Abstract
Background: Transdermal drug delivery system (TDDS) curing rheumatoid arthritis (RA) for long-term treatment can improve patients’ compliance and reduce the accumulation of drug side effects. However, TDDS is constrained by the tight junction of the stratum corneum and low permeation efficiency. It is necessary to adopt proper permeation methods to ensure the therapeutic effect. The transethosome (TE), which is derived from transfersome and ethosome (E), containing a high content of ethanol along with an edge activator or permeation enhancer, has superior deformability and higher permeation efficiency.
Methods and Results: In this study, sinomenine hydrochloride-loaded TE was decorated with ascorbic acid to form antioxidant surface transethosome (AS-TE). It was revealed that TE and AS-TE containing sodium deoxycholate can effectively increase the entrapment efficiency of hydrophilic drug, and has superior deformability and higher permeation efficiency than E group. The plasma pharmacokinetics of rabbits showed that TE group and AS-TE group had similar blood concentration and bioavailability; however, micro-dialysis on synovial fluid demonstrated that AS-TE group had higher drug concentration. In RA rat models, the alleviation of the joint swell of AS-TE group was more obvious in the course of 3 weeks of treatment. The inflammatory cytokines and erythrocyte sedimentation rate were significantly lower than those in the negative control group and TE1 group.
Conclusion: AS-TE, which can enhance transdermal permeability and drug deposition for the oxidant stress of RA, had further research potential to serve as a TDDS of RA.
Keywords: transdermal drug delivery system, antioxidant surface, transethosome, oxidant stress, micro-dialysis
Introduction
Rheumatoid arthritis (RA) is a chronic disease characterized by persistent synovitis, systemic inflammation, and autoantibody changes.1 In developed countries, the RA incidence in adults accounts for within 0.5–1.0%, and the incidence of females is 2–3 times higher than that of males.2 In the presence of limited treatments and poor prognosis, uncontrolled RA may lead to cause injury, disability, cardiovascular disease, and other complications, as well as decreasing the quality of life. Therefore, RA is one of the diseases that can cause joints to deform and shift out of place and even may affect the entire body.2
Sinomenine hydrochloride (Figure 1A) is a disease modifying anti-rheumatic drug approved by China Food and Drug Administration, widely used clinically treating RA in China for a long time.3 It is a morphinane alkaloid extracted from the dried roots of Sinomenium acutum Rehderett Wilson, is soluble in ethanol, acetone, chloroform, water, and possesses low permeability, as well as involving a nerve palsy effect and cardiotoxicity, short half-life, low oral bioavailability, and fluctuating drug concentrations in blood.4 Oral administration of sinomenine hydrochloride may lead to gastrointestinal irritation and first-pass effect. Long-term oral treatment by using traditional chemical drugs (eg, sinomenine hydrochloride) may lead to the accumulation of toxicity and side effects, and some transdermal formulations have been successfully developed to overcome these problems, and the early research had confirmed the feasibility of transdermal delivery.5,6 TDDS, as a non-invasive method of administration, can prevent the first-pass effect and gastrointestinal-tract irritation. TDDS can decrease the number of administrations, and maintain blood concentration in the treatment window for sustained-release effect, which is conducive to the long-term treatment of RA. However, the tight junction of the stratum corneum hinders freely penetration of all drugs into the body. It is generally believed that drug with a molecular weight of less than 500 Da, and Log P of 3~5 can easily permeate into the body through the skin.7,8 With the development of nanotechnology, nanocarrier is increasingly used in TDDS. Touitou et al, prepared ethosome (E), namely a nanocarrier containing a high content of ethanol (20–50%), which had superior deformability, and ethanol could be used as a chemical penetration enhancer as well.9 Transfersome is a trademark registered by the German company IDEAAG, which refers to its proprietary drug delivery technology. The carrier aggregate is composed of at least one amphiphat, which in aqueous solvents self-assembles into a lipid bilayer that closes into a simple lipid vesicle. By the addition of at least one bilayer softening component lipid bilayer flexibility and permeability are greatly increased.10 Then, the transethosome (TE), which is derived from transfersome and ethosome, contains a high content of ethanol along with an edge activator or permeation enhancer, possessing a greater deformability and entrapment efficiency (EE) for the hydrophilic drug than the ethosome and the transfersome.11
Figure 1.
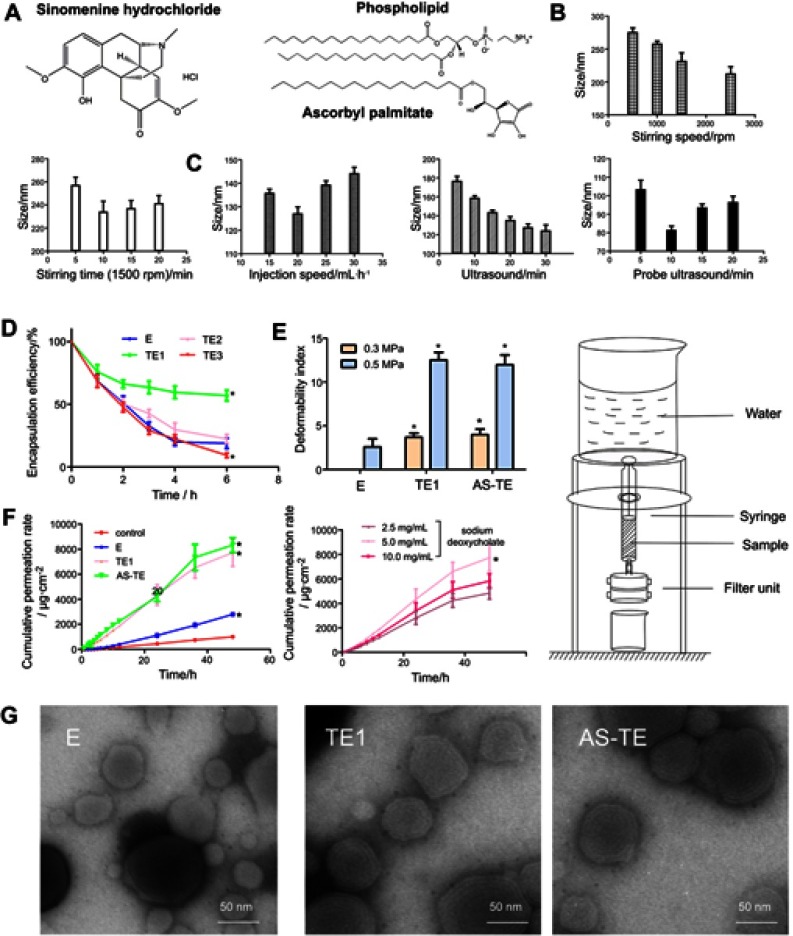
(A) The chemical structure of sinomenine hydrochloride, phospholipid, and ascorbyl palmitate. (B) The ethosomal sizes were prepared by different stirring velocities and time on the basis of the stirring-injection method. (C) The ethosomal sizes were prepared by different injection velocities, ultrasound time, and probe ultrasound time based on ultrasound-injection method. (D) The capsulation efficiency with different edge activators (*P<0.05 vs E). (E) The deformability indexes under 0.3 and 0.5 MPa were measured by self-made equipment on the right. (*P< 0.05 vs E). (F) The cumulative permeation rates in vitro of control (sinomenine hydrochloride normal saline solution), E, and TE1, containing different concentrations of sodium deoxycholate, and AS-TE. (*P <0.05 vs control and TE1 containing 5.0 mg/mL of sodium deoxycholate). (G) The TEM images of E, TE1, and AS-TE.
Abbreviations: E, ethosome; TE, transethosomes (ethosome containing sodium deoxycholate (TE1), tween-80 (TE2) and oleic acid (TE3)); AS-TE, antioxidant surface transethosome.
Oxidative stress reflects an imbalance relationship between the systemic manifestation of reactive oxygen species (ROS) and a biological system’s ability to readily detoxify the reactive intermediates or repair the damage resulted. Various cells can tolerate mild oxidative stress, as they have the ability of antioxidant defense as a repair system, and can recognize and remove molecules damaged by oxidation, including antioxidant enzyme and non-enzyme nutrients.12 By using the antioxidant non-enzyme nutrients, ascorbic acid, and coenzyme Q10, Jukanti et al, designed two kinds of liposomes targeting the inflammation site. Antioxidant surface liposomes could be preferentially localized at inflammatory sites via redox interaction in the presence of a high level of ROS.13 In our paper, sinomenine hydrochloride-loaded TE was decorated with ascorbic acid to form the antioxidant surface transethosome (AS-TE), which might enhance transdermal permeability and drug deposition.
Materials and animals
Sinomenine hydrochloride was purchased from Ark Pharm, Inc. (Chicago, IL, USA). Soy lecithin S100 was purchased from Lipoid GmbH. (Ludwigshafen, Germany). Sodium deoxycholate, tween 80, oleic acid, Na2S, and Triethanolamine were purchased from Beijing Innochem Science & Technology Co., Ltd. (Beijing, China). Ascorbyl palmitate was purchased from Alfa Aesar Co., Ltd (Heysham, UK); Pentobarbital sodium was purchased from Huaxia Reagent (Chengdu, China); Egg albumen was purchased from Acros Organics (Belgium); Complete Freund’s adjuvant (CFA) was purchased from Sigma–Aldrich Corporation (St. Louis, MO, USA); Carbomer 941 was purchased from Beijing Fengli Jingqiu Pharmaceutical Co., Ltd. (Beijing, China). Rat tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), and ROS ELISA Kit were purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd. (Shanghai, China). All other agents were analytically graded and used without further purification.
New Zealand rabbits and Sprague–Dawley rats were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). All the animals were used and cared for the experiment in accordance with the protocols established by Chinese Academy of Medical Sciences & Peking Union Medical College and approved by the experimental animal management committee, Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College.
Methods
Preparation of E, TE, and AS-TE
The E, TE, and AS-TE were prepared by ultrasound-guided injection method. The specific prescription is shown in Table 1. The soy lecithin S100, sinomenine hydrochloride, and anhydrous ethanol in the prescribed amounts were added into conical flasks sealed with a rubber stopper. The syringe pump (HK-400I, Shenzhen Hawk Medicinal Instrument Co., Ltd., Shenzhen, Guangdong, China) was used to inject the prescribed amount of distilled water into the conical flask at a rate of 20 mL·h–1 under 40 kHz sonication using an ultrasonic cleaner (ZQ-250DE, Kunshan Ultrasonic Instrument Co., Ltd, Jiangsu, China). After completion of the injection, ultrasound was continued for 10 mins, and the sample was transferred to an ultrasonic cell disrupter (ZQ-150Y, Shanghai Zhengqiao Scientific Instrument Co., Ltd, Shanghai, China) under ice bath for 10 mins. The sample was filtered by 0.22 μm filter and stored at 4°C. The preparation processes for TE and AS-TE were almost the same as well. The edge activator or ascorbyl palmitate was co-dissolved in the anhydrous ethanol with soy lecithin S100 and sinomenine hydrochloride, and the above-mentioned steps were continued as well.
Table 1.
The composition of nanocarrier in 1 mL aqueous solution and physicochemical properties (Mean ± SD, n=3)
| Items | E | TE1 | TE2 | TE3 | AS-TE |
|---|---|---|---|---|---|
| Lipoid S100/mg | 30 | 30 | 30 | 30 | 30 |
| Sodium deoxycholate/mg | 5 | 5 | |||
| Tween-80/mg | 5 | ||||
| Oleic acid/mg | 5 | ||||
| Sinomenine hydrochloride/mg | 5 | 5 | 5 | 5 | 5 |
| Ascorbyl palmitate/mg | 5 | ||||
| Ethanol/μL | 300 | 300 | 300 | 300 | 300 |
| Water/μL | 700 | 700 | 700 | 700 | 700 |
| Size/nm | 82.5±7.9 | 88.7±9.6 | 130.8 ±18.2* | 172.3±19.8* | 93.2±7.2 |
| PDI | 0.142±0.031 | 0.152±0.036 | 0.256±0.062 | 0.302±0.082 | 0.168±0.023 |
| Zeta potential/mV | −4.5±1.8 | −20.6±3.2* | −18.6±5.9* | −12.9±4.2* | −17.6±3.6* |
| Entrapment efficiency/% | 18.9±2.9 | 60.2±6.3* | 10.8±2.2 | 24.8±4.2 | 59.9±4.5* |
Note: * p < 0.05 vs E.
Abbreviations: E, ethosome; TE, transethosomes (ethosome containing sodium taurocholate (TE1), tween-80 (TE2) and oleic acid (TE3)); AS-TE, antioxidant surface transethosome.
Characterization of E, TE, and AS-TE
The particle size and zeta potential were measured using a laser particle size analyzer (Malvern Zetasizer Nano ZS, Malvern Instruments Ltd., Worcestershire, UK). Morphological examination was performed using a transmission electron microscope (TEM, HT7700, Hitachi, Japan), and samples testing with TEM were made by casting a drop of aqueous sample on carbon-coated copper grids, and positively stained with 2.0% phosphotungstic acid. The amount of sinomenine hydrochloride entrapped in nanocarrier was determined by dialysis method. Then, 1 mL of sample was added into a 5 cm 3,500 Da dialysis bag, which was placed into 500 mL of distilled water under stirring of 100 rpm. For each experiment, 5 mL dialysis medium was sampled at predetermined time intervals (0.5, 1, 2, 3, 4, 5, and 6 hrs), and then the same volume of pure medium was immediately added into the beaker. All samples were filtered through a 0.22 μm membrane filter and analyzed by high-pressure liquid chromatography (HPLC).
The quantification of sinomenine hydrochloride was carried out using the Agilent 1100 series HPLC system, which was equipped with the Agilent 1200 series DAD detector and a reversed-phase C18 column (4.6 mm×250 mm, 5 μm, Dikma). The data were captured and processed using Agilent ChemStation for LC 3D systems acquisition software. The mobile phase was a mixture of methanol and 0.5% ethylenediamine aqueous solution (60:40, v/v), and eluted at a flow rate of 1.0 mL/min. Effluents were detected at 276 nm. This method has been validated for selectivity, linearity, limit of detection (30 ng·mL–1), and quantification (94 ng·mL–1), accuracy, precision, as well as repeatability.
Measurement of deformability index
The deformability index was defined by Eq. (1) to characterize the deformability of nanocarrier, which was measured by an extrusion method as reported earlier and self-made equipment (Figure 1E).14 Briefly, the nanocarriers were extruded through a Whatman™ 50 nm Nuclepore™ Polycarbonate Track-Etched Membrane Filter (Whatman, UK) by applying pressures of 0.3 and 0.5 MPa for 15 and 5 mins, respectively.
where E denotes the deformability index of the vesicles bilayer; j is the rate of penetration through a membrane filter (the weight of suspension extruded in 5 or 15 mins); rv represents vesicle size (after extrusion); and rp denotes pore size of the membrane.
In vitro skin permeation and deposition studies
The abdominal hair of about 200 g of Sprague–Dawley rats was shaved, and the abdominal skin was gently treated with Na2S solution. After 24 hrs, rats were sacrificed and the abdominal skin was quickly excised and subcutaneous tissue was removed. The excised skin was washed with normal saline, and store at –20°C. Valia-Chien diffusion cells (TP-6, Tianjin Xinzhou Technology Co., Ltd., China) with an effective diffusion area of 2.0 cm2 were used at 37.0°C to perform the in vitro skin permeation and deposition studies. The excised skin samples were clamped between the donor and the receptor chamber of diffusion cells with the stratum corneum (SC) facing the donor chamber. The 7 mL of aqueous solution, E, TE containing a different concentration of sodium deoxycholate and AS-TE (1 mL is equal to 5 mg of sinomenine hydrochloride) were applied on the donor compartment. Receptor chamber was filled with 7 mL of normal saline. The receptor medium was stirred at 400 rpm throughout the experiment. For each experiment, 1 mL of receptor medium was sampled at predetermined time intervals (0.5, 1, 2, 3, 4, 5, 6, 8, 10, 12, 24, 36, and 48 hrs), and then the same volume of pure medium was immediately added into the receptor chamber. All samples were filtered through a 0.22 μm membrane filter and analyzed by 3.2 HPLC method.
After 48 hrs of the skin permeation experiment, the surface of skin specimens was washed with methanol five times to remove skin bound formulations and drug. The effective surface area of the skin was separated, and the SC layer was removed by stripping 20 times using cellophane adhesive tape (3M Company, St. Paul, MN, USA). The drug content in the SC was measured by extracting the drug with methanol from the adhesive tapes. The remaining epidermis/dermis layers were eventually homogenized in a vial filled with methanol (1 mL·cm–2) by using a high-speed homogenizer (FA-25, FLUKO, Germany) at 20,000 rpm for 5 mins on ice bath (0°C). The tissue suspension was centrifuged for 5 mins at 10,000 rpm (TGL-16G, Shanghai Anting Scientific Instrument Factory, China), and then the supernatants were filtered and assayed for their sinomenine hydrochloride content by 3.2 HPLC method.
Plasma pharmacokinetics in rabbits
2.5 g of Carbomer 941 was added into 50 mL of distilled water and stayed overnight. Triethanolamine was added into Carbomer dropwise under stirring until the colorless transparent semi-solid gel arose. The mixture of Carbomer gel and formulation in equal volumes was centrifuged at 5,000 rpm for 30 mins (H1850, Hunan Xiangyi Laboratory Instrument Development Co. Ltd., China) to remove the bubbles. Then, the semi-solid gels containing nanocarrier was obtained at 2.5 mg·mL–1.
Healthy Japanese white rabbits (males, 2.0±0.5 kg) were selected in this experiment, and their hair on the hind knee was shaved before formulation administration. The rabbits were randomly divided into control (sinomenine hydrochloride solution gel), i.g., TE1, and AS-TE group (n=3 for each group). I.g. group was intragastrically administrated with a dose of 5 mg·kg–1. AS-TE was administered transdermally with the dose of 5 mg·kg–1 by applying gel evenly on hind knee site. The administrative area was covered by the SCOTCHPAKTM polyester membrane (3M, St. Paul, MN, USA) and fixed by a medical adhesive tape (Qingdao Hainuo Biological Engineering Co., Ltd., China). Control and TE1 were administered to another group of rabbit with the same operation and dose. At predetermined time points of 2, 4, 6, 8, 10, 12, 24, 36, and 48 hrs after the administration, 1.0 mL of blood samples was collected from the marginal ear vein and removed into the centrifugal tube containing heparin sodium. All blood samples were centrifuged at 3,000 rpm for 10 mins. Supernatants were separated and frozen immediately in a deep freezer at –80°C until analysis. The amount of sinomenine hydrochloride in the plasma samples was detected by HPLC as well.
The extraction of sinomenine hydrochloride from plasma samples was carried out by using liquid–liquid extraction. In brief, 200 μL of ammonia-ammonium chloride solution (pH =11.0) was added into the plasma samples (400 μL) in centrifuge tubes. The tubes were vortexed for 2 mins, and then 4 mL of ethyl acetate was added as an extraction solvent. After 5 min vortex-mixing, the mixture was centrifuged at 5,000 rpm for 10 mins. The organic layer was then separated and evaporated to dryness at 50°C under a stream of nitrogen (DC-24 nitrogen blowing instrument, Shanghai ANPU Science Instrument Co. Ltd., Shanghai, China). After that, the residue was dissolved into 100 μL of mobile phase and then 20 μL was injected into the HPLC system.
Sinomenine hydrochloride analysis was performed using Agilent 1100 series HPLC with an UV/Vis detector at 276 nm. Separation was performed on Wondasil C18 for herbal medicine column (250 mm×4.6 mm, 5 μm). The mobile phase was a mixture of methanol, 0.01 mol·L–1 of sodium dihydrogen phosphate and triethylamine (60:40:1, v/v/v) with a flow rate of 1.0 mL·min–1. The injection volume was 20 μL and column was thermostated at 3°C.
Micro-dialysis in knee cavity of rabbits
Nine healthy Japanese white rabbits (males, 2.0±0.5 kg) were selected in this experiment, and RA model was induced by egg albumen and CFA.15 Allergy reagent was prepared by mixing egg albumen normal saline solution of 20 mg·mL–1 and CFA in equal volumes. Allergy of rabbits was induced by subcutaneous injection of allergy reagent at five sites in the rabbit scapular region once a week for three consecutive weeks. Then, the RA models were eventually induced by joint injection of 0.5 mL egg albumen normal saline solution of 10 mg·mL–1. After 24 hrs, 1.0 mL of blood samples was collected from the marginal ear vein and removed into the centrifugal tube. The levels of TNF-α and IL-6 were determined by ELISA kit. The joint was swollen and inflammatory cytokines was significantly increased indicating that RA model has been induced successfully.
Nine RA model rabbits were anesthetized with pentobarbital sodium (30 mg·kg–1, i.v.). A micro-dialysis probe (tip length: 4 mm; MWCO: 5,000 Da, CMA, Sweden) was implanted in the joint cavity with the help of a steel needle introducer and split tubing. I.g. group was intragastrically administrated with a dose of 5 mg·kg–1. AS-TE was administered transdermally with the dose of 5 mg·kg–1 by smearing gel evenly on ankle area. The administrative area was covered by the SCOTCHPAKTM polyester membrane and fixed by medical adhesive tape. As a control, TE1 was administered to another group of rabbit with the same operation and dose. After a stabilization period of 1 hr post-operation, the Ringer’s solution was perfused through the probe at the flow of 5.0 μL·min–1. Micro-dialysis samples were then collected at 60 mins intervals for up to 660 mins post-dose. The extraction of sinomenine hydrochloride from Ringer’s solution samples was carried out by using liquid–liquid extraction, as same as those of plasma pharmacokinetics. Eventually, 20 μL of extracted sample was injected into the HPLC system by the HPLC method.
Micro-dialysis probe recovery was measured by in vivo calibration. Rabbits were anesthetized with pentobarbital sodium. The same micro-dialysis probe was implanted in the ankle. After a stabilization period of 1 hr post-operation, the Ringer’s solution containing different concentrations (from 20 to 500 ng/mL) of sinomenine hydrochloride was perfused through the probe at the flow rate of 5.0 μL·min–1 to ensure that probe recovery was independent of concentration. In addition, we examined the probe recovery at 12 hrs to ensure that the recovery could be kept stable during the study period. The in vivo relative recovery was calculated by Eq. (2):
where Rdial represents in vivo relative recovery of microdialysis probe at the flow rate of 5.0 μL·min–1; Cperf and Cdial are the perfusate and dialysate concentrations of sinomenine hydrochloride, respectively.
Treatment of the RA rat ankle
The Sprague–Dawley rats were injected on plantar with 100 μL of CFA. The girth of the ankle was measured by wrapping the string around the ankle joint.16,17 The model animals were randomly divided into the following four groups (n=6 per group, treatment was applied once daily for 3 weeks): (i) negative control (blank gel), (ii) i.g. with sinomenine hydrochloride normal saline solution (2.5 mg·mL–1), (iii) TE1 Carbomer gel (2.5 mg·mL–1), and (iv) AS-TE Carbomer gel (2.5 mg·mL–1).
The girth of all rats was measured twice a week for four consecutive weeks. After the experiment was completed, 1.0 mL of blood samples was collected from the venous plexus in the eye fundus and removed into the centrifugal tube. The inflammatory cytokines, TNF-α, IL-6, and ROS, were determined by ELISA kit. The rats were anesthetized with 8% chloral hydrate and 8.0 mL of blood sample was collected from aorta abdominals. The erythrocyte sedimentation rate (ESR) was measured using Westergren’s method.18 All rats were sacrificed and the paws were excised for further assessment. The paws were scanned with the IVIS Spectrum CT (PerkinElmer Inc., Waltham, MA, USA) with Medium Res CT acquisition mode, a voxel size of 75 μm and resolution of 225 μm, and were reconstructed into a 3D structure using Mimics software (Materialise, Belgium). The bone volume was calculated by the marching cubes method to triangulate the surface of the bone, and the bone surface was calculated using polyhedrons corresponding to the enclosed volume of the triangulated surface.
Statistical analysis
All experiments in this study were repeated at least three times, and data were expressed as mean ± standard deviation (SD). The statistical analysis was assessed by a two-tailed Student’s t-test using “GraphPad Prism 5” software and a P-value <0.05 was statistically considered significant.
Results and discussion
Preparation and characterization of E, TE, and AS-TE
The smaller particle size and better deformability were beneficial for enabling nanocarrier to easily pass through a narrow interstitial space. The smaller particle cannot be obtained by stirring-guided injection method (Figure 1B). For ultrasound-guided injection method, diameter was influenced by injection time, ultrasound and probe ultrasound time (Figure 1C). After prescription optimization, the particle size of E can be controlled below 100 nm, which was an advantage for transdermal permeability. Since the sinomenine hydrochloride was easily soluble in water and ethanol, the EE of E was less than 20%, which meant that sinomenine hydrochloride was mainly present in a free state, and the permeability of E was difficult to be exerted. Generally, hydrophilic drugs had poor transdermal permeability and low encapsulation efficiency, which had key problems need to be solved timely.
The pH gradient method can increase the EE utilization of the weak acids and bases that can cross the lipid bilayer in an electrically neutral state, however, its ionized state cannot cross the lipid bilayer. According to the Henderson-Hasselbalch theory, the change of each pH unit produces a difference of 10 times on the concentration of electrically neutral and ionized state. However, for sinomenine hydrochloride, its stability under alkaline conditions was poor. It was difficult to use a pH gradient method, and thus a new method is required to increase the EE of a hydrophilic drug. Therefore, 5 mg·mL–1 of surfactants, such as tween-80, sodium deoxycholate, and oleic acid were added into the ethosome (Table 2). While the size of TE2 and TE3 significantly increased, size of TE1 kept almost unchanged, and the absolute value of zeta potential increased, indicating that the stability of the TE1 increased. The most important concern was that and EE of TE1 increased by nearly three times, probably resulting from the reduction of polarity by a combination between deoxycholate anion and the sinomenine cation (Figure 1D). The size, zeta potential, and EE of TE1 were modified by ascorbic acid that was kept almost unchanged due to the similar amphipathic structure between phospholipid and ascorbyl palmitate. The morphology of the carriers was observed by TEM that showed E, TE1, and AS-TE had a multi-layer structure with a nearly spherical overall shape (Figure 1G).
Table 2.
Rat abdominal skin permeation parameters of sinomenine hydrochloride (Mean ± SD, n=3)
| Group | Permeation rate/μg·cm–2·h–1 | Amount deposited after 24 hrs/μg·cm–2 | |
|---|---|---|---|
| Stratum corneum | Dermis/epidermis | ||
| Control | 10.5±3.2 | 32.1±8.8 | 29.7±6.9 |
| E | 40.2±6.8* | 58.1±12.1 | 23.6±8.2 |
| TE1 | 79.6±7.3* | 100.7±15.2* | 54.3±12.2* |
| AS-TE | 73.9±8.2* | 120.7±23.2* | 33.2±15.2 |
Note: * p < 0.05 vs control, control: sinomenine hydrochloride normal saline solution.
Abbreviations: E, ethosome; TE1, transethosomes (ethosome containing sodium taurocholate); AS-TE, antioxidant surface transethosome.
Deformability of the lipid bilayers is an important factor for the permeation-enhancing effect of the lipid vesicles. Penetration of lipid vesicles through the skin is related to the deformability of the vesicle membrane.11 At a pressure of 0.3 MPa, E cannot pass through the 50 nm filtration within 15 mins. At the pressure of 0.5 MPa, the deformability index of TE1 was 5.2 times greater than that of E, which showed the better deformability of the TE (Figure 1E). Having the same size, TE1 can pass through the smaller skin pore and has better transdermal permeability. Moreover, deformability regularity of AS-TE was in accordance with TE1, resulting from similar nanostructure. It has been reported that surfactants and ethanol changed the packing characteristics of the lipids in the bilayer, thereby resulting in higher deformability. Tween-80, sodium deoxycholate, and oleic acid can be intercalated between the lipid bilayer, leading to a decreased phase transition temperature of skin lipids, as well as an increase in their fluidity.11
In vitro skin permeation and deposition studies
In order to further evaluate the differences in transdermal permeability between E and TE1, the Valia-Chien diffusion cells were used. The transdermal permeability indicated that the transdermal permeability efficiency of TE1 and AS-TE in 48 hrs was 98% higher than that of E, which is 7.6 times more than that of control. The order of the permeation profile obtained from the in vitro study was AS-TE ≈ TE1 > E > control (sinomenine hydrochloride aqueous solution) (Figure 1F). The different concentrations of sodium deoxycholate can affect transdermal permeability and TE1 with 5 mg·mL–1 of sodium deoxycholate had higher permeability. In the diffusion experiment, drug also can retain in the dermis and SC. The retention content of TE1 was significantly higher than that of control and E (Table 2). It was reported that ethanol can be used as a chemical permeation enhancer as well. Several studies showed that interaction of surfactants of nanocarrier and layers of the SC can promote the open of barrier function of these layers to less well-packed intercellular lipid structure forms, and thereby subsequently increasing permeation efficiency of the drug. TE containing ethanol and surfactants had a superposition of advantages, as well as including additional better deformability than ethosome.
Plasma pharmacokinetics in rabbits
Sinomenine hydrochloride was a morphinane alkaloid, and the ideal internal standard substance was morphine. However, the control of morphine was very strict, accordingly, it is difficult to be obtained. It was reported by Wu et al, that the external standard method was used to determine the plasma concentration of sinomenine hydrochloride.19 The representative chromatograms of blank rabbit plasma, and blank rabbit plasma spiked with sinomenine hydrochloride were shown in Figure 2A. The retention time of sinomenine hydrochloride was 5.85 mins, and signals of sinomenine hydrochloride and plasma could be effectively separated. Sinomenine hydrochloride showed a proper linearity in the range of 20–200 ng·mL–1 and the linear regression equation was A = (15.464 C – 3.584) × 10–3 (r = 0.9991); the mean absolute recovery and relative recovery were 80.23± 2.3% and 98.6 ±3.1%, respectively; the lower limit of detection was 10 ng·mL–1; the RSD of intra-day and inter-day precision was 2.8% and 3.9%, respectively. In summary, the external standard method had sufficient accuracy to determine the concentration of sinomenine hydrochloride in rabbit plasma.
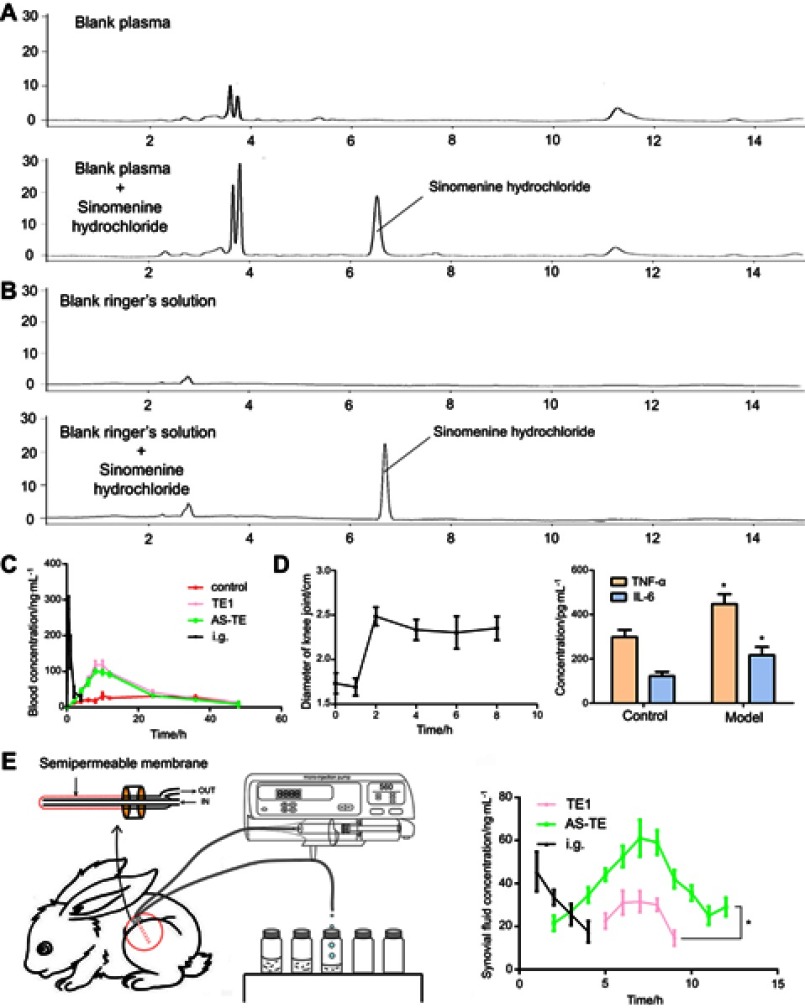
Figure 2.
The representative chromatograms of (A) plasma pharmacokinetic and (B) micro-dialysis sample. (C) The blood concentration-time profiles of healthy rabbits (control: sinomenine hydrochloride solution gel). (D) The diameters of knee joint and inflammation cytokines of rheumatoid arthritis model rabbits (*P < 0.05 vs control: healthy rabbits). (E) The synovial fluid concentration-time profiles of rheumatoid arthritis model rabbits (*P < 0.05 vs TE1).
Abbreviations: E, ethosome; TE1, transethosomes (ethosome containing sodium deoxycholate (TE1)); AS-TE, antioxidant surface transethosome.
The plasma sinomenine hydrochloride concentration–time profiles were shown in Figure 2C. It was revealed that the plasma sinomenine hydrochloride concentration from AS-TE group was almost similar to the TE1 group. The plasma concentration from TE1 and AS-TE group was higher than that of E group, because TE1 and AS-TE had superior deformability, and higher in vitro permeation efficiency than those of E. Several important pharmacokinetic parameters were calculated using “DAS 3.2.8” software and are accordingly shown in Table 3. The Cmax of i.g. the group was highest but drug concentration quickly fell below the limit of detection. The AUC, Cmax, and Tmax values of TE1 and AS-TE group were similar, and AUC of TE1 group had about two times higher than that of E (1158.3±30.0 vs 531.8±17.5 ng·h·mL–1), which clearly demonstrated that the TE1 and AS-TE enhanced bioavailability of sinomenine hydrochloride. Compared with previous studies on sinomenine hydrochloride microneedles, the nanocarrier had significantly sustained-release effect.
Table 3.
Plasma pharmacokinetic parameters (Mean ± SD, n=3)
| Group | AUC(0–t)/ ng·h·mL–1 |
AUC(0–∞)/ ng·h·mL–1 |
t1/2z/ h |
Tmax/ h |
CLz/F/ L·h–1·kg–1 |
Cmax/ ng·mL–1 |
|---|---|---|---|---|---|---|
| Control | 579.1±4.2 | 810.4±98.8 | 21.0±5.9 | 18.7±15.0 | 6.2±0.71 | 17.4±3.2 |
| TE1 | 1158.3±30.0* | 1271.2±40.0* | 12.2±1.4* | 8.7±1.2 | 3.9±0.1* | 62.8±5.6* |
| AS-TE | 1098.2±45.2* | 1135.2±32.8* | 11.2±0.9* | 9.0±0.9 | 4.1±0.5* | 57.8±3.2* |
* p < 0.05 vs control, control: sinomenine hydrochloride aqueous solution.
Micro-dialysis in the knee cavity of RA model rabbits (Figure 2E)
The degree of knee swelling and related inflammatory cytokines (TNF-α and IL-6) can be used for evaluating the RA model. After final inducing, the knee of rabbits can swell to a maximum within 24 hrs. The diameter and inflammatory cytokines had a higher increase than normal rabbits, indicating that RA model rabbits were successful (Figure 2D).
The recovery profile for sinomenine hydrochloride showed a non-concentration-dependency from 20 to 500 ng/mL, and the average recovery was 22.3% at 5.0 μL·min–1 with steady loss of sinomenine for 12 hrs. It has been reported that recoveries of the probes increased with the decreased perfuse rate. However, it took a long time to obtain sufficient sample volume at a low flow rate, and the perfuse rate of 5.0 μL·min–1 was set for the sample collection.
The representative chromatograms of blank Ringer’s solution and blank Ringer’s solution spiked with sinomenine hydrochloride were shown in Figure 2B. The amount of sinomenine hydrochloride in SF in TE1 group was very low to obtain a continuous profile and only Cmax and nearby concentrations were obtained by HPLC-UV (Figure 2E). The drug concentration in synovial fluid reached a peak after 1 hr and fell below the limit of detection after 5 hrs. The Tmax and Cmax of AS-TE group were 0.7-fold shorter and 2.8-fold higher relative to TE1 group. And drug concentration The results indicated that the AS-TE group might have its pharmacological effects faster than TE1 group. The sinomenine hydrochloride by transdermal administration on ankle site can firstly penetrate the skin, and then it must cross the synovial membrane to get into the intra-articular space. Although TE1 and AS-TE had almost the same in vitro permeation efficiency and plasma pharmacokinetic parameters, the results showed that sinomenine hydrochloride of AS-TE group can easily deposit on RA ankle site, because AS-TE could preferentially localize at inflammatory sites via redox interaction where high-level of ROS exists.
Treatment of the RA rat ankle
The main characteristics of the RA adjuvant model were joint swelling and inflammatory reactions. The degree of ankle swelling and related inflammatory cytokines (TNF-α, IL-6, and ROS) can be used as key indicators for evaluating the therapeutic effect. After plantar injection of 150 μL CFA, the ankle of rats can swell to the maximum within 24 hrs. During the 4 weeks of treatment, there was no significant change on the ankle girth in the negative control group (Figure 3A). However, after 4 weeks, the inflammatory reaction of the ankle was very intense and ulceration began to appear. The experiment was terminated considering the welfare of experimental animals.
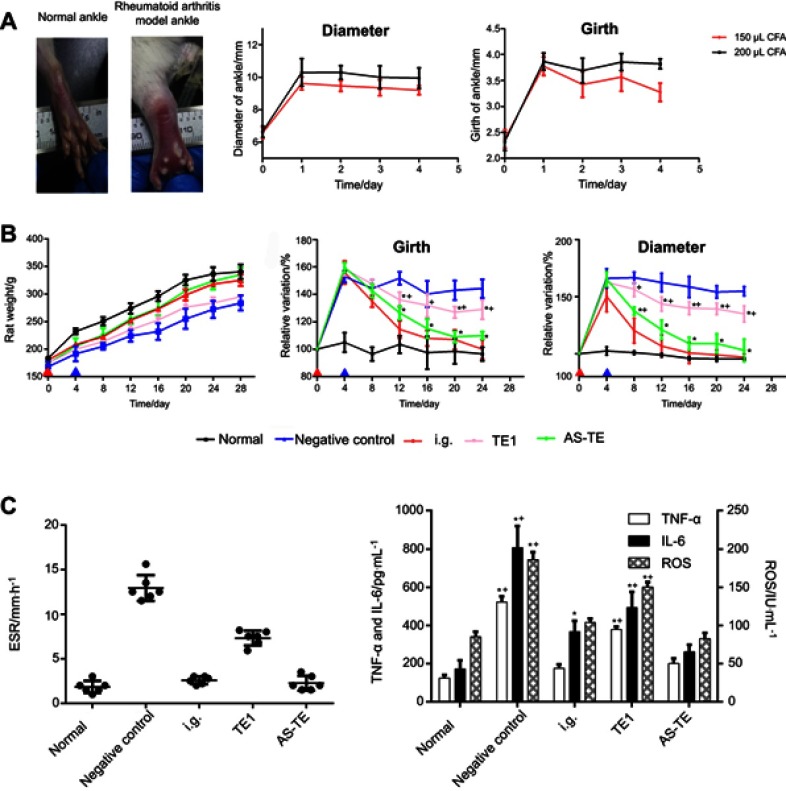
Figure 3.
(A) The photographs of normal and rheumatoid arthritis ankles and the diameter and girth-time profiles of rheumatoid arthritis ankles injected with 150 and 200 μL of complete Freund’s adjuvant (CFA). (B) The rat weight, diameter, and girth-time profiles with different treatments. (*P < 0.05 vs negative control group and +P < 0.05 vs normal control) (C) The erythrocyte sedimentation rate (ESR) and inflammation cytokines after 24 days of treatment. (*P < 0.05 vs negative control group and +P < 0.05 vs normal control) (Red triangle: the time of modeling, blue triangle: the time of treatment).
Abbreviations: E, ethosome; TE1, transethosomes (ethosome containing sodium deoxycholate (TE1)); AS-TE, antioxidant surface transethosome.
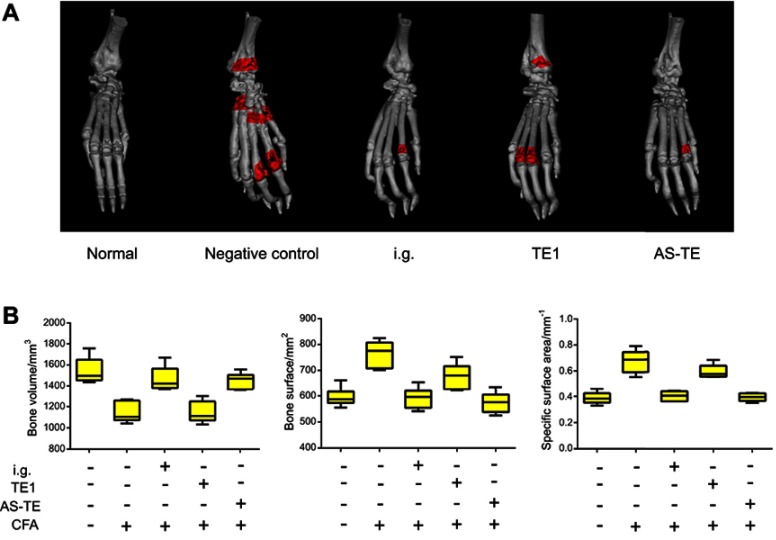
Ankle girth variations relative to normal girth and time were plotted on the ordinate and abscissa, respectively (Figure 3B). The average ankle girth of AS-TE group and TE1 group was 35% and 60% shorter, respectively, relative to those of normal rats. Similarly, the ESR and inflammatory cytokines data matched the changes in girth (Figure 3C). TNF-α, IL-6, and ROS of AS-TE group significantly decreased compared with other groups, especially ROS, due to the reducibility of ascorbyl palmitate. In addition, the ROS level of other groups except for AS-TE had no significant difference. The ESR was a significant index for clinical diagnose of RA. The ESR of the negative control group was extremely high and ESR of i.g. and AS-TE group significantly decreased. Besides, the micro-computed tomography scan images showed that paws in the negative control group had multiple bone defects and i.g. and AS-TE group can restrain the trend (Figure 4A). Even more specifically, the rats were treated using i.g. and AS-TE had higher bone volume and lower bone surface, as well as a specific surface area. Furthermore, the specific surface area had lower error because of removing the impact of different paws` sizes (Figure 4B). In summary, it was considered that AS-TE had a superior therapeutic effect than TE1, in according with micro-dialysis results.
Figure 4.
(A) The bone micro-computed tomography (CT) scan images and the red areas showed the bone injury. (B) The bone volumes, bone surfaces, and specific surface areas after 24 days of treatment.
Abbreviations: E, ethosome; TE1, transethosomes (ethosome containing sodium deoxycholate (TE1)); AS-TE, antioxidant surface transethosome; i.g., intragastric administration.
Conclusions
A novel sinomenine hydrochloride-loaded AS-TE was fabricated using ascorbyl palmitate as an antioxidant and TE as a basic transdermal carrier. AS-TE had an appropriate particle size, zeta potential, morphology, and encapsulation efficiency, and Valia-Chien diffusion cells showed that AS-TE and TE had a higher permeation rate. Pharmacokinetics and micro-dialysis demonstrated that AS-TE preferentially localized at inflamed joints via redox interaction where a high-level of ROS exists. Then, AS-TE can be transdermally delivered to inflamed joints of CFA rats with similar therapeutic efficacy to that of gastric administration of sinomenine hydrochloride. Furthermore, it was expected that AS-TE can be effectively used to treat other inflammatory diseases such as gout, synovitis, etc.
Author contributions
Hui Song, Jin Wen, He Li, Ya Meng, Yujia Zhang, Nan Zhang, and Wensheng Zheng participated in conducting the study. Wensheng Zheng and Hui Song designed and performed the majority of the study. Hui Song mainly drafted the manuscript. He Li provided language help and with writing assistance. Wensheng Zheng mainly proofread the article. All authors contributed to analysing data and drafting the article, gave final approval of version to be published, and agreed to be accountable for all aspects of the work.
Acknowledgments
This work was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences [Grant numbers CAMS- 2017-I2M-1-011].
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Scott DL, Symmons DP, Coulton BL, et al. Long-term outcome of treating rheumatoid arthritis: results after 20 years. Lancet. 1987;1:1108–1111. [DOI] [PubMed] [Google Scholar]
- 2.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4 [DOI] [PubMed] [Google Scholar]
- 3.Xu M, Liu L, Qi C, et al. Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: a systematic review and meta‑analysis. Planta Med. 2008;74:1423‑1429. doi: 10.1055/s-0028-1088319 [DOI] [PubMed] [Google Scholar]
- 4.Chan K, Liu ZQ, Jiang ZH, et al. The effects of sinomenine on intestinal absorption of paeoniflorin by the everted rat gut sac model. J Ethnopharmacol. 2006;103:425–432. doi: 10.1016/j.jep.2005.08.020 [DOI] [PubMed] [Google Scholar]
- 5.Yan H, Yan M, Li HD, et al. Pharmacokinetics and penetration into synovial fluid of systemical and electroporation administered sinomenine to rabbits. Biomed Chromatogr. 2015;29:883–889. doi: 10.1002/bmc.3369 [DOI] [PubMed] [Google Scholar]
- 6.Qian SS, Chen YL, Gui SY, et al. Enhanced penetration of sinomenine fomulations following skin pretreatment with a polymer microneedle patch. Lat Am J Pharm. 2014;33:464–469. [Google Scholar]
- 7.Guy RH, Hadgraft J. The effect of penetration enhancer on the kinetics of percutaneous absorption. J Control Release. 1987;5:43–51. doi: 10.1016/0168-3659(87)90036-8 [DOI] [Google Scholar]
- 8.Guy RH, Hadgraft J. Physicochemical aspects of percutaneous penetration and its enhancement. Pharm Res-Dordr. 1988;5:753–758. doi: 10.1023/A:1015980516564 [DOI] [PubMed] [Google Scholar]
- 9.Touitou E, Dayan N, Bergelson L, et al. Ethosomes—novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release. 2000;65:403–418. [DOI] [PubMed] [Google Scholar]
- 10.Gregor C, Schätzlein AG, Richardsen H, et al. Overcoming semipermeable barriers, such as the skin, with ultradeformable mixed lipid vesicles, transfersomes, liposomes, or mixed lipid micelles. Langmuir. 2003;26:10753–10763. [Google Scholar]
- 11.Song CK, Balakrishnan P, Shim CK, et al. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: characterization and in vitro/in vivo evaluation. Colloids Surf B. 2012;92:299–304. doi: 10.1016/j.colsurfb.2011.12.004 [DOI] [PubMed] [Google Scholar]
- 12.Reuter S, Gupta SC, Chaturvedi MM, et al. Oxidative stress, inflammation, and cancer: how are they linked? Free Radical Bio Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jukanti R, Devaraj G, Devaraj R, et al. Drug targeting to inflammation: studies on antioxidant surface loaded diclofenac liposomes. Int J Pharm. 2011;414:179–185. doi: 10.1016/j.ijpharm.2011.05.031 [DOI] [PubMed] [Google Scholar]
- 14.Gupta PN, Mishra V, Rawat A, et al. Non-invasive vaccine delivery in transfersomes, niosomes and liposomes: a comparative study. Int J Pharm. 2005;293:73–82. doi: 10.1016/j.ijpharm.2004.12.022 [DOI] [PubMed] [Google Scholar]
- 15.Largo R, Roman-Bias JA, Moreno-Rubio J, et al. Chondroitin sulfate improves synovitis in rabbits with chronic antigen-induced arthritis. 13th World Congress of the Osteoarthritis-Research-Society-International, Rome, Italy. 2008;18(Supply):17–23. [DOI] [PubMed] [Google Scholar]
- 16.Pearson CM. Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proc Soc Exp Biol Med. 1956;91:95–101. [DOI] [PubMed] [Google Scholar]
- 17.Holmdahl R, Lorentzen JC, Lu SM, et al. Arthritis induced in rats with non-immunogenic adjuvants as models for rheumatoid arthritis. Immunol Rev. 2001;184:184–202. [DOI] [PubMed] [Google Scholar]
- 18.International Council for Standardization in Haematology (Expert Panel on Blood Rheology). ICSH recommendations for measurement of erythrocyte sedimentation rate. J Clin Pathol. 1993;46:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu XX, Chen YL, Gui SY, et al. Sinomenine hydrochloride-loaded dissolving microneedles enhanced its absorption in rabbits. Pharm Dev Technol. 2015;21:787–793. doi: 10.3109/10837450.2015.1055766 [DOI] [PubMed] [Google Scholar]