Abstract
BACKGROUND
Nonalcoholic fatty liver disease (NAFLD) includes two distinct conditions, with different histologic features and prognosis: non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH). Furthermore, NASH is the more aggressive necro-inflammatory form, which may accumulate fibrosis and result in End stage liver disease (ESLD). NAFLD is also linked to systemic inflammatory conditions such as psoriasis. NAFLD is currently the most common cause of ESLD in Western countries, becoming a serious public health concern. Hidradenitis suppurativa (HS) is a systemic inflammatory/autoinflammatory disease of the terminal follicular epithelium of the apocrine gland with a prevalence of 0.05% to 4.10%. Due to its systemic inflammatory behavior several comorbidities were recently associated, however liver ones were scarcely assessed.
AIM
To evaluate the prevalence and characteristics of NASH/NAFL in HS patients.
METHODS
This retrospective study is a sub-analysis of a larger study carried out in 4 Italian dermatological centers. In this cohort, there were 83 patients: 51 patients with HS only, 20 patients with HS/NAFL and 12 with HS/NASH.
RESULTS
Inflammatory comorbidities were present in 3.9% of HS only patients, 25% of HS/NAFL patients and 58.3% of HS/NASH patients (P < 0.001). Similarly, mean Autoinflammatory Disease Damage Index (ADDI) was significantly higher among patients with HS/NASH (5.3 ± 2.2, P < 0.001) compared to patients with HS/NAFL or HS only (2.8 ± 1.6 and 2.6 ± 1.4 respectively). Furthermore, ADDI correlates with IHS4 in HS, HS/NAFL and HS/NASH. Diabetic patients have higher Hurley score than not diabetic ones. Ultrasound examination was significantly different in the three groups.
CONCLUSION
HS patients displayed a high prevalence of NASH/NAFLD and ultrasound examination should be particularly addressed to patients that display high ADDI scores.
Keywords: Non-alcoholic steatohepatitis, Non-alcoholic fatty liver, Nonalcoholic fatty liver disease, End stage liver disease, Hidradenitis suppurativa
Core tip: Nonalcoholic fatty liver disease (NAFLD), in its two variants non-alcoholic fatty liver and non-alcoholic steatohepatitis, is often linked to systemic inflammatory conditions, such as psoriasis. Remarkably, hidradenitis suppurativa (HS) is a new affirming systemic inflammatory disorder of the follicular epithelium of skin apocrine glands with a prevalence in normal population ranging from 0.05% to 4.10%. Furthermore, HS patients display a significant comorbidities burden (e.g., cardiovascular risk, metabolic syndrome, diabetes, and spondyloarthritis) but the association with NAFLD was not previously investigated. This is the first study which evaluated NAFLD prevalence and its characteristics in HS patients.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) includes two distinct entities, with different histologic clues and prognosis: non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH), the more aggressive necro-inflammatory form, which may accumulate fibrosis and result in End stage liver disease (ESLD) and its complications, including hepatocellular carcinoma (HCC)[1].
Nowadays, NAFLD represents the main cause of chronic liver disease in Europe and North America, where is found in 17%-30% of the population, worldwide the prevalence is 2%-4% of the population becoming a serious public health concern[2]. Evidences suggest that NAFLD is the hepatic sign of metabolic syndrome; therefore, is linked not only with an increase of liver-related mortality, but also of the overall mortality. Noninvasive techniques, such as biological tests and elastography can be used for the evaluation of NAFLD patients. Today, liver biopsy (diagnostic gold standard) should be recommended in selected cases.
Patients with NAFLD would benefit from their lifestyle changes by progressive weight loss through exercise and low sugar and fat intake. Pharmacotherapy should be reserved for patients with significant fibrosis. Unfortunately, there are no Food and Drug Administration (FDA) approved therapies[3].
Hidradenitis suppurativa (HS) is a systemic, chronic, inflammatory/autoinflam-matory disease with a relapsing remitting behavior and a deep impact on patient's quality of life. Despite its elusive pathogenesis, clinical manifestations are clear and space from painful nodules to fistula, mainly involving areas rich in apocrine gland-bearing, such as armpits, inguinal and anogenital regions (Dessau definition)[4-6]. HS is an affirming systemic inflammatory disease and this idea was sustained by the recent acquisitions in the pathogenesis[7], epidemiology[8] and therapy[9]. Until recently, it was considered to be a rare disease with a prevalence cited as approximately 1%[10]. Actually, the prevalence of HS seems to be greater varying from 0.05% to 4.10%; this variability is intrinsically affected by study type, being lower in retrospectively designed studies and the higher in prospective or self-reported ones[11].
European guidelines for the management of HS have been published[12]: No therapy is actually able to guaranty a high rate of complete disease remission. As for patients with NAFLD also patients with HS would benefit from their lifestyle changes by losing weight. Furthermore, topical and systemic antibiotics, injected corticosteroids, or biologics and other systemic treatments may be used. Oral antibiotics may be used to help prevent new lesions. Moderate stages may be treated with oral antibiotics, oral retinoids such as isotretinoin, hormonal therapy, and/or surgery[11,12]. For moderate to severe disease, target therapy directed against TNF-alpha proteins which are involved in the inflammation process are used: adalimumab has been approved by the FDA as orphan product for HS treatment. Adalimumab, a TNF blocker, is actually the only biologic drug approved in Italy for HS patients and notable in October 2018 it received an extension also for children over 12 years old[13]. Due to the increased body of comorbidities currently associated with HS[14,15], the liver metabolic comorbidities were neglected.
NAFLD is considered a multisystem pathology increasing the risk of diabetes mellitus, cardiovascular and chronic renal disorders, diseases with an increased incidence in HS patients[16].
Over the last decade, it has been growing the evidence that NAFLD is associated with psoriasis, another systemic chronic inflammatory disease[17-19]. Despite the high incidence of NAFLD and the current evidence that HS is not an uncommon disease, there are currently no studies in the literature investigating the association between NAFLD and chronic skin diseases other than psoriasis.
MATERIALS AND METHODS
Study population
This retrospective study is a sub-analysis of a larger one carried out in the Department of Dermatology of Ospedale Maggiore Policlinico at the beginning and after extended to other 3 primary dermatological Italian centers, namely San Donato Hospital, San Gallicano Hospital and Galeazzi Hospital. The study started in January 2018 and ended in December 2018. Patients were recruited by filling the recently proposed visual-aided questionnaire for the self- assessment of HS[20]. The positive patients were after assessed in a dedicated HS-Lab. The diagnosis of HS was performed by two independent board-certified dermatologists following the Dessau criteria[21]. The inclusion criteria comprehended HS diagnosis, Alcohol Use Disorders Identification Test (AUDIT) < 8[22], last 3 complete blood count (CBC) available with transaminases. The exclusion criteria comprehended AUDIT score > 7, pre-existent hepatic cirrhosis, viral hepatitis (B, C and E) and recent drug-related hepatitis (< 5 years), congenital hepatic malformations, hepatic or cholangitic autoimmune conditions.
All patients underwent a hepatologic visit and ultrasonographic (US) evaluation of the liver. Patients with raised liver enzymes underwent liver biopsy to evaluate the presence of NAFLD according the European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) and European Association for the Study of Obesity (EASO) Clinical Practice Guidelines for the management of NAFLD[23]. Patients were also screened for diabetes, a predisposing factor for NASH and NAFLD. Diabetes diagnosis was performed following these criteria: a random blood sugar level of equal or greater than 200 mg/dL or 11.1 mmol/L or fasting blood sugar test of 126 mg/dL (7 mmol/L) or higher on two separate tests or oral sugar test of equal of higher than 200 mg/dL (11.1 mmol/L) after two hours.
Outcomes of the study
During dermatological assessment, besides demographics, drug-history and comorbidities, were collected HS clinical phenotypes[24], static score as Hurley[25], dynamic score as international HS 4 (iHS4)[26], the Autoinflammatory Disease Damage Index (ADDI)[27,28] and Dermatology Life Quality Index (DLQI)[29].
Statistical analysis
Variables were described as number and/or percentages. All variables were preliminarily assessed with Shapiro-Wilk test to establish the parametric behavior. The Wilcoxon-Mann-Whitney test was employed to deal with quantitative variables, whilst Fisher’s exact test was applied with qualitative variables comparison. A P value < 0.05 was considered significant. The analysis was performed with the statistical software SPSS ver. 20.0 (Armonk, NY: IBM Corp.).
RESULTS
Demographics and clinical characteristics were summarized in Table 1. Interestingly from the pool of 86 patients that had a positive visual-aided questionnaire for the self- assessment of HS, after clinical assessment we enrolled 83 HS patients with the above HS clinical phenotypes: 54 regular type, 6 frictional type, 10 scarring folliculitis type, 5 conglobata type, 5 syndromic type, 3 ectopic type.
Table 1.
Characteristics of 83 patients with hidradenitis suppurative, Nonalcoholic fatty liver and Nonalcoholic steatohepatitis and intercalsses charactersitics
| HS only | NAFLD | P value | NASH | P value | NAFL | P value | |
| n | 51 | 32 | 12 | 20 | |||
| Age, mean(SD) | 43.04 (8.9) | 41.32 (9.0) | 0.564 | 41.58 (7.4) | 0.602 | 40.55 (10.3) | 0.315 |
| Age cat (%) | 0.646 | 0.463 | 0.629 | ||||
| < 30 | 3 (5.9) | 4 (12.5) | 1 (8.3) | 3 (15.0) | |||
| 30-39 | 16 (31.4) | 8 (25.0) | 2 (16.7) | 6 (30) | |||
| 40-49 | 18 (35.3) | 14 (43.8) | 7 (58.3) | 7 (35.0) | |||
| > 50 | 14 (27.5) | 6 (18.8) | 2 (16.7) | 4 (20.0) | |||
| Male, n (%) | 17 (33.3) | 14 (43.8) | 0.623 | 5 (41.7) | 0.835 | 9 (45.0) | 0.52 |
| Diabetes, n (%) | 12 (23.5) | 9 (28.1) | 0.853 | 3 (25.0) | 0.764 | 6 (30.0) | 0.794 |
| BMI, mean(SD) | 28.31 (2.5) | 27.56 (1.9) | 0.381 | 27.58 (2.7) | 0.376 | 27.55 (1.7) | 0.218 |
| bmi_cat (%) | 0.559 | 0.515 | 0.384 | ||||
| Normal Weight | 4 (7.8) | 4 (12.5) | 2 (16.7) | 2 (10.0) | |||
| Overweight | 38 (74.5) | 26 (81.3) | 9 (75.0) | 17 (85.0) | |||
| Obese | 9 (17.6) | 2 (6.3) | 1 (8.3) | 1 (5.0) | |||
| IHS4, mean(SD) | 9.57 (3.6) | 11.32 (2.8) | 0.025 | 12.67 (3.6) | 0.009 | 9.40 (3.9). | 0.861 |
| IHS4 cat (%) | 0.028 | 0.007 | 0.97 | ||||
| Mild | 5 (9.8) | 3 (9.4) | 1 (8.3) | 2 (10.0) | |||
| Moderate | 24 (47.1) | 10 (31.3) | 0 (0) | 10 (50.0) | |||
| Severe | 22 (43.1) | 19 (59.4) | 11 (91.7) | 8 (40) | |||
| Hurley (%) | 0.494 | 0.197 | 0.785 | ||||
| 1 | 5 (9.8) | 3 (9.4) | 1 (8.3) | 2 (10.0) | |||
| 2 | 24 (47.1) | 6 (18.8) | 1 (8.3) | 5 (25.0) | |||
| 3 | 22 (43.1) | 23 (71.9) | 10 (83.3) | 13 (65.0) | |||
| Elevated_liver_enzymes, n (%) | 19 (37.3) | 9 (28.1) | 0.617 | 4 (33.3) | 0.998 | 5 (25.0) | 0.482 |
| ADDI_score, mean(SD) | 2.55 (1.4) | 3.72 (1.8) | < 0.001 | 5.33 (2.2) | < 0.001 | 2.75 (1.6) | 0.603 |
| Inflammatory comorbidities, n (%) | 2 (3.9) | 12 (37.5) | < 0.001 | 7 (58.3) | < 0.001 | 5 (25.0) | 0.025 |
| In detail (%) | 0.001 | < 0.001 | 0.047 | ||||
| Acne conglobata | 1 (2.0) | 0 (0) | 0 (0) | 0 (0) | |||
| Crohn disease | 0 (0) | 2 (6.3) | 1 (8.3) | 1 (5.0) | |||
| Lichen sclerosus | 1 (2.0) | 0 (0) | 0 (0) | 0 (0) | |||
| PASH | 0 (0) | 5 (15.6) | 4 (33.3) | 1 (5.0) | |||
| Psoriasis | 0 (0) | 2 (6.3) | 1 (8.3) | 1 (5.0) | |||
| Spondyloarthritis | 0 (0) | 2 (6.3) | 1 (8.3) | 1 (5.0) | |||
| Uveitis | 0 (0) | 1 (3.1) | 0 (0) | 1 (5.0) | |||
| Positive ultrasound, n (%) | 11 (21.6) | 32 (100.0) | < 0.001 | 12 (100.0) | < 0.001 | 20 (100.0) | < 0.001 |
| NASH | 0 (0) | 12 (37.5) | < 0.001 | 12 (100.0) | 0 (0) | ||
| NAFL | 0 (0) | 20 (62.5) | < 0.001 | 0 (0) | 20 (100.0) |
ADDI: Autoinflammatory disease damage index; BMI: Body mass index; HS: Hidradenitis suppurativa; IHS4: International Hidradenitis Suppurativa Severity Scoring System, NASH: NonAlcoholic SteatoHepatitis, NAFL: NonAlcoholic Fatty Liver, PASH: Pyoderma gangrenosum, Acne, and hidradenitis suppurativa; SD: Standard deviation. Normal weight: 18.5–24.9 kg/m2, Overweight: 25–29.9 kg/m2 Obese: >29. 9 kg/m2.
In this cohort, there were 51 patients with HS only, 20 patients with HS and NAFL (HS/NAFL) and 12 with HS and NASH (HS/NASH)(Table 1). The groups were predominantly composed by females, in fact males were 33.3% of HS only, 43.8% HS/NAFLD, 41.7% HS/NAFL and 45% HS/NASH patients being female and did not display significant difference (P = 0.62, P = 0.52, P = 84). The average age between groups was similar (HS only 43 ± 8.9; HS/NAFLD 41.3 ± 9.0; HS/NAFL 40.6 ± 10.3; HS/NASH 41.6 ± 7.4, P = 0.56). Patients also had similar Body Mass Index (BMI) with HS only having an average BMI of 28.3 ± 2.5 kg/m2, HS/NAFLD patients being 27.6 ± 1.9 kg/m2 (P = 0.38), HS/NAFL 27.6 ± 1.7 kg/m2 (P = 0.22) and HS/NASH having 27.6 ± 2.7 kg/m2 (P = 0.38). Diabetes was present in 24% of HS only patients, 30% of HS/NAFL and 25% of HS/NASH patients. Inflammatory comorbidities (Table 1) were present in 3.9% of HS only patients, 37.5% of HS/NAFLD, 25% of HS/NAFL patients and 58.3% of HS/NASH patients with a statistically different prevalence (P < 0.001). Specifically, in HS only patients one had acne conglobata and 1 patient had lichen sclerosus; while in HS/NAFL there was one patient with Crohn’s disease, 1 with Pyoderma gangrenosum, Acne, and Hidradenitis Suppurativa (PASH), 1 with psoriasis, 1 with spondyloarthritis and 1 with uveitis. Finally, of the HS/NASH patients, 1 had Crohn’s disease, 4 had PASH, 1 had psoriasis and 1 had spondyloarthritis.
The average IHS4 score among HS/NASH patients (12.7 ± 3.6, P = 0.03) was the highest, while it was similar among those with HS only and HS/NAFL patients (9.6 ± 3.6 and 9.4 ± 3.9 respectively, P = 0.86). Likewise, mean ADDI was significantly higher among HS/NASH patients (5.3 ± 2.2, P < 0.001) compared to HS only and HS/NAFL patients (2.8 ± 1.6 and 2.6 ± 1.4 respectively) (Table 1). There were no significant differences in Hurley score, however 83% of HS/NASH patients had a Hurley score of 3, whereas only 65% of HS/NAFL and 57% of HS only patients had a Hurley score of 3 (P = 0.49). Presence of elevated liver enzymes was similar among the three groups (HS only 37.3%; HS/NAFL 25%; HS/NASH 33.3%, P = 0.62). Finally, ultrasound revealed a bright liver in 22% of HS only patients and all HS/NAFL and HS/NASH patients (P < 0.001).
HS only and HS/NAFL patients displayed a significant difference in inflammatory comorbidities (P = 0.025) and positivity of ultrasound (P < 0.001) (Table 1).
HS/NASH compared with patients with HS only displayed a significant difference in IHS4 (P = 0.009), ADDI (P < 0.001), inflammatory comorbidities rate (P < 0.001) and ultrasound positivity (P < 0.001) (Table 1).
HS patients with and without diabetes had a significant difference only in Hurley stage (P = 0.022) (Table 2).
Table 2.
Differences among hidradenitis suppurativa patients with and without diabetes
| Non diabetes | Diabetes | P value | |
| n | 62 | 21 | |
| Age, mean(SD) | 42.44 (9.4) | 41.62 (8.2) | 0.722 |
| Age cat (%) | 0.203 | ||
| < 30 | 4 ( 6.5) | 3 (14.3) | |
| > 50 | 17 (27.4) | 3 (14.3) | |
| 30-39 | 20 (32.3) | 4 (19.0) | |
| 40-49 | 21 (33.9) | 11 (52.4) | |
| Male, n (%) | 21 (33.9) | 10 ( 47.6) | 0.387 |
| Diabetes, n (%) | 0 ( 0.0) | 21 (100.0) | <0.001 |
| BMI, mean(SD) | 28.10 (2.6) | 27.81 (1.4) | 0.636 |
| bmi_cat (%) | 0.161 | ||
| Normal Weight | 8 (12.9) | 0 ( 0.0) | |
| Obese | 9 (14.5) | 2 ( 9.5) | |
| Overweight | 45 (72.6) | 19 ( 90.5) | |
| IHS4, mean(SD) | 10.37 (3.6) | 8.81 (4.1) | 0.101 |
| IHS4 cat, n (%) | 0.051 | ||
| Mild | 4 ( 6.5) | 4 ( 19.0) | |
| Moderate | 23 (37.1) | 11 ( 52.4) | |
| Severe | 35 (56.5) | 6 ( 28.6) | |
| Hurley, n (%) | 0.022 | ||
| 1 | 4 ( 6.5) | 4 ( 19.0) | |
| 2 | 14 (22.6) | 9 ( 42.9) | |
| 3 | 44 (71.0) | 8 ( 38.1) | |
| Elevated_liver_enzymes, n (%) | 21 (33.9) | 7 ( 33.3) | 1 |
| ADDI_score, mean(SD) | 3.13 (1.8) | 2.62 (2.0) | 0.275 |
| Inflammatory comorbidities, n (%) | 9 (14.5) | 5 ( 23.8) | 0.518 |
| In.detail, n (%) | 0.563 | ||
| 53 (85.5) | 16 ( 76.2) | ||
| Acne conglobata | 1 ( 1.6) | 0 ( 0.0) | |
| Crohn | 1 ( 1.6) | 1 ( 4.8) | |
| Lichen sclerosus | 0 ( 0.0) | 1 ( 4.8) | |
| PASH | 4 ( 6.5) | 1 ( 4.8) | |
| Psoriasis | 1 ( 1.6) | 1 ( 4.8) | |
| Spondyloarthritis | 1 ( 1.6) | 1 ( 4.8) | |
| Uveitis | 1 ( 1.6) | 0 ( 0.0) | |
| Positive_ultrasound, n (%) | 31 (50.0) | 12 ( 57.1) | 0.754 |
| NASH, n (%) | 9 (14.5) | 3 ( 14.3) | 1 |
| NAFL, n (%) | 14 (22.6) | 6 ( 28.6) | 0.795 |
| Disease, n (%) | 0.853 | ||
| HS only | 39 (62.9) | 12 ( 57.1) | |
| NAFL | 14 (22.6) | 6 ( 28.6) | |
| NASH | 9 (14.5) | 3 ( 14.3) |
ADDI: Autoinflammatory Disease Damage Index; BMI: Body mass index; HS: Hidradenitis suppurativa; IHS4: International Hidradenitis Suppurativa Severity Scoring System; NASH: Non-alcoholic steatohepatitis; NAFL: Nonalcoholic fatty liver; PASH: Pyoderma gangrenosum, Acne, and hidradenitis suppurativa; SD: Standard deviation. Normal weight: 18.5–24.9 kg/m2; Overweight: 25-29.9 kg/m2; Obese: > 29.9 kg/m2.
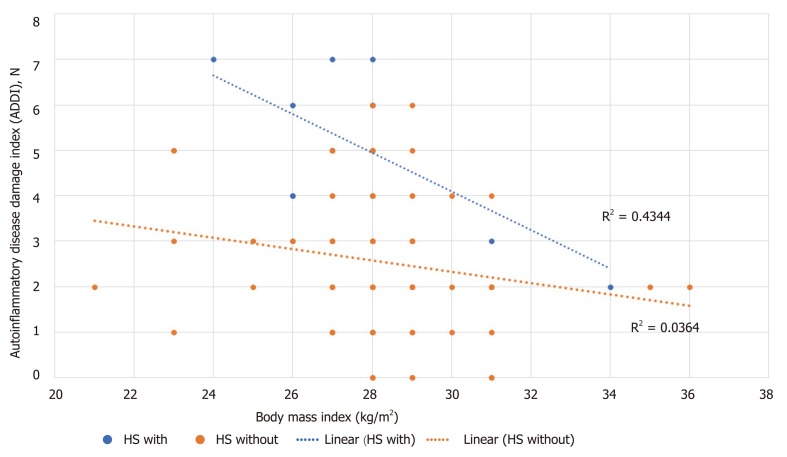
Age had a significant moderately positive correlation with ADDI among HS/NAFL patients (r = 0.57, P = 0.05). Next, BMI and ADDI were moderately negatively correlated in HS patients with inflammatory comorbidities (R2 = 0.43, Figure 1).
Figure 1.
Correlation between body mass index and Autoinflammatory Disease Damage Index among patients with hidradenitis suppurative (HS) only, or HS with other inflammatory comorbidity.
BMI and ADDI were weakly negatively correlated in patients with HS only (r = -0.25, P = 0.05) and in those who had HS and diabetes (r = -0.46, P = 0.04). Correlation between BMI and IHS4, age and IHS4, BMI and ADDI, among the three groups was not significant. In addition, correlation between BMI and IHS4, age and IHS4, age and ADDI based on presence of other inflammatory comorbidity was not significant. Finally, BMI and IHS4, age and IHS4, age and ADDI based on diabetes status was not significant.
Hurley score and categorical IHS4 score had good overlap in Hurley 1 and 2 scores, with 8 Hurley 1 patients also having mild IHS4 categorical score, 22 (96%) Hurley 2 patients having a moderate IHS4 categorical score, and 1 (4%) Hurley 2 patient having a severe IHS4 categorical score. However, among 52 patients with Hurley score 3, 12 (23%) were considered moderate IHS4, and 40 (77%) were considered severe (P < 0.001). Average ADDI score among Hurley 1 patients was 0.75 ± 1.2, 1.9 ± 1.4 among Hurley 2 patients and 3.8 ± 1.6 among Hurley 3 patients (P < 0.001).
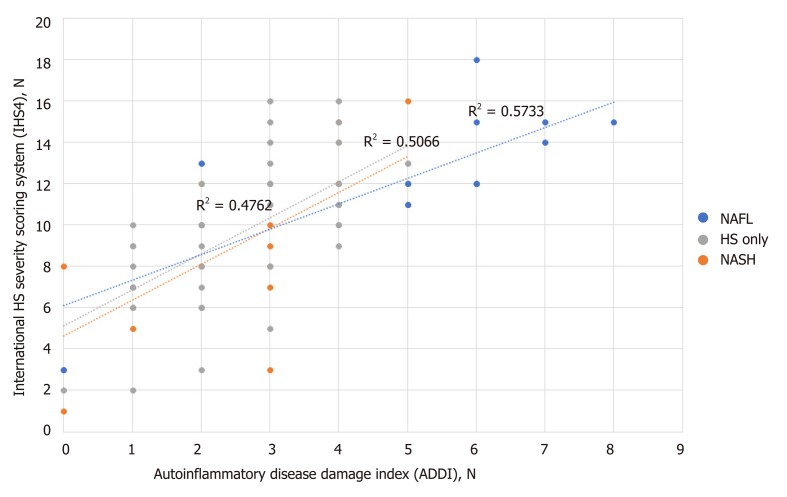
There was a moderate correlation between IHS4 and ADDI scores among all 3 groups [R2 = 0.48 (P < 0.001) for HS only; R2 = 0.51(P < 0.001), for HS/NAFL; R2 = 0.57 (P < 0.001), for HS/NASH, Figure 2].
Figure 2.
Correlation between international hidradenitis suppurativa severity scoring system and autoinflammatory Disease Damage Index among patients with hidradenitis suppurative only, or non-alcoholic staetohepatitis or non-alcoholic fatty liver.
DISCUSSION
In our cohort of HS patients, for the first time, was described a 38,5% NAFLD prevalence: 24% of NAFL and 14,5% of NASH. Likewise, in psoriasis, HS patients with NAFLD displayed the higher severity scores, namely IHS4 and ADDI. These findings, together with pathogenetic[7], epidemiologic[8] and therapeutic[9] evidences, further confirm the recent idea that HS is a systemic inflammatory disease. NAFLD is the main entity to cause ESLD in Europe and North America, this is easy to predict that it will become the most frequent liver transplantation indication by 2030[16]. Although the weight of the disease is so overwhelming, there are no really effective drugs in treatment[3]. Therefore, it is essential to investigate all co-morbidities that can worsen the prognosis, among these in our study emerges the role of HS, whose treatment is a controversial issue[30-32]. Microbiological data show that HS is associated with polymicrobial flora, including anaerobic microorganisms[33,34]. On this point, Guet-Revillet et al[33], in a French prospective microbiological study on 102 HS lesions from 82 patients, found that Staphylococcus lugdunensis was cultured in 58% of HS lesions and anaerobic microorganisms, including actinomycetes, were observed in 24% of abscesses or nodules and in 87% of chronic lesions. More recently, in a prospective metagenomic study, the same Author, using high-throughput sequencing, confirmed the high prevalence of polymicrobial anaerobic flora in HS[35]. Overall, topical or oral antibiotics (monotherapy or combination therapy) is commonly suggested for the management of HS flares[12,36]. The most common antibiotic regimens used for the treatment of HS included topical clindamycin, oral tetracyclines, oral clindamycin-rifampicin combination and parenteral ertapenem followed by oral rifampicin-moxifloxacin-metronidazole combination[12,37]. No data are available for the antibiotic management of HS with the newer drugs, including dalbavancin, daptomycin and tigecycline[38-40]. Moreover, there are insufficient data to support intravenous antibiotics[41-43]. A major concern of the antibiotic use in HS is the increasing of antimicrobial resistance[44,45]. Finally, a clinical monitoring and a dose adjustment in patients with liver disease can be required[46,47] in view of the fact that NAFLD remains the main source of ESLD in Western countries[1]. It is clear, with these premises, that the available therapeutic armamentarium for the treatment of both diseases is very inadequate. Of our findings, the most obvious appears the US finding of bright liver in 22% of HS only and all HS/NAFL or HS/NASH patients (P < 0.001). However liver biopsy (histology) remains the gold standard in the diagnosis of NAFLD, as recently suggested in a meta-analysis that compared US and histology quantifying diagnostic sensitivity to 84.8% (79.5-88.9), specificity to 93.6% (87.2-97.0), positive likelihood ratio to 13.3 (6.4-27.6) and negative likelihood ratio to 0.16 (0.12-0.22)[48].
Interestingly the newly proposed ADDI score displayed a clinically meaning in addressing ultrasound examination in patients with NASH. PASH syndrome patients all displayed NAFLD and this confirm that higher levels of inflammations trigger the development of liver disease. Adipose tissue is not inert but metabolically active and release pro-inflammatory cytokines, furthermore the metabolic syndrome is a recognized comorbidity of both NASH and HS. Thus, the finding is that ADDI correlates with BMI in patients with inflammatory comorbidities. To further enforce its clinical capability, ADDI and IHS4, the dynamic severity index, correlated in the examined groups. Therefore ADDI, a composite index derived from the global examination of monogenic autoinflammatory diseases and applied to HS[27], is related to the dynamic index that monitor HS skin inflammation. This assumption empowers the thesis that HS should be considered an autoinflammatory polygenic disease and treated by physicians as a systemic condition. From this point of view, is it really correct talk about comorbidities or it is more proper define them as different manifestations of a common spectrum of disease, namely HS as a systemic disease. As for psoriasis, the real goal for the newly introduced biological therapy will be to act on both cutaneous and systemic manifestation of HS.
In conclusion high prevalence of NAFLD was found in HS patients and an US screening to exclude liver abnormalities should be performed especially in HS patients with active disease and inflammatory comorbidities.
ARTICLE HIGHLIGHTS
Research background
Nonalcoholic fatty liver disease (NAFLD), in its two variants non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH), is the main cause of End stage liver disease (ESLD) and its complications, including hepatocellular carcinoma (HCC) in North America and Europe. Due to its impact on morbility and mortality, the identification of population with high risk of NAFLD is mandatory and in literature some systemic inflammatory diseases are described to be linked with NAFLD. Hidradenitis suppurativa (HS) is a new affirming systemic inflammatory disorder of the follicular epithelium of skin apocrine glands with a prevalence in normal population ranging from 0.05% to 4.10%. No data are present in literature towards the prevalence of NAFLD in HS.
Research motivation
The estimation of NAFLD in HS patients may lead to an early and optimized treatment.
Research objectives
This study aimed first to evaluate the overall prevalence of NAFLD and specifically of NAFL and NASH. Secondary aims were the clinical characterization of these patients. Depict a profile of HS patients with NAFLD will be crucial in optimizing clinical and therapeutic management.
Research methods
This retrospective multicenter carried out 4 primary dermatological Italian centers started in January 2018 and ended in December 2018. Patients were recruited by filling the recently proposed visual-aided questionnaire for the self- assessment of HS and after underwent a dermatologic visit that evaluate HS with static (Hurley score) and dynamic indexes (ADDI: Autoinflammatory Disease Damage Index, IHS4: International Hidradenitis Suppurativa Severity Scoring System). Transaminases were assessed and all patients underwent liver sonography (US). NASH suspected cases were biopsied.
Research results
We included 83 HS patients, in detail 51 patients with HS only and 32 with NAFLD (20 with NAFL, 12 NASH). Inflammatory comorbidities were present in 3.9% of HS only patients, 37.5% of HS/NAFLD, 25% of HS/NAFL patients and 58.3% of HS/NASH patients (P < 0.001). The average IHS4 score among HS/NASH patients (12.7 ± 3.6, P = 0.03) was the highest, while it was similar among those with HS only and HS/NAFL patients (9.6 ± 3.6 and 9.4 ± 3.9 respectively, P = 0.86). Likewise, mean ADDI was significantly higher among HS/NASH patients (5.3 ± 2.2, P < 0.001) compared to HS only and HS/NAFL patients (2.8 ± 1.6 and 2.6 ± 1.4 respectively). While no significant differences were found in Hurley score. There was a significant positive correlation between IHS4 and ADDI scores among all 3 groups (r = 0.7, P < 0.001 for HS only; r = 0.71, P = 0.0004 for HS/NAFL; r = 0.76, P = 0.004 for HS/NASH). Finally, BMI and ADDI were weakly negatively correlated in patients with HS only (r = -0.25, P = 0.05) and in those who had HS and diabetes (r = -0.46, P = 0.04).
Research conclusions
HS patients have a high prevalence of NAFLD. In particular clinicians should sonographically assess HS patients with more active disease (high IHS4 score) and with other inflammatory comorbidities (high ADDI).
Research perspectives
The present study highlighted the association between HS and NAFLD. However other issues remain still open to future investigations. In particular related issues,that should be addressed to optimize patient management are the prevalence of NAFLD HS-related in different ethnicity and the impact of systemic therapies on NAFLD development in HS patients.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Institutional review board statement: This study was approved by the Milan Area 2 Ethics Committee (Milan, Italy).
Informed consent statement: Informed consent was obtained from all HS patients after a careful explanation of the nature of the disease and possible complications.
Conflict-of-interest statement: All authors declare no conflict of interest.
Data sharing statement: No additional data are available.
Peer-review started: January 4, 2019
First decision: January 23, 2019
Article in press: March 16, 2019
P-Reviewer: Hernanda PY, Luo GH S-Editor: Cui LJ L-Editor: A E-Editor: Zhang YL
Contributor Information
Giovanni Damiani, Department of Dermatology, Case Western Reserve University, Cleveland, OH 44195, United States; Young Dermatologists Italian Network (YDIN), Centro Studi GISED, Bergamo 24100, Italy; Clinical Dermatology, IRCCS Galeazzi Orthopaedic Institute, Milan 20100, Italy; Department of Biomedical, Surgical and Dental Sciences, University of Milan, Milan 20161, Italy.
Sebastiano Leone, Division of Infectious Diseases, “San Giuseppe Moscati” Hospital, Avellino 83100, Italy.
Kristen Fajgenbaum, University of North Carolina School of Medicine, Chapel Hill, NC 27516, United States.
Nicola L Bragazzi, School of Public Health, Department of Health Sciences (DISSAL), University of Genoa, Gevova 16132, Italy.
Alessia Pacifico, San Gallicano Dermatological Institute, IRCCS, Rome 00144, Italy.
Rosalynn RZ Conic, Department of Dermatology, Case Western Reserve University, Cleveland, OH 44195, United States.
Paolo DM Pigatto, Clinical Dermatology, IRCCS Galeazzi Orthopaedic Institute, Milan 20100, Italy; Department of Biomedical, Surgical and Dental Sciences, University of Milan, Milan 20161, Italy.
Carlo Maiorana, Center for Jawbone Atrophies Policlinico Hospital, University of Milan School of Dentistry, Milan 20123, Italy.
Pierpaolo Poli, Center for Jawbone Atrophies Policlinico Hospital, University of Milan School of Dentistry, Milan 20123, Italy.
Emilio Berti, Dipartimento di Fisiopatologia Medico-Chirurgica e dei Trapianti, Università degli Studi di Milano, Unità Operativa di Dermatologia, IRCCS Fondazione Ca’ Granda, Ospedale Maggiore Policlinico, Milan 20122, Italy.
Maria C Pace, Department of Women, Child and General and Specialized Surgery, University of Campania “Luigi Vanvitelli”, Naples 80138, Italy.
Piergiorgio Malagoli, Dermatology Unit, Azienda Ospedaliera San Donato Milanese, Milan 20097, Italy.
Vincenzo Bettoli, Department of Clinical and Experimental Dermatology, O.C. of Dermatology, Azienda Ospedaliero-Universitaria di Ferrara, Ferrara 44121, Italy.
Marco Fiore, Department of Women, Child and General and Specialized Surgery, University of Campania “Luigi Vanvitelli”, Naples 80138, Italy. marco.fiore@hotmail.it.
References
- 1.Calzadilla Bertot L, Adams LA. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17050774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kneeman JM, Misdraji J, Corey KE. Secondary causes of nonalcoholic fatty liver disease. Therap Adv Gastroenterol. 2012;5:199–207. doi: 10.1177/1756283X11430859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sporea I, Popescu A, Dumitrașcu D, Brisc C, Nedelcu L, Trifan A, Gheorghe L, Fierbințeanu Braticevici C. Nonalcoholic Fatty Liver Disease: Status Quo. J Gastrointestin Liver Dis. 2018;27:439–448. doi: 10.15403/jgld.2014.1121.274.quo. [DOI] [PubMed] [Google Scholar]
- 4.Fimmel S, Zouboulis CC. Comorbidities of hidradenitis suppurativa (acne inversa) Dermatoendocrinol. 2010;2:9–16. doi: 10.4161/derm.2.1.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurzen H, Kurokawa I, Jemec GB, Emtestam L, Sellheyer K, Giamarellos-Bourboulis EJ, Nagy I, Bechara FG, Sartorius K, Lapins J, Krahl D, Altmeyer P, Revuz J, Zouboulis CC. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455–456; discussion 457-472. doi: 10.1111/j.1600-0625.2008.00712_1.x. [DOI] [PubMed] [Google Scholar]
- 6.Marzano AV, Damiani G, Ceccherini I, Berti E, Gattorno M, Cugno M. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis) Br J Dermatol. 2017;176:1588–1598. doi: 10.1111/bjd.15226. [DOI] [PubMed] [Google Scholar]
- 7.Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis Suppurativa: A Systematic Review Integrating Inflammatory Pathways Into a Cohesive Pathogenic Model. Front Immunol. 2018;9:2965. doi: 10.3389/fimmu.2018.02965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shlyankevich J, Chen AJ, Kim GE, Kimball AB. Hidradenitis suppurativa is a systemic disease with substantial comorbidity burden: a chart-verified case-control analysis. J Am Acad Dermatol. 2014;71:1144–1150. doi: 10.1016/j.jaad.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Włodarek K, Ponikowska M, Matusiak Ł, Szepietowski JC. Biologics for hidradenitis suppurativa: an update. Immunotherapy. 2019;11:45–59. doi: 10.2217/imt-2018-0090. [DOI] [PubMed] [Google Scholar]
- 10.McMillan K. Hidradenitis suppurativa: number of diagnosed patients, demographic characteristics, and treatment patterns in the United States. Am J Epidemiol. 2014;179:1477–1483. doi: 10.1093/aje/kwu078. [DOI] [PubMed] [Google Scholar]
- 11.Saunte DML, Jemec GBE. Hidradenitis Suppurativa: Advances in Diagnosis and Treatment. JAMA. 2017;318:2019–2032. doi: 10.1001/jama.2017.16691. [DOI] [PubMed] [Google Scholar]
- 12.Gulliver W, Zouboulis CC, Prens E, Jemec GB, Tzellos T. Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord. 2016;17:343–351. doi: 10.1007/s11154-016-9328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Food and Drug Administration (FDA) Rare Disease and Orphan Drug Designated Approvals. Available from URL: https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/DrugandBiologicApprovalReports/NDAandBLAApprovalReports/UCM500987.pdf. [Google Scholar]
- 14.Matusiak Ł. Profound consequences of hidradenitis suppurativa: a review. Br J Dermatol. 2018 doi: 10.1111/bjd.16603. [DOI] [PubMed] [Google Scholar]
- 15.Damiani G, di Meo N, Marzano AV. A unique pneumopathy in a patient with skin nodules and abscesses. Intern Emerg Med. 2017;12:637–640. doi: 10.1007/s11739-017-1658-0. [DOI] [PubMed] [Google Scholar]
- 16.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Awosika O, Eleryan MG, Rengifo-Pardo M, Doherty L, Martin LW, Ehrlich A. A Case-control Study to Evaluate the Prevalence of Nonalcoholic Fatty Liver Disease Among Patients with Moderate-to-severe Psoriasis. J Clin Aesthet Dermatol. 2018;11:33–37. [PMC free article] [PubMed] [Google Scholar]
- 18.Prussick RB, Miele L. Nonalcoholic fatty liver disease in patients with psoriasis: a consequence of systemic inflammatory burden? Br J Dermatol. 2018;179:16–29. doi: 10.1111/bjd.16239. [DOI] [PubMed] [Google Scholar]
- 19.Fiore M, Leone S, Maraolo AE, Berti E, Damiani G. Liver Illness and Psoriatic Patients. Biomed Res Int. 2018;2018:3140983. doi: 10.1155/2018/3140983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cazzaniga S, Naldi L, Damiani G, Atzori L, Patta F, Guidarelli G, Bettoli V. Validation of a visual-aided questionnaire for the self-assessment of hidradenitits suppurativa. J Eur Acad Dermatol Venereol. 2018;32:1993–1998. doi: 10.1111/jdv.15050. [DOI] [PubMed] [Google Scholar]
- 21.Zouboulis CC, Del Marmol V, Mrowietz U, Prens EP, Tzellos T, Jemec GB. Hidradenitis Suppurativa/Acne Inversa: Criteria for Diagnosis, Severity Assessment, Classification and Disease Evaluation. Dermatology. 2015;231:184–190. doi: 10.1159/000431175. [DOI] [PubMed] [Google Scholar]
- 22.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 23.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 24.van der Zee HH, Jemec GB. New insights into the diagnosis of hidradenitis suppurativa: Clinical presentations and phenotypes. J Am Acad Dermatol. 2015;73:S23–S26. doi: 10.1016/j.jaad.2015.07.047. [DOI] [PubMed] [Google Scholar]
- 25.Hurley HJ. Roenigk Roenigk’s Dermatologic Surgery: Principles and Practice (Roenigk RK, Roenigk HH Jr, eds), 2nd edn. New York: Marcel Dekker, Inc; 1996. Hidradenitis suppurativa; p. 623–645. [Google Scholar]
- 26.European Hidradenitis Suppurativa Foundation Investigator Group. Zouboulis CC, Tzellos T, Kyrgidis A, Jemec GBE, Bechara FG, Ingram JR, Kanni T, Karagiannidis I, Martorell A, Pinter A, Prens EP, Presser D, Szepietowski JC, Wilden SM, Sabat R, Giamarellos-Bourboulis EJ, Schneider-Burrus S, von Stebut E, van der Zee HH, Matusiak Ł. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol. 2017;177:1401–1409. doi: 10.1111/bjd.15748. [DOI] [PubMed] [Google Scholar]
- 27.Damiani G, Della Valle V, Iannone M, Dini V, Marzano AV. Autoinflammatory Disease Damage Index (ADDI): a possible newborn also in hidradenitis suppurativa daily practice. Ann Rheum Dis. 2017;76:e25. doi: 10.1136/annrheumdis-2016-210901. [DOI] [PubMed] [Google Scholar]
- 28.Ter Haar NM, van Delft ALJ, Annink KV, van Stel H, Al-Mayouf SM, Amaryan G, Anton J, Barron KS, Benseler S, Brogan PA, Cantarini L, Cattalini M, Cochino AV, de Benedetti F, Dedeoglu F, de Jesus AA, Demirkaya E, Dolezalova P, Durrant KL, Fabio G, Gallizzi R, Goldbach-Mansky R, Hachulla E, Hentgen V, Herlin T, Hofer M, Hoffman HM, Insalaco A, Jansson AF, Kallinich T, Kone-Paut I, Kozlova A, Kuemmerle-Deschner JB, Lachmann HJ, Laxer RM, Martini A, Nielsen S, Nikishina I, Ombrello AK, Özen S, Papadopoulou-Alataki E, Quartier P, Rigante D, Russo R, Simon A, Trachana M, Uziel Y, Ravelli A, Schulert G, Gattorno M, Frenkel J. In silico validation of the Autoinflammatory Disease Damage Index. Ann Rheum Dis. 2018;77:1599–1605. doi: 10.1136/annrheumdis-2018-213725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 30.Esposito S, Noviello S, Leone S. Skin and soft tissue infections: current therapeutic options. Infez Med. 2008;16:65–73. [PubMed] [Google Scholar]
- 31.Esposito S, Leone S, Petta E, Noviello S, Iori I. [Skin and soft tissue infections: classification and epidemiology] Infez Med. 2009;17 Suppl 4:6–17. [PubMed] [Google Scholar]
- 32.Esposito S, Leone S, Noviello S, Ianniello F. [Analysis of current guidelines for the treatment of skin and soft tissue infections] Infez Med. 2009;17 Suppl 4:58–63. [PubMed] [Google Scholar]
- 33.Guet-Revillet H, Coignard-Biehler H, Jais JP, Quesne G, Frapy E, Poirée S, Le Guern AS, Le Flèche-Matéos A, Hovnanian A, Consigny PH, Lortholary O, Nassif X, Nassif A, Join-Lambert O. Bacterial pathogens associated with hidradenitis suppurativa, France. Emerg Infect Dis. 2014;20:1990–1998. doi: 10.3201/eid2012.140064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esposito S, Noviello S, Leone S. Epidemiology and microbiology of skin and soft tissue infections. Curr Opin Infect Dis. 2016;29:109–115. doi: 10.1097/QCO.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 35.Guet-Revillet H, Jais JP, Ungeheuer MN, Coignard-Biehler H, Duchatelet S, Delage M, Lam T, Hovnanian A, Lortholary O, Nassif X, Nassif A, Join-Lambert O. The Microbiological Landscape of Anaerobic Infections in Hidradenitis Suppurativa: A Prospective Metagenomic Study. Clin Infect Dis. 2017;65:282–291. doi: 10.1093/cid/cix285. [DOI] [PubMed] [Google Scholar]
- 36.Esposito S, Bassetti M, Borre' S, Bouza E, Dryden M, Fantoni M, Gould IM, Leoncini F, Leone S, Milkovich G, Nathwani D, Segreti J, Sganga G, Unal S, Venditti M Italian Society of Infectious Tropical Diseases; International Society of Chemotherapy. Diagnosis and management of skin and soft-tissue infections (SSTI): a literature review and consensus statement on behalf of the Italian Society of Infectious Diseases and International Society of Chemotherapy. J Chemother. 2011;23:251–262. doi: 10.1179/joc.2011.23.5.251. [DOI] [PubMed] [Google Scholar]
- 37.Bettoli V, Join-Lambert O, Nassif A. Antibiotic Treatment of Hidradenitis Suppurativa. Dermatol Clin. 2016;34:81–89. doi: 10.1016/j.det.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Leone S, Noviello S, Boccia G, De Caro F, Esposito S. Methicillin-resistant Staphylococcus aureus infections: role of daptomycin/β-lactams combination. Infez Med. 2015;23:99–104. [PubMed] [Google Scholar]
- 39.Esposito S, Noviello S, Leone S. Dalbavancin for the treatment of acute bacterial skin and skin structure infections. Infez Med. 2015;23:313–317. [PubMed] [Google Scholar]
- 40.Noviello S, Ianniello F, Leone S, Fiore M, Esposito S. In vitro activity of tigecycline: MICs, MBCs, time-kill curves and post-antibiotic effect. J Chemother. 2008;20:577–580. doi: 10.1179/joc.2008.20.5.577. [DOI] [PubMed] [Google Scholar]
- 41.Ingram JR, Collier F, Brown D, Burton T, Burton J, Chin MF, Desai N, Goodacre TEE, Piguet V, Pink AE, Exton LS, Mohd Mustapa MF. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol. 2018 doi: 10.1111/bjd.17537. [DOI] [PubMed] [Google Scholar]
- 42.Esposito S, Leone S, Noviello S, Ianniello F, Russo M, Foti G, Carpentieri MS, Cellesi C, Zanelli G, Cellini A, Girmenia C, De Lalla F, Maiello A, Maio P, Acone N, Marranconi F, Sabbatani S, Pantaleoni M, Ghinelli F, Soranzo ML, Vigano P, Re T, Viale P, Scudeller L, Registro Nazionale OPAT. Outpatient parenteral antibiotic therapy in the elderly: an Italian observational multicenter study. J Chemother. 2009;21:193–198. doi: 10.1179/joc.2009.21.2.193. [DOI] [PubMed] [Google Scholar]
- 43.Esposito S, Leone S, Petta E, Noviello S, Ianniello F. Treatment options for skin and soft tissue infections caused by meticillin-resistant Staphylococcus aureus:oralvs.parenteral; home vs. hospital. Int J Antimicrob Agents. 2009;34 Suppl 1:S30–S35. doi: 10.1016/S0924-8579(09)70547-3. [DOI] [PubMed] [Google Scholar]
- 44.Ippolito G, Leone S, Lauria FN, Nicastri E, Wenzel RP. Methicillin-resistant Staphylococcus aureus: the superbug. Int J Infect Dis. 2010;14 Suppl 4:S7–11. doi: 10.1016/j.ijid.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Fiore M, Taccone FS, Leone S. Choosing the appropriate pharmacotherapy for multidrug-resistant Gram positive infections. Expert Opin Pharmacother. 2018;19:1517–1521. doi: 10.1080/14656566.2018.1512584. [DOI] [PubMed] [Google Scholar]
- 46.Leone S, Rossi M, Bisi L, Gori A, Esposito S. Letter: antibiotic dose adjustment in patients with advanced liver disease. Aliment Pharmacol Ther. 2013;38:561–562. doi: 10.1111/apt.12411. [DOI] [PubMed] [Google Scholar]
- 47.Leone S, Bisi L, Rossi M, Gori A. Comment on "Management of infections in cirrhotic patients: report of a consensus conference" S Fagiuoli et al. [Dig liver dis 2014;46:204-212] Dig Liver Dis. 2014;46:573–574. doi: 10.1016/j.dld.2014.01.155. [DOI] [PubMed] [Google Scholar]
- 48.Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, Clark JM. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082–1090. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]