Abstract
Emerging evidence suggests that the intestinal microbiota is a source of sleep-promoting signals. Bacterial metabolites and components of the bacterial cell wall are likely to provide important links between the intestinal commensal flora and sleep-generating mechanisms in the brain. Butyrate is a short-chain fatty acid produced by the intestinal bacteria by the fermentation of nondigestible polysaccharides. We tested the hypothesis that butyrate may serve as a bacterial-derived sleep-promoting signal. Oral gavage administration of tributyrin, a butyrate pro-drug, elicited an almost 50% increase in non-rapid-eye movement sleep (NREMS) in mice for 4 hours after the treatment. Similarly, intraportal injection of butyrate led to prompt and robust increases in NREMS in rats. In the first 6 hours after the butyrate injection, NREMS increased by 70%. Both the oral and intraportal administration of butyrate led to a significant drop in body temperature. Systemic subcutaneous or intraperitoneal injection of butyrate did not have any significant effect on sleep or body temperature. The results suggest that the sleep-inducing effects of butyrate are mediated by a sensory mechanism located in the liver and/or in the portal vein wall. Hepatoportal butyrate-sensitive mechanisms may play a role in sleep modulation by the intestinal microbiota.
Subject terms: Sleep, Circadian regulation
Introduction
Sleep is greatly affected by peripheral metabolic signals, such as satiety and orexigenic hormones1–3, increased lipolysis4,5, systemic pro-inflammatory signals6, activation of brown adipose tissue7 or the liver8. Recent evidence points to the importance of the intestinal microbiota in metabolic signaling (reviewed in9). Microbiota-derived signals modulate complex brain-related functions and various behaviors (reviewed in10).
The brain sleep mechanisms and the gut flora are linked through a dynamic bidirectional relationship. Depletion of intestinal microbiota induces significant reduction in sleep suggesting that the gut flora is a source of sleep-inducing signals11,12, while circadian disruption and chronic sleep fragmentation promote intestinal dysbiosis13,14. Cell wall components of bacteria induce sleep when injected systemically15–18 suggesting that fragments of disintegrating intestinal bacteria, once translocated into the portal circulation, could serve in sleep signaling. In addition to the cell wall fragments, live intestinal bacteria are also a source of biologically active metabolites, such as short-chain fatty acids (SCFAs), secondary bile acids, indole-derivatives, succinate or hormones and neurotransmitters (reviewed in19). The role of metabolites produced by live bacteria in sleep regulation is, however, poorly understood.
Butyrate, a SCFA, is a product of anaerobic bacterial fermentation of non-digestible carbohydrates in the hindgut, a major metabolic product of the clostridial clusters of intestinal flora20. Mammalian cells do not produce significant amounts of butyrate, the only significant sources are the microbiota and ingestion of dairy products21,22. Butyrate binds to the FFAR2, FFAR3 and GPR109A receptors23–27, and it also acts as a histone deacetylase inhibitor and affects gene transcription28. It is readily absorbed into the portal circulation and transported directly to the liver29. The liver represents a major sink for intestinally-produced butyrate as evidenced by the steep concentration gradient between portal and systemic levels of butyrate29–31. All three butyrate receptors are expressed in the liver23,32,33.
We hypothesized that intestinally-produced butyrate acts on hepatoportal sensory mechanisms to promote sleep. To test this, we investigated the effects of oral administration, direct intraportal injection, as well as systemic injection of butyrate and tributyrin, a butyrate-yielding pro-drug, in mice and rats. Our results demonstrate that oral and intraportal administration of butyrate induces robust increases in non-rapid-eye movement sleep (NREMS), while systemic butyrate treatment has no effect on sleep. These findings indicate the existence of a butyrate-sensitive hepatoportal sleep-inducing sensory mechanism.
Results
Oral gavage administration of tributyrin
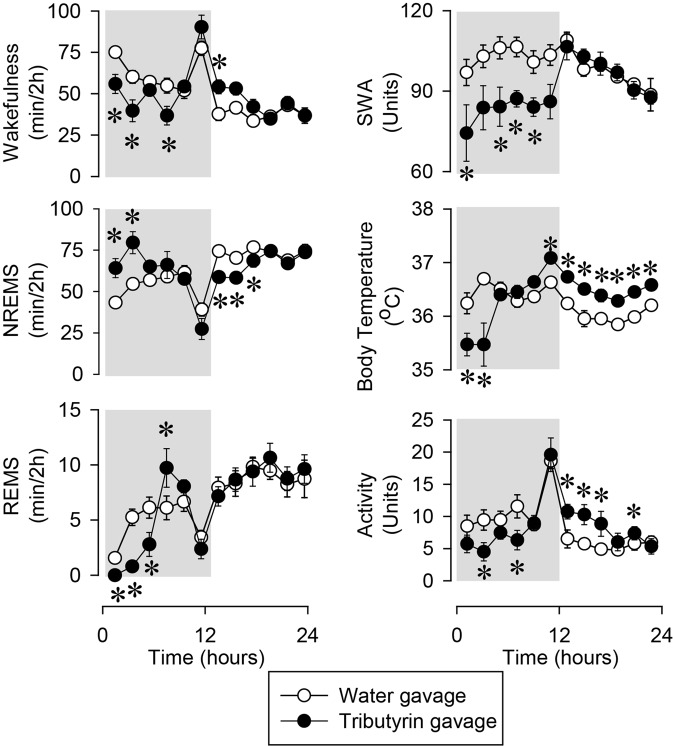
Oral gavage administration of tributyrin at the beginning of the dark phase elicited robust sleep responses in mice (Fig. 1, Table 1). In the first four hours after the treatment, time spent in NREMS increased by 47% above baseline at the expense of rapid-eye movement sleep (REMS) and wakefulness (NREMS baseline: 97.9 ± 3.3 min/4 h, tributyrin: 143.7 ± 10.0 min/4 h, p < 0.01; REMS baseline: 6.8 ± 1.0 min/4 h, tributyrin: 0.8 ± 0.3 min/4 h, p < 0.001). The increases in NREMS time were due to significantly longer NREMS episodes, while REMS decreases were the consequence of a significant decrease in the number of REMS episodes [average episode numbers in the first 6 h on the baseline day: NREMS 34 ± 1.7, REMS 9 ± 1.1; after tributyrin treatment: NREMS 32 ± 3.2, REMS 2 ± 0.7 (vs. baseline p < 0.001); average episode durations in the first 6 h on the baseline day: NREMS 273 ± 14.2 s, REMS 89 ± 5.7 s; after tributyrin treatment: NREMS 407 ± 40.9 min (vs. baseline p < 0.001), REMS 66 ± 15.5 s]. Sleep latency was not affected (baseline: 18.3 ± 4.0 min, tributyrin: 21.3 ± 3.0 min).
Figure 1.
The effects of oral administration of tributyrin on wakefulness, non-rapid-eye movement sleep (NREMS), rapid-eye movement sleep (REMS), electroencephalographic (EEG) slow-wave activity (SWA) motor activity and body temperature. Data are presented in 2-h time blocks; shaded area represents the dark period. Time “0”: time of the treatments. Asterisks: significant difference from baseline, Tukey’s HSD test; error bar: SE.
Table 1.
Oral administration of tributyrin.
| NREMS | REMS | Temperature | Activity | SWA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | df | F | p | df | F | p | |
| Treatment | 1,7 | 0.7 | n.s. | 1,7 | 1.4 | n.s. | 1,7 | 2.4 | n.s. | 1,7 | 0.0 | n.s. | 1,7 | 14.6 | <0.01 |
| Time | 11,77 | 15.12 | <0.001 | 11,77 | 18.3 | <0.001 | 11,77 | 7.5 | <0.001 | 11,77 | 11.7 | <0.001 | 11,77 | 2.7 | <0.01 |
| Treatment x Time | 11,77 | 6.1 | <0.001 | 11,77 | 4.0 | <0.001 | 11,77 | 12.8 | <0.001 | 11,77 | 3.2 | <0.01 | 11,77 | 3.4 | <0.001 |
Non-rapid eye movement sleep (NREMS), rapid-eye movement sleep (REMS), body temperature, motor activity and electroencephalographic slow-wave activity (SWA): statistical results.
The NREMS increase was accompanied by a 0.8–1.2 °C drop in body temperature and greatly suppressed electroencephalographic slow-wave activity (EEG SWA) and motor activity. Subsequently, there was a short rebound increase in REMS during the second half of the dark, and a decrease in NREMS in the first half of the light period. Body temperature and activity were slightly, but significantly, elevated in the light period.
Intraportal administration of sodium butyrate
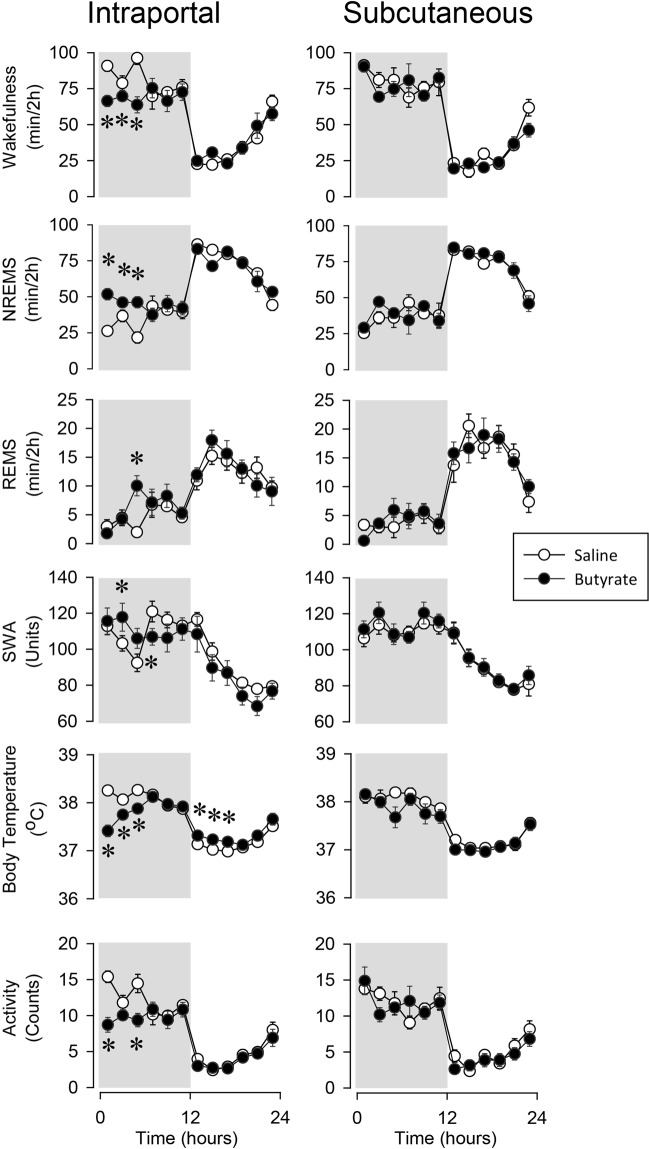
Intraportal injection of butyrate induced prompt and robust NREMS increases in rats (Fig. 2, Table 2). Sleep latency decreased from the baseline value of 36.1 ± 6.7 to 2.7 ± 2.0 min after butyrate treatment (p < 0.01). Overall, NREMS increased by 70% in the first 6 hours after the butyrate injection (baseline: 84.6 ± 6.7 min/6 h, butyrate: 144.0 ± 4.2 min/6 h). The animals showed the behavioral signs of normal sleep, they were easily arousable, and actively engaged with their environment in response to mild tactile or auditory stimuli. The NREMS response was due to the increased duration of NREMS episodes, while the number of sleep episodes was not affected (average NREMS episode duration in the first 6 h on the baseline day: 186 ± 16.2 s, after butyrate treatment: 301 ± 17.4 s, p < 0.001; number of NREMS episodes in the first 6 h on the baseline day: 28 ± 2.7, after butyrate treatment: 29 ± 1.9).
Figure 2.
The effects of intraportal and subcutaneous administration of butyrate on wakefulness, NREMS, REMS, EEG SWA, motor activity and body temperature. See legend to Fig. 1 for details.
Table 2.
Intra-portal administration of butyrate.
| NREMS | REMS | Temperature | Activity | SWA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | df | F | p | df | F | p | |
| Treatment | 1,7 | 67.7 | <0.001 | 1,7 | 7.8 | <0.05 | 1,8 | 2.0 | n.s. | 1,7 | 0.2 | n.s. | 1,7 | 0.2 | n.s. |
| Time | 11,77 | 38.6 | <0.001 | 11,77 | 10.2 | <0.001 | 11,88 | 54.0 | <0.001 | 11,77 | 37.2 | <0.001 | 11,77 | 37.2 | <0.001 |
| Treatment x Time | 11,77 | 4.3 | <0.001 | 11,77 | 2.5 | <0.01 | 11,88 | 14.0 | <0.001 | 11,77 | 3.7 | <0.001 | 11,77 | 3.7 | <0.001 |
NREMS, REMS, body temperature, motor activity and EEG SWA: statistical results.
REMS also increased after butyrate treatment as indicated by significant treatment and treatment x time interactions in ANOVA; post hoc analysis revealed significant REMS increase in the 5–6 h time block. REMS increase was due to the combined effects of slightly elevated episode numbers and episode durations; neither change, by itself, was significant (average REMS episode duration in the first 6 h on the baseline day: 79 ± 9.7 s, after butyrate treatment: 95 ± 8.0 s; number of REMS episodes in the first 6 h on the baseline day: 6 ± 1.3, after butyrate treatment: 10 ± 1.4).
Motor activity decreased by 39% and body temperature by 0.4–1 °C in the first 6 h. The effects on EEG SWA were biphasic. In the first 6 h after the butyrate injection, there was a tendency towards increased SWA, which was followed by a slight, but significant decline below baseline.
Systemic administration of sodium butyrate
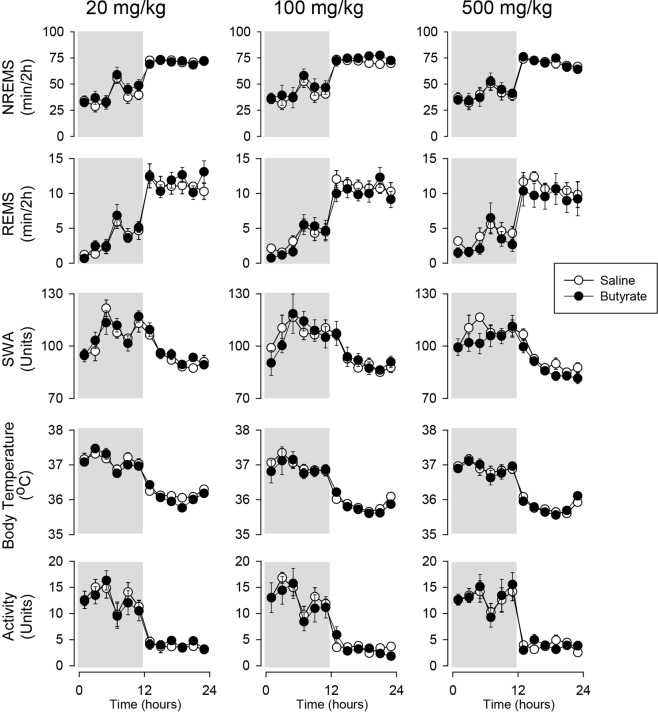
Systemic injection of butyrate did not have any significant effects on sleep, EEG SWA, body temperature and motor activity. In rats, subcutaneous injection of 1 g/kg, the same dose that promotes sleep after intraportal administration, did not affect any of the measured parameters (Fig. 2, Table 3). Similarly, in mice, intraperitoneal injection of 0.02, 0.1 and 0.5 g/kg butyrate was void of any significant effects (Fig. 3, Table 4).
Table 3.
Subcutaneous administration of butyrate.
| NREMS | REMS | Temperature | Activity | SWA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | df | F | p | df | F | p | |
| Treatment | 1,4 | 0.2 | n.s. | 1,4 | 2.1 | n.s. | 1,4 | 6.6 | 0.06 | 1,4 | 1.4 | n.s. | 1,4 | 0.4 | n.s. |
| Time | 11,44 | 42.9 | <0.001 | 11,44 | 18.1 | <0.001 | 11,44 | 53.1 | <0.001 | 11,44 | 21.1 | <0.001 | 11,44 | 16.6 | <0.001 |
| Treatment x Time | 11,44 | 1.0 | n.s. | 11,44 | 1.2 | n.s. | 11,44 | 1.6 | n.s. | 11,44 | 1.4 | n.s. | 11,44 | 0.5 | n.s. |
NREMS, REMS, body temperature, motor activity and EEG SWA: statistical results.
Figure 3.
The effects of intraperitoneal administration of butyrate on NREMS, REMS, EEG SWA, motor activity and body temperature. See legend to Fig. 1 for details.
Table 4.
Intraperitoneal administration of butyrate.
| NREMS | REMS | Temperature | Activity | SWA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | df | F | p | df | F | p | |
| 20 mg/kg | |||||||||||||||
| Treatment | 1,6 | 4.6 | n.s. | 1,6 | 1.8 | n.s. | 1,6 | 0.8 | n.s. | 1,5 | 0.9 | n.s. | 1,7 | 0.5 | n.s. |
| Time | 11,66 | 26.4 | <0.001 | 11,66 | 47.1 | <0.001 | 11,66 | 60.7 | <0.001 | 11,55 | 17.1 | <0.001 | 11,77 | 14.2 | <0.001 |
| Treatment x Time | 11,66 | 0.8 | n.s. | 11,66 | 0.8 | n.s. | 11,66 | 1.8 | n.s. | 11,55 | 0.4 | n.s. | 11,77 | 0.8 | n.s. |
| 100 mg/kg | |||||||||||||||
| Treatment | 1,6 | 2.1 | n.s. | 1,6 | 1.8 | n.s. | 1,6 | 0.3 | n.s. | 1,6 | 0.6 | n.s. | 1,5 | 0.0 | n.s. |
| Time | 11,66 | 19.8 | <0.001 | 11,66 | 26.4 | <0.001 | 11,66 | 43.5 | <0.001 | 11,66 | 33.6 | <0.001 | 11,55 | 7.7 | <0.001 |
| Treatment x Time | 11,66 | 0.4 | n.s. | 11,66 | 1.1 | n.s. | 11,66 | 0.5 | n.s. | 11,66 | 0.6 | n.s. | 11,55 | 0.8 | n.s. |
| 500 mg/kg | |||||||||||||||
| Treatment | 1,6 | 0.0 | n.s. | 1,6 | 1.5 | n.s. | 1,6 | 0.2 | n.s. | 1,6 | 0.0 | n.s. | 1,5 | 3.9 | n.s. |
| Time | 11,66 | 1.1 | n.s. | 11,66 | 6.5 | <0.001 | 11,66 | 36.5 | <0.001 | 11,66 | 18.1 | <0.001 | 11,55 | 9.4 | <0.001 |
| Treatment x Time | 11,66 | 0.6 | n.s. | 11,66 | 1.8 | n.s. | 11,66 | 1.3 | n.s. | 11,66 | 0.3 | n.s. | 11,55 | 0.8 | n.s. |
NREMS, REMS, body temperature, motor activity and EEG SWA: statistical results.
Discussion
Evidence suggests that the gut bacteria are a source of sleep-inducing signals11,12, and we hypothesized that SCFAs may serve as such signal. Our major finding is that orally or intraportally administered tributyrin and butyrate, respectively, robustly increases NREMS in rats and mice. These observations are consistent with prior reports that intravenous injection of butyrate induces slow, high-amplitude EEG waves and behavioral signs of sleep in rabbits34 or EEG-defined NREMS in cats35.
Butyrate is a four-carbon SCFA, produced by the microbiota in mice and rats. It is the product of the anaerobic fermentation of non-digestible carbohydrates by gut bacteria and also a component of dairy products, such as butter, milk and cheese36. Tributyrin is an ester, composed of three butyric acid molecules and glycerol. It is considered a pro-drug to deliver biologically active butyrate as lipases in the host organism hydrolyze it resulting in the release of butyrate37. SCFAs, including butyrate, are readily absorbed from the intestines into the portal circulation and directly reach the liver29. Plasma levels of butyric acid in the portal circulation after oral administration of tributyrin are higher and more prolonged without detectable toxicity in mice and rats as compared to administration of butyrate itself38. To mimic the effects of intestinally produced butyrate, we administered tributyrin orally to mice. This treatment elicited an almost 50% increase in NREMS in the first 4 h supporting the notion that butyrate from the intestinal tract may potentially serve as a sleep-inducing signal molecule. NREMS increased at the expense of both REMS and wakefulness. REMS suppression may be due to the mutual inhibitory interaction between NREMS- and REMS-promoting mechanisms39 or it could be a NREMS-independent effect of tributyrin. The treatments were administered at dark onset. Since the latency to increased sleep is very short and the duration of the sleep increases did not exceed 8 hours, the effects of butyrate on sleep were manifested predominantly during the dark, active, phase.
Sleep-inducing doses of butyrate also elicited a 0.4–1.2 °C drop in body temperature. Since naturally occurring NREMS is associated by decreased energy expenditure and body temperature (reviewed in40), it is possible that the slight hypothermic response is simply the thermic manifestation of enhanced NREMS after butyrate treatment. It has been proposed that a drop in core body temperature prompts sleepiness41, thus an alternative interpretation is also possible, i.e., an initial drop in body temperature in response to butyrate may invoke the sleep responses.
Baseline sleep recordings were performed on the day before the butyrate treatment. In thoroughly-habituated rats and mice, such as our experimental animals, sleep and body temperature are remarkably stable across two successive days. This is evidenced, for example, by the experiments where mice received ip injection of saline on day 1 and butyrate on the following day (Fig. 3). Thus, it is highly unlikely that the observed sleep-promoting effects of intraportally or orally administer butyrate are confounded by order of the treatments.
There is a steep concentration gradient between the high portal levels of butyrate and very low butyrate concentration in the systemic circulation29,30,42,43, and orally administered tributyrin increases portal, but not systemic, levels of butyrate44. These observations indicate that the liver removes almost all butyrate from the portal blood31. Thus, the most likely target for butyrate to induce sleep is the hepatoportal system. To investigate this possibility, we injected butyrate directly into the portal vein in rats. Intra-portal butyrate treatment greatly reduced NREMS latency and increased the time spent in NREMS. To investigate, if a potential butyrate overflow from the liver into the systemic circulation could be responsible for the sleep effects, we injected the same amount of butyrate systemically. Systemic administration of butyrate did not have any effect on sleep in rats. Similarly, none of the systemically-administered doses of butyrate had any effect on sleep-wake activity in mice. These findings indicate that the sleep effects of orally or intraportally administered butyrate are not due to the actions of butyrate that possibly escaped the hepatic sink. We conclude that butyrate acts on the liver and/or the portal vein to promote NREMS. There is prior evidence that the liver is involved in peripheral sleep signaling, since local warming of the liver increases NREMS8, and depletion of liver Kupffer cells impairs recovery sleep responses after sleep loss and sleep in a cold environment45.
Hepatoportal sensors have been described for several gut-derived molecules, e.g., glucose, cholecystokinin, and amino acids46–48. They are located in the wall of the portal vein and the liver and have been implicated in the regulation of glucose and energy homeostasis (reviewed in49). There is also evidence for hepatoportal SCFA sensors. Butyrate receptors are present in the hepatoportal region. Butyrate signals through the receptors FFAR2, FFAR3 and GPR109A, all of which is expressed in the liver32,33,50, thus may serve as hepatic sensors. FFAR3 is also expressed by the portal vein wall in close proximity to neuronal markers51. The activation of these receptors affects brain circuits as evidenced by the observation that the effects of dietary SCFAs on the activity of the nucleus tractus solitarius and parabrachial nucleus are abolished by the selective sensory denervation of the periportal area51. The sensory innervation of the hepatoportal region is provided by the vagus and spinal afferents, both of which have been implicated in sleep signaling52–55. Butyrate directly activates vagal afferents56 and the effects of butyrate on feeding is suppressed by hepatic vagotomy57.
Several bacterial-derived sleep-inducing molecules, such as lipopolysaccharide and fragments of peptidoglycans, have been described before (reviewed in58). All these molecules are components of the bacterial cell wall, they are released from disintegrating bacteria or from bacteria during cell division. They have pronounced inflammatory actions via the stimulation of the production of pro-inflammatory cytokines (reviewed in59). Sleep responses to systemic bacterial infection are linked to these pro-inflammatory processes. The properties of butyrate are, however, fundamentally different. Butyrate is produced by live bacteria in the intestines, and it has strong anti-inflammatory properties. It suppresses colonic and liver inflammation and lipopolysaccharide-induced production of pro-inflammatory cytokines and NF-κB activation26,60–63. This indicates that not only systemic pro-inflammatory signals related to bacterial infections, but also bacterial-derived anti-inflammatory signals from the intestinal tract have the potential to modulate sleep.
Methods
Animals
Male Sprague-Dawley rats and breeding pairs of C57BL/6 J mice were purchased from The Jackson Laboratories, Inc.; the mice were further bred at Washington State University. During the experiments, the animals were housed in temperature-controlled (mice: 30 ± 1 °C, rats: 23 ± 1 °C), sound-attenuated isolation chambers on a 12:12-hour light-dark cycle (lights on at 3 AM). Food and water were available ad libitum throughout all experiments. Animals were provided regular lab chow (Harlan Teklad, Product no. 2016), in which fats, proteins, and carbohydrates comprise 12%, 22%, and 66% of calories, respectively. All animal procedures were conducted in compliance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All animal protocols were approved by the Institutional Animal Care and Use Committees at Washington State University.
Surgery
All surgical procedures were performed using ketamine-xylazine anesthesia (87 and 13 mg/kg, respectively). For sleep-wake activity recordings, 3-month old mice (25.5 ± 0.9 g) and rats (325–350 g) were implanted with three cortical EEG electrodes, placed over the frontal and parietal cortices, and two nuchal electromyographic (EMG) electrodes. The EEG and EMG electrodes were anchored to the skull with dental cement. Telemetry transmitters were implanted intraperitoneally for body temperature and motor activity recordings. In addition, the rats were implanted with an intraportal cannula64 three weeks prior to the sleep surgery. Briefly, a biocompatible polyurethane was inserted into the superior mesenteric vein and the tip of the cannula routed to the main stream of the portal vein. The free end of the cannula was routed subcutaneously to the dorsal surface of the neck and exteriorized. The cannula was sutured to the portal vein, the abdominal muscles and the neck skin. The patency was maintained by daily flushing with 0.2 ml isotonic saline followed by 0.08 ml of lock solution containing 500 IU/ml heparin in 50% glycerol solution. The animals were allowed to recover from surgery for at least 10 days before any experimental manipulation started and handled daily to adapt them to the experimental procedures.
Sleep-wake activity recordings and analyses
The animals were tethered to commutators, which were further routed to Grass Model 15 Neurodata amplifier system (Grass Instrument Division of Astro-Med, Inc., West Warwick, RI). The amplified EEG and EMG signals were digitized at 256 Hz and recorded by computer. The high-pass and low-pass filters for EEG signals were 0.5 and 30.0 Hz, respectively. The EMG signals were filtered with low and high cut-off frequencies at 100 and 10,000 Hz, respectively. The outputs from the 12A5 amplifiers were fed into an analog-to-digital converter and collected by computer using Sleep Wave software (Biosoft Studio, Hersey, PA). Sleep-wake states were scored visually off-line in 10-s segments. The vigilance states were defined as NREMS, REMS and wakefulness according to standard criteria as described previously1. EEG power data from each artifact free 10-s segment were subjected to off-line spectral analysis by fast Fourier transformation. EEG power data in the range of 0.5 to 4.0 Hz during NREMS were used to compute EEG SWA. EEG SWA data were normalized for each animal by using the average EEG SWA across 24 h on the baseline day as 100.
Telemetry recordings
Core body temperature and locomotor activity were recorded by MiniMitter telemetry system (Starr Life Sciences Corp.) using VitalView software. Temperature and activity values were collected every 1 and 10 min, respectively, throughout the experiment and were averaged over 2-h time blocks.
Experimental procedures
Experiment 1: The effects of oral gavage administration of tributyrin in mice
Eight mice were habituated to the gavage procedure by administering 0.3 ml water for 7 days 5–15 min before dark onset. After the habituation period, a baseline day was recorded after the oral gavage of 0.3 ml water. This treatment controls for the non-specific effects of the gavage administration, such as changes in the gastric volume. The following day, 0.3 ml tributyrin was administered (Millipore Sigma). The treatments were performed 5–10 min before dark onset. Sleep and telemetric recordings started at dark onset and continued for 23.5 h.
Experiment 2: The effects of intraportal administration of sodium butyrate in rats
Ten rats were habituated to the injection procedure by daily flushing of the cannula with isotonic saline 5–20 min before dark onset. On the baseline day, 2 ml/kg isotonic NaCl (vehicle) was administered through the cannula. On the test day, the animals received 1 g/kg sodium butyrate, dissolved in isotonic NaCl, in a volume of 2 ml/kg. The pH of the butyrate solution was set to 7.4 by using NaOH. The treatments were performed 5–20 min before dark onset. Sleep and telemetric recordings started at dark onset and continued for 23.5 h. Due to the malfunction of some of the implants, EEG/EMG was obtained only from 8 animals, and body temperature from 9 rats.
Experiment 3: The effects of subcutaneous administration of sodium butyrate in rats
Ten days after Experiment 2, five rats were used again to test the effects of sc administration of 1 g/kg butyrate. The animals were habituated to the treatment by daily sc administration of isotonic saline. On the baseline day, the animals were injected sc with 2 ml/kg isotonic NaCl (vehicle). On the test day, the animals received 1 g/kg buffered sodium butyrate subcutaneously in a volume of 2 ml/kg. The treatments took place 5–10 min before dark onset. Sleep and telemetric recordings started at dark onset and continued for 23.5 h.
Experiment 4: The effects of intraperitoneal administration of sodium butyrate in mice
Three doses of butyrate were tested in the same group of mice (n = 7). After the habituation period, the animals received 10 ml/kg isotonic NaCl (vehicle) ip to obtain baseline values. On the test day, the mice were injected with 20 mg/kg sodium butyrate intraperitoneally. One week later, a new vehicle baseline day was recorded followed by the test day of 100 mg/kg butyrate. Finally, after one additional week of recovery, a third vehicle baseline and test day (500 mg/kg butyrate) were recorded. The treatments took place 5–10 min before dark onset. Sleep and telemetric recordings started at dark onset and continued for 23.5 h.
Statistics
Time spent in wakefulness, NREMS and REMS, as well as, EEG SWA, body temperature and motor activity were calculated in 2-h blocks. Two-way repeated measures ANOVA was performed across 24 h between test days and the corresponding baselines (factors: treatment and time, both repeated). When appropriate, Tukey’s HSD test was applied post hoc. An α-level of P < 0.05 was considered to be significant.
Acknowledgements
This work was supported by the National Institute of Health, National Heart, Lung, and Blood Institute, Grant Number R01HL122390, to É.S.
Author Contributions
É.S. & L.K. conceived and designed the experiments, N.S.M., A.R.M. and É.S. conducted the experiments, É.S. and L.K. analyzed the data. All authors reviewed the manuscript.
Data Availability
All data generated or analyzed during this study are included in this published article.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shemyakin A, Kapás L. L-364,718, a cholecystokinin-A receptor antagonist, suppresses feeding-induced sleep in rats. Am. J. Physiol. Regul. Integr. Comp. Physio.l. 2001;280:R1420–R1426. doi: 10.1152/ajpregu.2001.280.5.R1420. [DOI] [PubMed] [Google Scholar]
- 2.Szentirmai É, Hajdu I, Obal F, Jr., Krueger JM. Ghrelin-induced sleep responses in ad libitum fed and food-restricted rats. Brain Res. 2006;1088:131–140. doi: 10.1016/j.brainres.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 3.Szentirmai É, Kapás L, Krueger JM. Ghrelin microinjection into forebrain sites induces wakefulness and feeding in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R575–R585. doi: 10.1152/ajpregu.00448.2006. [DOI] [PubMed] [Google Scholar]
- 4.Danguir J, Nicolaidis S. Circadian sleep and feeding patterns in the rat: possible dependence on lipogenesis and lipolysis. Am. J. Physiol. 1980;238:E223–E230. doi: 10.1152/ajpendo.1980.238.3.E223. [DOI] [PubMed] [Google Scholar]
- 5.Szentirmai É, Kapás L. The role of the brown adipose tissue in β3-adrenergic receptor activation-induced sleep, metabolic and feeding responses. Sci. Rep. 2017;7:958. doi: 10.1038/s41598-017-01047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szentirmai É, Kapás L. Brown adipose tissue plays a central role in systemic inflammation-induced sleep responses. PLoS. One. 2018;13:e0197409. doi: 10.1371/journal.pone.0197409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szentirmai É, Kapás L. Intact brown adipose tissue thermogenesis is required for restorative sleep responses after sleep loss. Eur. J. Neurosci. 2014;39:984–998. doi: 10.1111/ejn.12463. [DOI] [PubMed] [Google Scholar]
- 8.El Hajjaji FZ, et al. Sleep structure and feeding pattern changes induced by the liver’s thermal status in the rat. J. Sleep Res. 2012;21:204–211. doi: 10.1111/j.1365-2869.2011.00973.x. [DOI] [PubMed] [Google Scholar]
- 9.Heiss CN, Olofsson LE. Gut microbiota-dependent modulation of energy metabolism. J. Innate Immun. 2018;10:163–171. doi: 10.1159/000481519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arneth BM. Gut-brain axis biochemical signalling from the gastrointestinal tract to the central nervous system: gut dysbiosis and altered brain function. Postgrad. Med. J. 2018;94:446–452. doi: 10.1136/postgradmedj-2017-135424. [DOI] [PubMed] [Google Scholar]
- 11.Brown R, Price RJ, King MG, Husband AJ. Are antibiotic effects on sleep behavior in the rat due to modulation of gut bacteria? Physiol. Behav. 1990;48:561–565. doi: 10.1016/0031-9384(90)90300-S. [DOI] [PubMed] [Google Scholar]
- 12.Millican, N. S., Massie, A. R., Szentirmai, É. & Kapás, L. The effects of antibiotic-induced but-microbiome depletion on sleep in mice. Program No. 596.16.2018 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2018. Online (2018).
- 13.Poroyko VA, et al. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci. Rep. 2016;6:35405. doi: 10.1038/srep35405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voigt RM, et al. The circadian clock mutation promotes intestinal dysbiosis. Alcohol Clin. Exp. Res. 2016;40:335–347. doi: 10.1111/acer.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadlecova O, Anochina IP, Bauer V, Masek K, Raskova H. Effect of Escherichia coli endotoxin on temperature and sleep cycles of rats. J. Infect. Dis. 1972;126:179–181. doi: 10.1093/infdis/126.2.179. [DOI] [PubMed] [Google Scholar]
- 16.Szentirmai É, Krueger JM. Sickness behaviour after lipopolysaccharide treatment in ghrelin deficient mice. Brain Behav. Immun. 2014;36:200–206. doi: 10.1016/j.bbi.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadlecova O, Masek K, Raskova H, Rotta J. Fever and sleep cycle impairment after streptococcal mucopeptide administration. Toxicon. 1972;10:473–477. doi: 10.1016/0041-0101(72)90172-9. [DOI] [PubMed] [Google Scholar]
- 18.Krueger JM, Pappenheimer JR, Karnovsky ML. Sleep-promoting effects of muramyl peptides. Proc. Natl. Acad. Sci. USA. 1982;79:6102–6106. doi: 10.1073/pnas.79.19.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholson JK, et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 20.Macfarlane, G. T. & Gibson, G. R. Carbohydrate fermentation, energy transduction and gas metabolism in the human large intestine. In: Mackie, R., White, B.A. (Eds), Gastrointestinal Microbiology, pp. 269–318, (Springer US, 1997).
- 21.Hoverstad T, Midtvedt T. Short-chain fatty acids in germfree mice and rats. J. Nutr. 1986;116:1772–1776. doi: 10.1093/jn/116.9.1772. [DOI] [PubMed] [Google Scholar]
- 22.Wichmann A, et al. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host. Microbe. 2013;14:582–590. doi: 10.1016/j.chom.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Brown AJ, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 24.Le Poul E, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson NE, Kotarsky K, Owman C, Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun. 2003;303:1047–1052. doi: 10.1016/S0006-291X(03)00488-1. [DOI] [PubMed] [Google Scholar]
- 26.Thangaraju M, et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009;69:2826–2832. doi: 10.1158/0008-5472.CAN-08-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taggart AK, et al. D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 2005;280:26649–26652. doi: 10.1074/jbc.C500213200. [DOI] [PubMed] [Google Scholar]
- 28.Candido EP, Reeves R, Davie JR. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978;14:105–113. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- 29.Rémésy C, Demigné C. Changes in availability of glucogenic and ketogenic substrates and liver metabolism in fed or starved rats. Ann. Nutr. Metab. 1983;27:57–70. doi: 10.1159/000176624. [DOI] [PubMed] [Google Scholar]
- 30.Rémésy C, Demigné C, Chartier F. Origin and utilization of volatile fatty acids in the rat. Reprod. Nutr. Dev. 1980;20:1339–1349. doi: 10.1051/rnd:19800725. [DOI] [PubMed] [Google Scholar]
- 31.Demigne C, Yacoub C, Remesy C. Effects of absorption of large amounts of volatile fatty acids on rat liver metabolism. J. Nutr. 1986;116:77–86. doi: 10.1093/jn/116.1.77. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Millar JS, Brownell N, Briand F, Rader DJ. Modulation of HDL metabolism by the niacin receptor GPR109A in mouse hepatocytes. Biochem. Pharmacol. 2010;80:1450–1457. doi: 10.1016/j.bcp.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilkes JJ, Lloyd DJ, Gekakis N. Loss-of-function mutation in myostatin reduces tumor necrosis factor alpha production and protects liver against obesity-induced insulin resistance. Diabetes. 2009;58:1133–1143. doi: 10.2337/db08-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samson FE, Jr., White RP. Effects of fatty acid anions on the electroencephalogram of unanesthetized rabbits. Am. J. Physiol. 1956;186:271–274. doi: 10.1152/ajplegacy.1956.186.2.271. [DOI] [PubMed] [Google Scholar]
- 35.Matsuzaki M, Takagi H. Sleep induced by sodium butyrate in the cat. Brain Res. 1967;4:206–222. doi: 10.1016/0006-8993(67)90006-6. [DOI] [PubMed] [Google Scholar]
- 36.Stilling RM, et al. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016;99:110–132. doi: 10.1016/j.neuint.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Heidor R, Ortega JF, de Conti A, Ong TP, Moreno FS. Anticarcinogenic actions of tributyrin, a butyric acid prodrug. Curr. Drug Targets. 2012;13:1720–1729. doi: 10.2174/138945012804545443. [DOI] [PubMed] [Google Scholar]
- 38.Schroder C, Eckert K, Maurer HR. Tributyrin induces growth inhibitory and differentiating effects on HT-29 colon cancer cells in vitro. Int. J. Oncol. 1998;13:1335–1340. doi: 10.3892/ijo.13.6.1335. [DOI] [PubMed] [Google Scholar]
- 39.Endo T, Schwierin B, Borbely AA, Tobler I. Selective and total sleep deprivation: effect on the sleep EEG in the rat. Psychiatry Res. 1997;66:97–110. doi: 10.1016/S0165-1781(96)03029-6. [DOI] [PubMed] [Google Scholar]
- 40.Parmeggiani PL. Thermoregulation and sleep. Front Biosci. 2003;8:s557–567. doi: 10.2741/1054. [DOI] [PubMed] [Google Scholar]
- 41.Gilbert SS, van den Heuvel CJ, Ferguson SA, Dawson D. Thermoregulation as a sleep signalling system. Sleep Med Rev. 2004;8:81–93. doi: 10.1016/S1087-0792(03)00023-6. [DOI] [PubMed] [Google Scholar]
- 42.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters SG, Pomare EW, Fisher CA. Portal and peripheral blood short chain fatty acid concentrations after caecal lactulose instillation at surgery. Gut. 1992;33:1249–1252. doi: 10.1136/gut.33.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyoshi M, et al. Oral administration of tributyrin increases concentration of butyrate in the portal vein and prevents lipopolysaccharide-induced liver injury in rats. Clin. Nutr. 2011;30:252–258. doi: 10.1016/j.clnu.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 45.Ames C, Boland E, Szentirmai É. Effects of Macrophage Depletion on Sleep in Mice. PLoS. One. 2016;11:e0159812. doi: 10.1371/journal.pone.0159812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niijima A. Afferent impulse discharges from glucoreceptors in the liver of the guinea pig. Nutrition. 1996;12:390, 392–390, 393. [PubMed] [Google Scholar]
- 47.Niijima A. Visceral afferents and metabolic function. Diabetologia. 1981;20(Suppl):325–330. doi: 10.1007/BF00254499. [DOI] [PubMed] [Google Scholar]
- 48.Niijima A, Meguid MM. An electrophysiological study on amino acid sensors in the hepato-portal system in the rat. Obes. Res. 1995;3(Suppl 5):741S–745S. doi: 10.1002/j.1550-8528.1995.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 49.Thorens B, Larsen PJ. Gut-derived signaling molecules and vagal afferents in the control of glucose and energy homeostasis. Curr. Opin. Clin. Nutr. Metab. Care. 2004;7:471–478. doi: 10.1097/01.mco.0000134368.91900.84. [DOI] [PubMed] [Google Scholar]
- 50.Zhuang P, et al. Arachidonic acid sex-dependently affects obesity through linking gut microbiota-driven inflammation to hypothalamus-adipose-liver axis. Biochim. Biophys. Acta Mol. Basis. Dis. 2017;1863:2715–2726. doi: 10.1016/j.bbadis.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 51.De Vadder. F, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 52.Chase MH, Nakamura Y, Clemente CD, Sterman MB. Afferent vagal stimulation: neurographic correlates of induced EEG synchronization and desynchronization. Brain Res. 1967;5:236–249. doi: 10.1016/0006-8993(67)90089-3. [DOI] [PubMed] [Google Scholar]
- 53.Kukorelli T, Juhász G. Electroencephalographic synchronization induced by stimulation of small intestine and splanchnic nerve in cats. Electroencephalogr. Clin. Neurophysiol. 1976;41:491–500. doi: 10.1016/0013-4694(76)90061-4. [DOI] [PubMed] [Google Scholar]
- 54.Kapás L, Hansen MK, Chang HY, Krueger JM. Vagotomy attenuates but does not prevent the somnogenic and febrile effects of lipopolysaccharide in rats. Am. J. Physiol. 1998;274:R406–R411. doi: 10.1152/ajpcell.1998.274.2.C406. [DOI] [PubMed] [Google Scholar]
- 55.Opp MR, Toth LA. Somnogenic and pyrogenic effects of interleukin-1beta and lipopolysaccharide in intact and vagotomized rats. Life Sci. 1998;62:923–936. doi: 10.1016/S0024-3205(98)00010-1. [DOI] [PubMed] [Google Scholar]
- 56.Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D. Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G907–G915. doi: 10.1152/ajpgi.2001.281.4.G907. [DOI] [PubMed] [Google Scholar]
- 57.Goswami C, Iwasaki Y, Yada T. Short-chain fatty acids suppress food intake by activating vagal afferent neurons. J. Nutr. Biochem. 2018;57:130–135. doi: 10.1016/j.jnutbio.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 58.Krueger JM, Opp MR. Sleep and Microbes. Int. Rev. Neurobiol. 2016;131:207–225. doi: 10.1016/bs.irn.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Motta V, Soares F, Sun T, Philpott DJ. NOD-like receptors: versatile cytosolic sentinels. Physiol. Rev. 2015;95:149–178. doi: 10.1152/physrev.00009.2014. [DOI] [PubMed] [Google Scholar]
- 60.Perez RV, et al. Selective targeting of Kupffer cells with liposomal butyrate augments portal venous transfusion-induced immunosuppression. Transplantation. 1998;65:1294–1298. doi: 10.1097/00007890-199805270-00002. [DOI] [PubMed] [Google Scholar]
- 61.Schwab M, et al. Involvement of different nuclear hormone receptors in butyrate-mediated inhibition of inducible NF kappa B signalling. Mol. Immunol. 2007;44:3625–3632. doi: 10.1016/j.molimm.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 62.Zimmerman MA, et al. Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302:G1405–G1415. doi: 10.1152/ajpgi.00543.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.D’Souza WN, et al. Differing roles for short chain fatty acids and GPR43 agonism in the regulation of intestinal barrier function and immune responses. PLoS. One. 2017;12:e0180190. doi: 10.1371/journal.pone.0180190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strubbe JH, Bruggink JE, Steffens AB. Hepatic portal vein cannulation for infusion and blood sampling in freely moving rats. Physiol. Behav. 1999;65:885–887. doi: 10.1016/S0031-9384(98)00248-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.