Abstract
Malignancy increases sepsis incidence by 10-fold and elevates sepsis-associated mortality. Advances in treatment have improved survival of cancer patients shortly after sepsis, but there is a paucity of information on how sepsis impacts cancer growth, development and prognosis. To test this, cecal-ligation-and-puncture (CLP) surgery was performed on B16 melanoma-bearing mice to show sepsis has detrimental effect in hosts with advanced tumors leading to increased mortality. Surprisingly, mice experiencing CLP-induced sepsis earlier during tumor development exhibited CD8 T cell-dependent attenuation of tumor growth. Sepsis-resistant CD8 TILs showed increased in vivo activation, effector IFN-γ cytokine production, proliferation and expression of activation/inhibitory PD-1/LAG-3 receptors due to a sepsis-induced liberation of tumor antigens. Sepsis reinvigorated CD8 TILs were also amenable to (anti-PD-L1/LAG-3) checkpoint-blockade therapy further prolonging cancer-associated survival in sepsis survivors. Thus, sepsis has the capacity to improve tumor-specific CD8 T cell responses leading to better cancer prognosis and increased survival.
Introduction
Sepsis is defined by the vigorous production of pro- and anti-inflammatory cytokines in response to a systemic pathogen resulting in injury or death of nearly 2 million US patients every year (1). Sepsis strikes regardless of age or health-status, but the majority of septic patients display comorbidities upon sepsis induction. Malignancy is the greatest risk factor, with a 10-fold increase in sepsis incidence regardless of cancer type (2, 3). The majority of cancer patients have active/progressing tumors at the time of sepsis and experience greater sepsis-associated mortality than patients without cancer (2, 4). These clinical outcomes have been reverse-translated in murine models using multiple sepsis models (e.g., cecal ligation and puncture (CLP)) and malignant cell types (e.g., pancreatic adenocarcinoma) (5–7). Improved treatment has increased survival of the entire septic population and has greatly benefitted cancer patients resulting in a 76% survival-rate after sepsis (2). Thus, cancer patients have high incidence of sepsis and it is important to understand how sepsis impacts cancer prognosis and long-term outcome of an increasing number of sepsis survivors.
Sepsis deleteriously impacts an array of immune cell subsets and contributes to the immunoparalysis phase of sepsis characterized by increased susceptibility to secondary infections and de novo tumor growth (8, 9). Using the CLP model of polymicrobial sepsis in mice lacking comorbidities, we and others have defined how sepsis affects CD8 T cell-mediated immunity that protects the host against intracellular bacteria, viruses, and tumors (10). In general, naïve and previously-established memory CD8 T cells undergo apoptotic death during a septic event that increases susceptibility to new- and previously-encountered pathogens, respectively (11). In addition, the circulating memory CD8 T cells (TCIRCM) in the blood and spleen that remain after sepsis have diminished Ag-dependent functions characterized by sub-optimal proliferative capacity and effector cytokine (IFN-γ) production (12, 13). In stark contrast, tissue-resident memory CD8 T cells (TRM) that reside in non-lymphoid tissues (NLT) do not experience loss in numbers or function after sepsis, possibly due to their secluded localization and inability of cytokines/chemokines produced during sepsis to reach them (13). Thus, it is becoming clear that sepsis-induced immunoparalysis is not as simple as previously suspected and can be compartmentalized (14). This apparent dichotomy in susceptibility of various CD8 T cell populations to sepsis in mice lacking comorbidities, make it difficult to predict the extent sepsis impacts tumor-infiltrating CD8 T cells (TILs) and cancer progression.
Materials and Methods
Mice, cell lines, CLP surgery, and tumor monitoring
Male and female C57Bl/6 mice >6-weeks old were used. Sepsis was induced with a single cecal puncture using a 25G needle to extrude a small amount of cecal content, leading to a septic state characterized by weight loss, shivering, and diarrhea with mortality rates between 0–10%, as previously described (13, 15, 16). Sham (control) mice underwent similar laparotomy omitting cecal ligation and puncture. Model advantages, limitations and relevance to human disease are discussed previously (10, 17). B16 and B16-OVA (gift from Dr. Lyse Norian, University of Alabama-Birmingham). B16 cells were grown in DMEM with 4.5g/L D-glucose, L-glutamine, 10% fetal calf serum and supplementum complementum. For implantation, 2×104 B16 cells were subcutaneously injected in the hind flank at 100μL volume with equal parts B16 medium and Matrigel Matrix (Corning).
Cell isolation, fluorescent labeling, and flow cytometric analysis
Tumors were cut into small pieces and incubated in DMEM medium +10% FCS + SC + 0.8mg/mL Collagenase type I (Worthington) + 60U/mL of DNase on a 37°C shaker at 300RPM. After 45–60 min, the tissue was mashed through a 70μm cell strainer using the plunger flange of a syringe and cell suspensions treated with ACK lysis solution to remove any erythrocytes. Cells were labeled with the following fluorescently-labeled mAb: CD8α (53–6.7), CD69 (H1.2F3), PD-1 (J43), LAG-3 (eBioC9B7W), IFN-γ (XMG1.2), CD45.2 (104), Thy1.1 (HIS51), Ki67 (51-36524X BD). Samples requiring intracellular labeling were subsequently treated with Cytofix/Cytoperm for 10 min at 4°C and washed in Perm/Wash (BD Biosciences) before labeling. For Ki67 analysis, Foxp3 staining fixation and permeabilization set (eBioscience) was used. CFSE labeling was performed as previously described (13). Samples were run on a FACSCanto flow cytometer (BD Biosciences) and analyzed using FlowJo software.
In vivo mAb administration
Mice received 200μg of αPD-L1 (10F.9G2) and αLAG-3 (C9B7W) mAb or control rat IgG every 3 days.
SPADE Analysis
Flow cytometric data of tumor samples were manually gated on CD8+ cells using FlowJo software and exported to specifically analyze the CD8 TIL population. The SPADE analysis separated cells into distinct nodes based on the marker panel analyzed, including PD-1, LAG-3, and Ki67. The parameter shown is LAG-3 (PE) in the Blue-Yellow color scheme using median metric and a symmetric scale that was global among samples.
Statistics
Statistical analyses were performed using GraphPad Prism software v7. Data are shown as ±SEM. Bar graphs, tumor growth graphs and survival curves were analyzed using unpaired t-test, 2-way ANOVA, and Log-rank (Mantel-Cox) tests, respectively.
Results and Discussion
Sepsis induced mortality in tumor-bearing mice is dependent on tumor size
Experimental data in mice using various sepsis and/or tumor models have recapitulated the increased rate of sepsis-induced mortality in cancer patients (5–7). To independently confirm and further develop this concept, B6 mice were injected with B16 cells subcutaneously (SQ) 21 days before undergoing laparotomy followed by ligation and puncture of the distal cecum to induce polymicrobial sepsis (CLP) or laparotomy alone (Sham; Fig 1A). High mortality rates in cancer hosts were observed in the 2 days following sepsis induction using a low-severity model of sepsis in which 100% of tumor-free mice survive (Fig 1B). Tumor-bearing hosts that underwent sham surgery did not succumb in the following 2 days indicating tumor burden alone cannot account for mortality observed in this experiment.
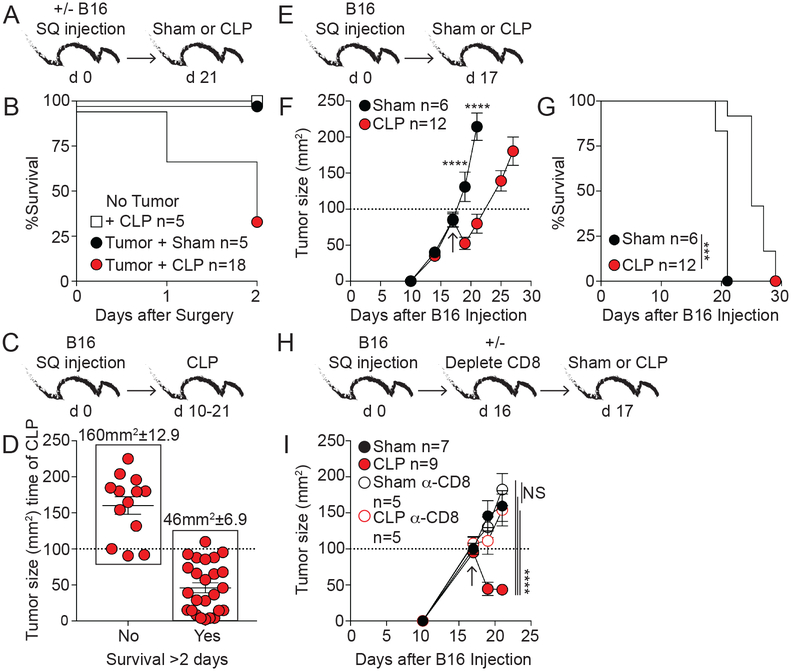
Fig 1. CD8 T cells contribute to slower tumor progression in sepsis survivors.
A) Experimental design. B) Survival after surgery. C) Experimental design; 10 to 21 days after tumor injection CLP surgery was performed. D) Mice were separated into survivors and non-survivors 2 days after surgery and tumor size (mm2) at the time of CLP induction for each mouse is shown. Average tumor size (mm2) is indicated above. E) Experimental design. F) Tumor size (mm2) at indicated time points after B16 injection. Arrow indicates time of surgery. G) Survival. H) Experimental design. One day before surgery mice were treated with 800μg αCD8 or rat IgG mAb. I) Tumor size (mm2). Arrow indicates time of surgery. Tumor volumes ±SEM are shown. Tumor growth and survival curves analyzed using two-way ANOVA and Log-rank (Mantel-Cox) tests, respectively. NS=not significant, ***p<0.001, ****p<0.0001. Data in panels A-D are pooled from at least 2 independent experiments; E-G are representative of greater than 13 independent experiments; H-I representative of 2 independent experiments.
Tumor-bearing hosts underwent CLP surgery at the same time after tumor inoculation but tumor size at the time of sepsis induction (Fig 1A,B) varied and potentially contributed to the differential susceptibility of these hosts to sepsis associated mortality. To determine if tumor size/progression at the time of sepsis induction influenced subsequent mortality, CLP was performed 10–21 days after B16 implantation to generate a cohort of mice with a range of tumor sizes (Fig 1C). Analysis of survivor and non-survivors 2 days after CLP surgery showed a clear correlation between acute mortality and tumor size at the time of sepsis onset (Fig 1D). Concomitant with other reports, these data indicate tumor-bearing hosts exhibit increased susceptibility to sepsis-induced mortality, but mice bearing B16 tumors ≤100mm2 in size rarely succumbed to sepsis. Thus, sepsis-induced mortality is dependent on size of the tumor.
CD8 T cells contribute to slower tumor progression in sepsis survivors
Having identified a subset of tumor-bearing hosts that survive the septic event, we next investigated the impact of sepsis on cancer progression and host survival. Mice were challenged with B16 melanoma and 17 days later (when tumors were ≤100mm2) underwent sham or CLP surgery (Fig 1E). Interestingly, tumor size was reduced shortly after sepsis induction and ultimately prolonged survival of previously-septic hosts (Fig 1F–G). Selective neutralization of sepsis-induced inflammatory cytokines (TNF or IL-12 and IFN-γ) was not sufficient to inhibit the capacity of sepsis to shrink the tumor (data not shown), suggesting the myriad of cytokines produced after sepsis might have functional redundancy. Alternatively, CD8 T cells contribute to partial control of B16 progression (18), and presence of CD8 TILs in clinical isolates indicates a positive prognosis (19). To determine to what extent CD8 T cells contributed to the sepsis-related capacity to slow tumor progression, B16-bearing hosts received anti-CD8 or rat IgG mAb 1 day prior to surgery and tumor progression was monitored (Fig 1H). Once again, sepsis led to a reduction in tumor size in CD8 T cell sufficient hosts compared to sham counterparts (Fig 1I). Intriguingly, the sepsis-induced influence on tumor progression was lost in hosts lacking CD8 T cells. It is important to note that CD8 T cell depletion in sham hosts did not alter tumor growth, potentially due to the late timing of depletion in this experiment. To test this possibility, B16 tumors were harvested early (day 13) and late (day 21) after implantation and the phenotype and function of CD8 TILs was analyzed (Supplemental Fig. 1). The activation/inhibitory receptor PD-1 is expressed on CD8 T cells shortly after cognate Ag recognition (20), and PD-1 expressing CD8 TILs are characterized by their close proximity to tumor Ag and ability to exert (or retain) some effector functions (e.g., IFN-γ production) in the B16 tumor model (21). Importantly, expression of PD-1, ability to produce IFN-γ directly ex vivo, and CD8 TIL proliferation (based on Ki67 expression) was highly dependent on the time of tumor inoculation and tumor size, suggesting the window of opportunity in which CD8 T cells can exert their function(s) is limited. Thus, the data in Figure 1 collectively show CD8 T cells contribute to sepsis capacity to diminish tumor size and prolong survival in tumor-bearing hosts that survive the septic event. Although activation of innate immune cells after sepsis may facilitate a CD8 T cell-mediated reduction in tumor size, we focused the following experiments on understanding the contribution of tumor-infiltrating CD8 T cells (CD8 TILs) and their role in anti-tumor immunity after sepsis induction.
Numerical maintenance and increased activation/effector functions of CD8 TILs in the post-septic environment
The post-septic environment is characterized by transient lymphopenia and diminished CD8 T cell-mediated immunity that increases susceptibility to secondary infections (10–12). However, the deleterious impact of sepsis on CD8 T cells is compartmentalized, with cells in circulation being much more susceptible to the sepsis-induced alterations compared to cells in non-lymphoid tissues (NLT) (13). The residence of cells in NLT can be defined by i.v. injection of mAb prior to tissue harvesting that differentially labels cells within parenchyma (i.v.−) and vasculature (i.v.+) (22). Using this technique, we recently showed that i.v.− tissue-resident CD8 T cells (TRM) in NLT are less susceptible to sepsis-induced apoptosis compared to circulatory (i.v.+) CD8 T cells within the same tissue (13). In a similar manner, the Ag-dependent ‘sensing and alarm’ function (capacity to produce IFN-γ) of infection-induced skin TRM remained optimal in contrast to CD8 TCIRCM in the septic environment.
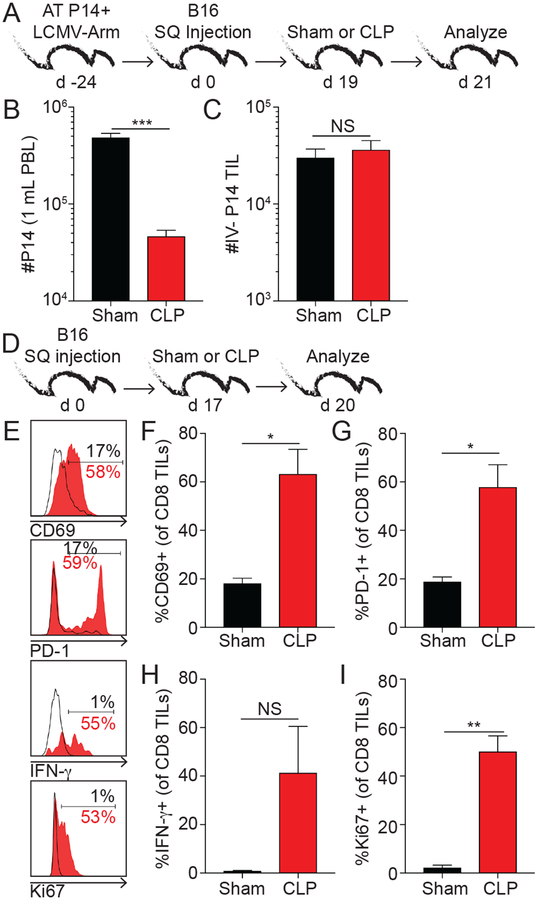
The i.v.-labeling technique in B16-bearing hosts revealed the majority (e.g., ~96%) of CD8 T cells harvested from the tumor were excluded from vasculature (Supplemental Fig. 2), similar to a previous report (23). The location of CD8 TILs within the tissue suggested these cells might not experience significant numerical loss after sepsis, similar to infection-induced CD8 TRM. To test this, hosts with detectable numbers of CD8 TCIRCM and TILs were generated (Fig 2A). Briefly, mice received adoptive transfer of congenic (Thy1.1+) naïve T-cell-transgenic (TCR-Tg) P14 CD8 T cells followed by LCMV-Armstrong infection. At a memory time point (day 24-post infection), B16 tumors were implanted and sham or CLP surgery performed 19 days later. Of note, virus-specific Thy1.1 P14 memory CD8 T cells generated using this approach infiltrate tumors (23). Two days after sham or CLP surgery, mice received an i.v. injection of fluorescently-labeled CD45.2 mAb prior to sacrifice and tissues were analyzed (Fig 2A). The number of P14 TCIRCM in the peripheral blood was dramatically reduced demonstrating classical post-septic lymphopenia (Fig 2B). Despite this, the number of tissue-resident (i.v.−) P14 TILs were numerically maintained confirming sepsis has limited capacity to influence number of cells embedded in NLT or tumors (Fig 2C).
Fig 2. Numerical maintenance and increased activation/effector functions of CD8 TILs in the post-septic environment.
A) Experimental design. Mice received adoptive transfer of 104 Thy1.1+ naïve P14 cells before LCMV-Arm infection (2×105 PFU i.p.). 24 days later B16 cells (2×104 SQ) were injected and after 19 days surgery was performed. i.v. labeling with CD45.2 mAb was performed 3 min before tissue harvesting. B) Number of P14 cells in PBL. C) Number of vasculature-excluded (i.v.−) P14 cells in tumor samples. D) Experimental design. E) Representative histograms. % of CD8 TILs expressing F) CD69, G) PD-1, H) IFN-γ and I) Ki67. Unpaired t-tests. NS=not significant, *p<0.05, **p<0.01, ***p<0.001. Summary data from at least 3 mice per group. Data in panels B-C and E-I are representative of 2 and 3 independent experiments, respectively.
Sepsis also has the ability to diminish Ag-dependent functions (e.g., effector cytokine production and proliferation) of circulatory CD8 T cells (12). To test the extent to which sepsis altered the Ag-dependent functions of CD8 TILs, B16-bearing mice underwent sham or CLP surgery 17 days after tumor inoculation and 3 days later CD8 TILs were analyzed (Fig 2D). Similar to the data in Fig 1F, sepsis led to a reduction in tumor size (data not shown). Expression of CD69 and PD-1, direct ex vivo IFN-γ production, and proliferative capacity of CD8 TILs increased after sepsis induction potentially suggesting increased interaction of the CD8 TILs with tumor Ag (Fig 2E–I). Collectively, sepsis has the capacity to decrease tumor size, maintain numbers and reinvigorate function of CD8 TILs that influences disease progression.
Sepsis increases Ag-dependent activation and proliferation of tumor-specific CD8 T cells
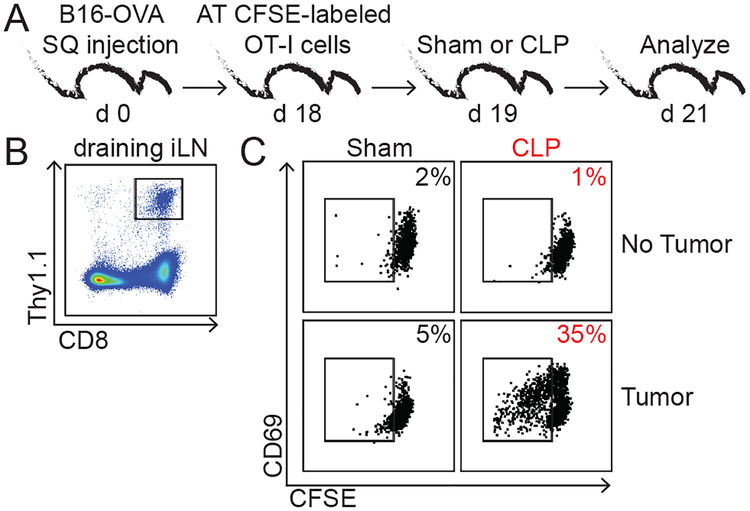
The reinvigorated Ag-dependent CD8 TIL responses suggest the liberation of existing, but inaccessible tumor Ag(s), following a septic event. To test if tumor Ag indeed becomes more accessible in the septic environment, mice bearing ovalbumin-expressing B16 (B16-OVA) tumors received CFSE-labeled Thy1.1+ OVA-specific TCR-Tg OT-I CD8 T cells 1 day before Sham or CLP surgery. A control group of tumor-free hosts also received OT-I CD8 T cells before surgery (Fig 3A). Donor OT-I cells in tumor draining lymph nodes 3 days after transfer were analyzed to determine if they encountered tumor Ag (Fig 3B). OT-I cells in tumor-free hosts showed minimal CD69 expression and CFSE dilution, suggesting lack of CD8 T cell activation and proliferation by homeostatic proliferation in the lymphopenic environment of septic hosts in the time frame analyzed (Fig 3C). Intriguingly, B16-OVA-bearing hosts that experienced CLP surgery had increased frequencies of proliferating OT-I cells (increased expression of CD69 and CFSE dilution) compared to sham counterparts. Thus, these data show tumor Ag is accessible to tumor-specific CD8 T cells in the post-septic environment and potentially contributes to their improved ability to function and provide a detectable level of anti-tumor immunity. Moreover, these data re-define the previous idea that sepsis universally impairs T cell-mediated immunity in mice lacking comorbidities (10, 17, 24).
Fig 3. Sepsis increases Ag-dependent activation and proliferation of tumor-specific CD8 T cells.
A) Experimental design. 18 days after B16-OVA injection mice received adoptive transfer of 5×106 Thy1.1+ CFSE-labeled naïve OT-I cells 1 day prior to sham/CLP surgery. B) Representative gating strategy of Thy1.1+ OT-I cells from tumor-draining inguinal lymph node. Representative dot plots of CD69 and CFSE of donor OT-I cells in tumor-free (top) and tumor-bearing (bottom) hosts that underwent sham (left) or CLP (right) surgery. Frequency of CFSE-diluted OT-I cells is indicated. Data are representative of 2 independent experiments.
PD-1 and LAG-3 blockade further reduces tumor progression after sepsis
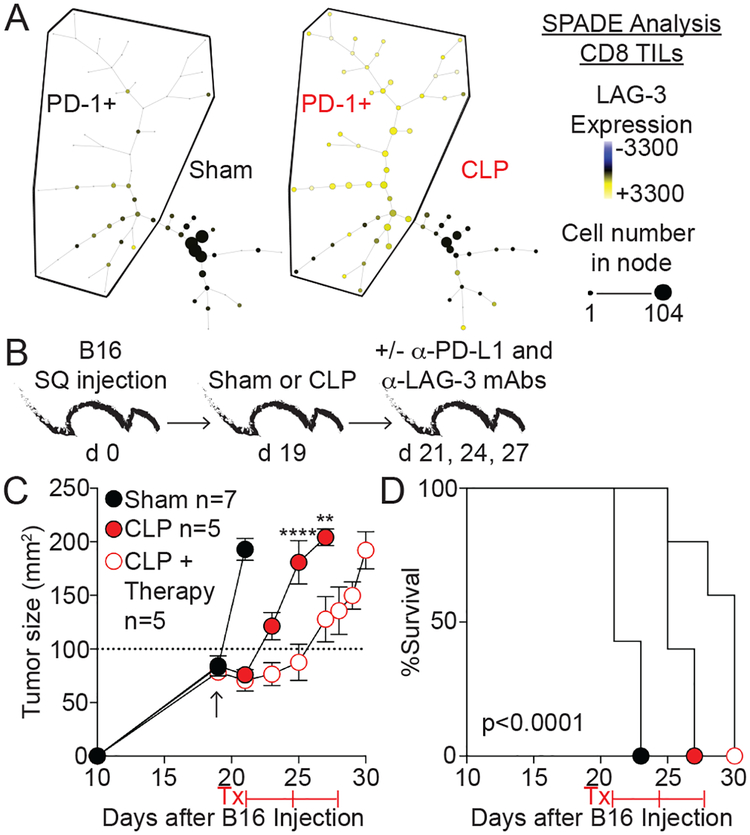
The capacity of sepsis to reinvigorate CD8 TILs indicated an opportunity to provide additional treatment to tumor-bearing hosts that survive sepsis using checkpoint inhibitors. Blockade of PD-1 signaling has benefitted melanoma patients when CD8 TIL infiltration is present (25), and dual blockade strategies provide additional benefit as shown using anti-PD-L1 and LAG-3 mAb in the B16 model (26). Expression of PD-1 and LAG-3 on CD8 TILs harvested after sham or CLP surgery was determined by Spanning-tree Progression Analysis of Density-normalized Events (SPADE) analyses to assess the feasibility of using combinatorial checkpoint blockade therapy to further prolong survival of cancer hosts that survive sepsis. SPADE analysis uses a non-biased approach to analyze flow-cytometric data and separate cells with similar characteristics into nodes. Here, PD-1-expression CD8 TILs are shown in the PD-1+ bubble (Fig 4A). The SPADE trees show the relative expression of LAG-3 in each node (low (blue) to high (yellow) expression), and LAG-3 expression was mainly seen on cells within the PD-1+ bubble. Additionally, the representation of PD-1+LAG-3+ CD8 TILs was dramatically increased after sepsis based on node size (cell number) (Fig 4A). These data indicate reinvigorated CD8 TILs co-express PD-1 and LAG-3 in sepsis survivors and are potentially amenable to therapy. To directly test this hypothesis, B16-bearing mice underwent sham or CLP surgery. After resolution of the acute phase of sepsis (day 2), the mice received anti-PD-L1 and -LAG-3 or control IgG mAb (Fig 4B). Of note, sham mice had to be removed from the experiment at the time of anti-PD-1/LAG-3 mAb treatment due to the size of the tumors. As shown previously, sepsis itself leads to diminished tumor progression and prolonged host survival compared to sham counterparts (Fig 4C–D). Importantly, septic mice receiving checkpoint blockade therapy displayed an even greater reduction in tumor progression and increased survival compared to septic mice that received control IgG. Thus, current cancer immunotherapy strategies could potentially increase the durability of reinvigorated CD8 TILs and further diminish tumor progression in hosts that survive sepsis.
Fig 4. PD-1 and LAG-3 blockade further reduces tumor progression after sepsis.
A) Representative SPADE trees of CD8 TILs 3 days after sham or CLP surgery was performed on mice that received 2×104 B16 cells 17 days earlier. PD-1 expressing cells are shown in the PD-1+ bubble. In an unbiased approach, cells sharing similar characteristics are grouped into nodes. Relative expression of LAG-3 is shown based on node color (Blue-Yellow). Node size indicates the number of cells. B) Mice received 2×104 B16 cells SQ and 19 days later underwent surgery. 2 days later therapy mice received αPD-L1 and αLAG-3 or rat IgG control Ab every 3 days. C) Tumor size (mm2). Arrow indicates time of surgery. D) Survival. Tumor growth and survival curves analyzed using two-way ANOVA and Log-rank (Mantel-Cox) tests, respectively. **p<0.01, ****p<0.0001.
Reports published in the 1800’s document first-hand observations of erysipelas diminishing tumor progression, driving the use of heat-killed bacteria (‘Coley’s toxins’) to treat multiple cancer types (27). Dr. Coley’s original therapy has evolved to modern-day approaches using attenuated L. monocytogenes as a platform to reinvigorate previously-established CD8 TIL responses in advanced cancer patients (28). Reductionist, non-infectious approaches using CpG oligonucleotides as an analog for prokaryotic bacterial DNA have also shown efficacy in diminishing tumor progression by promoting TLR9 responses (29). Considering the current knowledge of ‘hot’ and ‘cold’ tumors based on infiltrating immune cell responses and local cytokine environment, it is not surprising a stimulus that elicits pro-inflammatory cytokine responses can have beneficial effects on previously-established anti-tumor immune responses. What makes the data in this report intriguing is that sepsis – with its ability to lead to global alterations in immune responses, including well-documented detrimental effects on numbers and function of circulatory naïve and memory CD8 T cells – is able to reinvigorate dormant CD8 TILs and improve cancer prognosis. Importantly, recent case reports of intra-abdominal sepsis improving cancer prognosis further substantiate this observation (30, 31). In summary, clinical and experimental data suggest sepsis has the capacity to modify tumor-specific CD8 T cell responses and improve cancer prognosis in patients.
Supplementary Material
Key Points.
CLP-induced polymicrobial sepsis has the capacity to attenuate tumor growth.
Sepsis reinvigorates tumor-infiltrating CD8 T cells.
Checkpoint blockade after sepsis further improves survival of tumor-bearing hosts.
Acknowledgements
Badovinac lab for helpful discussion. Dr. John Harty and Lecia Epping (U Iowa) for reagents.
Footnotes
Supported by NIH Grants GM113961, P30CA08682 (VPB), GM115462 (TSG), T32AI007485 (DBD and IJJ), T32AI007511 (IJJ), The Holden Comprehensive Cancer Center and its NCI Award P30CA086862 (VPB) and a VA Merit Award I01BX001324 (TSG)
The authors declare no competing interests.
References
- 1.Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE, Jernigan JA, Martin GS, Septimus E, Warren DK, Karcz A, Chan C, Menchaca JT, Wang R, Gruber S, Klompas M, and Program CDCPE. 2017. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009–2014. JAMA 318: 1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danai PA, Moss M, Mannino DM, and Martin GS. 2006. The epidemiology of sepsis in patients with malignancy. Chest 129: 1432–1440. [DOI] [PubMed] [Google Scholar]
- 3.Williams MD, Braun LA, Cooper LM, Johnston J, Weiss RV, Qualy RL, and Linde-Zwirble W. 2004. Hospitalized cancer patients with severe sepsis: analysis of incidence, mortality, and associated costs of care. Crit Care 8: R291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torres VB, Azevedo LC, Silva UV, Caruso P, Torelly AP, Silva E, Carvalho FB, Vianna A, Souza PC, Godoy MM, Azevedo JR, Spector N, Bozza FA, Salluh JI, and Soares M. 2015. Sepsis-Associated Outcomes in Critically Ill Patients with Malignancies. Ann Am Thorac Soc 12: 1185–1192. [DOI] [PubMed] [Google Scholar]
- 5.Lyons JD, Chen CW, Liang Z, Zhang W, Chihade DB, Burd EM, Farris AB, Ford ML, and Coopersmith CM. 2018. Murine Pancreatic Cancer Alters T Cell Activation and Apoptosis and Worsens Survival After Cecal Ligation and Puncture. Shock doi: 10.1097/SHK.0000000000001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyons JD, Mittal R, Fay KT, Chen CW, Liang Z, Margoles LM, Burd EM, Farris AB, Ford ML, and Coopersmith CM. 2016. Murine Lung Cancer Increases CD4+ T Cell Apoptosis and Decreases Gut Proliferative Capacity in Sepsis. PLoS One 11: e0149069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox AC, Robertson CM, Belt B, Clark AT, Chang KC, Leathersich AM, Dominguez JA, Perrone EE, Dunne WM, Hotchkiss RS, Buchman TG, Linehan DC, and Coopersmith CM. 2010. Cancer causes increased mortality and is associated with altered apoptosis in murine sepsis. Crit Care Med 38: 886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotchkiss RS, Monneret G, and Payen D. 2013. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 13: 862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otto GP, Sossdorf M, Claus RA, Rodel J, Menge K, Reinhart K, Bauer M, and Riedemann NC. 2011. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit Care 15: R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen IJ, Sjaastad FV, Griffith TS, and Badovinac VP. 2018. Sepsis-Induced T Cell Immunoparalysis: The Ins and Outs of Impaired T Cell Immunity. J Immunol 200: 1543–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Condotta SA, Rai D, James BR, Griffith TS, and Badovinac VP. 2013. Sustained and incomplete recovery of naive CD8+ T cell precursors after sepsis contributes to impaired CD8+ T cell responses to infection. J Immunol 190: 1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duong S, Condotta SA, Rai D, Martin MD, Griffith TS, and Badovinac VP. 2014. Polymicrobial sepsis alters antigen-dependent and -independent memory CD8 T cell functions. J Immunol 192: 3618–3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danahy DB, Anthony SM, Jensen IJ, Hartwig SM, Shan Q, Xue HH, Harty JT, Griffith TS, and Badovinac VP. 2017. Polymicrobial sepsis impairs bystander recruitment of effector cells to infected skin despite optimal sensing and alarming function of skin resident memory CD8 T cells. PLoS Pathog 13: e1006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavaillon JM, and Giamarellos-Bourboulis EJ. 2018. << Immunosuppression >> is Inappropriately Qualifying the Immune Status of Septic and Sirs Patients. Shock doi: 10.1097/SHK.0000000000001266. [DOI] [PubMed] [Google Scholar]
- 15.Strother RK, Danahy DB, Kotov DI, Kucaba TA, Zacharias ZR, Griffith TS, Legge KL, and Badovinac VP. 2016. Polymicrobial Sepsis Diminishes Dendritic Cell Numbers and Function Directly Contributing to Impaired Primary CD8 T Cell Responses In Vivo. J Immunol 197: 4301–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen IJ, Winborn CS, Fosdick MG, Shao P, Tremblay MM, Shan Q, Tripathy SK, Snyder CM, Xue HH, Griffith TS, Houtman JC, and Badovinac VP. 2018. Polymicrobial sepsis influences NK-cell-mediated immunity by diminishing NK-cell-intrinsic receptor-mediated effector responses to viral ligands or infections. PLoS Pathog 14: e1007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danahy DB, Strother RK, Badovinac VP, and Griffith TS. 2016. Clinical and Experimental Sepsis Impairs CD8 T-Cell-Mediated Immunity. Crit Rev Immunol 36: 57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim MT, Richer MJ, Gross BP, Norian LA, Badovinac VP, and Harty JT. 2015. Enhancing Dendritic Cell-based Immunotherapy with IL-2/Monoclonal Antibody Complexes for Control of Established Tumors. J Immunol 195: 4537–4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fridman WH, Zitvogel L, Sautes-Fridman C, and Kroemer G. 2017. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol 14: 717–734. [DOI] [PubMed] [Google Scholar]
- 20.Ahn E, Araki K, Hashimoto M, Li W, Riley JL, Cheung J, Sharpe AH, Freeman GJ, Irving BA, and Ahmed R. 2018. Role of PD-1 during effector CD8 T cell differentiation. Proc Natl Acad Sci U S A 115: 4749–4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horton BL, Williams JB, Cabanov A, Spranger S, and Gajewski TF. 2018. Intratumoral CD8(+) T-cell Apoptosis Is a Major Component of T-cell Dysfunction and Impedes Antitumor Immunity. Cancer Immunol Res 6: 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, Qunaj L, Griffith TS, Vezys V, Barber DL, and Masopust D. 2014. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc 9: 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erkes DA, Smith CJ, Wilski NA, Caldeira-Dantas S, Mohgbeli T, and Snyder CM. 2017. Virus-Specific CD8(+) T Cells Infiltrate Melanoma Lesions and Retain Function Independently of PD-1 Expression. J Immunol 198: 2979–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cabrera-Perez J, Condotta SA, Badovinac VP, and Griffith TS. 2014. Impact of sepsis on CD4 T cell immunity. J Leukoc Biol 96: 767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, and Ribas A. 2014. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515: 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, Tangsombatvisit S, Grosso JF, Netto G, Smeltzer MP, Chaux A, Utz PJ, Workman CJ, Pardoll DM, Korman AJ, Drake CG, and Vignali DA. 2012. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 72: 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarthy EF 2006. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J 26: 154–158. [PMC free article] [PubMed] [Google Scholar]
- 28.Deng W, Lira V, Hudson TE, Lemmens EE, Hanson WG, Flores R, Barajas G, Katibah GE, Desbien AL, Lauer P, Leong ML, Portnoy DA, and Dubensky TW Jr. 2018. Recombinant Listeria promotes tumor rejection by CD8(+) T cell-dependent remodeling of the tumor microenvironment. Proc Natl Acad Sci U S A 115: 8179–8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jahrsdorfer B, and Weiner GJ. 2008. CpG oligodeoxynucleotides as immunotherapy in cancer. Update Cancer Ther 3: 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adashek M, Chan A, and Medina A. 2018. Gastrointestinal Perforation after Rituximab Therapy in Mantle Cell Lymphoma: A Case Report. Case Rep Oncol 11: 784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roelofsen T, Wefers C, Gorris MAJ, Textor JC, Massuger L, de Vries IJM, and van Altena AM. 2018. Spontaneous Regression of Ovarian Carcinoma After Septic Peritonitis; A Unique Case Report. Front Oncol 8: 562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.