Abstract
The circadian system regulates physiology and behavior. Acute challenges to the system, such as those experienced when traveling across time zones, will eventually result in re-synchronization to the local environmental time cues, but this re-synchronization is oftentimes accompanied by adverse short-term consequences. When such challenges are experienced chronically, adaptation may not be achieved, as for example in the case of rotating night shift workers. The transient and chronic disturbance of the circadian system is most frequently referred to as “circadian disruption”, but many other terms have been proposed and used to refer to similar situations. It is now beyond doubt that the circadian system contributes to health and disease, emphasizing the need for clear terminology when describing challenges to the circadian system and their consequences. The goal of this review is to provide an overview of the terms used to describe disruption of the circadian system, discuss proposed quantifications of disruption in experimental and observational settings with a focus on human research, and highlight limitations and challenges of currently available tools. For circadian research to advance as a translational science, clear, operationalizable, and scalable quantifications of circadian disruption are key, as they will enable improved assessment and reproducibility of results, ideally ranging from mechanistic settings, including animal research, to large-scale randomized clinical trials.
Keywords: circadian disruption, circadian misalignment, chronodisruption, chronic disease, circadian desynchrony, circadian desynchronization, phase, amplitude, rhythmicity, chronotype, health, social jetlag, chronobiology
To date, numerous studies have reported on the deleterious health consequences of “circadian disruption”, in many cases referring to circadian misalignment, circadian desynchrony, social jetlag, or chronodisruption. These terms are often used interchangeably, because their definitions are conflicting and vague, but within and between studies, terminology is sometimes also used to describe different phenomena. This review summarizes existing terminology and concepts and gives an overview of currently proposed metrics to quantify circadian disruption. Necessary next steps and future directions will be outlined, to help basic and translational researchers in choosing the appropriate metrics for the question at hand, and finally boost reproducibility, comparability, and robustness of findings across studies.
Studying challenges to the circadian system (see Table 1 Glossary for relevant biological rhythms terminology) is of critical importance due to its ubiquitous and fundamental role in regulating physiology (e.g., Pilorz et al., 2018), and therefore its potential role in disease etiology and progression. The circadian system (with its pacemaker in the suprachiasmatic nucleus, SCN) optimizes the daily timing of biochemical, physiological, and behavioral processes (Albrecht, 2012). The SCN also coordinates the peripheral circadian clocks in various tissues and organs. Interfering with the circadian system by environmental and/or genetic manipulations has been associated with endocrine disruption (Bedrosian et al., 2016), mental disorders (Barnard & Nolan, 2008; Takahashi et al., 2008; Foster et al., 2013; McClung, 2015), metabolic syndrome (Roenneberg et al., 2012; Thaiss et al., 2014; Parsons et al., 2015), neuropsychiatric disorders (Musiek & Holtzman, 2016), and cardiovascular deficits (Portaluppi et al., 2012). It has been hypothesized that these consequences are due to circadian disruption, including misalignment between environmental rhythms and the endogenous circadian rhythms, but also a misalignment or uncoupling between the different components of the circadian system (Martino et al., 2007; Feng et al., 2011).
Table 1.
Glossary of biological rhythms terms relevant to this manuscript.
| Adaptation. Change in a quality of an organism (structure or function, including behavior) that enhances its fitness. |
| Amplitude. The extent by which a sinusoidal oscillation deviates from its mean, defining its maximum (i.e., peak) or minimum (i.e., trough). |
| Biological clock or oscillator. A self-sustained oscillator-system (bridging single cells to organism) that produces rhythmic biological outputs even in the absence of external rhythmic cues, zeitgebers (see definition). Biological clocks exist across species in practically all phyla. |
| Biological rhythm: Rhythmic output of a biological clock or oscillator (see definition). |
| Chronotype. 1) proxy for phase angle of entrainment (see definition), as for example quantified by the mid-point of sleep on free days; 2) diurnal preference; and 3) sometimes subsumed under personality trait. In this review, we will only refer to 1) and 2). Used to described inter-individual differences in phenotypic expressions of circadian-regulated behavioral output. |
| Circadian rhythm. A biological rhythm with a period (see definition) of about 24h (derived from Latin, circa diem) that is generated by a biological clock (see definition) and that persists even in temporal isolation (i.e., in absence of all external time cues). Circadian rhythms are temperature-compensated (meaning that temperature does not change the rhythm’s period) and they can actively entrain (see definition). |
| Circadian system. Networks of circadian clocks within an organism. The mammalian circadian system comprises a central pacemaker (in the suprachiasmatic nucleus, SCN) and the so-called peripheral clocks in most of the cells of tissues and organs. It optimizes the daily timing of biochemical, physiological, and behavioral processes by optimizing their temporal interaction. |
| Diurnal. Active during the daytime, inactive during the nighttime (e.g., humans). Opposed to nocturnal (see definition). |
| Entrainment. Active process of synchronization of an oscillator to a zeitgeber (see definition), Entrainment will result in a stable phase relationship between the oscillator and the zeitgeber |
| Jet Lag. Transient misalignment between an individual’s circadian clock and the local time (e.g., the local light-dark cycle at the destination of a trans-meridian flight). The circadian system will gradually entrain to the local L/D cycle, in humans on average 1 day per hour time change. |
| Masking. An external cue influences rhythmic biological function, but without affecting the circadian oscillator, e.g. enhancing (positive masking) or suppressing (negative masking) effects of environmental light. Birds in a cage are a good example: they will hop from perch to perch under the control of the circadian clock, but will immediately stop if put into darkness. |
| Nocturnal. Active during the nighttime, inactive during the daytime (e.g., mice). Opposed to diurnal (see definition). |
| Period. Duration of one cycle, measured as for example the time elapsed between two peaks or troughs of a rhythm. |
| Phase. Timing of a cycle defined by a reference point such as its minimum or its maximum temperature, dim light melatonin onset, dawn or dusk. |
| Phase angle of entrainment. The difference between the phase (see definition) of a biological rhythm and and that of a zeitgeber (see definition). See also chronotype. |
| Range of entrainment. Range of zeitgeber periods (e.g. LD cycles) that an oscillator can stably entrain to. The experimental protocol of a ‘forced desynchrony’ specifically uses experimental light-dark cycles that are outside of the range of entrainment to distinguish investigate circadian and homeostatic regulations separately. |
| Zeitgeber. A rhythmic signal that circadian clocks use to actively synchronize (entrain) with the cyclic environment (normally 24 h). The light/dark (LD) cycle is the most important zeitgeber for circadian clock. |
Modern lifestyles increasingly deprive us of natural zeitgebers (dt. time-givers), and may alter inter-relationships between zeitgebers, such as the alternation between light and dark or warm and cold. We spend most of our time indoors, have drastically reduced daytime light exposure, and experience artificial light at night, together resulting in a weakened light/dark signal. Circadian clocks have to be synchronized (entrained) with their 24-h environment, because the intrinsic circadian period is on average slightly longer than 24h by about 11 min (Czeisler et al., 1999). The light/dark cycle is considered the most important zeitgeber for the human circadian system (Roenneberg et al., 2003a; Duffy & Wright, 2005). Depending on an individual’s endogenous circadian period and their light environment, synchronization to the 24h light/dark cycle can occur within a range of different phases of entrainment. This variability in phase of entrainment can also be approximated by inter-individual differences in sleep timing (also called chronotype) (Roenneberg et al., 2007a; Phillips et al., 2010; Fischer et al., 2017). It is noteworthy, however, that sleep timing is a behavioral, systemic output; while it is regulated by the circadian system, it is also influenced heavily by the homeostatic drive for sleep, as well as work and social constraints on when sleep can occur.
Electricity and associated constant accessibility to light, energy, and food, as well as 24/7 work schedules and travel across time zones have created an environment that is fundamentally different from the one 200 years ago. While technological and lifestyle changes have positive effects on daily life, it has been proposed that those changes in accessibility may significantly contribute to the increasing prevalence of so-called lifestyle diseases, such as metabolic and sleep disorders as well as psychiatric illnesses (Foster et al., 2013; Depner et al., 2014; Anothaisintawee et al., 2016; Broussard & Van Cauter, 2016; Potter et al., 2016; Smolensky et al., 2016; Sulli et al., 2018).
In this review, I will focus on circadian disruption as a modifiable risk factor, and omit in-depth discussion of genetic and lesion models of circadian disruption, which have been discussed in more detail elsewhere (e.g., Evans & Davidson, 2013). The first part of this review will summarize definitions and terminology most commonly associated with circadian disruption, namely circadian disruption itself, circadian misalignment, circadian desynchrony, circadian desynchronization, chronodisruption, and social jetlag (see Figure 1).
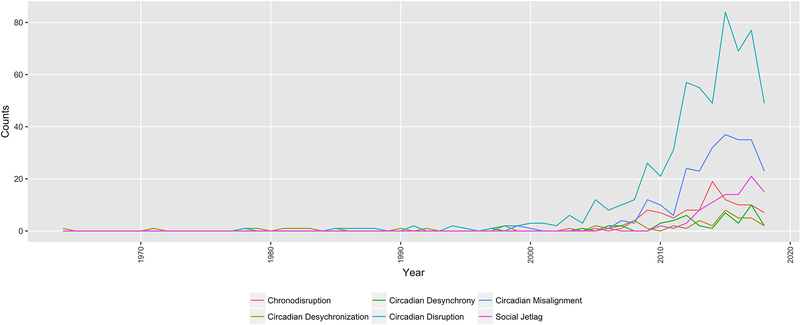
Figure 1. Forty years of circadian disruption terminology on Pubmed (https://www.ncbi.nlm.nih.gov/pubmed/; 1964–2018).
The search was conducted on July 5th 2018 and used the respective terms in quotation marks (i.e., “circadian disruption”). The absolute frequency is plotted across years.
The term circadian disruption has been used since the 1980s (de Castro et al., 1978; Farr et al., 1985), but gained popularity after it was used by Stevens and Rea in 2001 when they proposed a link between light at night, endocrine disruption, and breast cancer risk. It has since then been used extensively, but its definition remains nebulous. According to Rüger and Scheer (2009) circadian disruption can result from i) circadian misalignment due to external factors (e.g., shift work, jet lag) or internal ones (e.g., blindness); or ii) circadian dysfunction resulting from damage to the SCN caused by disease (e.g., tumor, lesion or disease) or deleterious genetic variations. While this typology is helpful, it concerns more the scenarios that potentially cause circadian disruption more than defining the phenomenon itself or providing guidance on how to quantify it. Qian and Scheer (2016) state that “(…) circadian disruption is a disturbance of biological timing, which can occur at different organizational levels and/or between different organizational levels, ranging from molecular rhythms in individual cells to misalignment of behavioral cycles with environmental changes”. This extends the Rüger and Scheer classification by differentiating between systemic, organismal, and cellular levels of misalignment. The cause of disruption, though, can be external or internal to the system. Qian and Scheer (2016) define environmental misalignment as occurring when an environmental signal, such as the light/dark cycle, is misaligned with the endogenous SCN phase. Behavioral misalignment is defined as occurring when behavioral cycles, such as the feeding/fasting cycle, or sleep and wake, are misaligned with the endogenous SCN phase. It is noteworthy though that we currently have little knowledge of the inter-relationships between behavioral and environmental signals, and their phase coherence (i.e. the extent to which their phase-relationship is fixed). On the organismal level, disruption is described as an internal misalignment or internal desynchrony (Qian & Scheer, 2016), where the rhythms between the central pacemaker and the peripheral clocks are abnormally aligned or running with different periods from one another, respectively. As an example, Wehrens et al. (2017) have recently reported that it is possible to decouple glucose rhythms from the timing of dim light melatonin onset (as a central phase marker) by shifting meal timing in laboratory settings, suggesting that the importance of measuring several environmental factors, as well as central and peripheral read-outs to correctly assess misalignment. Roenneberg and Merrow (2016) have proposed a similar idea, and have mapped circadian clock components and their possible states of synchronization across system levels, ranging from optimal entrainment to arrhythmicity. Finally, at the tissue and molecular level, desynchronization between cells is also referred to as circadian disruption (Qian & Scheer, 2016).
Circadian misalignment (Baron & Reid, 2014), circadian desynchrony (Sack & Lewy, 1997), and desynchronization (Aschoff, 1965) are widely used terms that can be considered specific types of circadian disruption, each of which can occur at different biological scales from cellular, tissue, and organismal level to the systemic one. Desynchrony and desynchronization are used interchangeably to refer to differing periods between two (or more) rhythms, whereas misalignment describes an abnormal phase angle between two (or more) rhythms. The two rhythms may be both internal (see internal desynchrony above, central vs. peripheral rhythms), or one may be internal and the other external (e.g., central vs. light/dark or peripheral vs. feeding/fasting). Both concepts are for example quantifiable by phase angle differences and a comparison of the rhythms’ period estimates. In 1965, Aschoff, for example, used the term desynchronization to describe the relationship between rhythms in core body temperature and sleep/wake, with periods of 24.7 and 32.6h.
Chronodisruption has been used in the literature in generic reference to a wide variety of settings and experimental protocols (e.g., Galdames et al., 2014; Froy & Garaulet, 2018; Madrid-Navarro et al., 2018). In this usage, it can be considered synonymous with circadian disruption. Chronodisruption has also been used to refer to a specific quantitative metric, which is based on computing the overlap between an individual’s biological night (approximated by sleep timing information) and work hours (i.e., work timing, including “associated activity windows” of usually 2h prior to work start and after work end, Erren & Reiter, 2009; Erren & Reiter, 2013; Erren & Morfeld, 2014). In this review, the term chronodisruption is reserved for its specific quantitative definition, which is reviewed below.
Social jetlag (Wittmann et al., 2006; Roenneberg et al., 2012) is often used to generally refer to the challenge to the circadian system humans experience in their everyday life, especially due to work and social constraints. However, like chronodisruption, it has a specific quantitative definition: social jetlag is the difference between sleep timing on work and free days, and represents a proxy for circadian misalignment. As noted above, sleep timing is not a pure circadian output, and on the other hand, circadian rhythms cannot be inferred from diurnal ones obtained in entrained conditions (i.e., outside of the lab), due to masking by behavioral and environmental factors (Aschoff et al., 1965; Aschoff et al., 1982; Rietveld et al., 1993; Mrosovsky, 1999). While social jetlag as originally proposed focused on sleep timing, there have been initial efforts to extend the approach to other behaviors relevant to the circadian system, such as meal timing (Gill & Panda, 2015). Social jetlag also is, like chronodisruption, a system-level metric, that does not allow for multi-level insights, potentially missing relevant organ-, tissue- or cellular-levels of misalignment.
So, what do we mean by circadian disruption?
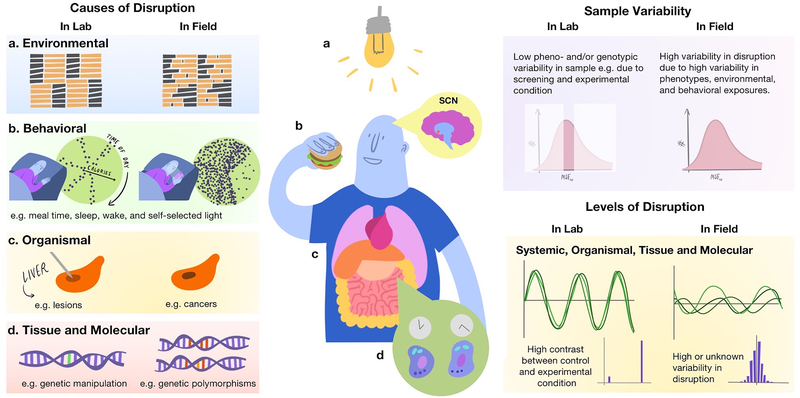
Taken together, circadian disruption seems to serve as an umbrella term for most of the other commonly used terms. Circadian disruption also is oftentimes considered the consequence of a temporal challenge or other perturbations, albeit rarely explicitly quantified. One reason for this may be that experiments examining the link between circadian disruption and physiology and behavior were done in the laboratory: laboratory settings are highly controlled and maximize the contrast between control and experimental condition. In this type of setting, the experimental manipulation (such as the inversion of a light/dark cycle) will equal the level of circadian disruption that is induced, especially in animals with identical genetic background. In field settings, if disruption is considered a consequence of the challenge the organism is exposed to, one would predict that the magnitude of disruption is a function that challenge, as well as the state of the organism (see Figure 2). This point will be critical for future considerations how to ideally quantify circadian disruption.
Figure 2. Circadian Disruption in laboratory and field settings.
The left panel displays potential causes of disruption and how they might differ between laboratory and field settings. On the upper part of the right panel, sample variability of the study population is illustrated. While laboratory studies usually target specific populations in a smaller number of individuals, field studies include larger and more heterogeneous samples. Both approaches are valid. Laboratory studies minimize noise in the signal by selecting homogenous samples, and thereby can detect even small effects. However, generalizability is limited. The resulting levels of disruption (lower right panel) should this differ between laboratory and field settings, with high contrast conditions and little inter-individual variability in laboratory settings, and higher variability in field ones. In view of our improved understanding of circadian organization, including the relevance of peripheral clocks, future work is needed to harmonize conceptual approaches and operationalization of circadian disruption in the context of mechanistic, observational and intervention studies. Figure credit: Olivia Walch, PhD, twitter: @oliviawalch).
The potpourri of terms for circadian disruption is useful, too, as it illustrates different viewpoints one can take. However, existing terms either lack generalizability, or do not yield specific and testable predictors for the involvement of the circadian system in disease etiology, across model systems and methodologies. The first part of this review clearly suggests that we will need not just one, but a set of metrics to systematically monitor and assess the magnitude of circadian disruption associated with challenges to the circadian system and its downstream consequences.
At this point, it is critical to note that the circadian system is inherently adaptive. Changes and alterations in circadian rhythms are not necessarily negative, as often suggested by circadian disruption terminology, but those transient changes can indicate and/or promote the attainment of a stable state of entrainment. Indeed, mice re-entrained faster, when intercellular synchrony in the SCN was disturbed, suggesting that transient phase alterations can also be beneficial (An et al., 2013). Using real-time bioluminescence in flies, Roberts et al. (2015) showed that after a brief desynchronization of the neurons, cells returned with stronger synchrony, further underscoring the importance of identifying the relevant cut-offs of circadian disruption. Understanding the relationship between alterations, adaptation, plasticity, flexibility and disruption, and building dose-response-relationships predictive of adaptation vs. disruption are critical next steps in the research agenda of how to advance translational chronobiology. Furthermore, environmental challenges, that are not necessarily zeitgebers, can also impact and alter circadian organization. Recent work by Dyar et al. (2018) suggests for example that a high fat diet, can modify circadian wiring and cohesion between cells and across tissues. We will need further work to identify how such environmental challenges exacerbate and interact with other zeitgebers, behavioral, and environmental signals, and affect system-wide (re-)synchronization.
Another important aspect that deserves attention is the use of the word circadian. Its formal usage refers to an endogenously sustained, about 24h rhythm generated by the circadian system (Dunlap & Loros, 2004). Rhythms observed under entrained conditions, outside of the laboratory, are oftentimes driven by behavioral and environmental rhythms, and therefore cannot be referred to as circadian (e.g., Broussard et al., 2017). Thus, terminology and metrics such as social jetlag and chronodisruption, which are entirely based on behaviors and environmental information, should be thought of as proxies for circadian disruption. Translational circadian research requires explicit and transparent quantification of circadian disruption, with an explicit specification of the level of description (and its limitation). Quantifying the extent of circadian disruption, and potential effects of interventions mitigating its effects on physiology and behavior will generate the necessary data to establish evidence-based classifications of circadian disruption as a disease risk factor. The next section gives an overview of currently available metrics, experimental protocols, and exposures that are used to probe the effects of circadian disruption on health.
Metrics and Protocols
Experimental human studies.
In human laboratory studies, several protocols allow the assessment of short-term circadian disruption on a variety of outcomes, including physiological function, performance, and mood. Circadian misalignment protocols include forced desynchrony protocols, where individuals are scheduled to daylengths outside of their range of entrainment (e.g., 20 or 28h days), while under dim light conditions, so that their behaviors such as sleep/wake and food intake will occur at every circadian phase (Czeisler et al., 1999; Broussard et al., 2017). This enables not only the computation of key circadian parameters, such as circadian period, phase or amplitude, but also to examine the effects of the misalignment between circadian rhythms (e.g., in melatonin) and behavioral cycles on physiology. Such protocols have, for example, shown that circadian misalignment will impair insulin sensitivity and can induce a pre-diabetic state in healthy participants (Scheer et al., 2009). Typically, such experiments report results for the most pronounced contrast (e.g., when being awake and eating during the biological day vs. biological night), which would be equivalent to a report of maximal or peak exposure in epidemiological studies. Inverted sleep schedules, simulated night shifts protocols, and circadian misalignment protocols (Archer & Oster, 2015; Qian & Scheer, 2016) do not vary phase relationships by regular assessments across all circadian phases (i.e., they keep the exposures fixed) and thus also compare the maximal difference between aligned/misaligned or day vs. nighttime sleep/work. Those protocols are indispensable to probe the physiological consequences of circadian disruption. Kervezee and colleagues (2018) recently showed that on a molecular level, simulated night shift work reduces the amplitude of peripheral RNA transcripts, while phase read-outs remain similar to habitual bedtimes (and therefore result in a misalignment with the shifted sleep/wake cycle). This study illustrates well the need for a comprehensive assessment of rhythmicity, including amplitude, when considering the consequences of strain to the circadian system. Finally, with regards to caloric intake, forced desynchrony, circadian misalignment, and simulated night shift protocols usually serve participants breakfast, lunch, dinner and snacks. Including those meal patterns arguably increases ecological validity, although meal patterns in real life are probably more erratic (Gill & Panda, 2015; Kant, 2018; Park et al., 2018). Skene et al. (2018) re-enforce the importance of studying circadian disruption across system levels, as they observed that while traditional markers of SCN phase (such as rhythms in melatonin or PER3 expression) hardly shifted during simulated night-shift work, many of the circulating plasma metabolites dissociated from the SCN rhythm and aligned with the shifted behavioral cycles of feeding/fasting and sleep/wake. This disruption in the circadian organization might represent a pathway through which shift work is associated with metabolic disease.
Experimental non-human studies.
Overall, most human laboratory studies examine the effects of misalignment by shifting sleep/wake, and meal cycles. In animals, however, the primary method to examine the effects of circadian disruption on physiology and behavior is manipulation of the light/dark cycle (Evans & Davidson, 2013). In their review on the consequences of circadian disruption on metabolic outcomes, Arble et al. (Arble et al., 2015) describe widely used jetlag, shift work, and non-24h models in animal research. In jetlag models, animals are exposed to a single or repeated shift in the environmental light/dark cycle (i.e., 2–10h); the directionality of the shift can be varied to study differential effects of phase delays or advances. Depending on the magnitude of the shift, which represents the challenge to the circadian system, adaptation can take up to several days. Yamazaki et al. (Yamazaki et al., 2000) showed that the SCN entrains faster to abrupt changes in the light/dark cycle as compared to locomotor rhythms or circadian rhythms in peripherical organs, such as the liver (see also Yamaguchi et al., 2013). Results were reported by plotting the rate of re-entrainment across tissues while showing the initial and the shifted light/dark cycle. Subsequent work using the same types of protocols showed that re-entrainment (also sometimes referred to as re-setting) of the liver is severely disrupted in older mice as compared to younger ones (Davidson et al., 2008), and that chronic phase shifts increase the risk of mortality in mice (Davidson et al., 2006). In experimental animal research, shift work is typically mimicked by inverting the light/dark cycle. Similar to human laboratory protocols, the key contrast that will be used to examine the association between circadian disruption and potential outcomes is “day” vs. “night” shift. A recent study in breast-cancer prone mutant mice showed that this inversion will not only result in disturbed sleep, but also reduce tumor suppression (Van Dycke et al., 2015), which is in line with the modestly increased breast cancer risk epidemiological studies have reported when comparing day-working women to those with a history of rotating night shift work, especially when shift work occurred pre-menopause (Lin et al., 2015; Cordina-Duverger et al., 2018). To increase comparability to the human shift work population, efforts have been made to also induce physical activity, while keeping light/dark cycles constant, for example by keeping nocturnal animals in slowly moving running wheels during the daytime (i.e., their inactive phase). This by itself is sufficient to shift activity and feeding cycles in rats (Arble et al., 2010). If the goal is to further increase ecological validity, and thus quantify exposure landscapes in field conditions more realistically, it will be useful to consider the extent to which changes in light/dark cycles, feeding/fasting cycles, sleep/wake cycles, as well as rest/activity cycles are correlated in humans, with more variability to be expected the less restrictive the work schedule (e.g. 8 vs. 12h shifts in humans). Variability in those behavioral and environmental rhythms are highly controlled in laboratory settings (see Fig. 2, “Causes of Disruption), a necessary step to isolate effects in experimental protocols. For translational work to gain a better understanding of causes and their effect (as well as their effect size), further work examining the independent and combined effects of those usually co-occurring behaviors on behavioral and physiological variables is warranted. So-called T7 or T20 cycles are commonly used examples of non-24h models in animal laboratory setting, and can be considered equivalents of the human forced desynchrony protocols. Using a T7 cycle (3.5h of light, 3.5h of darkness), where mice were exposed to light at all circadian phases, Altimus et al. (2008) reported no effect on sleep, nor circadian arrhythmicity or changes in phase, albeit circadian period appeared slightly lengthened (LeGates et al., 2012). In this setup, circadian rhythmicity was assessed by quantifying body temperature and activity rhythms, as well as by tracking phase changes in liver and SCN clock gene expression. Le Gates et al. (2012) showed that this T7 light exposure cycle impairs learning and mood – we now know that this association is linked to ipRGCs, with SCN-projections mediating the effects of light on learning (but independent of SCN function), while associations of light with mood appear to be mediated by thalamic pathways independent of the SCN (Fernandez et al., 2018). T20 cycles (10h of light alternating with 10h of darkness) have been shown to accelerate weight gain in mice, and also lead to loss of dendritic length and decreased complexity of neurons in the prefrontal cortex. Consistent with those changes the neural architecture in an area that is important for e.g. executive function, mice displayed reduced cognitive flexibility (Karatsoreos et al., 2011).
Observational, Field, and Ecological Studies.
Human circadian protocols are high cost, and therefore are typically conducted in a limited number of highly-screened participants. Screening criteria not only select healthy participants, but also typically restrict the study population to a selected range of sleep and circadian phenotypes. While this is useful and important to study basic mechanisms of circadian and sleep regulation and increase statistical power by reducing inter-individual variability, it introduces a fundamental disconnect between observational vs. experimental studies. As laid out above, experimental studies compare conditions (e.g. day vs. night; aligned vs. misalignment, see Fig. 2, “Causes of Disruption” in the lab), and this comparison is assumed to directly reflect the magnitude of disruption induced by a given protocol (see Fig. 2, “Levels of Disruption”). The implicit assumption here is that the degree of disruption on the circadian system is equal amongst study participants. Variability between participants is often kept to a minimum in human laboratory studies, mostly by following screening protocols and rigorous exclusion criteria. As in animal research, the goal of this procedure is to eliminate potential confounders, minimize variability, and thereby to maximize effectiveness of those often cost-intense protocols to test a given hypothesis. Such designs can also be necessary, when the hypothesized effects are small. However, this assumption, that the resulting level of circadian disruption will be equal amongst participants, cannot necessarily be made. Field studies usually include a wider range of geno- and phenotypes in the population (see Fig. 2, “Sample Variability”). From that, if follows that when studying the effect of an environmental challenge (such as shift work or irregular light/dark cycles, see “Causes of Disruption) on the circadian system, individual sample characteristics should be considered, as they may modify, exacerbate, or attenuate the ultimate level of disruption. In Figure 2, the example illustrates the distribution of mid-sleep on free days, corrected for sleep debt accrued during the work week [x-axis, MSFsc], and it is noteworthy that this distribution has several hours differences between the extremes in the general, day-working population (Roenneberg et al., 2007a; Roenneberg et al., 2012; Fischer et al., 2017). The hypothesis inherent to that working model is that the magnitude of disruption in a field or population-based study will depend on both: the environmental challenge and the phenotype. In case where the population of interest all had the same phenotype, the predicted level of disruption would be equivalent to the challenge to which they are exposed. However, in cases where there is variability in both the challenge and phenotype, a distribution in the level of disruption will result (see inset, Fig. 2, “Levels of Disruption” in the field). We have some indication that this working model is useful. For example, it has been shown that the effects of shift work on sleep duration, timing, and quality depend on both the type of shift (i.e., morning, evening, and night shift, or the environmental challenge) and the individual circadian phenotype, or chronotype (Juda et al., 2013a). Social jetlag, a proxy for circadian misalignment shows a slightly-skewed distribution in a day working population with a wide range of circadian and sleep phenotypes (Roenneberg et al., 2012), indicating that the same challenge can result in differing levels of circadian disruption depending on the individual phenotype. The next section will outline those most frequently used exposure metrics for circadian disruption in observational and intervention field studies.
Chronotype.
Chronotype has been defined by Roenneberg as the individual phase of entrainment, i.e., the phase at which an individual synchronizes to the 24h day (Roenneberg, 2012). Measuring the timing of melatonin rhythms is accepted as the key marker of human individual circadian phase (Arendt, 2006; Benloucif et al., 2008). Masking is minimized by collecting repeated plasma or saliva samples in dim light conditions – other physiological read-outs, such as core body temperature can also be used to estimate circadian phase, but are more sensitive to masking by additional factors such as physical activity or sleep (Lewy et al., 1999) as compared to the melatonin rhythm. This gold standard measure of circadian phase, though, can be costly and cumbersome to assess. An alternative, cost-efficient, and non-invasive approach consists in approximating circadian phase by assessing sleep timing on free days (i.e., when constraints on sleep timing are low). Sleep timing is however only in part regulated by the circadian system, so that this assessment does not reflect a “pure” clock output (Borbely et al., 2016). Sleep timing can be queried by sleep logs or questionnaires, such as the Munich ChronoType Questionnaire (MCTQ, Roenneberg et al., 2003b), from which one can extract mid-sleep on free days (which is then corrected for sleep debt accumulated during the work week, MSFsc). This mid-sleep variable correlates with dim light melatonin onset (DLMO, r=0.4–0.8), a gold-standard circadian marker (Burgess et al., 2003; Kitamura et al., 2014; Kantermann et al., 2015). Other questionnaire-based approaches comprise an assessment of diurnal preference (Horne & Ostberg, 1976; Adan & Almirall, 1991; Laborde et al., 2018); a comprehensive overview of the existing instruments can be found here (Levandovski et al., 2013; Putilov, 2017). Chronotype is sometimes used as a proxy for circadian disruption (e.g., Merikanto et al., 2013; Knutson & von Schantz, 2018), with the assumption that later types would be more misaligned due to daytime social commitments and activities than earlier types. This is valid, if it can be ascertained that the environmental challenges are equal across chronotypes. However, in most observational, field, population, and intervention studies, this assumption is unlikely to hold true. For example, work and school start times in the day working population (about 60% of the US population between 15 and 65 years, Bureau of Labor Statistics, 2018) are variable. The measurement error introduced by using chronotype as a proxy for disruption will be exacerbated by any shift workers in the study sample, as it will further increase variability of the environmental challenge (Roenneberg et al., 2012; Juda et al., 2013a). In the large, observational Nurses’ Health Study II, chronotype overall was not associated with type 2 diabetes (T2D, Vetter et al., 2015a). However, an interaction between chronotype and lifetime duration of night shift work exposure was observed, suggesting that early types were at higher odds of T2D as compared to intermediate ones, when women worked rotating night shifts for a longer duration. The inverse was true for late types: compared to intermediate types, late chronotypes were most likely to have T2D when they did not work rotating night shifts. It remains to be clarified whether this is due to more frequent early morning shifts exposure, and further replication studies are needed in prospective settings (Vetter et al., 2018). This modeling approach was feasible because of the large sample size of the size of this study; a composite metric capturing this interaction would, however, provide more statistical power.
Social jetlag.
Social jetlag was coined by Roenneberg and colleagues in 2006 (Wittmann et al., 2006) to describe the mismatch between internal and external time. To do so, they proposed assessments of sleep timing on work-free days and days that are constrained by work, school or other social obligations. This assessment can be performed via questionnaires such as the MCTQ (Roenneberg et al., 2003b), or its shift-work version (Juda et al., 2013b), but the same information could also be extracted from sleep logs or actigraphy data if daily work schedule information is gathered in addition to sleep behavior. Because sleep timing is in part regulated by the circadian system (Daan et al., 1984; Borbely et al., 2016), the idea is that sleep can be used as a scalable, behavioral read-out to approximate inter-individual differences in circadian phase, subject to the caveat noted above (i.e., sleep is not a pure circadian output). Sleep on work-free days is considered to reflect the natural sleep cycle, and thereby serves as a proxy for internal time, while workday sleep is thought to be more reflective of external, social time, due to its constrained nature. The difference between workday and free day sleep timing is used to compute social jetlag. Again, if societal constraints are identical across individuals, results will reflect inter-individual differences in sleep timing (or chronotype). In case of variable work and school start times, however, this metric incorporates potential variability in both, exposure and phenotype level, and provides a single-metric estimation of the individual level of disruption on a systemic level. In cross-sectional studies, social jetlag has been shown to be associated with body mass index (Roenneberg et al., 2012; Parsons et al., 2015), and biomarkers of cardiometabolic health (Rutters et al., 2014; Wong et al., 2015), but prospective studies are currently missing. While useful, this metric also has limitations: 1) it solely captures system-level output, and we have no insights into how social jetlag relates to internal phase relationships or molecular-level rhythms; 2) in individuals with sleep disorders, or otherwise disrupted sleep behavior, this metric is likely to be flawed (Suh et al., 2017); 3) as it is an aggregate measure, it omits potential informative time series information, such as variation within free days or within work days; and finally 4) while social jetlag approximates current levels of circadian disruption, and thereby is useful to track the effects of for example work schedule or lifestyle interventions (e.g., Vetter et al., 2015b), we currently have no formal way to estimate cumulative, lifetime exposure. This is an important challenge that will need to be addressed, as such a metric is crucial to identify biomarkers of chronic circadian disruption (Mullington et al., 2016). Changes in circadian phase over the lifespan (Roenneberg et al., 2004; Crowley et al., 2014), as well as changes in work schedules and other environmental challenges over time, contribute to the difficulty of estimating a cumulative lifetime exposure. In 2013, Erren and Morfeld (Erren & Morfeld, 2014) proposed an approach to compute a cumulative chronodisruption metric, in addition to quantifying acute chronodisruption.
Chronodisruption.
Chronodisruption is computed based on individual sleep timing, assuming an 8h sleep duration, and contrasting this information with work hours timing. Erren and Morfeld (Erren & Morfeld, 2014) expand the contrast by adding “shift-related activities” 2h prior work start time and subsequent to work end to account for activities such as time spent commuting. Broadly, the overlap between sleep timing and work hours is used to quantify chronodisruption. While very clearly defined, the metric has not been applied yet in its suggested form, as the extent of required information is usually not available, especially in existing datasets that are used to study the health risks associated with shift work. In an Australian case-control study, authors classified participants as being exposed to chronodisruption, either as a function of shift schedule information only, or while considering chronotype in their classification, and reported no added value of considering chronotype in this breast cancer study (Fritschi et al., 2018). The authors highlight the difficulty of obtaining the relevant information to compute the metric as proposed. Another limitation of this metric is its generalizability, as the conceptual framework and its operationalization are focused on occupational health and shift work research.
Behavioral entrainment or Phasor Analysis.
This metric was proposed in 2008 by Rea et al. (Rea et al., 2008; Miller et al., 2010). It uses circular cross-correlation functions to examine the correspondence between light exposure (as measured by the Daysimeter, Bierman et al., 2005) and activity patterns. The two time-varying signals are multiplied and integrated into a single value to determine the covariation of the two signals, resulting in a cross-correlation coefficient between −1 and 1. Using Fourier decomposition and spectral analysis of the behavioral entrainment correlation functions, Rea et al. (Rea et al., 2008) obtain an average phasor across seven days of recordings in day- and shift working nurses. Phasor length or magnitude represents how well light and activity correlate over time. As expected, nurses working rotating shifts showed shorter phasor length as compared to day-working nurses, suggesting a mismatch between physical activity patterns and the light/dark signal. Thereby, the metric provides a powerful tool to quantify the relationship between a continuous, environmental signal (such as the light/dark cycle) and a behavioral phenotype (in this case, physical activity). The angular direction of the phasor indicates the offset between light and activity patterns. As expected in diurnal species, a perfect alignment would correspond to 0; negative values would indicate activity occurring prior to light onset, while positive values would indicate activity after light onset. Rea and colleagues thereby also provide an alternative approach to quantify chronotype (late types would be expected to have an activity onset subsequent to lights on). As in the case of the other behavioral and environmental measures, no circadian measure is part of this metric, so that it can provide an approximation but not a direct measure of circadian disruption. Another noteworthy limitation of this approach is that humans can self-select their individual light exposure levels, so that the usage of blinds and dark shades can alter the angle between lights/activity onset. For example, one would predict that extremely dark bedrooms precluding natural light exposure will result in activity patterns preceding light onset (e.g., Skeldon et al., 2017; Swaminathan et al., 2017), while this is likely to result in a late circadian phenotype, and even exacerbate it. The authors also compute the same metric for rats that underwent a jetlag protocol and compared them to non-jetlagged controls, finding reduced phasor length, and altered phasor angles for the rats that underwent the jetlag protocol. This demonstrates the utility of their metrics across model organisms, which they stress is essential to identify acute and long-term effects of circadian disruption (Rea et al., 2008). Less anthropocentric approaches are indeed necessary to capture and understand the system-wide consequences of circadian disruption, and continuous metrics allow for better-powered studies by enabling examinations of dose-response relationships between the circadian disruption and health outcomes. This metric, along the ones described further below, also enriches computational possibilities, as its continuous nature allows more flexibility in modeling approaches. Another advantage of this metric is its integration of information across the entire time series. Conversely, it is limited in its application to longitudinal recordings, which can be challenging, especially because accurate and scalable (i.e., cost-effective, robust, low participants burden, and commercially available in large quantities) light measurement devices are currently scarce (Price et al., 2017). Overall, this approach is promising, but has not been extensively been used. While Rea et al. (2008) compared mean phasor magnitudes with other measures of entrainment, the extent of its prediction power regarding health outcomes and/or physiological and molecular function remains to be elucidated.
Composite Phase Deviations.
Fischer et al. (2016) proposed this novel metric to quantify circadian misalignment; composite phase deviations can be derived from multiple sources, such as questionnaires, sleep logs, continuous recordings of activity, or even physiological read-outs, such as body temperature or melatonin. Similar to social jetlag, it uses a single source of information, and does not integrate across environmental and behavioral measures as Behavioral Entrainment does. Unlike social jetlag, however, it provides an integration of intra-individual variability across longitudinal data. Specifically, the composite phase deviation metric quantifies the distance between mid-sleep on a given day and the designated reference (such as chronotype; but note that the authors propose that dynamic references could further improve the measurement), as well as distance between mid-sleep on a given day and mid-sleep on the day before. The vector length that is obtained as a function of those two variables is used as the metric’s indicator. Time series data can then be summarized by the sum, the average, or the maximum vector. As one would expect, shift workers in rotating schedules have the highest levels of deviations, and adapting work hours to individual sleep timing reduced the level of composite phase deviations in this population (Fischer et al., 2016). It is currently unclear how this metric relates to physiological markers of the circadian system and long-term health effects, warranting future studies.
Inter-day stability (IS) and intra-day variability (IV).
Similar to the CPD metric, these two statistics are computed solely based on activity counts (Witting et al., 1990; van Someren et al., 1996). They were proposed in the early 1990s and have since then been used as measures of circadian disruption. The IS estimates how stable the rest-activity cycle is across the time period of study, with higher IS indicating higher stability, while IV estimates the fragmentation of the 24h rest-activity cycle, so that disrupted rest-activity patterns will obtain a higher IV score. Zuurbier et al. (2015) have demonstrated that higher fragmentation and instability of activity patterns, as measured by IV and IS, are prospectively associated with increased risk of mortality in an elderly population in nursing homes. Aging is known to be associated with damped clock rhythmicity, as evidenced in many behavioral and physiological read-outs, such as physical activity, body temperature or melatonin rhythms (Duffy et al., 2015; Hood & Amir, 2017). Reduced zeitgeber strength (such as reduced daytime light exposure), reduced clock input attributed to lens yellowing in the retina, and decreased neuronal signaling in the SCN, are thought to contribute to this phenomenon. In a recent cross-sectional study, it was shown that neither IV nor IS were associated with body mass index (BMI), while low relative amplitude of the rest-activity rhythm was linked to higher BMI (Cespedes Feliciano et al., 2017). IV and IS presumably capture both variability in exposure and the phenotypical variability. The findings cited above also illustrate the dire need for comprehensive characterization of rhythmicity in future studies, including amplitude.
Comparison of Behavioral Entrainment, Composite Phase Deviation, and IS/IV metrics.
Two major differences between the phasor analysis, composite phase deviations, and IS/IV metrics are noteworthy. Rea et al. (2008) showed that both IS and phasor metrics are useful in separating day vs. rotating night shift nurses, and Fischer et al. (2016) indicate that composite phase deviations correlate with both phasor metrics (r = −0.48) and IS (r = −0.56). More work is warranted to systematically compare and evaluate the relative contribution, overlap, and redundancy of these metrics, especially in the context of health and disease. In addition, because circadian disruption usually co-occurs with disturbed sleep, and because several metrics use sleep behavior as a read-out and approximation of circadian disruption, it will be necessary to also consider the contribution of other sleep-centered metrics (Bei, Wiley, Trinder, & Manber, 2016), such as the Sleep Regularity Index (Phillips et al., 2017).
Shift Work.
The most common observational proxy for circadian disruption in human studies is shift work, and more specifically night shift work and rotational schedules involving night work. In general, humans are day active, so that working during the biological night is considered the maximal challenge to the circadian system. However, when individuals report on their night shift work history, assessments also implicitly capture multiple factors that are usually used independently as exposures in animal studies. Working during the night is correlated with light exposure during the night, nighttime eating and caloric intake, being awake and active at times when we usually sleep, daytime sleep (which in turn reduces sleep quality and reduces sleep duration (Åkerstedt, 2003), especially in individuals with an early chronotype (Juda et al., 2013a), as well as consuming stimulants at night, such as cigarettes and caffeine. While night shifts represent the most strenuous shift for most individuals, early morning shifts (i.e., shifts starting before or at 6am) can also be a burden: depending on commute and start times, individuals sometimes have to awaken as early as 3:30am to be at work at 6:00am. Such early wake up times end the sleep phase for the vast majority of individuals during their biological night, disrupt sleep (Åkerstedt et al., 2010), and have been shown to impair insulin sensitivity (Eckel et al., 2015). Identifying the contribution of the necessary and sufficient drivers of adverse health effects of shift work is a key to designing efficient and effective prevention and intervention strategies. Is it, for example, sufficient to shift meal and caloric intake entirely to the biological day to prevent disease or improve metabolic function, or is it necessary to couple interventions (e.g., adjunct sleep, light, and meal time interventions) to achieve the desired effects? Because shift work is necessary, especially in the health-care, transportation and security-sensitive sectors, the need to design effective prevention strategies is great.
At the same time, it is imperative from an occupational health standpoint that we gain a better understanding of the most strenuous aspects of shift schedules, ideally in conjunction with information on individual circadian and sleep phenotypes – an approach recommended in 2011 by the International Agency for Research on Cancer (Stevens et al., 2011). Shift work studies now increasingly assess and report the multiple dimensions of work schedules, including timing and duration of shifts, direction of rotation, number of consecutive shifts, weekly work hours, time between shift transitions. This level of descriptive and analytical detail is required for the development of medical guidelines and the refinement of individual and organizational prevention and intervention strategies (Papantoniou et al., 2016). Shift work assessment now increasingly reflects more adequately the variable nature of the exposure. Taken together, shift work in general has been widely used as a proxy for circadian disruption, but only recently have studies started to collect work schedule information reflecting the multi-dimensionality of schedules. These types of assessments, however, still do not account for inter-individual variability in circadian and sleep phenotypes. We have proposed to use social jetlag (Juda et al., 2013b) or composite phase deviations (Fischer et al., 2016) as a proxy for misalignment in shift workers to obtain a higher resolution, single-unit estimate of the disruption an individual experiences in a given schedule, while others have suggested that chronodisruption (Erren & Reiter, 2009; Erren & Reiter, 2013; Erren & Morfeld, 2014) might be a better way to incorporate inter-individual differences in sleep and circadian phenotypes into exposure assessment (Kantermann et al., 2010). Yet these remain activity-based proxies for circadian disruption, as we still lack direct measures of circadian clock disruption that are non-invasive, low burden, and scalable to epidemiological samples (DLMO is not feasible on cost and logistical grounds in such large samples), although there are clear and promising efforts in this direction (Laing et al., 2017; Braun et al., 2018; Wittenbrink et al., 2018). Until such measures are shown to be robust in field settings, future shift work studies should consider deriving multiple measures of circadian disruption to also capture much needed insights into organismal and molecular consequences of shift work, especially in real-life, community-based settings. A study in individuals working morning (6:00–15:00) and evening shifts (15:00–0:00) tracked average wake times, meal times and peak times of clock gene expression from hair follicle cells (Akashi et al., 2010), and showed a shift in meal and wake times by about 7h when transitioning from morning to evening shifts, but clock gene peaks shifted by 2h, and the authors conclude that the one week of evening shift was not sufficient for adaptation. This was a helpful step, but the number of hair follicle cells needed for such assessments, and the to-be-expected limited compliance has damped the success of this method. Including non-invasive sensor and mobile health technology will be essential for those efforts.
Artificial Light at Night.
Light at night has been proposed as a risk factor for breast cancer by Stevens and colleagues (Stevens et al., 2007; Stevens et al., 2014; Lunn et al., 2017). While the exact physiological mechanisms remain to be elucidated, they likely to involve melatonin suppression, and downstream alternations in immune function and endocrine disruption (Stevens & Rea, 2001; Stevens et al., 2014; Dominoni et al., 2016; Lunn et al., 2017). Experimental work in rats has shown that light at night affects therapy resistance of human breast cancer tumor tissue (Dauchy et al., 2014) and breast-cancer prone mice show reduce tumor suppression when exposed to a simulated shift work protocol that continuously inverses the light/dark cycle (Van Dycke et al., 2015). In epidemiological studies, results have been mixed and often limited by ecological or case-control study designs (Hurley et al., 2014; Portnov et al., 2016; James et al., 2017; Johns et al., 2018). A recent workshop organized by the National Institutes of Health summarizing the evidence on light and health stresses the importance of identifying markers of circadian disruption that can be used in epidemiological studies to study the associations between light and health (Lunn et al., 2017). Cancer is not the only outcome that has been linked to artificial light at night. Animal models have shown that aberrant light exposure patterns can induce depressive-like behavior, independent of circadian clock and sleep, and it has been suggested that this direct link between light and mood is mediated by intrinsically photosensitive retinal ganglion cells (ipRGCs, LeGates et al., 2012). Those photosensitive retinal ganglion cells are a constituent part of the retino-hypothalamic pathway that provides the SCN with information on environmental light exposure levels, and that together with rods and cones, determine the biological effects of light (Lucas et al., 2014; Hughes et al., 2015). Indeed, the association between light and mood is well established (Dumont & Beaulieu, 2007), and morning bright light exposure is a highly effective therapeutic approach in depression and mood disorders (Wirz-Justice et al., 2009). In a recent, prospective study, Obayashi et al. (Obayashi et al., 2018) reported that individuals who sleep in bedrooms that are brighter than 5lux at night are at higher risk for depression as compared to those sleeping in darker bedrooms. Daytime levels of light exposure were unknown, however. This is relevant, because we know that the biological effect of light on circadian physiology depend not only on timing (e.g. night vs. daytime), duration of exposure, spectral composition, but also on an individual’s light history (Chang et al., 2011). Future work is warranted to address this critical gap in knowledge, especially as taking into account an individual’s light history might help increase efficacy of interventions. Finally, the reduced zeitgeber strength associated with artificial light at night also results in a wider distribution of circadian phenotypes in humans (Roenneberg et al., 2007a; Fischer et al., 2017; Swaminathan et al., 2017), and it affects activity patterns and temporal niches in animals (Dominoni et al., 2013; Dominoni et al., 2016). Artificial light at night is therefore likely to induce further misalignment between environmental or behavior cycles and physiology, or exacerbate disruption. One additional, but crucial point to consider is the model organism chosen to study the effects of light at night. Humans are a diurnal species, i.e., we are mostly active during the day, while model organisms, such as rodents can be nocturnal, diurnal, and/or crespuscular (active in twilight). Rhythms in for example body temperature or serotonin peak during daytime in diurnal animals, while body temperature peaks at nighttime in nocturnal ones (Challet, 2007). Melatonin expression patterns are similar in nocturnal and diurnal species, with the typical increase in the dark phase of the 24h day. In contrast, circadian rhythms in glucose and lipid metabolism out of phase in diurnal vs. nocturnal animals (Kumar Jha et al., 2015). Mohawk and Lee (2005) for example have shown that stress slows down re-entrainment after a 6h phase advance jetlag protocol in both, degus, a diurnal rodent, and the nocturnal rat. Such a comparative approach using both diurnal and nocturnal species to this question is advantageous as it allows to identify universal mechanisms of entrainment across temporal niches (see Yan et al., 2018, for a recent review).
Position in Time Zone.
Time zones are regions that adhere to the same local time, in reference to Coordinated Universal Time (or UTC). Time zones were created by dividing the world in 24 time zones of approximately 15° each, with variation in this division being attributable to national and international borders. Using position in time zone as a proxy for circadian misalignment assumes that within a time zone, sunrise occurs increasingly later from east to west, while the local time remains constant. As the sun moves from east to west, residing in the west of a given time zone will result in less early morning light exposure, and more evening light exposure. It has been shown this gradient in solar time from east to west is associated with later sleep timing (Roenneberg et al., 2007b; Jankowski et al., 2014), but local time will continue to dictate work hours and other social constraints across the time zone. Consequently, the average mismatch between external, local time and internal time is assumed to be higher further west in a given time zone. We and others have shown modest, but significant associations between position in time zone and some forms of cancers in ecological study designs (Borisenkov, 2011; Gu et al., 2017; VoPham et al., 2018). Limitations of this proxy include the population-level exposure and covariate information, the lack of direct and objective assessments of individual phenotypes, and the absence of organismal or molecular read-outs. However, those exploratory approaches are useful to generate novel hypotheses and identify previously unknown risk factors for diseases, especially when a plausible biological mechanism has been proposed. Daylight Savings Time (DST) will not be discussed in depth in this review, as it is usually not used as a tool to study circadian disruption. However, the reported overall modest increased risk of adverse health and safety consequences associated with DST (Monk & Folkard, 1976; Coren, 1996; Kantermann et al., 2007; Janszky & Ljung, 2008; Barnes & Wagner, 2009; Janszky et al., 2012; Kirchberger et al., 2015; Prats-Uribe et al., 9000) has been argued to reflect sleep deprivation and the disruption of the circadian clock’s seasonal adjustment experienced especially during the spring transition (Kantermann et al., 2007).
Do we actually measure circadian disruption?
As elucidated above, it is likely that we need a set of metrics to capture circadian disruption, ideally at multiple levels. While laboratory studies have methods available to probe circadian rhythms across all levels of description, population- and field studies mainly rely on sleep- and activity-based metrics in entrained conditions rather than true circadian metrics, that would need to be obtained in temporal isolation. Diurnal rhythms in actigraphy data are rather used as a proxy, and reflect a highly systemic clock output (Broussard et al., 2017) that currently cannot directly inform on the endogenous clock function, nor capture the organismal or molecular interplay of clock-regulated processes. Alternative environmental measures, such as shift work, are approximations of zeitgeber intensity, variability and potentially constellation, and thereby also represent a proxy. Ideally, the zeitgeber landscape would be assessed, together with physiological read-outs at all system-levels, to capture the extent of disruption. A recent study by Skarke et al. (Skarke et al., 2017) characterized the “chronobiome”, using multiple sensors to track exposure and outcome information over several days. Linking high-dimensional, longitudinal zeitgeber recordings to circadian rhythms, and ultimately health, will provide critical information for the design of effective interventions inspired by circadian biology.
Laboratory-based protocols typically measure maximum disruption, and reduce inter-individual variability usually to a minimum, which limits comparability with ecological studies, where a continuum of disruption is assessed on the individual and environmental level. Common to both types of settings is that all protocol and metrics can only provide snapshots of the current level of circadian disruption of an individual and can therefore be biased and/or erroneous when estimating lifetime circadian disruption. Epidemiological studies of shift work and health however suggest that especially shift work exposure for 5 years or more is especially relevant for chronic disease development (e.g., Vyas et al., 2012; Gan et al., 2015; Lin et al., 2015). Prospective, longitudinal studies with repeated assessment at multiple levels of circadian disruption and zeitgeber environments are needed if the search for a cumulative, specific, and predictive biomarker of circadian disruption at any system-level ought to be successful. The utility of such an endeavor is not debated (Mullington et al., 2016), but the heterogeneity and in part absence of operationalizable definitions of circadian disruption have hampered efforts so far. Moving forward will require a consensus in the field defining a set of measures that can be employed when studying circadian disruption and its acute and chronic effects on cognition, health, and wellbeing.
Most measures of circadian disruption, especially in field-settings, do not consider amplitude as part of the assessment, even though it is an integral part of rhythmicity, together with period and phase, and should therefore be considered when studying circadian rhythm disruption. Activity rhythms more readily provide the opportunity to assess amplitude (as compared to sleep for example), and a recent, cross-sectional study within the UK Biobank has shown that reduced amplitude in physical activity is associated with adverse mood outcomes (Lyall et al., 2018). Activity patterns in real life, however, do not directly reflect circadian rhythms, as outlined above. Limitations of such approaches might be offset by their scalability, and advanced statistical tools will be useful to examine the magnitude of error associated with behavioral proxies, and depending on the research question and the setting (e.g. clinical vs. research), certain margins of errors will be acceptable, in view of scalable, cost-effective methods. It should be emphasized, however, that currently very little is known about the relationship between amplitude of organismal level outputs (e.g., activity) and amplitudes of any circadian rhythms at the molecular level, either centrally or peripherally.
In summary, the following points should be considered when studying effects of circadian disruption:
Distinction between concept and operationalization. Oftentimes, circadian disruption terminology is used interchangeably with the operationalization and measurement of the concept. Future work needs to clearly outline the concept it is aiming to capture, how this will be operationalized, as well as the exact quantification.
Field vs. laboratory studies. It is imperative to further test frameworks of circadian disruption research in field and laboratory settings. Assumptions inherent to the paradigms used, and their associated limitations, need to be explicitly acknowledged and discussed. For translational efforts to be effective, the sample characteristics, including age, sex, race, ethnicity, and sleep and circadian phenotypes, as well as their potential to modify the examined associations, need to be systematically assessed.
Better quantification of the circadian landscape and behavioral proxies. Technology now enables high-resolution and high-dimensional environmental and behavioral monitoring, so that tracking individuals and animals in-, but especially outside of the laboratory, is becoming increasingly feasible. This represents not only a unique opportunity to explore the temporal zeitgeber environment and embedded behaviors in relationship to health and disease, but will also allow identify intervention opportunities.
Circadian alterations vs. disruption. Changes or variations in phase and/or amplitude can be highly adaptive, and thus are not per se negative; the acute and long-term effects will depend on the frequency, extent and chronicity of those alterations. Systematic continuous measures of biological functions at multiple levels of description will be necessary to identify relevant thresholds for a given outcome. For this to be successful, concurrent quantification of magnitude, frequency, and chronicity of a potential cause of disruption, sample characteristics, as well as its resulting level of disruption need to be recorded.
Levels and duration of disruption. Rhythms within a human can be altered at the intracellular, cellular, tissue, organ, and systems-level. Alterations in rhythms comprise the relative phase and phase-relationships between and within each of those levels, in relationship to environmental signals and zeitgebers, as well as the amplitude of a given rhythm. Further work is not only needed to identify the level most relevant for prediction of adverse outcomes (on individual-level and population-level), but also to identify the level(s) most useful to track and intervene on. Differentiating between adaptation, variation, and disruption will be the major challenge of the field, and will require to consider outcome-wide association patterns in addition to single-outcome studies. Another major challenge is the objective assessment of long-term environmental and behavioral sources of disruption, and their changes over time, as it is this long-term exposure that appears to be carrying the risk. Recent work has suggested a plasma-based mRNA biomarker for chronic sleep deprivation (Laing et al., 2018), and similar approaches might be useful in the context of circadian disruption, albeit currently not available. Finally, new laboratory paradigms that vary levels of exposure (in magnitude and duration), show variation in the induced level of disruption, and a corresponding dose-response-relationship in the dependent variable of interest will be useful to better understand the physiological mechanisms underlying the long-term adverse health effects of circadian disruption.
Causality and prospective designs. Changes in rhythmicity (i.e. in phase and/or amplitude, incl. arrhythmicity) could be precursors, symptoms, correlates, or consequences of a given condition. To disentangle those possibilities, we need i) experimental animal and human work to establish mechanistic links and identify biological pathways that might underlie associations between circadian disruption and acute physiological and behavioral outcomes, and ii) prospective, longitudinal studies to establish the temporal relationship between exposure (i.e., circadian disruption) and outcomes (such as obesity, cancer, asthma, diabetes, depression, or cardiovascular disease). Randomized clinical trials are needed to quantify the effects of circadian interventions.
Conclusions
This review aimed to summarize current definitions and metrics used to quantify “circadian disruption”, discuss potential limitations, and describe the next steps needed to enable the systematic examination of acute and chronic consequences of circadian misalignment in laboratory-, population- and community-based studies. The heterogeneity and breadth in approaches and concepts associated with circadian disruption is immense, but also reflective of the fundamental role that circadian rhythms play in physiology and behavior. It follows that a single metric cannot fully encompass the circadian system, but that a set of complementary measures will be necessary to capture the acute and chronic effects of challenges to the circadian system on health and disease. Future work is warranted to generate a consensus on how to best define and assess circadian disruption.
Acknowledgements
I would like to thank Andrew J.K. Phillips, Till Roenneberg, Michael J. Parsons, Larissa Hunt, and Dorothee Fischer for their helpful comments on earlier versions of this manuscript.
Funding
This work was supported by R01 DK105072 and 5R21OH011052 of the National Institutes of Health.
Abbreviations
- BMI
Body Mass Index
- DLMO
Dim-Light Melatonin Onset
- DST
Daylight Savings Time
- ipRGCs
Intrinsically photosensitive retinal ganglion cells
- IS
Inter-day stability
- IV
Intra-day variability
- MCTQ
Munich ChronoType Questionnaire
- SCN
Suprachiasmatic nucleus
- UTC
Coordinated Universal Time
- T2D
Type 2 diabetes
Footnotes
Conflict of Interest Statement
I have no conflict of interest to declare.
References
- Adan A & Almirall H (1991) Horne & Östberg morningness-eveningness questionnaire: A reduced scale. Personality and Individual Differences, 12, 241–253. [Google Scholar]
- Akashi M, Soma H, Yamamoto T, Tsugitomi A, Yamashita S, Nishida E, Yasuda A, Liao JK & Node K (2010) Noninvasive method for assessing the human circadian clock using hair follicle cells. PNAS, 107, 15643–15648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åkerstedt T (2003) Shift work and disturbed sleep/wakefulness. Occupational Medicine, 53, 89–94. [DOI] [PubMed] [Google Scholar]
- Åkerstedt T, Kecklund G & Selén J (2010) Early morning work - prevalence and relation to sleep/wake problems: a national representative survey. Chronobiology International, 27, 975–986. [DOI] [PubMed] [Google Scholar]
- Albrecht U (2012) Timing to perfection: the biology of central and peripheral circadian clocks. Neuron, 74, 246–260. [DOI] [PubMed] [Google Scholar]
- Altimus CM, Guler AD, Villa KL, McNeill DS, Legates TA & Hattar S (2008) Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc Natl Acad Sci U S A, 105, 19998–20003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, Harang R, Meeker K, Granados-Fuentes D, Tsai CA, Mazuski C, Kim J, Doyle FJ 3rd, Petzold LR & Herzog ED (2013) A neuropeptide speeds circadian entrainment by reducing intercellular synchrony. Proc Natl Acad Sci U S A, 110, E4355–4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anothaisintawee T, Reutrakul S, Van Cauter E & Thakkinstian A (2016) Sleep disturbances compared to traditional risk factors for diabetes development: Systematic review and meta-analysis. Sleep Medicine Reviews, 30, 11–24. [DOI] [PubMed] [Google Scholar]
- Arble DM, Bass J, Behn CD, Butler MP, Challet E, Czeisler C, Depner CM, Elmquist J, Franken P, Grandner MA, Hanlon EC, Keene AC, Joyner MJ, Karatsoreos I, Kern PA, Klein S, Morris CJ, Pack AI, Panda S, Ptacek LJ, Punjabi NM, Sassone-Corsi P, Scheer FA, Saxena R, Seaquest ER, Thimgan MS, Van Cauter E & Wright KP (2015) Impact of Sleep and Circadian Disruption on Energy Balance and Diabetes: A Summary of Workshop Discussions. Sleep, 38, 1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arble DM, Ramsey KM, Bass J & Turek FW (2010) Circadian Disruption and Metabolic Disease: Findings from Animal Models. Best practice & research. Clinical endocrinology & metabolism, 24, 785–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer SN & Oster H (2015) How sleep and wakefulness influence circadian rhythmicity: effects of insufficient and mistimed sleep on the animal and human transcriptome. Journal of Sleep Research, 24, 476–493. [DOI] [PubMed] [Google Scholar]
- Arendt J (2006) Melatonin and Human Rhythms. Chronobiology International, 23, 21–37. [DOI] [PubMed] [Google Scholar]
- Aschoff J (1965) Circadian rhythms in man. Science, 148, 1427–1432. [DOI] [PubMed] [Google Scholar]
- Aschoff J, Daan S & Honma K-I (Year) Zeitgebers, Entrainment, and Masking: Some Unsettled Questions Proceedings of the Vertebrate Circadian Systems. Springer; Berlin Heidelberg, City: p. 13–24. [Google Scholar]
- Aschoff J, Klotter K & Wever RA (1965) Circadian Vocabulary. In Aschoff J (ed). North-Holland Publishing Company, Amsterdam. [Google Scholar]
- Barnard AR & Nolan PM (2008) When Clocks Go Bad: Neurobehavioural Consequences of Disrupted Circadian Timing. PLoS Genet, 4, e1000040–e1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CM & Wagner DT (2009) Changing to daylight saving time cuts into sleep and increases workplace injuries. The Journal of applied psychology, 94, 1305–1317. [DOI] [PubMed] [Google Scholar]
- Baron KG & Reid KJ (2014) Circadian misalignment and health. Int Rev Psychiatry, 26, 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrosian TA, Fonken LK & Nelson RJ (2016) Endocrine Effects of Circadian Disruption. Annu Rev Physiol, 78, 109–131. [DOI] [PubMed] [Google Scholar]
- Bei B, Wiley JF, Trinder J & Manber R (2016) Beyond the mean: A systematic review on the correlates of daily intraindividual variability of sleep/wake patterns. Sleep Medicine Reviews, 28, 108–124. [DOI] [PubMed] [Google Scholar]
- Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, Parry BL & Revell VL (2008) Measuring Melatonin in Humans. Journal of Clinical Sleep Medicine: JCSM: official publication of the American Academy of Sleep Medicine, 4, 66–69. [PMC free article] [PubMed] [Google Scholar]
- Bierman A, Klein TR & Rea MS (2005) The Daysimeter: A device for measuring optical radiation as a stimulus for the human circadian system. Measurement Science and Technology, 16. [Google Scholar]
- Borbely AA, Daan S, Wirz-Justice A & Deboer T (2016) The two-process model of sleep regulation: a reappraisal. J Sleep Res, 25, 131–143. [DOI] [PubMed] [Google Scholar]
- Borisenkov MF (2011) Latitude of residence and position in time zone are predictors of cancer incidence, cancer mortality, and life expectancy at birth. Chronobiol Int, 28, 155–162. [DOI] [PubMed] [Google Scholar]
- Braun R, Kath WL, Iwanaszko M, Kula-Eversole E, Abbott SM, Reid KJ, Zee PC & Allada R (2018) Universal method for robust detection of circadian state from gene expression. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard JL, Reynolds AC, Depner CM, Ferguson SA, Dawson D & Wright KP (2017) Circadian Rhythms Versus Daily Patterns in Human Physiology and Behavior. Springer; India, New Delhi, pp. 279–295. [Google Scholar]
- Broussard JL & Van Cauter E (2016) Disturbances of sleep and circadian rhythms: novel risk factors for obesity. Current Opinion in Endocrinology, Diabetes and Obesity, 23, 353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Savic N, Sletten T, Roach G, Gilbert SS & Dawson D (2003) The Relationship Between the Dim Light Melatonin Onset and Sleep on a Regular Schedule in Young Healthy Adults. Behavioral Sleep Medicine, 1, 102–114. [DOI] [PubMed] [Google Scholar]
- Cespedes Feliciano EM, Quante M, Weng J, Mitchell JA, James P, Marinac CR, Mariani S, Redline S, Kerr J, Godbole S, Manteiga A, Wang D & Hipp JA (2017) Actigraphy-Derived Daily Rest–Activity Patterns and Body Mass Index in Community-Dwelling Adults. Sleep, 40, zsx168–zsx168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challet E (2007) Minireview: Entrainment of the Suprachiasmatic Clockwork in Diurnal and Nocturnal Mammals. Endocrinology, 148, 5648–5655. [DOI] [PubMed] [Google Scholar]
- Chang A-M, Scheer FAJL & Czeisler CA (2011) The human circadian system adapts to prior photic history. The Journal of Physiology, 589, 1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordina-Duverger E, Menegaux F, Popa A, Rabstein S, Harth V, Pesch B, Bruning T, Fritschi L, Glass DC, Heyworth JS, Erren TC, Castano-Vinyals G, Papantoniou K, Espinosa A, Kogevinas M, Grundy A, Spinelli JJ, Aronson KJ & Guenel P (2018) Night shift work and breast cancer: a pooled analysis of population-based case-control studies with complete work history. Eur J Epidemiol, 33, 369–379. [DOI] [PubMed] [Google Scholar]
- Coren S (1996) Daylight Savings Time and Traffic Accidents. New England Journal of Medicine, 334, 924–925. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Van Reen E, LeBourgeois MK, Acebo C, Tarokh L, Seifer R, Barker DH & Carskadon MA (2014) A Longitudinal Assessment of Sleep Timing, Circadian Phase, and Phase Angle of Entrainment across Human Adolescence. PloS One, 9, e112199–e112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk D-J & Kronauer RE (1999) Stability, Precision, and Near-24-Hour Period of the Human Circadian Pacemaker. Science, 284, 2177–2181. [DOI] [PubMed] [Google Scholar]
- Daan S, Beersma DG & Borbely AA (1984) Timing of human sleep: recovery process gated by a circadian pacemaker. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology, 246, R161–R183. [DOI] [PubMed] [Google Scholar]
- Dauchy RT, Xiang S, Mao L, Brimer S, Wren MA, Yuan L, Anbalagan M, Hauch A, Frasch T, Rowan BG, Blask DE & Hill SM (2014) Circadian and Melatonin Disruption by Exposure to Light at Night Drives Intrinsic Resistance to Tamoxifen Therapy in Breast Cancer. Cancer Research, 74, 4099–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M & Block GD (2006) Chronic jet-lag increases mortality in aged mice. Current biology: CB, 16, R914–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Yamazaki S, Arble DM, Menaker M & Block GD (2008) Resetting of central and peripheral circadian oscillators in aged rats. Neurobiology of aging, 29, 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro JM, Stoerzinger A, Barkmeier D & Ellen P (1978) Medial septal lesions: disruptions of microregulatory patterns and circadian rhythmicity in rats. Journal of comparative and physiological psychology, 92, 71–84. [DOI] [PubMed] [Google Scholar]
- Depner CM, Stothard ER & Wright KP Jr (2014) Metabolic Consequences of Sleep and Circadian Disorders. Current Diabetes Reports, 14, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominoni DM, Borniger JC & Nelson RJ (2016) Light at night, clocks and health: from humans to wild organisms. Biology Letters, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominoni DM, Helm B, Lehmann M, Dowse HB & Partecke J (2013) Clocks for the city: circadian differences between forest and city songbirds. 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF & Wright KP (2005) Entrainment of the Human Circadian System by Light. J Biol Rhyth, 20, 326–338. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Zitting K-M & Chinoy ED (2015) Aging and Circadian Rhythms. Sleep Medicine Clinics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap JC & Loros JJ (2004) Chronobiology: Biological timekeeping. Sinauer Associates, Sunderland, MA, US. [Google Scholar]
- Dumont M & Beaulieu C (2007) Light exposure in the natural environment: Relevance to mood and sleep disorders. Sleep Medicine, 8, 557–565. [DOI] [PubMed] [Google Scholar]
- Dyar KA, Lutter D, Artati A, Ceglia NJ, Liu Y, Armenta D, Jastroch M, Schneider S, de Mateo S, Cervantes M, Abbondante S, Tognini P, Orozco-Solis R, Kinouchi K, Wang C, Swerdloff R, Nadeef S, Masri S, Magistretti P, Orlando V, Borrelli E, Uhlenhaut NH, Baldi P, Adamski J, Tschop MH, Eckel-Mahan K & Sassone-Corsi P (2018) Atlas of Circadian Metabolism Reveals System-wide Coordination and Communication between Clocks. Cell, 174, 1571–1585.e1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel Robert H., Depner Christopher M., Perreault L, Markwald Rachel R., Smith Mark R., McHill Andrew W., Higgins J, Melanson Edward L. & Wright Kenneth P. Jr (2015) Morning Circadian Misalignment during Short Sleep Duration Impacts Insulin Sensitivity. Current Biology, 25, 3004–3010. [DOI] [PubMed] [Google Scholar]
- Erren TC & Morfeld P (2014) Computing chronodisruption: How to avoid potential chronobiological errors in epidemiological studies of shift work and cancer. Chronobiology International, 31, 589–599. [DOI] [PubMed] [Google Scholar]
- Erren TC & Reiter RJ (2009) Defining chronodisruption. Journal of Pineal Research, 46, 245–247. [DOI] [PubMed] [Google Scholar]
- Erren TC & Reiter RJ (2013) Revisiting chronodisruption: when the physiological nexus between internal and external times splits in humans. Naturwissenschaften, 100, 291–298. [DOI] [PubMed] [Google Scholar]
- Evans JA & Davidson AJ (2013) Chapter Ten - Health Consequences of Circadian Disruption in Humans and Animal Models. In Martha UG (ed). Academic Press, pp. 283–323. [DOI] [PubMed] [Google Scholar]
- Farr LA, Campbell-Grossman C, Langenberg-Panzer SL, Mack JM, Petersen MJ & Smith TJ (1985) Reduction of circadian disruption following surgery by ingestion of caffeine in the laboratory rat. The Physiologist, 28, S173–174. [PubMed] [Google Scholar]
- Feng D, Liu T, Sun Z, Bugge A, Mullican SE, Alenghat T, Liu XS & Lazar MA (2011) A Circadian Rhythm Orchestrated by Histone Deacetylase 3 Controls Hepatic Lipid Metabolism. Science, 331, 1315–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez DC, Fogerson PM, Lazzerini Ospri L, Thomsen MB, Layne RM, Severin D, Zhan J, Singer JH, Kirkwood A, Zhao H, Berson DM & Hattar S (2018) Light Affects Mood and Learning through Distinct Retina-Brain Pathways. Cell, 175, 71–84.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, Lombardi DA, Marucci-Wellman H & Roenneberg T (2017) Chronotypes in the US – Influence of age and sex. PLOS ONE, 12, e0178782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, Vetter C & Roenneberg T (2016) A novel method to visualise and quantify circadian misalignment. Scientific Reports, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster RG, Peirson SN, Wulff K, Winnebeck E, Vetter C & Roenneberg T (2013) Sleep and circadian rhythm disruption in social jetlag and mental illness. Progress in Molecular Biology and Translational Science, 119, 325–346. [DOI] [PubMed] [Google Scholar]
- Fritschi L, Valerie Gross J, Wild U, Heyworth JS, Glass DC & Erren TC (2018) Shift work that involves circadian disruption and breast cancer: a first application of chronobiological theory and the consequent challenges. Occup Environ Med, 75, 231–234. [DOI] [PubMed] [Google Scholar]
- Froy O & Garaulet M (2018) The Circadian Clock in White and Brown Adipose Tissue: Mechanistic, Endocrine, and Clinical Aspects. Endocr Rev, 39, 261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdames HA, Torres-Farfan C, Spichiger C, Mendez N, Abarzua-Catalan L, Alonso-Vazquez P & Richter HG (2014) Impact of gestational chronodisruption on fetal cardiac genomics. Journal of molecular and cellular cardiology, 66, 1–11. [DOI] [PubMed] [Google Scholar]
- Gan Y, Yang C, Tong X, Sun H, Cong Y, Yin X, Li L, Cao S, Dong X, Gong Y, Shi O, Deng J, Bi H & Lu Z (2015) Shift work and diabetes mellitus: a meta-analysis of observational studies. Occupational and Environmental Medicine, 72, 72–78. [DOI] [PubMed] [Google Scholar]
- Gill S & Panda S (2015) A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F, Xu S, Devesa SS, Zhang F, Klerman EB, Graubard BI & Caporaso NE (2017) Longitude Position in a Time Zone and Cancer Risk in the United States. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 26, 1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood S & Amir S (2017) The aging clock: circadian rhythms and later life. J Clin Invest, 127, 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne JA & Ostberg O (1976) A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International journal of chronobiology, 4, 97–110. [PubMed] [Google Scholar]
- Hughes S, Jagannath A, Hankins MW, Foster RG & Peirson SN (2015) Chapter Six - Photic Regulation of Clock Systems. In Amita S (ed). Academic Press, pp. 125–143. [DOI] [PubMed] [Google Scholar]
- Hurley S, Goldberg D, Nelson D, Hertz A, Horn-Ross PL, Bernstein L & Reynolds P (2014) Light at Night and Breast Cancer Risk Among California Teachers. Epidemiology (Cambridge, Mass.), 25, 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P, Bertrand KA, Hart JE, Schernhammer ES, Tamimi RM & Laden F (2017) Outdoor Light at Night and Breast Cancer Incidence in the Nurses’ Health Study II. Environ Health Perspect, 125, 087010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski KS, Vollmer C, Linke M & Randler C (2014) Differences in sun time within the same time zone affect sleep–wake and social rhythms, but not morningness preference: Findings from a Polish–German comparison study. Time & Society, 23, 258–276. [Google Scholar]
- Janszky I, Ahnve S, Ljung R, Mukamal KJ, Gautam S, Wallentin L & Stenestrand U (2012) Daylight saving time shifts and incidence of acute myocardial infarction – Swedish Register of Information and Knowledge About Swedish Heart Intensive Care Admissions (RIKS-HIA). Sleep Medicine, 13, 237–242. [DOI] [PubMed] [Google Scholar]
- Janszky I & Ljung R (2008) Shifts to and from Daylight Saving Time and Incidence of Myocardial Infarction. New England Journal of Medicine, 359, 1966–1968. [DOI] [PubMed] [Google Scholar]
- Johns LE, Jones ME, Schoemaker MJ, McFadden E, Ashworth A & Swerdlow AJ (2018) Domestic light at night and breast cancer risk: a prospective analysis of 105 000 UK women in the Generations Study. British Journal Of Cancer, 118, 600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juda M, Vetter C & Roenneberg T (2013a) Chronotype modulates sleep duration, social jetlag and sleep quality in shift-workers. Journal of Biological Rhythms, 28, 141–151. [DOI] [PubMed] [Google Scholar]
- Juda M, Vetter C & Roenneberg T (2013b) The Munich ChronoType Questionnaire for Shift-workers (MCTQShift). Journal of Biological Rhythms, 28, 130–140. [DOI] [PubMed] [Google Scholar]
- Kant AK (2018) Eating patterns of US adults: Meals, snacks, and time of eating. Physiol Behav. [DOI] [PubMed] [Google Scholar]
- Kantermann T, Juda M, Merrow M & Roenneberg T (2007) The Human Circadian Clock’s Seasonal Adjustment Is Disrupted by Daylight Saving Time. Current Biology, 17, 1996–2000. [DOI] [PubMed] [Google Scholar]
- Kantermann T, Juda M, Vetter C & Roenneberg T (2010) Shift-work research: Where do we stand, where should we go? Sleep Biol Rhyth, 8, 95–105. [Google Scholar]
- Kantermann T, Sung H & Burgess HJ (2015) Comparing the Morningness-Eveningness Questionnaire and Munich ChronoType Questionnaire to the Dim Light Melatonin Onset. J Biol Rhythms, 30, 449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH & McEwen BS (2011) Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci U S A, 108, 1657–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kervezee L, Cuesta M, Cermakian N & Boivin DB (2018) Simulated night shift work induces circadian misalignment of the human peripheral blood mononuclear cell transcriptome. Proceedings of the National Academy of Sciences, 115, 5540–5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchberger I, Wolf K, Heier M, Kuch B, von Scheidt W, Peters A & Meisinger C (2015) Are daylight saving time transitions associated with changes in myocardial infarction incidence? Results from the German MONICA/KORA Myocardial Infarction Registry. BMC Public Health, 15, 778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura S, Hida A, Aritake S, Higuchi S, Enomoto M, Kato M, Vetter C, Roenneberg T & Mishima K (2014) Validity of the Japanese version of the Munich ChronoType Questionnaire. Chronobiology International, 31, 845–850. [DOI] [PubMed] [Google Scholar]
- Knutson KL & von Schantz M (2018) Associations between chronotype, morbidity and mortality in the UK Biobank cohort. Chronobiol Int, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Jha P, Challet E & Kalsbeek A (2015) Circadian rhythms in glucose and lipid metabolism in nocturnal and diurnal mammals. Molecular and Cellular Endocrinology, 418, 74–88. [DOI] [PubMed] [Google Scholar]
- Laborde S, Dosseville F, Aloui A, Ben Saad H, Bertollo M, Bortoli L, Braun B, Chamari K, Chtourou H, De Kort Y, Farooq A, Gordijn MC, Greco P, Guillen F, Haddad M, Hosang T, Khalladi K, Lericollais R, Lopes M, Robazza C, Smolders K, Wurm A & Allen MS (2018) Convergent and construct validity and test-retest reliability of the Caen Chronotype Questionnaire in six languages. Chronobiol Int, 1–11. [DOI] [PubMed] [Google Scholar]
- Laing EE, Moller-Levet CS, Dijk DJ & Archer SN (2018) Identifying and validating blood mRNA biomarkers for acute and chronic insufficient sleep in humans: a machine learning approach. Sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing EE, Möller-Levet CS, Poh N, Santhi N, Archer SN & Dijk D-J (2017) Blood transcriptome based biomarkers for human circadian phase. eLife, 6, e20214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Altimus CM, Wang H, Lee H-K, Yang S, Zhao H, Kirkwood A, Weber ET & Hattar S (2012) Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature, 491, 594–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levandovski R, Sasso E & Hidalgo MP (2013) Chronotype: a review of the advances, limits and applicability of the main instruments used in the literature to assess human phenotype. Trends Psychiatry Psychother, 35, 3–11. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Cutler NL & Sack RL (1999) The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms, 14, 227–236. [DOI] [PubMed] [Google Scholar]
- Lin X, Chen W, Wei F, Ying M, Wei W & Xie X (2015) Night-shift work increases morbidity of breast cancer and all-cause mortality: a meta-analysis of 16 prospective cohort studies. Sleep Med, 16, 1381–1387. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, Figueiro MG, Gamlin PD, Lockley SW, O’Hagan JB, Price LLA, Provencio I, Skene DJ & Brainard GC (2014) Measuring and using light in the melanopsin age. Trends in Neurosciences, 37, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn RM, Blask DE, Coogan AN, Figueiro MG, Gorman MR, Hall JE, Hansen J, Nelson RJ, Panda S, Smolensky MH, Stevens RG, Turek FW, Vermeulen R, Carreón T, Caruso CC, Lawson CC, Thayer KA, Twery MJ, Ewens AD, Garner SC, Schwingl PJ & Boyd WA (2017) Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Science of The Total Environment, 607–608, 1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall LM, Wyse CA, Graham N, Ferguson A, Lyall DM, Cullen B, Celis Morales CA, Biello SM, Mackay D, Ward J, Strawbridge RJ, Gill JMR, Bailey MES, Pell JP & Smith DJ (2018) Association of disrupted circadian rhythmicity with mood disorders, subjective wellbeing, and cognitive function: a cross-sectional study of 91 105 participants from the UK Biobank. The Lancet Psychiatry, 5, 507–514. [DOI] [PubMed] [Google Scholar]
- Madrid-Navarro CJ, Escamilla-Sevilla F, Minguez-Castellanos A, Campos M, Ruiz-Abellan F, Madrid JA & Rol MA (2018) Multidimensional Circadian Monitoring by Wearable Biosensors in Parkinson’s Disease. Frontiers in neurology, 9, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino TA, Tata N, Belsham DD, Chalmers J, Straume M, Lee P, Pribiag H, Khaper N, Liu PP, Dawood F, Backx PH, Ralph MR & Sole MJ (2007) Disturbed Diurnal Rhythm Alters Gene Expression and Exacerbates Cardiovascular Disease With Rescue by Resynchronization. Hypertension, 49, 1104–1113. [DOI] [PubMed] [Google Scholar]
- McClung CA (2015) Circadian Rhythms in Mood Disorders. John Wiley & Sons, Inc, pp. 249–269. [Google Scholar]
- Merikanto I, Lahti T, Puolijoki H, Vanhala M, Peltonen M, Laatikainen T, Vartiainen E, Salomaa V, Kronholm E & Partonen T (2013) Associations of Chronotype and Sleep With Cardiovascular Diseases and Type 2 Diabetes. Chronobiology International, 30, 470–477. [DOI] [PubMed] [Google Scholar]
- Miller D, Bierman A, Figueiro M, Schernhammer E & Rea M (2010) Ecological measurements of light exposure, activity and circadian disruption. Lighting Research & Technology, 42, 271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk JA & Lee TM (2005) Restraint stress delays reentrainment in male and female diurnal and nocturnal rodents. J Biol Rhythms, 20, 245–256. [DOI] [PubMed] [Google Scholar]
- Monk TH & Folkard S (1976) Adjusting to the changes to and from Daylight Saving Time. Nature, 261, 688. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N (1999) Masking: History, Definitions, and Measurement. Chronobiology International, 16, 415–429. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Abbott SM, Carroll JE, Davis CJ, Dijk DJ, Dinges DF, Gehrman PR, Ginsburg GS, Gozal D, Haack M, Lim DC, Macrea M, Pack AI, Plante DT, Teske JA & Zee PC (2016) Developing biomarker arrays predicting sleep and circadian-coupled risks to health. Sleep, 39, 727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES & Holtzman DM (2016) Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science, 354, 1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obayashi K, Saeki K & Kurumatani N (2018) Bedroom Light Exposure at Night and the Incidence of Depressive Symptoms: A Longitudinal Study of the HEIJO-KYO Cohort. Am J Epidemiol, 187, 427–434. [DOI] [PubMed] [Google Scholar]
- Papantoniou K, Vetter C & Schernhammer ES (2016) Shift work practices and opportunities for intervention. Occupational and Environmental Medicine. [DOI] [PubMed] [Google Scholar]
- Park MK, Freisling H, Huseinovic E, Winkvist A, Huybrechts I, Crispim SP, de Vries JHM, Geelen A, Niekerk M, van Rossum C & Slimani N (2018) Comparison of meal patterns across five European countries using standardized 24-h recall (GloboDiet) data from the EFCOVAL project. European journal of nutrition, 57, 1045–1057. [DOI] [PubMed] [Google Scholar]
- Parsons MJ, Moffitt TE, Gregory AM, Goldman-Mellor S, Nolan PM, Poulton R & Caspi A (2015) Social jetlag, obesity and metabolic disorder: investigation in a cohort study. International Journal of Obesity, 39, 842–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AJK, Chen PY & Robinson PA (2010) Probing the Mechanisms of Chronotype Using Quantitative Modeling. Journal of Biological Rhythms, 25, 217–227. [DOI] [PubMed] [Google Scholar]
- Phillips AJK, Clerx WM, O’Brien CS, Sano A, Barger LK, Picard RW, Lockley SW, Klerman EB & Czeisler CA (2017) Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Scientific Reports, 7, 3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilorz V, Helfrich-Forster C & Oster H (2018) The role of the circadian clock system in physiology. Pflugers Archiv: European journal of physiology, 470, 227–239. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Tiseo R, Smolensky MH, Hermida RC, Ayala DE & Fabbian F (2012) Circadian rhythms and cardiovascular health. Sleep Medicine Reviews, 16, 151–166. [DOI] [PubMed] [Google Scholar]
- Portnov BA, Stevens RG, Samociuk H, Wakefield D & Gregorio DI (2016) Light at night and breast cancer incidence in Connecticut: An ecological study of age group effects. Science of The Total Environment, 572, 1020–1024. [DOI] [PubMed] [Google Scholar]
- Potter GDM, Skene DJ, Arendt J, Cade JE, Grant PJ & Hardie LJ (2016) Circadian Rhythm and Sleep Disruption: Causes, Metabolic Consequences, and Countermeasures. Endocrine Reviews, 37, 584–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats-Uribe A, Tobías A & Prieto-Alhambra D (9000) Excess Risk of Fatal Road Traffic Accidents on the Day of Daylight Saving Time Change. Epidemiology, Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- Price LLA, Lyachev A & Khazova M (2017) Optical performance characterization of light-logging actigraphy dosimeters. J. Opt. Soc. Am. A, 34, 545–557. [DOI] [PubMed] [Google Scholar]
- Putilov AA (2017) Owls, larks, swifts, woodcocks and they are not alone: A historical review of methodology for multidimensional self-assessment of individual differences in sleep-wake pattern. Chronobiol Int, 34, 426–437. [DOI] [PubMed] [Google Scholar]
- Qian J & Scheer FAJL (2016) Circadian System and Glucose Metabolism: Implications for Physiology and Disease. Trends in Endocrinology & Metabolism, 27, 282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea MS, Bierman A, Figueiro MG & Bullough JD (2008) A new approach to understanding the impact of circadian disruption on human health. Journal of Circadian Rhythms, 6, 7–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld WJ, Minors DS & Waterhouse JM (1993) Circadian Rhythms and Masking: An Overview. Chronobiology International, 10, 306–312. [DOI] [PubMed] [Google Scholar]
- Roberts L, Leise TL, Noguchi T, Galschiodt AM, Houl JH, Welsh DK & Holmes TC (2015) Light evokes rapid circadian network oscillator desynchrony followed by gradual phase retuning of synchrony. Current biology: CB, 25, 858–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T (2012) What is chronotype? Sleep and Biological Rhythms, 10, 75–76. [Google Scholar]
- Roenneberg T, Allebrandt K, Merrow M & Vetter C (2012) Social Jetlag and Obesity. Current Biology, 22, 939–943. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Daan S & Merrow M (2003a) The Art of Entrainment. J Biol Rhyth, 18, 183–194. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M & Merrow M (2007a) Epidemiology of the human circadian clock. Sleep Medicine Reviews, 11, 429–438. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A & Merrow M (2004) A marker for the end of adolescence. Current Biology, 14, R1038–R1039. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kumar CJ & Merrow M (2007b) The human circadian clock entrains to sun time. Current Biology, 17, R44–R45. [DOI] [PubMed] [Google Scholar]
- Roenneberg T & Merrow M (2016) The Circadian Clock and Human Health. Current Biology, 26, R432–R443. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Wirz-Justice A & Merrow M (2003b) Life between Clocks: Daily Temporal Patterns of Human Chronotypes. Journal of Biological Rhythms, 18, 80–90. [DOI] [PubMed] [Google Scholar]
- Rüger M & Scheer FAJL (2009) Effects of circadian disruption on cardiometabolic system. Reviews in Endocrine & Metabolic Disorders, 10, 245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutters F, Lemmens SG, Adam TC, Bremmer MA, Elders PJ, Nijpels G & Dekker JM (2014) Is Social Jetlag Associated with an Adverse Endocrine, Behavioral, and Cardiovascular Risk Profile? Journal of Biological Rhythms. [DOI] [PubMed] [Google Scholar]
- Sack RL & Lewy AJ (1997) Melatonin as a chronobiotic: treatment of circadian desynchrony in night workers and the blind. J Biol Rhythms, 12, 595–603. [DOI] [PubMed] [Google Scholar]
- Scheer FAJL, Hilton MF, Mantzoros CS & Shea SA (2009) Adverse metabolic and cardiovascular consequences of circadian misalignement. Proc Natl Acad Sci USA, 106, 4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarke C, Lahens NF, Rhoades SD, Campbell A, Bittinger K, Bailey A, Hoffmann C, Olson RS, Chen L, Yang G, Price TS, Moore JH, Bushman FD, Greene CS, Grant GR, Weljie AM & FitzGerald GA (2017) A Pilot Characterization of the Human Chronobiome. Sci Rep, 7, 17141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeldon AC, Phillips AJK & Dijk D-J (2017) The effects of self-selected light-dark cycles and social constraints on human sleep and circadian timing: a modeling approach. Scientific Reports, 7, 45158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene DJ, Skornyakov E, Chowdhury NR, Gajula RP, Middleton B, Satterfield BC, Porter KI, Van Dongen HPA & Gaddameedhi S (2018) Separation of circadian- and behavior-driven metabolite rhythms in humans provides a window on peripheral oscillators and metabolism. Proceedings of the National Academy of Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolensky MH, Hermida RC, Reinberg A, Sackett-Lundeen L & Portaluppi F (2016) Circadian disruption: New clinical perspective of disease pathology and basis for chronotherapeutic intervention. Chronobiol Int, 33, 1101–1119. [DOI] [PubMed] [Google Scholar]
- Bureau of Labor Statistics (2018) https://data.bls.gov/pdq/SurveyOutputServlet.
- Stevens RG, Blask DE, Brainard GC, Hansen J, Lockley SW, Provencio I, Rea MS & Reinlib L (2007) Meeting report: the role of environmental lighting and circadian disruption in cancer and other diseases. Environ Health Perspect, 115, 1357–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RG, Brainard GC, Blask DE, Lockley SW & Motta ME (2014) Breast cancer and circadian disruption from electric lighting in the modern world. CA: A Cancer Journal for Clinicians, 64, 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RG, Hansen J, Costa G, Haus E, Kauppinen T, Aronson KJ, Castaño-Vinyals G, Davis S, Frings-Dresen MHW, Fritschi L, Kogevinas M, Kogi K, Lie J-A, Lowden A, Peplonska B, Pesch B, Pukkala E, Schernhammer E, Travis RC, Vermeulen R, Zheng T, Cogliano V & Straif K (2011) Considerations of circadian impact for defining ‘shift work’ in cancer studies: IARC Working Group Report. Occupational and Environmental Medicine, 68, 154–162. [DOI] [PubMed] [Google Scholar]
- Stevens RG & Rea MS (2001) Light in the built environment: potential role of circadian disruption in endocrine disruption and breast cancer. Cancer Causes Control, 12, 279–287. [DOI] [PubMed] [Google Scholar]
- Suh S, Ryu H, Kim S, Choi S & Joo EY (2017) Using Mid-Sleep Time to Determine Chronotype in Young Adults with Insomnia-Related Symptoms. Sleep Med Res, 8, 107–111. [Google Scholar]
- Sulli G, Manoogian ENC, Taub PR, & Panda S (2018). Training the Circadian Clock, Clocking the Drugs, and Drugging the Clock to Prevent, Manage, and Treat Chronic Disease. Trends Pharmacol Sci, 39, 812–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan K, Klerman EB & Phillips AJK (2017) Are Individual Differences in Sleep and Circadian Timing Amplified by Use of Artificial Light Sources? Journal of Biological Rhythms, 32, 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS, Hong H-K, Ko CH & McDearmon EL (2008) The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet, 9, 764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaiss Christoph A., Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler Anouk C., Abramson L, Katz Meirav N., Korem T, Zmora N, Kuperman Y, Biton I, Gilad S, Harmelin A, Shapiro H, Halpern Z, Segal E & Elinav E (2014) Transkingdom Control of Microbiota Diurnal Oscillations Promotes Metabolic Homeostasis. Cell, 159, 514–529. [DOI] [PubMed] [Google Scholar]
- Van Dycke Kirsten C.G., Rodenburg W, van Oostrom Conny T.M., van Kerkhof Linda W.M., Pennings Jeroen L.A., Roenneberg T, van Steeg H & van der Horst Gijsbertus T.J. (2015) Chronically Alternating Light Cycles Increase Breast Cancer Risk in Mice. Current Biology, 25, 1932–1937. [DOI] [PubMed] [Google Scholar]
- van Someren EJW, Hagebeuk EEO, Lijzenga C, Scheltens P, de Rooij SEJA, Jonker C, Pot A-M, Mirmiran M & Swaab DF (1996) Circadian rest—activity rhythm disturbances in alzheimer’s disease. Biological Psychiatry, 40, 259–270. [DOI] [PubMed] [Google Scholar]
- Vetter C, Dashti HS, Lane JM, Anderson SG, Schernhammer ES, Rutter MK, Saxena R & Scheer F (2018) Night Shift Work, Genetic Risk, and Type 2 Diabetes in the UK Biobank. Diabetes Care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter C, Devore EE, Ramin CA, Speizer FE, Willett WC & Schernhammer ES (2015a) Mismatch of Sleep and Work Timing and Risk of Type 2 Diabetes. Diabetes Care, 38, 1707–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter C, Fischer D, Matera JL & Roenneberg T (2015b) Aligning Work and Circadian Time in Shift Workers Improves Sleep and Reduces Circadian Disruption. Current Biology, 25, 907–911. [DOI] [PubMed] [Google Scholar]
- VoPham T, Weaver MD, Vetter C, Hart JE, Tamimi RM, Laden F & Bertrand KA (2018) Circadian misalignment and hepatocellular carcinoma incidence in the United States. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas MV, Garg AX, Iansavichus AV, Costella J, Donner A, Laugsand LE, Janszky I, Mrkobrada M, Parraga G & Hackam DG (2012) Shift work and vascular events: systematic review and meta-analysis. BMJ, 345:e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens SMT, Christou S, Isherwood C, Middleton B, Gibbs MA, Archer SN, Skene DJ & Johnston JD (2017) Meal Timing Regulates the Human Circadian System. Current Biology, 27, 1768–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz-Justice A, Benedetti F & Terman M (2009) Chronotherapeutics for Affective Disorders. Karger, Basel, Switzerland. [Google Scholar]
- Wittenbrink N, Ananthasubramaniam B, Münch M, Koller B, Maier B, Weschke C, Bes F, de Zeeuw J, Nowozin C, Wahnschaffe A, Wisniewski S, Zaleska M, Bartok O, Ashwal-Fluss R, Lammert H, Herzel H, Hummel M, Kadener S, Kunz D & Kramer A (2018) High-accuracy determination of internal circadian time from a single blood sample. The Journal of Clinical Investigation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witting W, Kwa IH, Eikelenboom P, Mirmiran M & Swaab DF (1990) Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biological Psychiatry, 27, 563–572. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M & Roenneberg T (2006) Social jetlag: misalignment of biological and social time. Chronobiology International, 23, 497–509. [DOI] [PubMed] [Google Scholar]
- Wong PM, Hasler BP, Kamarck TW, Muldoon MF & Manuck SB (2015) Social Jetlag, Chronotype, and Cardiometabolic Risk. The Journal of Clinical Endocrinology & Metabolism, 100, 4612–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Suzuki T, Mizoro Y, Kori H, Okada K, Chen Y, Fustin J-M, Yamazaki F, Mizuguchi N, Zhang J, Dong X, Tsujimoto G, Okuno Y, Doi M & Okamura H (2013) Mice Genetically Deficient in Vasopressin V1a and V1b Receptors Are Resistant to Jet Lag. Science, 342, 85–90. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M & Tei H (2000) Resetting central and peripheral circadian oscillators in transgenic rats. Science, 288, 682–685. [DOI] [PubMed] [Google Scholar]
- Yan L, Smale L & Nunez AA (2018) Circadian and photic modulation of daily rhythms in diurnal mammals. The European journal of neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuurbier LA, Luik AI, Hofman A, Franco OH, Van Someren EJW & Tiemeier H (2015) Fragmentation and Stability of Circadian Activity Rhythms Predict Mortality: The Rotterdam Study. American Journal of Epidemiology, 181, 54–63. [DOI] [PubMed] [Google Scholar]