Abstract
Background: Asphyxia is the most common cause of brain damage in newborns. Substantial evidence indicates that leukocyte recruitment in the cerebral vasculature during asphyxia contributes to this damage. We tested the hypothesis that superoxide radical () promotes an acute post-asphyxial inflammatory response and blood-brain barrier (BBB) breakdown. We investigated the effects of removing by superoxide dismutase (SOD) or C3, the cell-permeable SOD mimetic, in protecting against asphyxia-related leukocyte recruitment. We also tested the hypothesis that xanthine oxidase activity is one source of this radical.
Methods: Anesthetized piglets were tracheostomized, ventilated, and equipped with closed cranial windows for the assessment of post-asphyxial rhodamine 6G-labeled leukocyte-endothelial adherence and microvascular permeability to sodium fluorescein in cortical venules. Asphyxia was induced by discontinuing ventilation. SOD and C3 were administered by cortical superfusion. The xanthine oxidase inhibitor oxypurinol was administered intravenously.
Results: Leukocyte-venular adherence significantly increased during the initial 2 h of post-asphyxial reperfusion. BBB permeability was also elevated relative to non-asphyxial controls. Inhibition of production by oxypurinol, or elimination of by SOD or C3, significantly reduced rhodamine 6G-labeled leukocyte-endothelial adherence and improved BBB integrity, as measured by sodium fluorescein leak from cerebral microvessels.
Conclusion: Using three different strategies to either prevent formation or enhance elimination of during the post-asphyxial period, we saw both reduced leukocyte adherence and preserved BBB function with treatment. These findings suggest that agents which lower in brain may be attractive new therapeutic interventions for the protection of the neonatal brain following asphyxia.
Keywords: asphyxia, carboxyfullerene, endothelium, inflammation, leukocytes, oxypurinol, superoxide, xanthine oxidase
Introduction
Asphyxia is a relatively common source of neonatal brain damage (1), affecting ~2 in every 1,000 births (2). The hypoxic ischemia resulting from this oxygen deprivation can produce long-term sequelae, including intellectual disability and neurologic deficits, and nearly a quarter of neonatal deaths worldwide are attributable to birth asphyxia (2). A dominant response to ischemia is inflammation (2, 3), resulting from the accumulation of circulating leukocytes in the cerebral microvasculature and their subsequent extravasation into brain parenchyma (4–7). The mechanisms promoting the early increase in leukocyte-endothelial interactions in the cerebral circulation await further elucidation, but probable causes include the elaboration of cytokines and chemokines (8, 9), and other proinflammatory mediators (10). There is also consensus that reactive oxygen species (ROS), formed during reperfusion following transient ischemia, including in the neonatal brain, also modulate inflammation (11–13).
ROS are generated in multiple compartments within a cell and are produced by many sources, including mitochondria, peroxisomal lipid metabolism, NADPH oxidases, cyclooxygenase, and xanthine oxidase (14–16). Increased levels of ROS are involved in the pathophysiology of many neurodegenerative diseases, stroke, aging, and birth asphyxia (13, 17–20). In vitro, is increased in neurons (21) and cerebral endothelial cells (22) in response to anoxia-reoxygenation stimuli that simulate ischemia-reperfusion. Activated leukocytes can form copious amounts of from NADPH oxidase during their respiratory burst (23). Xanthine oxidase and cyclooxygenase are two enzymatic sources of , in addition to formation of the radical in the electron transport chain of mitochondria (23, 24).
Considerable evidence implicates ROS in playing a causal role in ischemic brain damage (12, 23, 25). A burst of during reoxygenation has been suggested to cause direct cellular damage to macromolecules and initiate cellular signaling pathways involved in cell survival and death, resulting in apoptosis in ischemic lesions. The neonatal brain is believed to be especially vulnerable to free radical damage, due in part to its low amount of antioxidants (1).
Although a number of therapies are under consideration for treating moderate to severe neonatal asphyxia (2, 26), therapeutic hypothermia currently remains the standard treatment, despite evidence showing that the long-range neuroprotective benefits of hypothermia treatment are modest (1). A new potential treatment for neonatal asphyxia is C3, a small, cell-permeable C60 fullerene compound with SOD mimetic properties: e,e,e tris malonic acid (e,e,e-C60(C(COOH2))3). Due to its arrangement of malonic acid groups, C3 is amphiphilic and cell-permeable, and can catalytically remove (27). C3 protected against ischemia-mediated release of the intracellular enzyme lactate dehydrogenase in mouse retinal endothelial cells (28). Additionally, C3 improved motor function and protected against striatal injury in parkinsonian non-human primates, a model known to involve inflammation (29). C3 has proven to be neuroprotective in other injury models (30, 31) and to improve cognitive function and survival in a murine model of aging (19). In contrast to these cytoprotective effects, the effects of C3 on inflammatory endpoints are unknown.
In the present study, we utilized SOD, C3, and the xanthine oxidase inhibitor oxypurinol to examine the involvement of in mediating leukocyte adherence to post-capillary cerebral venules and BBB permeability changes in response to asphyxia. We tested whether is produced in response to asphyxia, whether xanthine oxidase is a source of this radical, and whether the inflammatory response caused by asphyxia can be prevented by removing by SOD or C3 administration, or by preventing the formation of this radical.
Methods
Ethical Approval
Washington University Institutional Animal Care and Use Committee approved the experiments, which were consistent with Public Health Service guidelines. All experiments were conducted in compliance with the ARRIVE guidelines.
Animals
Experiments were performed on piglets that were 1–4 days of age, 1.5–3.0 kg, and of mixed sex. Animals were randomized into control, asphyxia, and asphyxia plus treatment groups. Fluorescently-labeled leukocytes within pial microvessels on the cortical surface of the piglet brain were imaged through a closed cranial window. Rhodamine 6G was used to label circulating leukocytes in situ (loading dose: 2 ml/kg of a filtered 0.06 mg/ml solution, 20 min prior to the first baseline measurement; Sigma Chemical, St. Louis, MO), as previously described (32–34). To enhance leukocyte labeling, additional rhodamine was infused (800 μl/min/kg for 30–45 s) before each imaging time point. Real-time, high-resolution images of rhodamine-labeled leukocytes were recorded to videotape (Super VHS) using an epifluorescence microscope (Olympus, Lake Success, NY) with a rhodamine-specific optical filter (535 nm/35 nm excitation, 565 nm dichroic, 610 nm/75 nm emission), 10 × immersion lens (0.4 numerical aperture, 3.1 mm working distance; Olympus), and a C-2400 Newvicon tube camera (Hamamatsu, Bridgewater, NJ).
Piglets were anesthetized with ketamine hydrochloride (20 mg/kg IM), tracheostomized, and ventilated with room air and oxygen. End-tidal CO2 and transcutaneous O2 were monitored continuously using a capnometer and forepaw sensor, respectively. Anesthesia was maintained by ventilating with isoflurane (1.0–1.5%) while paralysis was sustained using pancuronium (0.25 mg/kg/h IV). A cannula was placed in one femoral vein for administration of the paralytic agent and 5% dextrose in 0.45% normal saline (6 ml/kg/h) to maintain fluid status. Both femoral arteries were cannulated to record arterial blood pressure and to measure blood gases, blood glucose, and pH. Body temperature was maintained at 38–39°C using a thermoregulated heating pad. A closed Plexiglass cranial window was mounted over the right parietal cortex just posterior to the coronal suture and was slowly filled with artificial cerebral spinal fluid (aCSF) buffer after mounting. Through circumferentially placed ports on the window, intracranial pressure was monitored.
Asphyxia
After the cranial window was placed, the animal and surgical site were allowed to stabilize for 30 min, and then two baseline video recordings were obtained 30 min apart. Asphyxia was then induced by discontinuing ventilation for 9 min, resulting in severe hypoxia, hypercapnia, hypotension, bradycardia, and acidosis. These conditions resolved in the vast majority of animals within 30 min after resuming ventilation without the use of resuscitation (32–34). A very small minority of animals died in response to asphyxia. Arterial blood pressure was recorded and blood gases, blood glucose, and pH were measured at baseline and at 1 and 2 h of reperfusion.
In this study, C3 and SOD were administered by cortical superfusion through the cranial window, whereas oxypurinol was given intravenously. To determine whether window superfusion of aCSF affected leukocyte adhesion differently than allowing aCSF to remain static, we established two asphyxia groups. In one, the cranial window was filled with aCSF buffer before baseline measurements were initiated, and the fluid beneath the windows remained undisturbed. In another, the window was superfused at 50 μl/min with aCSF beginning 30 min before asphyxia and continuing throughout the 2 h of reperfusion. The leukocyte adherence and vascular permeability data from these 2 asphyxia groups were statistically analyzed and graphed in comparison to their respective drug-treated groups, and the mean arterial blood pressure, blood glucose, arterial pH, arterial partial pressure of CO2, and arterial partial pressure of O2 data from these 2 asphyxia groups were also statistically analyzed in comparison to their respective drug-treated groups. In a similar fashion, the cranial windows of 2 separate non-asphyxial control groups were either superfused with aCSF or allowed to remain static once filled, and were imaged over the same time period. No difference in the magnitude of leukocyte adherence or vascular permeability to fluorescein was noted between these 2 control groups, so the data from the 2 non-asphyxial control groups were merged into a single control group.
Drug Administration
Oxypurinol (5 mg/kg or 50 mg/kg) was dispensed as an intravenous bolus, 30 min before asphyxia. SOD (1,000 U/ml; Oxis International, Portland, OR) or C3 [pH 7.0, 300 μM; synthesized as described previously (27, 29, 35)] were dissolved in aCSF and administered by cortical superfusion (50 μl/min) through the cranial window starting 30 min before asphyxia and continuing throughout the 2 h of post-asphyxial reperfusion.
Quantification of Intravascular Leukocyte Dynamics
Leukocytes adhering to the endothelium of pial venules were quantified during off-line videotape playback on a high resolution monitor by an observer blind to experimental condition using OPTIMAS image analysis software (BioScan, Inc., Edmonds, WA). Initially, the two-dimensional surface areas of 2 separate contiguous groups of 2 or more small (20–80 μm) post-capillary venules and their primary branches were determined. Then the three-dimensional area of the venous network was calculated by multiplying the determined area by π. Leukocytes were manually counted and designated as adherent if they remained stationary within the venule for longer than 10 s. The number of leukocytes per millimeter vessel surface was calculated by dividing the raw leukocyte number by the 3-dimensional area of the venular network.
BBB Permeability
Intravenous sodium fluorescein (MW = 376) was used to assess the effects of different drug treatments on the increase in BBB permeability induced by asphyxia, as described in detail in earlier publications (32, 34). Briefly, after 2 h of reperfusion, 1 ml/kg of a 0.04% solution of sodium fluorescein was administered intravenously over 1 min.
Sodium fluorescein's extravascular presence was quantified using the OPTIMAS image analysis software with a linear intensity (0–255) scale. Video recordings were obtained just prior to the fluorescein injection and during the initial minute after its administration to obtain background and maximum/peak optical density (OD) values, respectively, and then again 20 minutes thereafter. The OD was measured offline in an experimental group-blinded manner at 3 separate extravascular locations along a large portion of the length of 2 different venules chosen from the video field vascular network (total of 6 perivenular locations). Changes in OD were normalized along a 0–100% scale based on the difference between the background OD and the maximum OD in each animal. The six perivascular OD values were typically similar, and were averaged to obtain a representative value for fluorescein leakage in the pial venular network for each animal.
Statistical Analysis
Sample sizes were determined based on data obtained in a previous publication (32), using a power of 0.80 and an alpha value of 0.05. Sample sizes were determined to be n = 5 per group. Since a limited number of animals could be studied in 1 day and control and asphyxia animals were included with every drug-treated group, control and asphyxia groups have larger sample sizes than drug-treated groups.
All statistics were performed using SigmaStat 4.0. One-way analysis of variance (ANOVA) and Tukey tests were performed for data shown in Table 1 and for Figures 2B,C. Two-way repeated measures ANOVA and Holm-Sidak tests were performed for Figures 1B,C. A Shapiro-Wilk test was performed and a Spearman's rank correlation coefficient was determined for Figure 2D. Significant differences were noted if p < 0.05.
Table 1.
Values are means ± S.E.M. Baseline values reported are averaged from two measurements recorded 30 min apart.
| Time point | Control | Asphyxia (+) | Asphyxia (−) | Asphyxia + SOD | Asphyxia + C3 | Asphyxia + Oxypurinol (5 mg/kg) | Asphyxia + Oxypurinol (50 mg/kg) |
|---|---|---|---|---|---|---|---|
| MEAN ARTERIAL BLOOD PRESSURE (mm Hg) | |||||||
| Baseline | 56 ± 1 | 57 ± 2 | 59 ± 1 | 55 ± 1 | 54 ± 1 | 55 ± 2 | 56 ± 1 |
| 1 h | 55 ± 1 | 54 ± 2 | 58 ± 1 | 53 ± 1 | 51 ± 1 | 54 ± 2 | 55 ± 1 |
| 2 h | 56 ± 1 | 52 ± 2 | 55 ± 1# | 53 ± 0 | 52 ± 2 | 51 ± 1 | 54 ± 1 |
| BLOOD GLUCOSE (mg/dL) | |||||||
| Baseline | 88 ± 7 | 104 ± 6 | 103 ± 5 | 95 ± 5 | 126 ± 3* | 111 ± 22 | 91 ± 4 |
| 1 h | 92 ± 7 | 120 ± 5* | 117 ± 10 | 106 ± 8 | 135 ± 8* | 112 ± 12 | 109 ± 10 |
| 2 h | 93 ± 7 | 122 ± 6 | 107 ± 10 | 93 ± 10 | 124 ± 15 | 88 ± 10 | 89 ± 8 |
| ARTERIAL pH | |||||||
| Baseline | 7.38 ± 0.01 | 7.36 ± 0.01 | 7.38 ± 0.01 | 7.34 ± 0.02 | 7.34 ± 0.02 | 7.41 ± 0.02 | 7.37 ± 0.02 |
| 1 h | 7.36 ± 0.01 | 7.31 ± 0.02 | 7.32 ± 0.02 | 7.29 ± 0.02 | 7.29 ± 0.01 | 7.35 ± 0.02 | 7.33 ± 0.02 |
| 2 h | 7.35 ± 0.01 | 7.35 ± 0.02 | 7.34 ± 0.02 | 7.33 ± 0.02 | 7.34 ± 0.02 | 7.40 ± 0.01 | 7.36 ± 0.01 |
| ARTERIAL PARTIAL PRESSURE OF CO2 (mm Hg) | |||||||
| Baseline | 40 ± 1 | 38 ± 1 | 38 ± 1 | 38 ± 1 | 43 ± 1 | 37 ± 1 | 37 ± 2 |
| 1 h | 41 ± 1 | 38 ± 2 | 40 ± 1 | 39 ± 1 | 41 ± 3 | 37 ± 2 | 40 ± 2 |
| 2 h | 40 ± 1 | 35 ± 1 | 39 ± 1 | 39 ± 2 | 41 ± 2 | 36 ± 2 | 38 ± 3 |
| ARTERIAL PARTIAL PRESSURE OF O2 (mm Hg) | |||||||
| Baseline | 94 ± 3 | 98 ± 3 | 95 ± 2 | 94 ± 3 | 88 ± 2 | 96 ± 3 | 91 ± 2 |
| 1 h | 96 ± 2 | 101 ± 3 | 105 ± 4 | 103 ± 4 | 98 ± 4 | 93 ± 3 | 102 ± 4 |
| 2 h | 92 ± 2 | 105 ± 4 | 100 ± 2 | 94 ± 2 | 112 ± 11* | 94 ± 3 | 99 ± 4 |
(+), with aCSF superfusion across the cranial window; (−), without aCSF superfusion across the cranial window.
p < 0.05 vs. control at the same time point;
p < 0.05 vs. baseline mean arterial blood pressure value for asphyxia without aCSF superfusion.
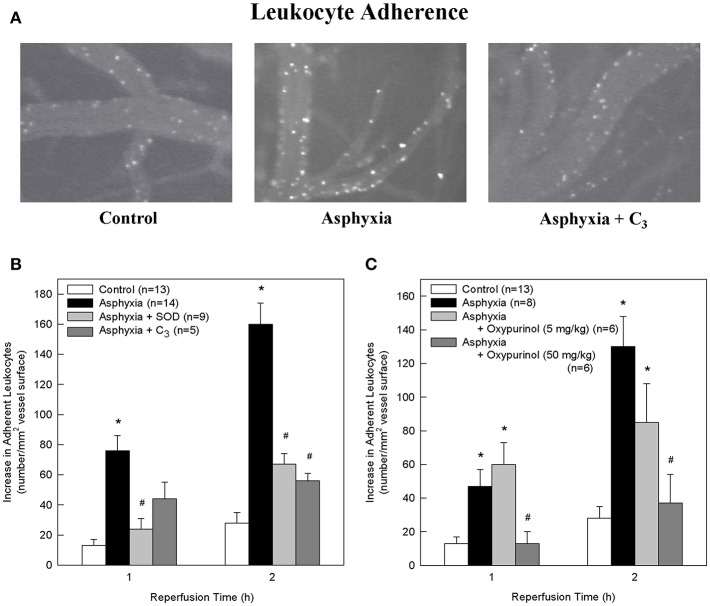
Figure 1.
SOD, the SOD mimetic C3, and oxypurinol block the asphyxia-mediated increase in leukocyte adherence. (A) Representative images of rhodamine 6G-labeled adherent leukocytes in control animals, asphyxial animals, and asphyxial animals administered C3, at 2 h of reperfusion. (B) Animals induced with asphyxia and concomitant window superfusion with aCSF (n = 14) exhibited a statistically significant increase in leukocyte adherence relative to control animals (n = 13) at both 1 and 2 h of reperfusion. Animals continuously administered 1,000 U/ml SOD at a rate of 50 μl/min by window superfusion beginning 30 min prior to asphyxia (n = 9) exhibited significantly decreased leukocyte adherence relative to asphyxial animals at both 1 and 2 h of reperfusion. Animals continuously administered 300 μM C3 at a rate of 50 μl/min by window superfusion beginning 30 min prior to asphyxia (n = 5) exhibited significantly decreased leukocyte adherence relative to asphyxial animals at 2 h of reperfusion. (C) Animals induced with asphyxia without aCSF superfusion across the window (n = 8) had a statistically significant increase in leukocyte adherence relative to control animals (n = 13) at both 1 and 2 h of reperfusion. Animals administered 50 mg/kg oxypurinol 30 min prior to undergoing the asphyxia protocol (n = 6) exhibited significantly decreased leukocyte adherence relative to asphyxial animals at both 1 and 2 h of reperfusion. Animals administered 5 mg/kg oxypurinol 30 min prior to undergoing the asphyxia protocol (n = 6) exhibited no change in leukocyte adherence relative to asphyxial animals at either time point. Values shown (mean ± S.E.M.) represent increases above baseline in the number of leukocytes adherent to cerebral venules after 1 and 2 h of post-asphyxial reperfusion. *p < 0.05 vs. control; #p < 0.05 vs. asphyxia.
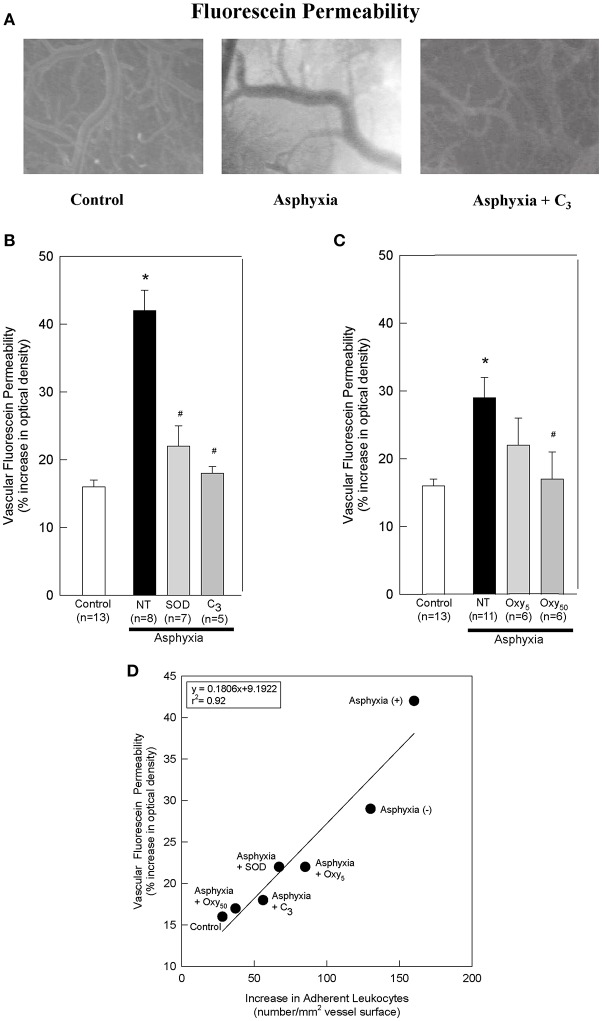
Figure 2.
SOD, C3, and oxypurinol block the asphyxia-mediated increases in vascular fluorescein permeability, and vascular fluorescein permeability directly correlates with leukocyte adherence. (A) Representative images of sodium fluorescein permeability in control animals, asphyxial animals, and asphyxial animals administered C3, at 2 h of post-asphyxial reperfusion and 20 min after fluorescein administration. Raw images were used for all data analyses, but for publication clarity, images had brightness and contrast enhanced (all images modified equally). (B) Animals induced with asphyxia and concomitant cranial window superfusion with aCSF (NT; n = 8) exhibited a statistically significant increase in vascular fluorescein permeability relative to control animals (n = 13). Animals continuously administered 1,000 U/ml SOD at a rate of 50 μl/min by window superfusion (n = 7) exhibited significantly decreased vascular fluorescein permeability relative to asphyxial animals. Similarly, animals continuously administered 300 μM C3 at a rate of 50 μl/min by window superfusion (n = 5) exhibited significantly decreased vascular fluorescein permeability relative to asphyxial animals. (C) Animals induced with asphyxia without aCSF superfusion across the cranial window (NT; n = 11) exhibited a statistically significant increase in vascular fluorescein permeability relative to control animals (n = 13). Animals administered 50 mg/kg oxypurinol (n = 6) exhibited significantly decreased vascular fluorescein permeability relative to asphyxial animals. Animals administered 5 mg/kg oxypurinol (n = 6) exhibited no change in vascular fluorescein permeability relative to asphyxial animals. (D) A linear regression was generated by matching leukocyte adherence to the corresponding vascular fluorescein permeability under the different experimental conditions. Values shown (mean ± S.E.M.) represent the percent increase in optical density relative to that measured before fluorescein administration. NT, not treated; (+), with aCSF superfusion; (–), without aCSF superfusion. *p < 0.05 vs. control; #p < 0.05 vs. asphyxia.
Results
Mean arterial blood pressure, blood glucose, arterial pH, arterial partial pressure of CO2, and arterial partial pressure of O2 at baseline and at 1 and 2 h of reperfusion are displayed in Table 1. Significant differences within and between groups are displayed in Table 1. While measures of blood glucose at 2 h, arterial pH at 1 h, and arterial partial pressure of CO2 at baseline and at 2 h demonstrated significant differences between the control, asphyxia with aCSF superfusion, asphyxia with SOD, and asphyxia with C3 groups overall (p < 0.05), post-hoc analyses revealed no individual between-group differences that were significant. No significant differences existed between the asphyxia groups and any of the corresponding treatment groups for any of the parameters at any time point.
Relative to non-asphyxial control animals, asphyxia resulted in progressive increases in the number of leukocytes adherent to cerebral venules during the initial 2 h of reperfusion (Figures 1A–C), consistent with our earlier studies (32, 34, 36). Adherence was 62% greater at 1 h and 23% greater at 2 h in asphyxial animals in which the window was superfused with aCSF (Figure 1B) relative to asphyxial animals in which the aCSF remained static during the experiment (Figure 1C).
After 2 h of post-asphyxial reperfusion, significant increases in sodium fluorescein permeability were also observed relative to non-asphyxial control animals (Figures 2A–C), indicative of a compromised BBB (32, 34, 36). In particular, in asphyxial animals with aCSF window superfusion, permeability was increased 42 ± 3% (Figure 2B), whereas in asphyxial animals with static aCSF under the window, permeability increased less (29 ± 3%; Figure 2C).
To determine if participated in promoting the elevated leukocyte adherence and/or the loss of BBB integrity that characterized the acute inflammatory response to asphyxia, we assessed the effects of removing by the extracellularly-confined enzyme SOD or by C3 (30). Topical superfusion of SOD significantly reduced leukocyte adherence at 1 and 2 h of reperfusion in asphyxial animals (Figure 1B). SOD also reduced asphyxia-induced elevations in BBB permeability to fluorescein (Figure 2B). Topical superfusion of C3 also reduced the post-asphyxial inflammatory response. As shown in Figures 1A,B and 2A,B, respectively, asphyxia-induced increases in both leukocyte adherence and BBB permeability were significantly attenuated by this scavenger.
We tested the hypothesis that xanthine oxidase is one source of following asphyxia by assessing the effects of oxypurinol on asphyxia-induced increases in leukocyte adherence and BBB permeability. Inhibition of endothelial and circulating xanthine oxidase activity with intravenous administration of oxypurinol significantly and dose-dependently reduced asphyxia-induced leukocyte adherence to post-capillary venules to levels that, at the higher dose, were not different from non-asphyxial controls (Figure 1C). Similarly, reductions in asphyxia-induced BBB breakdown were evidenced after administration of the higher dose of oxypurinol (Figure 2C).
When all experimental conditions were examined collectively, changes in leukocyte-endothelial adherence were highly correlated to changes in fluorescein permeability (r2 = 0.92; Figure 2D), suggesting a causal relationship between leukocyte adherence and BBB integrity.
Discussion
We demonstrated a marked, time-dependent increase in leukocyte adherence to cerebral venules and a loss in BBB integrity during the initial 2 h of reperfusion following asphyxia. Local superoxide scavenging with C3 or SOD, or systemic inhibition of xanthine oxidase activity with oxypurinol, significantly decreased these inflammatory responses. These results suggest that the formation of inflammation-promoting concentrations of results in part from the breakdown of high energy adenine nucleotides and the resulting elevation in purine catabolites, which in turn serve as substrates for endothelial and circulating xanthine oxidase.
Numerous studies in the brain have demonstrated that oxygen free radicals are produced upon reperfusion following ischemia, and that their generation through various biochemical pathways during reperfusion is associated with increases in BBB permeability (37). The significant reduction in leukocyte adherence observed when animals were administered C3 or SOD indicates that , generated from circulating neutrophils (38), cerebrovascular endothelial and smooth muscle cells (39), and/or the parenchyma surrounding the vasculature (38, 40–42), contributes to this vascular inflammatory response to asphyxia. The increase in BBB permeability following asphyxia is reduced by C3 or SOD treatment, which parallels the reduction in leukocyte adherence in asphyxial animals treated with either of these drugs. This is consistent with the notion that leukocyte adherence is a cause of BBB breakdown, at least in the acute post-asphyxial setting (43). Moreover, consistent with clinical studies of allopurinol in neonatal asphyxia (44), our results suggest that xanthine oxidase is a major source of produced in response to asphyxia, given that oxypurinol also significantly reduced asphyxia-mediated increases in leukocyte adherence and BBB permeability. The extent to which the latter inflammatory events result from oxypurinol-induced reductions in superoxide production and/or oxypurinol-induced increases in adenosine cannot be ascertained from the results of our study.
While all experimental treatments in this study were effective in reducing leukocyte adherence and BBB permeability, neither oxypurinol nor SOD is an ideal therapeutic compound. Oxypurinol first needs to be converted to an active state, and its potency is weak. SOD may lack substantial brain uptake due to its relatively large size. On the other hand, C3 has several features which may prove attractive clinically. It acts catalytically (27), and therefore retains activity for as long as it is present, has a plasma half-life of 8 h, and demonstrates significant brain uptake in both rodents and non-human primates (29, 45). C3 is also highly water soluble and has oral bioavailability (45). While C3 is roughly 100-fold less active than SOD itself (27), its ability to access mitochondria as well as other intracellular compartments may enhance its overall biological efficacy, especially as it appears to have a good safety profile with long-term (2 years, mice; 2 months, primates) chronic administration (19, 45). Newborn infants could theoretically be treated with C3 via oral fluids such as formula, or intravenously. Future investigation could help define the time window during which compounds such as C3 are effective in reducing the deleterious effects of neonatal asphyxia.
In this proof-of-concept study, we administered the superoxide scavenging agents C3 and SOD locally via cranial window superfusion, and the xanthine oxidase inhibitor oxypurinol systemically, 30 min prior to asphyxia, with the pharmacokinetics-based intention of providing some time for these agents to reach the intracellular and intercellular locations where superoxide might be generated rapidly during or shortly after asphyxia; whether their efficacy would have been as robust when administered after asphyxia, to more closely mimic the clinical situation, is unclear. We also did not explore the effects of oral or systemic C3 on post-asphyxial inflammation, as this would have involved dose-finding experiments requiring more C3 than was available. In addition, we focused on the acute response to asphyxia and did not attempt to quantify the extent of leukocyte diapedesis into brain parenchyma, nor measure endpoints, such as CA1 pyramidal neuron death, that are more indicative of asphyxial brain injury and the potential long-term neuroprotective effects of our interventions. Finally, while we found strong correlations between the extent of post-asphyxial leukocyte adherence to the venular endothelium and BBB breakdown under control conditions and in response to our interventions, we cannot conclude with certainty that the former caused 100% of the latter. It is possible that contributed to some portion of the observed BBB breakdown, not only independent of the extent of leukocyte adherence to the venular endothelium, but to BBB breakdown in other parts of the microvasculature as well.
In summary, we found that is produced in response to asphyxia, that xanthine oxidase is an important enzymatic source of this radical, and that scavenging of by administering C3 or SOD, or preventing its formation, can ameliorate this acute inflammatory response and lessen its potential to damage the neonatal cerebral microcirculation.
Ethics Statement
This study was carried out in accordance with the recommendations of Public Health Service guidelines. The protocol was approved by the Washington University Institutional Animal Care and Use Committee.
Author Contributions
JR wrote and revised substantial portions of the article. KQ prepared and tested C3 and wrote sections of the manuscript. EG and AS carried out the asphyxia experiments, including the fluorescent leukocyte labeling and imaging. TP contributed to initial experimental plans. NK contributed to writing portions of the article. LD and JG conceptualized the project, guided the experimental design and data analysis and interpretation, and contributed to writing portions of the article.
Conflict of Interest Statement
KQ is currently employed by company PerkinElmer, but he was not affiliated with a commercial company at the time of data collection. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Jennifer Meier, Ray Maceren, and Ronald Perez for their technical assistance in these studies.
Footnotes
Funding. This work is supported by NS37688 (LD), AG033679 (LD), the Larry L. Hillblom Foundation (LD), the Hartke Fund (LD), a Paul Beeson Physician Scholars Award (LD), VA Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC), Nashville, TN, USA (LD), a Jacob Javits Neuroscience Investigator Award (NS21045; TP), and T32 MH064913 (JR).
References
- 1.Negro S, Benders M, Tataranno ML, Coviello C, de Vries LS, van Bel F, et al. Early prediction of hypoxic-ischemic brain injury by a new panel of biomarkers in a population of term newborns. Oxid Med Cell Longev. (2018) 2018:7608108. 10.1155/2018/7608108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rocha-Ferreira E, Hristova M. Plasticity in the neonatal brain following hypoxic-ischaemic injury. Neural Plast. (2016) 2016:4901014. 10.1155/2016/4901014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rees S, Harding R, Walker D. The biological basis of injury and neuroprotection in the fetal and neonatal brain. Int J Dev Neurosci. (2011) 29:551–63. 10.1016/j.ijdevneu.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartl R, Schurer L, Schmid-Schonbein GW, del Zoppo GJ. Experimental antileukocyte interventions in cerebral ischemia. J. Cereb. Blood Flow Metab. (1996) 16:1108–19. 10.1097/00004647-199611000-00004 [DOI] [PubMed] [Google Scholar]
- 5.Jean WC, Spellman SR, Nussbaum ES, Low WC. Reperfusion injury after focal cerebral ischemia: the role of inflammation and the therapeutic horizon. Neurosurgery. (1998). 43:1382–96; discussion 1396–87. 10.1227/00006123-199812000-00076 [DOI] [PubMed] [Google Scholar]
- 6.Sughrue ME, Mehra A, Connolly ESJr, D'Ambrosio AL. Anti-adhesion molecule strategies as potential neuroprotective agents in cerebral ischemia: a critical review of the literature. Inflamm Res. (2004) 53:497–508. 10.1007/s00011-004-1282-0 [DOI] [PubMed] [Google Scholar]
- 7.Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci. (2017) 20:136–44. 10.1038/nn.4475 [DOI] [PubMed] [Google Scholar]
- 8.Kaur C, Ling EA. Blood brain barrier in hypoxic-ischemic conditions. Curr Neurovasc Res. (2008) 5:71–81. 10.2174/156720208783565645 [DOI] [PubMed] [Google Scholar]
- 9.Ransohoff RM, Schafer D, Vincent A, Blachere NE, Bar-Or A. Neuroinflammation: ways in which the immune system affects the brain. Neurotherapeutics. (2015) 12:896–909. 10.1007/s13311-015-0385-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu F, Liu L, Zhou H. Endothelial cell activation in central nervous system inflammation. J Leukoc Biol. (2017) 101:1119–32. 10.1189/jlb.3RU0816-352RR [DOI] [PubMed] [Google Scholar]
- 11.Altay T, Gonzales ER, Park TS, Gidday JM. Cerebrovascular inflammation after brief episodic hypoxia: modulation by neuronal and endothelial nitric oxide synthase. J Appl Physiol. (2004) 96:1223–30; discussion 1196. 10.1152/japplphysiol.00798.2003 [DOI] [PubMed] [Google Scholar]
- 12.Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. (2015) 6:524–51. 10.1016/j.redox.2015.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rocha-Ferreira E, Hristova M. Antimicrobial peptides and complement in neonatal hypoxia-ischemia induced brain damage. Front Immunol. (2015) 6:56. 10.3389/fimmu.2015.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenaz G, Baracca A, Fato R, Genova ML, Solaini G. New insights into structure and function of mitochondria and their role in aging and disease. Antioxid Redox Signal. (2006) 8:417–37. 10.1089/ars.2006.8.417 [DOI] [PubMed] [Google Scholar]
- 15.Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. (2006) 58:87–114. 10.1124/pr.58.1.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med Cell Longev. (2016) 2016:1245049. 10.1155/2016/1245049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. (1998) 78:547–81. 10.1152/physrev.1998.78.2.547 [DOI] [PubMed] [Google Scholar]
- 18.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. (2005) 308:1909–11. 10.1126/science.1106653 [DOI] [PubMed] [Google Scholar]
- 19.Quick KL, Ali SS, Arch R, Xiong C, Wozniak D, Dugan LL. A carboxyfullerene SOD mimetic improves cognition and extends the lifespan of mice. Neurobiol Aging. (2008) 29:117–28. 10.1016/j.neurobiolaging.2006.09.014 [DOI] [PubMed] [Google Scholar]
- 20.Thornton C, Baburamani AA, Kichev A, Hagberg H. Oxidative stress and endoplasmic reticulum (ER) stress in the development of neonatal hypoxic-ischaemic brain injury. Biochem Soc Trans. (2017) 45:1067–76. 10.1042/BST20170017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci. (2007) 27:1129–38. 10.1523/JNEUROSCI.4468-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beetsch JW, Park TS, Dugan LL, Shah AR, Gidday JM. Xanthine oxidase-derived superoxide causes reoxygenation injury of ischemic cerebral endothelial cells. Brain Res. (1998) 786:89–95. 10.1016/S0006-8993(97)01407-8 [DOI] [PubMed] [Google Scholar]
- 23.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. (2012) 298:229–317. 10.1016/B978-0-12-394309-5.00006-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstead WM. Cyclooxygenase-2-dependent superoxide generation contributes to age-dependent impairment of G protein-mediated cerebrovasodilation. Anesthesiology. (2003) 98:1378–83. 10.1097/00000542-200306000-00012 [DOI] [PubMed] [Google Scholar]
- 25.Sanderson TH, Reynolds CA, Kumar R, Przyklenk K, Huttemann M. Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol. (2013) 47:9–23. 10.1007/s12035-012-8344-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nair J, Kumar VHS. Current and emerging therapies in the management of hypoxic ischemic encephalopathy in neonates. Children. (2018) 5:e99. 10.3390/children5070099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali SS, Hardt JI, Quick KL, Kim-Han JS, Erlanger BF, Huang TT, et al. A biologically effective fullerene (C60) derivative with superoxide dismutase mimetic properties. Free Radic Biol Med. (2004) 37:1191–202. 10.1016/j.freeradbiomed.2004.07.002 [DOI] [PubMed] [Google Scholar]
- 28.Rieger JM, Shah AR, Gidday JM. Ischemia-reperfusion injury of retinal endothelium by cyclooxygenase- and xanthine oxidase-derived superoxide. Exp Eye Res. (2002) 74:493–501. 10.1006/exer.2001.1156 [DOI] [PubMed] [Google Scholar]
- 29.Dugan LL, Tian L, Quick KL, Hardt JI, Karimi M, Brown C, et al. Carboxyfullerene neuroprotection postinjury in Parkinsonian nonhuman primates. Ann Neurol. (2014) 76:393–402. 10.1002/ana.24220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dugan LL, Turetsky DM, Du C, Lobner D, Wheeler M, Almli CR, et al. Carboxyfullerenes as neuroprotective agents. Proc Natl Acad Sci USA. (1997) 94:9434–9. 10.1073/pnas.94.17.9434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dugan LL, Lovett EG, Quick KL, Lotharius J, Lin TT, O'Malley KL. Fullerene-based antioxidants and neurodegenerative disorders. Parkinsonism Relat Disord. (2001) 7:243–6. 10.1016/S1353-8020(00)00064-X [DOI] [PubMed] [Google Scholar]
- 32.Gidday JM, Park TS, Gonzales ER, Beetsch JW. CD18-dependent leukocyte adherence and vascular injury in pig cerebral circulation after ischemia. Am J Physiol. (1997) 272(6 Pt 2):H2622–9. 10.1152/ajpheart.1997.272.6.H2622 [DOI] [PubMed] [Google Scholar]
- 33.Gidday JM, Park TS, Shah AR, Gonzales ER. Modulation of basal and postischemic leukocyte-endothelial adherence by nitric oxide. Stroke. (1998) 29:1423–9; discussion 1429–30. 10.1161/01.STR.29.7.1423 [DOI] [PubMed] [Google Scholar]
- 34.Park TS, Gonzales ER, Gidday JM. Platelet-activating factor mediates ischemia-induced leukocyte-endothelial adherence in newborn pig brain. J Cereb Blood Flow Metab. (1999) 19:417–24. 10.1097/00004647-199904000-00007 [DOI] [PubMed] [Google Scholar]
- 35.Ali SS, Hardt JI, Dugan LL. SOD activity of carboxyfullerenes predicts their neuroprotective efficacy: a structure-activity study. Nanomedicine. (2008) 4:283–94. 10.1016/j.nano.2008.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaplan SS, Park TS, Gonzales ER, Gidday JM. Hydroxyethyl starch reduces leukocyte adherence and vascular injury in the newborn pig cerebral circulation after asphyxia. Stroke. (2000) 31:2218–23. 10.1161/01.STR.31.9.2218 [DOI] [PubMed] [Google Scholar]
- 37.Sun MS, Jin H, Sun X, Huang S, Zhang FL, Guo ZN, et al. Free radical damage in ischemia-reperfusion injury: an obstacle in acute ischemic stroke after revascularization therapy. Oxid Med Cell Longev. (2018) 2018:3804979. 10.1155/2018/3804979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosen GM, Pou S, Ramos CL, Cohen MS, Britigan BE. Free radicals and phagocytic cells. FASEB J. (1995) 9:200–9. 10.1096/fasebj.9.2.7540156 [DOI] [PubMed] [Google Scholar]
- 39.Kontos CD, Wei EP, Williams JI, Kontos HA, Povlishock JT. Cytochemical detection of superoxide in cerebral inflammation and ischemia in vivo. Am J Physiol. (1992) 263(4 Pt 2):H1234–42. 10.1152/ajpheart.1992.263.4.H1234 [DOI] [PubMed] [Google Scholar]
- 40.Terada LS, Willingham IR, Rosandich ME, Leff JA, Kindt GW, Repine JE. Generation of superoxide anion by brain endothelial cell xanthine oxidase. J Cell Physiol. (1991) 148:191–6. 10.1002/jcp.1041480202 [DOI] [PubMed] [Google Scholar]
- 41.Dugan LL, Lin TS, He YY, Hsu CY, Choi DW. Detection of free radicals by microdialysis/spin trapping EPR following focal cerebral ischemia-reperfusion and a cautionary note on the stability of 5,5-dimethyl-1-pyrroline N-oxide (DMPO). Free Radic Res. (1995) 23:27–32. 10.3109/10715769509064016 [DOI] [PubMed] [Google Scholar]
- 42.Reynolds IJ, Hastings TG. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci. (1995) 15(5 Pt 1):3318–27. 10.1523/JNEUROSCI.15-05-03318.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. (2013) 19:1584–96. 10.1038/nm.3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaandorp JJ, Benders MJ, Rademaker CM, Torrance HL, Oudijk MA, de Haan TR, et al. Antenatal allopurinol for reduction of birth asphyxia induced brain damage (ALLO-Trial); a randomized double blind placebo controlled multicenter study. BMC Pregnancy Childbirth. (2010) 10:8. 10.1186/1471-2393-10-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hardt JI, Perlmutter JS, Smith CJ, Quick KL, Wei L, Chakraborty SK, et al. Pharmacokinetics and toxicology of the neuroprotective e,e,e-methanofullerene(60)-63-tris malonic acid [C3] in mice and primates. Eur J Drug Metab Pharmacokinet. (2018) 43:543–54. 10.1007/s13318-018-0464-z [DOI] [PMC free article] [PubMed] [Google Scholar]