Abstract
Objective: In the present study, we aimed to investigate the potential role of fatty acid synthase (FASN) in the development and progression of colorectal cancer (CRC).
Materials and methods: FASN levels were analyzed in human CRC tissues and adjacent normal tissues by Western blots and immunohistochemistry. Potential roles of FASN in regulating CRC cell proliferation and migration were examined by genetic manipulation in vitro. The molecular signaling was determined to understand the mechanisms of observed FASN effects.
Results: FASN level was upregulated in CRC tissues and high expression of FASN was significantly associated with lymph node metastasis, TNM (Tumor, Node, Metastases) stage and poor prognosis in patients with CRC. Knockdown of FASN attenuated CRC cell proliferation and migration in vitro while FASN overexpression possessed the opposite effects. FASN regulated AMP-activated protein kinase (AMPK)/mechanistic target of rapamycin (mTOR) pathway in CRC cells.
Conclusion: FASN enhanced CRC cell proliferation and metastasis potentially through AMPK/mTOR pathway, indicating that FASN/AMPK/mTOR signaling axis may serve as a potential target for the treatment of CRC.
Keywords: FASN, proliferation, metastasis, AMPK/mTOR pathway, colorectal cancer
Introduction
Colorectal cancer (CRC) is the third most common malignant cancer and the fourth leading cause of cancer-related death in the world.1 In spite of emerging achievements in diagnosis and treatment of CRC, the incidence and mortality are still rampant.2 Hence, it is essential to identify effective and specific biomarkers of CRC and develop new treatment target to halt progression of CRC.
There are increasing evidence that metabolic reprogramming is a hallmark of cancer.3,4 Among all the metabolic changes, enhanced lipogenesis is considered as an important factor to support cancer cell unrestricted proliferation and metastasis.5–7 As a key enzyme involved in the de novo biogenesis of fatty acids, fatty acid synthase (FASN) plays a vital role in energy homeostasis by converting excess carbon intake into fatty acids for storage. It has been reported that FASN was overexpressed in several malignant diseases, such as ovarian cancer, breast cancer, prostate cancer and gastric cancer.8–11 Besides, overexpression of FASN was reported to be associated with tumor cell proliferation, metastasis, poor prognosis and high risk of recurrence.12–14 Recently, there were several studies focusing on FASN in CRC, which described that FASN was overexpressed in CRC and inhibition of FASN blocked the proliferation and metastasis of CRC cells,5,15,16 but the precise underlying mechanisms are equivocal and remain to be further explored.
In this study, we first detected the expression of FASN in CRC tissues and paired non-tumor tissues, and then investigated its effects on CRC cell proliferation and migration in vitro. Moreover, we also examined its effects on regulation of AMPK/mTOR pathway. Our studies demonstrate that FASN may function as a regulator for AMPK/mTOR pathway to mediate CRC cell proliferation and metastasis, which reveals a possible implication for new approaches to CRC therapy.
Materials and methods
CRC tissues and cell lines
A total of 18 pairs of primary CRC tumors and surrounding normal mucosal tissues were obtained from patients who underwent curative surgery from 2016 to 2017 at the Department of General Surgery, the First Affiliated Hospital of Soochow University (Suzhou, Jiangsu, People’s Republic of China). Another 113 formalin-fixed, paraffin-embedded tissues of CRC diagnosed between 2009 and 2014 at the First Affiliated Hospital of Soochow University were enrolled. The clinicopathologic information is shown in Table 1. Written informed consent was obtained from all patients. In addition, the study was approved by the First Affiliated Hospital of Soochow University Ethics Committee and was conducted in compliance with the Declaration of Helsinki.
Table 1.
The relationships between FASN and clinicopathological factors in 113 patients with colorectal cancer
| Clinic parameters | Total | FASN expression | χ2 | P-value | |
|---|---|---|---|---|---|
| None or low | High | ||||
| Total | 113 | 76 (67.2%) | 37 (32.8%) | ||
| Age (years) | |||||
| <65 | 46 | 31 (67.4%) | 15 (32.6%) | 0.001 | 0.980 |
| ≥65 | 67 | 45 (67.2%) | 22 (32.8%) | ||
| Gender | |||||
| Male | 70 | 47 (67.1%) | 23 (32.9%) | 0.001 | 0.974 |
| Female | 43 | 29 (67.4%) | 14 (32.6%) | ||
| Tumor size | |||||
| <5 cm | 66 | 46 (69.7%) | 20 (30.3%) | 0.429 | 0.512 |
| ≥5 cm | 47 | 30 (63.8%) | 17 (36.2%) | ||
| Tumor location | |||||
| Left-sided colon | 32 | 22 (68.8%) | 10 (31.2%) | 1.640 | 0.440 |
| Right-sided colon | 40 | 24 (60.0%) | 16 (40.0%) | ||
| Rectum | 41 | 30 (73.2%) | 11 (26.8%) | ||
| Depth of invasion | |||||
| T1-2 | 25 | 18 (72.0%) | 7 (28.0%) | 0.328 | 0.567 |
| T3-4 | 88 | 58 (65.9%) | 30 (34.1%) | ||
| Lymph node metastasis | |||||
| No | 69 | 56 (81.2%) | 13 (18.8%) | 15.553 | <0.001*** |
| Yes | 44 | 20 (45.5%) | 24 (54.5%) | ||
| TNM stage | |||||
| I | 18 | 15 (83.3%) | 3 (16.7%) | 19.559 | <0.001*** |
| II | 50 | 41 (82.0%) | 9 (18.0%) | ||
| III | 43 | 20 (46.5%) | 23 (53.5%) | ||
| IV | 2 | 0 (0.0%) | 2 (100.0%) | ||
Abbreviation: FASN, fatty acid synthase.
CRC cell lines (SW480, HCT116, SW620, LoVo, CaCo2) and normal colon epithelial cells (NCM460) were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, People’s Republic of China) and were cultured in Roswell Park Memorial Institute 1640 medium (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) or DMEM (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin G sodium and 100 µg/mL streptomycin sulfate (Gibco; Thermo Fisher Scientific, Inc.). All cells were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Western blot assay and immunohistochemistry (IHC)
Western blot assay and IHC were described in our previous study.17,18 Antibodies used in this study are as follows: anti-FASN (#3180, Cell Signaling Technology (CST)), anti-p-AMPKα (Thr172) (#2535, CST), anti-p-mTOR (S2448) (#9205, CST), anti-AMPKα (#2532, CST), anti-mTOR (#2983, CST) and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH, #AG019, Beyotime).
Construction of stable cell lines
SW480 and HCT116 cell lines stably expressing FASN-specific short hairpin RNA (shRNA) and a plasmid encoding human FASN were generated using a lentivirus technique (GenePharma, Shanghai, People’s Republic of China), as described in our previous study.17 The human FASN shRNA target sequences are 5′-TACGACTACGGCCCTCATT-3′.
Colony formation assay
In total, 1,000 cells were placed in 6-well plates for 7–10 days, and then fixed with 4% paraformaldehyde (Beyotime Institute of Biotechnology; Haimen, People’s Republic of China) and stained with 0.1% crystal violet (Beyotime Institute of Biotechnology). Detailed protocol was described in our previous study.11
Cell migration assay
Transwell migration assay was used in this study. Detailed protocol was described in our previous study.11,18
Statistical analysis
Data are presented as mean±standard error of the mean. Statistical significance was analyzed using a Student t-test (paired or unpaired, two-tailed). Pearson χ2 test was used to analyze the relationships between FASN expression and clinicopathologic factors. A value of P<0.05 was considered to indicate a statistically significant difference.
Result
FASN is highly expressed in human CRC tissues and is associated with LNM and TNM stage
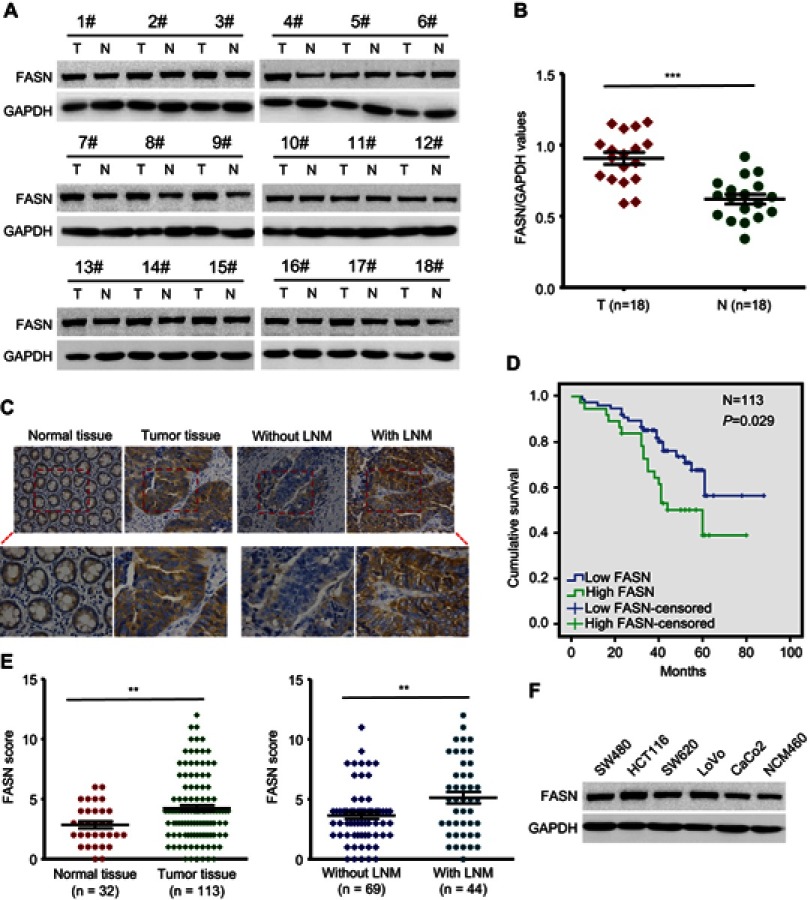
Previous studies have described that FASN was overexpressed in CRC and related to disease progression.5,15,16 To further confirm the role of FASN in CRC, we measured the level of FASN protein in 18 randomly selected pairs of CRC tissues and surrounding normal tissues by Western blots. Results showed that FASN expression was significantly higher in CRC tissues than in surrounding normal tissues (P<0.001, Figure 1A and B).
Figure 1.
FASN is upregulated in human CRC. (A) FASN protein level in 18 pairs of CRC tumors and surrounding normal tissues was measured by Western blot. GAPDH as a loading control. (B) Scatter plot analysis of FASN/GAPDH value in 18 CRC tumors and paired normal tissues. (C) Representative images of FASN IHC staining in CRC tissues and normal tissues (top, magnification, x200; bottom, magnification, x400). (D) Kaplan-Meier survival curve of CRC patients was analyzed according to FASN expression. The P-value was calculated by log-rank test. (E) Scatter plot analysis of FASN IHC score in CRC tissues and normal tissues. (F) The FASN protein level in five CRC cell lines and non-CRC NCM460 cell lines. Statistical significance was determined by a two-tailed, Student t-test. **P<0.01. ***P<0.001.
Abbreviations: FASN, fatty acid synthase; CRC, colorectal cancer; T, tumor; N, matched normal tissues; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. IHC, immunohistochemistry.
We then conducted IHC to assess the level of FASN protein in 113 CRC tissues and 32 adjacent nontumorous tissues. The CRC tissues presented FASN positive staining, whereas the normal tissues showed negative staining (Figure 1C). The difference was statistically significant (P<0.01, Figure 1E left). Our results also revealed that FASN expression in patients with LNM was further upregulated compared with those without LNM (P<0.01, Figure 1C and E). Besides, cytologic experiments showed that there was higher FASN expression in five CRC cell lines (SW480, HCT116, SW620, LoVo, CaCo2), compared to non-CRC NCM460 cells (Figure 1F).
Next, we studied the relationship between FASN expression and clinicopathological parameters in 113 patients suffered from CRC. We found that the increased expression of FASN was significantly correlated with LNM (P<0.001, Table 1) and TNM stage (P<0.001, Table 1). However, there was no significant difference regarding age, gender, tumor size, tumor location or depth of invasion (P>0.05, Table 1).
These data suggested that FASN expression is upregulated in human CRC tissues and enhanced FASN expression is associated with the development of CRC.
High expression of FASN leads to poor prognosis
To investigate the prognostic role of FASN protein in CRC patients, we next used the Kaplan–Meier method with log-rank test to plot the overall survival curve based on the expression level of FASN. The results showed that patients with high FASN expression had poorer overall survival rate (P=0.029, Figure 1D), which demonstrated the prognostic value of FASN in CRC.
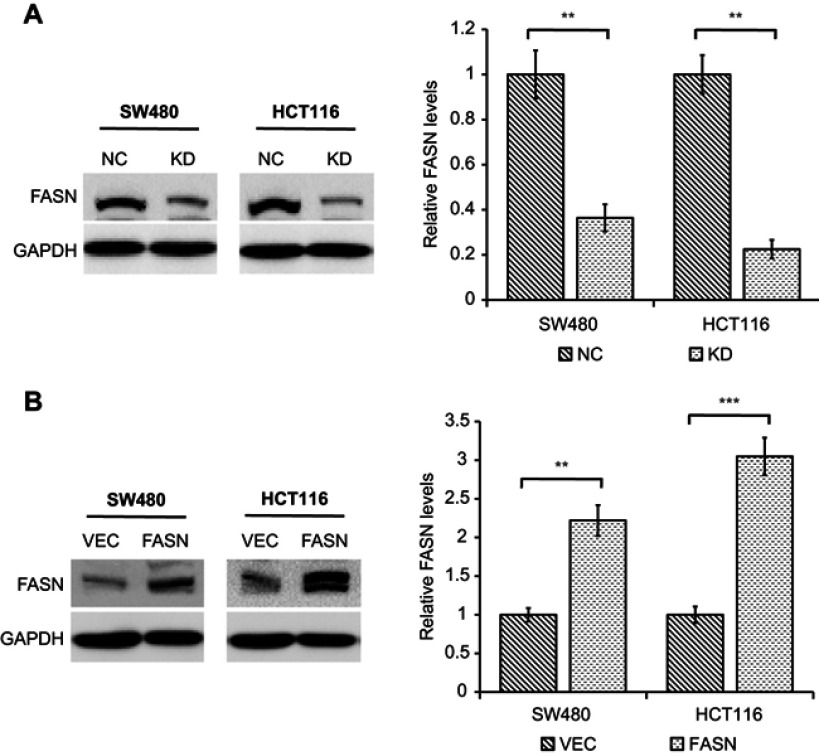
FASN knockdown and overexpression efficiency on CRC cells
To explore the roles of FASN in CRC cells, we used retroviral transduction to stably knockdown and overexpress FASN expression in SW480 and HCT116 cell lines. Western blot analysis was used to confirm the knockdown and overexpression efficiency. As shown in Figure 2A, FASN expression was statistically decreased at protein levels in cells stably transfected with shRNA against FASN (KD) compared with cells transfected with control-shRNA (NC) (P<0.01). Similarly, Western blot analysis showed that FASN expression was distinctly increased at protein levels in cells stably transfected with a plasmid encoding human FASN (FASN) compared with cells transfected with empty vector (VEC) (P<0.01, Figure 2B).
Figure 2.
Western blot analysis of FASN knockdown or overexpression efficiency in CRC cell lines. (A) The protein levels of FASN in CRC cells stably transfected with control-shRNA (NC) or shRNA against FASN (KD) were tested by Western blot. The bands were quantified and presented as the mean±SEM of three independent experiments (right). (B) The protein levels of FASN in CRC cells stably transfected with empty vector (VEC) or plasmid encoding human FASN (FASN) were tested by Western blot. The bands were quantified and presented as the mean±SEM of three independent experiments (right). Statistical significance was determined by a two-tailed, paired Student t-test. **P<0.01. ***P<0.001.
Abbreviations: FASN, fatty acid synthase; CRC, colorectal cancer; NC, control-shRNA; KD, shRNA against FASN; VEC, empty vector; FASN, plasmid encoding human FASN; SEM, standard error of the mean; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
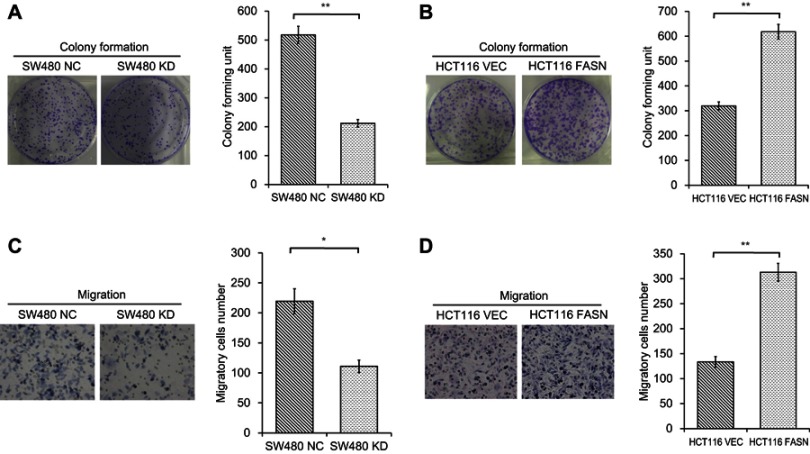
FASN promotes CRC cell proliferation and migration in vitro
Since FASN is overexpressed in CRC tissues, we then performed experiments to test the effects of FASN on CRC cell proliferation and metastasis. Colony formation assays showed that FASN knockdown resulted in the weakness of the ability to form foci in SW480 cells (P<0.01, Figure 3A). By contrast, FASN overexpression had the opposite effect in HCT116 cells (P<0.01, Figure 3B). Next, migration assays were conducted to assess the effects of FASN on CRC cell migration ability. Results showed that the migration ability of SW480 cells was greatly impaired when cells lacked FASN (P<0.05, Figure 3C). In contrary, FASN overexpression significantly enhanced the migration ability in HCT116 cells (P<0.01, Figure 3D). These results indicated that FASN enhances CRC cell proliferation and migration in vitro.
Figure 3.
Effects of FASN on CRC cell proliferation and migration in vitro. (A) Colony formation assays were performed in SW480 cells stably transfected with control-shRNA (NC) or shRNA against FASN (KD). Representative photographs are presented (left; magnification, x1) and the colonies were counted (right), presented as the mean±SEM (n=3). (B) Colony formation assays were performed in HCT116 cells stably transfected with empty vector (VEC) or plasmid encoding human FASN (FASN). Representative photographs are presented (left; magnification, x1) and the colonies were counted (right), presented as the mean±SEM (n=3). (C) Migration assays were conducted in SW480 NC and KD cells. Representative photographs are presented (left; magnification, x200) and the number of migratory cells (right) were counted, presented as the mean±SEM (n=3). (D) Migration assays were conducted in HCT116 VEC and FASN cells. Representative photographs are presented (left; magnification, x200) and the number of migratory cells (right) were counted, presented as the mean±SEM (n=3). Statistical significance was determined by a two-tailed, unpaired Student t-test. **P<0.01. *P<0.05.
Abbreviations: FASN, fatty acid synthase; CRC, colorectal cancer; NC, control-shRNA; KD, shRNA against FASN; VEC, empty vector; FASN, plasmid encoding human FASN; SEM, standard error of the mean.
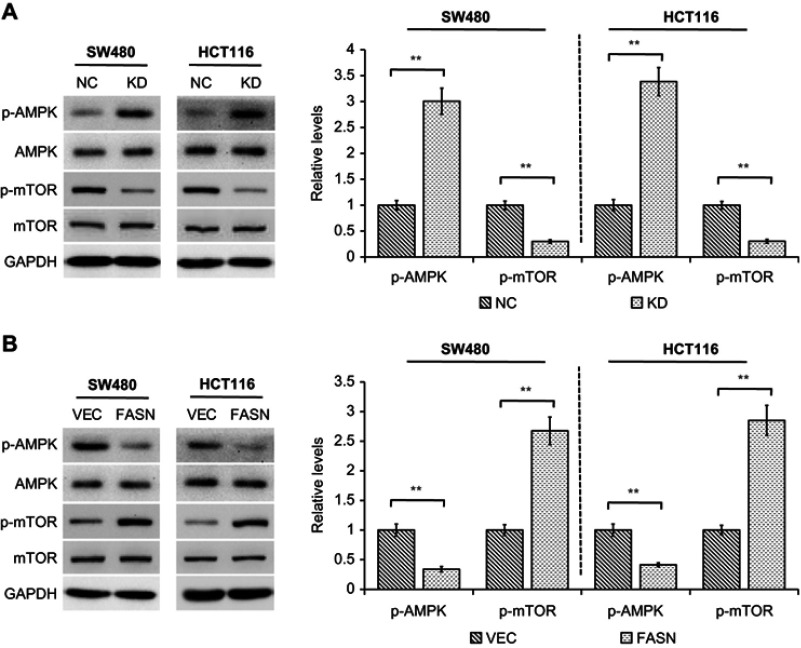
FASN regulates AMPK/mTOR pathway in CRC cells
Existing studies suggest that dysregulation of AMPK/mTOR signaling pathway is associated with a variety of cancers including non-small cell lung cancer,19 pancreatic cancer20 and CRC.21 Therefore, we sought to detect whether AMPK/mTOR pathway is implicated in the FASN-mediated CRC cell proliferation and metastasis. We found that knockdown of FASN significantly increased the level of phosphorylated AMPK (p-AMPK) in both SW480 and HCT116 cells, but not the total AMPK level (P<0.01, Figure 4A). At the same time, the phosphorylation of mTOR (p-mTOR) was markedly decreased by FASN silencing even though the total level of mTOR was not changed (P<0.01, Figure 4A). In contrast, overexpression of FASN led to an inhibition of AMPK phosphorylation while an increase of mTOR phosphorylation concomitantly (P<0.01, Figure 4B). These assays revealed that FASN acts as an important regulator on AMPK/mTOR pathway in CRC cells.
Figure 4.
FASN regulates AMPK/mTOR pathway. (A) Western blot analysis of indicated proteins in cells stably transfected with control-shRNA (NC) or shRNA against FASN (KD). GAPDH as a loading control. The bands were quantified and presented as the mean±SEM of three independent experiments (right). (B) Western blot analysis of indicated proteins in cells stably transfected with empty vector (VEC) or plasmid encoding human FASN (FASN). GAPDH as a loading control. The bands were quantified and presented as the mean±SEM of three independent experiments (right). Statistical significance was determined by a two-tailed Student t-test. **P<0.01.
Abbreviations: FASN, fatty acid synthase; NC, control-shRNA; KD, shRNA against FASN; VEC, empty vector; FASN, plasmid encoding human FASN; SEM, standard error of the mean; AMPK, AMP-activated protein kinase; mTOR, mechanistic target of rapamycin; p-, phosphorylated; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Discussion
It is widely accepted that metabolic reprogramming is considered as a hallmark of cancer and cancer cells acquire characteristic changes in metabolism to support their unrestricted proliferation and metastasis.5–7 Among all the metabolic alterations, activation of the de novo fatty acid synthesis pathway is indispensable for carcinogenesis.5,22 FASN, as a key enzyme of de novo fatty acid synthesis, has been commonly found overexpressed in cancers,8–11 providing cancer cells with an extra source of cellular fatty acids. Besides, studies showed that overexpression of FASN was associated with tumor cell proliferation, metastasis, poor prognosis and high risk of recurrence.12–14 In CRC, FASN was also shown to be highly expressed and associated with the development and progression of CRC.5,15,16 However, the precise mechanisms of the regulation of cell proliferation and metastasis by FASN are not well understood and remain to be further explored.
In the present study, we provided further evidence that FASN expression was higher in CRC tissues than that in the adjacent normal tissues. Moreover, clinical data analyses showed that high expression of FASN was significantly associated with LNM, TNM stage and poor prognosis in patients with CRC. We also evidenced that silencing FASN weakened the ability of CRC cell proliferation and migration in vitro while FASN overexpression had the opposite effects, highlighting the critical role of FASN in CRC.
Considering that dysregulation of AMPK/mTOR signaling pathway is associated with variety of cancers including non-small cell lung cancer,17 pancreatic cancer18 and CRC,19 we then asked whether AMPK/mTOR pathway is implicated in the FASN-mediated CRC cell proliferation and metastasis. In accordance with the previous studies,11,23 which indicated the effects of FASN on AMPK in gastric cancer and ovarian cancer, we also showed that FASN modulated AMPK/mTOR pathway in CRC cells. Evidence15 pointed out that inhibition of FASN led to a decrease of ATP production in CRC cell. ATP depletion is known to activate AMPK, which gauges the AMP and ATP levels of the cell.24 At the same time, AMPK, as a pivotal energy sensor governing normal and cancer cell metabolism, is a major negative regulator of the mTOR pathway, which intimately relates to cell proliferation, metastasis and invasion.25,26 Thus, it is plausible that in CRC cells, enhanced FASN expression may increase ATP production, resulting in AMPK inhibition and mTOR activation, thus promoting CRC cell proliferation and metastasis.
In summary, our present study indicated that FASN was upregulated in CRC tissues and high expression of FASN was significantly associated with LNM, TNM stage and poor prognosis in patients with CRC. We also emphasized the role of FASN in regulating CRC cell proliferation and migration in vitro. Moreover, we revealed a potential mechanism through which FASN could induce CRC cell proliferation and metastasis via its regulation of the AMPK/mTOR pathway, indicating that FASN/AMPK/mTOR signaling axis may serve as a potential target for treatment of CRC.
Acknowledgments
This study was supported by the Primary Research & Development Plan of Jiangsu Province Special Fund (grant no: BE2018659).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi: 10.1136/gutjnl-2015-310912 [DOI] [PubMed] [Google Scholar]
- 2.Bouvier AM, Launoy G, Bouvier V, et al. Incidence and patterns of late recurrences in colon cancer patients. Int J Cancer. 2015;137:2133–2138. doi: 10.1002/ijc.29578 [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 4.Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17(4):351–359. doi: 10.1038/ncb3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Xi Q, Wu G. Fatty acid synthase regulates invasion and metastasis of colorectal cancer via Wnt signaling pathway. Cancer Med. 2016;5(7):1599–1606. doi: 10.1002/cam4.711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov. 2011;10:671–684. doi: 10.1038/nrd3504 [DOI] [PubMed] [Google Scholar]
- 7.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gansler TS, Hardman W, Hunt DA, Schaffel S, Hennigar RA. Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Hum Pathol. 1997;28(6):686–692. [DOI] [PubMed] [Google Scholar]
- 9.Zielinska HA, Holly JMP, Bahl A, Perks CM. Inhibition of FASN and ERα signalling during hyperglycaemia-induced matrix-specific EMT promotes breast cancer cell invasion via a caveolin-1-dependent mechanism. Cancer Lett. 2018;419:187–202. doi: 10.1016/j.canlet.2018.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koochekpour S, Majumdar S, Azabdaftari G, et al. Serum glutamate levels correlate with gleason score and glutamate blockadedecreases proliferation, metastasis, and invasion and induces apoptosis in prostate cancer cells. Clin Cancer Res. 2012;18(21):5888–5901. doi: 10.1158/1078-0432.CCR-12-1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun L, Yao Y, Pan G, et al. Small interfering RNA-mediated knockdown of fatty acid synthase attenuates the proliferation and metastasis of human gastric cancer cells via the mTOR/Gli1 signaling pathway. Oncol Lett. 2018;16(1):594–602. doi: 10.3892/ol.2018.8648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alo PL, Visca P, Framarino ML, et al. Immunohistochemical study of fatty acid synthase in ovarian neoplasms. Oncol Rep. 2000;7(6):1383–1388. [DOI] [PubMed] [Google Scholar]
- 13.Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 2006;66(12):5977–5980. doi: 10.1158/0008-5472.CAN-05-4673 [DOI] [PubMed] [Google Scholar]
- 14.Migita T, Ruiz S, Fornari A, et al. Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer. J Natl Cancer Inst. 2009;101(7):519–532. doi: 10.1093/jnci/djp030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang L, Wu P, Senthilkumar R, et al. Loss of fatty acid synthase suppresses the malignant phenotype of colorectal cancer cells by down-regulating energy metabolism and mTOR signaling pathway. J Cancer Res Clin Oncol. 2016;142(1):59–72. doi: 10.1007/s00432-015-2000-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee KH, Lee MS, Cha EY, et al. Inhibitory effect of emodin on fatty acid synthase, colon cancer proliferation and apoptosis. Mol Med Rep. 2017;15(4):2163–2173. doi: 10.3892/mmr.2017.6254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun L, Yao Y, Lu T, et al. DAB2IP downregulation enhances the proliferation and metastasis of human gastric cancer cells by derepressing the ERK1/2 pathway. Gastroenterol Res Pract. 2018;2018:1–10. doi: 10.1155/2018/2968252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu T, Sun L, Zhu X. Yes-associated protein enhances proliferation and attenuates sensitivity to cisplatin in human gastric cancer cells. Biomed Pharmacother. 2018;105:1269–1275. doi: 10.1016/j.biopha.2018.06.031 [DOI] [PubMed] [Google Scholar]
- 19.Kang JI, Hong JY, Lee HJ, et al. Anti-tumor activity of yuanhuacine by regulating AMPK/mTOR signaling pathway and actin cytoskeleton organization in non-small cell lung cancer cell. PLoS One. 2015;10:e0144368. doi: 10.1371/journal.pone.0144368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipner MB, Marayati R, Deng Y, et al. Metformin treatment does not inhibit growth of pancreatic cancer patient-derived xenografts. PLoS One. 2015;10(12):e0144368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosono K, Endo H, Takahashi H, et al. Metformin suppresses azoxymethane-induced colorectal aberrant crypt foci by activating AMP-activated protein kinase. Mol Carcinog. 2010;49(7):662–671. doi: 10.1002/mc.20637 [DOI] [PubMed] [Google Scholar]
- 22.Fakih MG. Metastatic colorectal cancer: current state and future directions. J Clin Oncol. 2015;33:1809–1824. [DOI] [PubMed] [Google Scholar]
- 23.Wagner R, Stübiger G, Veigel D, et al. Multi-level suppression of receptor-PI3K-mTORC1 by fatty acid synthase inhibitors is crucial for their effcacy against ovarian cancer cells. Oncotarget. 2017;8:11600–11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhan P, Zhao S, Yan H, et al. α-enolase promotes tumorigenesis and metastasis via regulating AMPK/mTOR pathway in colorectal cancer. Mol Carcinog. 2017;56:1427–1437. doi: 10.1002/mc.22603 [DOI] [PubMed] [Google Scholar]
- 25.Qi D, Young LH. AMPK: energy sensor and survival mechanism in the ischemic heart. Trends Endocrinol Metab. 2015;26:422–429. doi: 10.1016/j.tem.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen MB, Zhang Y, Wei MX, et al. Activation of AMP-activated protein kinase (AMPK) mediates plumbagin-induced apoptosis and growth inhibition in cultured human colon cancer cells. Cell Signal. 2013;25(10):1993–2002. doi: 10.1016/j.cellsig.2013.05.026 [DOI] [PubMed] [Google Scholar]