Bone marrow contains adipose tissue (bmAT) that functions similar to an endocrine organ[128, 153] and is influenced by aging, obesity, and GH deficiency.[154, 155] After caloric restriction, bone marrow tissue expands in concert with adiponectin secretion in serum.[156, 157] Facial adipose tissue contributes to facial features and recognition and consists of both superficial and deep depots[158] that vary during aging, contributing to wrinkling.[159] In Grave’s disease, retro-orbital white adipose tissue expands and results in the bulging of the eyes.[160] Cardiovascular adipose tissue covers 80% of the heart’s surface[161] and functions to provide mechanical cushioning during beating, thermal protection for the heart,[128, 162] and production of free fatty acids that diffuse to the myocardium for energy storage.[163] During obesity, epicardial WAT hypertrophies, leading to an increase in the work required for heart contraction, cardiac hypertrophy, adipose infiltration into the myocardium, and release of proinflammatory signals.[163, 164] Finally, adipose tissue in the hands and feet helps to distribute weight and reduce stress concentrations.[134] In these highly loaded areas, adipocytes exhibit globules encapsulated by collagen and elastin, presumably for mechanical support.[165]
2.2. Cartilage Structure-Function Relationships
Cartilage is found throughout the body, provides cushioning and gliding within synovial joints (elbow, shoulder, hip, knee), and is a key biomechanical component to many tissues (e.g., ear, nose, intervertebral discs, rib cage). Lessons learned from development may be important when tissue engineering cartilage. Cartilage formed during embryogenesis serves as a template for bone growth during endochondral ossification.[166] During embryogenesis, MSCs derived from the mesoderm form the appendicular skeleton[167] (i.e., limbs) followed by cell condensation and differentiation into prechondrogenic cells, persistent chondrocytes that form the ECM and hyaline cartilage, and proliferating chondrocytes that form the growth plate.[168] There are three overall types of cartilage: elastic (ear flap, larynx), hyaline (joints, growth plate, nose, ears, trachea, larynx, respiratory tubes), and fibrous (spine, menisci). Cartilage contains cells termed chondrocytes that produce a type-II collagen-rich ECM and ground substance rich in proteoglycans. Native uninjured cartilage lacks a blood supply, which limits its ability for repair following injury. Nutrients are delivered via diffusion and this can be enhanced through mechanical loading.[67] In the knee joint, articular cartilage dissipates energy as the tibia, femur, and patella experience six degrees of freedom motion during limb proprioception, and supports forces greater than 6 times body weight during running.[67] In the mandibular condyle, cartilage prevents wear to the temporal mandibular joint during jaw movement activities (chewing, speaking). Although all of these may be termed “cartilage”, each has strikingly different structure-function features.
Articular cartilage in joints experiences multiaxial compressive and shear stresses together with hydrostatic and osmotic pressure during loading. This two-phase material contains both a solid collagen-II matrix and liquid phase of water (70–80%), electrolytes, and polyanionic proteoglycans (e.g., aggrecan, glycosaminoglycans) that attract interstitial fluid and give rise to osmotic pressure.[169] The matrix contains spatially graded chondrocytes that produce ECM.[170] This ECM is highly heterogeneous and anisotropic throughout its thickness and length, due in part to mechanical loading during formation. Cartilage is biphasic and viscoelastic, and experiences strain stiffening under applied stress as collagen fibers reorganize to accommodate joint loading.[171] The pericellular matrix embedding the chondrocytes is composed of collagen type-VI and proteoglycans, and attenuates stress.[172] During loading, the fluid movement is reduced by collagen-type II within the network, giving rise to mechanical stiffness and a Young’s modulus that ranges from 5 to 25MPa at the macroscale and 0.5–1MPa at the microscale from deep to superficial zones.[173–176] Mechanical loading also activates many mechanotransductive pathways that regulate chondrocyte anabolic and biosynthetic processes.[177] Joint gliding and mobility during loading is facilitated by synovial fluid in joints that contains a lubricating proteoglycan known as lubricin (PRG4).[178]
There are three main zones of cartilage: superficial, middle, and deep. The superficial zone contains type II collagen fibers parallel to the surface, low amounts of aggrecan, low hydraulic permeability, high amounts of lubricin, and more spindle-shaped chondrocytes.[170, 179] This arrangement of fibers contributes to their high tensile modulus, which helps to reduce Poisson effects during loading, stress shields deeper regions of cartilage, and creates extremely low friction (lowest in nature). The middle zone of cartilage has chondrocytes with a more rounded morphology, disorganized type II collagen, and increased amounts of aggrecan. The deep zone has collagen organized perpendicular to the underlying subchondral bone and increases in mineralization.[179] Structurally, collagen type II fibers have an ordered and graded structure at the macroscale, but appear highly disorganized at the microscale allowing entanglement of aggrecans.[180]
2.3. Dermis Structure-Function Relationships
Skin spans the entire surface of the body and plays many roles, including being an barrier for environmental threats (e.g., bacterial and viral infections, UV damage), regulating temperature, and sensing external stimuli.[72] Skin plays major roles in both heat regulation and excretion via perspiration.[71] Nearly 600–900mL of water are lost daily together with sebum and sweat used to lubricate the skin surface.[71] Skin is comprised of two primary layers termed the dermis and epidermis that also contain many additional tissues, including sweat glands, nerves, and blood vessels.
The dermis helps support the epidermis and connect it to the hypodermis. It is composed of collagen type III, elastin, proteoglycans, vessels, nerves, glands, and sensory receptors,[72] and contains an upper (papillary) layer and a lower (reticular) layer. In the papillary layer, organized parallel rows of microscopic structures termed papillae create unique ridges in the skin that result in fingerprints.[71] The deeper reticular layer contains white fibrous tissue and blood vessels.[71] Superficial to the dermis is the epidermis, which contains four cells types (keratinocytes, melanocytes, Merkel cells, Langerhans cells) that form the stratified squamous keratinized epithelium.[181] This thin layer has additional separate layers termed the stratum basale, spinosum, granulosum, and lucidum, and corneum that form due to continuous differentiation and cytomorphosis of keratinocytes. The hypodermis attaches to the deep fascia or periosteum of bone and contains pads of adipose tissue. Together, these components cover many diverse tissue surfaces ranging from the conjunctiva of the eyelids, external eardrum, urogenital system, and respiratory system.
Similar to other connective tissues, skin’s structure and function properties vary throughout the body. For example, the cheek and forehead contain sebaceous glands giving an oily texture, whereas skin beneath the eyebrows is thicker and contains coarse hairs. There are approximately 3000 sweat glands per square inch of skin.[71] Skin thickness may also vary throughout the body (0.07–5mm) and in disease states such as diabetes. The tensile modulus of naïve skin ranges from 2.5–8MPa.[182] Skin is also a stimuli responsive tissue due to mechanoreceptors (stretch, vibration, pressure, touch), thermoreceptors (hot, cold), and nociceptors (pain). Some receptors are encapsulated in layers of epineurium (e.g., Pacinian and Ruffini corpuscles) and some are unencapsulated (e.g., Merkel disc, peritrichial nerve).
Skin’s structure-function relationships vary drastically during the wound healing cascade, which consists of hemostasis, inflammation, proliferation/fibroplasia, and remodeling. Within the first hours after a skin injury, hemostasis occurs due to platelet aggregation and the assembly of a fibrin matrix[183] which promotes migration of cells to the injury site, and serves as a depot of growth factors.[74, 184] Chemotactic factors released by platelets initiate the inflammatory response[72] followed by re-epithelialization, neovascularization, and formation of granulation tissue (e.g., collagen III, hyaluronic acid, fibronectin).[185] Days to months post injury, ECM stress causes fibroblasts to produce collagen, generate a stiffer matrix, become more contractile, and differentiate into myofibroblasts[72, 74] that contract the wound and form a scar.[186]
2.4. Tendon Structure-Function Relationships
Tendons connect and transmit forces from muscle to bone,[187] and are composed of tenocytes (tendon fibroblasts) and tendon-derived stem/progenitor cells (TSPCs).[87] These cells are embedded in a heterogeneous matrix of collagens (types I, II, V, IX, and X), proteoglycans, elastin, fibronectin, and fluid (70% wet weight) surrounded by blood vessels.[188–194] Together, these molecules form a hierarchical network that contributes to strain transfer from the macro- (“fascicle”) to nano- (“fibril”) scales.[4, 124, 195–197] Native tendon contains highly organized collagen fibers that surround elongated tenocytes. Unlike normal tendon, injured and healing tendon is more disorganized in collagen structure and type (greater collagen type III:I), and contains neutrophils and macrophages, and other immune cells at the wound site,[198] that disrupt the native tissue environment. TSPCs may play important roles in tendon homeostasis and healing capacity following injury, due both to their ability to differentiate into tenocytes and participate in paracrine signaling.[87, 199] TSPCs are positive for stem cell markers (e.g., CD44, 90, and 146)[87, 200] and negative for markers of other cell populations (e.g., endothelial cells, leukocytes, CD34, 45).[87] Several transcription factors (e.g., Scleraxis, Mohawk, Tenomodulin) and ECM proteins (collagen I, and members of the SLRP family) may be particularly important to evaluate as are expressed in naïve tendon. However, it is difficult to accurately identify tendon cell types due to the limited number of markers that have been identified through development and aging.[201]
Individual tendons exhibit enormous spatial variation in material properties, as they originate from muscle (compliant material) and insert into bone (stiff material) through a fibrocartilaginous region that dissipates stress concentrations. This fibrocartilaginous insertion is lower in collagen and proteoglycan content than the midsubstance, and transitions in composition from type I to type II collagen. Disorganized ECM becomes more aligned and less wavy (termed “crimp”)[202] with mechanical loading, resulting in a distinct “toe-region” during mechanical loading due to this strain stiffening mechanism. In addition, tendons are viscoelastic and poroelastic, which results in rate- and time-dependent properties and fluid flow within the ECM.[203] During normal human motion, the stresses and strains that tendons experience vary based on anatomical position and activity level. Although many tendons operate in the toe-region of the tissue’s stress-strain curve (less than 5% of their load until rupture),[204] higher load bearing tendons, such as the Achilles, can experience forces nearly 70% of their maximum load and stress before rupture (~3500 N or ~55MPa).[205, 206] The primary type of loading in tendons is tensile, yet compressive, biaxial, and shear stresses may be present.[207, 208] Aging[93] and sex[209] may influence native tendon properties and their healing capacity.[210] Additionally, loading post injury[187, 211] and surgical intervention[212, 213] may impact tissue healing and joint recovery.
Both pathology and strength/endurance training in humans highlights the role of mechanical loading in tissue homeostasis, and its potential application in the design of functional biomaterial-based tissue replacements. Both low and high mechanical loading of tendon can disrupt homeostasis[214–221] Stress deprivation flattens and elongates fibroblasts, decreases cell number, decreases tensile modulus,[220] produces inflammatory cytokines, causes matrix degradation,[222] and causes the release of pro-inflammatory cytokines and vasodilators.[223] Certain loading regimes may promote optimal mechanical properties[219] through alterations in collagen synthesis.[217, 221]
3. Biomaterial Connective Tissue Mimics, Biologics, and Therapeutics
3.0. Overview of Biomaterial Connective Tissue Mimics
Major goals for engineering new biomaterials in this field are to replace or regenerate tissue and deliver therapeutics locally. Biomaterials can be engineered to mimic tissue structure in order to provide tissue function. Local drug release may overcome current limitations of oral administration, including the need for high or repeated doses, poor targeting, short circulation times, poor patient compliance, systemic toxicity, and the short half-lives of many drugs.[229–232] When designing biomaterials to mimic native tissues, the role of the extracellular matrix in regulating cell behavior should be considered. Biophysical signals incorporated into biomaterials, such as matrix elasticity,[233] viscoelasticity,[234] and porosity[235] may help control and regulate stem and stromal cell behavior. Fiber orientation is also critical for various cell behaviors, including stem cell differentiation,[236] cell alignment,[237–239] matrix deposition,[240, 241] and migration.[242] Many natural and synthetic materials are used as biomaterials to mimic and heal connective tissues (Table 3), and these may be synthesized into different geometries using a variety of techniques, including injection molding, electrospinning, 3D printing, and weaving. Using natural polymers is advantageous because they contain physiological components and comprise cell adhesive sequences that support cell adhesion and differentiation. However, natural polymers may be limited in mechanical strength, ability to control in vivo degradation, variation in production quality, and risks for contamination. Synthetic polymers are advantageous because of their high degree of mechanical, chemical, and structural tenability, well controlled composition and manufacturing, and capacity to incorporate bioactive compounds. However, synthetic polymers are limited in their biointegration, inability to remodel with surrounding tissue, and risk for inflammatory responses. Taken together, the chemical and biophysical features of materials may guide cell lineage specification in vitro and in vivo, and affect proliferation, motility, contractility, and many other cell functions by changing both acute signaling and transcriptional programs.[243–245]
Table 3:
Representative natural and synthetic polymers used in connective tissue biomaterials.
| Type | Material | Description | Degradation | Young’s Modulus (kPa) |
|---|---|---|---|---|
| Natural | Hyaluronic acid [251–255] | Glycosaminoglycan | Hyaluronidase, Hydrolysis[256] | T: - C: 4–95 [233, 253, 254] |
| Agarose[257] | Polysaccharide containing galactose residues (seaweed) | Agarase | T: - C: 7–16[257] |
|
| Alginate[258–261] | Polyanionic saccharide (algae, seaweed) | Alginate lyase, Requires modification (e.g., oxidation) | T: 3–25[262] C: 0.1–160[234, 263, 264] |
|
| Chitosan[259, 265, 266] | Polycationic saccharide (chitin) | Lysozyme, chitosanase, chitinase, NAGase[267] | T: 2500k[268] C: 2[259] |
|
| Collagen [297, 298][269–275] | Main structural protein in connective tissues | Protease | T: 6–40k[270, 276] C: 0.02–0.5[277] |
|
| Gelatin[255, 278–280] | Hydrolyzed from collagen (skin, tendon) | Protease | T: 50–175[280] C: 0.6–545[279, 281] |
|
| Silk[258, 282–286] | Fibroin (cocoons of larvae of silkworm) | Protease XIV and hydrolysis[287] | T: 515k-16000k[288] C: ~40–927[289, 290] |
|
| Fibrin | Fibrous, non-globular protein (fibrinogen) | Plasmin-mediated fibrinolysis | T: - C: 0.01–0.5[277] |
|
| Synthetic | Poly(α-esters) ▪ poly(glycolic acid) (PGA)[291] ▪ poly(lactic acid) (PLA)[292, 293] ▪ poly(ethylene glycol)[294, 295] |
Thermoplastics | Hydrolytic (aliphatic ester groups) | T:1.5k-100k[285, 292] C: 2–12[296] S: 1.8–10.2 [294] |
| Polyacrylamide | Polymer formed from acrylamide | High temperature or pH, shear stress, autooxidation | T: 10[297] C: 0.1–40[245] |
T: Tension; C: Compression; S: Shear
Biomaterials can provide biochemical signals to promote healing, as well as deliver therapeutics and cells to tissues. Biochemical signals arising from adhesive molecules such as laminin and fibronectin, as well as signaling ligands such as TGFβ and BMP may affect MSC attachment[246] and guide differentiation.[247] Since cell survival following implantation is often less than 26%, and in many situations lower than 1% after 24 hours,[248–250] biomaterials that can harbor cells in microenvironments to protect them from harsh conditions in vivo may have great utility. Materials can also release factors in response to environmental stimuli. For example, biomaterials can degrade in healing environments that exhibit high concentrations of proteases or non-neutral pH.
Many cell sources can be considered for connective tissue engineering, but MSCs have been the most widely explored. In 1999, Pittenger and colleagues isolated MSCs from bone marrow and demonstrated their multipotency, which has led to their application in every connective tissue discussed in this Review.[298] Adult MSCs are important in tissue homeostasis, remodeling, and regeneration due to their ability to differentiate down different lineages, modulate the immune system, and promote healing through paracrine signaling. With the right cues, MSCs can differentiate towards various mesenchymal lineages (e.g., bone, cartilage, fat, skin, tendon/ligament, muscle, bone marrow stroma). These cells are defined by the Mesenchymal and Tissue Stem Cell Committee at the International Society of Cellular Therapy[299] by being: (1) substrate adherent, (2) multipotent (differentiate into bone, fat, cartilage), and (3) express surface makers CD90/Thy-1, Cd73, and CD105. CD34 (hematopoietic and endothelial cell), CD45 (leukocyte), CD11b (monocyte and macrophage), CD79a (B-cell), and CD31 (platelet, monocyte, neutrophil) are common negative MSC markers. Similar stem/progenitor cells may also be derived from other sites throughout the body, including the perivasculature near blood vessels or within connective tissues such as in parallel collagen fibril chains in tendon.[87]
MSC-based cell therapies have been widely used to date; however, many therapies based on direct cell injection have produced conflicting results in vivo, potentially due to both complications in local delivery of cells to the site of injury and the absence of a scaffold environment protecting against harsh mechanical and inflammatory microenvironments. Unlike embryonic stem cells that may remain in equine tendon for 3 months post injection, MSCs had less than 5% survival after 10-days post-injection.[300] Quantification of MSC homing to specific locations following injection suggests that the number of cells reaching their desired location is limited.[301] Systemic delivery of MSCs also suffers from accumulation of MSCs in the lungs and potential complications such as arterial thrombosis if delivered through the blood.[302] Additionally, direct injection alone does not provide environmental cues to control MSC response to produce functional tissues. The normal microenvironment, or niche, of MSCs in the body provides important cellular, structural, and signaling cues that help maintain stemness, number, and activation. Therefore, much attention has been focused on designing biomaterial systems that not only harbor and protect these cells post injection/implantation, but also provide cues important for their regulation to regenerate functional tissues.
MSCs have limitations as cell sources given their weak definition and characterization in many studies. Although the term “MSC” was first introduced in 1991 to represent multipotent cells derived from bone marrow and periosteum, many studies use MSCs without investigating their multipotency. Given their ability to home to injury sites and secrete bioactive factors that aid in tissue healing, these cells may function more as “medicinal signaling cells.”[303] MSCs can suppress immune responses[304, 305] through the secretion of soluble factors,[306, 307] which may affect inflammatory cell infiltration post injury. MSCs may also promote fibroblast proliferation and ECM synthesis by releasing cytokines[308] important for regeneration (termed “paracrine signaling”). Additional cell types are also being considered for tissue regeneration. Induced pluripotent stem (iPS) cells are a particularly attractive cell type for regenerative medicine. These may be readily obtained by reprograming fibroblasts or other cells from a patient with transcription factors (Oct¾, Sox2, c-Myc, and KLf4) to be pluripotent, followed by differentiation down desired pathways.[309] Potential hurdles for iPSC translation include low reprogramming efficiency, low cell engraftment following transplantation, immunogenicity, and tumorigenicity.[310]
3.1. Adipose Tissue Biomaterials
Cosmetic reconstructions, congenital defects, trauma resections, and lumpectomy procedures to treat breast cancer have dramatically increased demand for biomaterials to replace adipose tissue.[311] FDA approved soft tissue fillers such as HA, collagen, and PMMA fill small volumes (~1–2ml) and their high costs and risk of foreign body reactions, inflammation, shape distortion, and resorption (40–60%+[312–314] within 6 months[315]) limit their application for larger volume applications (e.g., breast reconstructions) requiring up to 400ml. Current costs for soft tissue fillers for aesthetical surgeries have prices (USD/ml) ranging from $300 for collagen products (e.g., Cosmoderm©) to $1,000 for PLLA PMMA microspheres and collagen products (Artefill©).[311] Furthermore, many reconstructive procedures using prostheses, free flaps, or lipofilling cost between $2,300 and $23,000 in materials alone.[311] Although there are over 10 HA soft tissue fillers approved by the FDA (e.g., Restylane©), they are classified as temporary solutions because they require repeated injections and are not designed to induce regeneration. Therefore, methods aimed to improve degradation rates (e.g., crosslinking), and biological performance (e.g., factors to stimulate adipogenesis, bio-interactive signals) are being widely studied.
In the next sections, we highlight recent advances in both biologic free strategies and those that provide active cues to promote adipose tissue (Figure 3a).
Figure 3: Biomaterial strategies to mimic and heal connective tissues.
Several materials are engineered with varying structure and composition to recapitulate connective tissues, such as (a) adipose tissue, (b) cartilage, (c) dermis, and (d) tendon. In addition to using biologic-free approaches, methods incorporating cells, growth factors, and drugs are common.
3.1.1. Adipose Tissue Biomaterials: Biologic-Free Approaches
Work in the past decade to develop cell-free biomaterials for adipose tissue replacement has primarily focused on using decellularized adipose tissue (DAT). Biomaterials incorporating DAT have been able to retain native human adipose tissue mechanical properties,[316] contain similar ECM components (e.g., collagen type-I, IV, and laminin),[317] support adipogenic differentiation,[317–319] and stimulate angiogenesis.[320] Other techniques include using DAT microcarriers,[321] hydrogel composites,[318] and injectable gels.[322]
Another biologic-free approach involves materials that show similar porous structures to native adipose tissue.[319, 323] One technique incorporated lipoaspirate into a silk protein matrix to create a porous sponge suitable for tissue ingrowth after placement.[282, 323] Over 18 months, these materials led to regeneration of subcutaneous adipose tissue and maintained their original implanted volume.[282] In a different strategy, a zwitterionic poly (carboxybetaine-co-methylmethacrylate) co-polymer (CMMA) was electrospun to create nanofiber meshes with large interconnected pores and a low density structure to promote adipogenesis.[324]
3.1.2. Adipose Tissue Biomaterials: Biologic and Drug Approaches
Given the abundance of cells in adipose tissue, many biomaterial replacement strategies include a cellular component. The incorporation of ASCs in hydrogels is widely used to engineer adipose tissue due to the facile nature of their isolation via liposuction, expansion ability in culture, differentiation potential to pre-adipocytes, and opportunity for patient-specific cell products.[325] Matching scaffold and native tissue mechanical properties further promotes ASC differentiation into adipocytes,[326] which highlights the importance of evaluating native tissue structure function properties. From a materials perspective, use of natural polymers is advantageous for their biocompatibility and cell adhesion. However, bioactive properties can be incorporated into other materials through presentation of peptides (e.g., RGD to promote adhesion) from a hydrogel backbone.[327]
Injectable biomaterials and 3D porous implantable scaffolds are commonly used to deliver cells for adipose tissue regeneration. Injectable materials are advantageous because they allow minimally invasive delivery, can conform to any tissue geometry, and can gel in situ. Hydrogel properties may be tuned using chemical crosslinking (hyaluronic acid,[251–254] collagenase sensitive PEG,[294] DAT[251]) and physical and ionic crosslinking (alginate and O-carboxymethyl chitosan[259]), and can be made thermo-responsive (Pluronic F127,[328] HA-PNIPAAm-poly(amidoamine)[329]). Porous 3D scaffolds fabricated with solvent casting (gelatin[278]), freeze-drying (decellularized adipose tissue[319], PGS/PLLA,[330] PLGA[331]), cryogelation (HA and gelatin[279]), two-photon polymerization (gel-MOD[332]), and extrusion bioprinting (poly(D,L)-lactide,[333] DAT[334], alginate[260]) can support adipogenesis and angiogenesis, and possess similar mechanical properties to native tissue.[279] A limitation of some adipose tissue surrogate materials is their non-degradability. Therefore, it may be advantageous to tune material degradation to balance cellular infiltration with chronic inflammation caused by non-resorbable implants.[335]
Promoting neovascularization is important to enable engineered adipose tissue survival in vivo.[336, 337] Interestingly, ASCs alone demonstrated angiogenic activity for 21 days when seeded on a bioresorbable alginate scaffold.[261] Coculture of ASCs with endothelial cells, which absorb fatty acids and promote vascularization in vivo, on a porous silk substrate coated with laminin helped maintain tissue size and shape.[282] Sustained release of the angiogenic factors VEGF and Ang-1 contained in PLA microspheres promoted vascularization and differentiation of ASCs to endothelial cells.[293] Local delivery of dexamethasone also resulted in increased vascularization and greater retention of the initial tissue volume in vivo, likely due to its ability to downregulate preadipocyte factor 1 to prevent adipocyte differentiation.[338, 339] Taken together, this work highlights the potential of biomaterials approaches that incorporate biologics and drugs into scaffolds for adipose tissue regeneration.
3.2. Cartilage Biomaterials
Cartilage damage induced by intrinsic and extrinsic factors has motivated the design of biomaterials to improve or replace damaged tissue. The growth in the global orthopaedic device market is driven by worldwide aging and obesity, with markets exceeding $28B in 2014 and forecasted to reach $38B by 2019.[340] Although total joint replacement to replace osteoarthritic cartilage has remained one of the most successful surgical procedures of the 21st century, and represents a significant fraction of this market, there has been significant efforts to regenerate cartilage using biomaterial based strategies. Historically, the gold standard treatment to promote cartilage regeneration was microfracture, which dates back to the 1950s and involves debriding cartilage and exposing subchondral bone to promote a fibrin clot in order to promote new fibrocartilagenous tissue formation.[341, 342] Although biomaterials may be used to improve this technique,[343, 344] there is still a long post-op period during which joint loading must be minimal.[345] Importantly, a recent study has called into question the efficacy of microfracture in treating cartilage lesions following ACL rupture.[346]
Several design features may be considered when creating biomaterials to mimic and heal cartilage. Cartilage has minimal innervation and vascularization in adult tissue. Although this reduces the number of components necessary for engineering surrogate tissues, it creates a design challenge to maintain tissue nutrition. Furthermore, accurately recapitulating the mechanical, structural, and compositional changes of cartilage from development to maturation (18+ years) in a short time in tissue culture (1–2 months) to create replacements for adult patients has remained challenging.[67] Although approaches have advanced to clinical trials,[347] there still remains a need to more closely match native cartilage mechanical properties, provide improved surrounding tissue integration, and better mimic 3D multizonal architecture to provide spatial cues guiding cell phenotype. Furthermore, constructs must be biocompatible. An interconnected porous structure may also be desirable to allow nutrient/waste diffusion. Given the load bearing requirements of cartilage, material properties are often a major outcome metric evaluated. Engineering materials with very low friction to mimic native cartilage is also an important consideration.[348] Finally, the degradation rate of these materials is another important design consideration. Tuning biomaterial degradation to coincide with tissue healing is possible with breakdown mechanisms involving hydrolytic degradation (polymer chemistries including esters, ureas, urethanes, amides)[67] or enzymatic degradation.
In the next sections, we highlight recent advances in both biologic free strategies and those that provide active cues to promote cartilage tissue (Figure 3b). Non-biomaterial approaches, including osteochondral auto and allografts and total joint replacement are not reviewed.
3.2.1. Cartilage Biomaterials: Biologic-Free Approaches
Hydrogels, fibrous materials, and foams/sponges are common scaffold materials used as biologic-free cartilage biomaterials. 3D hydrogels are fabricated with covalent or ionic crosslinks and may contain interpenetrating networks[349] of other polymers or be further reinforced with fibers.[350, 351] Hydrogel materials offer a number of advantages, including high water content, chondrogenic potential, ready transduction of mechanical loads, and potential in situ scaffold formation. However, they also have challenges, including generally low mechanical properties (E ~200kPa)[350] and isotropy unless fiber reinforcement is used. Injectable materials that gel in situ[352] may allow filling of patient specific defects without requiring open surgery. Hydrogels containing an interpenetrating network of laponite nanoparticle-associate silated hydroxypropylmethyl cellulose was capable of in situ gelation and cytocompatibility, and promoted chondrogenesis.[353]
Fibrous materials have also been widely explored, including those produced via electrospinning.[354, 355] These can provide moduli on the order of tens to hundreds of MPa,[354, 355] and an interconnected porous structure. Electrospinning allows independent control[356] of fiber size, diameter, and stiffness and can be used with many materials, including PCL and PLGA.[357]
Foams and sponges are advantageous forms of cartilage biomaterials because they can possess highly interconnected porous structures with tunable mechanical properties (E ~ 12kPa-5MPa)[358, 359] and can be fabricated using porogens,[360, 361] freeze drying,[362] or 3D printing.[363, 364] Temperature gradients during freezing lead to unidirectional freezing, promoting ice crystal alignment and pores allowing improved infiltration of MSCs.[365]
Several biologic free approaches for cartilage repair are currently in clinical trials. In one approach, a bioceramic scaffold composed of multilayers with gradients of collagen type 1, HA, and magnesium enriched hydroxyapatite is being developed for osteochondral lesions of the knee (MaioRegen™). This biomimetic scaffold promotes differentiation of cells derived from the bone marrow and synovial fluid into osteocytes and chondrocytes, and integrates with surrounding tissues once implanted. Another gradient-based biologic-free approach contains gradients of aragonite (mineral) and HA to similarly mimic the osteochondral interface (Agili C™).[366]
3.2.2. Cartilage Biomaterials: Biologic and Drug Approaches
There are many challenges to engineer functional cartilage, including a source of appropriate cells and recapitulating native zonal heterogeneity. Due to their intrinsic low density in adult tissue, only 1–5% of the total tissue volume,[67] primary chondrocytes are often isolated and expanded in vitro prior to use. A major challenge here is that these cells are expected to proliferate, differentiate, and produce ECM, but chondrocytes in adult articular cartilage are metabolically quiescent. Many studies have explored the impact of cell culture in dynamic bioreactors, serum-free media conditions, added growth factors, hypoxic culture, and 3D culture.[367–370]
As chondrocyte expansion on 2D substrates may cause chondrocyte de-differentiation,[371] and cell expansion capability may be compromised by the fourth passage,[372] bone marrow and adipose derived progenitor cells have been explored for their ability to differentiate into chondrocytes. Although these cell sources are limited by differentiation heterogeneity (cells may hypertrophy, ossify, and be disorganized),[372] several methods have been developed to help guide differentiation. This may be particularly important in achieving native zonal mechanical properties in engineered tissues, as this has not been achieved in previous long-term studies.[373]
A number of cell-based strategies currently are used in the clinic for cartilage repair, are in clinical trials, or are in the pre-clinical stage. Autologous chondrocyte implantation was first introduced to repair cartilage in the early 1990s for cases where microfracture and debridement were unsuccessful.[374] In this procedure, a biopsy of healthy cartilage is taken, and isolated cells are expanded and reimplanted in a biomaterial scaffold.[375] Carticel® is the only FDA approved method of tissue engineering based on transplanting in vitro expanded autologous chondrocytes.[376] Although the efficacy of microfracture for treating cartilage lesions of the femoral condyle remains controversial,[346] biomaterials are now being used in combination with this procedure in an effort to improve outcomes. A chitosan-based solution mixed with autologous whole blood (CARGEL™) was shown to stabilize the clot induced during microfracture,[265, 266] and clinical trials demonstrated a greater percent lesion fill and a decreased T2 relaxation time compared to microfracture alone, out to 5-years post-surgery.[377] Additional biologic strategies for cartilage repair currently in clinical trials include chondrocytes mixed with fibrin (Chondron™) to treat ankle cartilage defects, human umbilical cord MSCs incorporated into a sodium hyaluronate solution (CARTISTEM®),[269] and the attachment of particulated juvenile cartilage rich in chondrocytes with a fibrin sealant (Denovo®).[269]
Engineering gradients in scaffold composition, structure, and mechanics can more closely mimic native cartilaginous tissue. Early approaches to tissue engineer zonal cartilage have included coculturing superficial and middle zone chondrocytes, using bone marrow and adipose derived progenitor cells, incorporating material mimics to promote zonal organization, and providing biochemical cues. Seeding superficial and middle zone chondrocytes in adjacent layers produced features more similar to native cartilage, including increased GAG and collagen in the middle zone and increased lubricin in the superficial zone.[378] Several material approaches, using PGA meshes and PLGA foams,[291] graded photocrosslinked hydrogels,[379] and 3D printing,[380] have since been developed to induce this organization. Providing chemical cues may also aid in promoting desirable cartilage phenotypes. For example, in a scaffold system consisting of decellularized bone and agarose, media perfusion improved cartilage to bone integration, chondrogenesis, and cartilage deposition.[381] Growth factor gradients formed from BMP2 and IGF1 released from PLGA-silk fibroin microspheres in alginate gels provided spatial and temporal gradients to enhance cartilage tissue formation.[258] Gradients in TGFβ concentration may also be important to create heterogeneous tissue growth.[382]
Several new biomaterial-based techniques to deliver agents to cartilage have been investigated. RUNX1, a cartilage-anabolic transcription factor, was delivered using PEG-poly(amino acid) block copolymer-based polyplex nanomicelles into mouse knee joints with OA.[295] OA progression was suppressed and expression of cartilage-anabolic markers and cell proliferation was augmented.[295] Delivery scaffolds may also mimic native tissue anatomy; large hemispherical scaffolds that mimic trochanter morphology were fabricated from 3D woven PCL fibers, seeded with ASCs, and used for anticytokine delivery.[383] MSC-seeded HA constructs were fabricated with rapid prototyping and used to fill large and anatomically complex chondral defects.[384] Manipulation of HA chemistry has been utilized to slow its in vivo degradation, increase GF retention, promote chondrogenesis, suppress hypertrophy of encapsulated hMSCs, and reduce cartilage abrasion in animal joints.[385] This approach may be particularly useful if therapeutics are coupled to positively charged nanocarriers prior to inclusion in HA gels to promote sustained intra-tissue delivery.[386] HA hydrogels may be further tuned to release TGFβ3, which was found to increase expression of collagen type II.[387] Additionally, HA and PCL scaffolds that released TGFβ3 led to improved histological scores of engineered cartilage, increased collagen type II content, and improved mechanical properties.[388]
3.3. Dermis Biomaterials
Skin disease affects 50% of Americans by age 65. As a result of its prevalence and $11B yearly cost in lost productivity, the market for prescription dermatological products (e.g., antiaging, psoriasis, skin cancer dermatitis) is growing at 10%/year, and comprises a significant market ($23.4B in 2016).[71] In addition to skin diseases, skin wounds and complications with skin healing have a $4.8B market worldwide.[72]
Common design criteria for biomaterials intended to replace or heal the dermis include biodegradation, macroporosity to allow for vascularization and cell recruitment, and biocompatibility. Certain features of native skin may be useful to mimic in dermal materials and to stimulate drug release from these materials. For example, the pH of skin ranges from 4 to 6[389] and is more acidic during healing to reduce infection risk and stimulate granulation tissue formation.[390] Lower limbs may be up to 5oC cooler than the 37oC core temperature,[391] and may increase in temperature during wound healing to promote vasodilation and increase nutrient and oxygen supply. Oxygen levels also vary during healing, and can modulate cell behavior.[391]
In the next sections, we review recent advances in biologic free strategies and those that provide active cues to engineer dermis tissue (Figure 3c). We focus on biomaterials strategies for non-cosmetic, more major injuries or defects, excluding strategies such as collagen-based fillers and Botox injection.[71]
3.3.1. Dermis Biomaterials: Biologic-Free Approaches
Several material systems and fabrication strategies are used to engineer dermal biomaterials. As with other connective tissue biomaterials, collagen, chitosan, alginate, silk, and HA are common material selections.[72] Decellularized scaffolds derived from animals or humans using cell removal procedures are also frequently used.[392] These materials are typically used with scaffold fabrication techniques such as electrospinning (gelatin, HA, sHA, and CS[255]; silk fibroin[283]), 3D printing (PLCL[393]), and photopolymerization (pectin[394]). For example, composite silk-collagen scaffolds were used to replace full skin and support nerve ingrowth and secretion of proinflammatory cytokines.[284] Additionally, decellularized human amniotic membrane was combined in a 3D bilayer scaffold with nanofibrous silk fibroin to promote cell adhesion and proliferation with the production of angiogenic growth factors.[283] Recombinant human-like collagen may be an alternative approach to collagen derived from animal tissue, but is currently limited by yield and biomimicry of native collagen.[395]
Adhesion of dermal biomaterials to surrounding tissue is important to promote biomaterial integration and prevent infection. In one study, gelatin methacrylate hydrogels incorporated tannic acid to promote tissue adhesion.[280] In another approach, tough adhesive hydrogels containing a dual interpenetrating network of alginate and polyacrylamide were adhered to skin tissue using chitosan as an adhesive bridging polymer.[396, 397] This strategy produced adhesion energies greater than commercially available products and showed good biocompatibility, highlighting its potential to promote skin healing.[396] As skin is a primary barrier to prevent unwanted infiltration of microorganisms into the body, dermal biomaterials are often designed to prevent infiltration. For example, hydrogels were designed to locally degrade and release iron ions to kill bacteria with exposure to hyaluronidase. Topical administration of the hydrogel on skin colonized with Staph Aureus inhibited infection and promoted tissue regeneration.[398] Silver nanoparticles can also reduce bacterial invasion via bacterial DNA and RNA denaturation.[399]
Several biomaterial strategies to engineer dermis have led to commercial products, including GraftJacket®,[400] Integra™,[401] Promogram™ ,[402] Talymed®,[403] and Algisite™.[404] These products utilize acellular, naturally derived ECM scaffolds from bovine, human, pig, and shark sources. GraftJacket® is made from decellularized human skin and consists of a matrix of collagen, elastin, and proteoglycans. TissueMend is derived from fetal bovine dermis and is similarly processed to produce an acellular dry material.[270] Additional non-animal sources of dermal materials include cellulose, silicone, and alginate.
3.3.2. Dermis Biomaterials: Biologic and Drug Approaches
The incorporation of cells and therapeutics into biomaterials for dermal regeneration has been actively pursued for decades. The first materials approved by the FDA for this purpose incorporated fibroblasts into collagen (Apligraf®[405]) and absorbable polyglactin mesh scaffolds (Dermagraft®[406]). More recent FDA approved biomaterials have incorporated a single layer of epithelial cells into tissues derived from human placenta (EpiFix®[407, 408]) and viable fibroblasts and keratinocytes within human skin cryogenically processed (TheraSkin®[409]). Preclinical work has also explored stem cells for dermal regeneration. For example, co-delivery of ASCs and umbilical cord blood CD34+ cells accelerated wound healing and skin regeneration.[410] Although stem cells may differentiate into dermal fibroblasts and promote repair, paracrine factors secreted by MSCs may be the primary mechanism for repair with these cells given the low efficiencies of engraftment (2.5%).[411] GFs and cytokines released by MSCs mediate fibroblast and keratinocyte migration, proliferation, and matrix remodeling.
As an alternative to cells, many dermal biomaterials have incorporated GFs and other agents to promote regeneration. This strategy is advantageous because injected growth factors have short half-lives in vivo (commonly minutes in serum).[412] GFs used for skin healing include VEGF[413] bFGF,[414] and PDGF.[415] Recombinant PDGF incorporated into a sodium carboxymethylcellulose gel (Regranex®) has been FDA approved and promotes granulation tissue formation for diabetic neuropathic ulcers.[416] Recent work inspired by scarless neonatal healing suggests that continuous protein kinase C inhibition and supply of growth factors (IGF, VEGF), Wnts, and MMPs can restore morphological transitions and rescue hair formation.[417] In addition to GF delivery, gene therapy approaches that deliver plasmid DNA encoding for growth factors via a functionalized scaffold may be used to tune local gene expression.[418] Finally, the release of calcium ions from materials may create tissue gradients that promote homeostasis, control proliferation and apoptosis, and accelerate wound healing.[419]
Mechanical and structural features of biomaterials can help guide the fate of embedded or recruited cells to promote skin healing. Mechanical stimuli such as biaxial stretching, vacuum, pressure relief, and passive forces can have cell, tissue, and protease targets that modulate tissue healing.[420–422] For example, vacuum pressure may increase ICAM-1, MIF, VEGF, and collagen type-I expression and stimulate angiogenesis.[423, 424] Additionally, high mechanical loading to fibroblasts can increase collagen formation, migration, proliferation, and formation of focal adhesions. Other sources of cell and tissue stimulation including electric fields can improve wound healing by stimulating VEGF, EGF receptors, integrins, and ion pumps.[425–427] In addition to mechanical loading, ECM structure also affects cell behavior. For example, nanogrooved matrices made of polyurethane acrylate increased hMSC migration.[428] Additionally, electrospun meshes of PLGA and silk fibroin that mimic skin’s random collagen organization were found to promote fibroblast adhesion and proliferation in diabetic wounds.[285] Recapitulating skin’s multiple layers in a scaffold has been a challenge to bioengineer. Recent advances in 3D printing, however, have enabled printing of skin layers and heterogeneity.[271, 429] For example, two distinct layers of fibroblasts and keratinocytes within collagen hydrogels were printed to create multilayered constructs.[271]
3.4. Tendon Biomaterials
Surgical repair is the primary treatment for tendon and ligament related injuries, with over 300,000 rotator cuff repair surgeries performed yearly.[430] However, many individuals suffer from post-operative complications including re-rupture,[431] elongation,[432] muscle atrophy,[433] reduced function,[431] and poor reconstitution of the tendon-bone interface.[434] Although tissue grafts have been used clinically, a Clinical Practice Guideline by the American Academy of Orthopaedic Surgeons has concluded that “inconclusive” evidence exists for their ability to augment cuff repair.[435] This motivates the design of new biomaterials to restore joint function and provide instructive cues to improve healing (Figure 3d). In addition to the multiscale structure-function relationships discussed in Section 2.4, additional criteria for tendon biomaterials may include delivering appropriate biological cues, providing mechanical augmentation for 12-weeks post-op,[432] having slow degradation, being biocompatible, allowing cell infiltration, and compatibility with arthroscopic approaches and sterilization technologies. Early work in the orthopaedic field highlighted the potential of demineralized bone matrix to promote bone formation.[436] As tendon connects muscle to bone, many similar strategies emerged, but no material has been engineered that fully recapitulates the human tendon or ligament.
3.4.1. Tendon Biomaterials: Biologic-Free Approaches
Many current commercially available products for tendon repair use naturally derived ECM such as dermis (GraftJacket®) and small intestine submucosa (CuffPatch®). Small intestine submucosa-derived ECM may be advantageous as it contains GFs that may promote healing.[270] Naturally derived ECMs incorporating collagen, hyaluronic acid, and silk offer excellent biocompatibility, but have inferior mechanical properties compared to native tissue.[270] This has, in part, motivated interest in synthetic scaffolds.
Several synthetic polymers are used to tissue engineer artificial tendon with increased mechanical material properties. This is particularly important as tendon can experience forces up to 3500N[206] during hopping and have Young’s moduli exceeding 800MPa.[205] Mimicking tendons’ aligned structure is also important to induce gene expression of tendon-related markers.[437] Pore size, anisotropy, and mechanical gradients affect tenogenic differentiation.[438–440] As tendon is primarily composed of collagen type I, collagen-based gels[273] and those reinforced with fibrous matrices to further increase scaffold mechanical properties have been developed. Silk-based scaffolds[286] and synthetic materials (e.g., PLLA, PLGA, PCL) have tunable structures to mimic tendon alignment,[441] fiber diameter,[442] and mechanics.[443] The most common method of scaffold construction uses electrospinning to create fibers of specific size (0.5–1.5 µm) and stiffness after a charged polymer solution is extruded from a needle onto a grounded collector. If rotated, the grounded collector can create materials with different fiber organization, which can have important effects on cell programming. Combinations of synthetic and natural ECM offer additional material opportunities. PLA fibers incorporated into collagen scaffolds achieved a modulus approaching the toe and linear moduli of native tendon.[292] Unlike many naturally derived ECM components such as elastin and collagen that have degradation half-lives on the order of decades, synthetic materials can provide superior mechanical properties and tunable degradation (e.g., PLGA). Another example is the Artelon® device composed of a bioabsorbable urethane urea for Achilles tendon repairs,[444] which can be modified to degrade in vivo.[445]
Given the heterogeneity and biomechanical gradients present in tendon, it is important that these constructs not only mimic the central midsubstance, but tendon’s insertion into bone (or muscle) if it is to be used as a complete replacement or serve as a template for new tendon tissue formation. Engineering tendon’s insertion has proven to be an immense design challenge as the transition from stiff bone to compliant tendon occurs over tens of microns. Strategies include using nanofibrous scaffolds with graded calcium phosphate[446] and collagen matrix submerged in simulated body fluid.[447] In another approach, a hierarchical scaffold was engineered with regions to guide cell ingrowth and collagen fiber alignment using interconnected gelatin beads infiltrated with hydroxyapatite and PLGA.[448] An array of channels was created to promote the formation of an aligned unmineralized scaffold prior to dissolving the gelatin to create a porous structure.[448] Together, this created a gradient in calcium content, modulus, and Scx expression, and showed that cells infiltrate with good viability.[448]
After devices are implanted, mechanical cues are important to promote tendon-like phenotypes in colonized cells. Applying physiological cyclic strain levels upregulates tenocyte markers (type I collagen and tenomodulin),[449] TGF-β,[450] N-cadherin,[451] cell organization,[451] and mechanical properties.[452] In contrast, applying higher strain levels upregulates biomarkers found in cartilage[449] and bone,[453, 454] disrupts gap junctions,[455] and induces apoptosis.[455] Lower loading levels are also unfavorable since they can decrease expression of tenomodulin, Mohawk, collagens, decorin, and matrix organization.[456] Taken together, this work highlights many biologic free ways to engineer tendon biomaterials that both serve as a material framework and provide tenogenic cues.
3.4.2. Tendon Biomaterials Biologic and Drug Approaches
Incorporation of biologics and drugs into tendon biomaterials provides an additional opportunity to control cell and tissue behavior. Adult tendon has relatively low cell numbers compared to other tissues,[457] but they play important roles in tensional homeostasis and remodeling.[458] Augmentation of injured tendon with tendon derived cells, tendon stem/progenitor cells, and other stem cells (ASCs, MSCs, bmMSCs, iPSCs) may therefore offer therapeutic approaches to augment diseased tendon.[199] Additionally, much work has incorporated lessons learned from in vitro culture experiments that have emphasized how substrate mechanics, mechanical stimulation, media conditions, and growth factor supplementation can affect tendon cell behavior. Together, these approaches offer exciting avenues to improve tendon healing at the expense of increased complexity and regulation, discussed in Section 4.
Many approaches have been investigated to control cell phenotype and deliver cells within biomaterials to tendon. Multipotent cells derived from bone marrow or adipose tissue seeded onto tendon biomaterials are typically differentiated towards tendon, fibrocartilage, and bone lineages to recapitulate the tendon-bone interface. Cell attachment, spreading, and tenogenic differentiation can be controlled through surface functionalization with cell adhesion ligands (e.g., collagen, fibronectin).[199] Cell fate may be controlled by priming cells in vitro by inducing transcription factor overexpression,[459] or subjecting cells to appropriate mechanical loading.[460] In a recent study, loading was induced by incorporating magnetically active components into biomaterials to promote differentiation in vivo.[461] Gradients in cell differentiation, to mimic interphases to bone or muscle, may be induced by immobilizing retroviruses encoding transcription factors to create spatial patterns of differentiation and tissue formation.[462] Combining chemical factors that induce epigenetic changes with structural cues may provide additional control over cell phenotype. Electrospun scaffolds loaded with histone deacetylation inhibitor promoted tenogenesis of stem cells.[463]
The role of GFs (e.g., bFGF,[464] TGFβ,[465] IGF1,[466] and PDGF[467]) during tendon healing underscore interest in their incorporation in tendon materials to control tenogenic differentiation of MSCs. Combining ASCs with BMP-12 altered macrophage polarization and improved flexor tendon healing.[468] CTGF delivered through porous sutures improved intrasynovial tendon healing.[469] Downregulation of miRNA29a increased expression of IL-33 and increased the ratio of collagen type III:I,[470] while supplementation of miRNA29a led to improved histological scores and decreased tendon CSA following injury.[471] In certain tendon microenvironments not exposed to the vasculature such as the intrasynovial ACL, healing is very limited. However, by combining a collagen sponge and fibrin to restore continuity between torn ends of ACLs, tissue healing was possible.[275] This promising approach is currently in clinical trials, and may lead to lower rates of adverse reactions and improved outcomes for ACL reconstruction.[275] Additionally, given the close relationships between biomechanical and chemical cues in tendon, some approaches utilize combined effects of biologics, mechanics, and topography. Tenogenic differentiation may be further promoted by combining ADSCs, PDGF-BB gradients, and nanofibers (collagen or PLLA).[467, 472] Aligned topography by microgrooves led to elongation of dermal fibroblasts and can be tuned by adding exogenous TGF-β1.[473]
There has been significant effort to deliver pharmaceutical agents, including corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDs) via materials to injured tendon in order to enhance healing.[474, 475] However, the appropriate timing for delivery of anti-inflammatories remains controversial; early delivery may result in inferior tendon healing[475] when compared to delayed delivery.[474] Nitric oxide (NO) delivered topically and with slow release patches has been suggested to improve tendinopathy by reducing pain and improving function.[476, 477]
4. Considerations in Translating Biomaterials
4.0. Overview in Translation of Biomaterials
Translating technology from bench to bedside presents an amazing opportunity to impact human health, but is also challenging from a regulatory, financial, and manufacturing perspective. Here, we address several challenges and considerations in the translation of biomaterials for connective tissues, specifically focusing on the utility and limitations of available guidance documents.
4.1. General Challenges and Considerations in Translating Biomaterials
Promising biomaterial-based therapies for connective tissue healing and regeneration typically are translated through small and large animal models before human application. Limitations in research funding, the desired timeline to translate a new drug or device to market, and expertise required beyond the infrastructure and scope of a laboratory or institution may hinder translation. For example, development and commercialization of a new hydrogel drug delivery system is estimated to cost between $50 to $800M[478] and taking a new drug molecule from bench to bedside may exceed $1.3B in cost.[479] The high cost burdens for translation are exacerbated by the long regulatory approval process timeline and fact that 1 in 10,000 drugs tested in pre-clinical tests succeeds in human testing.[479] The FDA approval process[480, 481] depends on whether the biomaterial is biologic/therapeutic free (typically 1–5 years) or carries a payload (typically 7–10 years).[482] Early consideration for whether the new biomaterial is a device, drug, or combination product therefore may have tremendous effects on its downstream translation and cost to develop. New technologies should seek guidance from the Center for Devices and Radiological Health (CDRH) or Center for Biologics Evaluation and Research (CBER), or the Office of Combinatorial Products (OCP) at the FDA early in the testing process to allow streamlined application submission.
Institutions that can bridge the gap between academics and industry may be necessary to provide funding, regulatory guidance, and insight into device manufacturing, packaging, sterility, and other testing procedures. Collaborations with an advanced technology team, clinical investigators, and corporate alliances may be important to identify interest and economics in the new technology, and ultimately drive it through concept validation, technology refinement, and commercialization.[483]
While there is no perfect rule book for translating biomaterials, we highlight some important strategies for success and examples of failed products. Broadly speaking, new products must treat clinical needs, be safe, and be adopted by end users. To achieve these criteria, it is often suggested that, as innovators, we “embrace complexity, engineer versatility, and deliver simplicity.”[484] Products reaching market quickly may do so by demonstrating lower costs compared to existing products, identifying the proper regulatory pathway, and securing funding. Since a major challenge on commercialization today is the added complexity of materials combined with therapeutic agents (small molecule drugs, biologics, genes, and cells), translation may be facilitated by using clinically approved materials (natural polymers, biodegradable thermoplastics (PGA, PLA, PCL, PLGA)).[484] Despite these strategies, barriers to translation often remain high and can include a lack of accurate pre-clinical models, clinical trial funding, lack of scalability to good manufacturing processes, intellectual property considerations, regulatory barriers, funding, and the dynamic nature of biomedical research environments across the globe.[485] Even the most promising academic discoveries may struggle to be quickly translated. For example, metal-metal hips were originally suggested to be beneficial for patients and provide more durability, but were later found to create debris particles that induced soft tissue necrosis.[484] Similarly, innovations in iPSCs in 2006 (2012 Nobel Prize) were expected to revolutionize regenerative medicine and result in rapid translation. However, safety concerns and high costs, among other issues, have limited translation into humans. Successfully translated products, such as GraftJacket™ have passed FDA trials and penetrated markets for tendon repair.[270] GraftJacket™ is made from decellularized human dermal collagen and its structure is retained during freeze drying to preserve vascular channels. Together, this approach may help reduce rejection, allow revascularization and cell infiltration, and minimizes inflammation. Alternatively, products that achieve FDA approval and reach the market may later encounter hurdles. In 2000, the Restore® hernia repair patch was extended for use in soft tissue repairs such as tendon and ligament. By proving substantial equivalence 510(k) extension through the FDA, the biocompatibility of the product was only required to be demonstrated in certain preclinical models. However, foreign body reactions were observed after implantation in humans and production was halted.[485] Other FDA approved products may receive recalls, but remain on the market. Infuse®, a combination product for bone grafts (collagen sponge loaded with rhBMP-2), was originally approved by the FDA in 2002 through the PMA pathway and showed initial promising results in nearly 800 patients.[485] In 2011, complications with rhBMP-2 were reported in many off-label uses and it was revealed that the original clinical trials for Infuse® contained errors in methodology and reporting.[485] The FDA later issued warnings, and additional litigation and study of product safety has followed as Infuse continues use in spine surgery indications.
4.2. Using Available Guidance Documents to Accelerate Translation
Several regulatory agencies provide published guidelines for important study considerations and endpoints. These documents are published by the American Society for Testing and Materials (ASTM), U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and International Standards Organization (ISO), and contain several tissue-specific recommendations that are re-reviewed regularly.[486] Guidance documents are particularly important because they provide a framework to validate safety and efficacy prior to testing in humans and highlight procedures for study standardization throughout disciplines. It is noted that although agencies recommend outcome metrics important for translation, pilot studies exploring early feasibility of a technology may not benefit from examining all possible endpoints. In smaller scale studies, it may still be best to focus on select criteria common across guidelines rather than devote excessive time and resources to a full comprehensive evaluation before initial safety and efficacy benchmarks are satisfied.
An important feature of biomaterials is their biocompatibility in vivo since they may induce a foreign body response after implantation. Foreign body responses may negatively affect material longevity and local tissue properties, and are most common among biomaterials containing nondegradable synthetic or metallic components.[487] Following implantation, biomaterials may interact with surrounding blood, which can lead to the formation of a protein film, acute and chronic inflammation, granulation tissue, foreign body reaction, and fibrotic capsule.[487] Histologically, a foreign body reaction will contain the presence of macrophages and foreign body giant cells near the interface of the biomaterial. This response may be due to the protein adsorption to the biomaterial, which can be caused by both chemical and topological factors.[487] Indeed, several guidance criteria are often included to emphasize that materials should not induce an immune response or cause infection (e.g., “biocompatible”, “histology”, “integration”, “immune response”, “biochemical analysis”). Biomaterial degradation, application of chemical and surface modifications on synthetic materials, and use of natural polymers may help avoid foreign body reactions.
While intended to provide useful guidance, adherence to these guidelines varies significantly in pre-clinical studies. For example, a recent study[488] highlighted the gap between recommendations and actual procedures followed by investigators in large animal studies of cartilage repair and regeneration. Although overall adherence increased slightly over a period of two decades, most studies followed only ~40% of the recommended guidelines.[488] The National Institutes of Health (NIH) is the major funding agency for studies designed to investigate “fundamental knowledge about the nature and behavior of living systems and the application of that knowledge to enhance health, lengthen life, and reduce illness and disability.” As the majority of NIH grants fund basic science research, it is unsurprising that 80–90% of projects do not proceed to testing in humans, and a long timeline (up to 15 years) is typical for those that do.[489] It may be possible to shorten this timeline and increase human translation by better incorporating themes from guidance documents that shift the focus of basic researchers towards considering translational feasibility earlier in the study planning process. This likely will require modifications to funding mechanisms, as funding for basic science research typically does not support the critical milestones that may be required before industry licensing.[489] It will also be important to appropriately coordinate the publication of guidelines with funding mechanisms that utilize these as criteria in study design. Variations in model recommendations between guidelines and tissues may also reflect the lack of predictive reliability for many preclinical disease models/standards, and will likely need to be addressed.
4.3. Comparison of Guidance Document Recommendations for Connective Tissue Biomaterials
We identified several general standards for connective tissue biomaterials (biomolecule release,[490] cell viability,[491] tissue engineered medical products,[492, 493] cell based products[494, 495]) and those specific to cartilage,[496–498] skin,[499, 500] and tendon.[501] Six guidelines for connective tissues were specifically compared for 39 study descriptors and outcomes included (Figure 4). To our surprise, although many guidelines contained similar study descriptors and outcomes, many of these were absent in guidelines for certain tissues. For example, ASTM and FDA guidelines for tendon and cartilage provide recommendations for injury models, species selection, study duration, and scaffold fixation, but these were absent from guidelines for skin. Depending on the study and routine outcome metrics for the tissue of interest, this discrepancy may solely be due to differences in field standards. However, some metrics including “injury model”, “species selection”, “demographics”, “biocompatibility”, “cell infiltration and maintenance”, and “statistical evaluation” may be considered a central feature of any study, yet were not consistently included. With many researchers conducting work in fields that may not be their original training, it may not be surprising that the lack of standardization in procedures across published standards propagates into variation in overall adherence to guidance documents. Of course, the maturity of a particular field may influence the timeframe that guidelines (and number of guidelines) are published. For example, for cartilage repair, guidelines from ASTM, FDA, and EMA were published in 2005, 2007, and 2008, respectively, whereas tendon repair was published in 2011. These findings further support the continued revision of these standards and importance of interdisciplinary diverse teams to tackle difficult research questions.[489]
Figure 4: Guidance document recommendations vary between connective tissue type and agency.
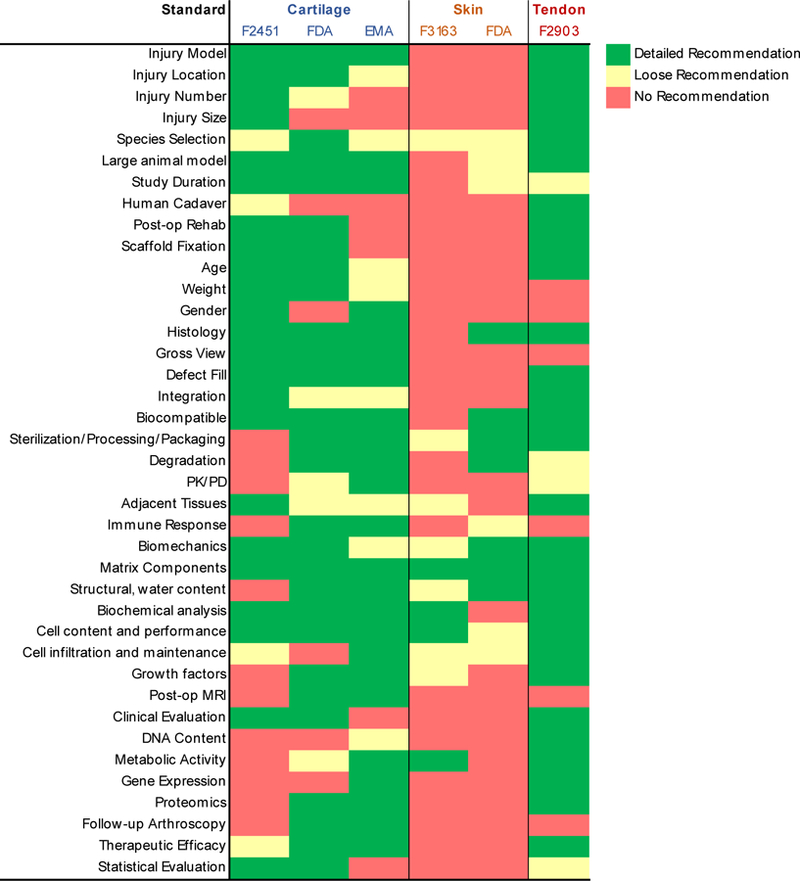
Six guidelines for connective tissues were compared for 39 study descriptors and outcomes included. Although many guidelines contained similar standards, many were absent and not all guidelines for a given tissue were in agreement. Recommended guidelines do not imply adherence in basic science studies.
Low adherence to published guidance documents has been attributed to infrastructure and expertise limitations, presence of pilot versus full studies, objectives of a study, differences in opinions between research teams (scientists, clinicians, engineers, translation experts) related to relative importance of outcome metrics, and the level of funding available.[488] Although many scientific journals and the National Institutes of Health require adherence of the ARRIVE guidelines, IACUC, and/or other checklists, adherence to larger agencies guidelines is not required for basic science research. Additional guidelines including MARIBEL (Minimum Information Reporting in Bio-Nano Experimental Literature) have been recently proposed for bio-nano research.[502] MARIBEL may be important within the biomedical field because it can be broadly applied to many biomaterials, and covers material characterization, biological characterization, and experimental protocol details.[502]
5. Summary and Future Perspectives
Connective tissue diseases are common and debilitating, and knowledge gained from understanding their multiscale structure function properties is informing the design and translation of new biomaterials used as treatments. Although specific multiscale structure-function relationships differ between adipose, cartilage, dermis, and tendon tissues, many biomaterials are fabricated from similar materials (some already used in FDA approved products), and use similar strategies to provide mechanical, structural, and biochemical cues to control cell behavior. Approaches that utilize cells as a component of the therapy share the challenge of cell engraftment and control over function in vivo. Recapitulation of the native cell microenvironment may enable better control over transplanted cell contributions to repair and regeneration but is complex given the current limited knowledge on the native niche and the dynamic nature of connective tissues and their crosstalk with neighboring tissues.
While significant strides have been made, many biomaterial mimics struggle to recapitulate basic structure-function criteria of native connective tissues. Although tissues are often classified as heterogeneous and have properties that vary based on anatomical location within the body, these design features are rarely discussed in outcome metrics of engineered biomaterials. As more specialized tissue biomaterials are engineered to function with proper physiology in vivo, new biomaterial monitoring strategies will be necessary to follow their impact. Several approaches to monitor wound healing in skin are already being developed which may be adapted to other connective tissues. For example, smart skin adhesive patches can monitor wound healing signals, including tissue temperature and thermal conductivity, calor or inflammation, strain, electrical impedance, glucose, and sweat.[503] Methods that integrate scaffolds with surrounding tissues are also critical for their long term efficacy in vivo, and may be achieved by designing materials with high material toughness that can dissipate interfacial stresses that would otherwise destabilize the interface.[396]
Our analysis of regulatory guidelines highlighted stark differences in required study deliverables between tissue types. This is further complicated by the varying availability of model systems to accurately study disease pathology across tissues. Although they are not perfect, the guidance documents help establish a set of best practices to improve standardization and straightforward comparison of biomaterial efficacy across studies.
Taken together, many studies highlighted in this review provide an outstanding platform for exciting innovation for new biomaterials that mimic and heal connective tissues. Biomaterials for tissues with fewer guidance standards may be easier to approach from a regulatory perspective, and may later provide important data to accelerate translation in other tissues. Collaborations between laboratories, institutions, industrial partners, and investors are ever important in biomaterial translation to clinical trials. The ongoing work exploring biomaterials in clinical trials and preclinical studies will ultimately improve the lives of many individuals.
Table 2:
Connective tissue structure function relationships in healthy adult tissue.
| Tissue | Purpose | Cells | Major ECM Components | Young’s Modulus (kPa) |
|---|---|---|---|---|
| Adipose | Store energy, cushion forces, heat insulation | Adipocytes | Collagen (IV), PG, laminin[130, 131] | Macro (C): 600[134] Micro (S): 1–3 [133] |
| Cartilage | Joint cushioning and gliding | Chondrocytes | Collagen (II)[224], PG (Agg, SLRP),[225] | Macro (T): 5k-25k[173–175]M Macro (C): 9k-13k[226] Micro (C): 500–1000[176] |
| Dermis | Skin flexibility and strength | Fibroblasts, macrophages, adipocytes | Collagen (I, III), elastin, PG, blood and lymphatic vessels, glands, hair follicles, nerves[72] | Macro (T): 2.5k-8k[182] Micro (T): 1–10[227] |
| Tendon | Transfer forces from muscle to bone | Tenocytes, tendon stem cells | Collagen (I)[188–191], elastin,[192] PG (SLRP),[228] surrounding blood vessels[194] | Macro (T): 200k-750k[206, 209] Micro (T): 20[124] |
C: Compression; S: Shear; T: Tension
Acknowledgments
This work was supported by the National Institute on Aging of the NIH (F32AG057135) and Novartis.
Grant Support: This review was supported by the National Institute on Aging at the NIH (F32AG057135) and Novartis.
Biography and Photo

David J. Mooney, Ph.D.
Dr. Mooney is the Robert P. Pinkas Family Professor of Bioengineering at Wyss Institute for Biologically Inspired Engineering at Harvard University. Dr. Mooney earned his Ph.D. from the Massachusetts Institute of Technology (Chemical Engineering) and B.S. from the University of Wisconsin, Madison (Chemical Engineering). Dr. Mooney’s laboratory aims to make cellular and molecular therapies more effective and practical to treat disease using novel biomaterials. Biomaterials developed in his laboratory are used in a variety of drug delivery, immunotherapy, and regenerative medicine projects to promote regeneration or targeted destruction of tissues and organs in the body.

Benjamin R. Freedman, Ph.D.
Dr. Freedman is a Postdoctoral Fellow in Dr. Mooney’s laboratory at the Wyss Institute for Biologically Inspired Engineering at Harvard University. Dr. Freedman earned his Ph.D. from the University of Pennsylvania (Bioengineering) and B.S. from the University of Rochester (Biomedical Engineering). His research focuses on developing new biomaterials to improve tissue healing, with a special focus on tendon.
Footnotes
Competing Interests Statement
The authors receive grant support through Novartis. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the position of the Wyss Institute for Biologically Inspired Engineering at Harvard University or Novartis.
References
- [1].P. C, Essentials of Pathophysiology: Concepts of Altered Health States, Lippincott Williams & Wilkins, 2011. [Google Scholar]
- [2].Shoulders MD, Raines RT, Annu Rev Biochem 2009, 78, 929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sorushanova A, Delgado LM, Wu Z, Shologu N, Kshirsagar A, Raghunath R, Mullen AM, Bayon Y, Pandit A, Raghunath M, Zeugolis DI, Adv Mater 2019, 31, e1801651. [DOI] [PubMed] [Google Scholar]
- [4].Ahmadzadeh H, Freedman B, Connizzo B, Soslowsky L, Shenoy V, Acta Biomaterialia 2014, In review. [DOI] [PMC free article] [PubMed]
- [5].Szczesny SE, Fetchko KL, Dodge GR, Elliott DM, J Orthop Res 2017, 35, 2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tanzer ML, Science 1973, 180, 561. [DOI] [PubMed] [Google Scholar]
- [7].Pritchard RH, Huang YY, Terentjev EM, Soft Matter 2014, 10, 1864. [DOI] [PubMed] [Google Scholar]
- [8].Daamen WF, Veerkamp JH, van Hest JC, van Kuppevelt TH, Biomaterials 2007, 28, 4378. [DOI] [PubMed] [Google Scholar]
- [9].Li B, Alonso DO, Bennion BJ, Daggett V, J Am Chem Soc 2001, 123, 11991. [DOI] [PubMed] [Google Scholar]
- [10].Pankov R, Yamada KM, J Cell Sci 2002, 115, 3861. [DOI] [PubMed] [Google Scholar]
- [11].Fruh SM, Schoen I, Ries J, Vogel V, Nat Commun 2015, 6, 7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hardingham TE, Fosang AJ, FASEB J 1992, 6, 861. [PubMed] [Google Scholar]
- [13].Iozzo RV, Schaefer L, Matrix Biol 2015, 42, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gordon JA, Freedman BR, Zuskov A, Iozzo RV, Birk DE, Soslowsky LJ, J Biomech 2015, 48, 2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Baldwin AK, Simpson A, Steer R, Cain SA, Kielty CM, Expert Rev Mol Med 2013, 15, e8. [DOI] [PubMed] [Google Scholar]
- [16].Verstraeten A, Alaerts M, Van Laer L, Loeys B, Hum Mutat 2016, 37, 524. [DOI] [PubMed] [Google Scholar]
- [17].De Maio F, Fichera A, De Luna V, Mancini F, Caterini R, Adv Orthop 2016, 2016, 8275391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dietz H, in GeneReviews((R)), (Eds: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A), Seattle (WA) 1993. [Google Scholar]
- [19].David TE, Nat Rev Cardiol 2013, 10, 375. [DOI] [PubMed] [Google Scholar]
- [20].Humphrey JD, Schwartz MA, Tellides G, Milewicz DM, Circ Res 2015, 116, 1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Van de Velde S, Fillman R, Yandow S, J Bone Joint Surg Am 2006, 88, 639. [DOI] [PubMed] [Google Scholar]
- [22].Brady AF, Demirdas S, Fournel-Gigleux S, Ghali N, Giunta C, Kapferer-Seebacher I, Kosho T, Mendoza-Londono R, Pope MF, Rohrbach M, Van Damme T, Vandersteen A, van Mourik C, Voermans N, Zschocke J, Malfait F, Am J Med Genet C Semin Med Genet 2017, 175, 70. [DOI] [PubMed] [Google Scholar]
- [23].Jeanne M, Gould DB, Matrix Biol 2017, 57–58, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vanakker O, Callewaert B, Malfait F, Coucke P, Annu Rev Genomics Hum Genet 2015, 16, 229. [DOI] [PubMed] [Google Scholar]
- [25].Bowen JM, Sobey GJ, Burrows NP, Colombi M, Lavallee ME, Malfait F, Francomano CA, Am J Med Genet C Semin Med Genet 2017, 175, 27. [DOI] [PubMed] [Google Scholar]
- [26].Forlino A, Marini JC, Lancet 2016, 387, 1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Spadaccio C, Mozetic P, Nappi F, Nenna A, Sutherland F, Trombetta M, Chello M, Rainer A, Basic Res Cardiol 2016, 111, 16. [DOI] [PubMed] [Google Scholar]
- [28].Abramowitz Y, Jilaihawi H, Chakravarty T, Mack MJ, Makkar RR, J Am Coll Cardiol 2015, 66, 1934. [DOI] [PubMed] [Google Scholar]
- [29].Koo HK, Lawrence KA, Musini VM, Cochrane Database Syst Rev 2017, 11, CD011103. [DOI] [PMC free article] [PubMed]
- [30].Scheres LJJ, van Dijk FS, Harsevoort AJ, van Dijk ATH, Dommisse AM, Janus GJM, Franken AAM, Bone Rep 2018, 8, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rudan I, Sidhu S, Papana A, Meng SJ, Xin-Wei Y, Wang W, Campbell-Page RM, Demaio AR, Nair H, Sridhar D, Theodoratou E, Dowman B, Adeloye D, Majeed A, Car J, Campbell H, Wang W, Chan KY, Global G Health Epidemiology Reference, J Glob Health 2015, 5, 010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Perl A, Nat Rev Rheumatol 2016, 12, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Burmester GR, Pope JE, Lancet 2017, 389, 2338. [DOI] [PubMed] [Google Scholar]
- [34].Streifler JY, Molad Y, Handb Clin Neurol 2014, 119, 463. [DOI] [PubMed] [Google Scholar]
- [35].Mok CC, Birmingham DJ, Ho LY, Hebert LA, Song H, Rovin BH, Lupus 2012, 21, 36. [DOI] [PubMed] [Google Scholar]
- [36].Murphy G, Isenberg D, Rheumatology (Oxford) 2013, 52, 2108. [DOI] [PubMed] [Google Scholar]
- [37].Mayes MD, Lacey JV Jr., Beebe-Dimmer J, Gillespie BW, Cooper B, Laing TJ, Schottenfeld D, Arthritis Rheum 2003, 48, 2246. [DOI] [PubMed] [Google Scholar]
- [38].Valanciene G, Jasaitiene D, Valiukeviciene S, Medicina (Kaunas) 2010, 46, 649. [PubMed] [Google Scholar]
- [39].Balbir-Gurman A, Braun-Moscovici Y, Best Pract Res Clin Rheumatol 2012, 26, 13. [DOI] [PubMed] [Google Scholar]
- [40].Salerni R, Rodnan GP, Leon DF, Shaver JA, Ann Intern Med 1977, 86, 394. [DOI] [PubMed] [Google Scholar]
- [41].Hughes P, Gelsthorpe K, Doughty RW, Rowell NR, Rosenthal FD, Sneddon IB, Clin Exp Immunol 1978, 31, 351. [PMC free article] [PubMed] [Google Scholar]
- [42].Freire M, Alonso M, Rivera A, Sousa A, Soto A, Gomez-Sousa JM, Baroja A, Vazquez-Trinanes C, Sopena B, Semin Arthritis Rheum 2015, 45, 294. [DOI] [PubMed] [Google Scholar]
- [43].Svanstrom H, Lund M, Melbye M, Pasternak B, Pharmacoepidemiol Drug Saf 2018. [DOI] [PubMed]
- [44].Kwon HH, Bang SY, Won S, Park Y, Yi JH, Joo YB, Lee HS, Bae SC, Lupus 2018, 961203318784648. [DOI] [PubMed]
- [45].Anastasiou C, Dulai O, Baskaran A, Proudfoot J, Verhaegen S, Kalunian K, Lupus Sci Med 2018, 5, e000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Terao M, Yang L, Matsumura S, Yutani M, Murota H, Katayama I, Dermatoendocrinol 2015, 7, e1010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D’Agostino RB Sr., O’Donnell CJ, Circulation 2007, 116, 39. [DOI] [PubMed] [Google Scholar]
- [48].Jensen MD, J Clin Endocrinol Metab 2008, 93, S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jerschow E, Anwar S, Barzilai N, Rosenstreich D, Journal of Allergy and Clinical Immunology 2007, 119, S179. [Google Scholar]
- [50].Lakowa N, Trieu N, Flehmig G, Lohmann T, Schon MR, Dietrich A, Zeplin PH, Langer S, Stumvoll M, Bluher M, Kloting N, Biochem Biophys Res Commun 2015, 457, 426. [DOI] [PubMed] [Google Scholar]
- [51].Darcy J, McFadden S, Bartke A, Adipocyte 2017, 6, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Caso G, McNurlan MA, Mileva I, Zemlyak A, Mynarcik DC, Gelato MC, Metabolism 2013, 62, 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL, Aging Cell 2010, 9, 667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Guo W, Pirtskhalava T, Tchkonia T, Xie W, Thomou T, Han J, Wang T, Wong S, Cartwright A, Hegardt FG, Corkey BE, Kirkland JL, Am J Physiol Endocrinol Metab 2007, 292, E1041. [DOI] [PubMed] [Google Scholar]
- [55].Cartwright MJ, Tchkonia T, Kirkland JL, Exp Gerontol 2007, 42, 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Mori MA, Thomou T, Boucher J, Lee KY, Lallukka S, Kim JK, Torriani M, Yki-Jarvinen H, Grinspoon SK, Cypess AM, Kahn CR, J Clin Invest 2014, 124, 3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Stout MB, Steyn FJ, Jurczak MJ, Camporez JG, Zhu Y, Hawse JR, Jurk D, Palmer AK, Xu M, Pirtskhalava T, Evans GL, de Souza Santos R, Frank AP, White TA, Monroe DG, Singh RJ, Casaclang-Verzosa G, Miller JD, Clegg DJ, LeBrasseur NK, von Zglinicki T, Shulman GI, Tchkonia T, Kirkland JL, J Gerontol A Biol Sci Med Sci 2017, 72, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Stout MB, Tchkonia T, Pirtskhalava T, Palmer AK, List EO, Berryman DE, Lubbers ER, Escande C, Spong A, Masternak MM, Oberg AL, LeBrasseur NK, Miller RA, Kopchick JJ, Bartke A, Kirkland JL, Aging (Albany NY) 2014, 6, 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR, N Engl J Med 2009, 360, 1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Pfannenberg C, Werner MK, Ripkens S, Stef I, Deckert A, Schmadl M, Reimold M, Haring HU, Claussen CD, Stefan N, Diabetes 2010, 59, 1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Drubach LA, Palmer EL 3rd, Connolly LP, Baker A, Zurakowski D, Cypess AM, J Pediatr 2011, 159, 939. [DOI] [PubMed] [Google Scholar]
- [62].Hioki C, Yoshida T, Kogure A, Takakura Y, Umekawa T, Yoshioka K, Shimatsu A, Yoshikawa T, Horm Metab Res 2004, 36, 607. [DOI] [PubMed] [Google Scholar]
- [63].Yanase T, Fan W, Kyoya K, Min L, Takayanagi R, Kato S, Nawata H, J Steroid Biochem Mol Biol 2008, 109, 254. [DOI] [PubMed] [Google Scholar]
- [64].Rodriguez-Cuenca S, Monjo M, Gianotti M, Proenza AM, Roca P, Am J Physiol Endocrinol Metab 2007, 292, E340. [DOI] [PubMed] [Google Scholar]
- [65].Loeser RF, Collins JA, Diekman BO, Nat Rev Rheumatol 2016, 12, 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, Kington RS, Lane NE, Nevitt MC, Zhang Y, Sowers M, McAlindon T, Spector TD, Poole AR, Yanovski SZ, Ateshian G, Sharma L, Buckwalter JA, Brandt KD, Fries JF, Ann Intern Med 2000, 133, 635. [DOI] [PubMed] [Google Scholar]
- [67].Camarero-Espinosa S, Rothen-Rutishauser B, Foster EJ, Weder C, Biomater Sci 2016, 4, 734. [DOI] [PubMed] [Google Scholar]
- [68].Griffin TM, Guilak F, Exerc Sport Sci Rev 2005, 33, 195. [DOI] [PubMed] [Google Scholar]
- [69].Friel NA, Chu CR, Clin Sports Med 2013, 32, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Forkel P, Reuter S, Sprenker F, Achtnich A, Herbst E, Imhoff A, Petersen W, Knee Surg Sports Traumatol Arthrosc 2015, 23, 112. [DOI] [PubMed] [Google Scholar]
- [71].Information K, (Ed: Information K), 2017, 434.
- [72].Castano O, Perez-Amodio S, Navarro-Requena C, Mateos-Timoneda MA, Engel E, Adv Drug Deliv Rev 2018. [DOI] [PubMed]
- [73].Silverstein P, Am J Med 1992, 93, 22S. [DOI] [PubMed] [Google Scholar]
- [74].Sanon S, Hart DA, EE T, Skin Tissue Engineering and Regenerative Medicine 2016, 28.
- [75].Reinke JM, Sorg H, Eur Surg Res 2012, 49, 35. [DOI] [PubMed] [Google Scholar]
- [76].Forde MS, Punnett L, Wegman DH, J Occup Environ Hyg 2005, 2, 203. [DOI] [PubMed] [Google Scholar]
- [77].Sein ML, Walton J, Linklater J, Appleyard R, Kirkbride B, Kuah D, Murrell GA, British journal of sports medicine 2010, 44, 105. [DOI] [PubMed] [Google Scholar]
- [78].in Bullitin No. 2368, August 1990., Bureau of Labor Statistics, 1988.
- [79].Longo UG, Ronga M, Maffulli N, Sports Med Arthrosc 2009, 17, 112. [DOI] [PubMed] [Google Scholar]
- [80].Rudavsky A, Cook J, J Physiother 2014, 60, 122. [DOI] [PubMed] [Google Scholar]
- [81].Factor D, Dale B, Int J Sports Phys Ther 2014, 9, 274. [PMC free article] [PubMed] [Google Scholar]
- [82].Jobe FW, Kvitne RS, Giangarra CE, Orthop Rev 1989, 18, 963. [PubMed] [Google Scholar]
- [83].McMaster WC, Troup J, Am J Sports Med 1993, 21, 67. [DOI] [PubMed] [Google Scholar]
- [84].Renstrom P, Johnson RJ, Sports Med 1985, 2, 316. [DOI] [PubMed] [Google Scholar]
- [85].Sommerich CM, McGlothlin JD, Marras WS, Ergonomics 1993, 36, 697. [DOI] [PubMed] [Google Scholar]
- [86].Malliaras P, Chan O, Simran G, Martinez de Albornoz P, Morrissey D, Maffulli N, Int J Sports Med 2012, 33, 480. [DOI] [PubMed] [Google Scholar]
- [87].Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF, Nat Med 2007, 13, 1219. [DOI] [PubMed] [Google Scholar]
- [88].Zhang J, Wang JH, J Orthop Res 2012, 30, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kannus P, Jozsa L, J Bone Joint Surg Am 1991, 73, 1507. [PubMed] [Google Scholar]
- [90].Slane LC, Thelen DG, Med Eng Phys 2015, 37, 712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Slane LC, DeWall R, Martin J, Lee K, Thelen DG, Physiol Meas 2015, 36, 1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Dudhia J, Scott CM, Draper ER, Heinegard D, Pitsillides AA, Smith RK, Aging Cell 2007, 6, 547. [DOI] [PubMed] [Google Scholar]
- [93].Pardes AM, Beach ZM, Raja H, Rodriguez AB, Freedman BR, Soslowsky LJ, J Biomech 2017, 60, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Stenroth L, Peltonen J, Cronin NJ, Sipila S, Finni T, J Appl Physiol (1985) 2012, 113, 1537. [DOI] [PubMed] [Google Scholar]
- [95].Svensson RB, Heinemeier KM, Couppe C, Kjaer M, Magnusson SP, J Appl Physiol (1985) 2016, 121, 1237. [DOI] [PubMed] [Google Scholar]
- [96].Cury DP, Dias FJ, Miglino MA, Watanabe IS, PLoS One 2016, 11, e0153568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Woo SL, Debski RE, Zeminski J, Abramowitch SD, Saw SS, Fenwick JA, Annu Rev Biomed Eng 2000, 2, 83. [DOI] [PubMed] [Google Scholar]
- [98]. A. Lab.
- [99]. Campnolo, 2007.
- [100].Rutkowski JM, Stern JH, Scherer PE, J Cell Biol 2015, 208, 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, Schwartz MA, Nature 2010, 466, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].VanBuren P, Work SS, Warshaw DM, Proc Natl Acad Sci U S A 1994, 91, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Swift J, Discher DE, J Cell Sci 2014, 127, 3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Janmey PA, Miller RT, J Cell Sci 2011, 124, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].You BM, Siy P, Anderst W, Tashman S, IEEE Trans Med Imaging 2001, 20, 514. [DOI] [PubMed] [Google Scholar]
- [106].Yao J, Snibbe J, Maloney M, Lerner AL, J Biomech Eng 2006, 128, 135. [DOI] [PubMed] [Google Scholar]
- [107].Freedman BR, Sheehan FT, J Orthop Res 2013, 31, 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Borotikar BS, Sheehan FT, Osteoarthritis Cartilage 2013, 21, 1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Cortes DH, Suydam SM, Silbernagel KG, Buchanan TS, Elliott DM, Ultrasound Med Biol 2015, 41, 1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Agres AN, Duda GN, Gehlen TJ, Arampatzis A, Taylor WR, Manegold S, Scand J Med Sci Sports 2015, 25, 860. [DOI] [PubMed] [Google Scholar]
- [111].Delp SL, Anderson FC, Arnold AS, Loan P, Habib A, John CT, Guendelman E, Thelen DG, IEEE Trans Biomed Eng 2007, 54, 1940. [DOI] [PubMed] [Google Scholar]
- [112].Freedman BR, Sarver JJ, Buckley MR, Voleti PB, Soslowsky LJ, J Biomech 2013. [DOI] [PMC free article] [PubMed]
- [113].Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, Discher DE, Science 2013, 341, 1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Dufrene YF, Ando T, Garcia R, Alsteens D, Martinez-Martin D, Engel A, Gerber C, Muller DJ, Nat Nanotechnol 2017, 12, 295. [DOI] [PubMed] [Google Scholar]
- [115].Han L, Grodzinsky AJ, Ortiz C, Annu Rev Mater Res 2011, 41, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Polacheck WJ, Chen CS, Nat Methods 2016, 13, 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wu PH, Aroush DR, Asnacios A, Chen WC, Dokukin ME, Doss BL, Durand-Smet P, Ekpenyong A, Guck J, Guz NV, Janmey PA, Lee JSH, Moore NM, Ott A, Poh YC, Ros R, Sander M, Sokolov I, Staunton JR, Wang N, Whyte G, Wirtz D, Nat Methods 2018. [DOI] [PMC free article] [PubMed]
- [118].Lipfert J, Skinner GM, Keegstra JM, Hensgens T, Jager T, Dulin D, Kober M, Yu Z, Donkers SP, Chou FC, Das R, Dekker NH, Proc Natl Acad Sci U S A 2014, 111, 15408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Freedman BR, Sheehan FT, Lerner AL, Knee 2015, 22, 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Akella SV, Regatte RR, Gougoutas AJ, Borthakur A, Shapiro EM, Kneeland JB, Leigh JS, Reddy R, Magn Reson Med 2001, 46, 419. [DOI] [PubMed] [Google Scholar]
- [121].Collins AT, Hatcher CC, Kim SY, Ziemian SN, Spritzer CE, Guilak F, DeFrate LE, McNulty AL, Ann Biomed Eng 2018. [DOI] [PMC free article] [PubMed]
- [122].Riggin CN, Sarver JJ, Freedman BR, Thomas SJ, Soslowsky LJ, J Biomech Eng 2013. [DOI] [PMC free article] [PubMed]
- [123].Freedman BR, Zuskov A, Sarver JJ, Buckley MR, LJ S, Journal of Orthopaedic Research 2014.
- [124].Freedman BR, Rodriguez AB, Hillin CD, Weiss SN, Han B, Han L, Soslowsky LJ, J R Soc Interface 2018, 15. [DOI] [PMC free article] [PubMed]
- [125].Connizzo BK, Sarver JJ, Han L, Soslowsky LJ, J Biomech 2014, 47, 3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Provenzano PP, Vanderby R Jr., Matrix Biol 2006, 25, 71. [DOI] [PubMed] [Google Scholar]
- [127].Palmer AK, Kirkland JL, Exp Gerontol 2016, 86, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Zwick RK, Guerrero-Juarez CF, Horsley V, Plikus MV, Cell Metab 2018, 27, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Ferrante AW Jr., Diabetes Obes Metab 2013, 15 Suppl 3, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Abrahamson DR, J Pathol 1986, 149, 257. [DOI] [PubMed] [Google Scholar]
- [131].Nakajima I, Yamaguchi T, Ozutsumi K, Aso H, Differentiation 1998, 63, 193. [DOI] [PubMed] [Google Scholar]
- [132].Mariman EC, Wang P, Cell Mol Life Sci 2010, 67, 1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Comley K, Fleck NA, Int J Solids Struct 2010, 47, 2982. [Google Scholar]
- [134].Fontanella CG, Nalesso F, Carniel EL, Natali AN, Med Biol Eng Comput 2016, 54, 653. [DOI] [PubMed] [Google Scholar]
- [135].Wojciechowicz K, Markiewicz E, Jahoda CA, Exp Dermatol 2008, 17, 675. [DOI] [PubMed] [Google Scholar]
- [136].Wojciechowicz K, Gledhill K, Ambler CA, Manning CB, Jahoda CA, PLoS One 2013, 8, e59811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Chase HB, Montagna W, Malone JD, Anat Rec 1953, 116, 75. [DOI] [PubMed] [Google Scholar]
- [138].Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, Horsley V, Cell 2011, 146, 761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Gallagher J, Tierney P, Murray P, O’Brien M, Knee Surg Sports Traumatol Arthrosc 2005, 13, 268. [DOI] [PubMed] [Google Scholar]
- [140].Sampatchalit S, Chen L, Haghighi P, Trudell D, Resnick DL, AJR Am J Roentgenol 2009, 193, W127. [DOI] [PubMed] [Google Scholar]
- [141].Draghi F, Ferrozzi G, Urciuoli L, Bortolotto C, Bianchi S, Insights Imaging 2016, 7, 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Distel E, Cadoudal T, Durant S, Poignard A, Chevalier X, Benelli C, Arthritis Rheum 2009, 60, 3374. [DOI] [PubMed] [Google Scholar]
- [143].Ioan-Facsinay A, Kloppenburg M, Arthritis Res Ther 2013, 15, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Hovey RC, Aimo L, J Mammary Gland Biol Neoplasia 2010, 15, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Anderson SM, Rudolph MC, McManaman JL, Neville MC, Breast Cancer Res 2007, 9, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Zangani D, Darcy KM, Shoemaker S, Ip MM, Exp Cell Res 1999, 247, 399. [DOI] [PubMed] [Google Scholar]
- [147].Wang X, Zhang X, Sun L, Subramanian B, Maffini MV, Soto A, Sonnenschein C, Kaplan DL, Tissue Eng Part A 2009, 15, 3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Hovey RC, MacKenzie DD, McFadden TB, In Vitro Cell Dev Biol Anim 1998, 34, 385. [DOI] [PubMed] [Google Scholar]
- [149].Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, Garrido I, Escourrou G, Valet P, Muller C, Cancer Res 2011, 71, 2455. [DOI] [PubMed] [Google Scholar]
- [150].DeFilippis RA, Chang H, Dumont N, Rabban JT, Chen YY, Fontenay GV, Berman HK, Gauthier ML, Zhao J, Hu D, Marx JJ, Tjoe JA, Ziv E, Febbraio M, Kerlikowske K, Parvin B, Tlsty TD, Cancer Discov 2012, 2, 826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Pascual G, Avgustinova A, Mejetta S, Martin M, Castellanos A, Attolini CS, Berenguer A, Prats N, Toll A, Hueto JA, Bescos C, Di Croce L, Benitah SA, Nature 2017, 541, 41. [DOI] [PubMed] [Google Scholar]
- [152].Tan J, Buache E, Chenard MP, Dali-Youcef N, Rio MC, Int J Dev Biol 2011, 55, 851. [DOI] [PubMed] [Google Scholar]
- [153].Horowitz MC, Berry R, Holtrup B, Sebo Z, Nelson T, Fretz JA, Lindskog D, Kaplan JL, Ables G, Rodeheffer MS, Rosen CJ, Adipocyte 2017, 6, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Menagh PJ, Turner RT, Jump DB, Wong CP, Lowry MB, Yakar S, Rosen CJ, Iwaniec UT, J Bone Miner Res 2010, 25, 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, Woelk L, Fan H, Logan DW, Schurmann A, Saraiva LR, Schulz TJ, Cell Stem Cell 2017, 20, 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Fazeli PK, Horowitz MC, MacDougald OA, Scheller EL, Rodeheffer MS, Rosen CJ, Klibanski A, J Clin Endocrinol Metab 2013, 98, 935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, Ning X, Bree AJ, Schell B, Broome DT, Soliman SS, DelProposto JL, Lumeng CN, Mitra A, Pandit SV, Gallagher KA, Miller JD, Krishnan V, Hui SK, Bredella MA, Fazeli PK, Klibanski A, Horowitz MC, Rosen CJ, MacDougald OA, Cell Metab 2014, 20, 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Rohrich RJ, Pessa JE, Plast Reconstr Surg 2007, 119, 2219. [DOI] [PubMed] [Google Scholar]
- [159].Gesta S, Tseng YH, Kahn CR, Cell 2007, 131, 242. [DOI] [PubMed] [Google Scholar]
- [160].Bahn RS, N Engl J Med 2010, 362, 726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Rabkin SW, Obes Rev 2007, 8, 253. [DOI] [PubMed] [Google Scholar]
- [162].Sacks HS, Fain JN, Holman B, Cheema P, Chary A, Parks F, Karas J, Optican R, Bahouth SW, Garrett E, Wolf RY, Carter RA, Robbins T, Wolford D, Samaha J, J Clin Endocrinol Metab 2009, 94, 3611. [DOI] [PubMed] [Google Scholar]
- [163].Iacobellis G, Nat Rev Endocrinol 2015, 11, 363. [DOI] [PubMed] [Google Scholar]
- [164].Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F, Am J Cardiol 2004, 94, 1084. [DOI] [PubMed] [Google Scholar]
- [165].Buschmann WR, Jahss MH, Kummer F, Desai P, Gee RO, Ricci JL, Foot Ankle Int 1995, 16, 254. [DOI] [PubMed] [Google Scholar]
- [166].Ortega N, Behonick DJ, Werb Z, Trends Cell Biol 2004, 14, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Hall BK, Miyake T, Anat Embryol (Berl) 1992, 186, 107. [DOI] [PubMed] [Google Scholar]
- [168].Pacifici M, Koyama E, Shibukawa Y, Wu C, Tamamura Y, Enomoto-Iwamoto M, Iwamoto M, Ann N Y Acad Sci 2006, 1068, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Lai WM, Hou JS, Mow VC, J Biomech Eng 1991, 113, 245. [DOI] [PubMed] [Google Scholar]
- [170].Aicher WK, Rolauffs B, Ann Rheum Dis 2014, 73, 645. [DOI] [PubMed] [Google Scholar]
- [171].Mow VC, Huiskes R, Basic Orthopaedic Biomechanics & Mechano-Biology, Lippincott Williams & Wilkins, Philadelphia: 2005. [Google Scholar]
- [172].Guilak F, Nims RJ, Dicks A, Wu CL, Meulenbelt I, Matrix Biol 2018. [DOI] [PMC free article] [PubMed]
- [173].Akizuki S, Mow VC, Muller F, Pita JC, Howell DS, Manicourt DH, J Orthop Res 1986, 4, 379. [DOI] [PubMed] [Google Scholar]
- [174].Woo SL, Lubock P, Gomez MA, Jemmott GF, Kuei SC, Akeson WH, J Biomech 1979, 12, 437. [DOI] [PubMed] [Google Scholar]
- [175].Kempson GE, Freeman MA, Swanson SA, Nature 1968, 220, 1127. [DOI] [PubMed] [Google Scholar]
- [176].Batista MA, Nia HT, Onnerfjord P, Cox KA, Ortiz C, Grodzinsky AJ, Heinegard D, Han L, Matrix Biol 2014, 38, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Lee W, Guilak F, Liedtke W, Curr Top Membr 2017, 79, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, Lefebvre V, Jay GD, Stewart M, Wang H, Warman ML, Carpten JD, J Clin Invest 2005, 115, 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Minns RJ, Steven FS, J Anat 1977, 123, 437. [PMC free article] [PubMed] [Google Scholar]
- [180].Han L, Dean D, Daher LA, Grodzinsky AJ, Ortiz C, Biophys J 2008, 95, 4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Arda O, Goksugur N, Tuzun Y, Clin Dermatol 2014, 32, 3. [DOI] [PubMed] [Google Scholar]
- [182].Zgheib C, Hodges M, Hu J, Beason DP, Soslowsky LJ, Liechty KW, Xu J, Wound Repair Regen 2016, 24, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Janmey PA, Winer JP, Weisel JW, J R Soc Interface 2009, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Goh ET, Kirby G, Jayukumar R, Liang XJ, T. A, Chapter 23 – accelerated wound healing using nanoparticles, 2016.
- [185].Bainbridge P, J Wound Care 2013, 22, 407. [DOI] [PubMed] [Google Scholar]
- [186].Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA, Nat Rev Mol Cell Biol 2002, 3, 349. [DOI] [PubMed] [Google Scholar]
- [187].Freedman BR, Gordon JA, Soslowsky LJ, Muscles Ligaments Tendons J 2014, 4, 245. [PMC free article] [PubMed] [Google Scholar]
- [188].Fukuta S, Oyama M, Kavalkovich K, Fu FH, Niyibizi C, Matrix Biol 1998, 17, 65. [DOI] [PubMed] [Google Scholar]
- [189].Sun M, Connizzo BK, Adams SM, Freedman BR, Wenstrup RJ, Soslowsky LJ, Birk DE, Am J Pathol 2015. [DOI] [PMC free article] [PubMed]
- [190].Connizzo BK, Freedman BR, Fried JH, Sun M, Birk DE, Soslowsky LJ, J Orthop Res 2015, 33, 882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Buckley MR, Sarver JJ, Freedman BR, Soslowsky LJ, J Biomech 2013. [DOI] [PMC free article] [PubMed]
- [192].Henninger HB, Underwood CJ, Romney SJ, Davis GL, Weiss JA, J Orthop Res 2013, 31, 1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Banes AJ, Link GW, Bevin AG, Peterson HD, Gillespie Y, Bynum D, Watts S, Dahners L, J Orthop Res 1988, 6, 73. [DOI] [PubMed] [Google Scholar]
- [194].Ahmed IM, Lagopoulos M, McConnell P, Soames RW, Sefton GK, J Orthop Res 1998, 16, 591. [DOI] [PubMed] [Google Scholar]
- [195].Freedman BR, Rodriguez AB, Leiphart RJ, Newton JB, Ban E, Sarver JJ, Mauck RL, Shenoy VB, Soslowsky LJ, Sci Rep 2018, 8, 10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Han WM, Heo SJ, Driscoll TP, Delucca JF, McLeod CM, Smith LJ, Duncan RL, Mauck RL, Elliott DM, Nat Mater 2016, 15, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Ahmadzadeh H, Connizzo BK, Freedman BR, Soslowsky LJ, Shenoy VB, J Biomech 2013, 46, 2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Kawamura S, Ying L, Kim HJ, Dynybil C, Rodeo SA, J Orthop Res 2005, 23, 1425. [DOI] [PubMed] [Google Scholar]
- [199].Bogdanowicz DR, Lu HH, Ann N Y Acad Sci 2017, 1410, 3.29265419 [Google Scholar]
- [200].Lee CH, Lee FY, Tarafder S, Kao K, Jun Y, Yang G, Mao JJ, J Clin Invest 2015, 125, 2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Huang AH, Lu HH, Schweitzer R, J Orthop Res 2015, 33, 800. [DOI] [PubMed] [Google Scholar]
- [202].Miller KS, Connizzo BK, Feeney E, Soslowsky LJ, J Biomech 2012, 45, 2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Yin L, Elliott DM, J Biomech 2004, 37, 907. [DOI] [PubMed] [Google Scholar]
- [204].Maganaris CN, Paul JP, J Physiol 1999, 521 Pt 1, 307. [DOI] [PMC free article] [PubMed]
- [205].Fukashiro S, Komi PV, Jarvinen M, Miyashita M, Eur J Appl Physiol Occup Physiol 1995, 71, 453. [DOI] [PubMed] [Google Scholar]
- [206].Wren TA, Yerby SA, Beaupre GS, Carter DR, Clin Biomech (Bristol, Avon) 2001, 16, 245. [DOI] [PubMed] [Google Scholar]
- [207].Fang F, Sawhney AS, Lake SP, J Biomech 2014, 47, 2869. [DOI] [PubMed] [Google Scholar]
- [208].Szczesny SE, Peloquin JM, Cortes DH, Kadlowec JA, Soslowsky LJ, Elliott DM, J Biomech Eng 2012, 134, 021004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Pardes AM, Freedman BR, Fryhofer GW, Salka NS, Bhatt PR, Soslowsky LJ, Ann Biomed Eng 2016, 44, 2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Fryhofer GW, Freedman BR, Hillin CD, Salka NS, Pardes AM, Weiss SN, Farber DC, Soslowsky LJ, J Appl Physiol (1985) 2016, 121, 1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Freedman BR, Fryhofer GW, Salka NS, Raja HA, Hillin CD, Nuss CA, Farber DC, Soslowsky LJ, J Biomech 2017, 56, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Freedman BR, Gordon JA, Bhatt PB, Pardes AM, Thomas SJ, Sarver JJ, Riggin CN, Tucker JJ, Williams AW, Zanes RC, Hast MW, Farber DC, Silbernagel KG, Soslowsky LJ, J Orthop Res 2016. [DOI] [PMC free article] [PubMed]
- [213].Freedman BR, Salka NS, Morris TR, Bhatt PR, Pardes AM, Gordon JA, Nuss CA, Riggin CN, Fryhofer GW, Farber DC, Soslowsky L, J Am Acad Orthop Surg 2017, 25, 635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Maeda E, Fleischmann C, Mein CA, Shelton JC, Bader DL, Lee DA, Connect Tissue Res 2010, 51, 434. [DOI] [PubMed] [Google Scholar]
- [215].Maeda E, Shelton JC, Bader DL, Lee DA, Biochem Biophys Res Commun 2007, 362, 399. [DOI] [PubMed] [Google Scholar]
- [216].Maeda E, Shelton JC, Bader DL, Lee DA, J Appl Physiol (1985) 2009, 106, 506. [DOI] [PubMed] [Google Scholar]
- [217].Screen HR, Shelton JC, Bader DL, Lee DA, Biochem Biophys Res Commun 2005, 336, 424. [DOI] [PubMed] [Google Scholar]
- [218].Yamamoto E, Kogawa D, Tokura S, Hayashi K, J Biomech Eng 2005, 127, 1168. [DOI] [PubMed] [Google Scholar]
- [219].Yamamoto E, Tokura S, Hayashi K, J Biomech Eng 2003, 125, 893. [DOI] [PubMed] [Google Scholar]
- [220].Hannafin JA, Arnoczky SP, Hoonjan A, Torzilli PA, J Orthop Res 1995, 13, 907. [DOI] [PubMed] [Google Scholar]
- [221].Legerlotz K, Jones GC, Screen HR, Riley GP, Scand J Med Sci Sports 2013, 23, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Thornton GM, Shao X, Chung M, Sciore P, Boorman RS, Hart DA, Lo IK, British journal of sports medicine 2010, 44, 698. [DOI] [PubMed] [Google Scholar]
- [223].Flick J, Devkota A, Tsuzaki M, Almekinders L, Weinhold P, Clin Biomech (Bristol, Avon) 2006, 21, 99. [DOI] [PubMed] [Google Scholar]
- [224].Eyre D, Arthritis Res 2002, 4, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Knudson CB, Knudson W, Semin Cell Dev Biol 2001, 12, 69. [DOI] [PubMed] [Google Scholar]
- [226].Roberts S, Weightman B, Urban J, Chappell D, J Bone Joint Surg Br 1986, 68, 278. [DOI] [PubMed] [Google Scholar]
- [227].Achterberg VF, Buscemi L, Diekmann H, Smith-Clerc J, Schwengler H, Meister JJ, Wenck H, Gallinat S, Hinz B, J Invest Dermatol 2014, 134, 1862. [DOI] [PubMed] [Google Scholar]
- [228].Dunkman AA, Buckley MR, Mienaltowski MJ, Adams SM, Thomas SJ, Satchell L, Kumar A, Pathmanathan L, Beason DP, Iozzo RV, Birk DE, Soslowsky LJ, Matrix Biol 2013, 32, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Ashley GW, Henise J, Reid R, Santi DV, Proc Natl Acad Sci U S A 2013, 110, 2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Florence AT, Jani PU, Drug Saf 1994, 10, 233. [DOI] [PubMed] [Google Scholar]
- [231].Cohen J, Science 1995, 270, 908. [DOI] [PubMed] [Google Scholar]
- [232].Langer R, Nature 1998, 392, 5. [PubMed] [Google Scholar]
- [233].Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA, Nat Mater 2013, 12, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee HP, Lippens E, Duda GN, Mooney DJ, Nat Mater 2016, 15, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Huebsch N, Lippens E, Lee K, Mehta M, Koshy ST, Darnell MC, Desai RM, Madl CM, Xu M, Zhao X, Chaudhuri O, Verbeke C, Kim WS, Alim K, Mammoto A, Ingber DE, Duda GN, Mooney DJ, Nat Mater 2015, 14, 1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Subramony SD, Dargis BR, Castillo M, Azeloglu EU, Tracey MS, Su A, Lu HH, Biomaterials 2013, 34, 1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Keller R, Shook D, Skoglund P, Phys Biol 2008, 5, 015007. [DOI] [PubMed] [Google Scholar]
- [238].Horne-Badovinac S, Integr Comp Biol 2014, 54, 667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Barocas VH, Tranquillo RT, J Biomech Eng 1997, 119, 137. [DOI] [PubMed] [Google Scholar]
- [240].Peach MS, Ramos DM, James R, Morozowich NL, Mazzocca AD, Doty SB, Allcock HR, Kumbar SG, Laurencin CT, PLoS One 2017, 12, e0174789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Orr SB, Chainani A, Hippensteel KJ, Kishan A, Gilchrist C, Garrigues NW, Ruch DS, Guilak F, Little D, Acta Biomater 2015, 24, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Perris R, Perissinotto D, Mech Dev 2000, 95, 3. [DOI] [PubMed] [Google Scholar]
- [243].Wen JH, Vincent LG, Fuhrmann A, Choi YS, Hribar KC, Taylor-Weiner H, Chen S, Engler AJ, Nat Mater 2014, 13, 979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Wells RG, Hepatology 2008, 47, 1394. [DOI] [PubMed] [Google Scholar]
- [245].Engler AJ, Sen S, Sweeney HL, Discher DE, Cell 2006, 126, 677. [DOI] [PubMed] [Google Scholar]
- [246].Kisiel M, Martino MM, Ventura M, Hubbell JA, Hilborn J, Ossipov DA, Biomaterials 2013, 34, 704. [DOI] [PubMed] [Google Scholar]
- [247].Re’em T, Witte F, Willbold E, Ruvinov E, Cohen S, Acta Biomater 2012, 8, 3283. [DOI] [PubMed] [Google Scholar]
- [248].Marquardt LM, Heilshorn SC, Curr Stem Cell Rep 2016, 2, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Emans PJ, Pieper J, Hulsbosch MM, Koenders M, Kreijveld E, Surtel DA, van Blitterswijk CA, Bulstra SK, Kuijer R, Riesle J, Tissue Eng 2006, 12, 1699. [DOI] [PubMed] [Google Scholar]
- [250].Quintavalla J, Uziel-Fusi S, Yin J, Boehnlein E, Pastor G, Blancuzzi V, Singh HN, Kraus KH, O’Byrne E, Pellas TC, Biomaterials 2002, 23, 109. [DOI] [PubMed] [Google Scholar]
- [251].Brown CF, Yan J, Han TT, Marecak DM, Amsden BG, Flynn LE, Biomed Mater 2015, 10, 045010. [DOI] [PubMed] [Google Scholar]
- [252].Booth BW, Yang CC, Burg KJ, J Biomater Sci Polym Ed 2012, 23, 2303. [DOI] [PubMed] [Google Scholar]
- [253].Fan M, Ma Y, Mao J, Zhang Z, Tan H, Acta Biomater 2015, 20, 60. [DOI] [PubMed] [Google Scholar]
- [254].Fan M, Ma Y, Zhang Z, Mao J, Tan H, Hu X, Mater Sci Eng C Mater Biol Appl 2015, 56, 311. [DOI] [PubMed] [Google Scholar]
- [255].Bhowmick S, Rother S, Zimmermann H, Lee PS, Moeller S, Schnabelrauch M, Koul V, Jordan R, Hintze V, Scharnweber D, Mater Sci Eng C Mater Biol Appl 2017, 79, 15. [DOI] [PubMed] [Google Scholar]
- [256].Patterson J, Siew R, Herring SW, Lin AS, Guldberg R, Stayton PS, Biomaterials 2010, 31, 6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F, Biomaterials 2004, 25, 3211. [DOI] [PubMed] [Google Scholar]
- [258].Wang X, Wenk E, Zhang X, Meinel L, Vunjak-Novakovic G, Kaplan DL, J Control Release 2009, 134, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Jaikumar D, Sajesh KM, Soumya S, Nimal TR, Chennazhi KP, Nair SV, Jayakumar R, Int J Biol Macromol 2015, 74, 318. [DOI] [PubMed] [Google Scholar]
- [260].Jia J, Richards DJ, Pollard S, Tan Y, Rodriguez J, Visconti RP, Trusk TC, Yost MJ, Yao H, Markwald RR, Mei Y, Acta Biomater 2014, 10, 4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Hirsch T, Laemmle C, Behr B, Lehnhardt M, Jacobsen F, Hoefer D, Kueckelhaus M, J Plast Reconstr Aesthet Surg 2018, 71, 101. [DOI] [PubMed] [Google Scholar]
- [262].Drury JL, Dennis RG, Mooney DJ, Biomaterials 2004, 25, 3187. [DOI] [PubMed] [Google Scholar]
- [263].Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ, Nat Mater 2010, 9, 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Kuo CK, Ma PX, Biomaterials 2001, 22, 511. [DOI] [PubMed] [Google Scholar]
- [265].Hoemann CD, Sun J, McKee MD, Chevrier A, Rossomacha E, Rivard GE, Hurtig M, Buschmann MD, Osteoarthritis Cartilage 2007, 15, 78. [DOI] [PubMed] [Google Scholar]
- [266].Stanish WD, McCormack R, Forriol F, Mohtadi N, Pelet S, Desnoyers J, Restrepo A, Shive MS, J Bone Joint Surg Am 2013, 95, 1640. [DOI] [PubMed] [Google Scholar]
- [267].Lim SM, Song DK, Oh SH, Lee-Yoon DS, Bae EH, Lee JH, J Biomater Sci Polym Ed 2008, 19, 453. [DOI] [PubMed] [Google Scholar]
- [268].Fernandez JG, Ingber DE, Macromol Mater Eng 2014, 299, 932. [Google Scholar]
- [269].Sridharan B, Sharma B, Detamore MS, Tissue Eng Part B Rev 2015. [DOI] [PMC free article] [PubMed]
- [270].Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP, J Bone Joint Surg Am 2006, 88, 2665. [DOI] [PubMed] [Google Scholar]
- [271].Lee W, Debasitis JC, Lee VK, Lee JH, Fischer K, Edminster K, Park JK, Yoo SS, Biomaterials 2009, 30, 1587. [DOI] [PubMed] [Google Scholar]
- [272].Killat J, Reimers K, Choi CY, Jahn S, Vogt PM, Radtke C, Int J Mol Sci 2013, 14, 14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Vunjak-Novakovic G, Altman G, Horan R, Kaplan DL, Annu Rev Biomed Eng 2004, 6, 131. [DOI] [PubMed] [Google Scholar]
- [274].Awad HA, Butler DL, Harris MT, Ibrahim RE, Wu Y, Young RG, Kadiyala S, Boivin GP, J Biomed Mater Res 2000, 51, 233. [DOI] [PubMed] [Google Scholar]
- [275].Murray MM, Flutie BM, Kalish LA, Ecklund K, Fleming BC, Proffen BL, Micheli LJ, Orthop J Sports Med 2016, 4, 2325967116672176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Loy C, Laine A, Mantovani D, Biotechnol J 2016, 11, 1673. [DOI] [PubMed] [Google Scholar]
- [277].Nam S, Hu KH, Butte MJ, Chaudhuri O, Proc Natl Acad Sci U S A 2016, 113, 5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Phull MK, Eydmann T, Roxburgh J, Sharpe JR, Lawrence-Watt DJ, Phillips G, Martin Y, J Mater Sci Mater Med 2013, 24, 461. [DOI] [PubMed] [Google Scholar]
- [279].Chang KH, Liao HT, Chen JP, Acta Biomater 2013, 9, 9012. [DOI] [PubMed] [Google Scholar]
- [280].Liu B, Wang Y, Miao Y, Zhang X, Fan Z, Singh G, Zhang X, Xu K, Li B, Hu Z, Xing M, Biomaterials 2018, 171, 83. [DOI] [PubMed] [Google Scholar]
- [281].Hu M, Azeloglu EU, Ron A, Tran-Ba KH, Calizo RC, Tavassoly I, Bhattacharya S, Jayaraman G, Chen Y, Rabinovich V, Iyengar R, Hone JC, He JC, Kaufman LJ, Sci Rep 2017, 7, 43934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Bellas E, Marra KG, Kaplan DL, Tissue Eng Part C Methods 2013, 19, 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Gholipourmalekabadi M, Samadikuchaksaraei A, Seifalian AM, Urbanska AM, Ghanbarian H, Hardy JG, Omrani MD, Mozafari M, Reis RL, Kundu SC, Biomed Mater 2018, 13, 035003. [DOI] [PubMed] [Google Scholar]
- [284].Vidal SEL, Tamamoto KA, Nguyen H, Abbott RD, Cairns DM, Kaplan DL, Biomaterials 2018. [DOI] [PMC free article] [PubMed]
- [285].Shahverdi S, Hajimiri M, Esfandiari MA, Larijani B, Atyabi F, Rajabiani A, Dehpour AR, Gharehaghaji AA, Dinarvand R, Int J Pharm 2014, 473, 345. [DOI] [PubMed] [Google Scholar]
- [286].Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL, Biomaterials 2003, 24, 401. [DOI] [PubMed] [Google Scholar]
- [287].Cao Y, Wang B, Int J Mol Sci 2009, 10, 1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Perez-Rigueiro J, Viney C, Llorca J, Elices M, J Appl Polym Sci 2000, 75, 1270. [Google Scholar]
- [289].Mandal BB, Kundu SC, Macromol Biosci 2008, 8, 807. [DOI] [PubMed] [Google Scholar]
- [290].Hofmann S, Knecht S, Langer R, Kaplan DL, Vunjak-Novakovic G, Merkle HP, Meinel L, Tissue Eng 2006, 12, 2729. [DOI] [PubMed] [Google Scholar]
- [291].Schaefer D, Martin I, Shastri P, Padera RF, Langer R, Freed LE, Vunjak-Novakovic G, Biomaterials 2000, 21, 2599. [DOI] [PubMed] [Google Scholar]
- [292].Mozdzen LC, Vucetic A, Harley BAC, J Mech Behav Biomed Mater 2017, 66, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].He Y, Li Z, Chen Z, Yu X, Ji Z, Wang J, Qian Y, Li L, Cell Biol Int 2018. [DOI] [PubMed]
- [294].Brandl FP, Seitz AK, Tessmar JK, Blunk T, Gopferich AM, Biomaterials 2010, 31, 3957. [DOI] [PubMed] [Google Scholar]
- [295].Aini H, Itaka K, Fujisawa A, Uchida H, Uchida S, Fukushima S, Kataoka K, Saito T, Chung UI, Ohba S, Sci Rep 2016, 6, 18743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Yang C, DelRio FW, Ma H, Killaars AR, Basta LP, Kyburz KA, Anseth KS, Proc Natl Acad Sci U S A 2016, 113, E4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Sun JY, Zhao X, Illeperuma WR, Chaudhuri O, Oh KH, Mooney DJ, Vlassak JJ, Suo Z, Nature 2012, 489, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR, Science 1999, 284, 143. [DOI] [PubMed] [Google Scholar]
- [299].Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E, Cytotherapy 2006, 8, 315. [DOI] [PubMed] [Google Scholar]
- [300].Guest DJ, Smith MR, Allen WR, Equine Vet J 2010, 42, 636. [DOI] [PubMed] [Google Scholar]
- [301].Sole A, Spriet M, Padgett KA, Vaughan B, Galuppo LD, Borjesson DL, Wisner ER, Vidal MA, Equine Vet J 2013, 45, 726. [DOI] [PubMed] [Google Scholar]
- [302].Becerra P, Valdes Vazquez MA, Dudhia J, Fiske-Jackson AR, Neves F, Hartman NG, Smith RK, J Orthop Res 2013, 31, 1096. [DOI] [PubMed] [Google Scholar]
- [303].Caplan AI, Stem Cells Transl Med 2017, 6, 1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M, Stem Cells 2006, 24, 74. [DOI] [PubMed] [Google Scholar]
- [305].Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM, Blood 2002, 99, 3838. [DOI] [PubMed] [Google Scholar]
- [306].Ryan JM, Barry F, Murphy JM, Mahon BP, Clin Exp Immunol 2007, 149, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y, Cell Stem Cell 2008, 2, 141. [DOI] [PubMed] [Google Scholar]
- [308].Baraniak PR, McDevitt TC, Regen Med 2010, 5, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Takahashi K, Yamanaka S, Cell 2006, 126, 663. [DOI] [PubMed] [Google Scholar]
- [310].Youssef AA, Ross EG, Bolli R, Pepine CJ, Leeper NJ, Yang PC, JACC Basic Transl Sci 2016, 1, 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Van Nieuwenhove I, Tytgat L, Ryx M, Blondeel P, Stillaert F, Thienpont H, Ottevaere H, Dubruel P, Van Vlierberghe S, Acta Biomater 2017, 63, 37. [DOI] [PubMed] [Google Scholar]
- [312].Smith P, Adams WP Jr., Lipschitz AH, Chau B, Sorokin E, Rohrich RJ, Brown SA, Plast Reconstr Surg 2006, 117, 1836. [DOI] [PubMed] [Google Scholar]
- [313].Nguyen A, Pasyk KA, Bouvier TN, Hassett CA, Argenta LC, Plast Reconstr Surg 1990, 85, 378. [PubMed] [Google Scholar]
- [314].Niechajev I, Sevcuk O, Plast Reconstr Surg 1994, 94, 496. [DOI] [PubMed] [Google Scholar]
- [315].Coleman SR, Saboeiro AP, Plast Reconstr Surg 2007, 119, 775. [DOI] [PubMed] [Google Scholar]
- [316].Omidi E, Fuetterer L, Reza Mousavi S, Armstrong RC, Flynn LE, Samani A, J Biomech 2014, 47, 3657. [DOI] [PubMed] [Google Scholar]
- [317].Flynn LE, Biomaterials 2010, 31, 4715. [DOI] [PubMed] [Google Scholar]
- [318].Cheung HK, Han TT, Marecak DM, Watkins JF, Amsden BG, Flynn LE, Biomaterials 2014, 35, 1914. [DOI] [PubMed] [Google Scholar]
- [319].Yu C, Bianco J, Brown C, Fuetterer L, Watkins JF, Samani A, Flynn LE, Biomaterials 2013, 34, 3290. [DOI] [PubMed] [Google Scholar]
- [320].Adam Young D, Bajaj V, Christman KL, J Biomed Mater Res A 2014, 102, 1641. [DOI] [PubMed] [Google Scholar]
- [321].Turner AE, Yu C, Bianco J, Watkins JF, Flynn LE, Biomaterials 2012, 33, 4490. [DOI] [PubMed] [Google Scholar]
- [322].Wu I, Nahas Z, Kimmerling KA, Rosson GD, Elisseeff JH, Plast Reconstr Surg 2012, 129, 1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Choi JS, Yang HJ, Kim BS, Kim JD, Lee SH, Lee EK, Park K, Cho YW, Lee HY, Tissue Eng Part C Methods 2010, 16, 387. [DOI] [PubMed] [Google Scholar]
- [324].Unnithan AR, Sasikala ARK, Thomas SS, Nejad AG, Cha YS, Park CH, Kim CS, Sci Rep 2018, 8, 5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Gimble JM, Katz AJ, Bunnell BA, Circ Res 2007, 100, 1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [326].Young DA, Choi YS, Engler AJ, Christman KL, Biomaterials 2013, 34, 8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].Rossi E, Gerges I, Tocchio A, Tamplenizza M, Aprile P, Recordati C, Martello F, Martin I, Milani P, Lenardi C, Biomaterials 2016, 104, 65. [DOI] [PubMed] [Google Scholar]
- [328].Vashi AV, Keramidaris E, Abberton KM, Morrison WA, Wilson JL, O’Connor AJ, Cooper-White JJ, Thompson EW, Biomaterials 2008, 29, 573. [DOI] [PubMed] [Google Scholar]
- [329].Tan H, Ramirez CM, Miljkovic N, Li H, Rubin JP, Marra KG, Biomaterials 2009, 30, 6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Frydrych M, Roman S, MacNeil S, Chen B, Acta Biomater 2015, 18, 40. [DOI] [PubMed] [Google Scholar]
- [331].Zhang K, Song L, Wang J, Yan S, Li G, Cui L, Yin J, Acta Biomater 2017, 51, 246. [DOI] [PubMed] [Google Scholar]
- [332].Ovsianikov A, Deiwick A, Van Vlierberghe S, Pflaum M, Wilhelmi M, Dubruel P, Chichkov B, Materials (Basel) 2011, 4, 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333].Chhaya MP, Melchels FP, Holzapfel BM, Baldwin JG, Hutmacher DW, Biomaterials 2015, 52, 551. [DOI] [PubMed] [Google Scholar]
- [334].Pati F, Ha DH, Jang J, Han HH, Rhie JW, Cho DW, Biomaterials 2015, 62, 164. [DOI] [PubMed] [Google Scholar]
- [335].Young DA, Christman KL, Biomed Mater 2012, 7, 024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Frye CA, Wu X, Patrick CW, In Vitro Cell Dev Biol Anim 2005, 41, 160. [DOI] [PubMed] [Google Scholar]
- [337].Liu Z, Kobayashi K, van Dinther M, van Heiningen SH, Valdimarsdottir G, van Laar T, Scharpfenecker M, Lowik CW, Goumans MJ, Ten Dijke P, Pardali E, J Cell Sci 2009, 122, 3294. [DOI] [PubMed] [Google Scholar]
- [338].Mahoney CM, Kelmindi-Doko A, Snowden MJ, Peter Rubin J, Marra KG, Acta Biomater 2017, 58, 26. [DOI] [PubMed] [Google Scholar]
- [339].Kelmendi-Doko A, Rubin JP, Klett K, Mahoney C, Wang S, Marra KG, J Tissue Eng 2017, 8, 2041731417735402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340].Medical M, (Ed: Report KIMI), 2015.
- [341].Ficat RP, Ficat C, Gedeon P, Toussaint JB, Clin Orthop Relat Res 1979, 74. [PubMed]
- [342].KH P, G. G, Journal of Bone and Joint Surgery 1959, 41, 11.13620684 [Google Scholar]
- [343].Dorotka R, Windberger U, Macfelda K, Bindreiter U, Toma C, Nehrer S, Biomaterials 2005, 26, 3617. [DOI] [PubMed] [Google Scholar]
- [344].Breinan HA, Martin SD, Hsu HP, Spector M, J Orthop Res 2000, 18, 781. [DOI] [PubMed] [Google Scholar]
- [345].Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG, Arthroscopy 2003, 19, 477. [DOI] [PubMed] [Google Scholar]
- [346].Ulstein S, Aroen A, Engebretsen L, Forssblad M, Lygre SHL, Rotterud JH, Orthop J Sports Med 2018, 6, 2325967118787767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [347].Huey DJ, Hu JC, Athanasiou KA, Science 2012, 338, 917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [348].Milner PE, Parkes M, Puetzer JL, Chapman R, Stevens MM, Cann P, Jeffers JRT, Acta Biomater 2018, 65, 102. [DOI] [PubMed] [Google Scholar]
- [349].DeKosky BJ, Dormer NH, Ingavle GC, Roatch CH, Lomakin J, Detamore MS, Gehrke SH, Tissue Eng Part C Methods 2010, 16, 1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [350].Liao IC, Moutos FT, Estes BT, Zhao X, Guilak F, Adv Funct Mater 2013, 23, 5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [351].Visser J, Melchels FP, Jeon JE, van Bussel EM, Kimpton LS, Byrne HM, Dhert WJ, Dalton PD, Hutmacher DW, Malda J, Nat Commun 2015, 6, 6933. [DOI] [PubMed] [Google Scholar]
- [352].Tan H, Chu CR, Payne KA, Marra KG, Biomaterials 2009, 30, 2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [353].Boyer C, Figueiredo L, Pace R, Lesoeur J, Rouillon T, Visage CL, Tassin JF, Weiss P, Guicheux J, Rethore G, Acta Biomater 2018, 65, 112. [DOI] [PubMed] [Google Scholar]
- [354].Torricelli P, Gioffre M, Fiorani A, Panzavolta S, Gualandi C, Fini M, Focarete ML, Bigi A, Mater Sci Eng C Mater Biol Appl 2014, 36, 130. [DOI] [PubMed] [Google Scholar]
- [355].Subramanian A, Vu D, Larsen GF, Lin HY, J Biomater Sci Polym Ed 2005, 16, 861. [DOI] [PubMed] [Google Scholar]
- [356].Li WJ, Mauck RL, Cooper JA, Yuan X, Tuan RS, J Biomech 2007, 40, 1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [357].Li WJ, Cooper JA Jr., Mauck RL, Tuan RS, Acta Biomater 2006, 2, 377. [DOI] [PubMed] [Google Scholar]
- [358].Kang Y, Yang J, Khan S, Anissian L, Ameer GA, J Biomed Mater Res A 2006, 77, 331. [DOI] [PubMed] [Google Scholar]
- [359].Gong Y, He L, Li J, Zhou Q, Ma Z, Gao C, Shen J, J Biomed Mater Res B Appl Biomater 2007, 82, 192. [DOI] [PubMed] [Google Scholar]
- [360].Lee CT, Huang CP, Lee YD, Biomacromolecules 2006, 7, 2200. [DOI] [PubMed] [Google Scholar]
- [361].Yan LP, Oliveira JM, Oliveira AL, Caridade SG, Mano JF, Reis RL, Acta Biomater 2012, 8, 289. [DOI] [PubMed] [Google Scholar]
- [362].Yang Q, Peng J, Guo Q, Huang J, Zhang L, Yao J, Yang F, Wang S, Xu W, Wang A, Lu S, Biomaterials 2008, 29, 2378. [DOI] [PubMed] [Google Scholar]
- [363].Zhang W, Lian Q, Li D, Wang K, Hao D, Bian W, He J, Jin Z, Biomed Res Int 2014, 2014, 746138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [364].Hung KC, Tseng CS, Hsu SH, Adv Healthc Mater 2014, 3, 1578. [DOI] [PubMed] [Google Scholar]
- [365].Rowland CR, Colucci LA, Guilak F, Biomaterials 2016, 91, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [366].Kon E, Filardo G, Shani J, Altschuler N, Levy A, Zaslav K, Eisman JE, Robinson D, J Orthop Surg Res 2015, 10, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [367].Cooper JA Jr., Li WJ, Bailey LO, Hudson SD, Lin-Gibson S, Anseth KS, Tuan RS, Washburn NR, Acta Biomater 2007, 3, 13. [DOI] [PubMed] [Google Scholar]
- [368].Grimshaw MJ, Mason RM, Osteoarthritis Cartilage 2000, 8, 386. [DOI] [PubMed] [Google Scholar]
- [369].Mandl EW, Jahr H, Koevoet JL, van Leeuwen JP, Weinans H, Verhaar JA, van Osch GJ, Matrix Biol 2004, 23, 231. [DOI] [PubMed] [Google Scholar]
- [370].Anderson DE, Johnstone B, Front Bioeng Biotechnol 2017, 5, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [371].Benya PD, Shaffer JD, Cell 1982, 30, 215. [DOI] [PubMed] [Google Scholar]
- [372].Chawla K, Klein TJ, Schumacher BL, Jadin KD, Shah BH, Nakagawa K, Wong VW, Chen AC, Masuda K, Sah RL, Tissue Eng 2007, 13, 1525. [DOI] [PubMed] [Google Scholar]
- [373].Griffin DJ, Bonnevie ED, Lachowsky DJ, Hart JC, Sparks HD, Moran N, Matthews G, Nixon AJ, Cohen I, Bonassar LJ, J Biomech 2015, 48, 1944. [DOI] [PubMed] [Google Scholar]
- [374].Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L, N Engl J Med 1994, 331, 889. [DOI] [PubMed] [Google Scholar]
- [375].Armiento AR, Stoddart MJ, Alini M, Eglin D, Acta Biomater 2018, 65, 1. [DOI] [PubMed] [Google Scholar]
- [376].Hauselmann HJ, Flura T, Marti C, Hauser N, Hedbom E, Schweiz Med Wochenschr 1998, 128, 824. [PubMed] [Google Scholar]
- [377].Shive MS, Stanish WD, McCormack R, Forriol F, Mohtadi N, Pelet S, Desnoyers J, Methot S, Vehik K, Restrepo A, Cartilage 2015, 6, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [378].Klein TJ, Schumacher BL, Schmidt TA, Li KW, Voegtline MS, Masuda K, Thonar EJ, Sah RL, Osteoarthritis Cartilage 2003, 11, 595. [DOI] [PubMed] [Google Scholar]
- [379].Steinmetz NJ, Aisenbrey EA, Westbrook KK, Qi HJ, Bryant SJ, Acta Biomater 2015, 21, 142. [DOI] [PubMed] [Google Scholar]
- [380].Sherwood JK, Riley SL, Palazzolo R, Brown SC, Monkhouse DC, Coates M, Griffith LG, Landeen LK, Ratcliffe A, Biomaterials 2002, 23, 4739. [DOI] [PubMed] [Google Scholar]
- [381].Grayson WL, Bhumiratana S, Grace Chao PH, Hung CT, Vunjak-Novakovic G, Osteoarthritis Cartilage 2010, 18, 714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [382].Albro MB, Nims RJ, Durney KM, Cigan AD, Shim JJ, Vunjak-Novakovic G, Hung CT, Ateshian GA, Biomaterials 2016, 77, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [383].Moutos FT, Glass KA, Compton SA, Ross AK, Gersbach CA, Guilak F, Estes BT, Proc Natl Acad Sci U S A 2016, 113, E4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [384].Saxena V, Kim M, Keah NM, Neuwirth AL, Stoeckl BD, Bickard K, Restle DJ, Salowe R, Wang MY, Steinberg DR, Mauck RL, Tissue Eng Part A 2016, 22, 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [385].Feng Q, Lin S, Zhang K, Dong C, Wu T, Huang H, Yan X, Zhang L, Li G, Bian L, Acta Biomater 2017, 53, 329. [DOI] [PubMed] [Google Scholar]
- [386].Bajpayee AG, Grodzinsky AJ, Nat Rev Rheumatol 2017, 13, 183. [DOI] [PubMed] [Google Scholar]
- [387].Fisher MB, Belkin NS, Milby AH, Henning EA, Soegaard N, Kim M, Pfeifer C, Saxena V, Dodge GR, Burdick JA, Schaer TP, Steinberg DR, Mauck RL, Cartilage 2016, 7, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [388].Kim IL, Pfeifer CG, Fisher MB, Saxena V, Meloni GR, Kwon MY, Kim M, Steinberg DR, Mauck RL, Burdick JA, Tissue Eng Part A 2015, 21, 2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [389].Lambers H, Piessens S, Bloem A, Pronk H, Finkel P, Int J Cosmet Sci 2006, 28, 359. [DOI] [PubMed] [Google Scholar]
- [390].Kruse CR, Singh M, Targosinski S, Sinha I, Sorensen JA, Eriksson E, Nuutila K, Wound Repair Regen 2017, 25, 260. [DOI] [PubMed] [Google Scholar]
- [391].Fierheller M, Sibbald RG, Adv Skin Wound Care 2010, 23, 369. [DOI] [PubMed] [Google Scholar]
- [392].Shevchenko RV, James SL, James SE, J R Soc Interface 2010, 7, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [393].Im H, Kim SH, Kim SH, Jung Y, Tissue Eng Part A 2018. [DOI] [PubMed]
- [394].Pereira RF, Barrias CC, Bartolo PJ, Granja PL, Acta Biomater 2018, 66, 282. [DOI] [PubMed] [Google Scholar]
- [395].Wang T, Lew J, Premkumar J, Poh CL, Naing MW, IET, Engineering Biology 2017, 1, 5. [Google Scholar]
- [396].Li J, Celiz AD, Yang J, Yang Q, Wamala I, Whyte W, Seo BR, Vasilyev NV, Vlassak JJ, Suo Z, Mooney DJ, Science 2017, 357, 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [397].Blacklow S, Li J, Freedman B, Chen C, Mooney D, (in review) [DOI] [PMC free article] [PubMed]
- [398].Tian R, Qiu X, Yuan P, Lei K, Wang L, Bai Y, Liu S, Chen X, ACS Appl Mater Interfaces 2018, 10, 17018. [DOI] [PubMed] [Google Scholar]
- [399].Zulkifli FH, Hussain FSJ, Zeyohannes SS, Rasad M, Yusuff MM, Mater Sci Eng C Mater Biol Appl 2017, 79, 151. [DOI] [PubMed] [Google Scholar]
- [400].Turner NJ, Badylak SF, Adv Wound Care (New Rochelle) 2015, 4, 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [401].Burke JF, Yannas IV, Quinby WC Jr., Bondoc CC, Jung WK, Ann Surg 1981, 194, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [402].Hart J, Silcock D, Gunnigle S, Cullen B, Light ND, Watt PW, Int J Biochem Cell Biol 2002, 34, 1557. [DOI] [PubMed] [Google Scholar]
- [403].Scherer SS, Pietramaggiori G, Matthews J, Perry S, Assmann A, Carothers A, Demcheva M, Muise-Helmericks RC, Seth A, Vournakis JN, Valeri RC, Fischer TH, Hechtman HB, Orgill DP, Ann Surg 2009, 250, 322. [DOI] [PubMed] [Google Scholar]
- [404].Dumville JC, O’Meara S, Deshpande S, Speak K, Cochrane Database Syst Rev 2013, CD009110. [DOI] [PMC free article] [PubMed]
- [405].Veves A, Falanga V, Armstrong DG, Sabolinski ML, Apligraf S Diabetic Foot Ulcer, Diabetes Care 2001, 24, 290. [DOI] [PubMed] [Google Scholar]
- [406].Harding K, Sumner M, Cardinal M, Int Wound J 2013, 10, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [407].Zelen CM, Serena TE, Denoziere G, Fetterolf DE, Int Wound J 2013, 10, 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [408].Koob TJ, Lim JJ, Massee M, Zabek N, Denoziere G, J Biomed Mater Res B Appl Biomater 2014, 102, 1353. [DOI] [PubMed] [Google Scholar]
- [409].Landsman AS, Cook J, Cook E, Landsman AR, Garrett P, Yoon J, Kirkwood A, Desman E, Foot Ankle Spec 2011, 4, 29. [DOI] [PubMed] [Google Scholar]
- [410].Whiteley J, Chow T, Adissu H, Keating A, Rogers IM, Stem Cells Transl Med 2018. [DOI] [PMC free article] [PubMed]
- [411].Wu Y, Chen L, Scott PG, Tredget EE, Stem Cells 2007, 25, 2648. [DOI] [PubMed] [Google Scholar]
- [412].Guo R, Xu S, Ma L, Huang A, Gao C, Biomaterials 2011, 32, 1019. [DOI] [PubMed] [Google Scholar]
- [413].Banerjee I, Mishra D, Das T, Maiti TK, J Biomater Sci Polym Ed 2012, 23, 111. [DOI] [PubMed] [Google Scholar]
- [414].Yang HS, Shin J, Bhang SH, Shin JY, Park J, Im GI, Kim CS, Kim BS, Exp Mol Med 2011, 43, 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [415].Xie Z, Paras CB, Weng H, Punnakitikashem P, Su LC, Vu K, Tang L, Yang J, Nguyen KT, Acta Biomater 2013, 9, 9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [416].Smiell JM, Am J Surg 1998, 176, 68S. [DOI] [PubMed] [Google Scholar]
- [417].Lei M, Schumacher LJ, Lai YC, Juan WT, Yeh CY, Wu P, Jiang TX, Baker RE, Widelitz RB, Yang L, Chuong CM, Proc Natl Acad Sci U S A 2017, 114, E7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [418].Liu X, Ma L, Liang J, Zhang B, Teng J, Gao C, Biomaterials 2013, 34, 2038. [DOI] [PubMed] [Google Scholar]
- [419].Kawai K, Larson BJ, Ishise H, Carre AL, Nishimoto S, Longaker M, Lorenz HP, PLoS One 2011, 6, e27106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [420].Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G, Am J Pathol 2001, 159, 1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [421].Humbert P, Fanian F, Lihoreau T, Jeudy A, Elkhyat A, Robin S, Courderot-Masuyer C, Tauzin H, Lafforgue C, Haftek M, Clin Interv Aging 2015, 10, 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [422].Kessler D, Dethlefsen S, Haase I, Plomann M, Hirche F, Krieg T, Eckes B, J Biol Chem 2001, 276, 36575. [DOI] [PubMed] [Google Scholar]
- [423].Wang W, Pan Z, Hu X, Li Z, Zhao Y, Yu AX, Exp Ther Med 2014, 7, 1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [424].Labler L, Rancan M, Mica L, Harter L, Mihic-Probst D, Keel M, J Trauma 2009, 66, 749. [DOI] [PubMed] [Google Scholar]
- [425].Pullar CE, Isseroff RR, J Cell Sci 2005, 118, 2023. [DOI] [PubMed] [Google Scholar]
- [426].Zhao M, Dick A, Forrester JV, McCaig CD, Mol Biol Cell 1999, 10, 1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [427].Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM, Nature 2006, 442, 457. [DOI] [PubMed] [Google Scholar]
- [428].Kim J, Kim HN, Lim KT, Kim Y, Seonwoo H, Park SH, Lim HJ, Kim DH, Suh KY, Choung PH, Choung YH, Chung JH, Sci Rep 2013, 3, 3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [429].Cubo N, Garcia M, Del Canizo JF, Velasco D, Jorcano JL, Biofabrication 2016, 9, 015006. [DOI] [PubMed] [Google Scholar]
- [430].Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL, J Bone Joint Surg Am 2012, 94, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [431].Iannotti JP, J Am Acad Orthop Surg 1994, 2, 87. [DOI] [PubMed] [Google Scholar]
- [432].McCarron JA, Derwin KA, Bey MJ, Polster JM, Schils JP, Ricchetti ET, Iannotti JP, Am J Sports Med 2013, 41, 134. [DOI] [PubMed] [Google Scholar]
- [433].Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S, J Shoulder Elbow Surg 2003, 12, 550. [DOI] [PubMed] [Google Scholar]
- [434].Rodeo SA, J Shoulder Elbow Surg 2007, 16, S191. [DOI] [PubMed] [Google Scholar]
- [435]. A. A. o. O. Surgeons, (Ed: AAOS), 2013.
- [436].Urist MR, Strates BS, Clin Orthop Relat Res 1970, 71, 271. [PubMed] [Google Scholar]
- [437].Yin Z, Chen X, Chen JL, Shen WL, Hieu Nguyen TM, Gao L, Ouyang HW, Biomaterials 2010, 31, 2163. [DOI] [PubMed] [Google Scholar]
- [438].Grier WK, Iyoha EM, Harley BAC, J Mech Behav Biomed Mater 2017, 65, 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [439].Caliari SR, Gonnerman EA, Grier WK, Weisgerber DW, Banks JM, Alsop AJ, Lee JS, Bailey RC, Harley BA, Adv Healthc Mater 2015, 4, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [440].Caliari SR, Harley BA, Adv Healthc Mater 2014, 3, 1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [441].Murugan R, Ramakrishna S, Tissue Eng 2007, 13, 1845. [DOI] [PubMed] [Google Scholar]
- [442].Pham QP, Sharma U, Mikos AG, Biomacromolecules 2006, 7, 2796. [DOI] [PubMed] [Google Scholar]
- [443].Czaplewski SK, Tsai TL, Duenwald-Kuehl SE, Vanderby R Jr., Li WJ, Biomaterials 2014, 35, 6907. [DOI] [PubMed] [Google Scholar]
- [444].Shoaib A, Mishra V, Foot Ankle Surg 2017, 23, 179. [DOI] [PubMed] [Google Scholar]
- [445].Dempsey DK, Robinson JL, Iyer AV, Parakka JP, Bezwada RS, Cosgriff-Hernandez EM, J Biomater Sci Polym Ed 2014, 25, 535. [DOI] [PubMed] [Google Scholar]
- [446].Li X, Xie J, Lipner J, Yuan X, Thomopoulos S, Xia Y, Nano Lett 2009, 9, 2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [447].Smith LJ, Deymier AC, Boyle JJ, Li Z, Linderman SW, Pasteris JD, Xia Y, Genin GM, Thomopoulos S, Interface Focus 2016, 6, 20150070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [448].Zhu C, Pongkitwitoon S, Qiu J, Thomopoulos S, Xia Y, Adv Mater 2018, 30, e1707306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [449].Zhang J, Wang JH, PLoS One 2013, 8, e71740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [450].Mendias CL, Gumucio JP, Lynch EB, J Appl Physiol (1985) 2012, 113, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [451].Ralphs JR, Waggett AD, Benjamin M, Matrix Biol 2002, 21, 67. [DOI] [PubMed] [Google Scholar]
- [452].Juncosa-Melvin N, Matlin KS, Holdcraft RW, Nirmalanandhan VS, Butler DL, Tissue Eng 2007, 13, 1219. [DOI] [PubMed] [Google Scholar]
- [453].Rui YF, Lui PP, Ni M, Chan LS, Lee YW, Chan KM, J Orthop Res 2011, 29, 390. [DOI] [PubMed] [Google Scholar]
- [454].Shi Y, Fu Y, Tong W, Geng Y, Lui PP, Tang T, Zhang X, Dai K, J Cell Biochem 2012, 113, 3133. [DOI] [PubMed] [Google Scholar]
- [455].Qi J, Chi L, Bynum D, Banes AJ, J Appl Physiol (1985) 2011, 110, 1425. [DOI] [PubMed] [Google Scholar]
- [456].Bayer ML, Schjerling P, Herchenhan A, Zeltz C, Heinemeier KM, Christensen L, Krogsgaard M, Gullberg D, Kjaer M, PLoS One 2014, 9, e86078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [457].Freedman BR, Bade ND, Riggin CN, Zhang S, Haines PG, Ong KL, Janmey PA, Biochim Biophys Acta 2015, 1853, 3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [458].Gardner K, Lavagnino M, Egerbacher M, Arnoczky SP, J Orthop Res 2012, 30, 1695. [DOI] [PubMed] [Google Scholar]
- [459].Chen X, Yin Z, Chen JL, Liu HH, Shen WL, Fang Z, Zhu T, Ji J, Ouyang HW, Zou XH, Tissue Eng Part A 2014, 20, 1583. [DOI] [PubMed] [Google Scholar]
- [460].Nirmalanandhan VS, Dressler MR, Shearn JT, Juncosa-Melvin N, Rao M, Gooch C, Bradica G, Butler DL, J Biomech Eng 2007, 129, 919. [DOI] [PubMed] [Google Scholar]
- [461].Goncalves AI, Rodrigues MT, Gomes ME, Acta Biomater 2017, 63, 110. [DOI] [PubMed] [Google Scholar]
- [462].Phillips JE, Burns KL, Le Doux JM, Guldberg RE, Garcia AJ, Proc Natl Acad Sci U S A 2008, 105, 12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [463].Zhang C, Wang X, Zhang E, Yang L, Yuan H, Tu W, Zhang H, Yin Z, Shen W, Chen X, Zhang Y, Ouyang H, Acta Biomater 2018, 66, 141. [DOI] [PubMed] [Google Scholar]
- [464].Sahoo S, Toh SL, Goh JC, Biomaterials 2010, 31, 2990. [DOI] [PubMed] [Google Scholar]
- [465].Jenner JM, van Eijk F, Saris DB, Willems WJ, Dhert WJ, Creemers LB, Tissue Eng 2007, 13, 1573. [DOI] [PubMed] [Google Scholar]
- [466].Li J, Weber E, Guth-Gundel S, Schuleit M, Kuttler A, Halleux C, Accart N, Doelemeyer A, Basler A, Tigani B, Wuersch K, Fornaro M, Kneissel M, Stafford A, Freedman BR, Mooney DJ, Adv Healthc Mater 2018, 7, e1701393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [467].Cheng X, Tsao C, Sylvia VL, Cornet D, Nicolella DP, Bredbenner TL, Christy RJ, Acta Biomater 2014, 10, 1360. [DOI] [PubMed] [Google Scholar]
- [468].Gelberman RH, Linderman SW, Jayaram R, Dikina AD, Sakiyama-Elbert S, Alsberg E, Thomopoulos S, Shen H, Clin Orthop Relat Res 2017, 475, 2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [469].Linderman SW, Shen H, Yoneda S, Jayaram R, Tanes ML, Sakiyama-Elbert SE, Xia Y, Thomopoulos S, Gelberman RH, J Orthop Res 2017. [DOI] [PMC free article] [PubMed]
- [470].Millar NL, Gilchrist DS, Akbar M, Reilly JH, Kerr SC, Campbell AL, Murrell GA, Liew FY, Kurowska-Stolarska M, McInnes IB, Nat Commun 2015, 6, 6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [471].Watts AE, Millar NL, Platt J, Kitson SM, Akbar M, Rech R, Griffin J, Pool R, Hughes T, McInnes IB, Gilchrist DS, Mol Ther 2017, 25, 2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [472].Madhurakkat Perikamana SK, Lee J, Ahmad T, Kim EM, Byun H, Lee S, Shin H, Biomaterials 2018, 165, 79. [DOI] [PubMed] [Google Scholar]
- [473].Wang W, Li J, Wang K, Zhang Z, Zhang W, Zhou G, Cao Y, Ye M, Zou H, Liu W, Am J Physiol Cell Physiol 2016, 310, C357. [DOI] [PubMed] [Google Scholar]
- [474].Blomgran P, Hammerman M, Aspenberg P, Sci Rep 2017, 7, 12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [475].Connizzo BK, Yannascoli SM, Tucker JJ, Caro AC, Riggin CN, Mauck RL, Soslowsky LJ, Steinberg DR, Bernstein J, Clin Orthop Relat Res 2014, 472, 2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [476].Kane TP, Ismail M, Calder JD, Am J Sports Med 2008, 36, 1160. [DOI] [PubMed] [Google Scholar]
- [477].Paoloni JA, Murrell GA, Foot Ankle Int 2007, 28, 1064. [DOI] [PubMed] [Google Scholar]
- [478].Hunziker E, Spector M, Libera J, Gertzman A, Woo SL, Ratcliffe A, Lysaght M, Coury A, Kaplan D, Vunjak-Novakovic G, Tissue Eng 2006, 12, 3341. [DOI] [PubMed] [Google Scholar]
- [479].Van Norman GA, JACC Basic Transl Sci 2016, 1, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [480].Proffen BL, Perrone GS, Roberts G, Murray MM, Ann Biomed Eng 2015, 43, 805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [481].Van Norman GA, JACC Basic Transl Sci 2016, 1, 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [482].Li J, Mooney DJ, Nat Rev Mater 2016, 1. [DOI] [PMC free article] [PubMed]
- [483].Tolikas M, Antoniou A, Ingber DE, Bioeng Transl Med 2017, 2, 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [484].Prestwich GD, Bhatia S, Breuer CK, Dahl SL, Mason C, McFarland R, McQuillan DJ, Sackner-Bernstein J, Schox J, Tente WE, Trounson A, Sci Transl Med 2012, 4, 160cm14. [DOI] [PubMed]
- [485].Stace ET, Dakin SG, Mouthuy PA, Carr AJ, J Cell Physiol 2016, 231, 36. [DOI] [PubMed] [Google Scholar]
- [486].Sah RL, Ratcliffe A, Tissue Eng Part B Rev 2010, 16, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [487].B. B.N., B. S.F., Principles of Tissue Engineering, Academic Press, 2014. [Google Scholar]
- [488].Pfeifer CG, Fisher MB, Carey JL, Mauck RL, Sci Transl Med 2015, 7, 310re9. [DOI] [PMC free article] [PubMed]
- [489].Duda GN, Grainger DW, Frisk ML, Bruckner-Tuderman L, Carr A, Dirnagl U, Einhaupl KM, Gottschalk S, Gruskin E, Huber C, June CH, Mooney DJ, Rietschel ET, Schutte G, Seeger W, Stevens MM, Urban R, Veldman A, Wess G, Volk HD, Sci Transl Med 2014, 6, 264cm12. [DOI] [PubMed]
- [490].ASTM,”Evaluation of in vitro Release of Biomolecules from Biomaterials Scaffolds for TEMPs”, F3142.22156 2017, 11.
- [491].ASTM,”Quantifying Cell Viability within Biomaterial Scaffolds”, F2739.24263 2016, 7.
- [492].ASTM,”Interpreting Images of Polymeric Tissue Scaffolds”, F2603.13030 2012, 10.
- [493].ASTM,”Tissue Engineered Medical Products (TEMPs)”, F2211.5069 2013, 8.
- [494].EMA,”GUIDELINE ON HUMAN CELL-BASED MEDICINAL PRODUCTS”, EMEA/CHMP/410869/2006 2008, 25.
- [495].FDA,”Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use”, ucm585403, 28.
- [496].ASTM,”Evaluating Growth of Engineered Cartilage Tissue using Magnetic Resonance Imaging”, F3224.10185 2017, 10.
- [497].ASTM,”in vivo Assessment of Implantable Devices Intended to Repair or Regenerate Articular Cartilage”, F2451.13396 2010, 10.
- [498].FDA,”Preparation of IDEs and INDs for Products Intended to Repair or Replace Knee Cartilage”, ucm288011 2012.
- [499].ASTM,”Classification of Cellular and/or Tissue-Based Products (CTPs) for Skin Wounds”, F3163.20836 2016, 6.
- [500].FDA,”Guidance for Industry and FDA Staff Class II Special Controls Guidance Document: Tissue Adhesive with Adjunct Wound Closure Device Intended for the Topical Approximation of Skin”, 1683 2010. [Google Scholar]
- [501].ASTM,”Tissue Engineered Medical Products (TEMPs) for Reinforcement of Tendon and Ligament Surgical Repair”, F2903.3753 2011, 7.
- [502].Faria M, Bjornmalm M, Thurecht KJ, Kent SJ, Parton RG, Kavallaris M, Johnston APR, Gooding JJ, Corrie SR, Boyd BJ, Thordarson P, Whittaker AK, Stevens MM, Prestidge CA, Porter CJH, Parak WJ, Davis TP, Crampin EJ, Caruso F, Nat Nanotechnol 2018, 13, 777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [503].Hwang I, Kim HN, Seong M, Lee SH, Kang M, Yi H, Bae WG, Kwak MK, Jeong HE, Adv Healthc Mater 2018, e1800275. [DOI] [PubMed]