Microvascular stasis in course of sepsis might be a consequence of hemolysis
There are various studies demonstrating that hemolysis or the presence of cell-free hemoglobin and heme causes microvascular stasis (Figure 1). Buurman and his team of researchers already showed that acute hemolysis (with plasma levels of cfHb ~20–30 µmol/L) – induced by infusion of water or pre-lysed red blood cells – was associated with an impaired renal, hepatic and intestinal microvasculature.2 They further showed that intraoperative hemolysis (with plasma levels of cfHb ~20 µmol/L) during major aortic surgery was associated with postoperative acute kidney injury.3 The offset times between occurring hemolysis and intestinal microvascular changes or renal microvascular changes were approximately 15–30 mins2 respective 120 mins3. Moreover, Vinchi and co-workers could reduce liver damage in a mouse model of heme overload in wild-type mice compared to hemopexin-null mice.4 They concluded that hemopexin prevents from hemolysis-induced hepatic microvascular stasis.4 Belcher et al compared different treatments to induce hemolysis and heme overload (eg, infusion of water, hemoglobin or heme) in transgenic sickle mice and found a relationship between microvascular stasis and total plasma heme concentrations (with plasma levels of heme ~25–80 µmol/L).5 They further proved that intravascular hemolysis during sickle cell disease elicits microvascular stasis via Toll-like receptor 4 signaling.5 Further studies of the researchers around Belcher and Vercellotti showed inhibition of hemoglobin-induced microvascular stasis in transgenic sickle mice by hemopexin and haptoglobin supplementation,6 overexpression of hemopexin7 or overexpression of ferritin heavy chain ferrioxidase.8
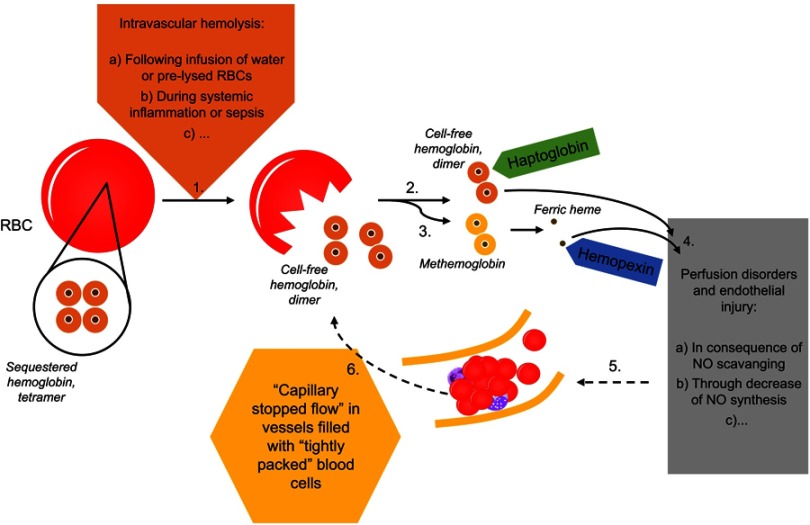
Figure 1.
In the course of intravascular hemolysis (1), eg, induced by infusion of water or pre-lysed red blood cells2–5 or as a consequence of systemic inflammation,15 hemoglobin will be released from the red blood cells (RBCs) into the plasma.1 Normally, cell-free hemoglobin or the during oxidation released ferric heme rapidly will be bound by its scavengers haptoglobin (2) and hemopexin (3). Massive hemolysis may result in saturation and depletion of these hemoglobin removal systems and consequently in an accumulation of hemoglobin and heme in plasma.1 Both, cell-free heme and hemoglobin mediate endothelial injury (4).1 Among others, cell-free hemoglobin is able to effectively scavenge nitric oxide (NO), which in turn leads to perfusion disorders (4).1 Microcirculatory disorders will be associated with a reduced perfused capillary density and red blood cell velocity (5).14 An increased amount of capillaries with either a low or a blocked flow is called as “capillary stopped-flow” or microvascular stasis (5).13,14 One consequence of changes in vessel diameter and concomitant rheological changes to blood cells will be the release of cell components (eg, hemoglobin) from red blood cells (6).13 Causality seems to apply in both directions (1–4 vs 4–1).
Based on these studies, plasma concentrations of cell-free hemoglobin of at least 20–30 µmol/L, which could be expected during acute hemolysis,2 may induce microcirculatory disorders in liver, kidneys and intestines.
Hemolysis in course of sepsis might be a consequence of microvascular stasis
Various other studies showed that microvascular stasis leads to hemolysis (Figure 1). Already, in 1940, Mumme described that renal stasis causes hemolysis.9 McKay and Whitaker found hemolysis during epinephrine infusion in rabbits, monkeys and dogs to be ultimately due to fragmentation of red blood cells in consequence of stasis.10 Similar to that, Dale and co-workers described intravascular hemolysis during lethal canine endotoxin shock as result of red blood cell accumulation in liver sinusoids and their subsequent fragmentation.11 Dao and Eberhard found pronounced vascular stasis with red blood cell sequestration in spleen, liver, lungs, kidneys and brain, and intravascular hemolysis during acute fatal babesiosis in hamsters.12 Moreover, in his overview of the mechanisms occurring in microcirculation during septic shock, Hinshaw figured out a release of cell components from blood cells (eg, hemoglobin) as a consequence of changes in vessel diameter and concomitant rheological changes to blood cells.13 So, red blood cells are mechanically damaged by altered flow properties in microvessels. Since capillary stopped flow is characterized by vessels filled with “tightly packed” blood cells, resting time and close contact of red blood cells to white blood cells are increased in low respective no flow areas. In addition to mechanical damage, an enzymatic damage of red blood cells by white blood cells would be possible, too.
Based on our own work using an animal sepsis model, it is not possible to clarify completely whether microvascular stasis causes hemolysis or vice versa.13,14 However, the time course pointed up that small intestinal microvasculature was affected first, while microvasculature in large intestines and liver changed simultaneously with occurring hemolysis. The release of hemoglobin from red blood cells was associated with microvascular stasis in both liver and intestines, but not with renal microvascular stasis.14 However, a concentration of cell-free hemoglobin of 20 µmol/L was exceeded only after an observation period of 240–360 mins. Since the offset time between hemolysis and renal microvascular changes was approximately 120 mins3, the effect of cell-free hemoglobin on renal microvasculature could also be expected at prolonged observation periods.
Conclusion
In any case, there is a relationship between the release of hemoglobin from red blood cells and the microvascular stasis in intestines and liver during sepsis and systemic inflammation. Causality seems to apply in both directions. One the one hand, microvascular stasis is one of the many triggers to release cell-free hemoglobin during sepsis.15 On the other hand, cell-free hemoglobin appears to be a kind of amplifier of microvascular disorders.
Since hemolysis is an easily measurable parameter, it could serve as a marker to indicate changes to abdominal organ microcirculation (difficult to measure and difficult to monitor). So, hemolysis might predict small intestinal microvascular stasis. Further studies are thus required to verify the link between microvascular stopped flow and intravascular hemolysis.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653 [DOI] [PubMed] [Google Scholar]
- 2.Hanssen SJ, Lubbers T, Hodin CM, Prinzen FW, Buurman WA, Jacobs MJ. Hemolysis results in impaired intestinal microcirculation and intestinal epithelial cell injury. World J Gastroenterol. 2011;17(2):213–218. doi: 10.3748/wjg.v17.i2.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vermeulen Windsant IC, Snoeijs MG, Hanssen SJ, et al. Hemolysis is associated with acute kidney injury during major aortic surgery. Kidney Int. 2010;77(10):913–920. doi: 10.1038/ki.2010.24 [DOI] [PubMed] [Google Scholar]
- 4.Vinchi F, Gastaldi S, Silengo L, Altruda F, Tolosano E. Hemopexin prevents endothelial damage and liver congestion in a mouse model of heme overload. Am J Pathol. 2008;173(1):289–299. doi: 10.2353/ajpath.2008.071130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belcher JD, Chen C, Nguyen J, et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123(3):377–390. doi: 10.1182/blood-2013-04-495887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belcher JD, Chen C, Nguyen J, et al. Haptoglobin and hemopexin inhibit vaso-occlusion and inflammation in murine sickle cell disease: role of heme oxygenase-1 induction. PLoS One. 2018;13(4):e0196455. doi: 10.1371/journal.pone.0196455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vercellotti GM, Zhang P, Nguyen J, et al. Hepatic overexpression of hemopexin inhibits inflammation and vascular stasis in murine models of sickle cell disease. Mol Med. 2016;22:437–451. doi: 10.2119/molmed.2016.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vercellotti GM, Khan FB, Nguyen J, et al. H-ferritin ferroxidase induces cytoprotective pathways and inhibits microvascular stasis in transgenic sickle mice. Front Pharmacol. 2014;5:79. doi: 10.3389/fphar.2014.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mumme C. Zur Klinik und Pathologie der Endokarditis und Aortitis fibroplastica sowie Thromboendarteritis obliterans mit hochgradiger Eosinophilie im Blut, Knochenmark und in den Organen. Z Klin Med. 1940;138(1):22. German. [Google Scholar]
- 10.McKay DG, Whitaker AN. Studies of catecholamine shock. I. Disseminated intravascular coagulation. J Am Pathol. 1969;56(2):153–176. [PMC free article] [PubMed] [Google Scholar]
- 11.Dale J, Ohlsson K, Nordstoga K, Aasen AO. Intravascular hemolysis and ultrastructural changes of erythrocytes in lethal canine endotoxin shock. Eur Surg Res. 1980;12(1):39–51. doi: 10.1159/000128108 [DOI] [PubMed] [Google Scholar]
- 12.Dao AH, Eberhard ML. Pathology of acute fatal babesiosis in hamsters experimentally infected with the WA-1 strain of Babesia. Lab Invest. 1996;74(5):853–859. [PubMed] [Google Scholar]
- 13.Hinshaw LB. Sepsis/septic shock: participation of the microcirculation: an abbreviated review. Crit Care Med. 1996;24:1072–1078. [DOI] [PubMed] [Google Scholar]
- 14.Oude Lansink M, Patyk V, de Groot H, Effenberger-Neidnicht K. Melatonin reduces changes to small intestinal microvasculature during systemic inflammation. J Surg Res. 2017;211:114–125. doi: 10.1016/j.jss.2016.11.055 [DOI] [PubMed] [Google Scholar]
- 15.Effenberger-Neidnicht K, Hartmann M. Review: mechanisms of hemolysis during sepsis. Inflammation. 2018;41(5):1569–1581. doi: 10.1007/s10753-018-0810-y [DOI] [PubMed] [Google Scholar]