Abstract
Background: Transcatheter arterial chemoembolization (TACE) is one of the local therapies most commonly used to treat intermediate-stage or advanced-stage hepatocellular carcinoma (HCC). However, the clinical benefits of PA-TACE (postoperative adjuvant TACE) for improving prognosis (progress-free survival [PFS] or overall survival [OS]) of low-risk HCC patients with R0-stage HCC after hepatectomy were not very clear.
Methods: From January 2005 to December 2012, 180 patients who underwent hepatectomy for HCC treatment were enrolled in this study, and the follow-up of these patients was ended in December 2017. Among these patients, 102 patients were performed PA-TACE 1 month later after R0 hepatectomy and 78 patients without adjuvant TACE after R0 hepatectomy. Survival analysis was calculated using the Kaplan–Meier statistical method. Differences between survival curves of different groups were tested using the univariate log-rank test. Multivariate Cox model was used to search for independent prognostic factors for progression or death and to acquire the adjusted HR.
Results: PA-TACE significantly improved the survival of HCC patients received surgical resection. The PFS (progress-free survival) of PA-TACE group (median PFS 52.0 months; 95% CI: 14.0–90.0) was significantly longer than the control group (median PFS 11.1 months; 95% CI: [7.9–14.3]; log-rank P<0.001); and the OS (in PA-TACE group (median OS 90.7 months; 95% CI: 84.4–97.0 months) was also much longer than that of control group (median OS 54.4 months; 95% CI: 38.2–70.6 months; log-rank p<0.001). Moreover, the benefits of PA-TACE are greater for low-risk patients than high-risk patients.
Conclusion: In patients with HCC, PA-TACE can significantly prolong progression-free survival and long-term OS. For low-risk patients, the benefits might be greater.
Keywords: R0 hepatocellular carcinoma, postoperative adjuvant transcatheter arterial chemoembolization, recurrence after hepatectomy, progress-free survival, overall survival
Introduction
The high infection rate of the hepatitis virus (such as HBV or HCV) has caused a large number of patients with hepatocellular carcinoma (HCC) in China, and HCC has been an urgent medical burden in the public medical system in China. 1–5 Although early diagnosis or effective treatment approaches will relieve the progress of HCC and prolong patients’ survival, most proportion of patients are often diagnosed as advanced stage of HCC (advanced HCC) which is not suitable for surgical resection/operation (hepatectomy) or liver transplantation.6–8 Liver resection remains the main curative option for early stage of HCC.8,9 Patients with early stage of HCC often have a good 5-year overall survival (OS) rate (about 50–70%) after curative hepatectomy.9,10 Unfortunately, these patients with HCC who are able to receive surgical resection are prone to recurrence after surgery.11 The long-term prognosis of patients with advanced HCC is still unsatisfactory due to the high rate of recurrence after surgical operation.12,13
Transcatheter arterial chemoembolization (TACE) is a kind of local therapies treating patients with intermediate-stage HCC or advanced HCC.14–17 By administration of embolizing agents or chemotherapeutic agents via arterial injection, TACE treatment could decrease blood flow of the HCC lesions in liver organ and lead to ischemic necrosis of HCC lesions.18,19 Usage of TACE as an adjuvant approach (PA-TACE) for HCC treatment has been performed in some clinical trials.20 Although results from Ke-Wei et al (2016) indicated that PA-TACE could improve survival (OS or recurrence-free survival) of patients, cohorts including in this study are not big enough to achieve statistical significance between treatment group or control group.20,21 Therefore, the usage or application of PA-TACE in HCC treatment needs further investigations.
In the present work, we retrospectively compared the progress-free survival (PFS) and long-term OS between two groups of patients treated with or without PA-TACE after R0 hepatectomy for HCC, to identify if PA-TACE after R0 hepatectomy is necessary.
Material and methods
Subject and study design
The methods and protocols of this work were all approved by the Ethics Committee of Chinese PLA General Hospital and this study was conducted in accordance with the Declaration of Helsinki. All patients included in this work signed written informed consent before the treatment. Since 2005–2012, a total of 196 patients were performed hepatectomy by one group of hepatobiliary surgeons of our hospital, 180 of which were proved to be R0 resection postoperative. Of the 180 cases, according to whether preventive interventional therapy was performed or not, 102 patients and 78 patients were divided into PA-TACE group and control group. To the patient’s decision to receive adjuvant TACE, they were also required to have a WHO performance status 0–1, Child–Pugh Class A or B, normal kidney function, white blood cell count 3.0*109/L and platelet count 50*109/L. In addition, high-risk patients were defined as lesions larger than 3 cm, multiple lesions, portal branch or surrounding tissues invasion. While low-risk patients were defined as lesions less than 3 cm, single lesion, no blood vessels and surrounding tissue invasion, Child A.
Patients underwent PA-TACE procedures with concentrated chemotherapeutic and Ethiodol (doxorubicin alone). Follow-up was regularly performed at the section for outpatients. The patients without data from the section for outpatients were collected through telephone inquiry. The endpoint of the study was the OS. All followed-up investigation was carried out until November 2017.
Data collections
Patients were with a diagnosis of primary HCC by imaging examination by computed tomography(CT), positron emission tomography or magnetic resonance imaging (MRI) . PA-TACE and data collection were performed following methods described by Sun et al (2016) and Li et al (2015).22,23
Detailed history and complete physical examination were conducted for all patients who were admitted to the Eastern Hepatobiliary Surgery Hospital with a diagnosis of primary liver malignancy. They were routinely investigated with immunological indexes of hepatitis B and C, hepatitis B virus-DNA load, liver function test, and serum tumor markers including α-fetoprotein (AFP), carbohydrate antigen 19–9 (CA19-9) and carcinoembryonic antigen. Imaging studies with chest CT and abdominal MRI were conducted.
Operation
All TACE procedures were performed using digital subtraction angiography guidance (9). At 4 weeks after RH, when the liver function of the patient had recovered, a hepatic arterial catheter was placed into the proper hepatic artery through the femoral artery using the Seldinger technique, and TACE was performed for the entire remnant liver. Hepatic angiography and dyna-CT were performed to detect any obvious tumor stains in the remnant liver. An emulsion of pharmorubicin (20–40 mg) and lipiodol (2–10 mL) (Lipiodol Ultrafluide, Guerbet, AulnaySous-Bois, France) then was infused through the catheter.
The dosage of lipiodol and doxorubicin was determined by body surface area and underlying liver function. After 1 month of follow-up evaluation, a CT scan was performed to determine the effects of TACE.
All procedures were technically successful with no major procedural complications requiring additional hospitalization or intervention.
Statistical analysis
Data were described as frequencies and proportions and continuous variables were converted into binary variables. Survival curves of the two groups of patients treated with or without PA-TACE were calculated using the Kaplan–Meier method. Differences between groups were tested by univariate log-rank tests. Multivariate Cox model was used to search for independent prognostic factors for progression or death and to acquire the adjusted HR. P-values <0.05 were considered statistically significant between groups. Calculations were performed using the Statistical Package for Social Sciences Program, version 22.0 (SPSS, Chicago, IL, USA).
Results
Characteristics
The overall median progression time of the study included was 18.9 months (95% CI, 14.6–23.2 months) and the median follow-up time was 56 months (range 4–157 months). Baseline characteristics are summarized in Table 1. Median international normalized ratio (INR) of PA-TACE group was 1.10 s (0–3.48 s) significantly higher than that of the control group (median 1.08 s, 0–1.33; p=0.003). Moreover, PA-TACE treatment significantly decreased AFP level of patients compared with the control group (Table 2). The two groups were operated by the same group of hepatobiliary surgeons. There was no significant difference in hepatectomy methods between different subgroups, such as vascular invasion and satellite nodules. Other indexes were balanced and comparable between the two groups, and the difference was not statistically significant.
Table 1.
Baseline patient characteristics
| Characteristics | PA-TACE | NonPA-TACE | P-value |
|---|---|---|---|
| Sex, M/F | 94/8 | 67/11 | 0.176 |
| Age, years, median (range) | 55 (32–82) | 56 (24–78) | 0.6 |
| Child-Pugh status A/B/C | 62/5/1 | 57/4/0 | 0.233 |
| MELD score | 7 (6–10) | 7 (6–9) | 0.813 |
| Laboratory values, median (range) | |||
| WBC count, 109/L | 5.2 (2.0–9.6) | 5.5 (2.3–13.4) | 0.215 |
| Platelet count, 109/L | 154 (54–319) | 154 (34–367) | 0.561 |
| Hemoglobin, g/dL | 135 (84–169) | 142 (103–169) | 0.089 |
| Serum total bilirubin, mg/dL | 11.5 (6.9–135) | 13.5 (4.7–47.2) | 0.784 |
| Serum albumin, g/dL | 39.6 (30.8-59.3) | 40.9 (31.8-89.3) | 0.074 |
| INR | 1.10 (0–3.48) | 1.08 (0–1.33) | 0.003* |
| Serum creatinine, mg/dL | 67.7 (35.6–123.2) | 70.4 (47.3–97.4) | 0.516 |
| Serum alpha-fetoprotein, ng/mL | |||
| <20/≥20 | 30/42 | 40/27 | 0.034* |
| Tumor burden and distribution | |||
| Unifocal/multifocal | 78/24 | 62/16 | 0.63 |
| Maximal lesion diameter (cm) | 5.94±2.95 | 6.23±4.34 | 0.595 |
Note: *Significant difference (P<0.05).
Abbreviations: INR, international normalized ratio; PA-TACE, postoperative adjuvant TACE; MELD, Model of End-stage Liver Disease; WBC, white blood cell.
Table 2.
PFS subgroup analysis
| Variables | n | PFS (median survival, 95%CI) | |||
|---|---|---|---|---|---|
| PA-TACE (n=102) | NonPA-TACE (n=78) | P-value | |||
| Total cohort | 180 | 52.0 (14.0–90.0) | 11.1 (7.9–14.3) | <0.001* | |
| Gender | Male | 161 | 52.0 (14.8–89.2) | 12.7 (9.4–16.0) | <0.001* |
| Female | 19 | 16.0 (7.0–67.5) | 8.4 (7.1–9.8) | 0.121 | |
| Alcohol intake | Yes | 86 | 52.0 (16.4–87.6) | 11.9 (9.1–14.7) | <0.001* |
| No | 92 | 48.8 (5.2–92.4) | 10.0 (7.2–12.8) | <0.001* | |
| Low-risk patients | Noninvade | 169 | 55.7 (19.1–92.3) | 11.1 (8.2–14.0) | <0.001* |
| Nonportal vein invasion | 167 | 55.7 (21.1–90.3) | 11.9 (9.1–14.6) | <0.001* | |
| Child-Pugh A | 119 | 52.0 (21.0–83.0) | 11.1 (6.4–15.8) | <0.001* | |
| Single-lesion | 140 | 48.8 (0–98.7) | 11.1 (8.0–14.2) | <0.001* | |
| High-risk patients | Invade | 11 | 8.1 (2–19.2) | 5.5 (0–16.1) | 0.629 |
| Nonportal vein invasion | 10 | 5.9(0–15.4) | 7.5 (7.1–7.9) | 0.806 | |
| Child-Pugh B | 9 | 5.9 (4.4–7.5) | 8.1 (5.1–11.1) | 0.852 | |
| Multi-lesion | 40 | 52.0 (8.9–95.1) | 9.7 (0–20.0) | 0.003* | |
| Blood supply | Rich | 51 | 90.0 (42.5–137.5) | 18.9 (11.7–26.1) | 0.001* |
| Lack | 121 | 44 (17.5–70.5) | 10.0 (7.6–12.4) | <0.001* | |
| AFP | <20 | 70 | 64.1 (39.3–88.9) | 12.8 (10.2–15.5) | <0.001* |
| ≥20 | 69 | 48.8 (11.1–86.6) | 9.0 (6.3–11.7) | 0.002* | |
| Maximal lession diameter (cm) | <3 | 30 | / | 15.8 (9.7–21.8) | 0.002* |
| ≥3 | 145 | 48.8 (4.0–93.7) | 10.9 (8.6–13.3) | <0.001* | |
Note: *Significant difference (P<0.05).
Abbreviations: AFP, α-fetoprotein; PA-TACE, postoperative adjuvant TACE; PFS, progress-free survival; hqTACE, quadra sphere TACE; cTACE, conventional TACE.
PFS and OS subgroup analysis
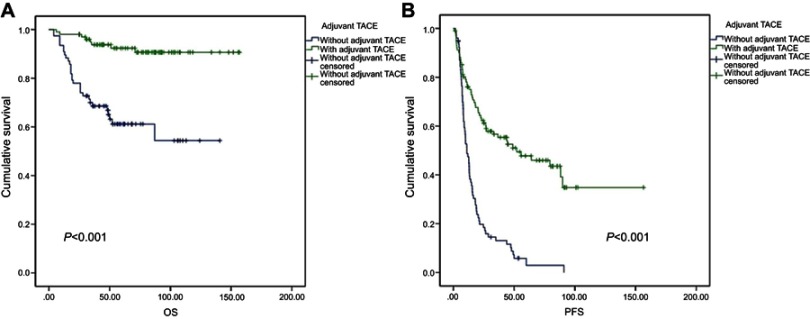
PA-TACE significantly improved the survival rate of patients with HCC after resection (Tables 2 and 3, and Figure 1). The PFS (progress-free survival) of PA-TACE group (median PFS 52.0 months; 95% CI: 14.0–90.0) was significantly longer than the control group (median PFS 11.1 months; 95% CI: [7.9–14.3]; log-rank P<0.001); and the OS in PA-TACE group (median OS 90.7 months; 95% CI: 84.4–97.0 months) was also much longer than that of control group (median OS 54.4 months; 95% CI: 38.2–70.6 months; log-rank p<0.001). Moreover, as shown in Tables 2 and 3, the benefits of PA-TACE are greater for low-risk patients than high-risk patients.
Table 3.
OS subgroup analysisa
| Variables | n | OS (survival rate, 95%CI) | |||
|---|---|---|---|---|---|
| PA-TACE (n=102) | NonPA-TACE (n=78) | P-value | |||
| Total cohort | 180 | 90.7 (84.4–97.0) | 54.4 (38.2–70.6) | <0.001* | |
| Gender | Male | 161 | 91.2 (84.8–97.6) | 54.5 (36.5–72.5) | <0.001* |
| Female | 19 | 87.5 (64.6–100) | 54.5 (25.1–83.6) | 0.104 | |
| Alcohol intake | Yes | 88 | 88.5 (78.7–98.3) | 61.9 (44.3–79.5) | 0.006* |
| No | 92 | 92.6 (84.4.2–100) | 49.4 (27.4–61.4) | <0.001* | |
| Low-risk patients | Noninvade | 169 | 90.1 (83.4–96.9) | 52.8 (35.2–70.4) | <0.001* |
| Nonportal vein invasion | 167 | 91.3 (85.0–97.6) | 52.2 (33.4–71.0) | <0.001* | |
| Child-Pugh A | 169 | 91.3 (82.3–100) | 65.8 (52.5–79.1) | <0.001* | |
| single-lesion | 140 | 91.4 (84.7–98.6) | 47.2 (27.0–67.4) | <0.001* | |
| High-risk patients | Invade | 11 | 100 (32.0–122.3) | 62.5 (29.0–96.0) | 0.254 |
| Non-portal vein Invasion | 13 | 83.3 (0.535–1.00) | 50.0 (1.0–99.0) | 0.239 | |
| Child-Pugh B | 11 | 67.2 (43.6–76.8) | 25.0 (0–67.5) | 0.071 | |
| Multi-lesion | 40 | 88.5 (73.8–100.0) | 81.3 (62.1–100.0) | 0.237 | |
| Blood supply | Rich | 131 | 92.1 (87.0–97.2) | 77.8 (60.4–95.2) | 0.194 |
| Lack | 49 | 91.5 (84.1–98.9) | 40.9 (15.4–76.4) | <0.001* | |
| AFP | <20 | 70 | 100 (78.2–134.2) | 62.2 (45.3–79.1) | 0.001* |
| ≥20 | 110 | 85.7 (73.2–97.9) | 29.4 (0–70.3) | 0.001* | |
| Maximal lesion diameter (cm) | <3 | 35 | 90.9 (73.8–100) | 70.7 (46.4–95.0) | 0.059 |
| ≥3 | 145 | 90.8 (84.1–97.7) | 50.4 (29.2–71.5) | <0.001* | |
Notes: aUni- and multivariate analyses of recurrence-free survival and OS. *Significant difference (P<0.05).
Abbreviations: AFP, α-fetoprotein; OS, overall survival; PA-TACE, postoperative adjuvant TACE.
Figure 1.
The survival analysis of patients who received PA-TACE after surgical resection.
Note: (A) OS analysis; (B) PFS analysis.
Abbreviations: OS, overall survival; PA-TACE, postoperative adjuvant TACE; PFS, progress-free survival.
In addition, PA-TACE treatment did not significantly prolong the survival of female patients compared with the control group (Tables 2 and 3). Although the PFS of female patients received PA-TACE group (median PFS 16.0 months; 95% CI: 7.0–67.5) or the OS (median OS 87.5 months; 95% CI: 64.6–100.0) seemed longer than PFS (median PFS 8.7 months; 95% CI: 7.1–9.8) or OS (median OS 54.5 months; 95% CI: 25.1–83.6) of control group female patients, there was no significant difference between these two group patients (for PFS analysis, log-rank P=0.121; for OS analysis, log-rank P=0.104).
Univariate analysis of PFS and OS
Table 4 shows the univariate analysis of PFS: INR was a risk factor for PFS prognosis in patients (HR 95% CI=1.454 (1.056–2.001), p=0.022), while tumors did not directly invade surrounding tissues (HR 95% CI=0.378 [0.197–0.727], p=0.004) and PA-TACE (HR 95% CI=0.293 [0.202–0.425], p<0.001) can significantly improve PFS in patients after tumor resection, and prevent intervention is also to improve liver tumor patients protective factors for OS after surgery (HR 95% CI=0.164 [0.074–0.360], p<0.001).
PFS and OS multi-factor analysis
Moreover, the multivariate COX regression analysis of factors affecting postoperative PFS and OS in patients with liver tumors was also examined. As shown in Table 4, female gender is an independent risk factor for PFS (HR 95% CI=1.887 [1.029–3.462], p=0.04), MELD (HR 95% CI=1.292; HR 95% CI=2.592 [1.223–5.493], p=0.013), and PA-TACE is an independent protective factor for PFS (HR 95% CI=0.293 [0.179–0.481], p<0.001) and OS. Results indicated that the PFS and OS in the intervention–prevention group were significantly better than those in the nonprophylaxis group.
Table 4.
PFS and OS single factor and multi-factor cox regression
| Variables | Univariate cox analysis results | Multi-factor cox analysis results | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI of HR | P-value | HR | 95% CI of HR | P-value | |
| PFS | ||||||
| Age | 0.984 | 0.966–1.003 | 0.102 | |||
| Gender (female) | 1.54 | 0.896–2.645 | 0.118 | 1.887 | 1.029–3.462 | 0.04* |
| Alcohol intake (non) | 1.214 | 0.851–1.731 | 0.285 | |||
| MELD score | 1.154 | 0.970–1.371 | 0.105 | 1.292 | 1.054–1.582 | 0.014* |
| Maximal lesion diameter (≥3cm) | 1.283 | 0.786–2.095 | 0.319 | 2.592 | 1.223–5.493 | 0.013* |
| TB | 0.994 | 0.979–1.010 | 0.459 | |||
| Albumin | 1.006 | 0.996–1.016 | 0.249 | |||
| Creatinine | 0.996 | 0.979–1.012 | 0.616 | |||
| INR | 1.454 | 1.056–2.001 | 0.022* | |||
| Noninvade | 0.378 | 0.197–0.727 | 0.004* | |||
| Blood-supply (poor) | 1.141 | 0.935–1.392 | 0.193 | 1.258 | 0.994–1.593 | 0.057 |
| Portal invasion | 1.539 | 0.749–3.161 | 0.24 | |||
| AFP (≥20) | 1.077 | 0.725–1.600 | 0.714 | |||
| Child-Pugh grade (B) | 1.879 | 0.862–4.099 | 0.113 | |||
| PA-TACE | 0.293 | 0.202–0.425 | <0.001* | 0.293 | 0.179–0.481 | <0.001* |
| OS | ||||||
| Age | 0.988 | 0.954–1.023 | 0.494 | |||
| Gender (female) | 1.896 | 0.790–4.553 | 0.152 | |||
| Alcohol intake (non) | 1.095 | 0.573–2.093 | 0.783 | |||
| MELD score | 1.459 | 0.968–2.198 | 0.071 | |||
| Maximal lesion diameter (≥3cm) | 1.39 | 0.539–2.587 | 0.496 | |||
| TB | 1.005 | 0.982–1.028 | 0.682 | |||
| Albumin | 0.999 | 0.982–1.016 | 0.87 | |||
| Creatinine | 0.996 | 0.967–1.025 | 0.77 | |||
| INR | 0.906 | 0.497–1.652 | 0.747 | |||
| Noninvade | 0.716 | 0.219–2.335 | 0.579 | |||
| Blood-supply (poor) | 1.37 | 0.905–2.075 | 0.137 | 1.861 | 1.006–3.443 | 0.048* |
| Portal invasion | 1.737 | 0.533–5.660 | 0.36 | |||
| AFP (≥20) | 1.321 | 0.640–2.728 | 0.452 | 2.363 | 0.913–6.113 | 0.076 |
| Child-Pugh grade (B) | 1.94 | 0.579–6.506 | 0.283 | |||
| PA-TACE | 0.164 | 0.074–0.360 | <0.001* | 0.159 | 0.047–0.537 | 0.003* |
Note: *Significant difference (P<0.05).
Abbreviations: AFP, α-fetoprotein; INR, international normalized ratio; OS, overall survival; PA-TACE, postoperative adjuvant TACE; PFS, progress-free survival; TB, total bilirubin; MELD, Model of End-Stage Liver Disease.
Adverse events
Clinical adverse events included fever, pain, nausea and fatigue, but were mostly limited to grades 1 and 2. Changes in laboratory values within 1 month after TACE were mostly mild, expected,and transient.
Discussion
Surgical resection is still considered to be the first choice for early liver cancer to achieve disease-free survival.24 However, even if the tumor is completely removed at an early stage, the recurrence rate after surgical resection remains high.25 Recurrence after surgical operation may be related to the characteristics of the HCC lesions themselves, basic liver diseases or surgical operations.26,27 TACE is considered to be the preferred standard treatment for nonsurgical treatment of patients with primary liver cancer, because liver cancer is mainly supplied by the hepatic artery.24,28 Selective hepatic artery embolism can cause ischemia and necrosis of tumor tissue, but has little effect on normal liver tissue. TACE is not only a topical treatment strategy for advanced liver cancer, but also helps to reduce recurrence and prolong survival. 29 Previous studies often focused on high-risk patients, and there is a lack of relevant research for preventive intervention in low-risk populations. Some randomized controlled studies have been indicated that adjuvant PA-TACE treatment could archive clinical benefits for patients suffering from HCC larger than 5 cm (<5 cm) in diameter, macroscopic vascular invasion or multiple nodules.30–32 Similar results were observed in clinical studies focused on prognosis analysis of patients with m-PVTT (macroscopic portal vein tumor thrombus) after surgical operation.33–35
For better understanding the application of PA-TACE in relieving postoperative recurrence and improving patients’ live quality, this study aims to reveal the effect of PA-TACE on HCC patients. Our results showed that PA-TACE can effectively improve the survival of low-risk HCC patients with postoperative tumors, especially for patients with low risk (nonsurvival lesions in the liver). This work included 180 patients received liver tumor resection. Patients received PA-TACE treatment that doxorubicin (20–40 mg) and lipiodol (2–10 mL) were injected into tumor tissues during embolization. PA-TACE can effectively prolong tumor-free survival and OS, indicating that preventive intervention is equally effective in preventing recurrence in low-risk patients. In this study, the surgical indications are strictly controlled,35,36 so the relatively early staging of selected patients may be the reason why the results are different from those of previous studies. Some factors that seem to obviously affect the results, like multi-lesions, show no significant difference in the statistical results of this study. Moreover, in this study no significant effect was observed on preventing tumor recurrence and prolonging their survival in high-risk patients received PA-TACE. Previous literature suggests that multiple lesions, invasion of blood vessels and invasion of the liver capsule are dangerous factors (risks) that are related with high tumor recurrence after surgical resection of liver cancer.34,37 Therefore, the possible reason for this result is that for high-risk patients, the significance of PA-TACE is mainly to deal with potentially surviving tumors and the sample size of high-risk patients in this study is smaller. In addition, the patients involved in this type of surgery are likely to have the risk of distant metastasis rather than recurrence. It may be less statistically efficient due to the failure to find validity of high-risk patients who received PA-TACE.
PA-TACE TACE can prevent possible micro-metastasis to prevent possible recurrence. In conventional TACE treatment, iodized oil is commonly used as a carrier for anticancer drugs to achieve preferential absorption and well deposition of chemotherapeutic drugs in HCC nodules. The concentration of chemotherapeutic drugs in liver tissue (100–400 folds to the whole body concentration chemotherapeutic drugs) through hepatic artery perfusion is much higher than through oral administration or intravenous injection. Moreover, administration of anticancer drugs via TACE make drugs accumulating in HCC lesions and drug concentrations in the tumor area can archive 5–10 times than that of normal liver tissue which not only enhances the anti-tumor effect but also reduces systemic side effects. In the present work, the time point of patients who received PA-TACE was 4 weeks after surgery, which is consistent with some previous work. 38,39 Chosen this time point could be beneficial to meet the heavier period of postoperative immunosuppression of liver cancer leading to more active proliferation of HCC cells in precancerous lesions. On this basis, proliferating cells are more sensitive to anti-tumor treatment strategies.40–43 Inhibiting or attenuating the growth of precancerous lesions to form new cancerous foci which is an important reason for the recent recurrence and metastasis of liver cancer.44–47 Therefore, PA-TACE treatment after hepatectomy is an important approach to inhibit the survival of cancer cells in precancerous lesions and prevent possible metastasis and recurrence. 48
Moreover, results in the present work showed that there was no significant difference in liver function scores and MELD scores between the patients 1 month after surgery and in the long-term follow-up. It may be because the patients in this study only received once TACE treatment. Increasing evidence has been confirmed that repeated TACE leading to different degrees of liver damage in patients with liver cancer. The limitations of this study included: the proportion of high-risk patients and female patients in the study sample was low. Meanwhile, this study is a retrospective study and high risk or female patients would be much fewer than low-risk patients. In addition, patients included in this study received only single PA-TACE and further study is needed for the efficacy and side effects of multiple PA-TACE
Conclusion
In conclusion, PA-TACE can effectively reduce the recurrence of patients with liver tumors and prolong the tumor-free survival and OS of patients. For low-risk patients, the benefits might be greater.
Acknowledgment
This work has been sponsored by Beijing Talents Foundation (2016000021223ZK25).
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zhang S, Wang F, Zhang Z. Current advances in the elimination of hepatitis B in China by 2030. Front Med. 2017;11(4):490–501. doi: 10.1007/s11684-017-0598-4 [DOI] [PubMed] [Google Scholar]
- 2.Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099–2108. doi: 10.1002/hep.27406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3(6):383–403. doi: 10.1016/S2468-1253(18)30056-6 [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 5.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 6.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2 [DOI] [PubMed] [Google Scholar]
- 7.Hyun MH, Lee YS, Kim JH, et al. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: a meta-analysis of high-quality studies. Hepatology. 2018;68(3):977–993. doi: 10.1002/hep.29883 [DOI] [PubMed] [Google Scholar]
- 8.Riaz A, Lewandowski R, Salem R. Radioembolization in advanced hepatocellular carcinoma. J Clin Oncol. 2018;36(19):1898–1901. doi: 10.1200/JCO.2018.77.7227 [DOI] [PubMed] [Google Scholar]
- 9.Sotiropoulos GC, Machairas N, Kostakis ID, Kouraklis G. The struggle for intensive care coverage after hepatic resections: the Greek reality. Lancet. 2017;389(10067):364–365. doi: 10.1016/S0140-6736(17)30151-4 [DOI] [PubMed] [Google Scholar]
- 10.Chen HS, Joo DJ, Shaheen M, et al. Randomized trial of spheroid reservoir bioartificial liver in porcine model of post-hepatectomy liver failure. Hepatology. 2018. [Epub ahead of print]. doi: 10.1002/hep.30184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Huang Z, Guo B, Liu S, Xiao W, Liang J. Short- and long-term outcomes of laparoscopic hepatectomy in elderly patients with hepatocellular carcinoma. J BUON. 2018;23(4):971–978. [PubMed] [Google Scholar]
- 12.Zheng X, Chen B, Wu JX, et al. Benefit of adjuvant radiotherapy following narrow-margin hepatectomy in patients with intrahepatic cholangiocarcinoma that adhere to major vessels. Cancer Manag Res. 2018;10:3973–3981. doi: 10.2147/CMAR.S172940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, Lei Z, Xia Y, et al. Association of preoperative antiviral treatment with incidences of microvascular invasion and early tumor recurrence in hepatitis B virus-related hepatocellular carcinoma. JAMA Surg. 2018;153(10):e182721. doi: 10.1001/jamasurg.2018.2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo GH. The use of steroid for transcatheter arterial chemoembolization: to relieve symptoms or to mask adverse events? Hepatology. 2018;68(3):1207. doi: 10.1002/hep.30112 [DOI] [PubMed] [Google Scholar]
- 15.Piscaglia F, Tovoli F, Pini P, Salvatore V. A new horizon in the prevention of the postembolization syndrome after transcatheter arterial chemoembolization for hepatocellular carcinoma. Hepatology. 2017. [Epub ahead of print] No abstract available. doi: 10.1002/hep.29517 [DOI] [PubMed] [Google Scholar]
- 16.Xie H, Yu H, Tian S, et al. What is the best combination treatment with transarterial chemoembolization of unresectable hepatocellular carcinoma? A systematic review and network meta-analysis. Oncotarget. 2017;8(59):100508–100523. doi: 10.18632/oncotarget.20119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng F, Jiang Q, Jia H, et al. Which is the best combination of TACE and sorafenib for advanced hepatocellular carcinoma treatment? A systematic review and network meta-analysis. Pharmacol Res. 2018;135:89–101. doi: 10.1016/j.phrs.2018.06.021 [DOI] [PubMed] [Google Scholar]
- 18.Wu XM, Wang JF, Ji JS, Chen MG, Song JG. Evaluation of efficacy of transcatheter arterial chemoembolization for hepatocellular carcinoma using magnetic resonance diffusion-weighted imaging. Onco Targets Ther. 2017;10:1637–1643. doi: 10.2147/OTT.S115568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang B, Li CL, Guo WH, et al. Intra-arterial ethanol embolization augments response to TACE for treatment of HCC with portal venous tumor thrombus. BMC Cancer. 2018;18(1):101. doi: 10.1186/s12885-018-4242-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun JJ, Wang K, Zhang CZ, et al. Postoperative adjuvant transcatheter arterial chemoembolization after R0 hepatectomy improves outcomes of patients who have hepatocellular carcinoma with microvascular invasion. Ann Surg Oncol. 2016;23(4):1344–1351. doi: 10.1245/s10434-015-5008-z [DOI] [PubMed] [Google Scholar]
- 21.Peng BG, He Q, Li JP, Zhou F. Adjuvant transcatheter arterial chemoembolization improves efficacy of hepatectomy for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Surg. 2009;198(3):313–318. doi: 10.1016/j.amjsurg.2008.09.026 [DOI] [PubMed] [Google Scholar]
- 22.Sun JJ, Wang K, Zhang CZ, et al. Postoperative adjuvant transcatheter arterial chemoembolization after R0 hepatectomy improves outcomes of patients who have hepatocellular carcinoma with microvascular invasion. Ann Surg Oncol. 2016;23:1344–1351. doi: 10.1245/s10434-015-5008-z [DOI] [PubMed] [Google Scholar]
- 23.Li J, Wang Q, Lei Z, et al. Adjuvant transarterial chemoembolization following liver resection for intrahepatic cholangiocarcinoma based on survival risk stratification. Oncologist. 2015;20:640–647. doi: 10.1634/theoncologist.2014-0470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labgaa I, Demartines N, Melloul E. Surgical resection vs. transarterial chemoembolization for intermediate stage hepatocellular carcinoma (BCLC-B): an unsolved question. Hepatology. 2018. Epub ahead of print. doi: 10.1002/hep.30338 [DOI] [PubMed] [Google Scholar]
- 25.Liao M, Chen P, Liao Y, et al. Preoperative high-sensitivity C-reactive protein to lymphocyte ratio index plays a vital role in the prognosis of hepatocellular carcinoma after surgical resection. Onco Targets Ther. 2018;11:5591–5600. doi: 10.2147/OTT.S167857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calderaro J, Petitprez F, Becht E, et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J Hepatol. 2018:pii: S0168-8278(18)32373–0 [Epub ahead of print]. doi: 10.1016/j.jhep.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 27.Pinna AD, Yang T, Mazzaferro V, et al. Liver transplantation and hepatic resection can achieve cure for hepatocellular carcinoma. Ann Surg. 2018;268(5):868–875. doi: 10.1097/SLA.0000000000002889 [DOI] [PubMed] [Google Scholar]
- 28.Kudo M. Proposal of primary endpoints for TACE combination trials with systemic therapy: lessons learned from 5 negative trials and the positive TACTICS trial. Liver Cancer. 2018;7(3):225–234. doi: 10.1159/000492535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogl TJ, Naguib NN, Nour-Eldin NE, et al. Review on transarterial chemoembolization in hepatocellular carcinoma: palliative, combined, neoadjuvant, bridging, and symptomatic indications. Eur J Radiol. 2009;72(3):505–516. doi: 10.1016/j.ejrad.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 30.Ye JZ, Chen JZ, Li ZH, et al. Efficacy of postoperative adjuvant transcatheter arterial chemoembolization in hepatocellular carcinoma patients with microvascular invasion. World J Gastroenterol. 2017;23(41):7415–7424. doi: 10.3748/wjg.v23.i41.7415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang JH, Zhong XP, Zhang YF, et al. Cezanne predicts progression and adjuvant TACE response in hepatocellular carcinoma. Cell Death Dis. 2017;8(9):e3043. doi: 10.1038/cddis.2017.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tong Y, Li Z, Liang Y, et al. Postoperative adjuvant TACE for patients of hepatocellular carcinoma in AJCC stage I: friend or foe? A propensity score analysis. Oncotarget. 2017;8(16):26671–26678. doi: 10.18632/oncotarget.15793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akkiz H, Carr BI, Kuran S, et al. Macroscopic portal vein thrombosis in HCC patients. Can J Gastroenterol Hepatol. 2018;2018:3120185. doi: 10.1155/2018/3120185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukumoto T, Kido M, Takebe A, et al. New macroscopic classification and back-flow thrombectomy for advanced hepatocellular carcinoma with portal vein tumor thrombus invading the contralateral second portal branch. Surg Today. 2017;47(9):1094–1103. doi: 10.1007/s00595-017-1507-9 [DOI] [PubMed] [Google Scholar]
- 35.Lim KC, Chow PK, Allen JC, Siddiqui FJ, Chan ES, Tan SB. Systematic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria. Br J Surg. 2012;99(12):1622–1639. doi: 10.1002/bjs.8915 [DOI] [PubMed] [Google Scholar]
- 36.Rodrigues TFDC, Silveira B, Tavares FP, et al. Open, laparoscopic, and robotic-assisted hepatectomy in resection of liver tumors: a non-systematic review. Arq Bras Cir Dig. 2017;30(2):155–160. doi: 10.1590/0102-6720201700020017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carr BI, Guerra V. Low alpha-fetoprotein levels are associated with improved survival in hepatocellular carcinoma patients with portal vein thrombosis. Dig Dis Sci. 2016;61(3):937–947. doi: 10.1007/s10620-015-3922-3 [DOI] [PubMed] [Google Scholar]
- 38.Liu S, Guo L, Li H, et al. Postoperative adjuvant trans-arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Ann Surg Oncol. 2018;25(7):2098–2104. doi: 10.1245/s10434-018-6438-1 [DOI] [PubMed] [Google Scholar]
- 39.Nakamura K, Beppu T, Hayashi H, et al. Recurrence-free survival of a hepatocellular carcinoma patient with tumor thrombosis of the inferior vena cava after treatment with sorafenib and hepatic resection. Int Surg. 2015;100(5):908–914. doi: 10.9738/INTSURG-D-14-00133.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martins-Neves SR, Cleton-Jansen AM, Gomes CMF. Therapy-induced enrichment of cancer stem-like cells in solid human tumors: where do we stand? Pharmacol Res. 2018;137:193–204. doi: 10.1016/j.phrs.2018.10.011 [DOI] [PubMed] [Google Scholar]
- 41.Cevatemre B, Erkısa M, Aztopal N, et al. A promising natural product, pristimerin, results in cytotoxicity against breast cancer stem cells in vitro and xenografts in vivo through apoptosis and an incomplete autopaghy in breast cancer. Pharmacol Res. 2018;129:500–514. doi: 10.1016/j.phrs.2017.11.027 [DOI] [PubMed] [Google Scholar]
- 42.Hou J, Hong Z, Feng F, et al. A novel chemotherapeutic sensitivity-testing system based on collagen gel droplet embedded 3D-culture methods for hepatocellular carcinoma. BMC Cancer. 2017;17(1):729. doi: 10.1186/s12885-017-3706-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skoda J, Veselska R. Cancer stem cells in sarcomas: getting to the stemness core. Biochim Biophys Acta Gen Subj. 2018;1862(10):2134–2139. doi: 10.1016/j.bbagen.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 44.Bu W, Liu Z, Jiang W, et al. Mammary precancerous stem and non-stem cells evolve into cancers of distinct subtypes. Cancer Res. 2018:pii: canres.1087.2018 [Epub ahead of print]. doi: 10.1158/0008-5472.CAN-18-1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsakogiannis D, Moschonas GD, Daskou M, et al. Polymorphic variability in the exon 19 of the RB1 gene and its flanking intronic sequences in HPV16-associated precancerous lesions in the Greek population. J Med Microbiol. 2018;67(11):1638–1644. doi: 10.1099/jmm.0.000843 [DOI] [PubMed] [Google Scholar]
- 46.Yu B, Wu K, Wang X, et al. Periostin secreted by cancer-associated fibroblasts promotes cancer stemness in head and neck cancer by activating protein tyrosine kinase 7. Cell Death Dis. 2018;9(11):1082. doi: 10.1038/s41419-018-1111-y [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Voeltzel T, Flores-Violante M, Zylbersztejn F, et al. A new signaling cascade linking BMP4, BMPR1A, ΔNp73 and NANOG impacts on stem-like human cell properties and patient outcome. Cell Death Dis. 2018;9(10):1011. doi: 10.1038/s41419-018-1111-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hazzah HA, Farid RM, Nasra MM, et al. A new approach for treatment of precancerous lesions with curcumin solid-lipid nanoparticle-loaded gels: in vitro and clinical evaluation. Drug Deliv. 2016;23(4):1409–1419. doi: 10.3109/10717544.2015.1065524 [DOI] [PubMed] [Google Scholar]