Oury et al. show that the scaffolding protein MACF1 links Rapsyn, which binds acetylcholine receptors, to the microtubule- and actin-network at neuromuscular synapses. MACF1 thereby plays a role in synaptic maturation in mice, and mutations of MACF1 are associated with congenital myasthenia in humans.
Abstract
Complex mechanisms are required to form neuromuscular synapses, direct their subsequent maturation, and maintain the synapse throughout life. Transcriptional and post-translational pathways play important roles in synaptic differentiation and direct the accumulation of the neurotransmitter receptors, acetylcholine receptors (AChRs), to the postsynaptic membrane, ensuring for reliable synaptic transmission. Rapsyn, an intracellular peripheral membrane protein that binds AChRs, is essential for synaptic differentiation, but how Rapsyn acts is poorly understood. We screened for proteins that coisolate with AChRs in a Rapsyn-dependent manner and show that microtubule actin cross linking factor 1 (MACF1), a scaffolding protein with binding sites for microtubules (MT) and actin, is concentrated at neuromuscular synapses, where it binds Rapsyn and serves as a synaptic organizer for MT-associated proteins, EB1 and MAP1b, and the actin-associated protein, Vinculin. MACF1 plays an important role in maintaining synaptic differentiation and efficient synaptic transmission in mice, and variants in MACF1 are associated with congenital myasthenia in humans.
Introduction
The neuromuscular synapse is a highly specialized junction, which is formed by motor nerve terminals and skeletal muscle fibers (Burden, 1998; Sanes and Lichtman, 2001). The synapse controls the movement of all skeletal muscles, including the diaphragm muscle that is essential for respiration and life. Key to rapid, robust, and reliable synaptic transmission, acetylcholine receptors (AChRs), the muscle receptors for the neurotransmitter, are highly enriched in the postsynaptic membrane (Fertuck and Salpeter, 1976). The high density of synaptic AChRs, approaching 20,000 molecules/µm2, ensures that acetylcholine will bind and activate a sufficient number of AChRs to reliably initiate a muscle action potential and muscle contraction (Wood and Slater, 2001). Although the neuromuscular synapse forms and functions before birth in mice, the structure and function of the synapse is modified during the first few postnatal weeks, increasing the reliability of neuromuscular transmission, and then maintained throughout life (Slater, 1982; Wood and Slater, 2001; Tintignac et al., 2015).
Two signaling pathways, one transcriptional and a second post-translational, are necessary to form and maintain synapses, ensuring that AChRs are expressed at a high concentration in the postsynaptic membrane (Burden, 1998; Sanes and Lichtman, 2001; Wu et al., 2010). The transcriptional pathway occurs selectively in myofiber nuclei situated near the synaptic site, termed subsynaptic nuclei, and stimulates the expression of key genes, which encode for proteins that are essential to build and maintain the synapse (Burden, 1993; Schaeffer et al., 2001). The post-translational pathway acts to redistribute and anchor AChRs, as well as other key muscle-derived proteins, at the postsynaptic membrane (Burden, 1998; Sanes and Lichtman, 2001; Wu et al., 2010; Tintignac et al., 2015). Both pathways require Agrin, which is secreted by motor nerve terminals (McMahan, 1990; Gautam et al., 1996), as well as Lrp4, the muscle receptor for Agrin (Kim et al., 2008; Zhang et al., 2008). Agrin-binding to Lrp4 leads to activation of MuSK, the transducing receptor tyrosine kinase (Burden et al., 2013), leading to recruitment of Dok-7, a cytoplasmic adaptor protein that assists in activating MuSK and also functions downstream from MuSK (Yamanashi et al., 2012; Burden et al., 2013). In contrast to these core components, which function both in the synapse-specific transcriptional pathway and the post-translational anchoring pathway, Rapsyn, a 43-kd peripheral membrane protein that binds to the main intracellular loop in the AChRs subunits, is crucial for anchoring AChRs in the postsynaptic membrane but does not act in the transcriptional pathway (Sobel et al., 1978; Neubig et al., 1979; Burden et al., 1983; Gautam et al., 1995; Banks et al., 2003).
In addition to their roles in forming synapses, each of these synaptic proteins is also essential for maintaining neuromuscular synapses, as inactivation of these genes in adult mice leads to synaptic disassembly (Li et al., 2018). Consistent with these findings, autoantibodies to MuSK, found in a subset of individuals with myasthenia gravis, cause neuromuscular dysfunction, demonstrating that these pathways also function throughout life in humans (Koneczny et al., 2014; Gilhus and Verschuuren, 2015). Moreover, in humans, hypomorphic mutations in any one of these key genes are responsible for congenital myasthenia (CM), a heterogeneous group of neuromuscular diseases characterized by muscle weakness and fatigue (Engel et al., 2015).
Although these core molecules are required both for forming and maintaining synapses, there is evidence that a distinct set of molecules selectively regulates the postnatal transition in structure and function of the neuromuscular synapse (Tintignac et al., 2015; Li et al., 2018). For example, α-Dystrobrevin, a component of the dystroglycan complex, is not required for synapse formation but plays an important role in synaptic maturation (Grady et al., 2000).
The molecules and mechanisms that act downstream from MuSK and regulate synaptic differentiation, including clustering of AChRs, during development and later during synaptic maturation are poorly understood. Very few proteins, namely, Rapsyn, Src-family kinases, and APC, have been reported to bind directly to AChRs (Sobel et al., 1978; Fuhrer and Hall, 1996; Bartoli et al., 2001; Wang et al., 2003). As described above, Rapsyn has an essential role in forming and maintaining neuromuscular synapses, while Src-family kinases, similar to α-Dystrobrevin, play a role only later in synaptic differentiation (Smith et al., 2001). Rapsyn was identified as an AChR-associated protein nearly 40 yr ago (Sobel et al., 1978; Neubig et al., 1979), but surprisingly little is known about how Rapsyn functions to anchor postsynaptic proteins (Li et al., 2016, 2018). Our poor understanding of Rapsyn function is due in part to difficulties in purifying soluble and well-folded Rapsyn, impeding knowledge of its structure and understanding how Rapsyn mediates synaptic differentiation. Moreover, few proteins are known to bind directly to Rapsyn, and two Rapsyn-interactors, α-Actinin and β-Catenin, have important roles in skeletal muscle differentiation and function, complicating study of their potential role at neuromuscular synapses (Zhang et al., 2007a; Dobbins et al., 2008; Li et al., 2008). We sought to identify additional Rapsyn-associated proteins in the hope that study of their function would lead to insights into the mechanisms by which Rapsyn regulates synaptic differentiation.
We performed a biochemical screen to identify proteins that associate with Rapsyn by isolating AChRs from skeletal muscle and using mass spectrometry (MS) to compare the protein composition of the AChR complex isolated from WT and Rapsyn mutant muscles. These experiments identified proteins that require Rapsyn for their association with AChR. Here, we describe one such protein, microtubule actin cross linking factor 1 (MACF1), a 650-kD scaffolding protein that contains spectrin-like repeats as well as binding sites for microtubules (MTs) and actin (Suozzi et al., 2012; Zhang et al., 2017). MACF1 (also known as ACF7) regulates MT and actin dynamics in epithelial cells as well as the shape and position of nuclei in muscle and additional cell types (Brown, 2008; Wang et al., 2015; Escobar-Aguirre et al., 2017).
A yeast two-hybrid screen had identified MACF1 as a Rapsyn-interacting protein over 10 yr ago (Antolik et al., 2007), but it remained unclear whether MACF1 was present at synapses and whether MACF1 had a role in synaptic differentiation and function. We demonstrate that MACF1 is concentrated at the postsynaptic membrane at neuromuscular synapses, where it serves as a synaptic scaffold and organizing center for MT-associated proteins, including End-binding protein 1 (EB1) and MT-associated protein 1b (MAP1b), as well the actin-associated protein, Vinculin. We show that MACF1 plays an important role in maintaining differentiation of neuromuscular synapses, efficient synaptic transmission, and motor performance in adult mice. In addition, in the absence of MACF1, the structure of the sarcoplasmic reticulum (SR) and mitochondria are perturbed, suggesting a role for MACF1, separate from its role at the synapse, in skeletal muscle. Further, we show that two patients diagnosed with CM carry missense changes in MACF1, suggesting that these variants in MACF1 cause CM in humans.
Results
MACF1 coisolates with AChRs in a Rapsyn-dependent manner
We performed a biochemical screen to identify proteins that associate with AChRs in a Rapsyn-dependent manner. We isolated AChRs from skeletal muscle tissue of WT or Rapsyn−/− mice, as well as from immortalized muscle cell lines derived from WT or Rapsyn−/− mice. AChRs and associated proteins were isolated using biotin-conjugated α-bungarotoxin (α-BGT), which binds tightly and specifically to AChRs, and streptavidin-agarose beads (Fig. 1 A). Proteins that coisolated with AChRs were identified by liquid chromatography (LC)/MS, and their abundance was quantified by measuring the mass spectral signal intensity of the identified tryptic peptides, the number of identified peptides, and the percentage of sequence coverage for each protein (Table S1). We performed two control experiments to ensure that each identified protein coisolated specifically with AChRs: (1) biotin-conjugated α-BGT was omitted from the isolation scheme, or (2) AChRs were saturated with unlabeled α-BGT before incubation with biotin-conjugated α-BGT. Proteins that were recovered in the absence of biotin-conjugated α-BGT binding were considered to bind nonspecifically to the streptavidin-agarose resin.
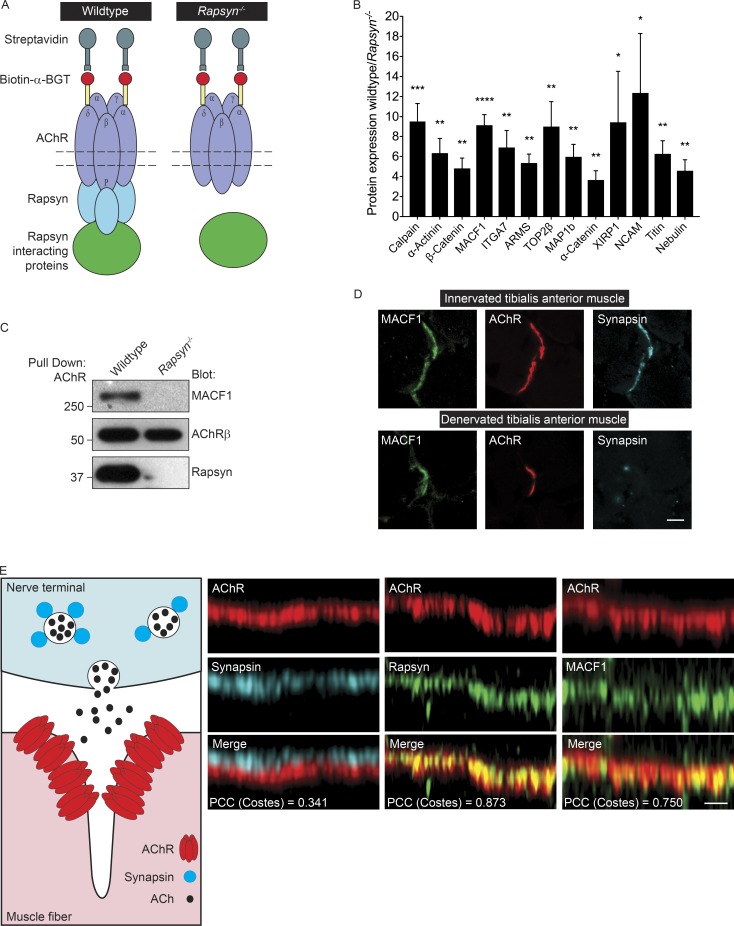
Figure 1.
MACF1 coisolates with AChRs in a Rapsyn-dependent manner. (A) The cartoon illustrates the procedure for isolating proteins that depend on Rapsyn for their coisolation with AChRs. (B) The proteins that associated with AChRs in a Rapsyn-dependent manner were 3- to 12-fold more abundant in AChR complexes isolated from WT than Rapsyn mutant muscle cells. The mean ± SEM values from six independent experiments are shown (*, P < 0.05; **, P < 0.005; ***, P < 0.0005; ****, P < 0.00005). (C) MACF1 coisolates with AChRs in a Rapsyn-dependent manner. (D) MACF1 (green) colocalizes with AChRs (red) and Synapsin (cyan) in cross-sections of innervated adult tibialis anterior muscle. MACF1 staining persists at denervated synaptic sites. Bar, 5 µm. (E) The cartoon illustrates that presynaptic Synapsin is separated by postsynaptic AChRs by >50 nm. As such, the positions of AChRs (red) and Synapsin (cyan) can be resolved in optical sections of the neuromuscular synapse. MACF1 (green) is present at the AChR-rich postsynaptic membrane (red) and not in nerve terminals. Bar, 2 µm. Colocalization of AChRs with Rapsyn (0.873 ± SEM 0.004), MACF1 (PCC = 0.750 ± SEM 0.021), and Synapsin (PCC = 0.341 ± SEM 0.021) were determined using PCC.
As expected, AChR subunits were isolated from WT and Rapsyn mutant cells at similar levels, and we therefore normalized the abundance of all other proteins to the level of AChR subunits (Fig. 1 B and Table S1). Yeast two-hybrid studies had identified four Rapsyn partners, including Calpain, α-Actinin, β-Catenin, and MACF1 (Antolik et al., 2007; Chen et al., 2007; Zhang et al., 2007a; Dobbins et al., 2008). The MS data confirmed that all four proteins coisolate with AChRs in a Rapsyn-dependent manner (Fig. 1 B and Table S1). In addition to these known Rapsyn partners, we identified nine proteins that associated with AChRs in a Rapsyn-dependent manner both in skeletal muscle tissue and in a muscle cell line (Fig. 1 B and Table S1). These proteins were 3- to 12-fold more abundant in AChR complexes isolated from WT than Rapsyn mutant muscle cells (Fig. 1 B and Table S1). In each instance, restoring Rapsyn expression in Rapsyn mutant cells rescued their normal association with AChRs (data not shown). The newly identified Rapsyn-dependent partners included Integrin α7, XIRP1, Titin, MAP1b, Nebulin, α-Catenin, TOP2β, ARMS, and NCAM (Fig. 1 B and Table S1). Earlier studies reported that ITGA7 and ARMS are expressed at neuromuscular synapses (Martin et al., 1996; Burkin et al., 1998; Luo et al., 2005), and we confirmed the presence of ITGA7, and ARMS, as well as TOP2β at neuromuscular synapses by immunostaining (Fig. S1). The function of these proteins, as well as the known Rapsyn-interacting protein at neuromuscular synapses, is poorly understood, in large part because their in vivo function at synapses has not been investigated. Because MACF1 is a scaffolding protein, containing domains that can associate with MT- as well as actin-based filament systems, which have been implicated in synaptic differentiation, we chose to study MACF1 further.
MACF1, a member of the Spectraplakin family of proteins that cross-link MTs and actin, contains CH1 and CH2 domains near the amino terminus, which serve as binding sites for actin, and a binding site for EB1, a MT end-binding protein, near the carboxy terminus (Suozzi et al., 2012; Zhang et al., 2017). A Plakin domain and multiple Spectrin-like repeats are contained between the actin- and MT-binding regions, yielding a rod-like, 650-kD molecule that assists in stabilizing MT- and actin-associated filament systems (Suozzi et al., 2012; Zhang et al., 2017). The yeast two-hybrid study demonstrated that the actin-binding domain in MACF1 binds the tetratricopeptide repeats in Rapsyn (Antolik et al., 2007).
To confirm the findings from our MS studies, we isolated AChRs from muscle of WT and Rapsyn−/− embryos and probed Western blots with antibodies to MACF1, Rapsyn, and the AChR β-subunit. Fig. 1 confirms that Rapsyn is required for MACF1 to coisolate with AChRs (Fig. 1 C). In addition, we stained cross-sections of skeletal muscle with antibodies to MACF1, AChRs, and Synapsin, a synaptic vesicle protein present in presynaptic motor nerve terminals, and found that MACF1 is concentrated at neuromuscular synapses in innervated and denervated muscle, demonstrating that MACF1 is a postsynaptic protein (Fig. 1 D). To determine whether MACF1 is additionally expressed in nerve terminals, we optically sectioned neuromuscular synapses from stained whole mounts of muscle, which demonstrated that MACF1 is present at the postsynaptic membrane but is not detectable in motor nerve terminals (Fig. 1 E).
MACF1 is not essential for neuromuscular synapse formation
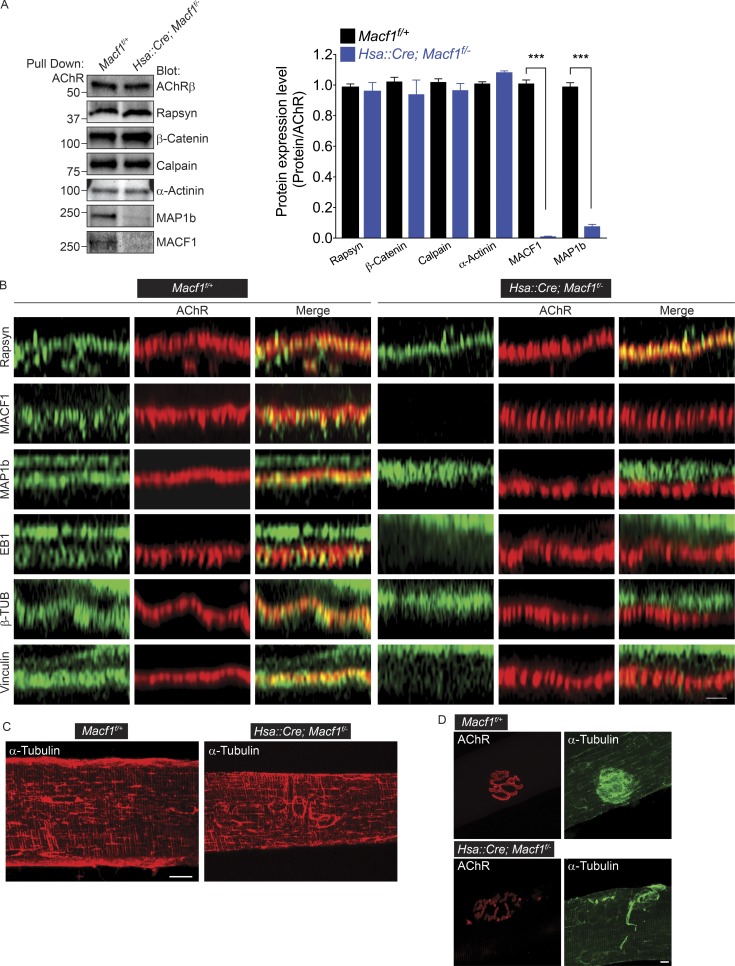
We next sought to determine the role that MACF1 might play at neuromuscular synapses. Macf1−/− mice die at gastrulation, precluding analysis of neuromuscular synapse formation (Chen et al., 2006). To conditionally inactivate Macf1 in skeletal muscle, we generated mice that carried null and floxed alleles of Macf1 and a transgene in which Cre recombinase expression was controlled by the regulatory region from the human skeletal muscle actin (Hsa) gene. The floxed allele of Macf1 contains loxP sites flanking exons 6 and 7, which encode the actin-binding domain in MACF1, leading to a frame shift and premature truncation of MACF1 (Wu et al., 2008). Mice carrying two floxed alleles of Macf1 (Macf1f/f) were crossed with mice that were heterozygous for Macf1 and carried the Hsa::Cre transgene (Hsa::Cre; Macf1+/−), generating the conditionally mutant and control mice. Hsa::cre; Macf1f/- mice were recovered postnatally at the expected Mendelian ratio (Fig. 2 A), indicating that MACF1 is not essential in skeletal muscle for the formation and function of neuromuscular synapses, which are required for respiration and survival at birth. We quantified the loss of MACF1 by measuring the amount of MACF1 in muscle tissue lysates and in complex with AChRs. Because muscle tissue contains multiple cell types and MACF1 is widely expressed, the amount of MACF1 associated with AChRs provides a better estimate of the reduction in MACF1 expression specifically in myofibers. MACF1 levels were reduced more than 10-fold in muscle tissue, and MACF1 was undetectable in AChR complexes from Hsa::Cre; macf1f/− mice, indicating that MACF1 is efficiently removed from myofibers in Macf1 muscle-conditional mutant mice (Fig. 2 B). Further, we confirmed the loss of MACF1 from synapses by immunostaining muscle fibers from Hsa::Cre; Macf1f/− mice (Fig. 2 C).
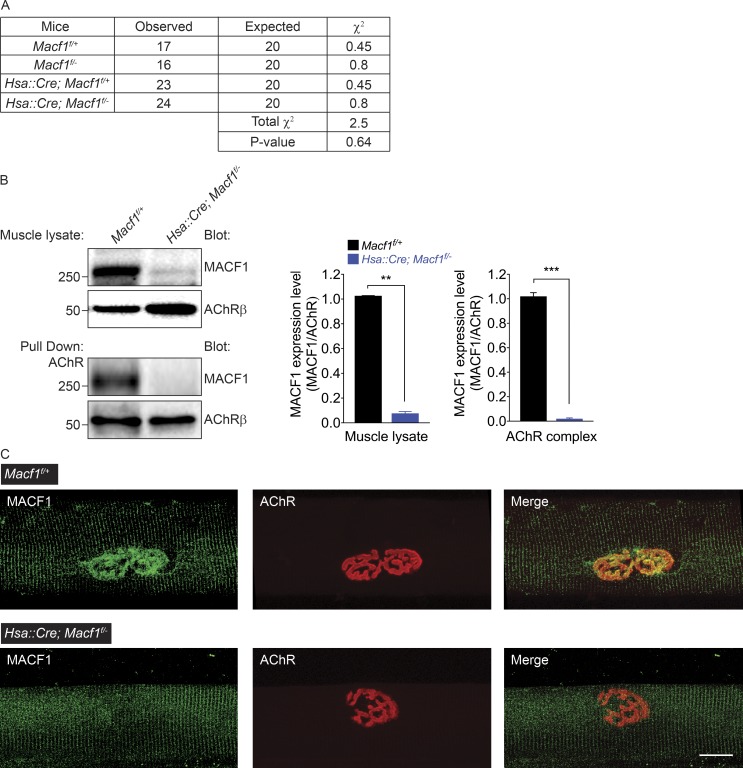
Figure 2.
MACF1 is efficiently inactivated in skeletal muscle using a Human skeletal actin (Hsa)::Cre transgene. (A) We determined the genotype of the progeny at P5–P10. χ2 analysis of progeny from a cross of Macf1f/f mice with Hsa::Cre; Macf1+/− mice shows that the occurrence of genotypes is unlikely to occur by chance, indicating that mice lacking MACF1 in muscle survive postnatally. (B) MACF1 expression is reduced by 14-fold in muscle lysates and undetectable (reduced by >98%) in AChR complexes isolated from Macf1 conditionally mutant mice. The mean ± SEM values from three independent experiments are shown (**, P < 0.005; ***, P < 0.0005). (C) Antibody staining shows that MACF1 (green) is enriched at synapses, marked by staining for AChRs (red), in Macf1f/+ control mice but absent from synapses in Hsa::Cre; Macf1f/− mice. The striated, sarcomeric staining is nonspecific, as this staining persists in Macf1 mutant mice. Bar, 10 µm.
During development, motor axons branch and terminate in a band of synapses that are adjacent to the main intramuscular nerve. Each muscle fiber is innervated at a single synaptic site, which is marked by the accumulation of synaptic vesicles in nerve terminals and a high concentration of AChRs in the postsynaptic membrane (Sanes and Lichtman, 2001). To determine whether MACF1 has a role in organizing presynaptic and postsynaptic differentiation, we analyzed whole mounts of diaphragm muscles from embryonic day 18. 5 (E18.5) mice, 4 to 5 d after synapses first form, by staining with probes that mark motor axons, nerve terminals, and AChRs. Neuromuscular synapses, shaped as ovoid plaques of AChRs that are apposed by motor nerve terminals, form in Hsa::Cre; Macf1f/− mice (Fig. 3 A). Although major features of synapse formation occurred normally in mice deficient in MACF1 muscle expression, the density of synaptic AChRs was reduced by 13% (Fig. 3 B). Consistent with these findings, although the number and size of AChR clusters induced by Agrin were normal in Macf1 mutant myotubes grown in cell culture, the density of AChRs in these clusters was reduced by 29% (Fig. S2). Thus, MACF1 participates but is not essential for early steps in postsynaptic differentiation.
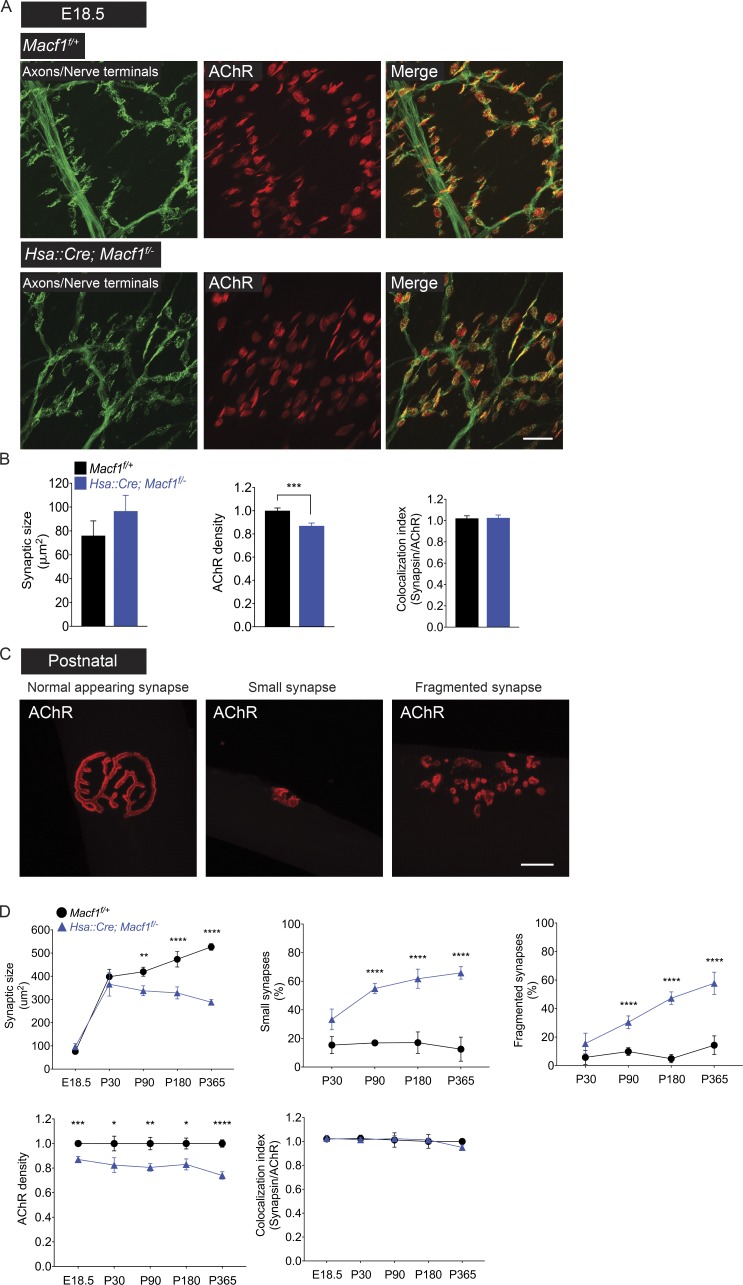
Figure 3.
The postnatal morphological maturation of the neuromuscular synapse is impaired in mice lacking MACF1 in muscle. (A) Diaphragm muscles from Macf1f/+ control and Hsa::Cre; Macf1f/− mice at E18.5 were stained with Alexa Fluor 488–α-BGT to label AChRs (red) and antibodies to Neurofilament/Synapsin to label motor axons/nerve terminals (green). Bar, 50 µm. (B) The size of the synapse, the density of synaptic AChRs, and percent of AChR-rich areas that are apposed by nerve terminals were quantified. At E18.5, synaptic size is normal, but the density of synaptic AChRs is reduced by 13% in Macf1 mutant muscle. The mean ± SEM values from three mice (100 synapses per mouse) are shown (***, P < 0.0005). (C) At P90, neuromuscular synapses in Macf1 muscle-conditional mutant mice show signs of deterioration. Synapses, marked by staining for AChRs (red), are smaller in size, and their pretzel-like shape is less elaborate. Bar, 10 µm. (D) Neuromuscular synapses in Macf1 muscle-conditional mutant mice deteriorate further, as synaptic size fails to increase between P90 and P365, and the extent of synaptic fragmentation worsens in Macf1 mutant mice. Moreover, the density of synaptic AChRs is reduced by 20%. The mean ± SEM values from three mice (100 synapses per mouse) are shown (*, P < 0.05; **, P < 0.005; ***, P < 0.0005; ****, P < 0.00005).
The postnatal morphological maturation of neuromuscular synapses is impaired in mice lacking MACF1 in muscle
Once formed, neuromuscular synapses mature over a 3-wk postnatal period. During this maturation period, synaptic size increases by four- to fivefold, and the ovoid plaque-like arrangement of AChRs is transformed and sculpted to generate a pretzel-like shape with a nearly contiguous arrangement of AChRs that is maintained throughout life (Fig. 3 C). Thereafter, synaptic size increases more modestly, while the density of synaptic AChRs, established by birth, is maintained (Slater, 1982; Fig. 3 D). To determine whether MACF1 is required for this postnatal structural reorganization, we quantified synaptic size, as described above, and fragmentation, a feature often observed at diseased synapses, by measuring the number of contiguous AChR-stained units at each synapse. In Macf1 conditionally mutant mice, like Macf1f/+ control mice, nerve terminals remained reliably aligned and apposed to AChRs (Fig. 3 D). Moreover, synaptic size increased normally in Macf1 conditionally mutant mice from E18.5 to postnatal day 30 (P30; Fig. 3 D). Thereafter, however, synaptic size failed to increase further in Macf1 muscle-conditionally mutant mice (Fig. 3 D). Moreover, Macf1 mutant synapses became fragmented after P30, and the extent of fragmentation increased over the next several months (Fig. 3 D). The density of synaptic AChRs, already reduced at birth, remained diminished and reduced by nearly 20% from P30 through P180 (Fig. 3 D). Thus, MACF1 is required for the morphological maturation and maintenance of neuromuscular synapses.
In addition, we examined neuromuscular synapses in older mice to learn whether the deficits, evident at P180, persisted or worsened with age. We studied muscle from mice at 1 yr of age and found that synaptic size and synaptic AChR density remained diminished and excessive synaptic fragmentation persisted (Fig. 3 D). Moreover, these deficits were apparent in multiple limb muscles as well as the diaphragm muscle (Fig. S3). These data further demonstrate that MACF1 is required to maintain neuromuscular synapses in adult mice.
Macf1 muscle-conditional mutant neuromuscular synapses are functionally impaired
Muscle fibers become atrophic as muscle use is diminished. We wondered whether the morphological changes at the neuromuscular synapse in Macf1 mutant mice might lead to decreased muscle activity and cause a reduction in muscle fiber size. Fig. 4 A shows that muscle fiber size was normal in Macf1 muscle-conditional mutant mice at P30, when synaptic morphology appeared normal. However, by P180, when morphological changes at the synapse were evident (Fig. 3, C and D), muscle fibers in Macf1 mutant mice were atrophic, as the cross-sectional area of muscle fibers decreased by 30% (Fig. 4 A).
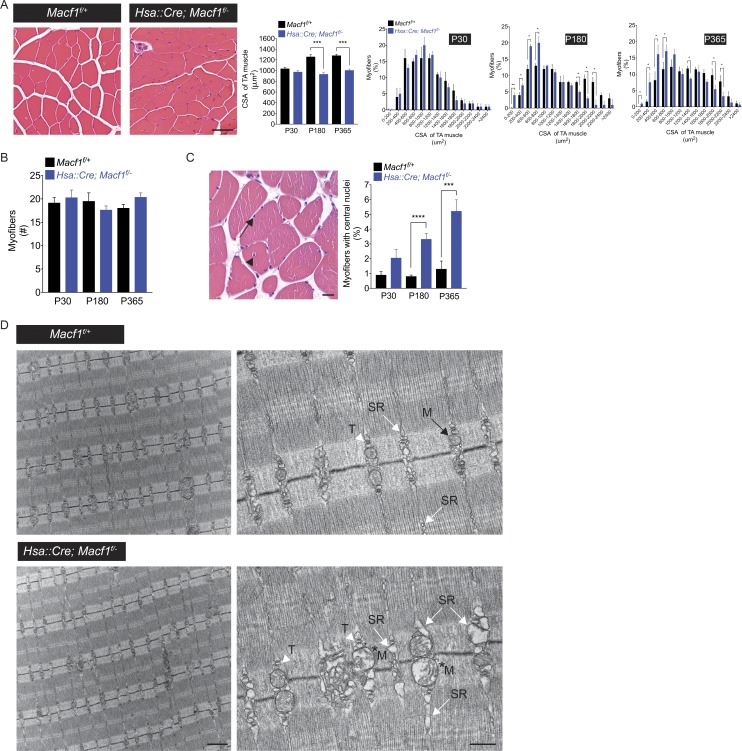
Figure 4.
Muscle fibers are atrophic and show aberrations in the structure of the SR and mitochondria in Macf1 mutant mice. (A) Cross-sections of P30, P180, and P365 tibialis anterior muscles were stained with hematoxylin and eosin. The cross-sectional area (CSA) of myofibers is reduced by 30% in Hsa::Cre; Macf1f/− mice at P180 and P365. The mean ± SEM values from three mice (100 myofibers per mouse) are shown (***, P < 0.0005). Bar, 50 µm. (B) The number of myofibers is normal in Macf1 mutant mice. The mean ± SEM values from three mice (measured area: 40,000 µm2) are shown. (C) Macf1 mutant mice exhibit a low level of muscle degeneration/regeneration. 1% of myofibers in control mice and 3–5% of myofibers in Macf1 muscle-conditional mutants have centrally located nuclei (arrow). The mean ± SEM values from three mice (100 myofibers per mouse) are shown (***, P < 0.0005, ****, P < 0.00005). Bar, 10 µm. (D) The organization of sarcomeres, Z-discs, and T-tubule/SR triads (arrowhead) appears normal in the absence of MACF1. In Macf1 mutant mice, the SR (white arrow) is distended, ranging from modestly to severely enlarged, and a subset of mitochondria (*M) shows signs of damage. Bar, 1 µm for the left images and 500 nm for the right images.
We measured the number of muscle fibers and the number of myofibers with central nuclei to determine whether myofibers were lost or showed signs of degeneration/regeneration. Fig. 4 B shows that the number of myofibers is unchanged in Macf1 mutant mice (Fig. 4 B). Nonetheless, a low level of muscle degeneration/regeneration is evident in Macf1 mutant mice (Fig. 4 C), as 1% of myofibers in control mice and 3–5% of myofibers in Macf1 mutant mice have centrally located nuclei at P180 and P365, respectively.
We used electron microscopy to study the ultrastructure of muscle in Macf1 mutant mice and learn whether the organization of sarcomeres or myofibrils was altered. Fig. 4 D shows that the general organization of muscle, notably the registered alignment of Z-discs, A-bands and I-bands, as well as the presence of triads, formed by transverse tubules (T-tubules) and sarcoplasmic reticulum (SR), appeared normal in the absence of MACF1. However, the SR appeared distended, ranging from modestly to severely enlarged, and a subset of mitochondria showed signs of damage. Together, these findings indicate that the structures of the SR and mitochondria are perturbed in the absence of MACF1 in ways that are unlikely to be a consequence of the synaptic defects (Fig. 4 D). Although we do not know how MACF1 regulates the structure of the SR and mitochondria, changes in their function may contribute to the muscle atrophy and diminished muscle function in Macf1 mutant mice (see below).
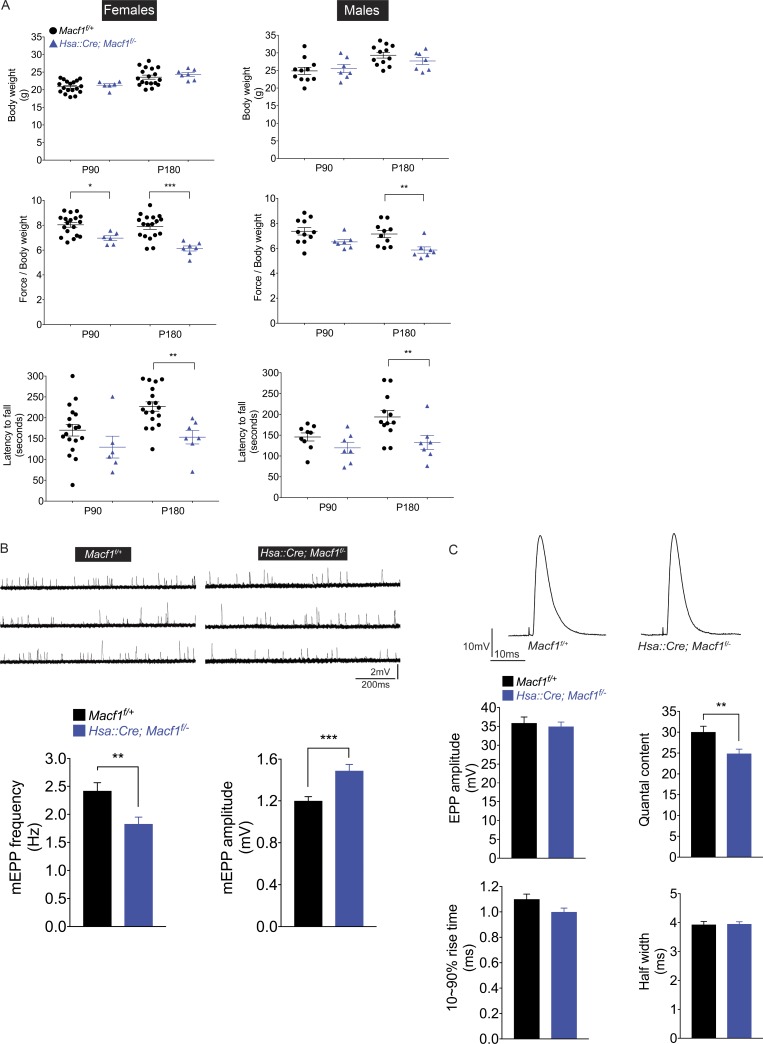
We next evaluated the motor performance of Macf1 muscle-conditional mutant mice in order to more directly determine whether muscle function was impaired in Macf1 mutant mice. We measured body weight, limb grip strength, and behavior on an accelerating rotarod at P90 and P180. Fig. 5 A shows that the motor performance of male and female Macf1 mutant mice was impaired, as their limb grip strength was reduced by 25%, and their performance on the rotarod was similarly diminished at P180 (Fig. 5 A). Together, these findings suggest that alterations in synaptic structure, the function of the SR and mitochondria, or a combination of these deficits is responsible for the decreased muscle use and muscle atrophy.
Figure 5.
Synaptic transmission and motor performance are impaired in Macf1 muscle-conditional mutant mice. (A) Motor performance of male and female Macf1 muscle-conditional mutant mice was impaired at P180, as assessed by grip strength and rotarod assays. The body weight of Macf1 mutant mice was normal. The mean ± SEM values from more than five mice are shown (*, P < 0.05; **, P < 0.005; ***, P < 0.0005). (B) The frequency of mEPPs was reduced and the mEPP amplitude was increased in P180 Macf1 muscle-conditional mice. The mean ± SEM values from four mice are shown. (C) Quantal content was reduced by 20% in Macf1 muscle-conditional mutant mice. The mean ± SEM values from four mice are shown (**, P < 0.005). The rise time and half width of the EPP were normal in Macf1 mutant mice. Likewise, the muscle resting membrane potential was similar in Hsa::Cre; Macf1f/− (70.3 ± 1.3 mV, n = 4 mice, n = 37 myofibers) and Macf1f/+ control mice (71.2 ± 0.8 mV, n = 4 mice, n = 38 myofibers).
We used intracellular microelectrodes to record synaptic activity to learn whether synaptic transmission was impaired in Macf1 mutant mice. The frequency of miniature endplate potentials (mEPPs) in the lumbrical muscle was reduced by 25%, while the mEPP amplitude was increased by 25% (Fig. 5 B). The 25% decrease in mEPP frequency is likely attributed to the similar decrease in synaptic size (Kuno et al., 1971). MEPP amplitude is determined in part by the number of AChR channels that open in response to acetylcholine, the conductance properties of individual AChRs, and the input resistance of the muscle fibers, which is inversely correlated with muscle fiber size (Katz, 1966). The 20% decrease in density of synaptic AChRs should result in a similar decrease in mEPP amplitude, and the 30% decrease in muscle fiber size is predicted to lead to a 40% increase in input resistance and mEPP amplitude (Katz and Thesleff, 1957). Together, the decreases in AChR density and muscle fiber size are calculated to produce a 20% increase in mEPP amplitude, close to the recorded value (Fig. 5 B). Thus, synaptic transmission is impaired in a manner that corresponds closely to the value calculated from the decreased density of synaptic AChRs and size of the atrophic muscle fibers.
We stimulated the plantar nerve to the lumbrical muscle and recorded evoked endplate potentials (EPPs) from individual myofibers, which revealed a nearly 20% decrease in quantal content, a measure of presynaptic release properties, including synaptic size (Fig. 5 C). Thus, synaptic transmission is impaired in mice lacking muscle-derived MACF1, which likely contributes to the decreased motor performance and muscle fiber atrophy.
MACF1 anchors MAP1b and EB1 at the postsynaptic membrane at neuromuscular synapses
MACF1 binds EB1 and thereby serves as a scaffold for MTs and MT-binding proteins (Suozzi et al., 2012; Zhang et al., 2017). Consistent with this role, we identified MAP1b in our MS screen of AChR-associated proteins (Fig. 1 B and Table S1), suggesting that MAP1b and possibly EB1 may be concentrated at neuromuscular synapses in a manner that depends on MACF1. We isolated AChRs from muscle of WT, Macf1f/+ control mice, Macf1 conditionally mutant mice, and Rapsyn mutant mice and probed Western blots with antibodies to MAP1b. MAP1b coisolates with AChRs from WT and Macf1f/+ control muscles but not from muscle lacking MACF1 (Fig. 6 A) or Rapsyn (Fig. S4). In contrast, Rapsyn, as well as Rapsyn-associated proteins, including β-Catenin, α-Actinin, and Calpain, associate with AChRs independent of MACF1 (Fig. 6 A). These results indicate that MACF1 plays an important role in recruiting a MT network, including MAP1b, to the postsynaptic membrane at neuromuscular synapses. Moreover, these findings indicate that Rapsyn anchors additional proteins, including certain components of the actin network, independent of MACF1.
Figure 6.
MACF1 anchors actin and MT networks at the postsynaptic membrane at neuromuscular synapses. (A) MAP1b coisolates with AChRs in a MACF1-dependent manner in P180 mice. Association between AChR and Rapsyn is not dependent on MACF1. The mean ± SEM values from three independent experiments are shown (*, P < 0.05; ***, P < 0.0005). (B) MACF1 (green) is required for the accumulation of MAP1b, EB1, β-TUB, and Vinculin (each shown in green) at the postsynaptic membrane of neuromuscular synapses, marked by staining for AChRs (red). We did not detect EB1 in the AChR complex by MS or Western blotting, suggesting that the association between EB1 and MACF1 is labile. MAP1b, EB1, and β-TUB, unlike MACF1 or Vinculin, are also expressed in nerve terminals, and their presynaptic expression is not dependent on muscle-derived MACF1. Rapsyn (green) is expressed exclusively at the postsynaptic membrane independent of MACF1. Bar, 1 µm. (C) MACF1 is dispensable for organizing MTs, stained with antibodies to α-TUB (red), in a grid-like pattern in the nonsynaptic regions of the tibialis anterior muscle. Bar, 20 µm. (D) MACF1 is necessary for concentrating MTs (green) in the postsynaptic region, marked by AChRs (red). Bar, 10 µm.
We stained neuromuscular synapses with antibodies to MAP1b, as well as EB1 and β-Tubulin (β-TUB), to further study expression of the MT network at synapses in Macf1 conditionally mutant mice. In addition, we stained synapses with antibodies to Vinculin, an actin-associated protein that is enriched at the postsynaptic membrane at neuromuscular synapses (Bloch and Hall, 1983), to determine whether MACF1 is required for the enrichment of this actin-binding protein at the postsynaptic membrane. Optical sectioning of stained neuromuscular synapses shows that MAP1b, EB1, β-TUB, and Vinculin are present both in presynaptic nerve terminals and at the postsynaptic membrane in Macf1f/+ control mice (Fig. 6 B). In the absence of muscle-derived MACF1, MAP1b, EB1, β-TUB, and Vinculin remain in presynaptic nerve terminals, but their expression at the postsynaptic membrane is absent (Fig. 6, B and D). In contrast, the complex grid-like arrangement of MTs in the nonsynaptic region of muscle appears unperturbed in the absence of MACF1 (Fig. 6 C), suggesting that the aberrations in the SR and mitochondria of Macf1 mutant mice are not due to major changes in the MT lattice. Thus, expression of MAP1b, EB1, β-TUB, and Vinculin at the postsynaptic membrane depends on MACF1.
MACF1 is a synaptic podosomal protein
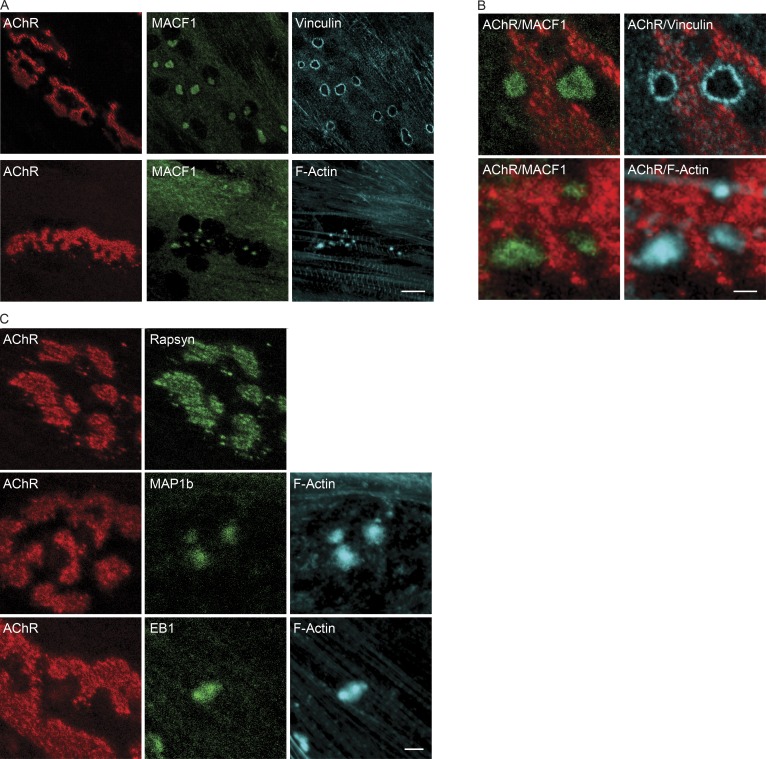
Myotubes that are grown in cell culture on Laminin-coated substrates develop AChR clusters that are perforated and form complex shapes, resembling the changing shapes of synapses found during postnatal synaptic maturation (Kummer et al., 2004). The AChR-poor perforations within these AChR clusters contain proteins found in the core and cortex of podosomes, dynamic, actin-rich, adhesive structures (Proszynski et al., 2009). Inhibition of Src family kinases leads to disassembly of the synaptic podosomes and disruption in the organization of AChRs, consistent with the idea that podosomal proteins promote the remodeling in the arrangement of AChRs during postnatal development (Proszynski et al., 2009). Because MACF1 has a role in maintaining the elaborate, pretzel-like shape of the synapse, we reasoned that MACF1 might be a component of synaptic podosomes. We therefore stained C2C12 myotubes, grown on a Laminin matrix, for AChRs, MACF1, and podosomal proteins. Fig. 7 shows that MACF1 is present in synaptic podosomes, where it colocalizes with core podosomal proteins, such as F-actin, and is excluded from the cortex of the podosome, marked by Vinculin (Fig. 7, A and B). We also stained for the MT-associated proteins, MAP1b and EB1, and found that MAP1b and EB1 colocalize with MACF1 within the core of synaptic podosomes (Fig. 7 C).
Figure 7.
MACF1 is a synaptic podosomal protein. (A) MACF1 (green) concentrates within perforations of AChR clusters (red). Bar, 10 µm. (B) Higher magnification images show that MACF1 (green) is enriched in the core of synaptic podosomes, like F-actin (cyan) and not found at the cortex of the podosome, marked by Vinculin (cyan). Bar, 1 µm. (C) Together with MACF1, MAP1b and EB1 (each shown in green) are enriched in the core of the synaptic podosome. Rapsyn (green) is not found in the perforations but together with AChRs. Bar, 4 µm.
Synaptic podosomes represent a model for the transitional period when synapses convert from a plaque- to a pretzel-like shape. Because MACF1 colocalizes with MT-associated proteins and not with Rapsyn and AChRs in synaptic podosomes, the association of MACF1 may shift from a primary association with MTs to an additional association with Rapsyn during this transitional period, directing MACF1 to the postsynaptic membrane. The structure and function of MACF1 is regulated by association of MACF1 with actin, which stimulates an intrinsic ATPase activity in MACF1, directing MTs to focal adhesions in epithelial cells (Wu et al., 2008). These findings raise the possibility that changes in the structure and activity of MACF1 may occur as the shape of AChR clusters transitions from a plaque to a pretzel-like shape, as modeled in synaptic podosomes, redirecting MACF1 to different binding partners, including Rapsyn.
Muscle fiber nuclei are enriched in the synaptic region of Macf1-conditional mutant mice
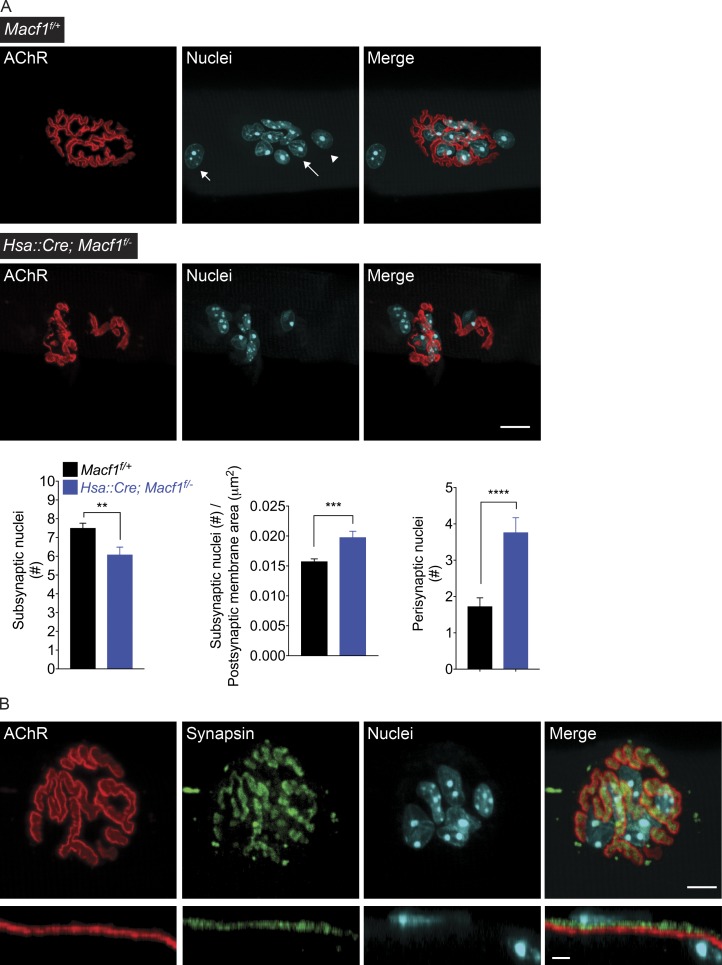
Individual, adult myofibers contain several hundred nuclei. A subset, ∼1%, of these nuclei is positioned and concentrated within the synaptic compartment of muscle fibers, which allows these nuclei to respond to local synaptic signals and boost transcription of genes that encode for synaptic proteins (Merlie and Sanes, 1985; Burden, 1993; Schaeffer et al., 2001). Spectraplakins are known to regulate nuclear positioning (Wang et al., 2015), so we wondered whether the positioning of synaptic nuclei might be perturbed in Macf1 conditionally mutant mice. We stained single, dissected myofibers from Macf1f/+ control and Macf1 conditionally mutant mice for AChRs, nerve terminals, and nuclei, and analyzed optical sections of neuromuscular synapses (Grady et al., 2005). This procedure allowed us to avoid counting Schwann cell nuclei and to selectively quantify myofiber nuclei in order to determine whether the arrangement of myonuclei was perturbed in muscle lacking MACF1 (Fig. 8, A and B). We measured the number of myonuclei at each AChR-stained area and normalized the number of synaptic nuclei to the postsynaptic area at 90 synapses in three mice. Fig. 8 A shows that myonuclei accumulated in the subsynaptic region of muscle from control and Macf1 mutant mice. At P180, Macf1 mutant synapses are 32% smaller than synapses in control mice (Fig. 3 D), and the number of subsynaptic myonuclei is similarly reduced, resulting in a comparable number of synaptic nuclei, normalized to postsynaptic membrane area, in control and Macf1 mutant mice (Fig. 8 A). Concomitant with this reduction in the total number of subsynaptic nuclei, the number of myofiber nuclei in the nearby perisynaptic region was increased by twofold in Macf1 mutant mice. Together, these data indicate that MACF1 is dispensable for anchoring subsynaptic nuclei and suggest that a subset of “synaptic nuclei,” unopposed by nerve terminals, become positioned in the perisynaptic region.
Figure 8.
Muscle fiber nuclei are enriched in the synaptic region of Macf1-conditional mutant mice. (A) Myonuclei accumulate in the subsynaptic region of muscle from control and Macf1 mutant mice. The total number of subsynaptic myonuclei is reduced by 18% in Macf1 muscle-conditional mutant mice at P180. The number of subsynaptic nuclei, normalized to synaptic size, is increased by 22% in Macf1 mutant mice. The number of nuclei in the perisynaptic region is increased by twofold. Synaptic sites were marked by staining for AChRs (red), and nuclei were stained with Hoechst 33342 (cyan). The mean ± SEM values from three mice (30 synapses per mouse) are shown (**, P < 0.005; ***, P < 0.0005). A long arrow indicates a subsynaptic nucleus, an arrowhead indicates a perisynaptic nucleus, which is adjacent to the synaptic site, and a short arrow indicates a nonsynaptic nucleus. Bar, 10 µm. (B) We distinguished between nuclei of perisynaptic cells, including Schwann cells, and myofiber nuclei by optical sectioning of muscle tissue stained for nuclei (cyan), AChRs (red) and Synapsin (green); we only counted nuclei within the myofiber. Bar, 5 µm.
Missense variants in MACF1 in humans are associated with CM
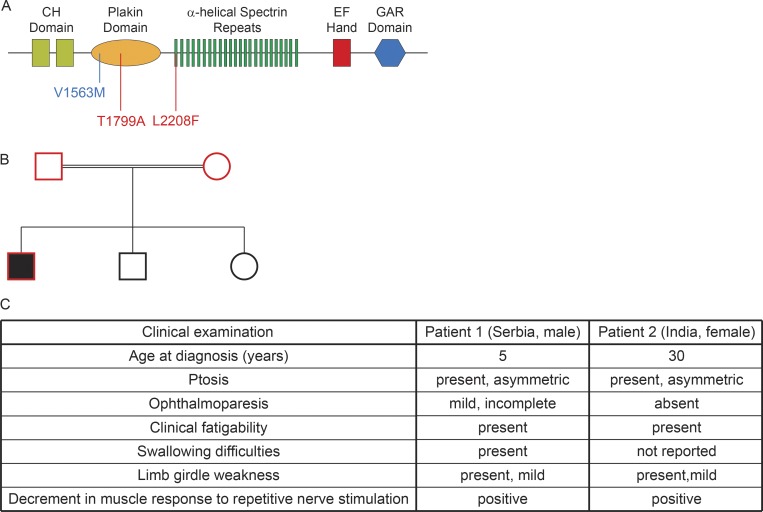
Mutations in genes that are essential for the formation and function of neuromuscular synapses are responsible for CM, a heterogeneous group of diseases that causes muscle weakness and fatigue (Müller et al., 2007; Engel et al., 2015). We used exome sequencing to learn whether mutations in MACF1 might be responsible for disease in patients diagnosed with CM. We identified one patient from Serbia who carries heteroallelic missense variants in residues that are conserved in mouse and human MACF1. The first variant is within a small exon encoding part of the Plakin domain (Chr1: 39802863 c.5395A>G; p.Thr1799Ala), and the second variant is within the adjacent exon encoding the first Spectrin-like repeat domain (Chr1: 39818781 c.6622C>T; p.Leu2208Phe [ENST00000289893]; Fig. 9 A). Alternatively spliced transcripts encoding both residues are expressed in skeletal muscle, heart muscle, whole blood, and esophagus (https://gtexportal.org). Neither variant has been seen in homozygosity in a control population of >125,000 individuals (http://gnomad.broadinstitute.org/). p.Thr1799Ala is predicted to be damaging by in silico tools, SIFT (http://sift.bii.a-star.edu.sg/) and MutationTaster (http://www.mutationtaster.org/), with a Combined Annotation Dependent Depletion score of 21.6 (top 0.5% most deleterious variants). As expected, the unaffected parents carried one allele of each variant and transmitted these two variants to the patient, while the unaffected siblings carry only one variant each (Fig. 9 B). No other pathogenic variants in genes previously associated with myasthenic syndromes or other neuromuscular disorders have been identified in this patient. Clinical examination identified symptoms of ptosis, weakness of extraocular muscles, weakness of neck flexors, weakness and fatigue of tongue, and swallowing difficulties starting at 5 yr old (Fig. 9 C). Needle electromyography was normal with no signs of nerve or muscle dysfunction, although the response to repetitive stimulation at 3 Hz in proximal muscles showed pathological decrement, indicating failure of neuromuscular transmission as typically found in myasthenic syndromes (Fig. 9 C).
Figure 9.
Two patients diagnosed with congenital myasthenia carry alleles with missense variants in MACF1. (A) Two missense variants of MACF1 were identified in the Serbian patient diagnosed with CM. The first variant is found in a small exon encoding part of the Plakin domain (Chr1: 39802863 c.5395A>G; p.Thr1799Ala), and the second variant is found in an exon encoding the first Spectrin repeat (Chr1: 39818781 c.6622C>T; p.Leu2208Phe; indicated in red). These domains are interposed between the calponin-homology (CH) domain and the Gas2-related (GAR) domain. The Indian patient is homozygous for a MACF1 variant in a large exon encoding the bulk of the Plakin domain (Chr1: 39801627 c.4687G>A; p.Val1563Met; indicated in blue). (B) Each parent carried one variant and transmitted this MACF1 variant to the patient; none of the unaffected siblings carried both MACF1 variants. In red: Samples submitted to whole exome sequencing. (C) Clinical examination identified the indicated symptoms.
We then interrogated a database, which contains more than 3,000 exomes from rare disease patients, together with clinical information from the RD-Connect genome phenome analysis platform, in order to determine whether additional cases of CM may be associated with variants in MACF1. By these means we found a second CM patient with a homozygous MACF1 variant. The missense variant (Chr1: 39801627 c.4687G>A; p.Val1563Met) is located in a large exon encoding the majority of the Plakin domain (Fig. 9 A). c.4687G>A is predicted to alter an exonic splice enhancer and potentially result in abnormal splicing (http://www.umd.be/HSF3/index.html). This variant has been observed four times among 125,725 healthy control individuals but never in homozygosity (http://gnomad.broadinstitute.org). The patient, who does not carry pathogenic variants in other myasthenia-associated genes, presented with late-onset fatigable weakness of the limb girdle musculature and mild, asymmetric ptosis, without ophthalmoparesis. Similar to the Serbian patient, recordings from the quadriceps, deltoid, and tibialis anterior muscles in this Indian patient showed a pathological decrement following stimulation at 3 Hz (Fig. 9 C). The patient responded well to acetylcholinesterase inhibitors and salbutamol. Together, these clinical findings are consistent with the synaptic deficits found in mice deficient in muscle-derived MACF1 and indicate that CM can be caused by mutations in MACF1.
Discussion
Here, we show that MACF1 coisolates with AChRs in a Rapsyn-dependent manner and recruits a MT network, including EB1 and MAP1b, as well as the actin-binding protein Vinculin, to the postsynaptic membrane to regulate the maintenance of neuromuscular synapses. The loss of MACF1 does not recapitulate the loss of Rapsyn, demonstrating that MACF1 does not mediate all roles for Rapsyn, including the clustering of AChRs at synapses during development. Instead, MACF1 plays a role in maintaining neuromuscular synapses, in part by promoting the postnatal growth of synapses and maintaining synaptic AChR density at normal levels. These findings point to a distinction between mechanisms and molecules to form neuromuscular synapses and those required for the maintenance of synapses. Thus, MACF1 joins Utrophin, Dystrobrevin, and Src-family kinases as synaptic proteins that have selective roles in the postnatal maturation and maintenance of neuromuscular synapses (Grady et al., 2000; Smith et al., 2001; Sadasivam et al., 2005). Importantly, our findings reveal that deficits in these later steps lead to an impairment in synaptic transmission and motor performance in mice and suggest that these deficits are a cause for CM in humans.
Major features of synapse differentiation occur normally during embryonic development and early postnatal life in mice deficient in muscle expression of MACF1. Neuromuscular synapses form in the central region of muscle and appear normal in size and shape. However, even at early stages, the density of synaptic AChRs is reduced by 13% in Macf1 muscle-conditional mutant mice, indicating that MACF1 has a role, though modest, at developing synapses. A failure to detect a deficit in motor performance in young Macf1 mutant mice may be due to the threefold excess of synaptic AChRs at neuromuscular synapses, which contributes to the safety factor for synaptic transmission (Wood and Slater, 2001). Over the next month, synaptic size increases and the shape of the synapse is transformed from an ovoid plaque to a complex pretzel-like shape in normal mice and mice deficient in muscle-derived MACF1. However, after 1 mo, synaptic size fails to increase further, and signs of deterioration, including synaptic fragmentation, become evident in Macf1 mutant mice.
In addition, a loss of MACF1 leads to distension of the SR and aberrations in mitochondria. We do not know how MACF1 regulates the structure of the SR and mitochondria, but perturbations in SR or mitochondrial function, including calcium homeostasis, may contribute to the muscle atrophy and decreased motor performance evident in Macf1 mutant mice. These perturbations may also contribute to the motor deficits of CM patients carrying variants in MACF1.
In Drosophila melanogaster, Shot, the homologue of MACF1, interacts with EB1 and MSP-300, a nuclear scaffold protein, and the proteins play important roles in positioning myofiber nuclei and stabilizing the shape of myonuclei during muscle contraction (Wang et al., 2015). SYNE/Nesprin, the mouse homologue of MSP-300, plays a similar role in positioning nuclei in muscle, including the accumulation of myonuclei in the subsynaptic region (Grady et al., 2005; Zhang et al., 2007b). SYNE-1 interacts with MuSK (Apel et al., 2000), and this association may contribute to tethering SYNE-1–decorated myonuclei in the synaptic region. Considering the findings in Drosophila, SYNE-1 may also associate with MACF1, which is attached to Rapsyn. As such, redundant mechanisms may function to tether SYNE-1–decorated nuclei in the myofiber subsynaptic region: one mechanism mediated by MACF1, which is attached to Rapsyn, and a second mediated by MuSK, which binds directly to SYNE-1. Such redundancy would provide a possible explanation for a near normal number of nuclei in the synaptic region of Macf1 mutant mice.
MACF1 plays an important role in recruiting MTs and MT-binding proteins, EB1 and Map1b, to neuromuscular synapses. A second pathway for recruiting MTs to the postsynaptic membrane is stimulated by Agrin and mediated by binding of MTs to CLASP2/CLIP170, which are recruited to the postsynaptic membrane by binding to LL5β (Schmidt et al., 2012; Basu et al., 2015). The deficits in synaptic size and synaptic AChR density appear similar in Clasp2 mutant and Macf1 muscle-conditional mutant mice (Basu et al., 2015). Although Clasp2 mutant mice failed to show gross deficits in motor behavior (Basu et al., 2015), it is unclear whether Clasp2 mutant mice have defects in synaptic transmission or motor performance. Moreover, it is unclear whether the reduction in synaptic size, evident in Macf1 muscle-conditionally mutant mice only after P30, is apparent in Clasp2 mutant mice throughout development or only after synaptic maturation.
The CLASP2 and MACF1 pathways do not appear to act in a redundant manner to recruit MTs and MT-binding proteins to the neuromuscular synapse. Notably, MAP1b, EB1, and β-TUB are lost from the postsynaptic membrane at neuromuscular synapses in Macf1 muscle-conditionally mutant mice. These findings suggest that CLASP2 is either absent or insufficient to tether these proteins to the postsynaptic membrane in the absence of MACF1.
Elegant studies have demonstrated a deficit in transport and insertion of AChR-containing secretory vesicles to the postsynaptic membrane in Clasp2 mutant mice (Basu et al., 2015), a role previously proposed for synaptic MTs (Jasmin et al., 1990; Marchand et al., 2002). Measurements of AChR synthesis will be necessary to determine whether impairment of this synthetic pathway contributes to the deficits found in Macf1 conditionally mutant mice.
We provide evidence that several proteins, including ITGA7, ARMS, and TOP2β, associate with AChRs in a Rapsyn-dependent manner. Prior studies demonstrated that ITGA7 and ARMS are concentrated at neuromuscular synapses (Martin et al., 1996; Luo et al., 2005), but it was unclear how these proteins become concentrated at synapses. Here, we show that their association with AChRs depends on Rapsyn. Surprisingly, we find that TOP2β, a nuclear protein known for its role in unknotting DNA during cell division and assisting in transcription of long transcripts (Wang, 2002; Ju et al., 2006; Austin et al., 2018), is also found outside the nucleus and at the postsynaptic membrane. Two different antibodies to TOP2β stain neuromuscular synapses, and staining is absent in muscle-conditional Top2β mutant mice, confirming that the antibodies recognize TOP2β. These findings raise several interesting questions: (1) whether a novel form of TOP2β is found outside the nucleus and at the postsynaptic membrane, (2) whether TOP2β binds directly to Rapsyn, (3) what role TOP2β plays at the postsynaptic membrane, (4) whether postsynaptic TOP2β is associated with nucleic acids, and (5) whether the catalytic activity of TOP2β is required for its function at the synapse.
CM is a heterogeneous group of neuromuscular diseases, often caused by recessive, hypomorphic mutations in genes that are responsible for building neuromuscular synapses or for the function of neuromuscular synapses. Mutations in these genes, including AGRIN, LRP4, MUSK, DOK-7, RAPSYN, ACHR, and CHAT, compromise synaptic transmission and lead to fatigable muscle weakness (Engel, 2018). We identified one patient with CM who carries two variants in MACF1 and a second patient who is homozygous for a different MACF1 variant. An earlier study identified a patient carrying a partial duplication of the MACF1 gene, leading to a decrease in MACF1 expression and presenting with diminished motor skills (Jørgensen et al., 2014). Although this patient was diagnosed with a novel myopathy, dubbed a “spectraplakinopathy type I” (Jørgensen et al., 2014), our findings, showing that variants in MACF1 are associated with CM, raise the possibility that the patient with the MACF1 partial duplication exhibits synaptic deficits, typical for CM. Here, we present a clinical case of CM in a patient carrying two variant missense alleles of MACF1. These findings suggest that mutations in MACF1 may lead to deficits in synaptic transmission and motor performance in humans, like mice, and cause CM. The patients were identified at 5 and 30 yr of age, comparatively late for a diagnosis of CM when caused by mutations in genes, such as RAPSYN or ACHR subunit genes, which have important roles in synapse formation. The late appearance of symptoms is consistent with a role for MACF1 in synaptic maturation and maintenance. The patients exhibited ptosis, limb girdle weakness, clinical fatigue, and a decrement in the muscle response to repetitive nerve stimulation. Surprisingly, defects in other organ systems were not evident. Two variants are present in an exon encoding the Plakin domain, and the second variant is present in an exon encoding the first Spectrin-like repeat. Macf1 is alternatively spliced (Hu et al., 2016), and it is possible that the variant forms are expressed from splice forms produced preferentially by muscle.
Consistent with a role for MACF1 as a scaffold for both actin and MT networks (Wu et al., 2008), we found that synaptic expression of Vinculin, an actin-binding protein, is dependent on MACF1. Thus, MACF1 serves as an organizing center at the postsynaptic membrane for both MT- and actin-based filament systems. Synaptic podosomes, which share features with synapses that are transitioning from an immature to a mature shape, contain MACF1, MT-associated proteins, and actin in their core and Vinculin at the cortex. How these proteins become rearranged as synapses mature and change shape is not understood, but MACF1 may participate and coordinate this reorganization.
Materials and methods
Mice
The following mice have been described previously: Rapsyn mutant mice (Gautam et al., 1995; MMRRC015108-UCD, RRID:MMRRC_015108-UCD); mice that carry a loxP-flanked allele of Macf1 (Wu et al., 2008); mice that carry a loxP-flanked allele of Top2β (Lyu and Wang, 2003); H2Kb-tsA58 transgenic mice (Jat et al., 1991; IMSR CRL:237, RRID:IMSR_CRL:237); and Hsa::Cre transgenic mice (Miniou et al., 1999). Mice, all maintained on a C57BL6 background, were housed and maintained according to Institutional Animal Use and Care Committee guidelines.
Growth of cultured skeletal muscle cells
C2C12 mouse muscle cells (ATCC CRL-1772, RRID:CVCL_0188) were grown at 37°C in growth medium: DMEM containing 4.5 g/l glucose, L-glutamine, and sodium pyruvate (Corning cellgro), supplemented with 10% FBS (GemCell). Myoblast fusion and myotube differentiation were induced when myoblasts were 70% confluent by switching to differentiation medium: DMEM with 4.5 g/liter glucose and 1 mM L-glutamine, supplemented with 2% heat-inactivated horse serum. Immortalized myoblasts were isolated from WT and Rapsyn mutant embryos and grown as described previously (Smith et al., 2001).
Isolation of AChR complexes
Whole leg muscles or immortalized muscle cells were homogenized at 4°C in lysis buffer (50 mM sodium chloride, 30 mM triethanolamine, pH 7.5, 50 mM sodium fluoride, 5 mM EDTA, 5 mM EGTA, 2 mM sodium orthovanadate, 1 mM benzamidine, 1 mM N-ethylmaleimide, 1 mM sodium tetrathionate, 5 µg/ml leupeptide, 5 µg/ml aprotinin, and 10 µM pepstatin, plus complete protease inhibitors; Roche). NP-40 was added to a final concentration of 1%, and the extract was incubated with rocking for 30 min at 4°C. In another experiment, we substituted digitonin for NP-40 (Table S2). Insoluble proteins were removed by centrifugation at 12,000 rpm for 20 min at 4°C. The supernatant was precleared for 1 h at 4°C with streptavidin-agarose beads (Sigma-Aldrich) before incubation for 1 h at 4°C with biotin–α-BGT (10−8 M; α-BGT; Invitrogen). Biotin–α-BGT/AChR complexes were incubated for 4 h with streptavidin-agarose beads. The beads were subsequently washed (three times for 9 min) in lysis buffer containing 1% NP-40. Proteins were eluted from the beads with 1% SDS in lysis buffer.
MS
To identify the proteins that require Rapsyn for their association with AChRs, we used skeletal muscle tissue from E18.5 WT and Rapsyn−/− mice and immortalized muscle cell lines. We isolated AChRs, as described above. To ensure that the isolated proteins were bound specifically to the AChR, we performed two control experiments. First, we omitted biotin–α-BGT from the incubation with cell lysates. As such, AChRs were unable to specifically bind to the streptavidin-agarose beads. Second, we preincubated cell lysates with a saturating dose (10−7 M) of unlabeled α-BGT to block binding of biotin–α-BGT and prevent binding of AChRs to the streptavidin-agarose beads. To confirm that the reduced recovery of MACF1 from Rapsyn mutant muscle was due to a loss of Rapsyn, we restored Rapsyn expression in Rapsyn muscle cells and confirmed by MS that restoring Rapsyn expression rescued coisolation of MACF1 with AChRs (data not shown). AChR complexes from WT and Rapsyn−/− mice and muscle cell lines were allowed to briefly enter (5 min at 180 V) SDS-polyacrylamide gels (4–12% acrylamide; Invitrogen). A single gel slice containing the unseparated proteins was excised from gels stained overnight with Coomassie blue, and the proteins were digested using MS-grade trypsin (Promega). To identify AChR partners, tryptic peptides were analyzed using nanoflow LC/ESI-MS/MS with a Q-Exactive HF quadrupole-Orbitrap coupled to a NanoEasy UPLC system (Thermo Fisher Scientific) running a gradient of 3–90% acetonitrile in 0.1% formic acid over 60 min (3–40% for 50 min, 40–90% for 5 min, and 90% for 5 min) at a 250 nl/min LC flow rate. The following acquisition parameters were specified: resolution 120,000, AGC target 3e6, MS1 scan range 300–1,750 Th, profile mode. The collision induced dissociation (MS2) parameters were as follows: top 15 data dependent acquisition (DDA) was selected, resolution was set to 15,000, the automatic gain control (AGC) target was set to 1e6, injection time was limited to 60 ms, normalized collision energy (NCE/stepped) was set to NCE 27, dynamic exclusion was set to 30 s, and ions with charge 1 or unassigned were excluded. Peptides were identified using MaxQuant/Andromeda version (Cox et al., 2011) using standard parameters (Gauthier et al., 2017) to search the Mouse fasta (date: 01092015, containing 16,699 protein sequences). Once identified, relative abundance of proteins was determined by integrating the mass spectral signal intensity for the identified peptides. The number of identified peptides and the percentage sequence coverage for each protein were also used to confirm the intensity-based relative quantitation of proteins. We required the identification of five or more peptides and sequence coverage greater than 4% to consider a protein further. The relative abundance of each identified protein based on spectral intensity was normalized to the abundance level of AChR subunits, and the ratio of relative protein abundance in AChR-bound complexes from WT/Rapsyn−/− muscle was calculated. MS data represent the mean values from three experiments with muscle tissues and three experiments with immortalized muscle cell lines. The Wilcoxon–Mann–Whitney test was used to determine statistical significance and was conducted using GraphPad Prism 6.0 software.
Western blotting
Proteins were fractionated by SDS-PAGE (6% or 8% acrylamide; Invitrogen) and transferred to polyvinylidene fluoride membranes. Blots were probed with antibodies to the AChR β-subunit (N8283, RRID:AB_262151; Sigma-Aldrich), Rapsyn (ab156002, RRID:AB_298028; Abcam), MACF1 (gift from R. Liem, Columbia University Medical Center, New York, NY; Chen et al., 2006), β-Catenin (ab32572, RRID:AB_725966; Abcam), α-Actinin (ab18061, RRID:AB_444218; Abcam), Calpain (ab28258, RRID:AB_725819; Abcam), or MAP1b (ab11266, RRID:AB_297884; Abcam). We used antibodies to the AChR β-subunit to ensure equal loading of proteins in whole-cell lysates and proteins isolated with biotin–α-BGT. We quantified the band intensities with a ChemiDoc imaging system (BioRad), as described previously (Remédio et al., 2016). The band intensity for each protein was indexed to the band intensity of the AChR β-subunit, and the graphs show the mean values from three separate experiments. The Wilcoxon–Mann–Whitney test was used to determine statistical significance and was conducted using GraphPad Prism 6.0 software.
Whole-mount muscle immunohistochemistry
Innervated and denervated hindlimb muscles from P60 mice were fixed in 1% PFA (in PBS) for 1 h at 4°C, rinsed twice at 4°C in PBS, cryoprotected overnight at 4°C (in PBS with 30% sucrose), and embedded in TissueTek (Sakura). Frozen sections (10 µm) were stained with antibodies to MACF1 (ab117418, RRID:AB_10898474; Abcam), TOP2β (MAB6348, RRID:AB_10889629; R&D Systems; 109524, RRID:AB_10859793; Abcam), Integrin α7 (sc-27706, RRID:AB_2265042; Santa Cruz Biotechnology), MAP1b (ab11266, RRID:AB_297884; Abcam) or Synapsin (106002, RRID:AB_887804; Synaptic Systems), and AChRs were labeled with Alexa Fluor 594–α-BGT (Invitrogen). Images were acquired with a Zeiss LSM 700 confocal microscope equipped with a 40× (1.4 NA) objective. Hindlimb muscles were denervated by cutting the sciatic nerve, as described previously by Simon et al. (1992), 1 wk before the dissection.
Diaphragm muscles were dissected from E18.5 embryos and postnatal mice in oxygenated L-15 medium. The muscles were pinned onto Sylgard-coated dissection dishes, fixed for 1.5 h in 1% PFA, and blocked for 1 h in PBS with 3% BSA (IgG free; Sigma-Aldrich) and 0.5% Triton X-100 (PBT). Primary antibodies, diluted in PBT, were force-pipetted into the muscle, and the muscles were incubated overnight at 4°C on an orbital shaker in a humidified chamber. Diaphragm muscles were washed 10 times over the course of 5 h with PBT at room temperature and rinsed in PBS before the muscle was whole-mounted in 50% glycerol. Muscles from at least three mice of each genotype were analyzed for each experiment. Diaphragm muscles were stained with Alexa Fluor 488–conjugated α-BGT (Invitrogen) to label AChRs and with antibodies to Neurofilament-L (171002, RRID:AB_887743; Synaptic Systems), β-TUBIII (302302, RRID:AB_10637424; Synaptic Systems), or Synapsin 1/2 (106002, RRID:AB_887804; Synaptic Systems) to label motor axons and nerve terminals, respectively (Kim and Burden, 2008). Images were acquired with a Zeiss LSM 700 confocal microscope, and the size of synapses, density of synaptic AChRs, and colocalization (Synapsin/AChRs) from at least three mice were measured as described previously (Jaworski and Burden, 2006). We defined small synapses as those that were ≥1 SD smaller than the mean size of synapses in Macf1f/+ control mice. Fragmented synapses contained five or fewer fragments, as defined by others (Valdez et al., 2010). The Wilcoxon–Mann–Whitney test was used to determine statistical significance and was conducted using GraphPad Prism 6.0 software.
Isolation and staining single muscle fibers
Tibialis anterior and lumbrical muscles were dissected in oxygenated L-15 medium and pinned to a Sylgard-coated dish and fixed in 2% PFA (in PBS) for 2 h. After several rinses in PBS, one to three myofibers were manually teased with fine forceps (Ralston et al., 1999). Fixed myofibers were blocked for 2 h at room temperature in PBS containing 5% BSA, 1% normal goat serum, and 0.04% saponin. Fibers were then incubated with primary antibodies for 2 h at room temperature (or overnight at 4°C), washed three times for 5 min with PBS containing 0.04% saponin, incubated with secondary antibodies for 2 h at room temperature, washed again, counterstained with Hoechst 33342 (4082S, RRID:AB_10626776; Cell Signaling Technology), and mounted in VectaShield (Vector Laboratories). Antibodies to Synapsin (106002, RRID:AB_887804; Synaptic Systems) were used to mark synaptic vesicles in nerve terminals. We used antibodies to MAP1b (ab11266, RRID:AB_297884; Abcam), EB1 (610534, RRID:AB_397891; BD Biosciences), Vinculin (V4505, RRID:AB_477617; Sigma-Aldrich), α-TUB (05–829, RRID:AB_310035; Millipore), and β-TUB (T5293, RRID:AB_477580; Sigma-Aldrich) to label these MT- and actin-binding proteins. The postsynaptic membrane was visualized by staining with Alexa Fluor 488–α-BGT (Invitrogen), antibodies to Rapsyn (MA1-746, RRID:AB_2177611; Thermo Fisher Scientific), and antibodies to MACF1 (ab117418, RRID:AB_10898474; Abcam). We quantified the number of synaptic and perisynaptic myonuclei by staining optically sectioned dissected single myofibers with antibodies to Synapsin 1/2 (106002, RRID:AB_887804; Synaptic Systems) and with a nuclear stain, Hoechst 33342 (4082S, RRID:AB_10626776; Cell Signaling Technology). This procedure allowed us to distinguish myofiber subsynaptic nuclei from Schwann cell nuclei as described previously (Grady et al., 2005).
Histology of muscle sections
Tibialis anterior muscles were fixed in 4% PFA (in PBS) for 72 h, dehydrated, and embedded in paraffin. 5-µm sections were stained with hematoxylin and eosin. Images were captured using a Zeiss Axiophot microscope. Cross-sectional areas of tibialis anterior muscles, the number of myofibers per area (40,000 µm2), and the number of central nuclei were measured using ImageJ software. The Wilcoxon–Mann–Whitney test was used to determine statistical significance and was conducted using GraphPad Prism 6.0 software. Muscles were prepared for electron microscopy as described previously (Wang et al., 2005; Friese et al., 2007).
Induction of AChR clusters and podosomes by Laminin
35-mm tissue culture dishes were coated with 5 µg/ml polyornithine (Sigma-Aldrich) in distilled water for 30 min and air-dried. Engelbreth-Holm-Swarm laminin (10 µg/ml in L-15 medium supplemented with 0.2% NaHCO3; Invitrogen) was applied to the polyornithine-coated dishes, which were incubated overnight at 37°C. Following removal of the laminin solution, myoblasts were plated and grown to 70% confluency before they were switched to differentiation medium and allowed to differentiate for 3–5 d. After a 45-min incubation with Alexa Fluor 488–conjugated α-BGT (Invitrogen) to stain AChRs, cells were washed in PBS and fixed in 1% PFA in PBS. Free aldehydes were blocked with 0.1 M glycine (pH 7.3), and cells were incubated with primary antibodies overnight at 4°C in PBS with 4% BSA and 0.2% Triton X-100 (PBT2). Cells were washed three times in PBS for 5 min, incubated for 2 h with secondary antibodies in PBT2, washed three times in PBS for 5 min, and mounted in VectaShield (Vector Labs).
Agrin-induced formation of AChR clusters in primary myotubes
Myoblasts were isolated from E17.5–18.5 embryos and grown in culture as described previously (Remédio et al., 2016). Myotubes were treated for 8 h with 500 pM Agrin (R&D Systems), rinsed three times in PBS, and fixed for 10 min with 1% PFA (in PBS). After washing twice for 10 min in PBS, the cultures were incubated in 0.1 M glycine (in PBS) for 10 min, rinsed twice in PBS, and incubated for 30 min in PBS with 1% BSA. Cultures were incubated with Alexa Fluor 594–α-BGT (1:1,000, in 1% BSA-PBS; Invitrogen) for 1 h, washed in PBS (three times for 30 min), permeabilized with PBS containing 0.1% Triton X-100 for 5 min, stained with antibodies to myosin heavy chain (in PBS with 1% BSA; M4276, RRID:AB_477190; Sigma-Aldrich) overnight at 4°C in PBS with 4% BSA, washed three times in PBS for 5 min, incubated for 2 h with secondary antibodies in 4% BSA-PBS, washed three times in PBS for 5 min, and mounted in VectaShield (Vector Labs). Images were captured using a Zeiss LSM 700 confocal microscope, and the number and size of AChR clusters were determined using ImageJ software, as described previously (Wang et al., 2005; Friese et al., 2007). We analyzed triplicate cultures from three mice, and >40 AChR clusters in each culture dish were analyzed. The Wilcoxon–Mann–Whitney test was used to determine statistical significance and was conducted using GraphPad Prism 6.0 software.
Image analysis
Images were acquired with a Zeiss LSM 700 or LSM 800 confocal microscope. Optical sections were acquired with a confocal laser scanning microscope Zeiss LSM 880 equipped with an Airyscan detection unit. To maximize the resolution enhancement, we used a high NA oil immersion α Plan-Apochromat 100×/1.46 Oil Corr M27 objective (Zeiss). All images were collected using Immersol 518F immersion media with a matched refractive index (nc = 1.518; 30°C; Zeiss). Detector gain and pixel dwell times were adjusted for each dataset, keeping them at their lowest values in order to avoid saturation and bleaching. Laser intensity values at the sample plane were measured with a Microscope Slide Thermal Sensor (Thorlabs). Zen Black 2.1 (Version 13.0.0.0) software, which processes each of the 32 Airy detector channels separately by performing filtering, deconvolution, and pixel reassignment, was used to process the acquired datasets and obtain images with enhanced spatial resolution. To determine the degree of colocalization between proteins stained with spectrally distinct fluorophores, we calculated the Pearson’s correlation coefficient (PCC) as described (Costes et al., 2004).
Whole exome sequencing and human data analysis/sharing
DNA samples isolated from blood were prepared for exome sequencing using the Nextera Exome Enrichment method from Illumina, using 50 ng of gDNA. Whole exome sequencing samples were sequenced on Illumina HiSeq 2500 instruments. Reads were aligned to NCBI Build 37 of the human reference sequence using Burrows-Wheeler Aligner 0.7.8, and variants were identified using Genome Analysis Toolkit HaplotypeCaller 3.6 generating a genomic Variant Call Format. All positions with genotype quality score (GQ) > 19 and overall read depth (DP) > 7 were uploaded onto the RD-Connect Genome-Phenome Analysis Platform (https://platform.rd-connect.eu/genomics/). Data were initially analyzed applying standard filtering criteria (high/moderate variant effect predictor and minor allele frequency < 0.01) and a gene list of all known CM-causing genes. As no variants in known CM genes were identified, the full genome was interrogated in search of a novel candidate gene. Candidate variants were confirmed and segregated in unaffected family members by Sanger sequencing. To identify additional cases, a large cohort of more than 3,000 exomes was interrogated for homozygous or compound heterozygous rare variants in MACF1.
Electrophysiology
Intracellular recordings were performed in lumbrical muscles from muscle-conditional Macf1 mutant mice (n = 4, Hsa::Cre; Macf1f/-) and their littermate controls (n = 4, Macf1f/+) at 7 to ∼8 mo of age, using methods described previously (Chen et al., 2010; Sugiura et al., 2011). Briefly, lumbrical muscles with plantar nerves attached were acutely isolated in oxygenated (95% O2, 5% CO2) Ringer’s solution (136.8 mM sodium chloride, 5 mM potassium chloride, 12 mM sodium bicarbonate, 1 mM sodium phosphate, 1 mM magnesium chloride, 2 mM calcium chloride, and 11 mM D-glucose, pH 7.3; Liley, 1956). Endplate regions, identified under a water-immersion objective (Olympus BX51WI), were impaled with glass micropipettes (resistance 20–40 MΩ) filled with 2 M potassium citrate and 10 mM potassium chloride. EPPs were elicited by supra-threshold stimulation (2 V, 0.1 ms) of the nerve via a suction electrode connected to an extracellular stimulator (SD9; Grass-Telefactor). A muscle-specific sodium channel blocker, μ-Conotoxin GIIIB (2 µM; Peptides International), was added to the bath solution 30 min before recording to prevent muscle contraction during recording. mEPPs and EPPs were acquired using an intracellular amplifier (AxoClamp-2B) and digitized with Digidata 1332 (Molecular Devices). Data were analyzed with pClamp 10.7 (Molecular Devices) and Mini Analysis Program (Synaptosoft, Inc.). To determine the quantal content, a direct method was applied by dividing EPPs over mEPPs as described previously (Boyd and Martin, 1956; Hubbard et al., 1969). The Wilcoxon–Mann–Whitney test was used to determine statistical significance and was conducted using GraphPad Prism 6.0 software.
Behavior
Grip strength was measured using a grip strength apparatus (Bioseb), which measures both forelimb and all-limb grip strength. To measure forelimb grip strength, the mouse was positioned in the center of a metal grid and held gently at the base of its tail so that only its front paws were able to grip the grid. The mouse was pulled back steadily, until the forelimb grip was released from the grid. The grip strength meter digitally displayed the maximum force applied (in grams) as the grasp was released. For the all-limb measurements, the mouse was allowed to grip the grid with both forelimbs and hindlimbs, and the mouse was pulled back steadily, until the mouse lost grip with the grid. The means of six consecutive trials of both forelimb and all-limb measurements were taken as an index of forelimb or all-limb grip strength. Mice were given an interval of 10–15 s between trials and 1–3 h between forelimb and all-limb testing. Body weight was determined after all grip strength measurements to analyze for potential covariability. To enhance the robustness and reliability of the grip strength assessment, all measurements were taken by the same experimenter (Mandillo et al., 2008).
Motor function of male and female mice at P90 and P180 was assessed on a rotarod (AccuRotor four-channel, Omnitech Electronics, Inc.). Mice were placed on the rotarod (3.0-cm rotating cylinder) rotating at 2.5 rpm, and the speed of rotation was increased linearly to 40 rpm over the course of 5 min. The time to fall from the rod was measured. Each mouse was subjected to three trials in 5-min intervals, and we recorded the longest latency to fall from the three trials. The Wilcoxon–Mann–Whitney test was used to determine statistical significance and was conducted using GraphPad Prism 6.0 software.
Online supplemental material
In Fig. S1, ARMS, Integrin α7, and Topoisomerase2β are enriched at the neuromuscular synapse. In Fig. S2, MACF1 is not essential for the Agrin-stimulated formation of AChRs clusters in muscle primary cultures. In Fig. S3, neuromuscular synapses in Macf1 muscle-conditional mutant mice are deteriorated in both tibialis anterior and lumbrical muscles at P365. In Fig. S4, MAP1b coisolates with AChRs in a Rapsyn-dependent manner. Table S1 shows a summary of Rapsyn-dependent AChR partners that were identified by MS both in muscle tissue and in cultured myotubes. Table S2 shows Rapsyn-dependent AChR partners that were identified by MS in muscle tissue using Digitonin as a detergent.
Supplementary Material
Acknowledgments
We thank Dr. Adam Mar, director of the Rodent Behavior Core at New York University Medical School, and Begona Gamallo-Lana for their assistance with the motor performance tests; the Histology Core for their assistance with preparation and staining of paraffin sections; and the Imaging Core, notably Fengxia Liang, Kristen Dancel-Manning, and Chris Petzold, for their help with our electron microscopy studies. We are grateful to Dr. Ronald Liem for providing antibodies to MACF1, Evelyn Ralston for her help with staining protocols for MTs, and Clara Franzini-Armstrong for her insight and comments on the ultrastructure of muscle from Macf1 mutant mice. We thank Dr. Atchayaram Nalini for clinical data, and Dr. Vedrana Milic-Rasic for electromyography. We are grateful to Steve Laurie and Sergi Beltran from the Centro Nacional de Análisis Genómico for their help with the processing of whole exome sequencing data.
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (RO1 NS075124 to S.J. Burden, P30 NS050276 to T.A. Neubert, and RO1 NS055028 to W. Lin) and the National Center for Research Resources (S10 RR027990) as well as funding from the European Community’s Seventh Framework Program (FP7/2007-2013) under grant agreements RD-Connect and NeurOmics.
The authors declare no competing financial interests.
Author contributions: J. Oury, Y. Liu, T.A. Neubert, W. Lin, H. Lochmüller, and S.J. Burden were involved in the design, acquisition, and interpretation of the experiments. A. Töpf, S. Todorovic, and V. Preethish-Kumar were involved in clinical analysis of the patients with CM.
References
- Antolik C., Catino D.H., O’Neill A.M., Resneck W.G., Ursitti J.A., and Bloch R.J.. 2007. The actin binding domain of ACF7 binds directly to the tetratricopeptide repeat domains of rapsyn. Neuroscience. 145:56–65. 10.1016/j.neuroscience.2006.11.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel E.D., Lewis R.M., Grady R.M., and Sanes J.R.. 2000. Syne-1, a dystrophin- and Klarsicht-related protein associated with synaptic nuclei at the neuromuscular junction. J. Biol. Chem. 275:31986–31995. 10.1074/jbc.M004775200 [DOI] [PubMed] [Google Scholar]
- Austin C.A., Lee K.C., Swan R.L., Khazeem M.M., Manville C.M., Cridland P., Treumann A., Porter A., Morris N.J., and Cowell I.G.. 2018. TOP2B: The First Thirty Years. Int. J. Mol. Sci. 19:E2765 10.3390/ijms19092765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks G.B., Fuhrer C., Adams M.E., and Froehner S.C.. 2003. The postsynaptic submembrane machinery at the neuromuscular junction: requirement for rapsyn and the utrophin/dystrophin-associated complex. J. Neurocytol. 32:709–726. 10.1023/B:NEUR.0000020619.24681.2b [DOI] [PubMed] [Google Scholar]
- Bartoli M., Ramarao M.K., and Cohen J.B.. 2001. Interactions of the rapsyn RING-H2 domain with dystroglycan. J. Biol. Chem. 276:24911–24917. 10.1074/jbc.M103258200 [DOI] [PubMed] [Google Scholar]
- Basu S., Sladecek S., Martinez de la Peña y Valenzuela I., Akaaboune M., Smal I., Martin K., Galjart N., and Brenner H.R.. 2015. CLASP2-dependent microtubule capture at the neuromuscular junction membrane requires LL5β and actin for focal delivery of acetylcholine receptor vesicles. Mol. Biol. Cell. 26:938–951. 10.1091/mbc.E14-06-1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch R.J., and Hall Z.W.. 1983. Cytoskeletal components of the vertebrate neuromuscular junction: vinculin, alpha-actinin, and filamin. J. Cell Biol. 97:217–223. 10.1083/jcb.97.1.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd I.A., and Martin A.R.. 1956. The end-plate potential in mammalian muscle. J. Physiol. 132:74–91. 10.1113/jphysiol.1956.sp005503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N.H. 2008. Spectraplakins: the cytoskeleton’s Swiss army knife. Cell. 135:16–18. 10.1016/j.cell.2008.09.023 [DOI] [PubMed] [Google Scholar]
- Burden S.J. 1993. Synapse-specific gene expression. Trends Genet. 9:12–16. 10.1016/0168-9525(93)90066-Q [DOI] [PubMed] [Google Scholar]
- Burden S.J. 1998. The formation of neuromuscular synapses. Genes Dev. 12:133–148. 10.1101/gad.12.2.133 [DOI] [PubMed] [Google Scholar]
- Burden S.J., DePalma R.L., and Gottesman G.S.. 1983. Crosslinking of proteins in acetylcholine receptor-rich membranes: association between the beta-subunit and the 43 kd subsynaptic protein. Cell. 35:687–692. 10.1016/0092-8674(83)90101-0 [DOI] [PubMed] [Google Scholar]
- Burden S.J., Yumoto N., and Zhang W.. 2013. The role of MuSK in synapse formation and neuromuscular disease. Cold Spring Harb. Perspect. Biol. 5:a009167 10.1101/cshperspect.a009167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkin D.J., Gu M., Hodges B.L., Campanelli J.T., and Kaufman S.J.. 1998. A functional role for specific spliced variants of the alpha7beta1 integrin in acetylcholine receptor clustering. J. Cell Biol. 143:1067–1075. 10.1083/jcb.143.4.1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Qian L., Yang Z.H., Huang Y., Ngo S.T., Ruan N.J., Wang J., Schneider C., Noakes P.G., Ding Y.Q., et al. 2007. Rapsyn interaction with calpain stabilizes AChR clusters at the neuromuscular junction. Neuron. 55:247–260. 10.1016/j.neuron.2007.06.031 [DOI] [PubMed] [Google Scholar]
- Chen F., Sugiura Y., Myers K.G., Liu Y., and Lin W.. 2010. Ubiquitin carboxyl-terminal hydrolase L1 is required for maintaining the structure and function of the neuromuscular junction. Proc. Natl. Acad. Sci. USA. 107:1636–1641. 10.1073/pnas.0911516107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.J., Lin C.M., Lin C.S., Perez-Olle R., Leung C.L., and Liem R.K.. 2006. The role of microtubule actin cross-linking factor 1 (MACF1) in the Wnt signaling pathway. Genes Dev. 20:1933–1945. 10.1101/gad.1411206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes S.V., Daelemans D., Cho E.H., Dobbin Z., Pavlakis G., and Lockett S.. 2004. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys. J. 86:3993–4003. 10.1529/biophysj.103.038422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J., Neuhauser N., Michalski A., Scheltema R.A., Olsen J.V., and Mann M.. 2011. Andromeda: a peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 10:1794–1805. 10.1021/pr101065j [DOI] [PubMed] [Google Scholar]
- Dobbins G.C., Luo S., Yang Z., Xiong W.C., and Mei L.. 2008. alpha-Actinin interacts with rapsyn in agrin-stimulated AChR clustering. Mol. Brain. 1:18 10.1186/1756-6606-1-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A.G. 2018. Genetic basis and phenotypic features of congenital myasthenic syndromes. Handb. Clin. Neurol. 148:565–589. 10.1016/B978-0-444-64076-5.00037-5 [DOI] [PubMed] [Google Scholar]
- Engel A.G., Shen X.M., Selcen D., and Sine S.M.. 2015. Congenital myasthenic syndromes: pathogenesis, diagnosis, and treatment. Lancet Neurol. 14:420–434. 10.1016/S1474-4422(14)70201-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Aguirre M., Zhang H., Jamieson-Lucy A., and Mullins M.C.. 2017. Microtubule-actin crosslinking factor 1 (Macf1) domain function in Balbiani body dissociation and nuclear positioning. PLoS Genet. 13:e1006983 10.1371/journal.pgen.1006983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertuck H.C., and Salpeter M.M.. 1976. Quantitation of junctional and extrajunctional acetylcholine receptors by electron microscope autoradiography after 125I-alpha-bungarotoxin binding at mouse neuromuscular junctions. J. Cell Biol. 69:144–158. 10.1083/jcb.69.1.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese M.B., Blagden C.S., and Burden S.J.. 2007. Synaptic differentiation is defective in mice lacking acetylcholine receptor beta-subunit tyrosine phosphorylation. Development. 134:4167–4176. 10.1242/dev.010702 [DOI] [PubMed] [Google Scholar]
- Fuhrer C., and Hall Z.W.. 1996. Functional interaction of Src family kinases with the acetylcholine receptor in C2 myotubes. J. Biol. Chem. 271:32474–32481. 10.1074/jbc.271.50.32474 [DOI] [PubMed] [Google Scholar]
- Gautam M., Noakes P.G., Mudd J., Nichol M., Chu G.C., Sanes J.R., and Merlie J.P.. 1995. Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature. 377:232–236. 10.1038/377232a0 [DOI] [PubMed] [Google Scholar]
- Gautam M., Noakes P.G., Moscoso L., Rupp F., Scheller R.H., Merlie J.P., and Sanes J.R.. 1996. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 85:525–535. 10.1016/S0092-8674(00)81253-2 [DOI] [PubMed] [Google Scholar]
- Gauthier S.A., Pérez-González R., Sharma A., Huang F.K., Alldred M.J., Pawlik M., Kaur G., Ginsberg S.D., Neubert T.A., and Levy E.. 2017. Enhanced exosome secretion in Down syndrome brain - a protective mechanism to alleviate neuronal endosomal abnormalities. Acta Neuropathol. Commun. 5:65 10.1186/s40478-017-0466-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilhus N.E., and Verschuuren J.J.. 2015. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 14:1023–1036. 10.1016/S1474-4422(15)00145-3 [DOI] [PubMed] [Google Scholar]
- Grady R.M., Zhou H., Cunningham J.M., Henry M.D., Campbell K.P., and Sanes J.R.. 2000. Maturation and maintenance of the neuromuscular synapse: genetic evidence for roles of the dystrophin--glycoprotein complex. Neuron. 25:279–293. 10.1016/S0896-6273(00)80894-6 [DOI] [PubMed] [Google Scholar]
- Grady R.M., Starr D.A., Ackerman G.L., Sanes J.R., and Han M.. 2005. Syne proteins anchor muscle nuclei at the neuromuscular junction. Proc. Natl. Acad. Sci. USA. 102:4359–4364. 10.1073/pnas.0500711102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Su P., Li R., Yin C., Zhang Y., Shang P., Yang T., and Qian A.. 2016. Isoforms, structures, and functions of versatile spectraplakin MACF1. BMB Rep. 49:37–44. 10.5483/BMBRep.2016.49.1.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J.I., Wilson D.F., and Miyamoto M.. 1969. Reduction of transmitter release by D-tubocurarine. Nature. 223:531–533. 10.1038/223531a0 [DOI] [PubMed] [Google Scholar]
- Jasmin B.J., Changeux J.P., and Cartaud J.. 1990. Compartmentalization of cold-stable and acetylated microtubules in the subsynaptic domain of chick skeletal muscle fibre. Nature. 344:673–675. 10.1038/344673a0 [DOI] [PubMed] [Google Scholar]
- Jat P.S., Noble M.D., Ataliotis P., Tanaka Y., Yannoutsos N., Larsen L., and Kioussis D.. 1991. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc. Natl. Acad. Sci. USA. 88:5096–5100. 10.1073/pnas.88.12.5096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski A., and Burden S.J.. 2006. Neuromuscular synapse formation in mice lacking motor neuron- and skeletal muscle-derived Neuregulin-1. J. Neurosci. 26:655–661. 10.1523/JNEUROSCI.4506-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen L.H., Mosbech M.B., Færgeman N.J., Graakjaer J., Jacobsen S.V., and Schrøder H.D.. 2014. Duplication in the microtubule-actin cross-linking factor 1 gene causes a novel neuromuscular condition. Sci. Rep. 4:5180 10.1038/srep05180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju B.G., Lunyak V.V., Perissi V., Garcia-Bassets I., Rose D.W., Glass C.K., and Rosenfeld M.G.. 2006. A topoisomerase IIbeta-mediated dsDNA break required for regulated transcription. Science. 312:1798–1802. 10.1126/science.1127196 [DOI] [PubMed] [Google Scholar]
- Katz B. 1966. Nerve, Muscle, and Synapse. McGraw-Hill, New York. 193 pp. [Google Scholar]
- Katz B., and Thesleff S.. 1957. On the factors which determine the amplitude of the miniature end-plate potential. J. Physiol. 137:267–278. 10.1113/jphysiol.1957.sp005811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N., and Burden S.J.. 2008. MuSK controls where motor axons grow and form synapses. Nat. Neurosci. 11:19–27. 10.1038/nn2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N., Stiegler A.L., Cameron T.O., Hallock P.T., Gomez A.M., Huang J.H., Hubbard S.R., Dustin M.L., and Burden S.J.. 2008. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 135:334–342. 10.1016/j.cell.2008.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koneczny I., Cossins J., and Vincent A.. 2014. The role of muscle-specific tyrosine kinase (MuSK) and mystery of MuSK myasthenia gravis. J. Anat. 224:29–35. 10.1111/joa.12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer T.T., Misgeld T., Lichtman J.W., and Sanes J.R.. 2004. Nerve-independent formation of a topologically complex postsynaptic apparatus. J. Cell Biol. 164:1077–1087. 10.1083/jcb.200401115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Turkanis S.A., and Weakly J.N.. 1971. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J. Physiol. 213:545–556. 10.1113/jphysiol.1971.sp009399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Cao Y., Wu H., Ye X., Zhu Z., Xing G., Shen C., Barik A., Zhang B., Xie X., et al. 2016. Enzymatic Activity of the Scaffold Protein Rapsyn for Synapse Formation. Neuron. 92:1007–1019. 10.1016/j.neuron.2016.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Xiong W.C., and Mei L.. 2018. Neuromuscular Junction Formation, Aging, and Disorders. Annu. Rev. Physiol. 80:159–188. 10.1146/annurev-physiol-022516-034255 [DOI] [PubMed] [Google Scholar]
- Li X.M., Dong X.P., Luo S.W., Zhang B., Lee D.H., Ting A.K., Neiswender H., Kim C.H., Carpenter-Hyland E., Gao T.M., et al. 2008. Retrograde regulation of motoneuron differentiation by muscle beta-catenin. Nat. Neurosci. 11:262–268. 10.1038/nn2053 [DOI] [PubMed] [Google Scholar]
- Liley A.W. 1956. An investigation of spontaneous activity at the neuromuscular junction of the rat. J. Physiol. 132:650–666. 10.1113/jphysiol.1956.sp005555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S., Chen Y., Lai K.O., Arévalo J.C., Froehner S.C., Adams M.E., Chao M.V., and Ip N.Y.. 2005. alpha-Syntrophin regulates ARMS localization at the neuromuscular junction and enhances EphA4 signaling in an ARMS-dependent manner. J. Cell Biol. 169:813–824. 10.1083/jcb.200412008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu Y.L., and Wang J.C.. 2003. Aberrant lamination in the cerebral cortex of mouse embryos lacking DNA topoisomerase IIbeta. Proc. Natl. Acad. Sci. USA. 100:7123–7128. 10.1073/pnas.1232376100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandillo S., Tucci V., Hölter S.M., Meziane H., Banchaabouchi M.A., Kallnik M., Lad H.V., Nolan P.M., Ouagazzal A.M., Coghill E.L., et al. 2008. Reliability, robustness, and reproducibility in mouse behavioral phenotyping: a cross-laboratory study. Physiol. Genomics. 34:243–255. 10.1152/physiolgenomics.90207.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand S., Devillers-Thiéry A., Pons S., Changeux J.P., and Cartaud J.. 2002. Rapsyn escorts the nicotinic acetylcholine receptor along the exocytic pathway via association with lipid rafts. J. Neurosci. 22:8891–8901. 10.1523/JNEUROSCI.22-20-08891.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P.T., Kaufman S.J., Kramer R.H., and Sanes J.R.. 1996. Synaptic integrins in developing, adult, and mutant muscle: selective association of alpha1, alpha7A, and alpha7B integrins with the neuromuscular junction. Dev. Biol. 174:125–139. 10.1006/dbio.1996.0057 [DOI] [PubMed] [Google Scholar]
- McMahan U.J. 1990. The agrin hypothesis. Cold Spring Harb. Symp. Quant. Biol. 55:407–418. 10.1101/SQB.1990.055.01.041 [DOI] [PubMed] [Google Scholar]
- Merlie J.P., and Sanes J.R.. 1985. Concentration of acetylcholine receptor mRNA in synaptic regions of adult muscle fibres. Nature. 317:66–68. 10.1038/317066a0 [DOI] [PubMed] [Google Scholar]
- Miniou P., Tiziano D., Frugier T., Roblot N., Le Meur M., and Melki J.. 1999. Gene targeting restricted to mouse striated muscle lineage. Nucleic Acids Res. 27:e27 10.1093/nar/27.19.e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J.S., Mihaylova V., Abicht A., and Lochmüller H.. 2007. Congenital myasthenic syndromes: spotlight on genetic defects of neuromuscular transmission. Expert Rev. Mol. Med. 9:1–20. 10.1017/S1462399407000427 [DOI] [PubMed] [Google Scholar]
- Neubig R.R., Krodel E.K., Boyd N.D., and Cohen J.B.. 1979. Acetylcholine and local anesthetic binding to Torpedo nicotinic postsynaptic membranes after removal of nonreceptor peptides. Proc. Natl. Acad. Sci. USA. 76:690–694. 10.1073/pnas.76.2.690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proszynski T.J., Gingras J., Valdez G., Krzewski K., and Sanes J.R.. 2009. Podosomes are present in a postsynaptic apparatus and participate in its maturation. Proc. Natl. Acad. Sci. USA. 106:18373–18378. 10.1073/pnas.0910391106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston E., Lu Z., and Ploug T.. 1999. The organization of the Golgi complex and microtubules in skeletal muscle is fiber type-dependent. J. Neurosci. 19:10694–10705. 10.1523/JNEUROSCI.19-24-10694.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remédio L., Gribble K.D., Lee J.K., Kim N., Hallock P.T., Delestrée N., Mentis G.Z., Froemke R.C., Granato M., and Burden S.J.. 2016. Diverging roles for Lrp4 and Wnt signaling in neuromuscular synapse development during evolution. Genes Dev. 30:1058–1069. 10.1101/gad.279745.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadasivam G., Willmann R., Lin S., Erb-Vögtli S., Kong X.C., Rüegg M.A., and Fuhrer C.. 2005. Src-family kinases stabilize the neuromuscular synapse in vivo via protein interactions, phosphorylation, and cytoskeletal linkage of acetylcholine receptors. J. Neurosci. 25:10479–10493. 10.1523/JNEUROSCI.2103-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J.R., and Lichtman J.W.. 2001. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2:791–805. 10.1038/35097557 [DOI] [PubMed] [Google Scholar]
- Schaeffer L., de Kerchove d’Exaerde A., and Changeux J.P.. 2001. Targeting transcription to the neuromuscular synapse. Neuron. 31:15–22. 10.1016/S0896-6273(01)00353-1 [DOI] [PubMed] [Google Scholar]
- Schmidt N., Basu S., Sladecek S., Gatti S., van Haren J., Treves S., Pielage J., Galjart N., and Brenner H.R.. 2012. Agrin regulates CLASP2-mediated capture of microtubules at the neuromuscular junction synaptic membrane. J. Cell Biol. 198:421–437. 10.1083/jcb.201111130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A.M., Hoppe P., and Burden S.J.. 1992. Spatial restriction of AChR gene expression to subsynaptic nuclei. Development. 114:545–553. [DOI] [PubMed] [Google Scholar]
- Slater C.R. 1982. Postnatal maturation of nerve-muscle junctions in hindlimb muscles of the mouse. Dev. Biol. 94:11–22. 10.1016/0012-1606(82)90063-X [DOI] [PubMed] [Google Scholar]
- Smith C.L., Mittaud P., Prescott E.D., Fuhrer C., and Burden S.J.. 2001. Src, Fyn, and Yes are not required for neuromuscular synapse formation but are necessary for stabilization of agrin-induced clusters of acetylcholine receptors. J. Neurosci. 21:3151–3160. 10.1523/JNEUROSCI.21-09-03151.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel A., Heidmann T., Hofler J., and Changeux J.P.. 1978. Distinct protein components from Torpedo marmorata membranes carry the acetylcholine receptor site and the binding site for local anesthetics and histrionicotoxin. Proc. Natl. Acad. Sci. USA. 75:510–514. 10.1073/pnas.75.1.510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y., Chen F., Liu Y., and Lin W.. 2011. Electrophysiological characterization of neuromuscular synaptic dysfunction in mice. Methods Mol. Biol. 793:391–400. 10.1007/978-1-61779-328-8_26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suozzi K.C., Wu X., and Fuchs E.. 2012. Spectraplakins: master orchestrators of cytoskeletal dynamics. J. Cell Biol. 197:465–475. 10.1083/jcb.201112034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tintignac L.A., Brenner H.R., and Rüegg M.A.. 2015. Mechanisms Regulating Neuromuscular Junction Development and Function and Causes of Muscle Wasting. Physiol. Rev. 95:809–852. 10.1152/physrev.00033.2014 [DOI] [PubMed] [Google Scholar]
- Valdez G., Tapia J.C., Kang H., Clemenson G.D. Jr., Gage F.H., Lichtman J.W., and Sanes J.R.. 2010. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc. Natl. Acad. Sci. USA. 107:14863–14868. 10.1073/pnas.1002220107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.C. 2002. Cellular roles of DNA topoisomerases: a molecular perspective. Nat. Rev. Mol. Cell Biol. 3:430–440. 10.1038/nrm831 [DOI] [PubMed] [Google Scholar]
- Wang J., Jing Z., Zhang L., Zhou G., Braun J., Yao Y., and Wang Z.Z.. 2003. Regulation of acetylcholine receptor clustering by the tumor suppressor APC. Nat. Neurosci. 6:1017–1018. 10.1038/nn1128 [DOI] [PubMed] [Google Scholar]
- Wang S., Reuveny A., and Volk T.. 2015. Nesprin provides elastic properties to muscle nuclei by cooperating with spectraplakin and EB1. J. Cell Biol. 209:529–538. 10.1083/jcb.201408098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Blagden C., Fan J., Nowak S.J., Taniuchi I., Littman D.R., and Burden S.J.. 2005. Runx1 prevents wasting, myofibrillar disorganization, and autophagy of skeletal muscle. Genes Dev. 19:1715–1722. 10.1101/gad.1318305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S.J., and Slater C.R.. 2001. Safety factor at the neuromuscular junction. Prog. Neurobiol. 64:393–429. 10.1016/S0301-0082(00)00055-1 [DOI] [PubMed] [Google Scholar]
- Wu H., Xiong W.C., and Mei L.. 2010. To build a synapse: signaling pathways in neuromuscular junction assembly. Development. 137:1017–1033. 10.1242/dev.038711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Kodama A., and Fuchs E.. 2008. ACF7 regulates cytoskeletal-focal adhesion dynamics and migration and has ATPase activity. Cell. 135:137–148. 10.1016/j.cell.2008.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanashi Y., Tezuka T., and Yokoyama K.. 2012. Activation of receptor protein-tyrosine kinases from the cytoplasmic compartment. J. Biochem. 151:353–359. 10.1093/jb/mvs013 [DOI] [PubMed] [Google Scholar]
- Zhang B., Luo S., Dong X.P., Zhang X., Liu C., Luo Z., Xiong W.C., and Mei L.. 2007a Beta-catenin regulates acetylcholine receptor clustering in muscle cells through interaction with rapsyn. J. Neurosci. 27:3968–3973. 10.1523/JNEUROSCI.4691-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Luo S., Wang Q., Suzuki T., Xiong W.C., and Mei L.. 2008. LRP4 serves as a coreceptor of agrin. Neuron. 60:285–297. 10.1016/j.neuron.2008.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Yue J., and Wu X.. 2017. Spectraplakin family proteins - cytoskeletal crosslinkers with versatile roles. J. Cell Sci. 130:2447–2457. 10.1242/jcs.196154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Xu R., Zhu B., Yang X., Ding X., Duan S., Xu T., Zhuang Y., and Han M.. 2007b Syne-1 and Syne-2 play crucial roles in myonuclear anchorage and motor neuron innervation. Development. 134:901–908. 10.1242/dev.02783 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.