Abstract
On coral reefs, fishes can facilitate coral growth via nutrient excretion; however, as coral abundance declines, these nutrients may help facilitate increases in macroalgae. By combining surveys of reef communities with bioenergetics modeling, we showed that fish excretion supplied 25 times more nitrogen to forereefs in the Florida Keys, USA, than all other biotic and abiotic sources combined. One apparent result was a positive relationship between fish excretion and macroalgal cover on these reefs. Herbivore biomass also showed a negative relationship with macroalgal cover, suggesting strong interactions of top-down and bottom-up forcing. Nutrient supply by fishes also showed a negative correlation with juvenile coral density, likely mediated by competition between macroalgae and corals, suggesting that fish excretion may hinder coral recovery following large-scale coral loss. Thus, the impact of nutrient supply by fishes may be context-dependent and reinforce either coral-dominant or coral-depauperate reef communities depending on initial community states.
Two of the most pervasive anthropogenic impacts to aquatic ecosystems are the selective harvest of higher trophic level organisms1 and modified nutrient regimes that change patterns of productivity2. Dramatic changes in ecosystem function often follow changes to top-down and bottom-up forcing3. Importantly, alterations of both top-down and bottom-up processes may be mediated through changing the relative abundance of consumers. That is, in addition to altering primary producer abundance through consumption, consumers may be a significant source of limiting nutrients via their excretion4. In both freshwater and marine systems, differential exploitation of species can fundamentally change nutrient cycling regimes5,6, as well as modify patterns of predation and herbivory.
In coral reef systems, alterations to top-down and bottom-up forcing via overfishing and anthropogenic eutrophication are among the mechanisms mediating the decline in coral, and rise in algae, on reefs worldwide7. Following loss of coral after disturbance, such as coral bleaching or disease, algae rapidly colonize open space. Under conditions of reduced herbivory these algae then proliferate8,9. Increased algal cover can prevent the settlement and/or survivorship of juvenile corals10,11 and outcompete and kill established corals12. Although increasing nutrient availability often has minimal impact on algal abundance when herbivory is intense, once herbivore pressure is reduced, increasing nutrient concentrations can exacerbate the increase in algal abundance8. Thus, interactions among disturbance, eutrophication and overfishing can lead to coral-depauperate reefs with abundant algae13.
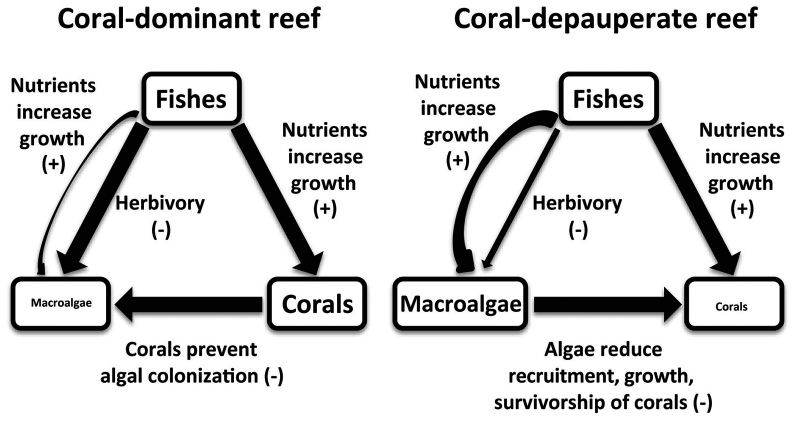
Despite the important role of both consumers and nutrients in influencing community dynamics on coral reefs, the role of bottom-up forcing by fish excretion has generally been ignored as a mechanism for altering benthic community structure. This is surprising given that nutrient input via consumers may be especially important in ecosystems, like coral reefs, that typically have low nutrient availability6,14,15,16. Further, previous studies show that nutrient excretion by fish schools has important effects on coral growth. Ambient water column ammonium and urea concentrations may be up to 3–4 times higher near dense schools of fish than in adjacent areas without fish17,18. Studies that have examined the impact of fish excretion on coral growth have consistently found positive effects, with corals often growing >50% faster when they host fishes (compared to corals that do not)17,19,20,21. Because nutrients from fish excretion can facilitate coral growth, consumer-driven nutrient supply may help reinforce coral dominance in reef ecosystems (Fig. 1), although this notion remains untested on a reef-wide scale.
Figure 1. Conceptual model of the effects of fish excretion on coral reefs.
Conceptual diagram of the proposed influence of fish excretion on corals and macroalgae in both coral-dominant and coral-depauperate reefs. Line thickness represents the strength of the interaction. Size of the words for fishes, corals, and macroalgae represents their relative abundance in the community in either scenario. The transition from coral-dominant to coral-depauperate could have resulted from any disturbance such as coral bleaching or a hurricane. Herbivory per unit area is assumed to decline from the coral-dominant to the coral-depauperate scenario, as a decline in coral cover results in more open space that herbivores are required to graze to keep algal populations low.
The positive effect of fish excretion may seem counterintuitive, as it is often assumed that increasing nutrient availability has negative effects on coral growth22. Yet, experiments suggest that nutrient enrichment can have either positive or negative effects on coral growth, with variation in responses driven by coral species, nutrient identity (N, P, or N and P), and ambient nutrient concentrations23,24,25. Thus, fish excretion could potentially affect corals in a different way than anthropogenic eutrophication due to differences in nutrient concentration or types of nutrients (e.g. ammonium and urea are often present in fish excretion vs. nitrate in anthropogenic eutrophication18). Further, fish excretion is likely spatially variable over time as fish move around a reef, or off the reef at night, while anthropogenic eutrophication is likely more spatially and temporally constant. Thus, the differences in nutrient identity, concentration, and consistency between fish excretion and anthropogenic eutrophication may result in fundamentally different effects on coral growth.
The impact of fish excretion may also be contingent on initial community composition. In areas where disturbance has reduced coral cover, nutrient input from fishes could increase primary production by macroalgae that tend to proliferate when free space is available (Fig. 1). Since macroalgae can have direct negative effects on corals10,11,12, this interaction could function as a negative feedback on coral abundance. Thus, the impact of fish excretion could be an important feedback mechanism that reinforces either coral-dominated or coral-depauperate community states depending on the initial starting conditions of the community [e.g.26].
Here, we investigate the roles of bottom-up (nutrient excretion from fishes) and top-down (herbivore biomass) forces on benthic community structure on coral reefs in the Florida Keys, USA, where coral cover has declined significantly over the past several decades27. We used field surveys across forereefs in the Florida Keys to quantify reef fish abundance, benthic community structure, and algal stoichiometry. Using bioenergetics models, we calculated the excretion rates of nitrogen and phosphorus by resident fishes as an estimate of bottom-up forcing on benthic communities. Using, these modeled fish excretion data and our field surveys of reef communities, we examined the relationships among herbivory, fish-derived nutrients, and reef community structure.
Results
Fish biomass varied widely across reef sites (Table S1). Herbivorous fish biomass (Scaridae and Acanthuridae) was high relative to many other sites in the wider Caribbean28 averaging 22.5 g/m2 (range: 10.9–66.6 g/m2). Parrotfishes (Scaridae; mean 16.3 g/m2, range: 5.6–49.7 g/m2) were often more abundant than surgeonfishes (Acanthuridae; mean 6.2 g/m2, range: 3.2–16.9 g/m2). Carnivorous fish biomass (e.g. Lutjanidae, Haemulidae, Serranidae, Labridae) was also high, but variable, averaging 87.8 g/m2 (range: 19.6–213.6 g/m2).
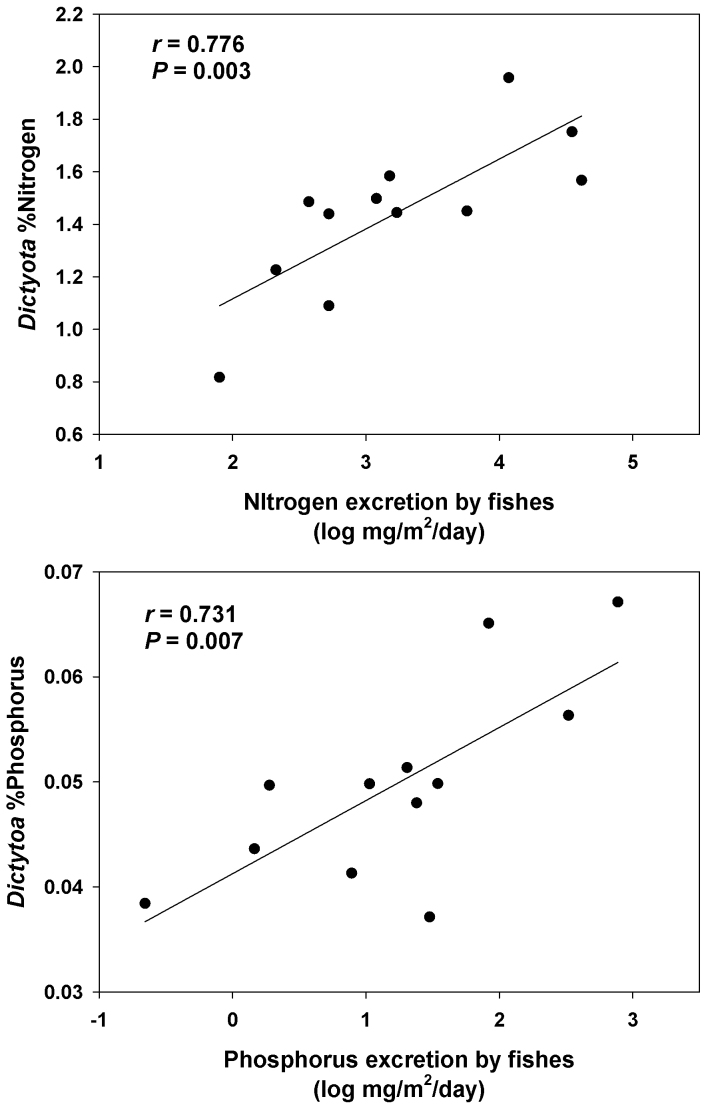
Bioenergetics modes (Table S2) showed that fishes were a significant source of nitrogen and phosphorus to reefs (Table 1). When compared to other sources of nitrogen input to the Florida Keys National Marine Sanctuary29, the modeled input of nitrogen from fishes on the average reef represent the most important source of nitrogen for the benthos – 25.3 times higher than all other sources combined (Table 1). Nutrient content of the brown alga Dictyota menstrualis across reef sites showed a positive correlation between algal tissue nutrient concentration and fish excretion rates for both nitrogen (r = 0.78, P = 0.003) and phosphorus (r = 0.73, P = 0.007; Fig. 2). These data suggest that fish biomass and fish-derived nutrients have a strong influence on nutrient availability on these reefs.
Table 1. Summary of fish nitrogen and phosphorus excretion data from our study compared with other nitrogen inputs in the Florida Keys National Marine Sanctuary (from Lamb-Wozniack 2007). Minima and maxima values represent the reefs with the lowest and highest excretion rates as calculated from the mean bioenergetics models.
| Type of fish excretion | Mean (mg/m2/day) | 95% CI for Mean | Min | Max |
|---|---|---|---|---|
| Phosphorus excretion - All fishes | 5.18 | 4.75–5.44 | 0.53 | 17.97 |
| Phosphorus excretion - Carnivores only | 4.93 | 4.52–5.17 | 0.37 | 17.71 |
| Phosphorus excretion - Herbivores only | 0.25 | 0.23–0.27 | 0.11 | 0.60 |
| Nitrogen excretion - All fishes | 35.67 | 31.84–37.75 | 6.70 | 101.11 |
| Nitrogen excretion - Carnivores only | 31.60 | 29.76–33.36 | 4.23 | 96.74 |
| Nitrogen excretion - Herbivores only | 4.07 | 2.08–4.40 | 1.84 | 9.63 |
| Other N sources to reefs in the Florida Keys – from Lamb-Wozniak (2007) | N mass (mg/m2/day) | |||
| Anthropogenic sources | 0.13 | |||
| Upwelling/internal bores | 0.17 | |||
| Atmospheric deposition | 0.11 | |||
| Influx of Florida Bay waters | 0.03 | |||
| Florida Current gyres | 0.15 | |||
| Nitrogen fixation | 0.71 | |||
| Ammonium efflux from sediments | 0.11 | |||
| Total | 1.41 |
Figure 2. Relationship between modeled fish excretion rates and tissue nutrients of algae.
Relationships between fish excretion and the percent nitrogen (A) and percent phosphorus (B) in the tissues of the brown alga Dictyota menstrualis.
Macroalgal cover averaged 38.9 ± 3.6% (mean ± SE) but varied over 3 fold (22–65% among reefs) (Table S1). The most abundant macroalgae were the brown algae Dictyota spp., which represented 90% of the total macroalgal cover across all reefs. Halimeda spp. (~5% of cover), Stypopodium zonale (~2% of cover), Amphiroa spp. (~1% of cover) and Laurencia spp. (~1% of cover) were other common algae. Results of multiple regression showed that fish excretion and parrotfish biomass were both important predictors of macroalgal cover, but in different directions (Table 2). Excretion rates of both nitrogen and phosphorus showed positive correlations with algal cover while parrotfish biomass showed negative correlations. The most informative models according to AICc contained both excretion and parrotfish biomass terms. There was moderately better support for the model containing nitrogen excretion and parrotfish biomass over the model containing phosphorus excretion and parrotfish biomass (ΔAICc = 2.74). Models including only single terms (e.g. parrotfish biomass or nutrient excretion terms only) had ΔAICc values >9.3 suggesting considerably less support. Importantly, results of multiple regression were quantitatively similar regardless of if we used the mean, lower 95%, or upper 95% derived values from bioenergetics models (compare Table 2 to Table S3). Thus, patterns were robust across a substantial gradient of modeled fish excretion rates that take into account potential natural variability in diet, consumption rates, and stoichiometry of these fishes. As another way to visualize the interaction of fish excretion and herbivory on macroalgal cover, Pearson's correlation showed a strong positive correlation between the ratio of total fish: parrotfish biomass and macroalgal cover (r = 0.81, P = 0.001; Fig. 3). Thus, as the overall biomass of fishes (and concomitant rates of nutrient excretion) increased per unit of parrotfish biomass, macroalgal cover also increased.
Table 2. Results of multiple linear regression using Akaike Information Criteria (AICc), assessing factors that may explain patterns in macroalgal cover or juvenile coral abundance. A positive estimate for a model term indicates a positive correlation bewteeen macroalgae or corals and that model term; a negative estimate indicates a negative correlation bewteeen macroalgae or corals and that model term.
| Macroalgal cover – Model terms (estimate) | r2 | AICc | ΔAICc |
|---|---|---|---|
| Log nitrogen excretion (10.84) | 0.83 | 85.51 | 0 |
| Log parrotfish biomass (−12.98) | |||
| Log phosphorus excretion (9.09) | 0.79 | 88.25 | 2.74 |
| Log parrotfish biomass (−13.18) | |||
| Log nitrogen excretion (9.76) | 0.46 | 94.84 | 9.33 |
| Log phosphorus excretion (7.99) | 0.41 | 95.97 | 10.46 |
| Log parrotfish biomass (−10.99) | 0.27 | 98.58 | 13.07 |
| Juvenile coral abundance - Model terms (estimate) | r2 | AICc | ΔAICc |
| Log nitrogen excretion (−1.15) | 0.76 | 44.23 | 0 |
| Log coral cover (1.11) | |||
| Log phosphorus excretion (−0.94) | 0.75 | 44.82 | 0.59 |
| Log coral cover (1.35) | |||
| Log nitrogen excretion (−1.51) | 0.50 | 48.16 | 3.93 |
| Log coral cover (1.46) | 0.49 | 48.42 | 4.19 |
| Log phosphorus excretion (−1.06) | 0.33 | 51.71 | 7.48 |
Figure 3. Relationship between patterns in fish biomass and macroalgal cover across reefs.
Correlation between the ratio of total fish biomass:parrotfish biomass and macroalgal cover.
Abundance of juvenile corals averaged 6.9 ± 0.6 individuals/m2 (mean ± SE) and varied over 2.3 fold (range 4.1–9.3 individuals/m2) (Table S1). The most common juvenile corals were Agaricia spp., Porites astreoides, Porites porites, and Siderastrea siderea which represented >90% of all juvenile corals. Multiple regression suggested nutrient excretion rates were negatively correlated with juvenile coral density, while coral cover showed a positive correlation with coral density (Table 2). Models including only single terms (e.g. coral cover or nutrient excretion terms only) had ΔAICc values >3.9 suggesting less support versus the model with both terms. As for regressions with algal cover, results of multiple regression with juvenile coral abundance were quantitatively similar regardless of if we used the mean, lower 95%, or upper 95% derived values from bioenergetics models (compare Table 2 to Table S3).
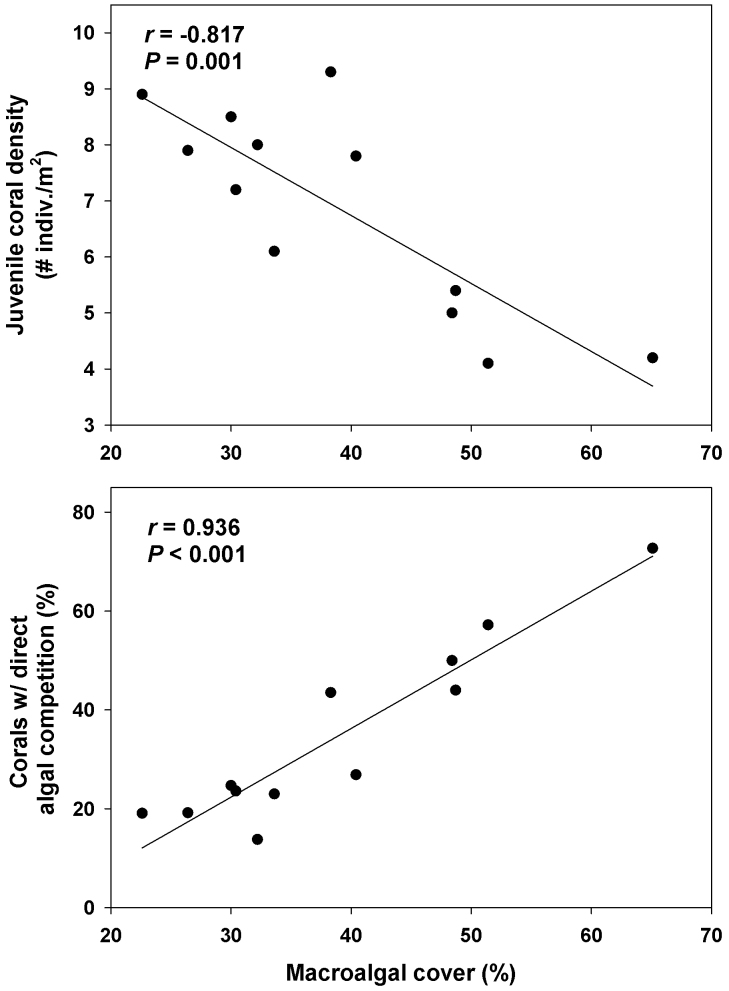
Further, there was a strong negative correlation between the abundance of juvenile corals and macroalgal cover (r = −0.82, P = 0.001, Fig. 4A). Although this pattern could result if macroalgae grew over juvenile corals and obscured them from view, we searched through macroalgae for juvenile corals during our survey making it unlikely that we overlooked corals when macroalgal cover was dense. Additionally, as macroalgal cover increased, the frequency of juvenile corals in direct physical contact with macroalgae, likely a proxy for direct competition, increased significantly (r = 0.94, P < 0.001, Fig. 4B).
Figure 4. Patterns in macroalgal cover and juvenile coral density and competition.
Relationships between macroalgal cover and the density of juvenile corals (A) and the percentage of juvenile corals in direct contact with macroalgae, a likely proxy for direct competition (B).
Discussion
Multiple interacting stressors, such as disturbance, overfishing, and eutrophication, have eroded the resilience of many coral reefs leading to coral-depauperate systems with high macroalgal cover13,30. Nutrient availability on coral reefs, particularly nutrients supplied by fishes, may be an important, context-dependent, force influencing transitions between coral- and algal-dominated states (Fig. 1). Previous studies suggest that nutrient loading from fishes may facilitate coral growth17,19,20,21, potentially reinforcing high coral cover. Further, when coral cover is high, little reef area is covered by algae, resulting in strong top-down control by herbivores and the reinforcement of coral dominance26,31. However, when disturbance reduces coral cover, as has happened over decades in the Florida Keys27, grazing rates per unit area of reef often decline as there is more open space available for algal colonization. Under conditions of low herbivory, nutrient loading may facilitate an increase in algae8. Our data suggests that once a coral-depauperate state is reached, fish excretion may act to reinforce this state by facilitating macroalgal growth, which, in turn, may limit coral recruitment and survivorship via direct competition. Thus, fish-mediated nutrient loading may act as a feedback that may either facilitate or inhibit corals depending on the initial starting condition of the community.
Unfortunately, we could not rigorously test both sides of our proposed conceptual model of how fish-derived nutrients may impact coral reef communities (Fig. 1). While we show strong evidence to support the idea that fish excretion can facilitate macroalgae and suppress coral recovery once coral cover is low, we cannot address if fishes facilitate coral growth and reinforce coral dominance in our system as coral cover is low across the Florida Keys. However, multiple studies of the positive feedbacks between fish excretion and coral growth at relatively small scales17,19,20,21,22 support the idea that abundant fishes could facilitate corals at the reef-wide scale. Rigorous experimental examination of how reductions in coral cover impact the nature of fish excretion likely are not logistically or ethically feasible on ecologically meaningful scales. However, simulation modeling studies (e.g.13,31) may be especially useful for examining how variations in fish biomass, fish excretion, and herbivory can interact to impact reef communities and potentially alter the dynamics of corals and macroalgae.
Nutrient supply from consumers should be most important in relatively nutrient-poor systems such as tropical freshwater streams15 and seagrass beds6,16. The reefs of the Florida Keys, like many reefs worldwide, are relatively oligotrophic (dissolved inorganic nitrogen < 0.25 μM and soluble reactive phosphorus < 0.05 μM)32. Macroalgae in the Florida Keys are often nitrogen limited33, and our data suggest that nitrogen may have been the most limiting nutrient (i.e., the model with the most support explaining algal cover included nitrogen excretion). When we compared estimates of the input of nitrogen from a variety of different biotic and abiotic processes to reefs in the Florida Keys29 with our modeled estimates of nitrogen excretion by fishes, the input of nitrogen from fishes is over two orders of magnitude higher than any other single source of nitrogen and 25 times higher than all other sources of nitrogen combined.
On the average reef in our study, carnivorous fishes were responsible for 7.8 times more nitrogen excretion and 19.7 times more phosphorus excretion than were herbivorous fishes. These differences were due to the higher abundance of carnivorous fishes, in general, as well as the higher rates of nutrient release by carnivores due to their more nutrient rich diets34. Herbivorous and carnivorous fishes also vary in their functional role because the nutrients they excrete may be derived from different places within the reef landscape. For herbivores, the majority of nutrients excreted would be derived from their diets of algae and detritus gleaned from the reef. Thus, they would mostly be recycling nitrogen and phosphorus back to the primary producer community on the reef. Many of the carnivorous fish species (Haemulidae and Mullidae), however, do not feed on reefs but forage in nearby seagrass beds and sand plains at night and shelter on reefs during the day35. Thus, much of the nitrogen and phosphorus excreted by many carnivorous fishes may be vectored to these reefs from nearby habitats, essentially acting to subsidize primary production on reefs.
There is a long-running debate over the relative roles of herbivory versus nutrient availability in determining algal abundance on coral reefs8. Most empirical work to date shows that herbivory is the primary determinant of overall algal abundance, but these two factors often interact with nutrient availability impacting algal species composition and abundance across a range of herbivory levels8,9,36. However, studies of the interaction of top-down and bottom-up forcing on reefs have focused on the impact of anthropogenic eutrophication, or variations in upwelling intensity, and have generally ignored the importance of nutrient supply from fishes. This lack of appreciation for the role of fishes as sources of limiting nutrients may lead to a gap in our knowledge regarding ecosystem function. Although our data suggest that herbivory and fish excretion both impact algal communities, fish excretion may exert as much or more influence on the reefs in our study. Although single variable models were much less informative than models including both parrotfish biomass and fish excretion terms, models of either nitrogen excretion or phosphorus excretion alone explained more variance in the algal cover data (r2 = 0.46, ΔAICc = 9.33 and r2 = 0.41, ΔAICc = 10.46, respectively) than did parrotfish biomass alone (r2 = 0.27, ΔAICc = 13.07). Thus, the nutrients excreted by fish may have more influence over algal cover than does herbivory in this reef system.
Our study represents a unique examination of the interplay between bottom-up forcing from fish excretion and top-down forcing from herbivores on reef community structure. Like other studies [e.g.37,38,39], we showed a significant negative correlation between herbivore biomass, particularly parrotfish biomass, and macroalgal cover. However, the strong positive correlation between the ratio of overall fish biomass:parrotfish biomass and macroalgal cover (Fig. 3) supports the notion the increasing nutrient availability from more abundant fishes (i.e. carnivores) can reduce the efficiency of top-down control by herbivores, at least on reefs where coral cover is already low. Granted, the absolute abundance of these fishes will matter as well. If herbivores are extremely overfished, it likely will not matter how much nutrients fish excrete as macroalgae will have little top-down control and will likely flourish. Yet, on reefs where fishes are abundant, the ratio of overall fish biomass:herbivorous fish biomass may be an important determinant of algal abundance.
As one example, the reef in our study with the highest biomass of herbivorous fishes (66.6 g/m2 total herbivore biomass; 49.7 g/m2 parrotfishes) had moderate macroalgal cover (31%) perhaps due to the supply of nutrients by the abundant carnivores (197.8 g/m2). If the nutrient supply of these carnivores were unimportant in this system, one would have expected this reef, with herbivore biomass that is higher than many well-protected reefs in the Caribbean28, to have little macroalgae. An alternative explanation is that increasing carnivore biomass could increase algal production via top-down regulation of herbivore biomass or alteration to herbivore grazing behavior40. Yet in our study system, the carnivore community is dominated by smaller bodied species (e.g. most grunts and snapper, as opposed to large-bodied grouper, other snapper, sharks etc.) that would be unlikely to feed on the herbivorous fishes present on these reefs.
Another potential alternative explanation for the positive relationship between total fish biomass (and therefore fish excretion) and macroalgal cover is that sites with higher algal abundance have higher delivery of nutrients from other sources. As such, the sites with higher primary productivity support greater fish biomass through a bottom-up mechanistic pathway. However, a long-term (1995–2010) water quality monitoring data set at the majority of our field sites41, that spans our gradient of fish biomass, does not support this hypothesis. Across our sites, there were no differences in productivity as measured by water column chlorophyll a concentration at 1 m depth (mean = 0.24 μg/l, range = 0.22–0.25 μg/l over the duration of our study41). Unfortunately, these water quality data were not available for all of our sites so we could not include them in potential regression models. Yet, the small range in chlorophyll a data for the sites that were available (0.22–0.25 μg/l) suggests that there would be little relationship between chlorophyll a concentrations and the strong gradients in macroalgal abundance and fish biomass that we document.
Fish excretion and coral cover combined to explain 74% of the variance in juvenile coral abundance across reefs, despite other potential local and regional scale factors that drive larval supply, recruitment, and survivorship42. The positive relationship between coral cover and juvenile coral abundance is not surprising, as more abundant adult corals would mean more supply of coral larvae especially for coral species producing brooded larvae that often recruit locally. However, the negative correlation between juvenile corals and fish excretion is a seeming paradox if fish excretion is an important facilitator of coral health, as was previously suggested17,19,20,21,22. This effect may be strongly dependent on coral colony size. Adult coral colonies might be more likely to benefit from fish excretion as these larger corals are relatively immune from competition with macroalgae. Yet, the juvenile corals we focused on are often more vulnerable to competition from macroalgae11. In fact, Dictyota spp., which represented >95% of the algal community on these reefs, may be a potent competitor against corals as they produce alleopathic compounds that can reduce survivorship and settlement of coral larvae43, cause tissue mortality in established corals44, and potentially alter the abundance of beneficial coral-associated bacteria45. Abundant macroalgae may also simply prevent coral larvae from settling to reefs by preventing contact with preferred larval settlement sites10. Thus, the seemingly paradoxical negative relationship that we see between fish excretion and juvenile coral abundance is perhaps mediated by the size-dependent effects of competition between corals and abundant macroalgae.
Having abundant fishes that excrete large amounts of nutrients does not mean that coral cover on reefs will necessarily decline. Surveys of coral reefs remote from human impacts show that both fish biomass and coral cover are often high46. Further, the recovery of both carnivorous and herbivorous fishes inside some marine protected areas (MPA's) have led to increased macroalgal removal and positive trajectories for coral recruitment and overall coral cover37,47. However, resilience to disturbance and recovery of corals are not uniformly high across MPA's, with corals recovering slowly or failing to recover at all in some protected areas48. Although there are likely a variety of mechanisms impacting reef resilience, our data suggest that nutrient supply from fishes should be considered as an important mechanism that may impact the recovery of reefs once low coral cover is reached. Thus, MPA's may promote mechanisms that can either increase (more herbivores) or decrease (more nutrients from fishes) a reef's resilience to frequent coral bleaching events, reduced coral growth rates, and lower recruitment rates that may accompany climate change7. We are not suggesting that MPA's should not be implemented; they are clearly one of, if not the most important, tool to help promote the resilience of reefs in the face of climate change and ocean acidification7,49. But, our data suggest that we need a better understanding of how fishes affect nutrient cycling in reef systems and how consumer-derived nutrients will impact the transition between coral-dominated and coral-depauperate reefs.
Methods
Field site and survey methods
The Florida Keys Reef Tract consists of a large bank reef system located approximately 8 km offshore of the Florida Keys, USA, and paralleling the island chain. Coral cover is low on reefs across the Florida Keys reef tract with most forereefs having ~10% coral cover. Macroalgal cover is quite variable on a reef-to-reef basis, ranging from ~0–70%50 but with an average of roughly 15% across all reefs27,50. Given that fishing pressure on herbivorous fishes is non-existent, there are abundant large herbivores (e.g. Sparisoma viride, Scarus vetula) throughout the reefs of the Florida Keys51. In July-August 2010, we surveyed 12 shallow forereefs in the northern Florida Keys reef tract to assess the relationships between fish community structure, algal cover, and juvenile coral abundance (Electronic Supplementary Material Table S4 includes more information about sampling sites). These sites represent the majority of the forereefs within the Florida Keys National Marine Sanctuary in the upper Florida Keys. Given that these reefs were all within ~50 km of one another, they likely have similar disturbance histories (as the main disturbances are hurricanes and warm water anomalies which would likely affect reefs regionally instead of affecting individual reefs). All surveys were conducted at depths of 5–8 m.
We quantified the fish community on each reef using 25 m × 4 m belt transects (n = 10 per reef). Transects were laid out parallel to the main reef formation. The starting point for each transect was determined by swimming a randomly chosen number of fin kicks away from the end point of the previous transect. As we slowly swam each transect, we counted all fishes and estimated their lengths to the nearest 1 cm. Divers carried 1 m long PVC T-bars to help estimate lengths. A second pass over the 25 m transect using a 2 m wide transect was made to quantify the abundance of territorial, herbivorous damselfishes (Pomacentridae) which can affect abundance and community structure of corals and macroalgae52. Although both fish abundance and benthic community structure may change somewhat over time, these single time point surveys commonly show strong and persistent relationships between fish abundance and benthic community structure37,38. We used published length:weight relationships53 to convert fish lengths into biomass. The herbivorous urchin Diadema antillarum was seen on approximately <1% of transects and were not quantified.
We quantified benthic community structure using photoquadrats. After we surveyed each 25 m transect to quantify the fish community, we photographed the benthic community at each meter of the transect. We used a digital camera in an underwater housing attached to 75 cm tall PVC stand resulting in 0.5 × 0.4 m (0.2 m2) photoquadrats of the benthos (250 photos per reef). We used Coral Point Count with Excel extensions to quantify the cover of benthic organisms within each photoquadrat. For each photograph, we placed 36 random points per picture and identified the organisms under each point. Organisms were identified to lowest taxonomic level possible (e.g. species or genus for corals, most macroalgae, and macroinvertebrates; functional group for crustose coralline algae and algal turfs). Because topographic complexity (also called rugosity) of a reef is an important correlate of community structure on reefs, we measured reef rugosity at two randomly chosen points along each 25 m transect using common methods54.
Although fish excretion may increase the growth of adult corals17,20, coral cover is very low (<10%) across most reefs in the Florida Keys making it difficult to test how fish excretion may reinforce coral dominance. However, we were interested primarily in how fish-derived nutrients impact benthic communities and coral recovery once adult corals become rare. As such, we focused on juvenile corals because their dynamics are key for understanding the potential of reefs to recover from disturbances, and because they are the most vulnerable to competition from macroalgae11. To quantify juvenile coral abundance, we performed counts of all corals <5 cm colony diameter55. At each reef, we identified and measured all juvenile corals in 10 randomly placed 0.5 × 0.5 m (0.25 m2) quadrats along each of four 25 m long transects (40 quadrats in total per reef). In each quadrat, we carefully searched in crevices and under macroalgae to identify juvenile corals. We did not include small corals that were clearly formed by the partial mortality of larger colonies.
We quantified levels of nitrogen and phosphorus in the tissues of the common brown alga Dictyota menstrualis to determine if one of the most common algae on the reef exhibited a signal of increased nutrient loading by fishes. We were not using these nutrient content data to assess specific rates of nutrient uptake by algae, but were using them as a proxy for the ambient nutrient conditions on each reef. Nutrient content of macroalgae are often used to assess nutrient availability in coastal ecosystems as it reflects ambient nutrient conditions over a relatively long time frame (i.e., weeks to months) as compared with ambient water nutrients56. Thus, we expected reefs with higher fish biomass, and therefore more fish-derived nutrients, to have algae with higher nutrient content. We chose D. menstrualis as it is common on these reefs and easily found at all sites. At each reef site, we collected portions of D. menstrualis within each benthic transect along the forereef (n = 10 algal samples per reef site). These samples were dried at 60°C for 48 hrs, ground using a mortar and pestle, and analyzed for concentrations of carbon, nitrogen, and phosphorous using standard methods (Electronic Supplementary Material Appendix S1).
Bioenergetics modeling
Since empirical nutrient excretion estimates exist for only one fish species in our data set, the gray snapper Lutjanus griseus6, we used bioenergetics modeling to estimate excretion rates for the fishes in our data set. Bioenergetics modeling allows for nutrient excretion rates of an organism to be estimated using a mass balance approach given a priori knowledge of the natural history (e.g., diet, feeding activity), physiology (e.g., stoichiometry of predator and prey, assimilation efficiency of nutrients) and environmental conditions (temperature). Bioenergetics models are commonly used to estimate growth, feeding, and excretion across a variety of marine fishes16,57,58,59. Because of the mass balance approach employed by the models the inherent model structure is similar across taxa whereas the parameter estimates cater to the specific taxonomic resolution desired58. We used this mass balance approach to generate linear models of fish mass (g) and excretion rate (nutrient (g) wet weight−1 day−1) for the 9 most common families in our surveys (Electronic Supplementary Material Appendix S1, Table S2). These families represented >95% of the biomass of resident fishes on these reefs. The stoichiometry of fish excretion has been shown to vary substantially among families but much less within families60. These equations were used in conjunction with calculations of fish biomass from each reef to estimate excretion rates per unit reef area (g nutrient day−1) for each survey site6. Models were generated using R software (R Core Development Team).
Models were parameterized using diet analyses of thousands of individuals61,62 in conjunction with nutrient stoichiometry data (C, N, P) for all 9 families of fish, including 181 individuals from 21 species (Electronic Supplementary Material Appendix S1, Table S5). Stoichiometry data of prey items were drawn from 22 fish species, 12 invertebrate species (179 individuals total), and 4 algal species (Electronic Supplementary Material Appendix S1, Table S5). Physiological parameters, such as growth rate and length-weight regressions, were drawn from published values in the literature (Electronic Supplementary Material Appendix S1, Table S6). All fish were assumed to be in constant water temperature (27°C). To account for inherent error that occurs when parameterizing such models, we propagated uncertainty associated with diet content and consumption rates, two of the most influentidal parameters for bioenergetics models34,57,58, through the models using Monte Carlo simulations (Electronic Supplementary Material Appendix S1). Using the mean values for the slope and regression coefficients for each family-level model as well as the upper and lower 95% confidence intervals of these parameters, we calculated a range of estimates of community-level nutrient supply for both N and P associated with the fish community of each reef site.
Excretion rates are influenced by diet stoichiometry, such that fish that consume food resources with high nutrient content relative to that of their own body often excrete greater quantities of nutrients relative to fish that consume lower quality diets34. Therefore, carnivores that eat animal prey relatively rich in nitrogen and phosphorus may contribute more to consumer-driven nutrient cycling than herbivores that are feeding on lower quality primary producers. As such, we calculated excretion rates as the sum of all fish biomass per reef, as well as separating excretion rates by carnivorous and herbivorous fishes. Excretion rates from carnivores included the families Haemulidae, Holocentridae, Labridae, Lutjanidae, Mullidae, and Serranidae ( >95% of the carnivore biomass). Excretion rates from herbivores included individuals from the families Scaridae, Acanthuridae, and Kyphosidae. We compared these modeled fish excretion data with modeled estimates of nitrogen input to the Florida Keys National Marine Sanctuary from both natural and anthropogenic sources29.
Statistics
The parameters potentially explaining patterns in macroalgal cover and juvenile coral density were: herbivorous fish biomass (overall herbivore biomass, scarid biomass, and acanthurid biomass were all examined as they often suggest different patterns38), corallivorous fish biomass (Sparisoma viride, Sparisoma aurofrenatum, Scarus vetula63), nutrient excretion by fishes (N and P), coral cover, territorial damselfish density, reef rugosity, and distance from shore (See Electronic Supplementary Material Table S7 for parameter explanations). We used Pearson's correlation coefficients to assess which parameters had the strongest correlations with macroalgal cover and juvenile coral density (Electronic Supplementary Material Table S8). For both macroalgal cover and juvenile coral density, we then chose the three parameters that had the strongest Pearson correlation coefficients to use in multiple linear regression. For macroalgal cover, the strongest correlations were for parrotfish biomass, N excretion, and P excretion. For juvenile coral density, the strongest correlations were for coral cover, N excretion, and P excretion (Electronic Supplementary Material Table S8). We then used multiple linear regression to assess which of the three parameters (or combinations of parameters) best explained patterns in macroalgal cover and juvenile coral density. Rates for N and P excretion were not included in models simultaneously due to collinearity and, thus, were modeled independently. Parrotfish biomass, nutrient excretion rates, and coral cover were all log transformed to meet the model assumptions. We evaluated the regression models using Akaike's Information Criteria corrected for small sample size (AICc) to determine the models that best explained the data structure for either macroalgal cover or juvenile coral density. Additionally, we used Pearson's correlation to assess the relationship between: (1) nutrient excretion rates of fishes and the nutrient content of the alga D. menstrualis, (2), the ratio of total fish biomass:parrotfish biomass and macroalgal cover, (3) macroalgal cover and the density of juvenile corals, and (4) macroalgal cover and the proportion of juvenile corals in direct competition with macroalgae.
Supplementary Material
Supplementary Info - Nutrient supply from fishes facilitates macroalgae and suppresses corals in a Caribbean coral reef ecosystem
Acknowledgments
The study was supported by a Faculty Development Grant from the College of Arts and Sciences at Florida International University to D.E.B., an EPA STAR Fellowship to J.E.A. and NSF grant OCE #0746164 to C.A.L. We thank the Florida Keys National Marine Sanctuary for facilitating our research via permit #FKNMS-2010-067.
Footnotes
The authors declare no competing financial interests.
Author Contributions D.E.B. designed the study; All authors contributed to data collection; J.E.A. conducted the bioenergetics modeling; D.E.B. conducted statistical analyses; D.E.B., J.E.A. and C.A.L. wrote the manuscript; all authors contributed to the manuscript revision.
References
- Estes J. A. et al. Trophic downgrading of planet Earth. Science 333, 301–306 (2011). [DOI] [PubMed] [Google Scholar]
- Smith V. H., Tilman G. D. & Nekola J. C. Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 100, 179–196 (1999). [DOI] [PubMed] [Google Scholar]
- Hillebrand H. et al. Consumer versus resource control of producer diversity depends on ecosystem type and producer community structure. Proc. Natl. Acad. Sci. U. S. A. 104, 10904–10909 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner R. W. & Elser J. J. Ecological Stoichiometry. (Princeton University Press, 2002). [Google Scholar]
- McIntyre P. B., Jones L. E., Flecker A. S. & Vanni M. J. Fish extinctions alter nutrient recycling in tropical freshwaters. Proc. Natl. Acad. Sci. U. S. A. 104, 4461–4466 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layman C. A., Allgeier J. E., Rosemond A. D., Dahlgren C. P. & Yeager L. A. Marine fisheries declines viewed upside down: human impacts on consumer-driven nutrient recycling. Ecol. Appl. 21, 343–349 (2011). [DOI] [PubMed] [Google Scholar]
- Hoegh-Guldberg O. et al. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742 (2007). [DOI] [PubMed] [Google Scholar]
- Burkepile D. E. & Hay M. E. Herbivore vs. nutrient control of marine primary producers: Context-dependent effects. Ecology 87, 3128–3139 (2006). [DOI] [PubMed] [Google Scholar]
- Burkepile D. E. & Hay M. E. Nutrient versus herbivore control of macroalgal community development and coral growth on a Caribbean reef. Mar. Ecol.-Prog. Ser. 389, 71–84 (2009). [Google Scholar]
- Kuffner I. B. et al. Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar. Ecol.-Prog. Ser. 323, 107–117 (2006). [Google Scholar]
- Box S. J. & Mumby P. J. Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar. Ecol.-Prog. Ser. 342, 139–149 (2007). [Google Scholar]
- Burkepile D. E. & Hay M. E. Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proc. Natl. Acad. Sci. U. S. A. 105, 16201–16206 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung T., Seymour R. M. & Johnson C. R. Alternative stable states and phase shifts in coral reefs under anthropogenic stress. Ecology 92, 967–982 (2011). [DOI] [PubMed] [Google Scholar]
- Vanni M. J. Nutrient cycling by animals in freshwater ecosystems. Annu. Rev. Ecol. Syst. 33, 341–370 (2002). [Google Scholar]
- McIntyre P. B. et al. Fish distributions and nutrient cycling in streams: Can fish create biogeochemical hotspots? Ecology 89, 2335–2346 (2008). [DOI] [PubMed] [Google Scholar]
- Allgeier J. E., Yeager L. A. & Layman C. A. Consumers regulate nutrient limitation regimes and primary production in seagrass ecosystems. Ecology (In Press). [DOI] [PubMed] [Google Scholar]
- Meyer J. L., Schultz E. T. & Helfman G. S. Fish schools: an asset to corals. Science 220, 1047–1049 (1983). [DOI] [PubMed] [Google Scholar]
- Crandall J. B. & Teece M. A. Urea is a dynamic pool of bioavailable nitrogen in coral reefs. Coral Reefs 31, 207–214 (2012). [Google Scholar]
- Meyer J. L. & Schultz E. T. Tissue condition and growth rate of corals associated with schooling fish. Limnol. Oceanogr. 30, 157–166 (1985). [Google Scholar]
- Holbrook S. J., Brooks A. J., Schmitt R. J. & Stewart H. L. Effects of sheltering fish on growth of their host corals. Mar. Biol. 155, 521–530 (2008). [Google Scholar]
- Liberman T., Genin A. & Loya Y. Effects on growth and reproduction of the coral Stylophora pistillata by the mutualistic damselfish Dascyllus marginatus. Mar. Biol. 121, 741–746 (1995). [Google Scholar]
- Fabricius K. E. Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar. Pollut. Bull. 50, 125–146 (2005). [DOI] [PubMed] [Google Scholar]
- Bucher D. J. The Effects of Experimentally Elevated Nutrient Concentrations on Growth Rate, Skeletal Architecture and Soft Tissue Morphology of Acroporid Corals (Scleractinia: Acroporidae). Ph.D. thesis, Southern Cross University, (2003). [Google Scholar]
- Koop K. et al. ENCORE: The effect of nutrient enrichment on coral reefs. Synthesis of results and conclusions. Mar. Pollut. Bull. 42, 91–120 (2001). [DOI] [PubMed] [Google Scholar]
- Davies P. S. A rapid method for assessing growth rates of corals in relation to water pollution. Mar. Pollut. Bull. 21, 346–348 (1990). [Google Scholar]
- Mumby P. J. Phase shifts and the stability of macroalgal communities on Caribbean coral reefs. Coral Reefs 28, 761–773 (2009). [Google Scholar]
- Schutte V. G. W., Selig E. R. & Bruno J. F. Regional spatio-temporal trends in Caribbean coral reef benthic communities. Mar. Ecol.-Prog. Ser. 402, 115–122 (2010). [Google Scholar]
- Newman M. J. H., Paredes G. A., Sala E. & Jackson J. B. C. Structure of Caribbean coral reef communities across a large gradient of fish biomass. Ecol. Lett. 9, 1216–1227 (2006). [DOI] [PubMed] [Google Scholar]
- Lamb-Wozniak K. Nitrogen cycling on coral reefs: a stable isotopic investigation of nutrient dynamics within the Florida Keys coral reef tract. Doctor of Philosophy thesis, University of Miami, Doctoral Dissertation, (2007). [Google Scholar]
- Hughes T. P., Graham N. A. J., Jackson J. B. C., Mumby P. J. & Steneck R. S. Rising to the challenge of sustaining coral reef resilience. Trends Ecol. Evol. 25, 633–642 (2010). [DOI] [PubMed] [Google Scholar]
- Mumby P. J., Hastings A. & Edwards H. J. Thresholds and the resilience of Caribbean coral reefs. Nature 450, 98–101 (2007). [DOI] [PubMed] [Google Scholar]
- Szmant A. M. & Forrester A. Water column and sediment nitrogen and phosphorus distribution patterns in the Florida Keys, USA. Coral Reefs 15, 21–41 (1996). [Google Scholar]
- Beach K. S., Walters L. J. & Borgeas H. B. Irradiance and nutrient limitation of Dicytota spp. populations on Conch Reef, Florida Keys, USA. J. Exp. Mar. Biol. Ecol. 329, 101–112 (2006). [Google Scholar]
- Schindler D. E. & Eby L. A. Stoichiometry of fishes and their prey: Implications for nutrient recycling. Ecology 78, 1816–1831 (1997). [Google Scholar]
- Heck K. L. et al. Trophic transfers from seagrass meadows subsidize diverse marine and terrestrial consumers. Ecosystems 11, 1198–1210 (2008). [Google Scholar]
- Smith J. E., Hunter C. L. & Smith C. M. The effects of top-down versus bottom-up control on benthic coral reef community structure. Oecologia 163, 497–507 (2010). [DOI] [PubMed] [Google Scholar]
- Mumby P. J. et al. Trophic cascade facilitates coral recruitment in a marine reserve. Proc. Natl. Acad. Sci. U. S. A. 104, 8362–8367 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams I. D. & Polunin N. V. C. Large-scale associations between macroalgal cover and grazer biomass on mid-depth reefs in the Caribbean. Coral Reefs 19, 358–366 (2001). [Google Scholar]
- Mumby P. J. The impact of exploiting grazers (scaridae) on the dynamics of Caribbean coral reefs. Ecol. Appl. 16, 747–769 (2006). [DOI] [PubMed] [Google Scholar]
- Madin E. M. P., Gaines S. D., Madin J. S. & Warner R. R. Fishing indirectly structures macroalgal assemlabes via altering herbivore behavior. Am. Nat. 176, 785–801 (2010). [DOI] [PubMed] [Google Scholar]
- Boyer J. N. & Briceno H. O. Annual report of the water quality monitoring project for the water quality protection program of the Florida Keys National Marine Sanctuary. Southeast Environmental Research Center, Florida International University, 82 (2010). [Google Scholar]
- Caley M. J. et al. Recruitment and the local dynamics of open marine populations. Annu. Rev. Ecol. Syst. 27, 477–500 (1996). [Google Scholar]
- Paul V. J. et al. Chemically mediated interactions between macroalgae Dictyota spp. and multiple life-history stages of the coral Porites astreoides. Mar. Ecol.-Prog. Ser. 426, 161–170 (2011). [Google Scholar]
- Rasher D. B. & Hay M. E. Chemically rich seaweeds poison corals when not controled by herbivores. Proceedings of the National Academy of Science 107, 9683–9688 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega Thurber R. et al. Macroalgae decrease growth and alter microbial community structure of the reef- building coral, Porites astreoides. PLoS ONE 7, e44246 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin S. A. et al. Baselines and degradation of coral reefs in the Northern Line Islands. PLoS ONE 3, e1548 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby P. J. & Harborne A. R. Marine reserves enhance the recovery of corals on Caribbean reefs. PLoS ONE 5, e8657 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham N. A. J., Nash K. L. & Kool J. T. Coral reef recovery dynamics in a changing world. Coral Reefs 30, 283–294 (2011). [Google Scholar]
- Edwards H. J. et al. How much time can herbivore protection buy for coral reefs under realistic regimes of hurricanes and coral bleaching? Glob. Change Biol. 17, 2033–2048 (2011). [Google Scholar]
- Maliao R. J., Turingan R. G. & Lin J. Phase-shift in coral reef communities in the Florida Keys National Marine Sanctuary (FKNMS), USA. Mar. Biol. 154, 841–853 (2008). [Google Scholar]
- Paddack M. J., Cowen R. K. & Sponaugle S. Grazing pressure of herbivorous coral reef fishes on low coral-cover reefs. Coral Reefs 25, 461–472 (2006). [Google Scholar]
- Ceccarelli D. M., Jones G. P. & McCook L. J. Territorial damselfishes as determinants of the structure of benthic communities on coral reefs. Oceanogr. Mar. Biol. 39, 355–389 (2001). [Google Scholar]
- Bohnsack J. A. & Harper D. E. Length-weight relationships of selected marine reef fishes from the southeastern United States and the Caribbean. NOAA Technical Memorandum NMFS-SEFC-215. (1988).
- Risk M. J. Fish diversity on a coral reef in the Virgin Islands. Atoll Res. Bull. 153, 1–6 (1972). [Google Scholar]
- Edmunds P. J., Aronson R. B., Swanson D. W., Levitan D. R. & Precht W. F. Photographic versus visual census techniques for the quantification of juvenile corals. Bull. Mar. Sci. 62, 937–946 (1998). [Google Scholar]
- Atkinson M. J. & Smith S. V. C:N:P ratios of benthic marine plants. Limnol. Oceanogr. 28, 568–574 (1983). [Google Scholar]
- Fish Bioenergetics 3.0 (University of Wisconsin System Sea Grant Institute, Madison, 1997).
- Schreck C. B. & Moyle P. B. Methods for Fish Biology. (American Fisheries Society, Bthesda, ML, 1990). [Google Scholar]
- MacFarlane R. B. Energy dynamics and growth of Chinook salmon (Oncorhynchus tshawytscha) from the Central Valley of California during the estuarine phase and first ocean year. Can. J. Fish. Aquat. Sci. 67, 1549–1565 (2010). [Google Scholar]
- Vanni M. J., Flecker A. S., Hood J. M. & Headworth J. L. Stoichiometry of nutrient recycling by vertebrates in a tropical stream: linking species identity and ecosystem processes. Ecol. Lett. 5, 285–293 (2002). [Google Scholar]
- Layman C. A., Quattrochi J. P., Peyer C. M. & Allgeier J. E. Niche width collapse in a resilient top predator following ecosystem fragmentation. Ecol. Lett. 10, 937–944 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layman C. A. & Silliman B. R. Preliminary survey and diet analysis of juvenile fishes of an estuarine creek on Andros Island, Bahamas. Bull. Mar. Sci. 70, 199–210 (2002). [Google Scholar]
- Burkepile D. E. Context-dependent corallivory by parrotfishes in a Caribbean reef ecosystem. Coral Reefs 31, 111–120 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Info - Nutrient supply from fishes facilitates macroalgae and suppresses corals in a Caribbean coral reef ecosystem